Abstract
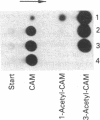
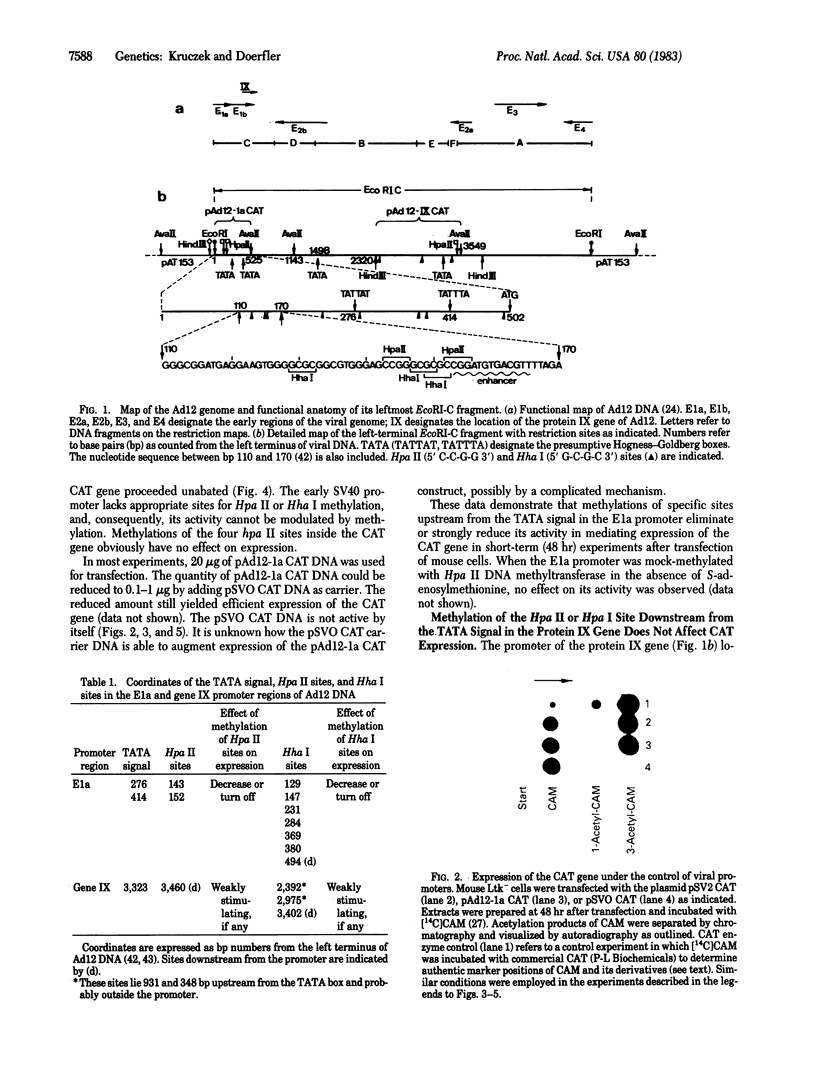
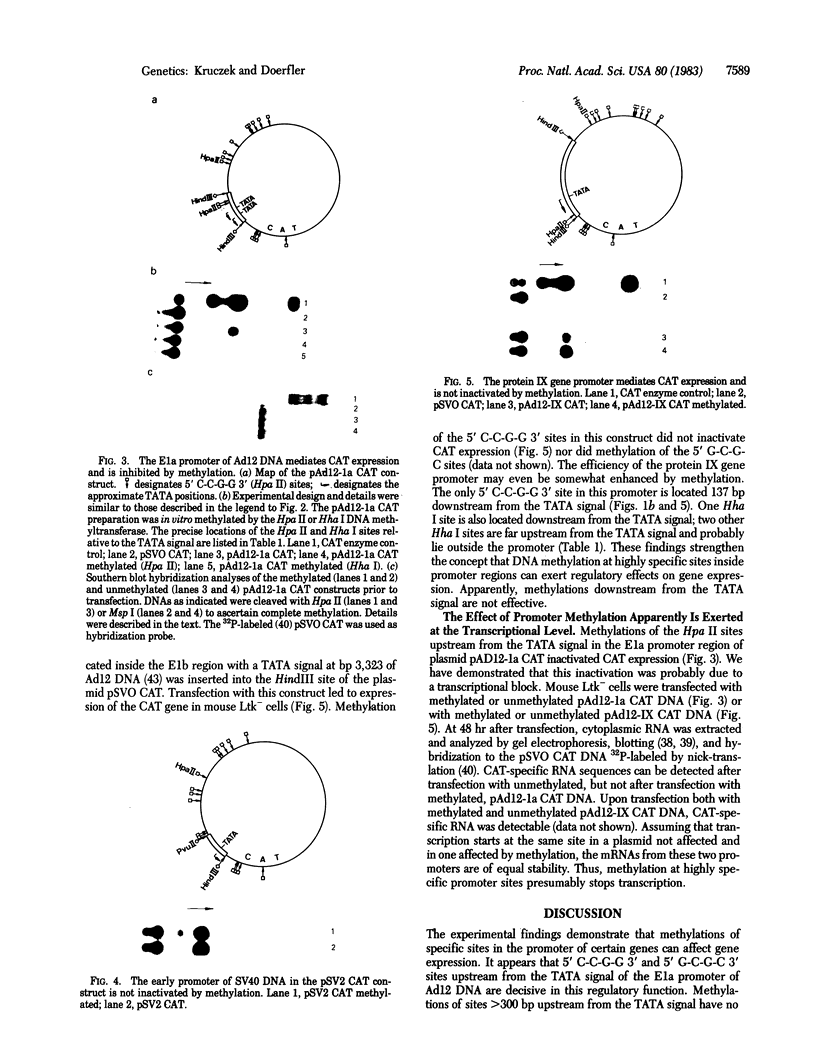
The effect of DNA methylation at specific promoter sites on gene expression was tested by using a sensitive and quantitative assay system. The plasmid pSVO CAT contains the prokaryotic gene chloramphenicol acetyltransferase (CAT) and a HindIII site in front of it for experimental promoter insertion. Upon insertion into pSVO CAT, the E1a and protein IX gene promoters from adenovirus type 12 (Ad12) DNA were capable of mediating CAT expression upon transfection in mouse cells. In many viral and nonviral eukaryotic genes, DNA methylation at highly specific sites in the promoter region can attain a regulatory function in gene expression. One of the important sites is the 5' C-C-G-G 3' sequence. The CAT-promoting activity of the early simian virus 40 promoter in plasmid pSV2 CAT is refractory to methylation by the Hpa II or Hha I DNA methyltransferase at 5' C-C-G-G 3' or 5' G-C-G-C 3' sequences, respectively, because this promoter lacks such sites. The CAT coding sequence of this plasmid carries four Hpa II and no Hha I sites. Methylation of the Hpa II sites in the coding region does not affect expression. The E1a promoter of Ad12 DNA comprising the leftmost 525 base pairs of the viral genome carries two 5' C-C-G-G 3' and three 5' G-C-G-C 3' sites upstream from the leftmost "TATA" signal. Methylation of the Hpa II or Hha I sites incapacitates this promoter. The promoter of protein IX gene of Ad12 DNA contains one 5' C-C-G-G 3' and one 5' G-C-G-C 3' site downstream and two 5' G-C-G-C 3' sites greater than 300 base pairs upstream from the TATA motif and probably outside the promoter. The protein IX promoter is not inactivated by methylation of these sites. These data demonstrate that critical 5' methylations in the promoter region decrease or eliminate transcription; methylations of sites too far upstream or probably any sites downstream from the TATA site do not affect expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M., Hurst J., Flavell R. A. DNA methylation and the regulation of globin gene expression. Cell. 1983 Aug;34(1):197–206. doi: 10.1016/0092-8674(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Colby W. W., Chen E. Y., Smith D. H., Levinson A. D. Identification and nucleotide sequence of a human locus homologous to the v-myc oncogene of avian myelocytomatosis virus MC29. Nature. 1983 Feb 24;301(5902):722–725. doi: 10.1038/301722a0. [DOI] [PubMed] [Google Scholar]
- Constantinides P. G., Jones P. A., Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977 May 26;267(5609):364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- Cordingley M. G., Campbell M. E., Preston C. M. Functional analysis of a herpes simplex virus type 1 promoter: identification of far-upstream regulatory sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2347–2365. doi: 10.1093/nar/11.8.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creusot F., Acs G., Christman J. K. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2'-deoxycytidine. J Biol Chem. 1982 Feb 25;257(4):2041–2048. [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation--a regulatory signal in eukaryotic gene expression. J Gen Virol. 1981 Nov;57(Pt 1):1–20. doi: 10.1099/0022-1317-57-1-1. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Stabel S., Ibelgaufts H., Sutter D., Neumann R., Groneberg J., Scheidtmann K. H., Deuring R., Winterhoff U. Selectivity in integration sites of adenoviral DNA. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):551–564. doi: 10.1101/sqb.1980.044.01.057. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Wang R. Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981 Jun 19;212(4501):1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- Everett R. D., Baty D., Chambon P. The repeated GC-rich motifs upstream from the TATA box are important elements of the SV40 early promoter. Nucleic Acids Res. 1983 Apr 25;11(8):2447–2464. doi: 10.1093/nar/11.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin A., Manley J. L., Prives C. L. Methylation of simian virus 40 Hpa II site affects late, but not early, viral gene expression. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5142–5146. doi: 10.1073/pnas.79.17.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973 Aug;54(2):536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M., Mohandas T., Shapiro L. J. Cell cycle-specific reactivation of an inactive X-chromosome locus by 5-azadeoxycytidine. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1215–1219. doi: 10.1073/pnas.79.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Sawada Y., Shinawawa M., Shimizu Y., Shiroki K., Shimojo H., Sugisaki H., Takanami M., Uemizu Y., Fujinaga K. Nucleotide sequence of the transforming early region E1b of adenovirus type 12 DNA: structure and gene organization, and comparison with those of adenovirus type 5 DNA. Nucleic Acids Res. 1981 Dec 11;9(23):6571–6589. doi: 10.1093/nar/9.23.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenow H., Overgaard-Hansen K., Patkar S. A. Proteolytic cleavage fo native DNA polymerase into two different catalytic fragments. Influence of assay condtions on the change of exonuclease activity and polymerase activity accompanying cleavage. Eur J Biochem. 1971 Oct 14;22(3):371–381. doi: 10.1111/j.1432-1033.1971.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Kruczek I., Doerfler W. The unmethylated state of the promoter/leader and 5'-regions of integrated adenovirus genes correlates with gene expression. EMBO J. 1982;1(4):409–414. doi: 10.1002/j.1460-2075.1982.tb01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann I., Doerfler W. Shifts in the extent and patterns of DNA methylation upon explanation and subcultivation of adenovirus type 12-induced hamster tumor cells. Virology. 1982 Apr 15;118(1):169–180. doi: 10.1016/0042-6822(82)90330-0. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., BESSMAN M. J., SIMMS E. S., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. I. Preparation of substrates and partial purification of an enzyme from Escherichia coli. J Biol Chem. 1958 Jul;233(1):163–170. [PubMed] [Google Scholar]
- Ley T. J., DeSimone J., Anagnou N. P., Keller G. H., Humphries R. K., Turner P. H., Young N. S., Keller P., Nienhuis A. W. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982 Dec 9;307(24):1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- Mann M. B., Smith H. O. Specificity of Hpa II and Hae III DNA methylases. Nucleic Acids Res. 1977 Dec;4(12):4211–4221. doi: 10.1093/nar/4.12.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeady M. L., Jhappan C., Ascione R., Vande Woude G. F. In vitro methylation of specific regions of the cloned Moloney sarcoma virus genome inhibits its transforming activity. Mol Cell Biol. 1983 Mar;3(3):305–314. doi: 10.1128/mcb.3.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L. Functional relationships between transcriptional control signals of the thymidine kinase gene of herpes simplex virus. Cell. 1982 Dec;31(2 Pt 1):355–365. doi: 10.1016/0092-8674(82)90129-5. [DOI] [PubMed] [Google Scholar]
- Niwa O., Yokota Y., Ishida H., Sugahara T. Independent mechanisms involved in suppression of the Moloney leukemia virus genome during differentiation of murine teratocarcinoma cells. Cell. 1983 Apr;32(4):1105–1113. doi: 10.1016/0092-8674(83)90294-5. [DOI] [PubMed] [Google Scholar]
- Ott M. O., Sperling L., Cassio D., Levilliers J., Sala-Trepat J., Weiss M. C. Undermethylation at the 5' end of the albumin gene is necessary but not sufficient for albumin production by rat hepatoma cells in culture. Cell. 1982 Oct;30(3):825–833. doi: 10.1016/0092-8674(82)90287-2. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schirm S., Doerfler W. Expression of viral DNA in adenovirus type 12-transformed cells, in tumor cells, and in revertants. J Virol. 1981 Sep;39(3):694–702. doi: 10.1128/jvi.39.3.694-702.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stabel S., Doerfler W. Nucleotide sequence at the site of junction between adenovirus type 12 DNA and repetitive hamster cell DNA in transformed cell line CLAC1. Nucleic Acids Res. 1982 Dec 20;10(24):8007–8023. doi: 10.1093/nar/10.24.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R., Razin A., Cedar H. In vitro methylation of the hamster adenine phosphoribosyltransferase gene inhibits its expression in mouse L cells. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3418–3422. doi: 10.1073/pnas.79.11.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R., Sciaky-Gallili N., Razin A., Cedar H. Pattern of methylation of two genes coding for housekeeping functions. Proc Natl Acad Sci U S A. 1983 May;80(9):2422–2426. doi: 10.1073/pnas.80.9.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugisaki H., Sugimoto K., Takanami M., Shiroki K., Saito I., Shimojo H., Sawada Y., Uemizu Y., Uesugi S., Fujinaga K. Structure and gene organization in the transformed Hind III-G fragment of Ad12. Cell. 1980 Jul;20(3):777–786. doi: 10.1016/0092-8674(80)90324-4. [DOI] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated viral DNA sequences in hamster cells transformed by adenovirus 12. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):565–568. doi: 10.1101/sqb.1980.044.01.058. [DOI] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Kressmann A., Cedar H., Maechler M., Doerfler W. Expression of a cloned adenovirus gene is inhibited by in vitro methylation. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1073–1077. doi: 10.1073/pnas.79.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardimon L., Kuhlmann I., Doerfler W., Cedar H. Methylation of adenovirus genes in transformed cells and in vitro: influence on the regulation of gene expression? Eur J Cell Biol. 1981 Aug;25(1):13–15. [PubMed] [Google Scholar]
- Vogel S., Brötz M., Kruczek I., Neumann R., Eick D., Winterhoff U., Doerfler W. Cloned fragments of human adenovirus type-12 DNA. Gene. 1981 Nov;15(2-3):273–278. doi: 10.1016/0378-1119(81)90136-0. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. MspI, an isoschizomer of hpaII which cleaves both unmethylated and methylated hpaII sites. Nucleic Acids Res. 1978 Sep;5(9):3231–3236. doi: 10.1093/nar/5.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter D. E., Baserga R. Effect of methylation on expression of microinjected genes. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1106–1110. doi: 10.1073/pnas.79.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]