Abstract
Phytopathogenic oomycetes, such as Phytophthora infestans, potentially secrete many RxLR effector proteins into plant cells to modulate plant immune responses and promote colonization. However, the molecular mechanisms by which these RxLR effectors suppress plant immune responses are largely unknown. Here we describe an RxLR effector PITG_22798 (Gene accession: XM_002998349) that was upregulated during early infection of potato by P. infestans. By employment of agroinfiltration, we observed that PITG_22798 triggers cell death in Nicotiana benthamiana. Confocal microscopic examination showed that PITG_22798-GFP (Green Fluorescent Protein) located in the host nucleus when expressed transiently in N. benthamiana leaves. A nuclear localization signal (NLS) domain of PITG_22798 is important for nuclear localization and cell death-inducing activity. Sequence alignment and transient expression showed that PITG_22798 from diverse P. infestans isolates are conserved, and transient expression of PITG_22798 enhances P. infestans colonization of N. benthamiana leaves, which suggests that PITG_22798 contributes to P. infestans infection. PITG_22798-triggered cell death is dependent on SGT1-mediated signaling and is suppressed by the P. infestans avirulence effector 3b (AVR3b). The present research provides a clue for further investigation of how P. infestans effector PITG_22798 associates with and modulates host immunity.
Keywords: cell death, effector, Nicotiana benthamiana, Phytophthora infestans, plant immunity
1. Introduction
Plants are attacked by various pathogens and defend themselves via multiple resistance mechanisms [1,2]. Generally, plant immunity can be divided into two types, pathogen-associated molecular pattern-triggered immunity (PTI) and effector-triggered immunity (ETI) [3]. Fungi and oomycetes can secrete a wide diversity of effectors into host cells to manipulate plant immunity. These effectors are thought to determine the outcome of plant–microbe interactions [4,5].
Two classes of oomycetes effectors target distinct sites in the host plant: apoplastic effectors, such as GIP1, EPI1, and EPICI, are secreted into plant extracellular space, whereas cytoplasmic effectors, such as CRNs (crinkling and necrosis induced protein) and RxLR effectors, are translocated inside the plant cell, where they target different subcellular compartments [6]. CRNs are generally considered as a class of cell death-inducing effectors in plant, such as CRN2 and CRN8 [7], whereas the RxLR type effectors have been shown to manipulate the plant immune network [8,9,10]. RxLR type effectors were classified based on a common sequence pattern identified in oomycete avirulence proteins. They are modular proteins that contain N-terminal signal peptides, RxLR-dEER motif, and diverse and rapidly evolving C-terminal effector domains [11,12]. It is documented that the genomes of P. infestans, P. sojae, P. ramorum, and Hyaloperonospora arabidopsidis have more than 550, 390, 370, and 130 genes, respectively, encoding potential effector proteins with RxLR-dEER motifs [8,10]. Some effectors of fungal pathogens may also contain functional variants of the RxLR motif [13]. The major function of RxLR effectors is believed to help pathogens to colonize plants by interacting with critical host targets to suppress plant basic defense responses [1,14,15,16,17]. For example, a P. infestans RXLR effector PITG_04314 targets plant PP1c isoforms by re-localizing PP1c isoforms from the nucleolus to the host nucleoplasm to promote late blight disease [18].
Increasing evidence indicates that effectors traffic to a range of subcellular localizations in plant cells and target diverse host proteins to execute their functions [6]. For example, the PITG_04097 nuclear localization is required for both suppression of MAMP (microbe-associated molecular pattern) signaling and virulence function [17]. The P. infestans RxLR effector PexRD2 interacts with MAPKKKe in the plant cytosol and specifically inhibits the MAPKKKe-dependent resistance [16]. Many pathogen effector proteins target the nucleus in order to modify host cell physiology, such as CRN8 from P. infestans [7].
During transient Agrobacterium-mediated expression studies of P. infestans RxLR effectors in Nicotiana benthamiana, we found that one RxLR-like effector, PITG_22798 (Gene accession: XM_002998349), could induce cell death. In this work, we found that PITG_22798 was localized in the host nucleus and its induction of cell death in N. benthamiana required nuclear localization. We also discovered that this induced cell death was dependent on the defense regulator SGT1 (suppressor of G2 allele of skp1) and was suppressed by the RxLR effector, avirulence 3b (AVR3b). The work described herein provides important foundations for further dissection of the roles of P. infestans RxLR effector PITG_22798 regulation in plant immunity.
2. Results
2.1. PITG_22798 Is Induced Early during Infection of P. infestans and Promotes Pathogen Colonization
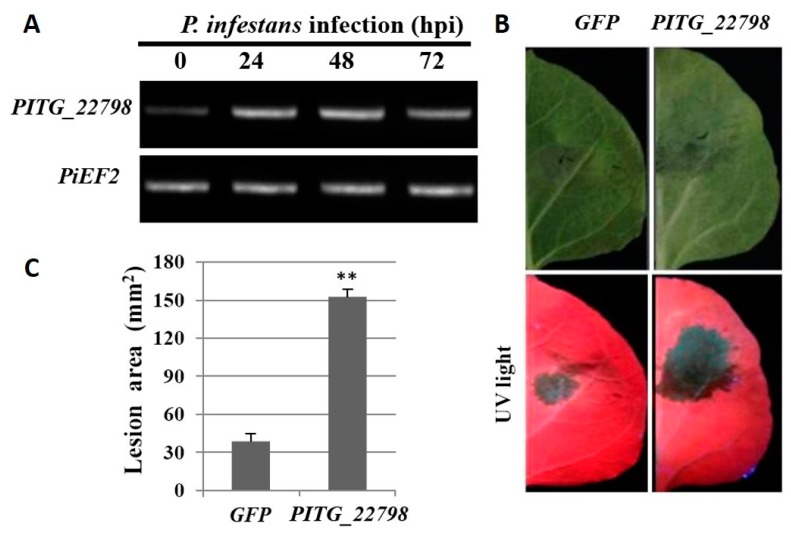
To investigate the expression pattern of PITG_22798 during P. infestans infection, we designed gene-specific primers (Table S1 in Supplementary Material) and performed reverse transcriptase PCR (RT-PCR) using cDNA reverse transcribed from potato leaf RNA isolated at 0, 24, 48, and 72 h after inoculation. The RT-PCR results revealed that PITG_22798 was upregulated at 24 h and 48 h after inoculation of potato plants with P. infestans (Figure 1A). The weak band at 0 h presumably reflects the very lower expression of the PITG_22798 in P. infestans zoospores.
Figure 1.
PITG_22798 is induced during infection of P. infestans and promotes colonization. (A) Semiquantitative RT-PCR was performed to test PITG_22798 expression during P. infestans infection in potato leaves at 0, 24, 48, 72 h post-inoculation (hpi). The elongation factor 2 gene (PiEF2) of P. infestans was used as a control to equalize cDNA amounts. (B) A typical leaf with larger lesions on the half of the leaf expressing PITG_22798 in N. benthamiana. Agrobacterium was used to transiently express PITG_22798 on one half of a leaf and GFP (Green Fluorescent Protein) on the other. Leaves were subsequently infected with P. infestans. The pictures were taken at 5 days after inoculation under white light (top) and UV light (bottom). The OD600 of Agrobacterium for transient express of PITG_22798 is 0.05. (C) Lesion area at 5 d after zoospore inoculation following infection of GFP- or PITG_22798-expressing leaf tissue with P. infestans 88069. Results are the mean ± SE of infections from three biological replicates using at least nine leaves each. The asterisk indicates a value significantly different from the GFP (p < 0.01, t-test).
To test whether PITG_22798 contributes to the virulence of P. infestans, we transiently expressed PITG_22798 in N. benthamiana followed by P. infestans inoculation. The graph in Figure 1B,C shows an increased P. infestans lesion size in the presence of the PITG_22798 relative to lesions occurring with the GFP (Green Fluorescent Protein gene) control, indicating that PITG_22798 enhances P. infestans leaf colonization.
2.2. PITG_22798 Causes Cell Death in N. benthamiana
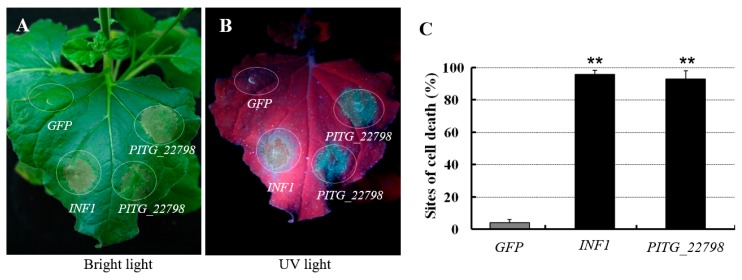
We noted that when we transiently expressed the PITG_22798 in N. benthamiana by agroinfiltration, cell death occurred at 6 days post-infiltration (dpi). The cell death observed was associated with accumulation of autofluorescent compounds. To better visualize the accumulation of such compounds, we examined the inoculated sites under UV light. Similar to INF1 (positive control), PITG_22798 resulted in increased autofluorescence in dead and dying cells, whereas GFP (negative control) did not lead to necrosis or autofluorescence in N. benthamiana (Figure 2). We also expressed PITG_22798 in two wild potato species, S. chacoense and S. hjertingii, by PVX (potato virus X vector)-agroinfection assay, and in N. tabacum leaves by agroinfiltration. The results revealed that expression of PITG_22798 induced cell death in N. tabacum but not in potato species (Figure S1), suggesting that PITG_22798-induced cell death may not be toxic-like due to the artificially high expression. Overall, the above data suggest that PITG_22798 could induce cell death in two tested Nicotiana species.
Figure 2.
PITG_22798-induced cell death in Nicotiana benthamiana. Images show cell death induced by PITG_22798 under white light (A) and UV light (B). Plant leaves were infiltrated with A. tumefaciens cells containing a potato virus X (PVX) vector carrying genes INF1 (positive control, OD600 = 0.4), PITG_22798 (OD600 = 0.4), or GFP (Green Fluorescent Protein) (negative control, OD600 = 0.4). All pictures were taken at 6 days post-infiltration (dpi). (C) The graph shows the percentage of inoculation sites showing cell death. Three N. benthamiana plants and four leaves per plant were used for each test. Values are means ± SD. The experiment has been repeated three times with similar results. Asterisks denote values significantly different from GFP (p < 0.01, t-test).
2.3. PITG_22798 Is Conserved in P. infestans Isolates
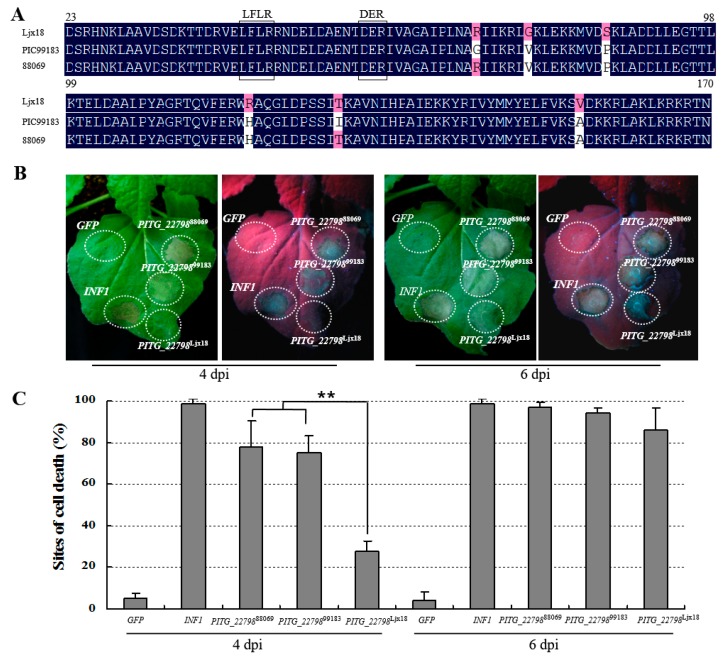
PITG_22798 encodes a protein of 170 amino acids (aa) with a signal peptide from 1–22 aa. It contains the RXLR-like motifs: LFLR-DER. To study the diversity of PITG_22798, its orthologues were cloned from 11 diverse P. infestans isolates, namely EC1, Ljx18, IPO-C, UK3928A, HB09-23, HB09-16-2, HB09-14-2, HB09-21, HB09-41, 88069, and PIC99183 (Table S2). Among 11 cloned sequences, 9 sequences are identical while the other 2 from Ljx18 and 99183 had only 4 and 2 aa differences, respectively. The alignment of predicted amino acid sequences showed a high conservation with 96% similarity (Figure 3A).
Figure 3.
Polymorphisms of PITG_22798 and the cell death induce ability. (A) Alignment of PITG_22798 orthologues from 11 P. infestans isolates. The amino acid sequences of other 9 orthologues are same as that from 88069. ClustalW was used to make the alignment. Polymorphic amino acids were marked with red. (B) PITG_22798Ljx18 induces a delayed cell death in N. benthamiana. A. tumefaciens containing GFP, INF1, PITG_2279888069, PITG_2279899183, or PITG_22798Ljx18 was infiltrated in N. benthamiana leaves with an OD600 of about 0.4. GFP was used as a negative control while INF1 was used as a positive control. PITG_2279888069 and PITG_2279899183 induce cell death at both 4 dpi and 6 dpi, while PITG_22798Ljx18 only induces cell death at 6 dpi. (C) Graphs show the cell death percentages. Six N. benthamiana plants and four leaves per plant were used for each test. Data were collected from 72 infiltration sites based on three independent experiments. Values are means ± SD. Asterisks denote values significantly different from the PITG_22798Ljx18 (p < 0.01, t-test). The experiment has been repeated three times with similar results.
To investigate whether sequence polymorphisms of PITG_22798 affect its ability to trigger cell death, PITG_22798 orthologues from P. infestans isolates 88069 (PITG_2279888069), 99183 (PITG_2279899183), and Ljx18 (PITG_22798Ljx18) were transiently expressed in N. benthamiana. The results showed that all of them triggered cell death, while a delayed symptom was observed with the orthologue from Ljx18 (Figure 3B,C).
2.4. Effector PITG_22798 Functions in the Nucleus to Induce Cell Death
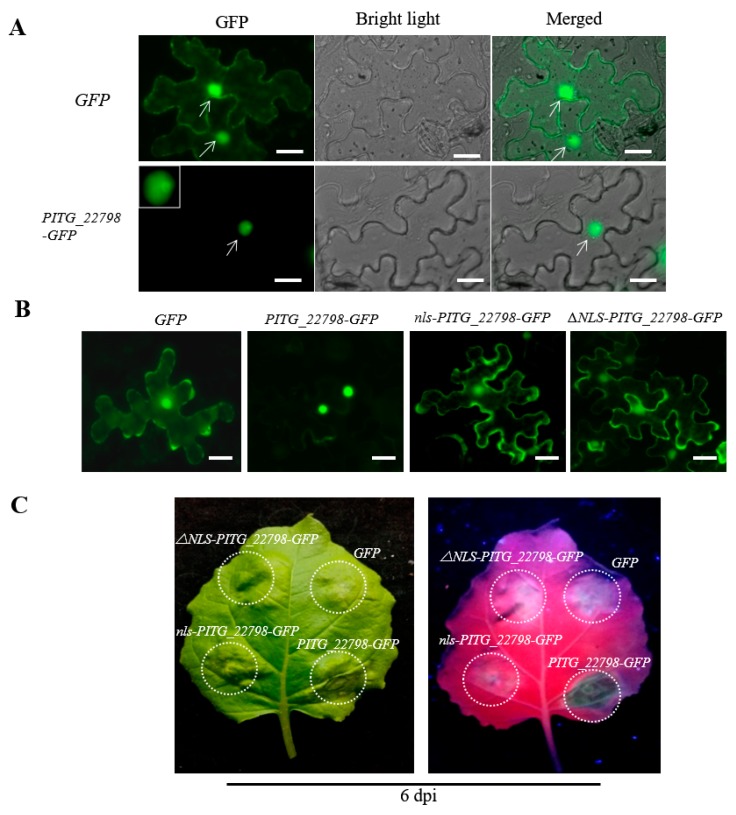
GFP was fused to the C-terminus of PITG_22798 (23–170, without signal peptide) and transiently expressed in N. benthamiana by agroinfiltration. To avoid quick cell death, a low-concentration agrobacteria suspension (OD600 = 0.01) was used for agroinfiltration. The results showed that PITG_22798-GFP localizes to the nucleus (Figure 4A). Meanwhile, immunoblots demonstrated that GFP and PITG_22798-GFP fusion proteins were intact (Figure S2), indicating that the fluorescence accurately reflects the localization of PITG_22798. These results strongly suggest that PITG_22798 is a nuclear localized effector.
Figure 4.
Localization and functional characterization of a nuclear localization signal (NLS) motif for PITG_22798. (A) PITG_22798-GFP fusion protein and control GFP were expressed in N. benthamiana leaves. Photos show confocal images of epidermal cells at 36 h after agroinfiltration. White arrows indicate the nucleus. PITG_22798-GFP is clearly visible in the nucleus (arrow). Left GFP channel, middle bright field channel, right GFP, and bright field channels merged. The white scale bars represent 20 μm. (B) Overview of the mutant construct. nls-PITG_22798-GFP, ΔNLS-PITG_22798-GFP, PITG_22798-GFP and control GFP were transiently expressed in N. benthamiana leaves using agroinfiltration. Confocal microscopy was used to investigate fusion protein distributions. The epidermal cells transformed by nls-PITG_22798-GFP and ΔNLS-PITG_22798-GFP showed the same amount of cytosolic fluorescence as the control GFP. Photos show confocal images of mesophyll cells at 36 h after agroinfiltration. The white scale bars represent 20 µm. The OD600 of the Agrobacterium culture for localization was 0.01. (C) Expression of PITG_22798 and mutants in N. benthamiana leaves by agroinfiltration. The OD600 of the Agrobacterium culture for infiltration was 0.4. Photographs of symptoms were taken 6 d after infiltration. Three repeats show similar results. Each repeat includes 24 infiltration sites on 6 N. benthamiana plants (4 leaves per plant).
A predicted nuclear localization signal (NLS) motif (157-KKRLAKLKRKR-167) was located in the C-terminus of PITG_22798. We mutated the NLS of PITG_22798 (nls-PITG_22798) and found that the NLS mutant (23–170, 157-KKRLAKLKAAA-167) and ΔNLS-PITG_22798 (23–170, deleted NLS) mainly accumulated in the cytoplasm rather than nucleus (Figure 4B). It was also observed that the NLS mutant and ΔNLS-PITG_22798 could not induce cell death (Figure 4C), indicating that nuclear localization is required for PITG_22798 to trigger cell death.
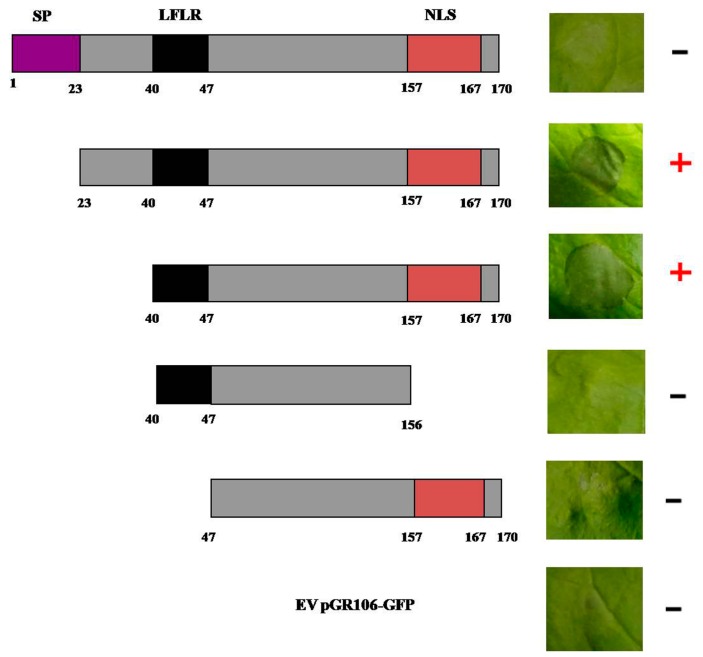
To further dissect the function of PITG_22798, we employed four deletion constructs of PITG_22798 and tested their ability to induce cell death in N. benthamiana (Figure 5). These experiments indicated that a region from 40 to 170 aa of PITG_22798 was necessary for triggering cell death. Deleted LFLR or NLS domain of PITG_22798 lost the ability to induce cell death in N. benthamiana (Figure 5). The results further revealed that both the RxLR-like motif (43-LFLR-47) and the predicted C-terminal NLS (157-KKRLAKLKRKR-167) were important domains for cell death induction.
Figure 5.
Deletion analysis of PITG_22798. Deletion mutants of PITG_22798 were transiently expressed in N. benthamiana by agroinfiltration. A schematic view of the different mutants and deletion constructs are shown on the left panel. Symptoms of infiltration sites are shown on the right panel. Plus (+) and minus (−) signs indicate the presence or absence, respectively, of cell death symptoms. The assays were repeated at least three times with similar results. Photograph of symptoms were taken at 6 dpi. SP, signal peptide. LFLR, an RxLR-like motif. NLS, nuclear localization signal sequence.
2.5. PITG_22798-Mediated Cell Death Requires SGT1, but Not HSP90
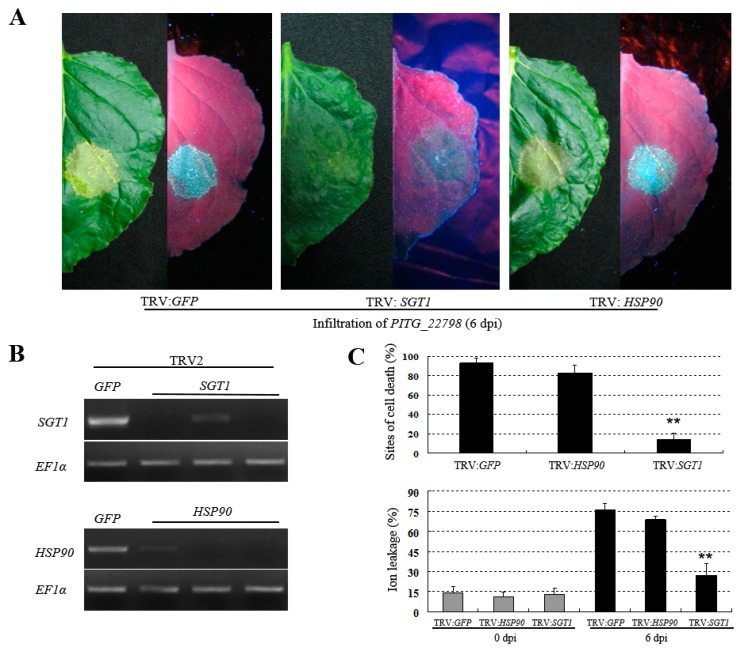
Heat shock protein 90 (HSP90) and SGT1 are considered components of signal-transduction pathways leading to cell death mediated by many NB-LRRs (nucleotide-binding site leucine-rich repeats) cell death. Here, we tested whether SGT1 and HSP90 are required for PITG_22798-induced cell death using virus-induced gene silencing (VIGS). Three weeks after infiltration of silencing constructs, newly grown developed leaves were infiltrated with A. tumefaciens strains containing PITG_22798 (Figure 6A). We confirmed that the SGT1 and HSP90 transcripts were reduced in the silenced plants compared with negative control infiltrated with tobacco rattle virus (TRV)-GFP (Figure 6B). Percentages of cell death were recorded at 6 dpi (days post-infiltration). In SGT1-silenced plants, less than 20% of PITG_22798-inoculated sites exhibited cell death, whereas over 80% of GFP- or HSP90-silenced leaves showed cell death (Figure 6C, upper part). This observation was verified by ion leakage analysis, which indicated a significant decline in percentage of total conductivity of SGT1-silenced leaves compared with GFP- or HSP90-silenced leaves (Figure 6C, lower part). These results suggested that cell death triggered by PITG_22798 in N. benthamiana requires the SGT1-mediated signaling pathway rather than the HSP90-mediated signaling pathway.
Figure 6.
SGT1 (suppressor of G2 allele of skp1) is required for PITG_22798-induced cell death. (A) Representative symptoms of PITG_22798-induced cell death on SGT1- and HSP90-silenced N. benthamiana leaves at 6 d post-infiltration. (B) RT-PCR was done to test the silencing efficiency of SGT1 and HSP90. EF1α was used to confirm equal total RNA amounts among samples. (C) Quantification of cell death and ion leakage on SGT1- and HSP90-silenced N. benthamiana leaves. Cell death differences were based on three independent experiments scored at 6 dpi (upper panel). Ion leakage was measured at 0 and 6 dpi (lower panel). Results were obtained from 120 infiltration sites based on five independent experiments. Values are means ± SD. Asterisks denote values significantly different from control TRV: GFP (p < 0.01, t-test).
2.6. AVR3b Suppresses PITG_22798-Triggered Cell Death in N. benthamiana
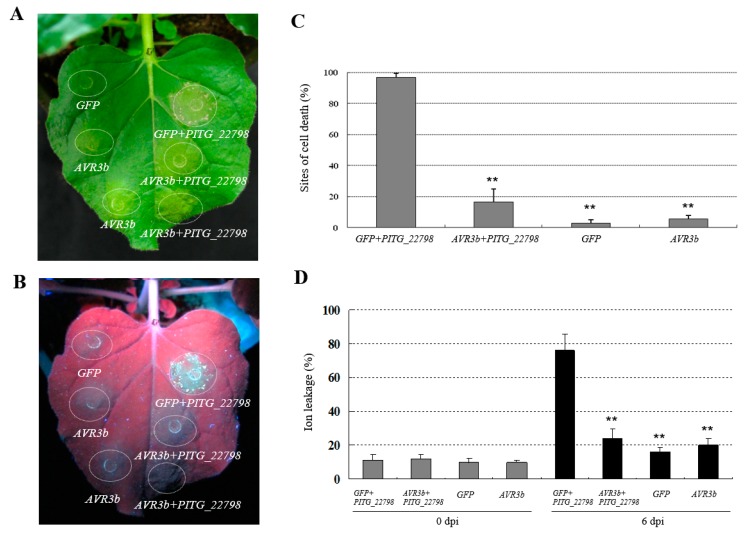
To study whether other RxLR effectors could interfere with PITG_22798-mediated cell death, we selected eight RxLR effectors which were co-agroinfiltrated with PITG_22798 in N. benthamiana. The results obtained at 6 dpi demonstrated that PITG_22798-induced cell death was significantly inhibited when it was coexpressed with AVR3b, whereas other effectors did not interfere with PITG_22798-triggered cell death (Figure S3). Considering percentage of the inoculation sites that showed cell death symptoms and ion leakage by percentage of total conductivity, AVR3b significantly suppressed PITG_22798-induced cell death (Figure 7).
Figure 7.
Avirulence 3b (AVR3b) suppresses the cell death induced by PITG_22798. PITG_22798 and AVR3b were agro-co-infiltrated in N. benthamiana leaves in a 1:1 ratio with a final OD600 of about 0.4. GFP and AVR3b were used as negative controls. Photos were taken at 6 dpi under normal light (A) and UV light (B). (C) PITG_22798-induced cell death is significantly reduced after co-infiltrated with AVR3b. Error bars represent SD. Results were obtained from 36 infiltration sites based on three independent experiments. Asterisks indicate significant difference from positive control (p < 0.01, t-test). (D) PITG_22798-induced ion leakage is significantly reduced after co-infiltration with AVR3b. Error bars represent SD. Asterisks denote values significantly different from positive control (p < 0.01, t-test). Experiments were repeated three times with similar results.
3. Discussion
Cell death plays a ubiquitous role in plant–microbe interactions and can be associated with both susceptible and resistance responses [19]. However, less is known about RxLR effectors of P. infestans that could directly induce cell death in N. benthamiana. Until now, only one example has been reported. PexRD2 is such an RxLR effector that can trigger cell death in N. benthamiana, and also can interact with host MAPKKK to perturb plant immunity [16,20]. In this work, we identified an RxLR-like effector PITG_22798 which can trigger cell death in N. benthamiana (Figure 2) and tobacco. However, expression of PITG_22798 in potato did not lead to cell death. Cell death is thus unlikely to be associated with the effector function of PITG_22798. Some RxLR effectors have been shown to act as avirulence (AVR) factors, triggering the ETI pathway due to recognition by cognate R proteins [2,12,21]. The cell death triggered by PITG_22798 may result from recognition by an unknown R protein in N. benthamiana. This is supported by the fact that PITG_22798-triggered cell death is dependent on SGT1, which is required for many R proteins to function. Even if this is in accordance with the view that N. benthamiana possibly possesses a broad-spectrum R protein against P. infestans [22], further experiments are needed to support the hypothesis.
The nucleus is an activity center for both PTI and ETI, and many critical regulators are trafficked there from various subcellular locations following pathogen perception [23,24]. By fusing it to GFP, we showed that PITG_22798 is a nuclear-localized protein (Figure 4A). Moreover, PITG_22798 requires a functional NLS for nuclear accumulation and cell death induction (Figure 4B and Figure 5). Therefore, our results suggest that PITG_22798 may implement its function in the nucleus.
Both SGT1 and HSP90 are critical signaling components required for R gene-mediated resistance in several plant species against various plant pathogens, including fungi and bacteria [25]. These proteins also play a role in stabilization of R proteins [26,27,28]. Additionally, non-RxLR effectors INF1- or INF2A-mediated cell death were also suppressed when NbSGT1 was silenced in N. benthamiana, indicating that SGT1 function is not only limited to the NB-LRR proteins but also required for the immune response that is triggered by non-NB-LRR-type proteins [26,29]. Our findings demonstrated that SGT1, rather than HSP90, is required for PITG_22798-triggered cell death in N. benthamiana (Figure 6). This finding is similar to the report that AVR-blb2, another P. infestans RxLR effector, triggered cell death, which needs SGT1 rather than HSP90 in the presence of the cognate Rpi-blb2 protein [28]. SGT1 was reported to act as an adaptor of plant NLR (nucleotide-binding domain- and leucine-rich repeat-containing) proteins (including R proteins) and protect them from degradation [27]. Although the question of how SGT1 regulates PITG_22798 cell death induction could not be addressed, we hypothesize that NbSGT1 may be required for a cognate R protein that recognizes PITG_22798 in N. benthamiana.
Hundreds of predicted RxLR effector-encoding genes exist in the genomes of sequenced oomycete plant pathogens. Surveys of Phytophthora RxLR effectors identified many that could suppress cell death and a few that could induce cell death, such as cell death inducers Avh147, Avh238, and Avh241 [30,31]. Many of the suppressors, such as AVR3aKI, AVR3b, and PITG_02680 of P. infestans, and Avr1b and Avh331 of P. sojae, can suppress cell death activated by PTI [17,18,32,33]. Here, we showed that AVR3b can suppress the cell death triggered by PITG_22798 (Figure 7 and Figure S3). It has been reported that AVR3b, as an avirulence factor, can trigger cell death due to recognition by the cognate R3b protein [34]. It also, as a virulence factor, can suppress INF1-triggered cell death and promote P. infestans infection in N. benthamiana [17]. AVR3a and AVR3b can all suppress INF1-triggered cell death [17]. However, only AVR3b can suppress PITG_22798-induced cell death. Therefore, it is possible that PITG_22798 cell death is a different pathway to INF1 cell death. It is also possible that AVR3b has more than one virulence function. It remains unclear, however, whether AVR3b directly blocks recognition of PITG_22798, or impacts on the expression, import, and stability of PITG_22798, or by another mode that may associate with different subcellular localization of PITG_22798 (nucleus) and AVR3b (cytosol and plasma membrane). A possible explanation could be that AVR3b may interfere with PITG_22798 through an intermediary signaling pathway that is required for PITG_22798 to trigger cell death, which merits further investigation. Further studies are needed to clarify the molecular mechanism underlying how AVR3b suppresses PITG_22798-mediated cell death. However, the fact that it is able to suppress this cell death explains why N. benthamiana is not resistant to P. infestans, and also why PITG_22798 is able to enhance P. infestans colonization (Figure 1B,C), which is likely associated with its virulence function.
4. Materials and Methods
4.1. Microbial Strains, Plants, and Culture Conditions
Escherichia coli DH5α was cultured at 37 °C in LB (Luria-Bertani) medium and used for cloning and propagation of recombinant plasmids. Agrobacterium tumefaciens strain GV3101 used for transient expression was cultured at 28 °C in YEB (Yeast Extract Broth) medium using appropriate antibiotics. P. infestans isolates used in this study are shown in Supplementary Material, Table S2. All isolates were cultured on Rye agar medium at 22 °C. Plants of N. benthamiana, N. tabacum, S. chacoense, S. hjertingii, and a Chinese potato variety “E-potato 3” (S. tuberosum cv.) were grown at 25 °C in greenhouse under 16/8 h light/dark photoperiod.
4.2. Construction of Plasmids
The deletion mutants were obtained by PCR amplifications using appropriate primers (Table S1). For protein fusions, PITG_22798 (23–170 aa, without signal peptide) was cloned from plasmid containing PITG_22798 using Gateway technology (Invitrogen, Carlsbad, CA, USA) with specific primers shown in Table S1. PITG_22798 was inserted into pK7FWG2 vector to produce C-terminal GFP fusion vector, which was then transformed into the A. tumefaciens strain GV3101.
To clarify whether other RxLR effectors could interfere with PITG_22798-triggered cell death, eight RxLR effector genes were cloned and constructed into pGR106 vector (Table S3). Eight RxLR effectors of P. infestans included AVR3aKI [35], AVR2 [36], AVR3b [34], PITG_21388 [37], PITG_14783, PITG_20303, and PITG_13959 [17], and PITG_23008 [10]. Primers used for plasmid construction and other constructs used in this study are documented in the Table S1. All above plasmids were validated by sequencing in Shanghai Sunny Biotechnology Co., Ltd. (Shanghai, China).
4.3. Expression Analysis of PITG_22798 and P. infestans Infection Assay
Detached leaves of potato (“E-potato-3”) of 8-week-old plants were inoculated with 10 µL zoospores from P. infestans isolate HB0914-2 at 5 × 104 mL−1 using the method of Vleeshouwers et al. [38]. Leaf discs (1 cm diameter) of equal sizes surrounding the inoculation sites were collected at 0, 24, 48, and 72 h after inoculation and frozen in liquid nitrogen for immediate use or stored at −80 °C for RNA extraction. The RNA extraction and cDNA synthesis were done as previously described [39]. Reverse transcriptase PCR (RT-PCR) was used to examine PITG_22798 expression at different time points. The constitutively expressed PiEF2 (Gene accession: XM_002901697.1) was used as a reference for equalizing cDNA amounts in each reaction. The RT-PCR primers used are listed in Table S1.
Agrobacterium tumefaciens transient assays (ATTA) in combination with P. infestans infection were carried out as described [15]. Briefly, Agrobacterium cultures were resuspended in agroinfiltration medium at a final concentration of OD600 = 0.05 and used for transient expression in N. benthamiana by agroinfiltration. After 1 day, each infiltration site was inoculated with 10 µL zoospores from P. infestans isolate 88069 at 5 × 104 mL−1. Lesions were measured and photographed at 5 days postinfection and data was collected from three biological replicates (each replicate with at least nine leaves).
4.4. Cloning and Sequence Analysis of PITG_22798
For cloning of PITG_22798, P. infestans genomic DNA was used as templates. P. infestans mycelia were scraped from the rye agar medium surface after cultured for about 14 d for DNA isolation using the DNA isolation kit (Sangon Biotech, Shanghai, China). High-fidelity Pfu polymerase (Vazyme Biotech Co, Ltd., Nanjing, China) was used for amplification of PITG_22798 orthologues (without signal peptide) and the primers are shown in Table S1. The PCR products were digested with both ClaI and NotI and ligated into a binary vector pGR106 vector (potato virus X vector), which was then transformed into A. tumefaciens strain GV3101 for Agrobacterium-mediated transient expression as described by Du et al. [40]. All the above plasmids were validated by sequencing in Shanghai Sunny Biotechnology Co., Ltd. (Shanghai, China).
The sequence analysis of PITG_22798 was conducted with ClustalW (http://clustalw.ddbj.nig. ac.jp/index.php?lang=ja) to create multiple sequence alignments, which were then manually adjusted to minimize the number of implied mutations. SignalP 4.1 server (http://www.cbs.dtu.dk/ services/SignalP/) was used for discriminating signal peptides. Sequence translation was done at http://web.expasy.org/translate/. NLS sequence domain of PITG_22798 was predicted by NLStradamus with a prediction cutoff value of 0.6. NLStradamus uses hidden Markov models (HMMs) (http://www.moseslab.csb.utoronto.ca/NLStradamus/) to predict novel NLSs in proteins [41].
4.5. Agroinfiltration and PVX-Agroinfection Assay
Agroinfiltration in N. benthamiana and PVX (potato virus X vector)-agroinfection in potato were performed as described elsewhere [40]. At least six plants were agroinfiltrated for each treatment and four apical expanded leaves were used per plant. The infiltrated plants were maintained in plant growth chambers at 22 °C with a 16/8 h light/dark photoperiod.
To check the different induction of PITG_22798 homologs in N. benthamiana plants, three independent tests were conducted, each contained 24 leaves of 6 plants. The cell death was measured at 4 and 6 dpi and t-test was used for comparison with PITG_22798Ljx18. The number of the positive cell deaths (i.e., more than 50% of the inoculated region produces clear cell death) were counted as described previously [36] and expressed as the mean percentage of total inoculations sites.
To look into the effects of other RxLR type effectors on PITG_22798-induced cell death in N. benthamiana leaves, A. tumefaciens cells carrying the cell death-inducing gene (PITG_22798) and each other RxLR effector genes were mixed in a 1:1 ratio and then infiltrated by following the method mentioned above. Long-wave UV lamp (Black Ray, B-100A, San Gabriel, CA, USA) was used to monitor the autofluorescence of dead cells where phenolic compounds were released.
4.6. Western Blot and Confocal Microscopic Examination
Nicotiana benthamiana leaves infiltrated with A. tumefaciens containing GFP (Green Fluorescent Protein gene) and PITG_22798-GFP were harvested at 36 h post-inoculation (hpi). The stability of GFP fusion protein was tested by Western blot as previously described [20]. Briefly, 100 mg leaf tissue was put into 1 mL protein RIPA lysis buffer. After centrifugation, the protein supernatant was suspended in 10 µL 2× SDS loading buffer, and then loaded onto a 12% SDS-PAGE gel for fractionation. After electrophoresis, gel was blotted onto a PVDF membrane for 1.5 h at 30 V. The membrane was blocked using TBS plus 4% nonfat dry milk (TBSM) for 2 h, followed by addition of the monoclonal GFP antibody (Sigma-Aldrich, St. Louis, MO, USA) at 1:3000 dilution and incubated at room temperature. After 1 h, the membrane was washed with TBS containing 0.05% Tween-20 (TBST) five times, and then the secondary antibody was added at 1:8000 dilution for 1 h at room temperature, followed by five washes. Protein gel-blotting chemiluminescence detection kit (Thermo Scientific Pierce, Rockford, IL, USA) was used according to the manufacturer’s instructions to develop the blots on light-sensitive autoradiograph films.
Subcellular localization of PITG_22798 was detected by confocal microscopy A. tumefaciens (OD600 = 0.01) containing GFP was pressure infiltrated in the left half of a leaf of 4-week-old N. benthamiana, while PITG_22798 was infiltrated in the right half. The infiltrated cells were observed using a LSM510 Meta confocal microscope (Carl Zeiss, Jena, Germany) at 36 hpi. The excitation wavelengths used for GFP was 488 nm.
4.7. Virus-Induced Gene-Silencing (VIGS) Assay
VIGS was performed as previously described [42,43]. TRV-PDS was used as a positive control and TRV-GFP was used as a negative control. At four-leaf stage, N. benthamiana leaves were infiltrated by A. tumefaciens containing TRV-GFP, TRV-PDS, TRV-SGT1, and TRV-HSP90. Three weeks later, when positive control leaves became totally white, RNA was extracted from the leaves using Trizol Reagent (Vazyme Biotech Co, Ltd., Nanjing, China) according to the manufacturer recommendations. Primers RT-NbSGT1 and RT-NbHSP90 were used for RT-PCR (Table S1). EF-1α was used as a control for equalizing cDNA amounts in each reaction.
4.8. Measurement of the Ion Leakage
Ion leakage was measured as previously described [44]. After infiltration, leaf discs (1 cm diameter) were rinsed three times (2–3 min per time) with distilled water and subsequently floated on 10 mL of distilled water. The electrolyte leakage in the solution was measured after 3 h of floating at room temperature using a conductivity meter (METTLER TOLEDO FE30, Zurich, Switzerland). Total conductivity was obtained after keeping the flasks in an oven (90 °C) for 30 min. Results were shown as percentage of total conductivity.
5. Conclusions
In summary, we report a nuclear-localized P. infestans RxLR effector PITG_22798 which could trigger cell death in N. benthamiana and enhance P. infestans colonization. PITG_22798-triggered cell death requires its nuclear localization and can be suppressed by the P. infestans effector AVR3b. PITG_22798-triggered cell death is dependent on the SGT1-mediated signaling pathway. Although the exact mechanisms of signaling crosstalk remain unclear, our results provide useful information for in-depth investigation of how PITG_22798 modulates defense of N. benthamiana. The next challenge is to better understand the role of PITG_22798 and its targets in the progression of disease in important host crop potato. Up to now, we have identified several interacting proteins in potato, which will help us further investigate its function involved in manipulating of potato immunity.
Acknowledgments
We thank Paul Birch for valuable discussion on the research and providing P. infestans isolates EC1_DC2005 and UK3928A, Geert Kessel for providing P. infestans isolates IPO-C and PIC99183. This work was partially supported by the National High Technology Project of China (Grant number 2013AA102603), National Natural Science Foundation of China (Grant number 31171603) and China Agriculture Research System (Grant number CARS-10-P06).
Abbreviations
| PTI | Pathogen-associated molecular pattern triggered immunity |
| ETI | Effector-triggered immunity |
| RT-PCR | Reverse transcriptase PCR |
| VIGS | Virus-induced gene silencing |
| NLS | Nuclear localization signal |
| hpi | Hours post inoculation |
| dpi | Days post infiltration |
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/18/2/409/s1.
Author Contributions
Hongyang Wang performed most experiments and analyzed data; Yajuan Ren did confocal microscopic examination and repeated cell death induction; Juan Du did western blotting for PITG_22798-GFP expression; Jing Zhou and Juan Hou repeated SGT1 VIGS assay; Rui Jiang and Haixia Wang designed experiments in PITG_22798-triggered cell death suppression. Zhendong Tian and Conghua Xie designed experiments, analyzed data and wrote the paper.
Conflicts of Interest
The authors declare no competing interests.
References
- 1.Chisholm S.T., Coaker G., Day B., Staskawicz B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Giraldo M.C., Valent B. Filamentous plant pathogen effectors in action. Nat. Rev. Microbiol. 2013;11:800–814. doi: 10.1038/nrmicro3119. [DOI] [PubMed] [Google Scholar]
- 3.Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 4.Panstruga R., Dodds P.N. Terrific protein traffic: The mystery of effector protein delivery by filamentous plant pathogens. Science. 2009;324:748–750. doi: 10.1126/science.1171652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodds P.N., Rathjen J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010;11:539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 6.Whisson S.C., Boevink P.C., Wang S., Birch P.R. The cell biology of late blight disease. Curr. Opin. Microbiol. 2016;34:127–135. doi: 10.1016/j.mib.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Damme M., Bozkurt T.O., Cakir C., Schornack S., Sklenar J., Jones A.M., Kamoun S. The Irish potato famine pathogen Phytophthora infestans translocates the CRN8 kinase into host plant cells. PLoS Pathog. 2012;8:e1002875. doi: 10.1371/journal.ppat.1002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxter L., Tripathy S., Ishaque N., Boot N., Cabral A., Kemen E., Thines M., Ah-Fong A., Anderson R., Badejoko W., et al. Signatures of adaptation to obligate biotrophy in the Hyaloperonospora arabidopsidis genome. Science. 2010;330:1549–1551. doi: 10.1126/science.1195203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouwmeester K., Meijer H.J., Govers F. At the Frontier; RXLR effectors crossing the Phytophthora-Host interface. Front. Plant Sci. 2011;2:75. doi: 10.3389/fpls.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas B.J., Kamoun S., Zody M.C., Jiang R.H., Handsaker R.E., Cano L.M., Grabherr M., Kodira C.D., Raffaele S., Torto-Alalibo T., et al. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature. 2009;461:393–398. doi: 10.1038/nature08358. [DOI] [PubMed] [Google Scholar]
- 11.Win J., Morgan W., Bos J., Krasileva K.V., Cano L.M., Chaparro-Garcia A., Ammar R., Staskawicz B.J., Kamoun S. Adaptive evolution has targeted the C-terminal domain of the RXLR effectors of plant pathogenic oomycetes. Plant Cell. 2007;19:2349–2369. doi: 10.1105/tpc.107.051037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vleeshouwers V.G., Raffaele S., Vossen J.H., Champouret N., Oliva R., Segretin M.E., Rietman H., Cano L.M., Lokossou A., Kessel G., et al. Understanding and exploiting late blight resistance in the age of effectors. Annu. Rev. Phytopathol. 2011;49:507–531. doi: 10.1146/annurev-phyto-072910-095326. [DOI] [PubMed] [Google Scholar]
- 13.Kale S.D., Gu B., Capelluto D.G., Dou D., Feldman E., Rumore A., Arredondo F.D., Hanlon R., Fudal I., Rouxel T., et al. External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell. 2010;142:284–295. doi: 10.1016/j.cell.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Schornack S., Huitema E., Cano L.M., Bozkurt T.O., Oliva R., Van Damme M., Schwizer S., Raffaele S., Chaparro-Garcia A., Farrer R., et al. Ten things to know about oomycete effectors. Mol. Plant Pathol. 2009;10:795–803. doi: 10.1111/j.1364-3703.2009.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLellan H., Boevink P.C., Armstrong M.R., Pritchard L., Gomez S., Morales J., Whisson S.C., Beynon J.L., Birch P.R. An RxLR effector from Phytophthora infestans prevents relocalisation of two plant NAC transcription factors from the endoplasmic reticulum to the nucleus. PLoS Pathog. 2013;9:e1003670. doi: 10.1371/journal.ppat.1003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King S.R., McLellan H., Boevink P.C., Armstrong M.R., Bukharova T., Sukarta O., Win J., Kamoun S., Birch P.R., Banfield M.J. Phytophthora infestans RXLR effector PexRD2 interacts with host MAPKKK epsilon to suppress plant immune signaling. Plant Cell. 2014;26:1345–1359. doi: 10.1105/tpc.113.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng X., McLellan H., Fraiture M., Liu X., Boevink P.C., Gilroy E.M., Chen Y., Kandel K., Sessa G., Birch P.R., et al. Functionally redundant RXLR effectors from Phytophthora infestans act at different steps to suppress early flg22-triggered immunity. PLoS Pathog. 2014;10:e1004057. doi: 10.1371/journal.ppat.1004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boevink P.C., Wang X., McLellan H., He Q., Naqvi S., Armstrong M.R., Zhang W., Hein I., Gilroy E.M., Tian Z., et al. A Phytophthora infestans RXLR effector targets plant PP1c isoforms that promote late blight disease. Nat. Commun. 2016;7:10311. doi: 10.1038/ncomms10311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanneganti T.D., Huitema E., Cakir C., Kamoun S. Synergistic interactions of the plant cell death pathways induced by Phytophthora infestans Nepl-like protein PiNPP1.1 and INF1 elicitin. Mol. Plant Microbe Interact. 2006;19:854–863. doi: 10.1094/MPMI-19-0854. [DOI] [PubMed] [Google Scholar]
- 20.Oh S.K., Young C., Lee M., Oliva R., Bozkurt T.O., Cano L.M., Win J., Bos J.I., Liu H.Y., van Damme M., et al. In planta expression screens of Phytophthora infestans RXLR effectors reveal diverse phenotypes, including activation of the Solanum bulbocastanum disease resistance protein Rpi-blb2. Plant Cell. 2009;21:2928–2947. doi: 10.1105/tpc.109.068247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dangl J.L., Jones J.D. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 22.Shibata Y., Kawakita K., Takemoto D. SGT1 and HSP90 are essential for age-related non-host resistance of Nicotiana benthamiana against the oomycete pathogen Phytophthora infestans. Physiol. Mol. Plant Pathol. 2011;75:120–128. doi: 10.1016/j.pmpp.2010.10.001. [DOI] [Google Scholar]
- 23.Rivas S. Nuclear dynamics during plant innate immunity. Plant Physiol. 2012;158:87–94. doi: 10.1104/pp.111.186163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deslandes L., Rivas S. The plant cell nucleus. Plant Signal Behav. 2011;6:42–48. doi: 10.4161/psb.6.1.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin M.J., Muskett P., Kahn K., Feys B.J., Jones J.D., Parker J.E. Regulatory role of SGT1 in early R gene-mediated plant defenses. Science. 2002;295:2077–2080. doi: 10.1126/science.1067747. [DOI] [PubMed] [Google Scholar]
- 26.Peart J.R., Lu R., Sadanandom A., Malcuit I., Moffett P., Brice D.C., Schauser L., Jaggard D.A., Xiao S., Coleman M.J. Ubiquitin ligase-associated protein SGT1 is required for host and nonhost disease resistance in plants. Proc. Natl. Acad. Sci. USA. 2002;99:10865–10869. doi: 10.1073/pnas.152330599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadota Y., Shirasu K., Guerois R. NLR sensors meet at the SGT1-HSP90 crossroad. Trends Biochem. Sci. 2010;35:199–207. doi: 10.1016/j.tibs.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Oh S.K., Kim H., Choi D. Rpi-blb2-mediated late blight resistance in Nicotiana benthamiana requires SGT1 and salicylic acid-mediated signaling but not RAR1 or HSP90. FEBS Lett. 2014;588:1109–1115. doi: 10.1016/j.febslet.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 29.Huitema E.V., Vleeshouwers V.G., Cakir C., Kamoun S., Govers F. Differences in intensity and specificity of hypersensitive response induction in Nicotiana spp. by INF1, INF2A, and INF2B of Phytophthora infestans. Mol. Plant Microbe Interact. 2005;18:183–193. doi: 10.1094/MPMI-18-0183. [DOI] [PubMed] [Google Scholar]
- 30.Yu X., Tang J., Wang Q., Ye W., Tao K., Duan S., Lu C., Yang X., Dong S., Zheng X., et al. The RxLR effector Avh241 from Phytophthora sojae requires plasma membrane localization to induce plant cell death. New Phytol. 2012;196:247–260. doi: 10.1111/j.1469-8137.2012.04241.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q., Han C., Ferreira A.O., Yu X., Ye W., Tripathy S., Kale S.D., Gu B., Sheng Y., Sui Y., et al. Transcriptional programming and functional interactions within the Phytophthora sojae RXLR effector repertoire. Plant Cell. 2011;23:2064–2086. doi: 10.1105/tpc.111.086082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L., McLellan H., Naqvi S., He Q., Boevink P.C., Armstrong M., Giuliani L.M., Zhang W., Tian Z., Zhan J., et al. Potato NPH3/RPT2-Like Protein StNRL1, targeted by a Phytophthora infestans RXLR effector, is a susceptibility factor. Plant Physiol. 2016;171:645–657. doi: 10.1104/pp.16.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dou D., Kale S.D., Wang X., Chen Y., Wang Q., Wang X., Jiang R.H., Arredondo F.D., Anderson R.G., Thakur P.B., et al. Conserved C-terminal motifs required for avirulence and suppression of cell death by Phytophthora sojae effector Avr1b. Plant Cell. 2008;20:1118–1133. doi: 10.1105/tpc.107.057067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G., Huang S., Guo X., Li Y., Yang Y., Guo Z., Kuang H., Rietman H., Bergervoet M., Vleeshouwers V.G. Cloning and characterization of R3b; members of the R3 superfamily of late blight resistance genes show sequence and functional divergence. Mol. Plant Microbe Interact. 2011;24:1132–1142. doi: 10.1094/MPMI-11-10-0276. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong M.R., Whisson S.C., Pritchard L., Bos J.I. B., Venter E., Avrova A.O., Rehmany A.P., Bohme U., Brooks K., Cherevach I., et al. An ancestral oomycete locus contains late blight avirulence gene Avr3a, encoding a protein that is recognized in the host cytoplasm. Proc. Natl. Acad. Sci. USA. 2005;102:7766–7771. doi: 10.1073/pnas.0500113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilroy E.M., Breen S., Whisson S.C., Squires J., Hein I., Kaczmarek M., Turnbull D., Boevink P.C., Lokossou A., Cano L.M., et al. Presence/absence, differential expression and sequence polymorphisms between PiAVR2 and PiAVR2-like in Phytophthora infestans determine virulence on R2 plants. New Phytol. 2011;191:763–776. doi: 10.1111/j.1469-8137.2011.03736.x. [DOI] [PubMed] [Google Scholar]
- 37.Halterman D.A., Chen Y., Sopee J., Berduo-Sandoval J., Sanchez-Perez A. Competition between Phytophthora infestans effectors leads to increased aggressiveness on plants containing broad-spectrum late blight resistance. PLoS ONE. 2010;5:e10536. doi: 10.1371/journal.pone.0010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vleeshouwers V.G., Dooijeweert W.V., Keizer L.C. P., Sijpkes L., Govers F., Colon L.T. A laboratory assay for Phytophthora infestans resistance in various Solanum species reflects the field situation. Eur. J. Plant Pathol. 1999;105:241–250. doi: 10.1023/A:1008710700363. [DOI] [Google Scholar]
- 39.Wang H., Sun C., Jiang R., He Q., Yang Y., Tian Z., Tian Z., Xie C. The dihydrolipoyl acyltransferase gene BCE2 participates in basal resistance against Phytophthora infestans in potato and Nicotiana benthamiana. J. Plant Physiol. 2014;171:907–914. doi: 10.1016/j.jplph.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Du J., Rietman H., Vleeshouwers V.G. Agroinfiltration and PVX agroinfection in potato and Nicotiana benthamiana. J. Vis. Exp. 2014:e50971. doi: 10.3791/50971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen Ba A.N., Pogoutse A., Provart N., Moses A.M. NLStradamus: A simple hidden markov model for nuclear localization signal prediction. BMC Bioinform. 2009;10:202. doi: 10.1186/1471-2105-10-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du J., Tian Z., Liu J., Vleeshouwers V.G., Shi X., Xie C. Functional analysis of potato genes involved in quantitative resistance to Phytophthora infestans. Mol. Biol. Rep. 2013;40:957–967. doi: 10.1007/s11033-012-2137-3. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y., Schiff M., Dinesh-Kumar S.P. Virus-induced gene silencing in tomato. Plant J. 2002;31:777–786. doi: 10.1046/j.1365-313X.2002.01394.x. [DOI] [PubMed] [Google Scholar]
- 44.Campos P.S., Quartin V., Ramalho J.C., Nunes M.A. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. Plant Physiol. 2003;160:283–292. doi: 10.1078/0176-1617-00833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.