Abstract
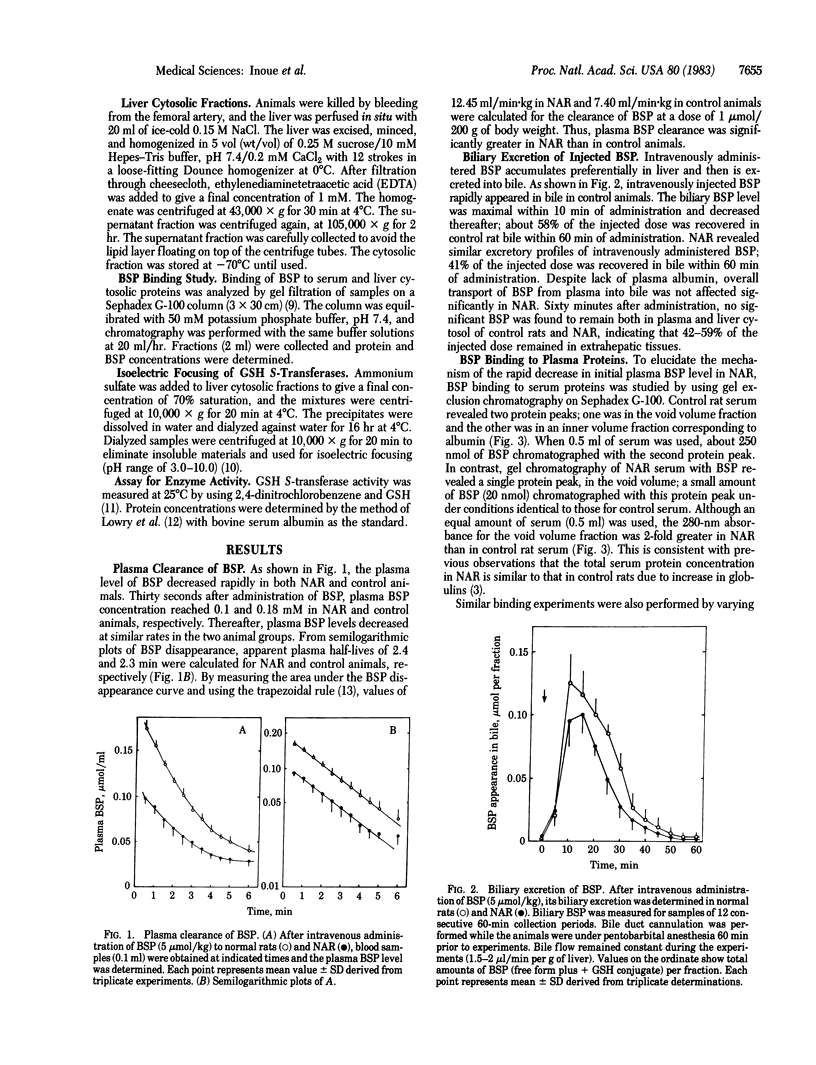
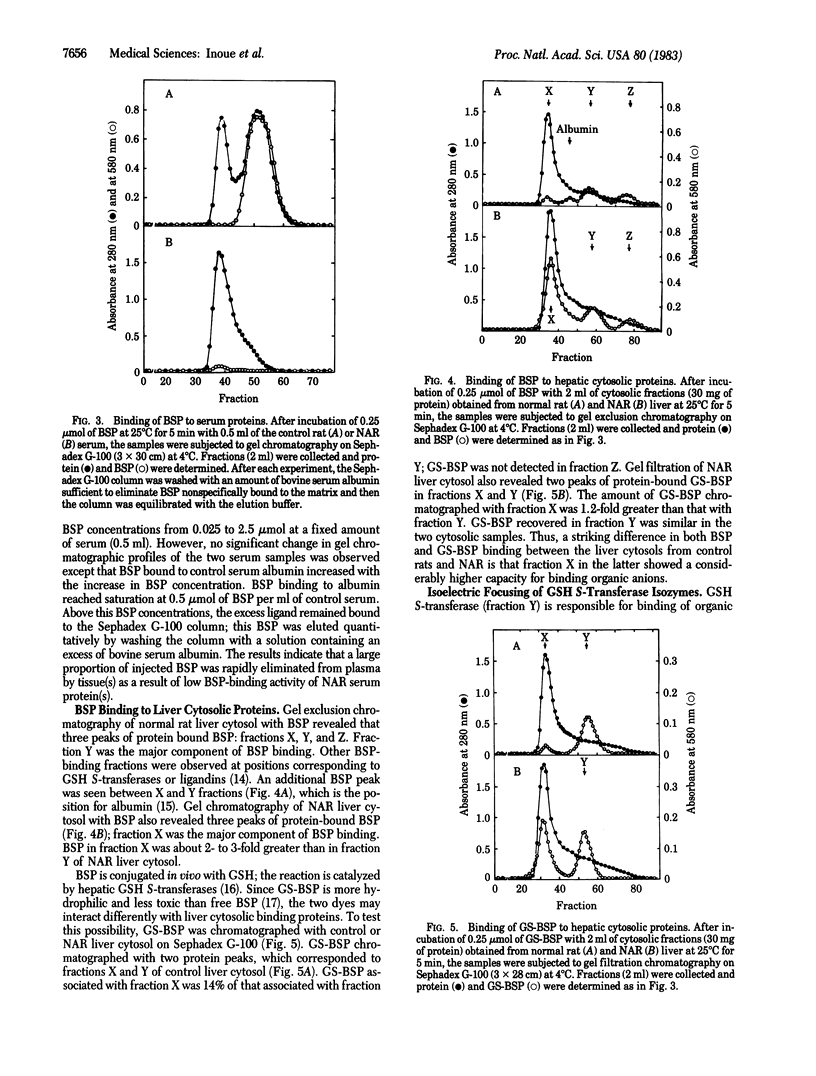
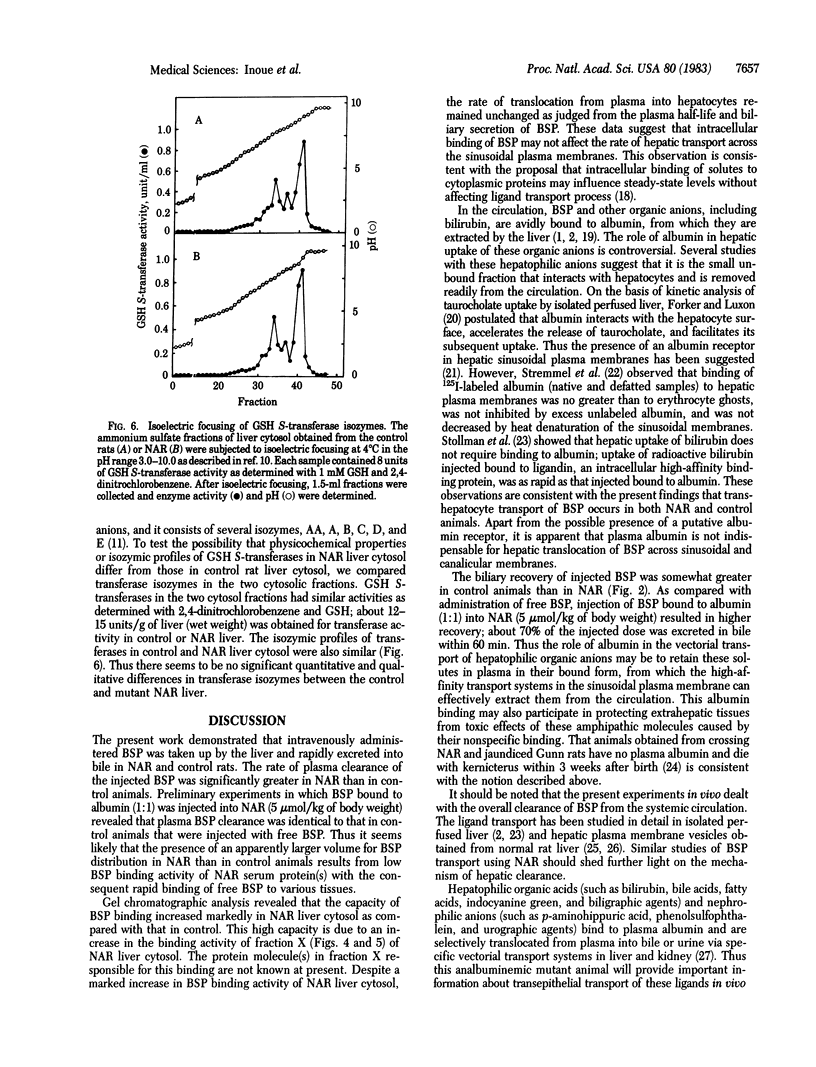
To investigate a possible function of plasma albumin in the vectorial transport of organic anions by the liver, the plasma disappearance of sulfobromophthalein (BSP) and its interaction with plasma and liver cytosolic proteins were studied in normal rats and mutant Nagase analbuminemic rats (NAR). After intravenous administration of BSP, plasma BSP decreased rapidly in both NAR and control animals: plasma clearance values of BSP in NAR and controls were 12.45 and 7.40 ml/min per kg, respectively. Gel exclusion Sephadex G-100 chromatography of BSP with control rat serum revealed a protein peak in the void volume and another in the albumin fraction. BSP chromatographed exclusively with the albumin fraction; binding of BSP to plasma albumin occurred stoichiometrically. Similar studies with NAR serum revealed a single protein peak, in the void volume; a small amount of BSP chromatographed with this protein peak. The amount of BSP that chromatographed with NAR serum protein(s) was 8% of that with control rat serum albumin. Sephadex G-100 chromatography of BSP with control rat liver cytosol revealed four peaks of protein-bound BSP in fractions corresponding to the void volume (fraction X), albumin, glutathione S-transferases (fraction Y, Mr 45,000), and fraction Z (Mr 12,000); fraction Y was the major component of BSP binding. Gel chromatography of NAR liver cytosol with BSP revealed three BSP peaks, fractions X, Y, and Z; fraction X was the major component of BSP binding. Total BSP binding by 30 mg of hepatic cytosolic proteins was 4.5 nmol for controls and 10.4 nmol for NAR. Isoelectric focusing of liver cytosol revealed no quantitative or qualitative differences in glutathione S-transferase isozymes between control and mutant animals. Intravenously administered BSP (5 mumol/kg) rapidly appeared in bile as the free form and the glutathione conjugate in normal rats and NAR; 41% and 57% of injected BSP was excreted within 60 min in NAR and control rat bile, respectively. These results indicate that binding of BSP to plasma albumin is not indispensable to transhepatocyte transport of BSP in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cormode E. J., Lyster D. M., Israels S. Analbuminemia in a neonate. J Pediatr. 1975 Jun;86(6):862–867. doi: 10.1016/s0022-3476(75)80215-0. [DOI] [PubMed] [Google Scholar]
- Esumi H., Okui M., Sato S., Sugimura T., Nagase S. Absence of albumin mRNA in the liver of analbuminemic rats. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3215–3219. doi: 10.1073/pnas.77.6.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi H., Takahashi Y., Sekiya T., Sato S., Nagase S., Sugimura T. Presence of albumin mRNA precursors in nuclei of analbuminemic rat liver lacking cytoplasmic albumin mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(3):734–738. doi: 10.1073/pnas.79.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forker E. L., Luxon B. A. Albumin helps mediate removal of taurocholate by rat liver. J Clin Invest. 1981 May;67(5):1517–1522. doi: 10.1172/JCI110182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grausz H., Schmid R. Reciprocal relation between plasma albumin level and hepatic sulfobromophthalein removal. N Engl J Med. 1971 Jun 24;284(25):1403–1406. doi: 10.1056/NEJM197106242842504. [DOI] [PubMed] [Google Scholar]
- Gärtner U., Stockert R. J., Levine W. G., Wolkoff A. W. Effect of nafenopin on the uptake of bilirubin and sulfobromophthalein by isolated perfused rat liver. Gastroenterology. 1982 Dec;83(6):1163–1169. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Inoue M., Hara M., Nagashima F., Matsui S., Mitsuyasu N., Morino Y. Affinity chromatography of hepatic glutathione S-transferases on omega-aminoalkyl sepharose derivatives of glutathione. Biochim Biophys Acta. 1981 Jun 15;659(2):362–369. doi: 10.1016/0005-2744(81)90062-0. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. Taurocholate transport by rat liver sinusoidal membrane vesicles: evidence of sodium cotransport. Hepatology. 1982 Sep-Oct;2(5):572–579. doi: 10.1002/hep.1840020510. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. The mechanism of biliary secretion of reduced glutathione. Analysis of transport process in isolated rat-liver canalicular membrane vesicles. Eur J Biochem. 1983 Aug 15;134(3):467–471. doi: 10.1111/j.1432-1033.1983.tb07590.x. [DOI] [PubMed] [Google Scholar]
- Inoue M., Okajima K., Morino Y. Renal transtubular transport of mercapturic acid in vivo. Biochim Biophys Acta. 1981 Feb 20;641(1):122–128. doi: 10.1016/0005-2736(81)90575-7. [DOI] [PubMed] [Google Scholar]
- Killenberg P. G., Hoppel C. L. Inhibition of rat liver mitochondrial oxidative phosphorylation by sulfobromophthalein. Mol Pharmacol. 1974 Jan;10(1):108–118. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest. 1969 Nov;48(11):2156–2167. doi: 10.1172/JCI106182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwack G., Ketterer B., Arias I. M. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature. 1971 Dec 24;234(5330):466–467. doi: 10.1038/234466a0. [DOI] [PubMed] [Google Scholar]
- Nagase S., Shimamune K., Shumiya S. Albumin-deficient rat mutant. Science. 1979 Aug 10;205(4406):590–591. doi: 10.1126/science.451621. [DOI] [PubMed] [Google Scholar]
- Nagase S., Shimamune K., Shumiya S. Albumin-deficient rat mutant: an animal model for analbuminemia. Jikken Dobutsu. 1980 Jan;29(1):33–38. doi: 10.1538/expanim1978.29.1_33. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Waggoner J. G., Berk P. D. Hepatic organic anion uptake in the rat. J Clin Invest. 1975 Nov;56(5):1280–1292. doi: 10.1172/JCI108204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumiya S., Nagase S. Establishment of an albumin-deficient and jaundiced strain of rats. Jikken Dobutsu. 1981 Jul;30(3):291–297. doi: 10.1538/expanim1978.30.3_291. [DOI] [PubMed] [Google Scholar]
- Stollman Y. R., Gärtner U., Theilmann L., Ohmi N., Wolkoff A. W. Hepatic bilirubin uptake in the isolated perfused rat liver is not facilitated by albumin binding. J Clin Invest. 1983 Aug;72(2):718–723. doi: 10.1172/JCI111021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Potter B. J., Berk P. D. Studies of albumin binding to rat liver plasma membranes. Implications for the albumin receptor hypothesis. Biochim Biophys Acta. 1983 Mar 15;756(1):20–27. doi: 10.1016/0304-4165(83)90019-3. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y., Yamada T., Kaplowitz N. Newly identified organic anion-binding proteins in rat liver cytosol. Biochim Biophys Acta. 1982 Dec 20;709(2):342–352. doi: 10.1016/0167-4838(82)90477-0. [DOI] [PubMed] [Google Scholar]
- WIEGAND R. G., SANDERS P. G. CALCULATION OF KINETIC CONSTANTS FROM BLOOD LEVELS OF DRUGS. J Pharmacol Exp Ther. 1964 Dec;146:271–275. [PubMed] [Google Scholar]
- Weisiger R., Gollan J., Ockner R. Receptor for albumin on the liver cell surface may mediate uptake of fatty acids and other albumin-bound substances. Science. 1981 Mar 6;211(4486):1048–1051. doi: 10.1126/science.6258226. [DOI] [PubMed] [Google Scholar]
- Whelan G., Hoch J., Combes B. A direct assessment of the importance of conjugation for biliary transport of sulfobromophthalein sodium. J Lab Clin Med. 1970 Apr;75(4):542–557. [PubMed] [Google Scholar]
- Wolkoff A. W., Goresky C. A., Sellin J., Gatmaitan Z., Arias I. M. Role of ligandin in transfer of bilirubin from plasma into liver. Am J Physiol. 1979 Jun;236(6):E638–E648. doi: 10.1152/ajpendo.1979.236.6.E638. [DOI] [PubMed] [Google Scholar]


