Abstract
Objective:
To characterize the temporal and spatial pattern of cerebral microbleeds (CMBs) after cranial irradiation in patients with medulloblastoma.
Methods:
We retrospectively identified patients with medulloblastoma treated with craniospinal irradiation at the Massachusetts General Hospital between 1999 and 2015. Longitudinal MRI including T2*-weighted gradient-recalled echo (GRE) sequences were reviewed, and the prevalence, spatial pattern, and risk factors associated with CMBs were characterized.
Results:
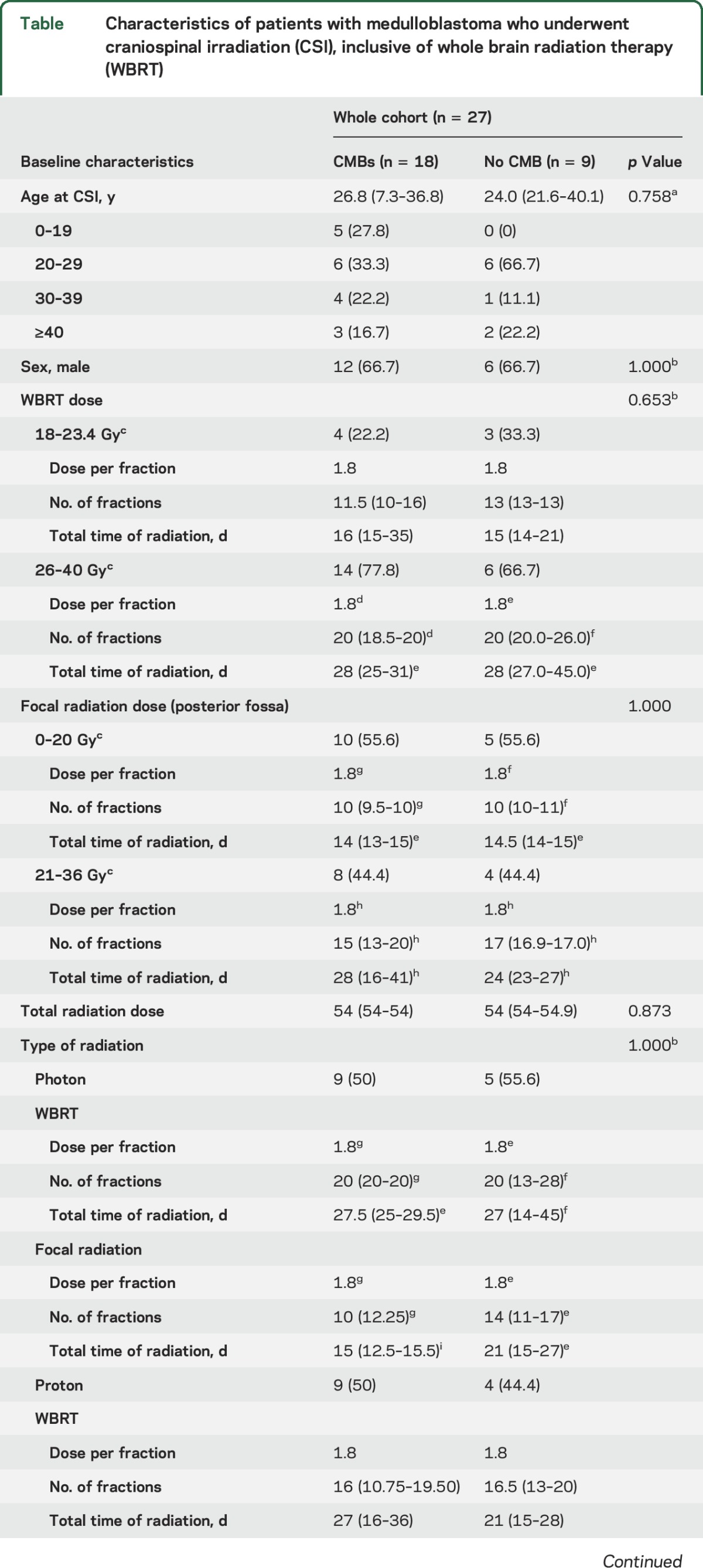
We identified a total of 27 patients; 5 patients were children (median age 6.3 years) and 22 patients were adults (median age 28.8 years). CMBs were found in 67% (18/27) of patients, who were followed for a median of 4.1 years. Patients with CMBs had longer GRE follow-up time compared to those without CMBs (4.9 vs 1.7 years, p = 0.035). The median latency of the appearance of CMBs was 2.79 years (interquartile range 1.76–4.26). The prevalence of CMBs increased with each year from time of radiation therapy, and the cumulative prevalence was highest in patients age <20 years (100% cumulative prevalence, vs 59% in adult patients treated at age ≥20 years). CMBs were mostly found in lobar distribution and predominately in bilateral occipital lobes. Patients using antithrombotic medications developed CMBs at a significantly higher rate (p = 0.041).
Conclusions:
Our data demonstrate a high prevalence of CMBs following cranial irradiation, progressively increasing with each year from time of radiation therapy.
Medulloblastoma is one of the most common malignant brain tumors in children, accounting for approximately 20% of all pediatric brain tumors, and the incidence rate in children is 10-fold higher than in adults.1 Standard treatment for medulloblastoma includes maximal safe surgical resection, followed by craniospinal irradiation (CSI) with a focal boost to the tumor bed or posterior fossa. Adjuvant chemotherapy is commonly offered to patients with high-risk features.2,3 Current overall survival rates at 5 years range from 50% to 80%.2–5 Neurotoxicity from cancer therapy remains an important issue in patient management and is in particular a concern in long-term survivors.
Cerebral microbleeds (CMBs) are a type of radiation-induced vascular complication thought to represent microvascular injury.6–8 Radiologically, CMBs are characterized by small foci of signal hypointensity visualized on T2*-weighted gradient-recalled echo (GRE) sequences on MRI.9,10 Previous pathologic studies have demonstrated that CMBs were iron-positive blood breakdown products from prior hemorrhage, most commonly in form of hemosiderin-laden macrophages, or evidence of vasculopathies, such as fibrinoid necrosis, microaneurysm, dissection in the wall of a distended vessel, or cavernoma.11,12
In the present study, we aimed to characterize the prevalence and spatial and temporal pattern of CMBs following cranial irradiation. Focusing our analysis on patients with medulloblastoma, considered a treatable disease with prolonged overall survival, allowed us to characterize the evolution of radiation-associated CMBs over an extended period of time.
METHODS
Patient recruitment.
We searched the clinical database at the Brain Tumor Center of the Massachusetts General Hospital between 1999 and 2015 for patients fulfilling the following eligibility criteria: (1) diagnosis of medulloblastoma; (2) treatment with CSI inclusive of whole brain radiation therapy (WBRT); (3) documented clinical follow-up for a minimum of 1 year; and (4) availability of serial MRI scans as part of cancer staging both at baseline (prior to radiation therapy) and during follow-up after cranial irradiation.
We identified a total of 44 patients with histologically proven medulloblastoma. Of these, 17 patients were excluded for various reasons, including insufficient clinical follow-up of less than 1 year (n = 4), lack of GRE-MRI sequences (n = 6), or other missing longitudinal data (n = 7).
Of the remaining 27 patients, we analyzed basic demographic data and clinical and treatment characteristics, including age at the time of diagnosis, sex, radiation dose and type of radiation, presence of cardiovascular risk factors, history of antithrombotic use (antiplatelet or anticoagulant), and history of adjuvant chemotherapy consisting of multiagent chemotherapy, including cisplatin, vincristine, cyclophosphamide, etoposide, carboplatin, lomustine, bevacizumab, and thiotepa.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review board of Massachusetts General Hospital.
MRI scanning.
To be eligible for this study, patients were required to have undergone brain MRI scans prior to and at various time points after radiation therapy. MRI sequences needed to include axial T2*-weighted GRE sequences for detection of CMBs, axial T1-weighted imaging, axial T2-weighted imaging, and axial fluid-attenuated inversion recovery (FLAIR) sequences.
All patients were scanned at a 1.5T MRI system (General Electric Medical Systems, Milwaukee, WI, or Siemens, Munich, Germany), or occasionally (10 of 132 MRI scans) at a 3.0T MRI system (General Electric Medical Systems or Siemens). The range of imaging parameters was as follows: for 1.5T MRI: T2*-weighted GRE, repetition time (TR) 450–12,000 ms, echo time (TE) 20–48.8 ms, flip angle 20°–90°, slice thickness 3–5 mm, inter slice gap 3.0–9.1 mm. For 3.0T MRI: T2*-weighted GRE, TR 359–500 ms, TE 4.9–19.9 ms, flip angle 10°–20°, slice thickness 3–5 mm, inter slice gap 4–6 mm.
MRI analysis.
CMBs were defined as small round or oval areas of homogenous signal void ranging in diameter from 2 to 10 mm, visualized on T2*-weighted GRE, and not visualized on T1-weighted imaging, T2-weighted imaging, and FLAIR sequences.9
A trained investigator, blinded to clinical data, reviewed all imaging datasets. The presence, number, and anatomical distribution of CMBs were classified based on recent published guidelines.9,10 Anatomical distribution of CMBs were classified into 3 regions: lobar (cortical and subcortical hemispheric white matter), deep (deep gray matter: basal ganglia, thalamus; and white matter of internal and external capsule), and infratentorial (brainstem and cerebellum).
To determine the spatial distribution of CMBs, we generated images, in which all CMBs of all patients at the last follow-up MRI after radiation therapy were combined. The original MRI were registered to the Montreal Neurological Institute 152 template13,14 using the elastix toolbox for rigid and nonrigid registration.15 The resulting transformation was applied to the microbleed annotations. The 3D visualization was created with MeVisLab.16
Statistical analysis.
Categorical variables are presented in frequency and percentage while quartiles are used to describe continuous variables. We calculated the prevalence of CMBs in yearly strata after cranial irradiation. The first, second, third, fourth, and fifth year strata are defined as the following intervals: time of radiation therapy–1.5 years, 1.5–2.5 years, 2.5–3.5 years, 3.5–4.5 years, and 4.5–5.5 years, respectively. The prevalence of CMBs is defined as number of CMBs divided by the sum of the follow-up times between radiation therapy and the last T2*-weighted GRE for each individual in each year strata. For individuals with more than 1 scan on a given stratum, the scan with the maximum number of CMBs was used to calculate prevalence.
We explored the individual rate of increases in the number of CMBs by using the slope from general linear models and classifying the rate of formation of CMBs into 3 categories, including no new CMBs (slope = 0), low rate CMBs (slope 1–3), and high rate CMBs (slope ≥10). Notably, no patient was identified with a rate of CMB formation between 4 and 10 per year. In order to compare risk factors between the groups, the Fisher exact test was used for categorical variables and a one-way analysis of variance or the Kruskal-Wallis test was used for continuous variables depending on the distribution of data. A 2-sided p value of <0.05 was considered statistically significant. All analyses were performed using the Windows SPSS (Chicago, IL) software package version 17.0.
RESULTS
Twenty-seven patients (18 male and 9 female) with histologically proven medulloblastoma were longitudinally evaluated in the present study. Patients had a median age of 25 years (range 2–50) at time of diagnosis, 5 patients were children with median age of 6.3 years (range 2.7–7.6), and 22 patients were adults with median age of 28.8 years (range 22.7–40.1).
Surgical resection was done in 26 patients and biopsy was done in 1 patient. All patients underwent WBRT as part of the CSI regimen. All patients except one (26/27; 96.3%) received an additional focal boost to the tumor bed. Nineteen patients (70.4%) received adjuvant chemotherapy.
Longitudinal MRI follow-up after radiation therapy was performed in all patients including T2*-weighted GRE sequences, with median time interval of 4.1 years (interquartile range [IQR] 1.7–7.5) between initial radiation therapy and last T2*-weighted GRE. Among these, 21/27 patients underwent T2*-weighted GRE after radiation therapy on at least 2 subsequent time points.
CMBs were identified in 18 of 27 patients (66.7%) during follow-up. The median latency of the appearance of CMBs was 2.79 years (IQR 1.76–4.26). The median age at time of cranial irradiation was 26.8 years (range 3–47). High cumulative prevalence of CMBs (5/5, 100%) was observed in patients at age <20 years at time of radiation therapy and with a median follow-up time of 9 years (IQR 4.5–27). The cumulative prevalence of CMBs among patients who underwent radiation therapy at age ≥ older than 20 years ranged from 50% to 80% with a median follow-up time of 4 years (IQR 1–6.25). The cumulative prevalence of CMBs was slightly higher in patients treated with a WBRT dose ≥25 Gy and adjuvant chemotherapy, compared to those patients with WBRT dose <25 Gy and no chemotherapy, but this did not reach statistical significance (70% vs 30%; p = 0.653 and 63.2% vs 36.8%; p = 0.676, respectively). The type of radiation (photon vs proton radiation) did not seem to influence the frequency of CMBs.
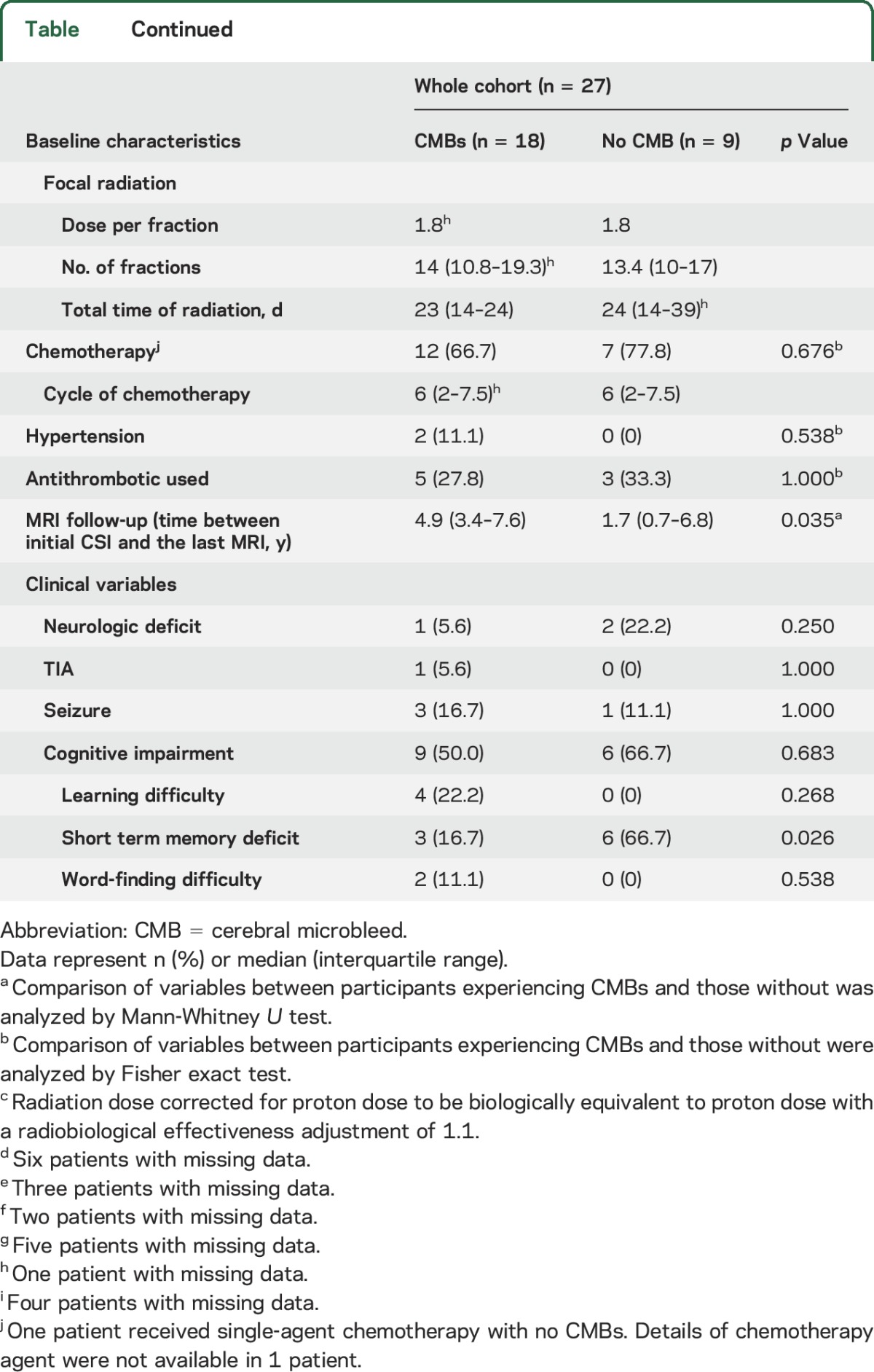
Clinical and demographic characteristics of patients with and without CMBs are presented in the table. The time interval from radiation therapy to the last follow-up T2*-weighted GRE was different between patients with and without CMBs (4.9 vs 1.7 years, p = 0.035). No statistically significant difference was identified between patients with and without CMBs in terms of sex, age at CSI, type of radiation, history of hypertension, use of anticoagulant or antithrombotic drugs, clinical variables such as neurologic deficit, TIA, seizure, learning difficulty, and word-finding difficulty. Neurocognitive deficits were present in >50% in all patients, independent of presence of CMBs; however, memory deficits were more common in patients without CMBs (16.7% vs 66.7%, p = 0.026).
Table.
Characteristics of patients with medulloblastoma who underwent craniospinal irradiation (CSI), inclusive of whole brain radiation therapy (WBRT)
The prevalence of CMBs per person-year stratified by years after radiation therapy was further analyzed. The prevalence of CMBs increased with time from cranial irradiation following an exponential pattern (figure 1A). The prevalence of lobar CMBs was higher than the prevalence of CMBs in deep and infratentorial regions (figure 1B). No CMBs were identified in deep gray matter within a 5-year follow-up period after radiation therapy. Five of 27 patients also had follow-up spine MRI with axial T2*-weighted GRE after CSI (2 patients had cervical MRI, 2 patients had lumbar MRI, and 1 patient had cervical and thoracic MRI). No spinal cord microbleeds were identified in any of these patients, who were followed for a median of 3.81 years (range 0.07–28.02).
Figure 1. Prevalence of cerebral microbleeds (CMBs) after cranial irradiation.
(A) Prevalence of CMBs stratified by years after radiation therapy. (B) Prevalence of CMBs after radiation therapy stratified by location. WBRT = whole brain radiation therapy.
CMBs were more frequently noted in lobar distributions, in particular in bilateral occipital lobes (figure 2). The individual number of CMBs at each time point is presented in figure 3. The overall rate in the number of new CMBs was 3.93 ± 5.4 lesions per year. Among these, the average rate in the low-rate group was 0.9 ± 0.8 lesions per year (n = 10) and in the high-rate group 12.5 ± 1.6 lesions per year (n = 4).
Figure 2. Topographic data of all cerebral microbleeds (CMBs) from the last follow-up MRI after cranial irradiation.

A red dot represents 1 CMB (n = 18, total number of CMBs = 457).
Figure 3. Total number of microbleeds during the 5-year observation period in the patients who developed cerebral microbleeds (CMBs).

Each line represents the change in number of CMBs for an individual.
We found that 75% (3 of 4 patients) within the high-rate CMB group had a history of antithrombotic use, compared to 10% (1 of 10 patients) within the low-rate CMB group (p = 0.041). In patients with high-rate CMBs, 2 patients had a history of acetylsalicylic acid use. Acetylsalicylic acid was started at 1.4 years prior to and at 0.9 years after CSI, and the duration of acetylsalicylic acid use was 1.4 and 2.8 years, respectively. One patient had history of low-molecular-weight heparin use, which was started at 5.3 years after CSI and was administered for 2 years. None of the other variables including age, sex, radiation dose, type of radiation, chemotherapy, and history of hypertension were identified as risk factors for CMBs. The pattern and distribution of CMBs in a representative patient is shown in figure 4.
Figure 4. Serial T2*-weighted gradient-recalled echo (GRE) demonstrating an increment of cerebral microbleeds (CMBs) over time in a characteristic patient.
T2*-weighted GRE were obtained at baseline before radiation therapy (A), at 21 months after radiation therapy (B), at 33 months after radiation therapy (C), at 44 months after radiation therapy (D), and 62 months after radiation therapy (E). Note the increase in CMBs over time.
To address a possible influence of a different MRI field strength on CMB count,17,18 only T2*-weighted GRE obtained at a 1.5T scanner were further analyzed and compared to the entire cohort. The results, including prevalence and risk factors of CMBs, remained consistent with the main analysis obtained from both 1.5T and 3.0T scanners (data not shown). With respect to other potential vascular adverse events associated with radiation therapy and in patients with CMBs, we identified 2 patients with development of a cavernoma and one patient with a TIA. None of the patients in this study had evidence of intracerebral hemorrhage (ICH) or development of a moyamoya-like syndrome.
DISCUSSION
CMBs are usually seen in patients with cerebral small vessel disease. CMBs in infratentorial and deep brain territory are associated with hypertensive vasculopathy,19 whereas CMBs seen in strictly lobar brain regions are commonly seen in cerebral amyloid angiopathy (CAA).10,20–22 CMBs are also associated with an increased risk of ICH23–25 in certain populations, and especially in the setting of antithrombotic therapy.26–28
Little is known about the pathophysiology of radiation-induced CMBs. Specifically, the temporal and spatial distribution of CMBs associated with cranial irradiation have so far not been systematically evaluated in a larger patient cohort.
Using longitudinal neuroimaging in a cohort of patients with medulloblastoma, we characterize the temporal and spatial pattern of CMBs associated with cranial irradiation. Our data show a high prevalence of CMBs following cranial irradiation. Most CMBs were detected in lobar distribution, particularly in occipital lobes. Furthermore, our findings reveal that the use of antithrombotic therapy might be a possible risk factor promoting the development of CMBs after cranial irradiation, and therefore may have important implications for patient management.
The existing literature suggests a prevalence of cerebral telangiectasias, or microbleeds, after cranial irradiation in the range of 20%, when assessed by conventional MRI without T2*-weighted GRE for detection.6,29 However, the prevalence of CMBs might be higher (in the range of 45%–50%) and detectable if more sensitive imaging modalities are used, such as phase-sensitive MRI.6 Regardless, the prevalence of CMBs after cranial irradiation and detected by T2*- weighted GRE remains poorly characterized.
We hypothesize that the actual prevalence of radiation-induced CMBs may be even higher than 66.7% since the median follow-up time of patients without detectable CMBs after radiation therapy in the present study was significantly shorter than in patients with CMBs (1.7 vs 5.0 years). Moreover, the prevalence of CMBs appeared to increase with each year after radiation therapy. This finding suggests cumulative and progressive small vessel injury following radiation therapy. The prevalence of radiation-associated CMBs also appears to be influenced by the age of the patient at time of radiation therapy, as younger individuals were at higher risk to develop CMBs. Specifically, we found that 100% of patients (n = 5) (age below 19 years) developed CMBs after cranial irradiation. A previous study demonstrated that age at time of cranial irradiation is a critically important factor in the development of CMBs, with 100% of individuals developing telangiectasias/microbleeds when treated with radiation at age 14 or younger.29 In line with these findings, another study found that only 33.3% of patients who underwent brain radiation at age 60 or older developed CMBs (4 of 12 patients) at a mean follow-up time of 29 months (range 3–169 months).6
Prevalence of radiation-induced CMBs in our series of 27 participants was 10-fold higher than in the general population in the age group of 45–50 years30 and 2-fold higher than in the general elderly population of individuals older than 80 years.19 These findings support the notion that the younger brain is more vulnerable to the effects of cranial irradiation.
Previous reports suggest that the radiation dose correlates with the frequency of CMBs. No CMBs were observed in brain regions exposed to a radiation dose of less than 25 Gy,6 suggesting a low risk to develop CMBs in patients treated with a radiation dose of 25 Gy or less. Our findings are consistent with this observation. We show that the frequency of CMBs in patients treated with a radiation dose of ≥25 Gy was higher than in patients treated with a radiation dose <25 Gy, although this finding was not statistically significant, which was likely due to insufficient patient numbers in both groups.
There are few reports suggesting that radiation-induced CMBs are associated with cognitive dysfunction and that the cognitive domains mostly affected are associated with the location of CMBs within the brain.31,32 We were unable to identify an association between CMBs and cognitive dysfunction in the present study; however, this was likely due to the limited number of patients and design of the study.
In terms of possible other risk factors for the development of CMBs, no significant correlations have been established for sex, type of radiation (proton vs photon), adjuvant chemotherapy, history of hypertension, history of antithrombotic use, and presence of CMBs.6,29 It is possible that genetic factors such as specific germline mutations in patients with medulloblastomas might represent an independent risk factor for neurovascular toxicity. However, we were not able to obtain sufficient molecular-genetic information in our patient cohort to address this issue, which could be evaluated in future prospective studies.
In regard to the spatial pattern of CMBs associated with cranial irradiation, CMBs were more commonly located in lobar areas and occipital regions. The lobar distribution of CMBs in these patients appears to be similar to the distribution of CMBs in CAA.20 Interestingly, in adult patients (mean age 53.2 years) treated with cranial irradiation, pathologic studies have shown an increased number of amyloid-containing blood vessels.33 Moreover, evidence from a postmortem study in patients who received brain irradiation for malignant neoplasm showed higher prevalence of cerebral β-amyloid (Aβ) deposition and amyloid angiopathy when compared with non-irradiated patients (22.2% vs 8.0%, p < 0.05).33
We hypothesize that radiation-induced small vessel injury might share at least some histologic features with the pathologic process that is driving amyloid angiopathy. Moreover, amyloid deposits might be even higher in posterior brain regions due to higher radiation exposure. Future pathology studies in patients who have been exposed to cranial irradiation are needed to better characterize such similarities. It might also be helpful to obtain brain histopathology data or amyloid-based PET imaging34 data to further delineate whether CMBs after cranial irradiation are associated with vascular Aβ deposition.
It is possible that the microcirculation in lobar brain areas is more susceptible to injury from radiation and thus may be the reason for our findings. Furthermore, a higher radiation dose administered to occipital brain regions is likely to play a role in the predominance of CMBs to this region.6
Notably, CMBs were less likely to occur in the cerebellum compared to the occipital lobes despite exposure to similar radiation doses within the posterior fossa.
While the underlying mechanism of this finding remains elusive, some investigators have hypothesized a higher threshold to radiation toxicity within the cerebellum.35,36
Collectively, our data demonstrate a remarkably high prevalence of CMBs after cranial irradiation with steady progression each year even after radiation therapy. CMBs were commonly found in lobar regions (predominantly in the occipital lobe) and resembled the distribution pattern seen in CAA. However, characterization of the detailed pathologic mechanisms and identification of risk factors to develop CMB after brain irradiation requires further investigation.
ACKNOWLEDGMENT
The authors thank Susanne J. van Veluw from the Department of Neurology, University Medical Center Utrecht, the Netherlands, for assistance with imaging analysis and Lily L. Altstein from the Biostatistics Center, Massachusetts General Hospital, Boston, for assistance with statistical analysis.
GLOSSARY
- Aβ
β-amyloid
- CAA
cerebral amyloid angiopathy
- CMB
cerebral microbleed
- CSI
craniospinal irradiation
- FLAIR
fluid-attenuated inversion recovery
- GRE
gradient-recalled echo
- ICH
intracerebral hemorrhage
- IQR
interquartile range
- TE
echo time
- TR
repetition time
- WBRT
whole brain radiation therapy
AUTHOR CONTRIBUTIONS
D. Roongpiboonsopit: involved in data collection, statistical analysis and interpretation of data, and drafting and revising the manuscript. H.J. Kuijf: involved in virtual imaging, technical support, and revising the manuscript. A. Charidimou: involved in data interpretation and revising the manuscript. L. Xiong: involved in data interpretation and revising the manuscript. A. Vashkevich: involved in data collection and revising the manuscript. S. Martinez-Ramirez: involved in data interpretation and revising the manuscript. H.A. Shih: involved in study concept and design, data collection, and revising the manuscript. C.M. Gill: involved in data collection and revising the manuscript. A. Viswanathan: involved in study concept and design, analysis and interpretation of data, revising the manuscript, and study supervision. J. Dietrich: involved in study concept and design, analysis and interpretation of data, revising the manuscript, and study supervision.
STUDY FUNDING
J.D. is a recipient of the American Academy of Neurology Clinical Research Training Fellowship. This work was supported by a K-12 institutional research award (to J.D.) and the American Cancer Society (to J.D.). J.D. received philanthropic support from the R. Tawil, S. McPhee, and B. Lockwood family foundations.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci 2012;19:1541–1544. [DOI] [PubMed] [Google Scholar]
- 2.Evans AE, Jenkin RD, Sposto R, et al. The treatment of medulloblastoma: results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J Neurosurg 1990;72:572–582. [DOI] [PubMed] [Google Scholar]
- 3.Tait DM, Thornton-Jones H, Bloom HJ, Lemerle J, Morris-Jones P. Adjuvant chemotherapy for medulloblastoma: the first multi-centre control trial of the International Society of Paediatric Oncology (SIOP I). Eur J Cancer 1990;26:464–469. [PubMed] [Google Scholar]
- 4.Rieken S, Gaiser T, Mohr A, et al. Outcome and prognostic factors of desmoplastic medulloblastoma treated within a multidisciplinary treatment concept. BMC Cancer 2010;10:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkin D, Goddard K, Armstrong D, et al. Posterior fossa medulloblastoma in childhood: treatment results and a proposal for a new staging system. Int J Radiat Oncol Biol Phys 1990;19:265–274. [DOI] [PubMed] [Google Scholar]
- 6.Tanino T, Kanasaki Y, Tahara T, et al. Radiation-induced microbleeds after cranial irradiation: evaluation by phase-sensitive magnetic resonance imaging with 3.0 tesla. Yonago Acta Med 2013;56:7–12. [PMC free article] [PubMed] [Google Scholar]
- 7.Roongpiboonsopit D, Chutinet A, Suwanwela N. Radiation-induced cerebral microbleeds. J Neurol Neurophysiol 2014;5:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shobha N, Smith EE, Demchuk AM, Weir NU. Small vessel infarcts and microbleeds associated with radiation exposure. Can J Neurol Sci 2009;36:376–378. [DOI] [PubMed] [Google Scholar]
- 9.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis 2011;32:528–534. [DOI] [PubMed] [Google Scholar]
- 12.van Veluw SJ, Biessels GJ, Klijn CJ, Rozemuller AJ. Heterogeneous histopathology of cortical microbleeds in cerebral amyloid angiopathy. Neurology 2016;86:867–871. [DOI] [PubMed] [Google Scholar]
- 13.Fonov VS, Evans AC, McKinstry RC, Almli C, Collins D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage 2009;47:S102. [Google Scholar]
- 14.Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 2011;54:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein S, Staring M, Murphy K, Viergever MA, Pluim JP. elastix: A toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 2010;29:196–205. [DOI] [PubMed] [Google Scholar]
- 16.Ritter F, Boskamp T, Homeyer A, et al. Medical image analysis. IEEE Pulse 2011;2:60–70. [DOI] [PubMed] [Google Scholar]
- 17.Stehling C, Wersching H, Kloska SP, et al. Detection of asymptomatic cerebral microbleeds: a comparative study at 1.5 and 3.0 T. Acad Radiol 2008;15:895–900. [DOI] [PubMed] [Google Scholar]
- 18.Nandigam RN, Viswanathan A, Delgado P, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol 2009;30:338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 2008;70:1208–1214. [DOI] [PubMed] [Google Scholar]
- 20.Rosand J, Muzikansky A, Kumar A, et al. Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann Neurol 2005;58:459–462. [DOI] [PubMed] [Google Scholar]
- 21.Pettersen JA, Sathiyamoorthy G, Gao FQ, et al. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol 2008;65:790–795. [DOI] [PubMed] [Google Scholar]
- 22.Shams S, Martola J, Granberg T, et al. Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis: the Karolinska imaging dementia study. AJNR Am J Neuroradiol 2015;36:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dannenberg S, Scheitz JF, Rozanski M, et al. Number of cerebral microbleeds and risk of intracerebral hemorrhage after intravenous thrombolysis. Stroke 2014;45:2900–2905. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa T, Ueba T, Kajiwara M, Fujisawa I, Miyamatsu N, Yamashita K. Cerebral microbleeds predict first-ever symptomatic cerebrovascular events. Clin Neurol Neurosurg 2009;111:825–828. [DOI] [PubMed] [Google Scholar]
- 25.Bokura H, Saika R, Yamaguchi T, et al. Microbleeds are associated with subsequent hemorrhagic and ischemic stroke in healthy elderly individuals. Stroke 2011;42:1867–1871. [DOI] [PubMed] [Google Scholar]
- 26.Gregoire SM, Jager HR, Yousry TA, Kallis C, Brown MM, Werring DJ. Brain microbleeds as a potential risk factor for antiplatelet-related intracerebral haemorrhage: hospital-based, case-control study. J Neurol Neurosurg Psychiatry 2010;81:679–684. [DOI] [PubMed] [Google Scholar]
- 27.Lovelock CE, Cordonnier C, Naka H, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke 2010;41:1222–1228. [DOI] [PubMed] [Google Scholar]
- 28.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 2010;75:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koike S, Aida N, Hata M, Fujita K, Ozawa Y, Inoue T. Asymptomatic radiation-induced telangiectasia in children after cranial irradiation: frequency, latency, and dose relation. Radiology 2004;230:93–99. [DOI] [PubMed] [Google Scholar]
- 30.Poels MM, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke 2010;41:S103–S106. [DOI] [PubMed] [Google Scholar]
- 31.Roddy E, Sear K, Felton E, et al. Presence of cerebral microbleeds is associated with worse executive function in pediatric brain tumor survivors. Neuro Oncol 2016;18:1548–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen Q, Lin F, Rong X, et al. Temporal cerebral microbleeds are associated with radiation necrosis and cognitive dysfunction in patients treated for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2016;94:1113–1120. [DOI] [PubMed] [Google Scholar]
- 33.Sugihara S, Ogawa A, Nakazato Y, Yamaguchi H. Cerebral beta amyloid deposition in patients with malignant neoplasms: its prevalence with aging and effects of radiation therapy on vascular amyloid. Acta Neuropathol 1995;90:135–141. [DOI] [PubMed] [Google Scholar]
- 34.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol 2007;62:229–234. [DOI] [PubMed] [Google Scholar]
- 35.Laissue JA, Blattmann H, Di Michiel M, et al., editors. Weanling piglet cerebellum: a surrogate for tolerance to MRT (microbeam radiation therapy) in pediatric neuro-oncology. Proc SPIE 2001;4508:65–73. [Google Scholar]
- 36.Laissue JA, Blattmann H, Wagner HP, Grotzer MA, Slatkin DN. Prospects for microbeam radiation therapy of brain tumours in children to reduce neurological sequelae. Dev Med Child Neurol 2007;49:577–581. [DOI] [PubMed] [Google Scholar]




