Supplemental Digital Content is available in the text.
Keywords: hypertension; rats, inbred SHR; sarcoplasmic reticulum calcium-transporting ATPases; stellate ganglion; sympathetic nervous system
Abstract
Reduced cardiomyocyte excitation–contraction coupling and downregulation of the SERCA2a (sarcoendoplasmic reticulum calcium ATPase 2a) is associated with heart failure. This has led to viral transgene upregulation of SERCA2a in cardiomyocytes as a treatment. We hypothesized that SERCA2a gene therapy expressed under a similar promiscuous cytomegalovirus promoter could also affect the cardiac sympathetic neural axis and promote sympathoexcitation. Stellate neurons were isolated from 90 to 120 g male, Sprague–Dawley, Wistar Kyoto, and spontaneously hypertensive rats. Neurons were infected with Ad-mCherry or Ad-mCherry-hATP2Aa (SERCA2a). Intracellular Ca2+ changes were measured using fura-2AM in response to KCl, caffeine, thapsigargin, and carbonylcyanide-p-trifluoromethoxyphenylhydrazine to mobilize intracellular Ca2+ stores. The effect of SERCA2a on neurotransmitter release was measured using [3H]-norepinephrine overflow from 340 to 360 g Sprague–Dawley rat atria in response to right stellate ganglia stimulation. Upregulation of SERCA2a resulted in greater neurotransmitter release in response to stellate stimulation compared with control (empty: 98.7±20.5 cpm, n=7; SERCA: 186.5±28.41 cpm, n=8; P<0.05). In isolated Sprague–Dawley rat stellate neurons, SERCA2a overexpression facilitated greater depolarization-induced Ca2+ transients (empty: 0.64±0.03 au, n=57; SERCA: 0.75±0.03 au, n=68; P<0.05), along with increased endoplasmic reticulum and mitochondria Ca2+ load. Similar results were observed in Wistar Kyoto and age-matched spontaneously hypertensive rats, despite no further increase in endoplasmic reticulum load being observed in the spontaneously hypertensive rat (spontaneously hypertensive rats: empty, 0.16±0.04 au, n=18; SERCA: 0.17±0.02 au, n=25). In conclusion, SERCA2a upregulation in cardiac sympathetic neurons resulted in increased neurotransmission and increased Ca2+ loading into intracellular stores. Whether the increased Ca2+ transient and neurotransmission after SERCA2A overexpression contributes to enhanced sympathoexcitation in heart failure patients remains to be determined.
Heart failure remains a predominant cause of mortality and morbidity globally and is characterized by a loss in efficient excitation–contraction coupling1,2 that leads to reduced inotropy. Downregulation of the SERCA2a (sarcoendoplasmic reticulum Ca2+ ATPase 2a), a key protein in cardiomyocyte excitation–contraction coupling, has been identified as a therapeutic target in both clinical1,3 and animal models of heart failure.4 Increasing myocyte SERCA2a levels by gene transfer in isolated human myocytes5 and preclinical animal models with heart failure6,7 restores cardiac inotropy and myocyte Ca2+ handling, without proarrhythmic side effects.4 Indeed, early small-scale clinical trials for the treatment of heart failure demonstrated positive results for outcome and biological safety after intracoronary injection of adeno-associated virus (AAV) type 1 SERCA2a. Prespecified clinical end points, including the 6-minute walk test, peak oxygen consumption, and left ventricular end-systolic pressure all improved.8,9 However, recent results from a larger phase 2 double-blind, placebo-controlled trial (CUPID2 [Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease]) failed to meet primary clinical end points.10,11
Adeno viruses (Ad) and AAV are powerful tools for altering gene expression because of their high transfection efficiency and low risk of pathogenicity.12 They also have increased efficiency at infecting multiple cell types, including myocytes, neurons, and retinal cells,13 if broad-spectrum promoters are used (eg, cytomegalovirus).10,14,15 Therefore, it is conceivable that overexpression of AAV SERCA2a when given into the coronary circulation might also transduce the neural cardiac axis, resulting in a deleterious performance. In particular, SERCA and impairment of its regulatory protein phospholamban have been implicated in modulating depolarization-induced Ca2+ transients in sympathetic neurons,16 thus promoting neurotransmission.17 This neural phenotype is a well-established negative prognostic indicator in patients with heart failure.18–21
We therefore tested the hypothesis that enhancing SERCA2a gene expression with a cytomegalovirus promoter facilitates cardiac sympathetic neurotransmission via abnormal endoplasmic reticulum (ER) and mitochondrial intracellular Ca2+ handling in normal stellate neurons. Furthermore, we tested whether dysregulation of SERCA contributes to Ca2+ impairment in a model of cardiac sympathetic dysautonomia.
Methods
Animals
Age- and weight-matched male 4- to 5-week (90–120 g), Sprague–Dawley (SD, n=46), spontaneously hypertensive rat (SHR, n=22) and normotensive Wistar Kyoto (WKY, n=20) rats, in addition adult 16- to 18-week (350–380 g) male SD rats (n=20), and 9- to 10-month SHR (n=3) and WKY rats (n=3), were purchased from Envigo (Harlan, Bicester, United Kingdom) and housed under standard laboratory conditions. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and the Animals (Scientific Procedures) Act 1986 (United Kingdom). Procedures were performed under British Home Office license requirements (PPL 30/3131).
Viral Constructs
Viral constructs were manufactured commercially (Vector BioLabs, Malvern, PA). Viruses were constructed under a nonspecific cell type cytomegalovirus promoter to the same construct of human ATP2Aa as used in the CUPID trials. Ad-mCherry used was for control experiments (stock: 1×1010 PFU/mL), and Ad-mCherry-hATP2Aa used to up regulate SERCA2a expression (human ATP2A2a, with mCherry driven under its own cytomegalovirus promoter; stock: 1.6×1010 PFU/mL).
Statistics
All statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA). Data are presented as means±SEM. Analysis was performed using paired or unpaired Student t test as appropriate after testing and confirming all data sets were normally distributed. For all experiments, statistical significance was accepted at P<0.05.
An expanded materials and methods section is available in the online-only Data Supplement.
Results
Confirmation of SERCA2a Gene Transfer Into the Right Atria by Western Blot
Percutaneous right atrial injection of SERCA2a or mCherry empty (3×109 PFU/mL) was confirmed by Western blot analysis (Figure 1A). Atrial myocytes endogenously express SERCA2a, and this expression level was significantly enhanced with atrial transfection with Ad-SERCA2a, when normalized to loading control (Figure 1C) (empty: 31.9±8.5%, n=6; SERCA: 60.9±4.1%, n=7; **P<0.01).
Figure 1.
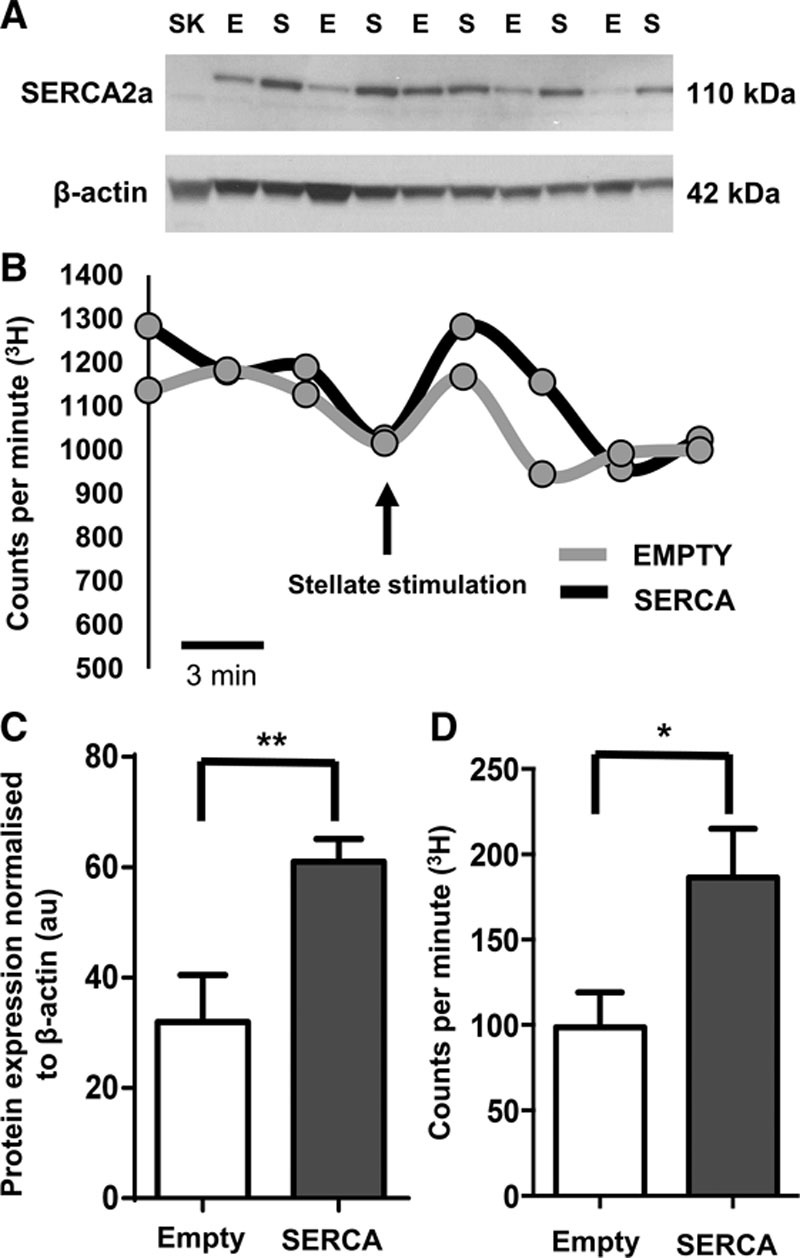
A, Western blot of right atrial tissue from adult (16 to 18 wk, 350–380 g) Sprague–Dawley (SD) rats who received right atrial percutaneous injection and viral gene transfer 5 d before dissection. B, Representative raw data traces showing [3H]-norepinephrine (NE) release from 350 to 380 g SD rat right atria in response to stellate stimulation (5 Hz, 1 minute), samples taken every 3 minutes, arrow indicates the time point at which the right stellate was stimulated, data point after stimulation taken as the peak in counts per minute (cpm). C, SERCA2a (sarcoendoplasmic reticulum calcium ATPase 2a) expression is significantly higher in atria receiving Ad-mCherry-SERCA2a gene transfer (S) than those receiving Ad-mCherry empty gene transfer (E) in which only endogenous SERCA2a is seen. No SERCA2A expression in skeletal muscle (SK) negative control, β-actin loading control expressed in all lanes. **P<0.01. D, Group mean data of delta CPM of [3H]-NE release (empty; n=7; SERCA; n=8). *P<0.05.
Effect of Right Stellate Stimulation on [3H]-NE Release After Gene Transfer
Right atrial injection of the SERCA2a viral vector transgene significantly increased [3H]-norepinephrine [3H]-NE) release in response to right stellate stimulation compared with atria that received injection of mCherry empty vector (Figure 1B and 1D; empty: 98.7±20.5 cpm, n=7; SERCA: 186.5±28.41 cpm, n=8; *P<0.05). This demonstrates that overexpression of SERCA2a can directly increase sympathetic neurotransmission.
Intracellular Free Ca2+ Transients in Ad-SERCA2a–Transduced Stellate Neurons of the SD Rat
Isolated stellate ganglia neurons from 4-week-old normotensive SD rats were transfected with either Ad-mCherry (empty) or Ad-mCherry-hATP2Aa (SERCA2a). Transfection of the desired gene was confirmed by only selecting cells for further experiments, which expressed the mCherry tag under 587nm excitation (Figure 2A) because gene transfer is not homogeneous. An example of the evoked intracellular free Ca2+ concentration change [Ca2+]i is shown in Figure 2B with group mean data (Figure 2C). SD stellate ganglion neurons overexpressing SERCA2a exhibit a significantly greater depolarization-induced Ca2+ transient than those infected with the mCherry empty vector (empty: 0.64±0.03 au, n=57; SERCA: 0.75±0.03 au, n=68; *P<0.05). The time taken for the peak of the [Ca2+]i to fall by 50% was also significantly shorter in the SERCA2a-treated neurons (empty: 0.88±0.06 s, n=37; SERCA: 0.73±0.04 s, n=68; *P<0.05; Figure 2D).
Figure 2.
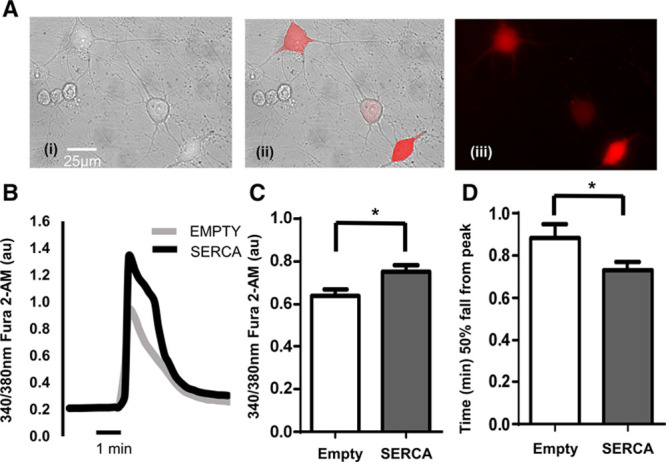
A, Ad-mCherry-hATP2Aa transfected stellate ganglia neurons from (4 to 5 wk, 90–120 g) Sprague–Dawley (SD) rat. (i) Bright field image, (ii) composite, and (iii) excitation at 587 nm to excite mCherry fluorescent tag. Only cells expressing mCherry fluorescence were used for experiments. B, Example raw data trace from isolated stellate ganglia neurons of the young SD rat (gray line, Ad-mCherry [empty]; black line, Ad-mCherry-hATP2Aa [SERCA (sarcoendoplasmic reticulum calcium ATPase)]) exposed to 50 mmol/L of KCl (30 s) to depolarize the neuron resulting in an increase in intracellular free Ca2+ ([Ca2+]i). C, Group mean data showing peak depolarization-evoked intracellular free Ca2+ increase between Ad-mCherry (gray; n=57) and Ad-mCherry-hATP2Aa (black; n=68) transfected stellate neurons. D, Group mean data of 50% fall time of ([Ca2+]i) from the peak (Ad-mCherry, gray; n=37; Ad-mCherry-hATP2Aa, black; n=42). *P<0.05.
ER Ca2+ Handling Within SD Stellate Neurons
Ca2+ concentrations from the ER were measured by monitoring [Ca2+]i change in response to caffeine (10 mmol/L for 30 seconds) to deplete ER Ca2+ stores and thapsigargin (1 μmol/L) to block ER Ca2+ reuptake. SERCA2a-treated cells had a significantly greater increases in [Ca2+]i in response to caffeine (Figure 3A and 3B; empty: 0.03±0.01 au, n=35; SERCA: 0.15±0.01 au, n=45) and thapsigargin (empty: 0.03±0.001 au, n=33; SERCA: 0.12±0.01 au, n=42; **P<0.01). This would support the idea that the increased depolarization-induced Ca2+ transients observed in the SERCA2a-treated neurons are likely because of greater SERCA2a expression, resulting in greater Ca2+ load in the ER which is in turn mobilized by calcium-induced calcium release. Not all neurons in wells incubated with the virus expressed the mCherry tag (efficiency ≈ 60–70%). In some experiments within one field of view, separate neurons with varying expression levels could be seen. Within dishes infected with the SERCA2a transgene, cells not expressing mCherry had caffeine and thapsigargin responses similar to empty vector–treated neurons.
Figure 3.
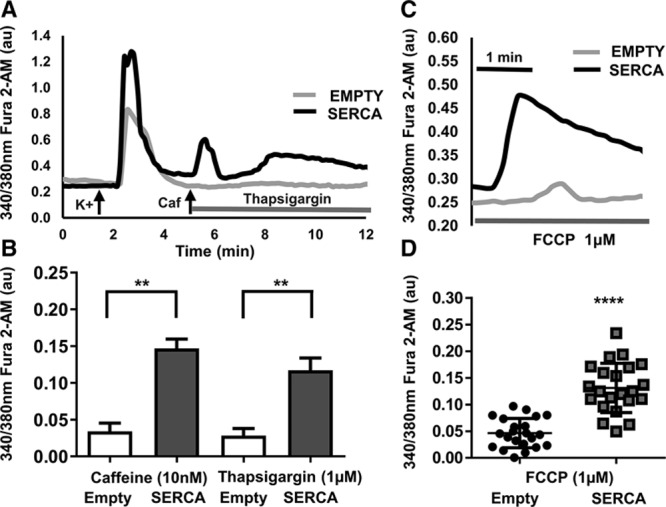
A, Representative raw data trace showing fluorescence ratio of fura-2AM to assess the effect of SERCA (sarco-endoplasmic reticulum calcium ATPase) gene transfection on endoplasmic reticulum (ER) Ca2+ handling. The effect of caffeine (10 mmol/L for 30 s at 5 min; to empty ER Ca2+ store) and thapsigargin (1 μmol/L thapsigargin from 5 min; to prevent ER Ca2+ reuptake) evoked intracellular Ca2+ changes in isolated Sprague–Dawley (SD) stellate neurons transfected with mCherry empty or SERCA. B, Group mean data of [Ca2+]i in response to caffeine (empty: n=35; SERCA: n=45) and thapsigargin (empty: n=33; SERCA: n=42). **P<0.01. C, Representative raw data trace showing the effect mCherry empty or SERCA2a gene transfection had on mitochondrial Ca2+ uncoupling by carbonylcyanide-p-trifluoromethoxyphenylhydrazine (FCCP; 1 μmol/L) in isolated SD rat stellate ganglia neurons. D, Group mean data of [Ca2+]i in response to FCCP (empty: n=22; SERCA: n=22). ****P<0.0001.
Effect on Mitochondrial Ca2+ Handling Within SD Stellate Neurons
The effect of SERCA2a overexpression on mitochondrial Ca2+ handling was observed by using the proton uncoupler carbonylcyanide-p-trifluoromethoxyphenylhydrazine (FCCP; 1 μmol/L) that causes depolarization of the mitochondrial membrane. This results in depletion of Ca2+ stores and inhibition of further mitochondrial Ca2+ uptake.16,22 Application of FCCP produced a transient increase in [Ca2+]i (Figure 3C). This change was significantly higher in the SERCA2a-transduced neurons compared with empty treated cells (Figure 3D; empty: 0.05±0.005 au, n=22; SERCA: 0.13±0.009 au, n=22; **P<0.01). This indicates that not only is ER Ca2+ loading increased by upregulating SERCA2a expression but that the concentration of whole cell bound intracellular Ca2+ had also increased.
Intracellular Free Ca2+ Transients in Ad-SERCA2a–Transduced Stellate Neurons of the SHR and WKY
SHR have previously been shown to exhibit high sympathetic drive, even before the onset of hypertension,16,17,23 and develop heart failure with increasing age,24 compared with the normotensive WKY. Therefore, the effect of SERCA2a upregulation was studied in these neurons to better reflect the disease model.
Representative raw data traces (Figure 4A) illustrate that [Ca2+]i transients were significantly greater in neurons of the SHR compared with the WKY rat (as previously reported17) in both experimental conditions, when the 2 cell types were carrying either the (i) empty or (ii) SERCA2a transgene (*P<0.05). Moreover, in concordance with the results seen in the SD stellate neurons, SERCA2a overexpression increased [Ca2+]i transients compared with empty control cells in both the WKY and the SHR, group mean data (Figure 4B; WKY, empty: 0.45±0.05 au, n=17; SERCA: 0.66±0.09 au, n=13; SHR, empty: 0.65±0.06 au, n=18; SERCA: 0.80±0.04 au, n=25; *P<0.05).
Figure 4.
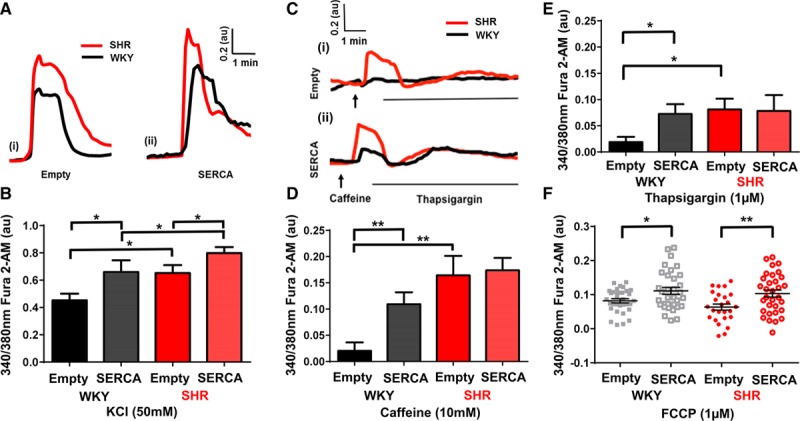
A, An example raw data traces showing free intracellular Ca2+ change in response to KCl (30 s; 50 mmol/L) depolarization of isolated stellate ganglia neurons from Wistar Kyoto (WKY; black) and spontaneously hypertensive rats (SHR; red), transfected with mCherry empty (i) or SERCA (sarco-endoplasmic reticulum calcium ATPase; ii). B, Group mean data of peak depolarization-induced free intracellular Ca2+ change (WKY: empty; n=17; SERCA; n=13; SHR: empty; n=18; SERCA; n=25). *P<0.05. C, Representative raw data trace showing the effect of SERCA gene transfection on endoplasmic reticulum (ER) Ca2+ handling, caffeine (10 mmol/L) and thapsigargin (1 μmol/L), in isolated WKY (black) and SHR (red) stellate neurons transfected with mCherry empty (i) or SERCA (ii). Group mean data of [Ca2+]i in response to caffeine (D) and thapsigargin (E; WKY: empty; n=17; SERCA; n=13; SHR: empty; n=18.;SERCA; n=25). F, Effect on mitochondrial Ca2+ uncoupling by carbonylcyanide-p-trifluoromethoxyphenylhydrazine (FCCP; 1 μmol/L) in isolated WKY and SHR rat stellate ganglia neurons with either mCherry empty or SERCA gene transfection. Group mean data of WKY: empty; n=32; SERCA; n=33; And SHR: empty; n=25; SERCA; n=32. **P<0.01.
Transmission Electron Microscopy of ER of Young 4-Week SHR and WKY
Interestingly, transmission electron microscopy images of the ER from stellate ganglia of 4-week-old SHR and WKY rats shows a striking difference in ER structure and organization (Figure 5). In SHR rats, the ER is organized into spatially compact sheets compared with the more disperse and varied ER form observed in WKY (Figure 5), suggesting that structural changes might underpin ER Ca2+ handling differences observed in the SHR compared with the WKY.
Figure 5.
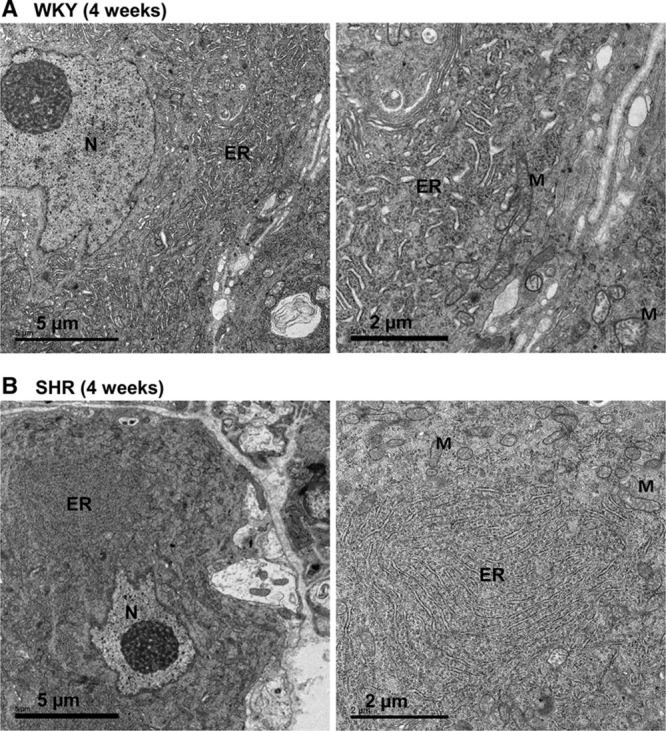
Transmission electron micrographs of stellate neurons in young Wistar Kyoto (WKY) rat (A) and spontaneously hypertensive rats (SHR; B). Endoplasmic reticulum (ER) morphology is affected in stellate neurons from SHRs. WKY rat shows a dispersed ER network, whereas SHR rats exhibit regions of highly enriched sheet-like ER. M indicates mitochondria; and N, nucleus.
ER Ca2+ Handling in Prehypertensive SHR and Age-Matched WKY Stellate Neurons
SERCA2a-treated cells of WKY neurons showed increased [Ca2+]i in response to mobilization of ER load with caffeine (WKY; empty: 0.02±0.02 au, n=17; SERCA: 0.11±0.02 au, n=13; Figure 4C and 4D) and thapsigargin (empty: 0.02±0.01 au, n=17; SERCA: 0.07±0.02 au, n=13; *P<0.05; **P<0.01; Figure 4E). Although mCherry empty-treated SHR neurons had a greater increase in [Ca2+]i in response to caffeine and thapsigargin compared with mCherry empty-treated WKY neurons, no further increase was observed after treatment with SERCA2a (SHR caffeine, empty: 0.16±0.04 au, n=18; SERCA: 0.17±0.02 au, n=25; thapsigargin, empty: 0.08±0.02 au, n=18; SERCA: 0.08±0.03 au, n=25; Figure 4C–4E). These data suggest that stellate neurons of the prehypertensive SHR present altered ER Ca2+ handling compared with normotensive WKY controls, even before upregulation of the SERCA2a transporter, in keeping with the observation that there is more ER per unit of cell volume in SHR neurons. Moreover, under basal conditions, upregulation of SERCA2a seems to have no further compounding effect on ER loading in the SHR, suggesting that the ER may already be working at full Ca2+ load.
Effect of Mitochondrial Ca2+ Handling in SHR and Age-Matched WKY Stellate Neurons
In SHR stellate neurons, transfection with SERCA2a was still able to increase the mitochondrial Ca2+ store as measured using the mitochondrial membrane uncoupler FCCP (Figure 4F), despite not increasing ER Ca2+ load. This may explain why SERCA2a transfection still leads to an increased overall depolarization-induced [Ca2+]i transient in these neurons. WKY neurons overexpressing SERCA2a also had a greater increase in [Ca2+]i in response to FCCP compared with mCherry empty controls (Figure 4F; WKY, empty: 0.08±0.006 au, n=32; SERCA: 0.11±0.01 au, n=33; SHR, empty: 0.06±0.009 au, n=25; SERCA: 0.10±0.01 au, n=32; **P<0.01).
Discussion
The key findings of this study are as follows: (1) The SERCA2a protein (predominantly thought to be the cardiomyocyte isoform of SERCA1,2) can be transduced into rat stellate neurons. (2) Upregulating SERCA2a in normal rat stellate neurons leads to greater depolarization-induced Ca2+ transients, as well as greater ER and mitochondrial Ca2+ load. (3) Right atrial percutaneous injection of the SERCA2a virus results in increased [3H]-NE release in response to right stellate stimulation. (4) Stellate neurons from SHRs have a greater ER calcium load than the WKY and have a greater abundance of ER per unit cell volume compared with WKY neurons. (5) SERCA2a overexpression does not increase ER load further in SHR neurons, but still increases the depolarization-induced Ca2+ transient, potentially through increased mitochondrial calcium loading.
SERCA2a Upregulation Results in Enhanced Intracellular Ca2+ Handling in Sympathetic Neurons
The predominant neuronal SERCA isoforms are SERCA3 in the cerebral cortex,25 SERCA2b in hippocampal pyramidal neurons,26 with low levels of SERCA2a expression in superior cervical ganglia neurons of young SHR and WKY rats, with no observed differences between the 2 strains.16 Incorporation of the SERCA2a isoform into cardiac stellate neurons resulted in increased depolarization-induced Ca2+ transients in normotensive and prehypertensive animal models and increased ER Ca2+ load within stellate neurons isolated from young SD and WKY rats. Increased depolarization-induced Ca2+ transients have previously been described in sympathetic neurons of the neonatal to adult SHR compared with age-matched WKY rat controls.17 Isolated neurons from the superior cervical ganglia have alluded to increased ER Ca2+ load in young prehypertensive SHR,16 analogous to the data shown here in stellate neurons exposed to the mCherry empty viral vector. Young prehypertensive SHR had larger ER Ca2+ stores and greater caffeine-evoked ER Ca2+ release compared with WKY neurons. This may be related to the activity of the SERCA transporter that is under the control of regulatory protein phospholamban. Phospholamban (PLN) is a small phosphoprotein that can regulate the activity of the SERCA. Dephosphorylated PLN is an inhibitor of SERCA, whereas phosphorylation of PLN relieves its inhibition.27 We have previously reported that expression of phosphorylated (Ser16) compared with total PLN is reduced in prehypertensive SHR superior cervical ganglion neurons. Therefore, less dephosphorylated PLN may increase SERCA activity, resulting in more rapid reuptake of calcium into the ER and faster recovery of the intracellular calcium transient in the prehypertensive SHR.16 We have now evaluated SERCA2a and PLN expression in stellate ganglia of WKYs and SHRs at 9 to 10 months of age when the SHR develops impaired left ventricular function.28 At this age, these preliminary data suggest there is no apparent statistical difference in the expression of both SERCA2a (WKY: 1.000±0.0003 au, n=3; SHR: 0.572±0.232 au, n=3) and PLN (WKY: 1.01±0.01 au, n=3; SHR: 1.52±0.52 au, n=3). However, given the small sample size and the difficulty in extracting sufficient levels of protein from this small ganglion, we cannot rule out that physiological reductions in SERCA2a occurred.
Although SERCA2a overexpression increased ER Ca2+ load in WKY stellate neurons to a level comparable with the SHR, no difference was seen in the ER load of the SHR between SERCA2a overexpression and control. This indicates that part of the faulty and heightened Ca2+ handling observed in stellate neurons of the SHR may be because of already maximal Ca2+ loading of the ER. Electron microscopy of the young WKY and prehypertensive SHR indicates that the ER is more densely and structurally organized within stellate neurons of the SHR. Although ER load was not altered in SHR neurons with SERCA2a overexpression, an increase in depolarization-induced Ca2+ transients in the SHR was still observed that may be because of both a greater ER and a mitochondrial Ca2+ load and release after subsequent depolarization. It remains to be seen whether sympathetic neuronal ER Ca2+ is already maximally loaded in a heart failure model, and whether upregulating SERCA2a in these neurons would also increase the depolarization-induced calcium transient and subsequent NE release. However, the fact that there is no difference in the expression of SERCA2a or PLN in 9- to 10-month-old SHR and WKYs and SERCA2a overexpression is still able to increase the depolarization-induced Ca2+ transient in prehypertensive SHRs when the ER is fully loaded makes potentiation of sympathetic neurotransmission likely.
Mitochondria are fundamentally important for maintaining cellular Ca2+ homeostasis, as well as energy production. Mitochondrial research has shown them to be necessary for regulating Ca2+ in many physiological processes, including vasomotion in blood vessels29 and accumulation of Ca2+ when cytosolic levels are low in synaptosomes.30 Functional or direct coupling of the ER and mitochondria has been suggested in many cell types, including sympathetic neurons,31,32and mitochondria have been indicated to be involved in the uptake of ER-released Ca2+, regulating neuronal excitability.32 FCCP depolarization within this study has been used as a means to assess mitochondrial Ca2+ load, although it does not rule out that part of the Ca2+ transient observed with FCCP could be because of a coupling between the mitochondria and the ER. The difference observed with FCCP-liberated free Ca2+ in SHR SERCA2a neurons indicates that FCCP is predominantly releasing Ca2+ from a non-ER store. The transient time scales of the application of FCCP reduce the chance that the observed Ca2+ transients are because of changes in energy production of the cell inhibiting SERCA activity by reducing ATP production.
Increased mitochondrial Ca2+ concentrations alter the mitochondrial membrane potential, with elevated mitochondrial Ca2+ levels being linked to impaired mitochondrial energetics33 and increased oxidative stress within cardiomyocytes.34,35 Previously, SERCA2a transgene in cardiomyocytes has been predicted to be protective at preventing mitochondrial stress by ensuring resting intracellular Ca2+ levels remain low,36 thereby protecting the myocardial energetics of the cell.37,38 We could not directly record the mitochondrial membrane potential in this study because of the mCherry fluorescent tag exhibiting cross fluorescent specter with tetramethylrhodamine ethyl ester (used to measure mitochondrial membrane potential16). Therefore, it remains to be established whether SERCA overexpression protects neuronal energetics.
SERCA2a Overexpression Results in Increased Neurotransmitter Release in Response to Right Stellate Stimulation
Right atrial injection of adeno- and lentiviral constructs has previously been established as viable tools to upregulate target genes of interest that can modulate neurotransmission in cardiac autonomic nerves.39–43 We established whether the increased depolarization-induced Ca2+ transients and elevated intracellular Ca2+ handling observed in isolated stellate neurons functionally translates. Direct stimulation of the isolated stellate ganglia infected with the SERCA2a gene construct resulted in significantly greater neurotransmitter release from the atria and potentially greater postsynaptic excitability. Although we have highlighted the effect that incorporation of atrial-injected SERCA2a gene transfer on sympathetic neurons may have, we cannot rule out that the viral construct could also be expressed in both cardiac afferent and vagal nerve fibers. This may alter local network processing and subsequent NE release, for example, via the release of other neurotransmitters and neuropeptides.
Limitations
As has previously been described,39 transfection rate with adenovirus is not 100% efficient (≈70%); therefore, it was vital that before Ca2+ imaging experiments, only cells expressing the target gene were used. Our mCherry fluorescent tag confirmed cells had integrated the transgene and were expressing the protein of interest. Inefficient transfection rate without a method of monitoring gene delivery could result in inconsistent or false-negative results.
Although within this study we have highlighted the effects of incorporation of SERCA2a into cardiac sympathetic nerves, the stellate ganglia contain a heterogeneous profile of sympathetic efferent cardiac and noncardiac neurons.44,45 The promiscuous nature of the cytomegalovirus-Ad viruses used means it is highly likely that all neurons of the stellate ganglia had the potential to overexpress SERCA2a after viral transfection. Overexpression of SERCA2a could have resulted in altered intracellular Ca2+ handling in neurons innervating noncardiac, as well as cardiac, tissue. Cardiac neurons and noncardiac neurons from the rat stellate ganglia do not have clearly distinct morphologies or resting membrane potentials45 and have been identified through their electrophysiological responses to cardiac nerve stimulation45 or through their endogenous activity in relation to the cardiac cycle,44 which cannot be assessed in isolated cultured neurons during calcium imaging. The inclusion of some noncardiac neurons may have introduced variability within our experimental groups although all sympathetic neurons responded similarly with SERCA2a overexpression.
To assess the functional significance of SERCA2a gene therapy coinfecting the cardiac autonomic axis, in vivo large mammal models would have to be studied to establish whether this gene transfer approach translated into more cardiac excitability. Specifically, this would need to be performed in an established heart failure model. Because adenovirus and AAV have high specificity of transfection for both myocytes and nerve cells,13 it is plausible that the intracoronary perfusion of SERCA2a gene constructs within the CUPID trials could have resulted in gene transfection into cardiac sympathetic neurons, as well as cardiomyocytes.9 Studies in spinal cord injury have shown that AAV–green fluorescent protein transduction close to the site of injury can result in the spread of a green fluorescent protein–tagged fluorescence throughout the spinal cord and into the central nervous system.46 This suggests the need for cell specific targeting in gene therapy.
Perspectives
Overexpression of SERCA2a using a promiscuous cytomegalovirus viral promoter resulted in increased neurotransmission and altered intracellular Ca2+ handling within neurons isolated from the stellate ganglia of normotensive rats. Recent use of gene therapy in clinical trials of heart failure failed to show a beneficial effect of SERCA2a overexpression targeted at myocytes, constructed under a similar promiscuous promoter. The potential for off target expression of the SERCA2a transgene in other cell types, including sympathetic neurons, may have compounded these results. Whether SERCA2a overexpression has a similar effect on the cardiac sympathetic neural axis in heart failure remains to be established.
Acknowledgments
Transmission electron microscopy work was undertaken in the Dunn School Electron Microscopy Facility, and we are grateful to Anna Pielach for preparing the samples for transmission electron microscopy analysis.
Sources of Funding
This work was supported by the British Heart Foundation (BHF) Centre of Research Excellence, Oxford. N.H. is a British Heart Foundation Intermediate Fellow (FS/15/8/3115).
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.116.08507/-/DC1.
Novelty and Significance
What Is New?
The first study to demonstrate that upregulating a myocyte isoform of SERCA (sarcoendoplasmic reticulum calcium ATPase) into isolated sympathetic neurons affects intracellular Ca2+ handling dynamics.
What Is Relevant?
Dysregulation of SERCA2a in heart failure has led to the development of therapeutic strategies to upregulate its activity in myocytes. It is feasibly possible these viruses may also infect sympathetic neurons altering their Ca2+ handling phenotypes.
An observed increase in endoplasmic reticulum (ER) Ca2+ capacity within the prehypertensive spontaneously hypertensive rats may contribute to the sympathetic hyperactivity.
Summary
An adenoviral gene transfer technique to upregulate SERCA2a in isolated sympathetic neurons resulted in altered intracellular Ca2+ handling, greater ER Ca2+ load, and greater depolarization-induced Ca2+ transients within 4- to 5-week Sprague–Dawley and Wistar Kyoto rats. Upregulation of SERCA2a expression had no effect on the ER Ca2+ load within 4- to 5-week prehypertensive spontaneously hypertensive rats, and transmission electron microscopy revealed a more disperse and varied ER appearance compared with the Wistar Kyoto, indicating that increased ER Ca2+ capacity and altered intracellular Ca2+ handling within the prehypertensive spontaneously hypertensive rats may contribute to the observed sympathetic hyperactivity. Therapeutic targets aimed at reducing rather than increasing ER load within sympathetic neurons in hypertension may be beneficial.
References
- 1.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ Res. 2012;110:1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 3.Limas CJ, Olivari MT, Goldenberg IF, Levine TB, Benditt DG, Simon A. Calcium uptake by cardiac sarcoplasmic reticulum in human dilated cardiomyopathy. Cardiovasc Res. 1987;21:601–605. doi: 10.1093/cvr/21.8.601. [DOI] [PubMed] [Google Scholar]
- 4.Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, Garcia E, O’Gara P, Liang L, Kohlbrenner E, Hajjar RJ, Peters NS, Poole-Wilson PA, Macleod KT, Harding SE. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol. 2011;4:362–372. doi: 10.1161/CIRCEP.110.961615. doi: 10.1161/CIRCEP.110.961615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del MonteF, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terracciano CM, Hajjar RJ, Harding SE. Overexpression of SERCA2a accelerates repolarisation in rabbit ventricular myocytes. Cell Calcium. 2002;31:299–305. doi: 10.1016/s0143-4160(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 7.Kawase Y, Ly HQ, Prunier F, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) Trial Investigators. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase ½ clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Investigators. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratner M. Heart failure gene therapy disappoints but experts keep the faith. Nat Biotechnol. 2015;33:573–574. doi: 10.1038/nbt0615-573a. doi: 10.1038/nbt0615-573a. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, Barnard D, Bouchard A, Jaski B, Lyon AR, Pogoda JM, Rudy JJ, Zsebo KM. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016;387:1178–1186. doi: 10.1016/S0140-6736(16)00082-9. doi: 10.1016/S0140-6736(16)00082-9. [DOI] [PubMed] [Google Scholar]
- 12.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 13.Lovric J, Mano M, Zentilin L, Eulalio A, Zacchigna S, Giacca M. Terminal differentiation of cardiac and skeletal myocytes induces permissivity to AAV transduction by relieving inhibition imposed by DNA damage response proteins. Mol Ther. 2012;20:2087–2097. doi: 10.1038/mt.2012.144. doi: 10.1038/mt.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong X, Engehausen DG, Freund CT, Agoulnik I, Guo Z, Oehler MK, Kim TE, Hasenburg A, Contant CF, Woo SL, Kieback DG. The efficacy of adenovirus-mediated gene therapy of ovarian cancer is enhanced by using the cytomegalovirus promoter. Anticancer Res. 1998;18:719–725. [PubMed] [Google Scholar]
- 15.Fukazawa T, Matsuoka J, Yamatsuji T, Maeda Y, Durbin ML, Naomoto Y. Adenovirus-mediated cancer gene therapy and virotherapy (Review). Int J Mol Med. 2010;25:3–10. [PubMed] [Google Scholar]
- 16.Li D, Lee CW, Buckler K, Parekh A, Herring N, Paterson DJ. Abnormal intracellular calcium homeostasis in sympathetic neurons from young prehypertensive rats. Hypertension. 2012;59:642–649. doi: 10.1161/HYPERTENSIONAHA.111.186460. doi: 10.1161/HYPERTENSIONAHA.111.186460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shanks J, Manou-Stathopoulou S, Lu CJ, Li D, Paterson DJ, Herring N. Cardiac sympathetic dysfunction in the prehypertensive spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2013;305:H980–H986. doi: 10.1152/ajpheart.00255.2013. doi: 10.1152/ajpheart.00255.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 19.Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler MD. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- 20.Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation. 1988;77:721–730. doi: 10.1161/01.cir.77.4.721. [DOI] [PubMed] [Google Scholar]
- 21.Zucker IH, Wang W, Brändle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis. 1995;37:397–414. doi: 10.1016/s0033-0620(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 22.Antigny F, Girardin N, Raveau D, Frieden M, Becq F, Vandebrouck C. Dysfunction of mitochondria Ca2+ uptake in cystic fibrosis airway epithelial cells. Mitochondrion. 2009;9:232–241. doi: 10.1016/j.mito.2009.02.003. doi: 10.1016/j.mito.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Shanks J, Mane S, Ryan R, Paterson DJ. Ganglion-specific impairment of the norepinephrine transporter in the hypertensive rat. Hypertension. 2013;61:187–193. doi: 10.1161/HYPERTENSIONAHA.112.202184. doi: 10.1161/HYPERTENSIONAHA.112.202184. [DOI] [PubMed] [Google Scholar]
- 24.Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OH. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation. 1995;91:161–170. doi: 10.1161/01.cir.91.1.161. [DOI] [PubMed] [Google Scholar]
- 25.Pottorf WJ, De Leon DD, Hessinger DA, Buchholz JN. Function of SERCA mediated calcium uptake and expression of SERCA3 in cerebral cortex from young and old rats. Brain Res. 2001;914:57–65. doi: 10.1016/s0006-8993(01)02773-1. [DOI] [PubMed] [Google Scholar]
- 26.Kopach O, Maistrenko A, Lushnikova I, Belan P, Skibo G, Voitenko N. HIF-1α-mediated upregulation of SERCA2b: The endogenous mechanism for alleviating the ischemia-induced intracellular Ca(2+) store dysfunction in CA1 and CA3 hippocampal neurons. Cell Calcium. 2016;59:251–261. doi: 10.1016/j.ceca.2016.02.014. doi: 10.1016/j.ceca.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998;78:921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- 28.Maskali F, Poussier S, Louis H, Boutley H, Lhuillier M, Thornton SN, Karcher G, Lacolley P, Marie PY. Assessment of the early stage of cardiac remodeling of spontaneously hypertensive heart failure rats using the quantitative 3-dimensional analysis provided by acipimox-enhanced FDG-PET. Int J Cardiovasc Imaging. 2014;30:449–456. doi: 10.1007/s10554-013-0350-3. doi: 10.1007/s10554-013-0350-3. [DOI] [PubMed] [Google Scholar]
- 29.Harhun MI. Mitochondrial Ca2+ handling is crucial for generation of rhythmical Ca2+ waves in vascular interstitial cells from rabbit portal vein. Cell Calcium. 2015;58:325–329. doi: 10.1016/j.ceca.2015.06.001. doi: 10.1016/j.ceca.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-Serrano A, Satrústegui J. Regulation of cytosolic free calcium concentration by intrasynaptic mitochondria. Mol Biol Cell. 1992;3:235–248. doi: 10.1091/mbc.3.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hongpaisan J, Pivovarova NB, Colegrove SL, Leapman RD, Friel DD, Andrews SB. Multiple modes of calcium-induced calcium release in sympathetic neurons II: a [Ca2+](i)- and location-dependent transition from endoplasmic reticulum Ca accumulation to net Ca release. J Gen Physiol. 2001;118:101–112. doi: 10.1085/jgp.118.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanden Berghe P, Kenyon JL, Smith TK. Mitochondrial Ca2+ uptake regulates the excitability of myenteric neurons. J Neurosci. 2002;22:6962–6971. doi: 10.1523/JNEUROSCI.22-16-06962.2002. doi: 20026707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2008;44:1–5. doi: 10.1016/j.ceca.2008.02.001. doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115:547–555. doi: 10.1172/JCI200524405. doi: 10.1172/JCI24405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Nat l Acad Sci USA. 2015;112:11389–11394. doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sikkel MB, Hayward C, MacLeod KT, Harding SE, Lyon AR. SERCA2a gene therapy in heart failure: an anti-arrhythmic positive inotrope. Br J Pharmacol. 2014;171:38–54. doi: 10.1111/bph.12472. doi: 10.1111/bph.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakata S, Lebeche D, Sakata Y, Sakata N, Chemaly ER, Liang LF, Padmanabhan P, Konishi N, Takaki M, del Monte F, Hajjar RJ. Mechanical and metabolic rescue in a type II diabetes model of cardiomyopathy by targeted gene transfer. Mol Ther. 2006;13:987–996. doi: 10.1016/j.ymthe.2006.01.002. doi: 10.1016/j.ymthe.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Lu CJ, Hao G, Nikiforova N, Larsen HE, Liu K, Crabtree MJ, Li D, Herring N, Paterson DJ. CAPON modulates neuronal calcium handling and cardiac sympathetic neurotransmission during dysautonomia in hypertension. Hypertension. 2015;65:1288–1297. doi: 10.1161/HYPERTENSIONAHA.115.05290. doi: 10.1161/HYPERTENSIONAHA.115.05290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li D, Nikiforova N, Lu CJ, Wannop K, McMenamin M, Lee CW, Buckler KJ, Paterson DJ. Targeted neuronal nitric oxide synthase transgene delivery into stellate neurons reverses impaired intracellular calcium transients in prehypertensive rats. Hypertension. 2013;61:202–207. doi: 10.1161/HYPERTENSIONAHA.111.00105. doi: 10.1161/HYPERTENSIONAHA.111.00105. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Li D, Dawson TA, Paterson DJ. Long-term effect of neuronal nitric oxide synthase over-expression on cardiac neurotransmission mediated by a lentiviral vector. J Physiol. 2009;587(pt 14):3629–3637. doi: 10.1113/jphysiol.2009.172866. doi: 10.1113/jphysiol.2009.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heaton DA, Li D, Almond SC, Dawson TA, Wang L, Channon KM, Paterson DJ. Gene transfer of neuronal nitric oxide synthase into intracardiac Ganglia reverses vagal impairment in hypertensive rats. Hypertension. 2007;49:380–388. doi: 10.1161/01.HYP.0000255792.97033.f7. doi: 10.1161/01.HYP.0000255792.97033.f7. [DOI] [PubMed] [Google Scholar]
- 43.Mohan RM, Heaton DA, Danson EJ, Krishnan SP, Cai S, Channon KM, Paterson DJ. Neuronal nitric oxide synthase gene transfer promotes cardiac vagal gain of function. Circ Res. 2002;91:1089–1091. doi: 10.1161/01.res.0000047531.75030.b5. [DOI] [PubMed] [Google Scholar]
- 44.Armour JA, Randall WC. Rebound cardiovascular responses following stimulation of canine vagosympathetic complexes or cardiopulmonary nerves. Can J Physiol Pharmacol. 1985;63:1122–1132. doi: 10.1139/y85-184. [DOI] [PubMed] [Google Scholar]
- 45.Mo N, Wallis DI, Watson A. Properties of putative cardiac and non-cardiac neurons in the rat stellate ganglion. J Auton Nerv Syst. 1994;47:7–22. doi: 10.1016/0165-1838(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 46.Klaw MC, Xu C, Tom VJ. Intraspinal AAV Injections Immediately Rostral to a Thoracic Spinal Cord Injury Site Efficiently Transduces Neurons in Spinal Cord and Brain. Mol Ther Nucleic Acids. 2013;2:e108. doi: 10.1038/mtna.2013.34. doi: 10.1038/mtna.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]


