Abstract
The c-Met receptor, also known as the HGF receptor, is one of the most studied tyrosine kinase receptors, yet its biological functions and activation mechanisms are still not fully understood. c-Met has been implicated in embryonic development and organogenesis, in tissue remodelling homeostasis and repair and in cancer metastasis. These functions are indicative of the many cellular processes in which the receptor plays a role, including cell motility, scattering, survival and proliferation. In the context of malignancy, sustained activation of c-Met leads to a signalling cascade involving a multitude of kinases that initiate an invasive and metastatic program. Many proteins can affect the activation of c-Met, including a variety of other cell surface and membrane-spanning molecules or receptors. Some cell surface molecules share structural homology with the c-Met extracellular domain and can activate c-Met via clustering through this domain (e.g., plexins), whereas other receptor tyrosine kinases can enhance c-Met activation and signalling through intracellular signalling cascades (e.g., EGFR). In this review, we provide an overview of c-Met interactions and crosstalk with partner molecules and the functional consequences of these interactions on c-Met activation and downstream signalling, c-Met intracellular localization/recycling and c-Met degradation.
Keywords: c-Met, HGF receptor, RTK, recycling, crosstalk
1. Introduction
c-Met, also known as the scatter factor receptor, is one of the major players in invasive growth [1] and morphogenetic events during embryogenesis and adult life [2]. c-Met was first identified as a member of the receptor tyrosine kinases family (RTKs) in 1987 [3] and has since attracted widespread interest because of its aberrant activation during the malignant progression of various cancers. Upon binding to its ligand, hepatocyte growth factor (HGF), c-Met triggers the induction of several downstream signalling cascades leading to a multitude of outcomes: increased survival, proliferation, anchorage-independent growth, enhanced motility, migration, scattering, metastasis and invasion [4,5,6,7,8,9,10].
2. c-Met Structure and Activation
c-Met is a heterodimer consisting of an extracellular α-chain bound through a disulphide bridge to a transmembrane β-chain [11]. The structure comprises several domains, including a SEMA domain (semaphorin like domain), a PSI domain (plexin-semaphorin-integrin domain) and four IPT domains (immunoglobulin–plexin–transcription factor domain) that share structural homologies with plexins, integrins, semaphorins and immunoglobulins [12]. As soon as HGF is recognized by the immunoglobulin-like domains, two c-Met heterodimers dimerize, leading to the autophosphorylation of two tyrosine residues within the catalytic loop (Tyr1234–Tyr1235). Subsequently, further autophosphorylation of two tyrosines (Tyr1349–Tyr1356) in the C-terminal domain provide the docking platform for the recruitment of other molecular interactors and signal conveyors [13] (Figure 1). Among these are the Grb2-associated binding protein 1 (Gab1) that supplies binding sites for Src–homology-2 domain (SH2)-containing effectors, like the SH2-transforming protein (SHC), “the phosphoinositide 3 kinase (PI3K), the SH2-domain containing protein tyrosine phosphatase (SHP2), the phospholipase Cγ1 (PLCγ1), the signal transducer and activator of transcription 3 (STAT3) and the Ras GTPase p120 [7,8,9,14,15,16]. Most of the cellular responses induced by c-Met activation are mediated by the action of the adaptor molecule, Gab1 [9,17,18]. A sequence of 13 amino acids, identified as the c-Met binding site, is responsible for the direct association of Gab1 to c-Met, leading to the assembly of the docking platform [7,17,18]. Gab1 activity and function are enhanced and prolonged upon HGF-dependent phosphorylation of tyrosine residues in Gab1 that provide extra binding sites for the recruitment of the aforementioned signal transducer and docking proteins. Consequently, by acting as a molecular bridge to unleash c-Met signalling, Gab1 functions as an upstream initiator of the Ras–ERKs/MAPKs cascade.
Figure 1.
Activation, signalling, internalization and recycling of c-Met. (A) Representation of the activation of the c-Met heterodimer illustrating the catalytic and docking tyrosine residues in the non-phosphorylated inactive state, non-HGF-bound state (left) or the active state (right). HGF-mediated c-Met activation triggers the sequential trans-phosphorylation of catalytic tyrosines (Tyr1234-1235, red residues) and docking tyrosines (Tyr1349-1356, blue residues), determining the biochemical signature for the further recruitment of amplifier and transducer molecules. Grb2 and STAT3 directly associate with the c-Met carboxy-terminal tail, while Gab1 interacts both indirectly (through Grb2) or directly providing a docking structure for SHC, PI3K, SHP2, PLCγ1 and p120, resulting in activation of the downstream signalling pathways; (B) Representation of the internalization and recycling of c-Met. To prevent c-Met over-stimulation, several protein-tyrosine phosphatases downregulate the c-Met signal, reverting the catalytic and docking tyrosines to a non-phosphorylated inactive state. The dissociation of the intermediate molecules from the complex refines the tuning and timing of the c-Met-mediated biological response and allows further internalization of c-Met. The Cbl E3 ubiquitin ligase mediates ubiquitination of c-Met, providing a signal for c-Met internalization, which has been shown to be clathrin- or caveolin-dependent. Internalization can be enhanced by Grb2, CIN85, SNX2, CD44v6 and PTP1B. Once internalized, the c-Met receptor can be delivered to lysosomes to be degraded or it can be recycled back to the plasma membrane through the endosomal compartments. PKCε is important for the delivery towards the endosomes, from which the c-Met receptor can signal to specific signalling routes, including STAT3. Recycling back to the plasma membrane has been shown to be dependent on RCP, GGA3 and/or TSN4.
To regulate the intensity of the c-Met signal, another group of proteins regulate the duration of c-Met activity through dephosphorylation of the catalytic and docking tyrosines. These phosphatase tyrosine proteins (PTPs), including the receptor, PTPs density-enhanced phosphatase 1 (DEP1), the non-receptor, PTPs PTP1B, leukocyte common antigen-related (LAR) and T-cell PTP (TCPTP), act as antagonistic c-Met partners in order to prevent prolonged c-Met signalling [19,20,21].
3. c-Met Signalling Cascade
Activation and crosstalk between several signalling pathways has been described following stimulation of the c-Met receptor with its ligand, HGF, leading to a global change in gene expression [22,23].
The mitogen-activated protein kinases (MAPKs) and the extracellular signal-regulated kinases (ERK1/2) are among the major c-Met executors downstream of c-Met activation and interaction with docking molecules [24]. Two main transducer pathways have been shown to link phosphorylated c-Met to the MAPK/ERK components; one involving activation of Ras small GTPase following the association of Grb2-son of sevenless protein (SOS) complex to the c-Met C-terminus [13] and one involving Ras inhibiting protein p120 deactivation upon c-Met–Gab1–SHP2 interaction [25]. Both of these steps result in translocation of ERKs to the nucleus to promote ETS/AP1-mediated transcriptional regulation of cell cycle modulators and adhesion proteins in order to control cell proliferation and motility [5,6,7,8,9,26]. c-Met can also activate the Jun amino-terminal kinases (JNKs) and p38 MAPKs via the same pathways, thereby regulating cytoskeleton-associated proteins that are important in cell migration and scattering [10,27]. Alternatively, PI3K was found to be directly activated by c-Met or indirectly by Ras-driven Akt/PKB signalling, which, in turn, leads to BCL2 antagonist of cell death (Bad) inactivation and to MDM2-mediated p53 degradation, promoting enhanced cell survival [28,29,30]. STAT3 associates with phosphorylated c-Met and undergoes phosphorylation itself, after which it translocates into the nucleus and increases transcription of genes involved in tumourigenesis [31,32].
4. c-Met Endocytosis and Recycling
As with all growth factor-induced receptors, ligand-induced signalling through c-Met is tightly controlled. One of the control mechanisms comprises the rapid internalization and degradation/recycling of the receptor in a process called receptor-mediated endocytosis [33]. The rapid removal of the receptor from the plasma membrane was described as an important mechanism by which cells quickly switch off further signalling to prevent sustained stimulation of the receptor, which can otherwise promote transformation [33]. Ligand stimulation of c-Met leads to polyubiquitinated c-Met and proteasomal degradation upon c-Met internalization [34]. Petrelli et al. demonstrated that internalization and degradation was dependent on c-Met binding to the endophilins, CIN85 and Cbl, through a process of clathrin-coated vesicle formation [35]. However, more recently, clathrin-independent endocytosis of the c-Met receptor through interaction with caveolin has also been demonstrated [36]. A pivotal role for Cbl in c-Met degradation was confirmed by Li et al. and provided evidence that the signalling adaptor protein, Grb2, is required for c-Met endocytosis [37]. Other factors involved in c-Met internalization are dorsal ruffle formation [38], sorting nexin 2 (SNX2) [39] and the c-Met interaction partner, CD44v6 [40] (see the CD44 section). Upon internalization, the endoplasmic reticulum-localized protein-tyrosine phosphatase 1B (PTP1B) and dynamin were found to coordinate the early events of endosome formation that dictate c-Met trafficking and degradation [34,41,42]. The fact that Cbl can promote degradation, but is not required for internalization [37], suggests that c-Met endocytosis and c-Met degradation are different processes that might serve different purposes. Indeed, it has been suggested that the compartmentalization of growth factor receptors plays a crucial role in the signal transduction process and that rather than being just destined for irreversible degradation, the endocytic vesicles might provide a modulatory substation that retains a targeted receptor to act at the right time at the right place with the right signalling output [43]. Evidence that c-Met localization determines signalling is provided by Kermogant et al., who showed that the translocation of c-Met from the endosomal compartment to the perinuclear compartment is dependent on PKCα, and inhibition of PKCα resulted in a decreased signalling of c-Met to STAT3 [44]. The same group also revealed that c-Met signalling to Rac1 is differently regulated from c-Met localized in peripheral endosomes than in perinuclear endosomes [45]. Trafficking to the perinuclear regions depends on an interaction with PKCε, which was demonstrated to be important for p-ERK1/2 accumulation at focal complexes upon c-Met activation [46].
Furthermore, various groups have provided evidence that endosomal-localized c-Met is not just destined to be degraded, but can be returned to the plasma membrane in a process that is called receptor recycling. Receptor recycling has long been demonstrated for another RTK, EGFR [47], and provides a means to sustain downstream signalling. Using c-Met mutant proteins that were found in renal cancers, Joffre et al. showed that the mutant versions of c-Met underwent an increased Cbl–Grb2-dependent recycling and a concomitant aberrant activation of GTPase Rac1, leading to enhanced cell migration, anchorage-independent cell growth and in vivo tumorigenesis [48]. Proteins that have been implicated in recycling back to the plasma membrane include Hrs, Golgi-localized γ-ear-containing Arf-binding protein 3 (GGA3), Tensin-4 and Rab coupling protein (RCP) [49,50,51,52] (Figure 1). The proteasomal inhibitor, lactacystin, promotes c-Met recycling back to the plasma membrane, possibly through preventing c-Met from entering the lysosomal degradation pathway. Lactacystin treatment coincided with a decrease in the endosomal sorting protein, Hrs, and siRNA-mediated Hrs inhibition promoted c-Met activity, suggesting that Hrs dictates c-Met degradation [49]. The adaptor protein, GGA3, interacts with activated c-Met to promote its recycling, and loss of GGA3 resulted in attenuated ERK1/2 activation and pronounced c-Met degradation [52]. Tensins are scaffold proteins that are known for their role in coupling integrin receptors to the cytoskeleton. TNS4, unlike the other tensins, abrogates this link and promotes cell migration. TNS4 was found to also interact with c-Met and to promote its recycling to the plasma membrane to prevent degradation in an integrin-independent manner [50]. Remarkably, we identified that another integrin binding partner, RCP, could also promote c-Met recycling in cells dependent on the expression of an oncogenic mutant p53 protein [53]. Loss of RCP in mutant p53-expressing cells decreased the recycling of c-Met back to the plasma membrane, thereby attenuating ERK1/2 signalling and decreasing cell invasion and cell scattering. These data suggest that the imbalance between recycling and degradation in favour of continuous endosomal trafficking contributes to the maintenance of the activated state of c-Met, leading to pro-malignant signalling.
5. c-Met and Its Membrane-Spanning Partner Molecules
c-Met signalling, degradation, activation and intracellular localization are not only determined by the docking and signalling molecules described so far, but can also be modulated by a large variety of c-Met interacting molecules comprising membrane spanning proteins and receptors, which include plexins, integrins, semaphorins and other RTKs (Table 1 and Figure 2). These proteins interact with c-Met and potentiate, inhibit or modulate the downstream signalling of c-Met, as explained below.
Table 1.
c-Met interacting proteins and their function on c-Met.
| Receptor | Cell System | Effect on c-Met | Biological Response | Reference | |
|---|---|---|---|---|---|
| Plexins | Plexin B1 | HUVEC | Inhibition | ↓ Angiogenesis | [54] |
| HT-29 | Activation | ↑ Invasion | [55] | ||
| SK-BR3, MLP29 | Activation | ↑ Migration ↑ Colony formation, ↑ Invasive growth |
[56] | ||
| YUSIK, MDA-MB 468, MCF-7 | Inhibition | ↓ Migration | [57,58,59,60] | ||
| Plexin B3 | HUVECs | Activation | ↑ Migration | [61] | |
| CD44 | CD44v9 | C4-2, LNCap | Activation | ↑ Resistance, invasion | [62] |
| CD44v6 | WM9, WM164, 1205Lu | Activation | ↑ Migration | [63] | |
| HeLa, HT29, HepG2 | Activation | ↑ c-Met internalization, signalling, scattering | [40,64,65] | ||
| fibroblasts | Activation | ↑ Proliferation | [66] | ||
| CD44v10 | Human pulmonary microvascular EC, B-cells | Activation | ↑ EC barrier enhancement, B-cell survival | [67,68] | |
| Tetraspanin | CD151 | AccM, Acc2 | Activation | ↑ Migration, proliferation | [69] |
| MDA-MB-231 | Activation | ↑ Branching morphogenesis | [70] | ||
| GTL-16 | Activation | ↑ Proliferation, anchorage-independent growth | [71] | ||
| CD82 | PC3, Hepa1-6 | Inhibition | ↓ Migration, invasion | [72,73] | |
| Oligodendrocytes (O4+ cells) | Inhibition | ↓ Differentiation | [74] | ||
| HCV29/YTS1 | Inhibition | ↓ Invasion | [75] | ||
| H1299 | Inhibition | ↓ Migration, lamellipodia formation | [76] | ||
| Integrin | α6β4 | GTL-16, A431, MDA-MB-435 | Activation | ↑ Invasive growth | [71,77] |
| MEFs | Activation | ↑ Colony formation, tumour growth | [78] | ||
| DU145 | Activation | ↑ Self-renewal, invasion | [79] | ||
| HLMVEC, HPAEC | Activation | EC barrier integrity | [80] | ||
| α5β1 | HMVEC | Activation | ↑ Migration, proliferation | [81] | |
| SKOV3ip1, HeyA8 | Activation | ↑ Metastasis | [82] | ||
| α3β1 | Mouse papillary cells | Activation | Kidney morphogenesis | [83] | |
| αxβ1 | PC9 | Activation | ↑ Proliferation | [84] | |
| α2β1 | PMCs | Activation | ↑ PMC activation | [85] | |
| RTKs | Ron | NIH3T3 | Reciprocal Activation | ↑ Colony formation | [86] |
| EGFR | A431, HepG2, AKN-1, HuH6, MRC5 | Activation | ↑ c-Met signalling | [87] | |
| PyVmT, MDA-MB231, 4T1, NCl H596, DLD1, HT29 | Activation | ↑ Motility, proliferation | [88,89,90] | ||
| PC-9, HCC827, SNU-16, MKN45, BT474, SKBR3 | Activation | ↑ Drug resistance | [91,92,93] | ||
| 5637 tumour bladder cell line | Activation | ↑ Survival, cell growth | [94] | ||
| ARPE-19 | Activation, Ecto-domain shedding | ↑ Wound healing | [95] | ||
| A549 | Ecto-domain shedding | NA | [96] | ||
| H1993, EBC1 | Activation | ↑ Survival, proliferation | [97] | ||
| H1975, H520, A549 | Activation | ↑ Tumour growth and survival | [98] | ||
| GEO-CR, SW48-CR | Activation | ↑ c-Met phosphorylation, ↑ Survival |
[99] | ||
| 32D, PC9 | Activation | ↑ c-Met phosphorylation, metastasis, invasion and colony formation | [100] | ||
| 201T, A549 | Activation | ↑ c-Met phosphorylation, xenograft growth | [101] | ||
| Her2 | SK-BR3, BT474 | Activation | ↑ Drug resistance | [102] | |
| H1993, EBC1 | Activation | ↑ Survival, proliferation, ↑ Migration | [97] | ||
| MDCK | Activation | ↑ EMT | [103] | ||
| Her3 | H1993, EBC1 | Activation | ↑ Survival, proliferation | [97] | |
| HCC827, | NA (HER3 activation) * | ↑ Drug resistance | [104] | ||
| MKN45, GTL16 | Activation | ↑ Drug resistance | [91,105] | ||
| IGFR | L3.6pl | Activation | ↑ Migration, invasion | [106] | |
| RET | H1993, EBC1 | Activation | ↑ Migration | [97] | |
| Death receptors | Fas | HepG2, Hepa1-6 | No effect | ↓ Apoptosis | [107,108] |
| HUVECs | NA | ↓ Apoptosis | [109] | ||
| DR5 | Medulloblastoma/glioma cell lines | NA | ↓ Apoptosis | [110] | |
| Mucins | Muc1 | Panc-1, HPAF2, MDA-MB-435, Mahlavu, SNU-449 | Inhibition | ↓ Invasion, EMT | [111,112,113] |
| Muc20 | HEK293, CHO-K1 | Inhibition | ↓ Invasion, EMT | [114] | |
| NRP1 | PCa cells | Activation | ↑ Bone metastasis | [115] | |
| ICAM1 | HT29, HepG2 | Activation | ↑ Proliferation | [116] | |
* Cooperation between c-Met and EGFR was seen, but c-Met activation was not directly determined.
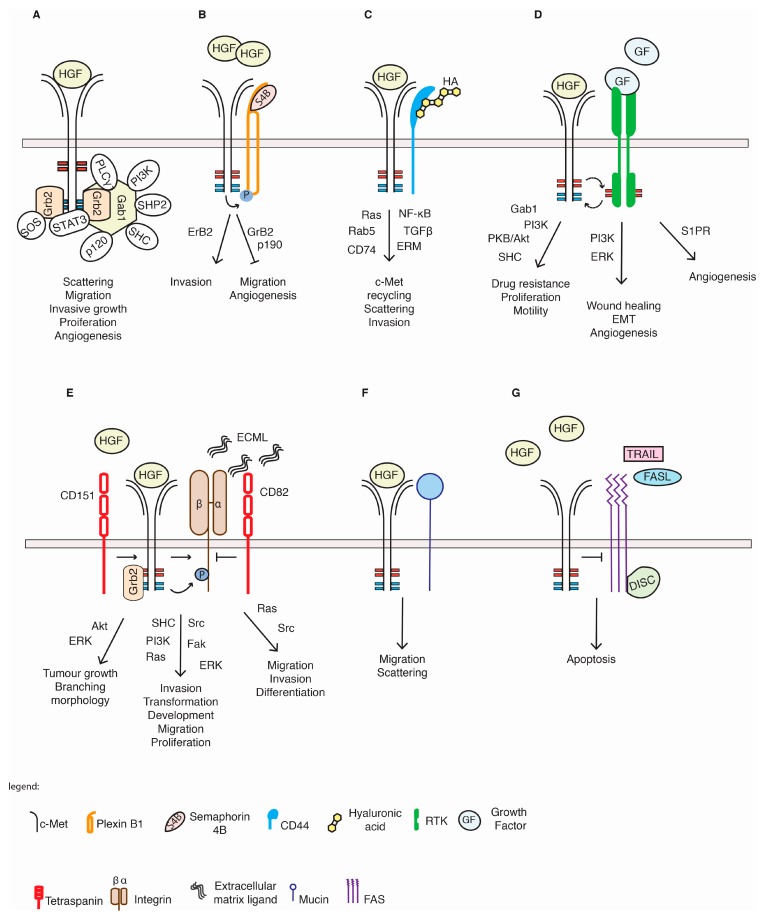
Figure 2.
Membrane molecules that interact with and influence c-Met function. c-Met interacts with a variety of other cell membrane molecules and receptors that can modulate c-Met signalling and cellular outcome. (A) Signalling of the c-Met dimer alone; (B) The plexin B1 extracellular domain has been found to associate with the c-Met counterpart, enhancing ErB2-mediated invasion or repressing c-Met through GrB2-p190 to promote invasion, migration and/or angiogenesis; (C) CD44 family members physically bind to c-Met to promote its internalization, which promoted c-Met-dependent invasion and migration; (D) Among the RTKs, the EGFR association with c-Met has been most studied. Many RTKs can transphosphorylate c-Met tyrosine residues and thereby amplify c-Met-signalling to promote drug resistance, EMT or wound healing. EGFR also promotes c-Met ectodomain shedding, which is not depicted in this figure; (E) Integrins associate with c-Met, enhancing its transforming potential. In detail, HGF and extracellular matrix proteins participate in c-Met/integrin binding, promoting migration and proliferation in a Ras- or Src-dependent manner. Tetraspanins were found to modulate integrin/c-Met function; (F) Mucins inhibit c-Met via unknown mechanisms, leading to decreased HGF-driven migration and scattering; (G) Inactive c-Met can prevent FasL- or TRAIL-driven Fas or DR5 complex formation, with TRAIL resulting in decreased apoptotic signalling.
5.1. Plexin Proteins
Plexins are transmembrane receptors originally discovered as main regulators of semaphorin signalling [117]. They activate downstream signalling pathways, related to cytoskeleton remodelling, via a variety of specific small Rho GTPases, determining cell migration and invasion [118,119]. In the context of RTK crosstalk, the high structural homology of the extracellular domain with plexins, which include a propeller structure involved in protein-protein interactions, make plexin family members good molecular partners for a variety of RTKs [55]. With respect to c-Met, plexin B1 and its ligand, semaphorin 4D, have been most studied, and both have been implicated in cancer progression [120]. The precise role of the plexin B1/Sema4D signalling axis in the malignant phenotype is still unknown, and several reports demonstrate functions both inhibiting and promoting malignancy [120]. This diversity is also reflected in the observation that plexins have opposing roles on c-Met function. Conrotto et al. and Giordano et al. both demonstrated a role for Sema4D to promote c-Met activation and signalling through binding with the plexin B1 receptor in epithelial cells, resulting in increased invasion and migration [55,56]. Others, however, revealed in various systems an inhibitory role for plexin B1 on c-Met function through direct binding, leading to decreased cell migration and decreased angiogenesis [54,57,58,59]. In breast cancer cell lines, plexin B1 was found to be phosphorylated by c-Met, leading to Grb2 (growth factor receptor bound-2) recruitment to the plexin B1/c-Met complex [59]. Grb2 subsequently recruited p190 RhoGAP, resulting in RhoA deactivation and suppression of cell migration. The differential function of Sema4D/plexin in tumour biology is also illustrated by the variable results in studies examining the expression levels of these molecules in the disease progression of human cancers with both low Sem4D and high plexin-B1, leading to poorer survival [55,56,60,61].
The differential role of the plexin B1/Sema4D/c-Met signalling axis in cell migration could be related to the expression of the RTK, ErbB2. While plexin B1 triggered increased cell invasion of LNCaP cells in an ErbB2-dependent manner, PC3 cells highly expressing c-Met displayed decreased motility after plexin B1 activation [121]. Similarly, MDA MB468 cells that express high levels of c-Met were inhibited in cell migration upon Sema4D-induced plexin B1 activation, while migration of MCF7 cells that express high levels of ErbB2 was increased under the same circumstances [60]. Interestingly, the effect on MCF7 could be reversed by knockdown of ErbB2 and overexpression of c-Met. In addition, overexpression of ErbB2 diminished the binding of c-Met to plexin B1, suggesting that in breast cancer cells and in prostate cancer cells, ErbB2 and c-Met are competing for plexin B1 binding, which largely determines the cellular outcome of Sema4D signalling. However, ErbB2 expression levels cannot explain the promoting effect of plexin B1 on c-Met function seen by Giordano et al. and Conrotto et al. [55,56], and it seems likely that other factors must play a role in determining c-Met function in relation to plexin B1 binding. c-Met was also found to interact with another plexin, plexin B3, leading to c-Met activation, leading to increased migration of HUVEC cells [61]. It will be interesting to see whether ErbB2 or other RTKs can influence this interaction and whether c-Met can be regulated by other members of the plexin family.
5.2. CD44 Proteins
CD44 glycoproteins are a family of transmembrane proteins transcribed from one mRNA that undergoes alternative splicing to generate multiple isoforms [122]. Ligands for CD44 are hyaluronic acid and osteopontin, which can promote tumour cell growth and chemotaxis, respectively [122]. A role for CD44 in the HGF signalling path was first described by van der Voort et al. [123], showing that CD44 promotes HGF signalling through c-Met. Mice that have been engineered to lose CD44 expression display haploinsufficiency for c-Met, demonstrating a role for CD44 and c-Met collaboration in vivo [124]. An interaction between c-Met and CD44 proteins was shown by Orian-Rousseau et al. revealing that HGF-dependent scattering and invasion of several cancer cell lines and primary cells require the function of the CD44v6 isoform [64]. This CD44v6 isoform was required for c-Met autophosphorylation and downstream signalling through interaction with ERM (Ezrin, radixin and moesin) proteins that link the actin cytoskeleton to the cellular membrane [64,65,125] and Ezrin phosphorylation indeed is required for HGF-induced cell scattering and morphogenesis [125]. Through this link with the ERM proteins, CD44v6 promotes the internalization of c-Met to a rab5-positive endosomal compartment from which c-Met promoted downstream signalling [40]. Some other studies have since confirmed that overexpression of this particular isoform of CD44 in different tumour cell systems promotes c-Met function and is involved in the crosstalk between c-Met and the NF-κB or the TGF-β1 signalling pathways [63,66]. The role of a co-receptor linking c-Met to ERM proteins is not exclusive to CD44v6, as more recently, ICAM1 was shown to fulfil a similar role in the absence of CD44v6 on c-Met function [116].
Other CD44 isoforms have similarly been shown to promote c-Met function. In prostate cancer cell lines, HGF-dependent activation of c-Met stimulated hyaluronan/CD44v9 signalling, which stabilized the androgen receptor and promoted its function [62]. Singleton et al. demonstrated that CD44 proteins are important regulators of c-Met-mediated vascular barrier enhancement. This process involves the association of CD44v10 with HGF-activated c-Met and its further translocation into Caveolin-enriched microdomains (CEMs, also named lipid rafts) on the cell membrane [68]. The CD44v10/c-Met then recruits Tiam1, cortactin and/or dynamin 2 proteins, providing the components for proper CEM scaffolding that preserves vascular endothelial cell barrier integrity. In summary, these data demonstrate that various CD44 isoforms have a positive role in c-Met functioning by promoting c-Met coupling to the ERM proteins, interaction with other signalling pathways and c-Met localization to specific microdomains on the cellular membrane.
5.3. Integrins
Integrins are heterodimeric membrane receptors responsible for the adhesive interactions between the cell and its surroundings, including the extracellular matrix and neighbouring cells [126]. They exert this scaffold function through the recruitment of a complex network of proteins that connect the actin cytoskeleton to extracellular components [127]. Besides promoting the assembly of tissues with specific physical and mechanical properties, they are also important molecular transducers mediating extracellular stimuli to intracellular signalling pathways. Integrins are composed of an α and β subunit, giving rise to a large number of different integrin molecules that respond to and interact with specific ligands. The heterogeneity in integrin molecules and their ligands is also reflected in the diverse biological functions of these molecules, including cell cycle regulation, modulation of the cell shape, cell motility, invasion and metastasis.
The crosstalk between c-Met and integrins is complex and involves a reciprocal regulation that can be ligand-dependent or -independent and signalling to similar downstream signalling molecules, leading to enhanced activation of those molecules and a c-Met-dependent transcriptional regulation of integrin expression [128].
The best-studied integrin receptor in relation to c-Met signalling is probably the α6β4 receptor, which binds laminin. Although both receptors can individually promote invasion [129], several reports suggest an association between this integrin and c-Met, which amplifies the signalling of both receptors [71,77,78,79,80]. Binding between c-Met and α6β4 promoted phosphorylation of the β4 integrin unit, enabling a subsequent activation of Shc and PI3K. c-Met activation occurred independent of integrin binding to laminin, as a truncated variant of β4 integrin unable to bind the ligand laminin still retained the ability to trigger invasion of cells in a HGF- and c-Met-dependent manner [62,77]. The β4 integrin/c-Met interaction can also impinge on other c-Met functions, as demonstrated in endothelial cells, in which HGF induced the formation of a β4 integrin/c-Met/sphingosine 1-phosphate receptor 1 (S1PR1) complex that was localized at CEMs and promoted vascular integrity [80] or in prostate tumour progenitor cells that underwent self-renewal [79].
c-Met was found to associate with other integrins, including the β1 integrins, and, thereby, promoted an invasive program or induced kidney development [81,82,83,84,85]. Mitra et al. reported that in epithelial cells, fibronectin promoted the association between α5β1 integrin and c-Met, leading to c-Met phosphorylation and increased Src and FAK activation [82]. Interestingly, in agreement with previous studies that reported c-Met activation upon cell adhesion in the absence of ligand, α5β1 integrin-mediated c-Met signalling was independent of the presence of HGF [82,130].
Together, these data demonstrate that a variety of integrins and, by implication, cell adhesion can positively influence c-Met signalling. The interaction between other RTKs and integrins has been studied in more detail and revealed more details on how integrins and the extracellular matrix can regulate RTK function [131]. For example, matrix attachment can exert inhibitory functions on EGFR [132], and EGFR signalling seems critically dependent on EGFR co-recycling with the α5β1 integrin in an RCP-dependent manner [133]. Given that RCP can promote recycling of c-Met [51], it is likely that α5β1 integrin can also promote c-Met recycling. Further research is needed to elucidate the precise functional and mechanistic details underlying the integrin and c-Met interaction and their interplay.
5.4. Tetraspanins
c-Met was also found to interact with members of the tetraspanin superfamily of membrane-spanning proteins. These molecules are known for their tight association with integrins and can modulate their function, possibly through compartmentalization of integrins [134]. It is therefore not surprising that some tetraspanin molecules can impinge on the c-Met/integrin interactions and regulate c-Met signalling. Klosek et al. first showed in human salivary gland cells that CD151 associates with c-Met and α3 or α6 integrin subunits, enhancing HGF/c-Met signalling to promote cell migration and proliferation [69]. Similarly, they found that the CD151 interaction with c-Met in MDA-MB-231 breast cancer cells triggered Akt activation, resulting in branching networks in Matrigel [70]. In GTL16 cells, Franco et al. revealed that CD151 associates with the c-Met receptor to drive β4 integrin phosphorylation and facilitated the coupling between c-Met and Gab1–Grb2 to promote MAPK phosphorylation and tumour growth [71].
Conversely, many publications have demonstrated an inhibitory role for the tetraspanin CD82 in c-Met signalling [72,73,74,75,76]. CD82, possibly in combination with gangliosides [73,75], prevents c-Met phosphorylation upon HGF activation, exposure to the extracellular matrix ligands or EGFR transactivation [72,74,76]. This leads to impaired binding of c-Met to Gab1/Grb2 [76], decreased Ras or Src activation [72,74] and a subsequent decrease in migration, invasion or differentiation. As the tetraspanins consist of a large family of proteins that can interact with different integrins, it is likely that other tetraspanin proteins will contribute to c-Met signalling. One likely candidate could be the tetraspanin, CD9, which was shown to contribute to cell migration, to facilitate FAK phosphorylation and to interact with EGFR [135,136].
5.5. Other RTKs
As signalling from RTKs is often amplified in human cancers, many therapeutics aim to target and inhibit RTKs. Using such inhibitors, it has become obvious that c-Met plays an important role in activating or potentiating the response of other RTKs or vice versa and that amplification or activation of c-Met contributes to chemoresistance. RTKs comprise a family of 58 proteins [137] and the closest related family member of c-Met that c-Met has been shown to associate with is RON [86]. Ligand-dependent hetero-dimerization induced specific transphosphorylation of the respective catalytic and docking sites on either RTK, leading to fully activated downstream signalling pathways and increased colony formation of NIH3T3 cells [86].
A large number of publications describe EGFR and c-Met crosstalk in which either RTK can promote the function of the other RTK and/or converge at similar hubs in their signalling pathways. One of the first reports by Jo et al. analysing this crosstalk revealed that in epithelial cancer cells, but not in “normal” liver cells, c-Met was constitutively phosphorylated upon TGFα and EGF exposure in the absence of HGF [87]. The authors proposed that in tumour cells, TGFα-mediated EGFR activation induces EGFR/c-Met interaction, detectable as co-immunoprecipitation and resulting in increased c-Met phosphorylation and signalling [87]. A cooperation between c-Met and EGFR interaction is even more apparent from the large number of reports that study drug resistance after inhibition of either RTK. Cells treated with EGFR inhibitors can acquire resistance through c-Met activation or amplification [93,138]. As an example, Troiani et al. confirmed the findings of Jo et al. and, in addition, revealed that overexpression of TGF-α could confer resistance to EGFR inhibitors through enhancing the EGFR/c-Met interaction and c-Met phosphorylation [99]. EGFR inhibitors can block HGF-mediated proliferation and motility [88,89], and several studies have highlighted the mutual synergism of c-Met and EGFR in promoting drug resistance, leading to the activation of PI3K/Akt and Gab1 [91,92,93,98,139]. The cellular effect of combination therapy can however be context- and cell-dependent, as demonstrated by Zhang et al. using xenograft models. Where H1993-derived tumours reacted to combination therapy by promoting apoptosis and suppressing proliferation, H1373-derived tumours only responded with decreased proliferation [140]. Several groups have investigated the molecular mechanisms underlying the crosstalk between EGFR and c-Met, and it has become apparent that several partner proteins can affect EGFR-mediated phosphorylation of c-Met, including Src, MAPK and β1 integrins [84,94,100,101]. Conversely, HGF stimulation could also induce EGFR phosphorylation [138,141], pointing out the reciprocal regulation of both receptors. Other molecules that have been implicated in the crosstalk include miR-27A, sprouty, the Wnt and the mTor signaling pathways, although the precise mechanisms underlying their role in the crosstalk are unknown [142,143]. EGFR was also found to promote c-Met oncogenic function via an alternative mechanism through promoting c-Met ecto-domain shedding, leading to enhanced wound healing [95,96], a process that has previously been described to promote the oncogenic function of c-Met [144].
Similar reciprocal relationships were found between c-Met and the EGFR family members, Her2 and Her3 [91,97,102,103,104,105], IGFR [106] and RET [97]. Interestingly, Tanizaki et al. revealed differential functional consequences in a cell line that overexpresses c-Met upon heterodimerization between c-Met and RET, EGFR, Her2 or Her3 [97]. Loss of EGFR and Her3 resulted in a decrease in cell proliferation and survival; loss of RET decreased cell migration; and loss of Her2 decreased proliferation, survival and cell migration [97]. These results suggest some specificity in the functional outcome of c-Met partnerships with other RTKs. As c-Met is often amplified in human tumours and is a target for drug strategies, a more thorough understanding of the crosstalk between c-Met and other RTKs is necessary.
5.6. c-Met and Others
Other membrane spanning molecules that affect c-Met function or that are affected by c-Met interaction are mucins and death receptors. Mucins are transmembrane glycoproteins, and tumour-associated Muc1 has been shown to be important in activating signal transduction pathways to promote invasion, metastasis, proliferation and chemoresistance [145]. As opposed to this pro-tumourigenic role, in pancreatic and liver cancer cells, muc1 interacting with c-Met was found to inhibit c-Met-dependent invasion and migration [111,113,114], possibly through enhanced c-Met turnover [113]. Muc20 similarly inhibited c-Met in Hek293 and CHO-K1 cells [114]. However, in breast cancer cells, Muc1 was shown to promote c-Met-driven migration and scattering through regulation of c-Met mRNA expression levels [112], suggesting that tissue conditional aspects determine the consequences of the relationship between Muc1 and c-Met.
Activation of RAF/MEK/ERK and the PI3K/PDK/Akt promotes proliferation and survival, while preventing apoptosis [146]. Via activation of these signalling routes, c-Met can inhibit apoptosis, but c-Met has also been shown to influence apoptosis through interaction with cell death receptors on the cell membrane. Through interacting with Fas, c-Met prevents Fas trimerization and recruitment of an active DISC complex and, therefore, acts as an antagonist for the Fas ligand (FasL) [107,108,109]. Activation of the c-Met receptor following HGF abrogated the c-Met/Fas interaction and promoted FasL or doxorubicin-induced apoptosis [107,147]. Fatty acids or anoikis also lead to c-Met dissociation from Fas and an increase in the sensitivity to apoptosis, suggesting that the external environment, possibly through the involvement of integrins, could modulate c-Met’s function on Fas [108,109]. Similarly to Fas, DR5 was also found to be inhibited upon c-Met interaction, preventing it from interacting with TRAIL and DISC association [110]. These reports are indicative of a direct role for the inactive c-Met receptor in preventing apoptosis.
6. Conclusions and Future Perspectives
It is clear that c-Met function is dynamically regulated and modulated by its localization, through protein modifications and interaction with a large variety of signalling molecules, co-receptors and other membrane molecules. These interactions can greatly affect the way c-Met reacts to its ligand, HGF, but can also change the way in which c-Met will react to drug strategies that are designed to inhibit c-Met function. Inhibiting c-Met alone might not be enough to fully inhibit the c-Met signalling cascade, as other RTKs might take over this function or RTKs or other cell membrane receptors simply transactivate c-Met in a ligand-independent manner. Inhibiting multiple cell surface molecules (e.g., multiple RTKs), including c-Met, might therefore have some therapeutic potential. Trials in which c-Met and the EGFR are targeted simultaneously reveal that some NSCLC (non-small cell lung cancer) patients responded with an increased progression-free survival and overall survival, although in some patients, dependent on the molecular alterations in their genome, worse outcomes were noted [148,149]. Other combinations of RTK inhibitors for NSCLC are also being explored (summarized in [150]), as well as targeting downstream convergent signaling routes of RTKs [151]. Given that c-Met interacts with other cell surface molecules and not just other RTKs, it might also be worthwhile to investigate combination therapies in which other cell surface molecules, e.g., integrins (for which some approved drugs exist [152]), are co-targeted together with c-Met. This is also interesting in the context of RTK crosstalk, as the interaction between c-Met and integrin β1 was demonstrated to mediate EGFR inhibitor resistance [84]. Alternatively, it might be worthwhile to target common features of cell surface molecules, such as recycling or endocytosis. We have previously identified that recycling of EGFR, c-Met and integrin α5β1 was enhanced in the oncogenic setting of p53 mutations by RCP [51], leading to enhanced signalling towards Akt and ERK1/2 [51,53]. Although a better mechanistic understanding of the mutant p53 functions on RCP is required, it will be interesting to explore RCP inhibition as a means to inhibit recycling of various receptors, including c-Met, integrins and EGFR, simultaneously.
In conclusion, a better understanding of the c-Met interactors and the consequences of these interactions on c-Met signaling will help in the design of novel therapeutic strategies and in understanding the shortcomings of current strategies, including drug resistance.
Acknowledgments
Patricia A. J. Muller is funded by a Sir Henry Dale fellowship from the Royal Society/Wellcome Trust.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 2.Birchmeier C., Birchmeier W., Gherardi E., vande Woude G.F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 3.Park M., Dean M., Kaul K., Braun M.J., Gonda M.A., vande Woude G. Sequence of met protooncogene CDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc. Natl. Acad. Sci. USA. 1987;84:6379–6383. doi: 10.1073/pnas.84.18.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoker M., Gherardi E., Perryman M., Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann G., Weidner K.M., Schwarz H., Birchmeier W. The motility signal of scatter factor/hepatocyte growth factor mediated through the receptor tyrosine kinase met requires intracellular action of ras. J. Biol. Chem. 1994;269:21936–21939. [PubMed] [Google Scholar]
- 6.Khwaja A., Lehmann K., Marte B.M., Downward J. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J. Biol. Chem. 1998;273:18793–18801. doi: 10.1074/jbc.273.30.18793. [DOI] [PubMed] [Google Scholar]
- 7.Maroun C.R., Holgado-Madruga M., Royal I., Naujokas M.A., Fournier T.M., Wong A.J., Park M. The gab1 ph domain is required for localization of gab1 at sites of cell–cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 1999;19:1784–1799. doi: 10.1128/mcb.19.3.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaeper U., Gehring N.H., Fuchs K.P., Sachs M., Kempkes B., Birchmeier W. Coupling of gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 2000;149:1419–1432. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weidner K.M., di Cesare S., Sachs M., Brinkmann V., Behrens J., Birchmeier W. Interaction between gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 10.Lamorte L., Kamikura D.M., Park M. A switch from p130Cas/Crk to Gab1/Crk signaling correlates with anchorage independent growth and jnk activation in cells transformed by the Met receptor oncoprotein. Oncogene. 2000;19:5973–5981. doi: 10.1038/sj.onc.1203977. [DOI] [PubMed] [Google Scholar]
- 11.Giordano S., Ponzetto C., di Renzo M.F., Cooper C.S., Comoglio P.M. Tyrosine kinase receptor indistinguishable from the c-Met protein. Nature. 1989;339:155–156. doi: 10.1038/339155a0. [DOI] [PubMed] [Google Scholar]
- 12.Stamos J., Lazarus R.A., Yao X., Kirchhofer D., Wiesmann C. Crystal structure of the HGF beta-chain in complex with the sema domain of the met receptor. EMBO J. 2004;23:2325–2335. doi: 10.1038/sj.emboj.7600243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponzetto C., Bardelli A., Zhen Z., Maina F., dalla Zonca P., Giordano S., Graziani A., Panayotou G., Comoglio P.M. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 14.Gual P., Giordano S., Williams T.A., Rocchi S., van Obberghen E., Comoglio P.M. Sustained recruitment of phospholipase C-gamma to Gab1 is required for hgf-induced branching tubulogenesis. Oncogene. 2000;19:1509–1518. doi: 10.1038/sj.onc.1203514. [DOI] [PubMed] [Google Scholar]
- 15.Maroun C.R., Naujokas M.A., Holgado-Madruga M., Wong A.J., Park M. The tyrosine phosphatase Shp-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 2000;20:8513–8525. doi: 10.1128/MCB.20.22.8513-8525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montagner A., Yart A., Dance M., Perret B., Salles J.P., Raynal P. A novel role for Gab1 and Shp2 in epidermal growth factor-induced ras activation. J. Biol. Chem. 2005;280:5350–5360. doi: 10.1074/jbc.M410012200. [DOI] [PubMed] [Google Scholar]
- 17.Sachs M., Brohmann H., Zechner D., Muller T., Hulsken J., Walther I., Schaeper U., Birchmeier C., Birchmeier W. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J. Cell Biol. 2000;150:1375–1384. doi: 10.1083/jcb.150.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lock L.S., Royal I., Naujokas M.A., Park M. Identification of an atypical Grb2 carboxyl-terminal Sh3 domain binding site in gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J. Biol. Chem. 2000;275:31536–31545. doi: 10.1074/jbc.M003597200. [DOI] [PubMed] [Google Scholar]
- 19.Machide M., Hashigasako A., Matsumoto K., Nakamura T. Contact inhibition of hepatocyte growth regulated by functional association of the c-Met/hepatocyte growth factor receptor and lar protein–tyrosine phosphatase. J. Biol. Chem. 2006;281:8765–8772. doi: 10.1074/jbc.M512298200. [DOI] [PubMed] [Google Scholar]
- 20.Palka H.L., Park M., Tonks N.K. Hepatocyte growth factor receptor tyrosine kinase Met is a substrate of the receptor protein–tyrosine phosphatase Dep-1. J. Biol. Chem. 2003;278:5728–5735. doi: 10.1074/jbc.M210656200. [DOI] [PubMed] [Google Scholar]
- 21.Sangwan V., Paliouras G.N., Abella J.V., Dube N., Monast A., Tremblay M.L., Park M. Regulation of the met receptor–tyrosine kinase by the protein-tyrosine phosphatase 1B and T-cell phosphatase. J. Biol. Chem. 2008;283:34374–34383. doi: 10.1074/jbc.M805916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai A.Z., Abella J.V., Park M. Crosstalk in met receptor oncogenesis. Trends Cell Biol. 2009;19:542–551. doi: 10.1016/j.tcb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Trusolino L., Comoglio P.M. Scatter-factor and semaphorin receptors: Cell signalling for invasive growth. Nat. Rev. Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 24.Trusolino L., Bertotti A., Comoglio P.M. Met signalling: Principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 25.Pelicci G., Giordano S., Zhen Z., Salcini A.E., Lanfrancone L., Bardelli A., Panayotou G., Waterfield M.D., Ponzetto C., Pelicci P.G., et al. The motogenic and mitogenic responses to hgf are amplified by the shc adaptor protein. Oncogene. 1995;10:1631–1638. [PubMed] [Google Scholar]
- 26.Paumelle R., Tulasne D., Kherrouche Z., Plaza S., Leroy C., Reveneau S., vanden Bunder B., Fafeur V. Hepatocyte growth factor/scatter factor activates the ets1 transcription factor by a ras-raf-mek-erk signaling pathway. Oncogene. 2002;21:2309–2319. doi: 10.1038/sj.onc.1205297. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Guzman M., Dolfi F., Zeh K., Vuori K. Met-induced jnk activation is mediated by the adapter protein crk and correlates with the Gab1–Crk signaling complex formation. Oncogene. 1999;18:7775–7786. doi: 10.1038/sj.onc.1203198. [DOI] [PubMed] [Google Scholar]
- 28.Fan S., Ma Y.X., Gao M., Yuan R.Q., Meng Q., Goldberg I.D., Rosen E.M. The multisubstrate adapter gab1 regulates hepatocyte growth factor (scatter factor)–c-Met signaling for cell survival and DNA repair. Mol. Cell. Biol. 2001;21:4968–4984. doi: 10.1128/MCB.21.15.4968-4984.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao G.H., Jeffers M., Bellacosa A., Mitsuuchi Y., vande Woude G.F., Testa J.R. Anti-apoptotic signaling by hepatocyte growth factor/met via the phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways. Proc. Natl. Acad. Sci. USA. 2001;98:247–252. doi: 10.1073/pnas.011532898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sipeki S., Bander E., Buday L., Farkas G., Bacsy E., Ways D.K., Farago A. Phosphatidylinositol 3-kinase contributes to Erk1/Erk2 map kinase activation associated with hepatocyte growth factor-induced cell scattering. Cell. Signal. 1999;11:885–890. doi: 10.1016/S0898-6568(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 31.Boccaccio C., Ando M., Tamagnone L., Bardelli A., Michieli P., Battistini C., Comoglio P.M. Induction of epithelial tubules by growth factor hgf depends on the stat pathway. Nature. 1998;391:285–288. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y.W., Wang L.M., Jove R., vande Woude G.F. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene. 2002;21:217–226. doi: 10.1038/sj.onc.1205004. [DOI] [PubMed] [Google Scholar]
- 33.Di Fiore P.P., Gill G.N. Endocytosis and mitogenic signaling. Curr. Opin. Cell Biol. 1999;11:483–488. doi: 10.1016/S0955-0674(99)80069-6. [DOI] [PubMed] [Google Scholar]
- 34.Hammond D.E., Urbe S., vande Woude G.F., Clague M.J. Down-regulation of Met, the receptor for hepatocyte growth factor. Oncogene. 2001;20:2761–2770. doi: 10.1038/sj.onc.1204475. [DOI] [PubMed] [Google Scholar]
- 35.Petrelli A., Gilestro G.F., Lanzardo S., Comoglio P.M., Migone N., Giordano S. The endophilin–CIN85–CBL complex mediates ligand-dependent downregulation of c-Met. Nature. 2002;416:187–190. doi: 10.1038/416187a. [DOI] [PubMed] [Google Scholar]
- 36.Korhan P., Erdal E., Kandemis E., Cokakli M., Nart D., Yilmaz F., Can A., Atabey N. Reciprocal activating crosstalk between c-Met and caveolin 1 promotes invasive phenotype in hepatocellular carcinoma. PLoS One. 2014;9:e105278. doi: 10.1371/journal.pone.0105278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li N., Lorinczi M., Ireton K., Elferink L.A. Specific Grb2-mediated interactions regulate clathrin-dependent endocytosis of the c-Met-tyrosine kinase. J. Biol. Chem. 2007;282:16764–16775. doi: 10.1074/jbc.M610835200. [DOI] [PubMed] [Google Scholar]
- 38.Abella J.V., Parachoniak C.A., Sangwan V., Park M. Dorsal ruffle microdomains potentiate met receptor tyrosine kinase signaling and down-regulation. J. Biol. Chem. 2010;285:24956–24967. doi: 10.1074/jbc.M110.127985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogi S., Fujita H., Kashihara M., Yamamoto C., Sonoda K., Okamoto I., Nakagawa K., Ohdo S., Tanaka Y., Kuwano M., et al. Sorting nexin 2-mediated membrane trafficking of c-Met contributes to sensitivity of molecular-targeted drugs. Cancer Sci. 2013;104:573–583. doi: 10.1111/cas.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasenauer S., Malinger D., Koschut D., Pace G., Matzke A., von Au A., Orian-Rousseau V. Internalization of met requires the co-receptor CD44V6 and its link to erm proteins. PLoS One. 2013;8:e62357. doi: 10.1371/journal.pone.0062357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeffers M., Taylor G.A., Weidner K.M., Omura S., vande Woude G.F. Degradation of the Met tyrosine kinase receptor by the ubiquitin-proteasome pathway. Mol. Cell. Biol. 1997;17:799–808. doi: 10.1128/mcb.17.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sangwan V., Abella J., Lai A., Bertos N., Stuible M., Tremblay M.L., Park M. Protein–tyrosine phosphatase 1B modulates early endosome fusion and trafficking of met and epidermal growth factor receptors. J. Biol. Chem. 2011;286:45000–45013. doi: 10.1074/jbc.M111.270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miaczynska M., Pelkmans L., Zerial M. Not just a sink: Endosomes in control of signal transduction. Curr. Opin. Cell Biol. 2004;16:400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Kermorgant S., Parker P.J. Receptor trafficking controls weak signal delivery: A strategy used by c-met for stat3 nuclear accumulation. J. Cell Biol. 2008;182:855–863. doi: 10.1083/jcb.200806076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menard L., Parker P.J., Kermorgant S. Receptor tyrosine kinase c-Met controls the cytoskeleton from different endosomes via different pathways. Nat. Commun. 2014;5:3907. doi: 10.1038/ncomms4907. [DOI] [PubMed] [Google Scholar]
- 46.Kermorgant S., Zicha D., Parker P.J. Pkc controls hgf-dependent c-Met traffic, signalling and cell migration. EMBO J. 2004;23:3721–3734. doi: 10.1038/sj.emboj.7600396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masui H., Castro L., Mendelsohn J. Consumption of EGF by A431 cells: Evidence for receptor recycling. J. Cell Biol. 1993;120:85–93. doi: 10.1083/jcb.120.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joffre C., Barrow R., Menard L., Calleja V., Hart I.R., Kermorgant S. A direct role for Met endocytosis in tumorigenesis. Nat. Cell Biol. 2011;13:827–837. doi: 10.1038/ncb2257. [DOI] [PubMed] [Google Scholar]
- 49.Hammond D.E., Carter S., McCullough J., Urbe S., vande Woude G., Clague M.J. Endosomal dynamics of met determine signaling output. Mol. Biol. Cell. 2003;14:1346–1354. doi: 10.1091/mbc.E02-09-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muharram G., Sahgal P., Korpela T., de Franceschi N., Kaukonen R., Clark K., Tulasne D., Carpen O., Ivaska J. Tensin-4-dependent met stabilization is essential for survival and proliferation in carcinoma cells. Dev. Cell. 2014;29:421–436. doi: 10.1016/j.devcel.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller P.A., Trinidad A.G., Timpson P., Morton J.P., Zanivan S., van den Berghe P.V., Nixon C., Karim S.A., Caswell P.T., Noll J.E., et al. Mutant p53 enhances met trafficking and signalling to drive cell scattering and invasion. Oncogene. 2013;32:1252–1265. doi: 10.1038/onc.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parachoniak C.A., Luo Y., Abella J.V., Keen J.H., Park M. Gga3 functions as a switch to promote met receptor recycling, essential for sustained erk and cell migration. Dev. Cell. 2011;20:751–763. doi: 10.1016/j.devcel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muller P.A., Caswell P.T., Doyle B., Iwanicki M.P., Tan E.H., Karim S., Lukashchuk N., Gillespie D.A., Ludwig R.L., Gosselin P., et al. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009;139:1327–1341. doi: 10.1016/j.cell.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 54.Conrotto P., Valdembri D., Corso S., Serini G., Tamagnone L., Comoglio P.M., Bussolino F., Giordano S. Sema4d induces angiogenesis through met recruitment by plexin b1. Blood. 2005;105:4321–4329. doi: 10.1182/blood-2004-07-2885. [DOI] [PubMed] [Google Scholar]
- 55.Conrotto P., Corso S., Gamberini S., Comoglio P.M., Giordano S. Interplay between scatter factor receptors and b plexins controls invasive growth. Oncogene. 2004;23:5131–5137. doi: 10.1038/sj.onc.1207650. [DOI] [PubMed] [Google Scholar]
- 56.Giordano S., Corso S., Conrotto P., Artigiani S., Gilestro G., Barberis D., Tamagnone L., Comoglio P.M. The semaphorin 4d receptor controls invasive growth by coupling with Met. Nat. Cell Biol. 2002;4:720–724. doi: 10.1038/ncb843. [DOI] [PubMed] [Google Scholar]
- 57.Soong J., Scott G. Plexin B1 inhibits met through direct association and regulates SHP2 expression in melanocytes. J. Cell Sci. 2013;126:688–695. doi: 10.1242/jcs.119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens L., McClelland L., Fricke A., Williamson M., Kuo I., Scott G. Plexin b1 suppresses c-Met in melanoma: A role for plexin b1 as a tumor-suppressor protein through regulation of c-Met. J. Investig. Dermatol. 2010;130:1636–1645. doi: 10.1038/jid.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun T., Krishnan R., Swiercz J.M. Grb2 mediates semaphorin-4D-dependent rhoa inactivation. J. Cell Sci. 2012;125:3557–3567. doi: 10.1242/jcs.101063. [DOI] [PubMed] [Google Scholar]
- 60.Swiercz J.M., Worzfeld T., Offermanns S. Erbb-2 and met reciprocally regulate cellular signaling via plexin-b1. J. Biol. Chem. 2008;283:1893–1901. doi: 10.1074/jbc.M706822200. [DOI] [PubMed] [Google Scholar]
- 61.Artigiani S., Conrotto P., Fazzari P., Gilestro G.F., Barberis D., Giordano S., Comoglio P.M., Tamagnone L. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep. 2004;5:710–714. doi: 10.1038/sj.embor.7400189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghatak S., Hascall V.C., Markwald R.R., Misra S. Stromal hyaluronan interaction with epithelial cd44 variants promotes prostate cancer invasiveness by augmenting expression and function of hepatocyte growth factor and androgen receptor. J. Biol. Chem. 2010;285:19821–19832. doi: 10.1074/jbc.M110.104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Damm S., Koefinger P., Stefan M., Wels C., Mehes G., Richtig E., Kerl H., Otte M., Schaider H. HGF-promoted motility in primary human melanocytes depends on CD44V6 regulated via NF-kappaB, EGR-1, and C/EBP-beta. J. Investig. Dermatol. 2010;130:1893–1903. doi: 10.1038/jid.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orian-Rousseau V., Chen L., Sleeman J.P., Herrlich P., Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16:3074–3086. doi: 10.1101/gad.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orian-Rousseau V., Morrison H., Matzke A., Kastilan T., Pace G., Herrlich P., Ponta H. Hepatocyte growth factor-induced ras activation requires erm proteins linked to both CD44V6 and F-actin. Mol. Biol. Cell. 2007;18:76–83. doi: 10.1091/mbc.E06-08-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghatak S., Bogatkevich G.S., Atnelishvili I., Akter T., Feghali-Bostwick C., Hoffman S., Fresco V.M., Fuchs J.C., Visconti R.P., Markwald R.R., et al. Overexpression of c-Met and CD44V6 receptors contributes to autocrine tgf-beta1 signaling in interstitial lung disease. J. Biol. Chem. 2014;289:7856–7872. doi: 10.1074/jbc.M113.505065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gordin M., Tesio M., Cohen S., Gore Y., Lantner F., Leng L., Bucala R., Shachar I. c-Met and its ligand hepatocyte growth factor/scatter factor regulate mature b cell survival in a pathway induced by cd74. J. Immunol. 2010;185:2020–2031. doi: 10.4049/jimmunol.0902566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singleton P.A., Salgia R., Moreno-Vinasco L., Moitra J., Sammani S., Mirzapoiazova T., Garcia J.G. CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, tiam1/rac1, dynamin 2, and cortactin. J. Biol. Chem. 2007;282:30643–30657. doi: 10.1074/jbc.M702573200. [DOI] [PubMed] [Google Scholar]
- 69.Klosek S.K., Nakashiro K., Hara S., Shintani S., Hasegawa H., Hamakawa H. CD151 forms a functional complex with c-Met in human salivary gland cancer cells. Biochem. Biophys. Res. Commun. 2005;336:408–416. doi: 10.1016/j.bbrc.2005.08.106. [DOI] [PubMed] [Google Scholar]
- 70.Klosek S.K., Nakashiro K., Hara S., Goda H., Hasegawa H., Hamakawa H. CD151 regulates HGF-stimulated morphogenesis of human breast cancer cells. Biochem. Biophys. Res. Commun. 2009;379:1097–1100. doi: 10.1016/j.bbrc.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 71.Franco M., Muratori C., Corso S., Tenaglia E., Bertotti A., Capparuccia L., Trusolino L., Comoglio P.M., Tamagnone L. The tetraspanin CD151 is required for Met-dependent signaling and tumor cell growth. J. Biol. Chem. 2010;285:38756–38764. doi: 10.1074/jbc.M110.145417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sridhar S.C., Miranti C.K. Tetraspanin KAI1/CD82 suppresses invasion by inhibiting integrin-dependent crosstalk with c-Met receptor and src kinases. Oncogene. 2006;25:2367–2378. doi: 10.1038/sj.onc.1209269. [DOI] [PubMed] [Google Scholar]
- 73.Li Y., Huang X., Zhang J., Li Y., Ma K. Synergistic inhibition of cell migration by tetraspanin cd82 and gangliosides occurs via the egfr or cmet-activated pl3k/akt signalling pathway. Int. J. Biochem. Cell Biol. 2013;45:2349–2358. doi: 10.1016/j.biocel.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 74.Mela A., Goldman J.E. Cd82 blocks cmet activation and overcomes hepatocyte growth factor effects on oligodendrocyte precursor differentiation. J. Neurosci. 2013;33:7952–7960. doi: 10.1523/JNEUROSCI.5836-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Todeschini A.R., dos Santos J.N., Handa K., Hakomori S.I. Ganglioside GM2–tetraspanin CD82 complex inhibits Met and its cross-talk with integrins, providing a basis for control of cell motility through glycosynapse. J. Biol. Chem. 2007;282:8123–8133. doi: 10.1074/jbc.M611407200. [DOI] [PubMed] [Google Scholar]
- 76.Takahashi M., Sugiura T., Abe M., Ishii K., Shirasuna K. Regulation of c-Met signaling by the tetraspanin KAI-1/CD82 affects cancer cell migration. Int. J. Cancer. 2007;121:1919–1929. doi: 10.1002/ijc.22887. [DOI] [PubMed] [Google Scholar]
- 77.Trusolino L., Bertotti A., Comoglio P.M. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell. 2001;107:643–654. doi: 10.1016/S0092-8674(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 78.Bertotti A., Comoglio P.M., Trusolino L. Beta4 integrin is a transforming molecule that unleashes met tyrosine kinase tumorigenesis. Cancer Res. 2005;65:10674–10679. doi: 10.1158/0008-5472.CAN-05-2827. [DOI] [PubMed] [Google Scholar]
- 79.Yoshioka T., Otero J., Chen Y., Kim Y.M., Koutcher J.A., Satagopan J., Reuter V., Carver B., de Stanchina E., Enomoto K., et al. Beta4 integrin signaling induces expansion of prostate tumor progenitors. J. Clin. Investig. 2013;123:682–699. doi: 10.1172/JCI60720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ephstein Y., Singleton P.A., Chen W., Wang L., Salgia R., Kanteti P., Dudek S.M., Garcia J.G., Jacobson J.R. Critical role of S1PR1 and integrin beta4 in HGF/c-Met-mediated increases in vascular integrity. J. Biol. Chem. 2013;288:2191–2200. doi: 10.1074/jbc.M112.404780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rahman S., Patel Y., Murray J., Patel K.V., Sumathipala R., Sobel M., Wijelath E.S. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified met-integrin induced signalling pathway in endothelial cells. BMC Cell Biol. 2005;6:8. doi: 10.1186/1471-2121-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mitra A.K., Sawada K., Tiwari P., Mui K., Gwin K., Lengyel E. Ligand-independent activation of c-Met by fibronectin and alpha5beta1-integrin regulates ovarian cancer invasion and metastasis. Oncogene. 2011;30:1566–1576. doi: 10.1038/onc.2010.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y., Chattopadhyay N., Qin S., Szekeres C., Vasylyeva T., Mahoney Z.X., Taglienti M., Bates C.M., Chapman H.A., Miner J.H., et al. Coordinate integrin and c-Met signaling regulate wnt gene expression during epithelial morphogenesis. Development. 2009;136:843–853. doi: 10.1242/dev.027805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ju L., Zhou C. Association of integrin beta1 and c-Met in mediating EGFR TKI gefitinib resistance in non-small cell lung cancer. Cancer Cell Int. 2013;13:15. doi: 10.1186/1475-2867-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCall-Culbreath K.D., Li Z., Zutter M.M. Crosstalk between the alpha2beta1 integrin and c-Met/HGF-R regulates innate immunity. Blood. 2008;111:3562–3570. doi: 10.1182/blood-2007-08-107664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Follenzi A., Bakovic S., Gual P., Stella M.C., Longati P., Comoglio P.M. Cross-talk between the proto-oncogenes met and ron. Oncogene. 2000;19:3041–3049. doi: 10.1038/sj.onc.1203620. [DOI] [PubMed] [Google Scholar]
- 87.Jo M., Stolz D.B., Esplen J.E., Dorko K., Michalopoulos G.K., Strom S.C. Cross-talk between epidermal growth factor receptor and c-met signal pathways in transformed cells. J. Biol. Chem. 2000;275:8806–8811. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- 88.Bonine-Summers A.R., Aakre M.E., Brown K.A., Arteaga C.L., Pietenpol J.A., Moses H.L., Cheng N. Epidermal growth factor receptor plays a significant role in hepatocyte growth factor mediated biological responses in mammary epithelial cells. Cancer Biol. Ther. 2007;6:561–570. doi: 10.4161/cbt.6.4.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y.W., Staal B., Essenburg C., Su Y., Kang L., West R., Kaufman D., Dekoning T., Eagleson B., Buchanan S.G., et al. Met kinase inhibitor SGX523 synergizes with epidermal growth factor receptor inhibitor erlotinib in a hepatocyte growth factor-dependent fashion to suppress carcinoma growth. Cancer Res. 2010;70:6880–6890. doi: 10.1158/0008-5472.CAN-10-0898. [DOI] [PubMed] [Google Scholar]
- 90.Huang P.H., Mukasa A., Bonavia R., Flynn R.A., Brewer Z.E., Cavenee W.K., Furnari F.B., White F.M. Quantitative analysis of egfrviii cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc. Natl. Acad. Sci. USA. 2007;104:12867–12872. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bachleitner-Hofmann T., Sun M.Y., Chen C.T., Tang L., Song L., Zeng Z., Shah M., Christensen J.G., Rosen N., Solit D.B., et al. Her kinase activation confers resistance to met tyrosine kinase inhibition in Met oncogene-addicted gastric cancer cells. Mol. Cancer Ther. 2008;7:3499–3508. doi: 10.1158/1535-7163.MCT-08-0374. [DOI] [PubMed] [Google Scholar]
- 92.Turke A.B., Zejnullahu K., Wu Y.L., Song Y., Dias-Santagata D., Lifshits E., Toschi L., Rogers A., Mok T., Sequist L., et al. Preexistence and clonal selection of met amplification in EGFR mutant nsclc. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yano S., Wang W., Li Q., Matsumoto K., Sakurama H., Nakamura T., Ogino H., Kakiuchi S., Hanibuchi M., Nishioka Y., et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–9487. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 94.Yamamoto N., Mammadova G., Song R.X., Fukami Y., Sato K. Tyrosine phosphorylation of p145Met mediated by egfr and src is required for serum-independent survival of human bladder carcinoma cells. J. Cell Sci. 2006;119:4623–4633. doi: 10.1242/jcs.03236. [DOI] [PubMed] [Google Scholar]
- 95.Xu K.P., Yu F.S. Cross talk between c-met and epidermal growth factor receptor during retinal pigment epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2007;48:2242–2248. doi: 10.1167/iovs.06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nath D., Williamson N.J., Jarvis R., Murphy G. Shedding of c-Met is regulated by crosstalk between a G-protein coupled receptor and the egf receptor and is mediated by a timp-3 sensitive metalloproteinase. J. Cell Sci. 2001;114:1213–1220. doi: 10.1242/jcs.114.6.1213. [DOI] [PubMed] [Google Scholar]
- 97.Tanizaki J., Okamoto I., Sakai K., Nakagawa K. Differential roles of trans-phosphorylated EGFR, Her2, Her3, and Ret as heterodimerisation partners of met in lung cancer with Met amplification. Br. J. Cancer. 2011;105:807–813. doi: 10.1038/bjc.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang Z., Du R., Jiang S., Wu C., Barkauskas D.S., Richey J., Molter J., Lam M., Flask C., Gerson S., et al. Dual Met–EGFR combinatorial inhibition against t790m–EGFR-mediated erlotinib-resistant lung cancer. Br. J. Cancer. 2008;99:911–922. doi: 10.1038/sj.bjc.6604559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Troiani T., Martinelli E., Napolitano S., Vitagliano D., Ciuffreda L.P., Costantino S., Morgillo F., Capasso A., Sforza V., Nappi A., et al. Increased TGF-alpha as a mechanism of acquired resistance to the anti-EGFR inhibitor cetuximab through EGFR–Met interaction and activation of met signaling in colon cancer cells. Clin. Cancer Res. 2013;19:6751–6765. doi: 10.1158/1078-0432.CCR-13-0423. [DOI] [PubMed] [Google Scholar]
- 100.Breindel J.L., Haskins J.W., Cowell E.P., Zhao M., Nguyen D.X., Stern D.F. EGF receptor activates Met through mapk to enhance non-small cell lung carcinoma invasion and brain metastasis. Cancer Res. 2013;73:5053–5065. doi: 10.1158/0008-5472.CAN-12-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dulak A.M., Gubish C.T., Stabile L.P., Henry C., Siegfried J.M. HGF-independent potentiation of EGFR action by c-Met. Oncogene. 2011;30:3625–3635. doi: 10.1038/onc.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shattuck D.L., Miller J.K., Carraway K.L., 3rd, Sweeney C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008;68:1471–1477. doi: 10.1158/0008-5472.CAN-07-5962. [DOI] [PubMed] [Google Scholar]
- 103.Khoury H., Naujokas M.A., Zuo D., Sangwan V., Frigault M.M., Petkiewicz S., Dankort D.L., Muller W.J., Park M. HGF converts ERBB2/Neu epithelial morphogenesis to cell invasion. Mol. Biol. Cell. 2005;16:550–561. doi: 10.1091/mbc.E04-07-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Engelman J.A., Zejnullahu K., Mitsudomi T., Song Y., Hyland C., Park J.O., Lindeman N., Gale C.M., Zhao X., Christensen J., et al. Met amplification leads to gefitinib resistance in lung cancer by activating erbb3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 105.Guo A., Villen J., Kornhauser J., Lee K.A., Stokes M.P., Rikova K., Possemato A., Nardone J., Innocenti G., Wetzel R., et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc. Natl. Acad. Sci. USA. 2008;105:692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bauer T.W., Somcio R.J., Fan F., Liu W., Johnson M., Lesslie D.P., Evans D.B., Gallick G.E., Ellis L.M. Regulatory role of c-Met in insulin-like growth factor-I receptor-mediated migration and invasion of human pancreatic carcinoma cells. Mol. Cancer Ther. 2006;5:1676–1682. doi: 10.1158/1535-7163.MCT-05-0175. [DOI] [PubMed] [Google Scholar]
- 107.Wang X., DeFrances M.C., Dai Y., Pediaditakis P., Johnson C., Bell A., Michalopoulos G.K., Zarnegar R. A mechanism of cell survival: Sequestration of fas by the HGF receptor Met. Mol. Cell. 2002;9:411–421. doi: 10.1016/S1097-2765(02)00439-2. [DOI] [PubMed] [Google Scholar]
- 108.Zou C., Ma J., Wang X., Guo L., Zhu Z., Stoops J., Eaker A.E., Johnson C.J., Strom S., Michalopoulos G.K., et al. Lack of Fas antagonism by met in human fatty liver disease. Nat. Med. 2007;13:1078–1085. doi: 10.1038/nm1625. [DOI] [PubMed] [Google Scholar]
- 109.Smyth L.A., Brady H.J. Cmet and Fas receptor interaction inhibits death-inducing signaling complex formation in endothelial cells. Hypertension. 2005;46:100–106. doi: 10.1161/01.HYP.0000167991.82153.16. [DOI] [PubMed] [Google Scholar]
- 110.Du W., Uslar L., Sevala S., Shah K. Targeting c-Met receptor overcomes trail-resistance in brain tumors. PLoS One. 2014;9:e95490. doi: 10.1371/journal.pone.0095490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bozkaya G., Korhan P., Cokakli M., Erdal E., Sagol O., Karademir S., Korch C., Atabey N. Cooperative interaction of muc1 with the HGF/c-Met pathway during hepatocarcinogenesis. Mol. Cancer. 2012;11:64. doi: 10.1186/1476-4598-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Horm T.M., Bitler B.G., Broka D.M., Louderbough J.M., Schroeder J.A. Muc1 drives c-Met-dependent migration and scattering. Mol. Cancer Res. 2012;10:1544–1554. doi: 10.1158/1541-7786.MCR-12-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Singh P.K., Behrens M.E., Eggers J.P., Cerny R.L., Bailey J.M., Shanmugam K., Gendler S.J., Bennett E.P., Hollingsworth M.A. Phosphorylation of muc1 by met modulates interaction with p53 and mmp1 expression. J. Biol. Chem. 2008;283:26985–26995. doi: 10.1074/jbc.M805036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Higuchi T., Orita T., Katsuya K., Yamasaki Y., Akiyama K., Li H., Yamamoto T., Saito Y., Nakamura M. Muc20 suppresses the hepatocyte growth factor-induced GRB2–Ras pathway by binding to a multifunctional docking site of met. Mol. Cell. Biol. 2004;24:7456–7468. doi: 10.1128/MCB.24.17.7456-7468.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang S., Zhau H.E., Osunkoya A.O., Iqbal S., Yang X., Fan S., Chen Z., Wang R., Marshall F.F., Chung L.W., et al. Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-Met signaling in human prostate cancer cells. Mol. Cancer. 2010;9:9. doi: 10.1186/1476-4598-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Olaku V., Matzke A., Mitchell C., Hasenauer S., Sakkaravarthi A., Pace G., Ponta H., Orian-Rousseau V. c-Met recruits ICAM-1 as a coreceptor to compensate for the loss of CD44 in CD44 null mice. Mol. Biol. Cell. 2011;22:2777–2786. doi: 10.1091/mbc.E11-02-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tamagnone L., Artigiani S., Chen H., He Z., Ming G.I., Song H., Chedotal A., Winberg M.L., Goodman C.S., Poo M., et al. Plexins are a large family of receptors for transmembrane, secreted, and gpi-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/S0092-8674(00)80063-X. [DOI] [PubMed] [Google Scholar]
- 118.Driessens M.H., Hu H., Nobes C.D., Self A., Jordens I., Goodman C.S., Hall A. Plexin-B semaphorin receptors interact directly with active rac and regulate the actin cytoskeleton by activating rho. Curr. Biol. 2001;11:339–344. doi: 10.1016/S0960-9822(01)00092-6. [DOI] [PubMed] [Google Scholar]
- 119.Tong Y., Chugha P., Hota P.K., Alviani R.S., Li M., Tempel W., Shen L., Park H.W., Buck M. Binding of Rac1, Rnd1, and Rhod to a novel Rho GTPase interaction motif destabilizes dimerization of the plexin-B1 effector domain. J. Biol. Chem. 2007;282:37215–37224. doi: 10.1074/jbc.M703800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ch’ng E.S., Kumanogoh A. Roles of SEMA4D and plexin-B1 in tumor progression. Mol. Cancer. 2010;9:251. doi: 10.1186/1476-4598-9-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Damola A., Legendre A., Ball S., Masters J.R., Williamson M. Function of mutant and wild-type plexinb1 in prostate cancer cells. Prostate. 2013;73:1326–1335. doi: 10.1002/pros.22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weber G.F., Ashkar S., Glimcher M.J., Cantor H. Receptor-ligand interaction between CD44 and osteopontin (ETA-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 123.Van der Voort R., Taher T.E., Wielenga V.J., Spaargaren M., Prevo R., Smit L., David G., Hartmann G., Gherardi E., Pals S.T. Heparan sulfate-modified CD44 promotes hepatocyte growth factor/scatter factor-induced signal transduction through the receptor tyrosine kinase c-Met. J. Biol. Chem. 1999;274:6499–6506. doi: 10.1074/jbc.274.10.6499. [DOI] [PubMed] [Google Scholar]
- 124.Matzke A., Sargsyan V., Holtmann B., Aramuni G., Asan E., Sendtner M., Pace G., Howells N., Zhang W., Ponta H., et al. Haploinsufficiency of c-met in CD44−/− mice identifies a collaboration of CD44 and c-Met in vivo. Mol. Cell. Biol. 2007;27:8797–8806. doi: 10.1128/MCB.01355-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Crepaldi T., Gautreau A., Comoglio P.M., Louvard D., Arpin M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J. Cell Biol. 1997;138:423–434. doi: 10.1083/jcb.138.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hynes R.O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-S. [DOI] [PubMed] [Google Scholar]
- 127.Winograd-Katz S.E., Fassler R., Geiger B., Legate K.R. The integrin adhesome: From genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 2014;15:273–288. doi: 10.1038/nrm3769. [DOI] [PubMed] [Google Scholar]
- 128.Chan P.C., Chen S.Y., Chen C.H., Chen H.C. Crosstalk between hepatocyte growth factor and integrin signaling pathways. J. Biomed. Sci. 2006;13:215–223. doi: 10.1007/s11373-005-9061-7. [DOI] [PubMed] [Google Scholar]
- 129.Chung J., Yoon S.O., Lipscomb E.A., Mercurio A.M. The met receptor and alpha6beta4 integrin can function independently to promote carcinoma invasion. J. Biol. Chem. 2004;279:32287–32293. doi: 10.1074/jbc.M403809200. [DOI] [PubMed] [Google Scholar]
- 130.Wang R., Kobayashi R., Bishop J.M. Cellular adherence elicits ligand-independent activation of the met cell-surface receptor. Proc. Natl. Acad. Sci. USA. 1996;93:8425–8430. doi: 10.1073/pnas.93.16.8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ivaska J., Heino J. Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu. Rev. Cell Dev. Biol. 2011;27:291–320. doi: 10.1146/annurev-cellbio-092910-154017. [DOI] [PubMed] [Google Scholar]
- 132.Mattila E., Pellinen T., Nevo J., Vuoriluoto K., Arjonen A., Ivaska J. Negative regulation of egfr signalling through integrin-alpha1beta1-mediated activation of protein tyrosine phosphatase tcptp. Nat. Cell Biol. 2005;7:78–85. doi: 10.1038/ncb1209. [DOI] [PubMed] [Google Scholar]
- 133.Caswell P.T., Chan M., Lindsay A.J., McCaffrey M.W., Boettiger D., Norman J.C. RAB-coupling protein coordinates recycling of alpha5beta1 integrin and egfr1 to promote cell migration in 3D microenvironments. J. Cell Biol. 2008;183:143–155. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bassani S., Cingolani L.A. Tetraspanins: Interactions and interplay with integrins. Int. J. Biochem. Cell Biol. 2012;44:703–708. doi: 10.1016/j.biocel.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 135.Chen S., Sun Y., Jin Z., Jing X. Functional and biochemical studies of CD9 in fibrosarcoma cell line. Mol. Cell. Biochem. 2011;350:89–99. doi: 10.1007/s11010-010-0685-1. [DOI] [PubMed] [Google Scholar]
- 136.Murayama Y., Shinomura Y., Oritani K., Miyagawa J., Yoshida H., Nishida M., Katsube F., Shiraga M., Miyazaki T., Nakamoto T., et al. The tetraspanin CD9 modulates epidermal growth factor receptor signaling in cancer cells. J. Cell. Physiol. 2008;216:135–143. doi: 10.1002/jcp.21384. [DOI] [PubMed] [Google Scholar]
- 137.Robinson D.R., Wu Y.M., Lin S.F. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 138.Mueller K.L., Hunter L.A., Ethier S.P., Boerner J.L. Met and c-Src cooperate to compensate for loss of epidermal growth factor receptor kinase activity in breast cancer cells. Cancer Res. 2008;68:3314–3322. doi: 10.1158/0008-5472.CAN-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Boccaccio C., Luraghi P., Comoglio P.M. Met-mediated resistance to EGFR inhibitors: An old liaison rooted in colorectal cancer stem cells. Cancer Res. 2014;74:3647–3651. doi: 10.1158/0008-5472.CAN-14-1088. [DOI] [PubMed] [Google Scholar]
- 140.Zhang Y.W., Staal B., Essenburg C., Lewis S., Kaufman D., vande Woude G.F. Strengthening context-dependent anticancer effects on non-small cell lung carcinoma by inhibition of both Met and EGFR. Mol. Cancer Ther. 2013;12:1429–1441. doi: 10.1158/1535-7163.MCT-13-0016. [DOI] [PubMed] [Google Scholar]
- 141.Reznik T.E., Sang Y., Ma Y., Abounader R., Rosen E.M., Xia S., Laterra J. Transcription-dependent epidermal growth factor receptor activation by hepatocyte growth factor. Mol. Cancer Res. 2008;6:139–150. doi: 10.1158/1541-7786.MCR-07-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Acunzo M., Romano G., Palmieri D., Lagana A., Garofalo M., Balatti V., Drusco A., Chiariello M., Nana-Sinkam P., Croce C.M. Cross-talk between Met and EGFR in non-small cell lung cancer involves Mir-27a and sprouty2. Proc. Natl. Acad. Sci. USA. 2013;110:8573–8578. doi: 10.1073/pnas.1302107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fong J.T., Jacobs R.J., Moravec D.N., Uppada S.B., Botting G.M., Nlend M., Puri N. Alternative signaling pathways as potential therapeutic targets for overcoming EGFR and c-Met inhibitor resistance in non-small cell lung cancer. PLoS One. 2013;8:e78398. doi: 10.1371/journal.pone.0078398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Merlin S., Pietronave S., Locarno D., Valente G., Follenzi A., Prat M. Deletion of the ectodomain unleashes the transforming, invasive, and tumorigenic potential of the met oncogene. Cancer Sci. 2009;100:633–638. doi: 10.1111/j.1349-7006.2008.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nath S., Mukherjee P. Muc1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014;20:332–342. doi: 10.1016/j.molmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chappell W.H., Steelman L.S., Long J.M., Kempf R.C., Abrams S.L., Franklin R.A., Basecke J., Stivala F., Donia M., Fagone P., et al. Ras/Raf/Mek/ERK and pi3k/pten/Akt/MTOR inhibitors: Rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–164. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Accordi B., Pillozzi S., Dell’Orto M.C., Cazzaniga G., Arcangeli A., Kronnie G.T., Basso G. Hepatocyte growth factor receptor c-met is associated with fas and when activated enhances drug-induced apoptosis in pediatric b acute lymphoblastic leukemia with tel-aml1 translocation. J. Biol. Chem. 2007;282:29384–29393. doi: 10.1074/jbc.M706314200. [DOI] [PubMed] [Google Scholar]
- 148.Sequist L.V., von Pawel J., Garmey E.G., Akerley W.L., Brugger W., Ferrari D., Chen Y., Costa D.B., Gerber D.E., Orlov S., et al. Randomized phase ii study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J. Clin. Oncol. 2011;29:3307–3315. doi: 10.1200/JCO.2010.34.0570. [DOI] [PubMed] [Google Scholar]
- 149.Spigel D.R., Ervin T.J., Ramlau R.A., Daniel D.B., Goldschmidt J.H., Jr., Blumenschein G.R., Jr., Krzakowski M.J., Robinet G., Godbert B., Barlesi F., et al. Randomized phase II trial of onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2013;31:4105–4114. doi: 10.1200/JCO.2012.47.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stinchcombe T.E. Novel agents in development for advanced non-small cell lung cancer. Ther. Adv. Med. Oncol. 2014;6:240–253. doi: 10.1177/1758834014532510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Parums D.V. Current status of targeted therapy in non-small cell lung cancer. Drugs Today. 2014;50:503–525. doi: 10.1358/dot.2014.50.07.2185913. [DOI] [PubMed] [Google Scholar]
- 152.Goodman S.L., Picard M. Integrins as therapeutic targets. Trends Pharmacol. Sci. 2012;33:405–412. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]