Patients with stage IV non‐small cell lung cancer have poor survival prognosis, but new therapies introduced since 2000 provide options for improved outcomes. This simulation modeling study sought to quantify survival gains from 1990, when standard treatment was best supportive care only, to 2015 and to estimate the impact of expanded use of systemic therapies in clinically appropriate patients.
Keywords: Non‐small cell lung cancer, Overall survival, Systemic therapy
Abstract
Background.
Approximately 190,000 Americans are diagnosed with non‐small cell lung cancer (NSCLC) annually, and about half have metastatic (Stage IV) disease. These patients have historically had poor survival prognosis, but several new therapies introduced since 2000 provide options for improved outcomes. The objectives of this study were to quantify survival gains from 1990, when best supportive care (BSC) only was standard, to 2015 and to estimate the impact of expanded use of systemic therapies in clinically appropriate patients.
Materials and Methods.
We developed a simulation model to estimate survival gains for patients with metastatic NSCLC from 1990–2015. Survival estimates were derived from major clinical trials and extrapolated to a lifetime horizon. Proportions of patients receiving available therapies were derived from the Surveillance, Epidemiology, and End Results database and a commercial treatment registry. We also estimated gains in overall survival (OS) in scenarios in which systemic therapy use increased by 10% and 30% relative to current use.
Results.
From 1990–2015, one‐year survival proportion increased by 14.1% and mean per‐patient survival improved by 4.2 months (32,700 population life years). Increasing treated patients by 10% or 30% increased OS by 5.1 months (39,700 population life years) and 6.9 months (53,800 population life years), respectively.
Conclusion.
Although survival remains poor in metastatic NSCLC relative to other common cancers, meaningful progress in per‐patient and population‐level outcomes has been realized over the past 25 years. These advances can be improved even further by increasing use of systemic therapies in the substantial proportion of patients who are suitable for treatment yet who currently receive BSC only.
Implications for Practice.
Approximately 93,500 Americans are diagnosed with metastatic non‐small cell lung cancer (NSCLC) annually. Historically, these patients have had poor survival prognosis, but newer therapies provide options for improved outcomes. This simulation modeling study quantified metastatic NSCLC survival gains from 1990–2015. Over this period, the one‐year survival proportion and mean per‐patient survival increased by 14.1% and 4.2 months, respectively. Though metastatic NSCLC survival remains poor, the past 25 years have brought meaningful gains. Additional gains could be realized by increasing systemic therapy use in the substantial proportion of patients who are suitable for treatment, yet currently receive only supportive care.
Introduction
Approximately 190,000 Americans will be diagnosed with non‐small cell lung cancer (NSCLC) in 2016 [1]. Of these patients, only about 30% will live one year. This dismal survival statistic is primarily due to the fact that about half of NSCLC cases are diagnosed at a metastatic stage (Stage IV), at which point the cancer has spread to the other lung, lymph nodes, fluid around the lungs or heart, and/or other parts of the body, and one‐year survival has historically been <20% in most settings [2]. As a result of this combination of factors, lung cancer is the leading cause of cancer death in the U.S., with an estimated 130,000 deaths from NSCLC each year [1]. Historically, only a fraction of patients who are diagnosed with metastatic NSCLC receive any systemic therapy [3], [4], [5], [6], and those that do usually have responses that last only a matter of months [7], [8], [9], [10].
The past decade brought a substantial number of new systemic therapy options for metastatic NSCLC, starting with platinum‐doublet therapy and continuing with the discovery of genetic biomarkers that predict clinical benefit from oral tyrosine kinase inhibitors, including erlotinib and crizotinib, for tumor activating mutations of the epidermal growth factor receptor (EGFR) gene [11], [12], [13] and rearrangements of the anaplastic lymphoma kinase (ALK) gene [14], [15], [16], respectively; monoclonal antibodies that target the vascular endothelial growth factor (VEGF) receptor pathway [17], [18]; and the development of immune checkpoint inhibitors [19], [20]. These therapies are improving the prognosis of patients with metastatic NSCLC, but the magnitude of survival improvement over this period of expansion remains uncertain.
The objective of this study is to quantify the survival impacts of changes in metastatic NSCLC therapeutic options over the past 25 years. Specifically, we estimate patient‐ and population‐level survival gains achieved through advances in systemic therapy for those diagnosed with metastatic NSCLC from 1990–2015 and project the potential survival impact of increasing the proportion of eligible patients who receive systemic therapy. Our findings provide stakeholders with quantitative estimates of the metastatic NSCLC survival gains of recent decades and examine the potential survival gains that may be realized in the coming 2–5 years as a result of increased treatment rates and new therapies.
Materials and Methods
Model Overview
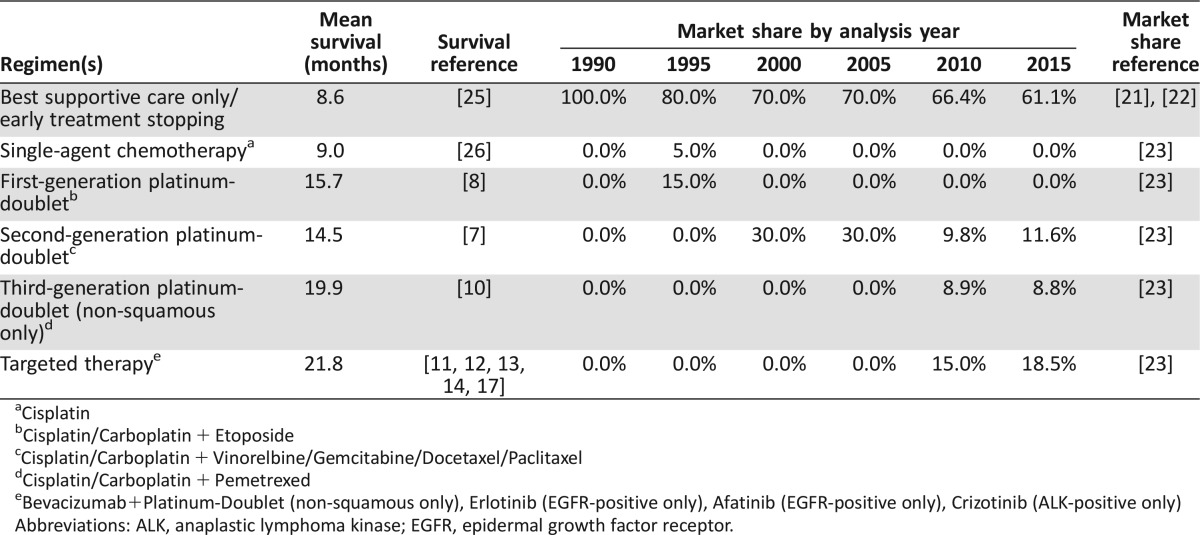
We developed a simulation model in Microsoft Excel (Version 14.5, Redmond, WA) to estimate overall survival (OS) for successive cohorts of patients diagnosed with metastatic NSCLC from 1990 to 2015. This analysis time horizon covers the period from when best supportive care (BSC) only was standard (i.e., 1990) to the present, when multiple cytotoxic and targeted therapies are available. We divided the analysis into five‐year increments to make it easier to gauge progress and because of the slow rate of development of U.S. Food and Drug Administration (FDA)‐approved therapies over the time horizon. Adult patients with metastatic NSCLC “enter” the model and receive BSC only or one of the first‐line systemic therapies available in the given year of the analysis (Table 1). In 2010 and 2015, treatments are additionally stratified by tumor histology (non‐squamous or squamous cell carcinoma) and mutation status (EGFR or ALK) to reflect emerging differences in standard of care in each of the subgroups.
Table 1. Treatment distributions by analysis year.
Cisplatin
Cisplatin/Carboplatin + Etoposide
Cisplatin/Carboplatin + Vinorelbine/Gemcitabine/Docetaxel/Paclitaxel
Cisplatin/Carboplatin + Pemetrexed
Bevacizumab+Platinum‐Doublet (non‐squamous only), Erlotinib (EGFR‐positive only), Afatinib (EGFR‐positive only), Crizotinib (ALK‐positive only)
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor.
OS was projected based on three data elements: (a) first‐line systemic therapy uptake from the peer‐reviewed literature [21], [22], (b) systemic therapy market shares conditional on receipt of treatment from a commercial database [23], and (c) survival duration from clinical trials referenced in National Comprehensive Cancer Network (NCCN) guidelines [7], [8], [10], [13], [16], [17], [24], [25], [26], [27]. Clinical trial survival outcomes were extrapolated to a lifetime horizon by fitting Weibull and log‐logistic curves to in‐trial Kaplan–Meier OS curves. Proportions of patients receiving any systemic therapies were derived from prior analyses of the Surveillance, Epidemiology, and End Results (SEER) database, and the proportion receiving specific systemic therapies was derived from 500 physician‐reported monthly audits of patient charts that were nationally projected from 2002 to 2015 [23]. Survival results for each analysis year were validated by comparing projected survival with that from the SEER database (see supplemental online Appendix, Figs. 2–6).
Proportion Receiving Any Systemic Therapy
We derived the proportion of patients in each analysis year that received any systemic therapy from several prior analyses of the SEER–Medicare linked lung cancer database and other observational studies that reported the proportion of patients diagnosed with metastatic NSCLC (Tumor‐Node‐Metastasis (TNM) staging system stage IV disease) that received systemic therapy within 3–6 months of diagnosis (varied by study) [21], [22], [28]. Given a lack of reports about systemic therapy uptake in 2015, we assumed an extrapolation of the linear trend in systemic therapy use from the preceding analysis years.
Systemic Therapies
Systemic therapies were grouped into five broad categories: (a) single agent platinum therapies, (b) first‐generation platinum‐doublet therapies, (c) second‐generation platinum‐doublet therapies, (d) third‐generation platinum‐doublet therapies, and (e) targeted therapies (both biomarker‐directed therapies targeting EGFR and ALK and monoclonal antibody strategies targeting VEGF). The specific regimens that were considered are noted in Table 1.
Survival Model
To estimate OS by treatment, we identified publications for the major clinical trials for all included first‐line systemic therapy regimens from NCCN guidelines [24]. Each publication included a Kaplan–Meier OS curve for the given regimen over the duration of the trial (the follow‐up range was 6 to 24 months). We used Engauge Digitizer (Version 4.1) to extract monthly survival proportions from the curves and exported these values to the simulation model for analysis. A variety of curve fits (Weibull, log‐logistic log‐normal, gamma, and Gompertz) were considered to extrapolate survival beyond the trial period, and fits were selected to minimize the sum of squared deviations from the mean [29]. To obtain OS for patients diagnosed in each analysis year, we used the market shares for available therapies and BSC only to calculate a weighted average of OS.
Model Outcomes
Model outcomes included one‐ and three‐year OS proportions, mean per‐patient OS life years, and mean OS life years for the entire population of metastatic NSCLC patients diagnosed in each analysis year. To project population‐level outcomes, we assumed 220,000 annual lung cancer diagnoses in the U.S. (all histologies) [30] and that 85% (n = 187,000) were NSCLC (versus small cell) [30]. Lastly, we assumed that 50% of NSCLC diagnoses (n = 93,500) were Stage IV based on the mean proportion of NSCLC diagnoses in the SEER database from 2004–2013 [2]. This annualized incidence figure was applied to all analysis years to allow comparison of outcomes between analysis years based on systemic therapy market shares (i.e., removing differences in size of the U.S. population and prevalence of smoking and other lung cancer risk factors as drivers of incidence).
Among incident metastatic NSCLC cases, we assumed 30% to be squamous cell carcinoma and 70% to be non‐squamous cell carcinoma histology [30].These proportions only impact the 2010 and 2015 analysis years, in which treatments were stratified by tumor histology to reflect widespread changes in clinical approaches to treating each subgroup. Using these outcomes, we estimated OS gains between 1990 and 2015, as well as incremental survival gains between each five‐year analysis year increment.
Outcome Validation
Estimated survival was compared with SEER OS curves for analysis years for which SEER outcomes were available (1990–2010) [2]. For each analysis year, we plotted model‐projected survival and SEER survival and qualitatively evaluated the alignment of curves (see supplemental online Figs. 2–6).
Scenario Analyses
Scenario analyses evaluated the survival gains that could be achieved by expanding the proportion of patients receiving systemic therapy by 10% or 30% (additive) relative to estimated use in 2015. In these scenarios, we assumed that the incremental gains in the proportion treated with systemic therapy (versus the 2015 proportion) were driven by increased use of biomarker testing (e.g., through next‐generation sequencing), discovery of an additional proportion with targetable biomarkers, and use of appropriate targeted therapies in that proportion (versus the status quo, in which there is less biomarker testing and many cannot tolerate platinum‐based therapy).
Lastly, we considered a scenario in which a new first‐line therapy is available in addition to all 2015 regimens, the new therapy has overall and progression‐free survival hazard ratios of 0.6 and 0.6 relative to second‐generation platinum‐doublet chemotherapy, and 20% of metastatic NSCLC patients that are not eligible for ALK‐ or EGFR‐targeted therapy receive it (with all 2015 market shares shifted proportionately to the 2015 base case values). This scenario provides an estimate of potential survival gains that can be achieved in the near future (2–5 years) related to the development of new therapies (e.g., first‐line immune checkpoint inhibitors).
Results
Base Case Results
Among metastatic NSCLC patients diagnosed since 1990, the estimated proportion receiving systemic therapy increased from none in 1990 to 20% in 1995, 30% in 2000, 34% in 2010, and 39% in 2015. Among the 34% of patients receiving systemic therapies for metastatic NSCLC in 2010, the use of targeted therapies expanded substantially beyond platinum doublets, such that 44% of those received targeted therapy in the first‐line setting. In 2015, targeted therapies are estimated to account for 48% of all systemic therapy for metastatic NSCLC (Table 1).
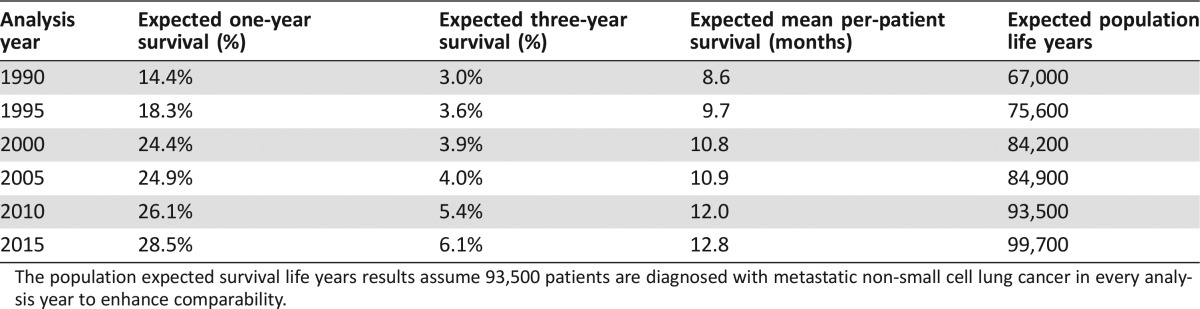
The base case analysis demonstrated that one‐year survival approximately doubled from 14.4% to 28.5% between 1990 and 2015—amounting to a change from a mean OS of 8.6 months to a mean of 12.7 months (Table 2). Similarly, the three‐year OS proportion increased approximately twofold from 3.0% to 6.1%. For all metastatic NSCLC cases, this gain amounted to 32,700 additional life years per annual cohort of diagnosed patients over the period of analysis.
Table 2. Base case overall survival results by analysis year.
The population expected survival life years results assume 93,500 patients are diagnosed with metastatic non‐small cell lung cancer in every analysis year to enhance comparability.
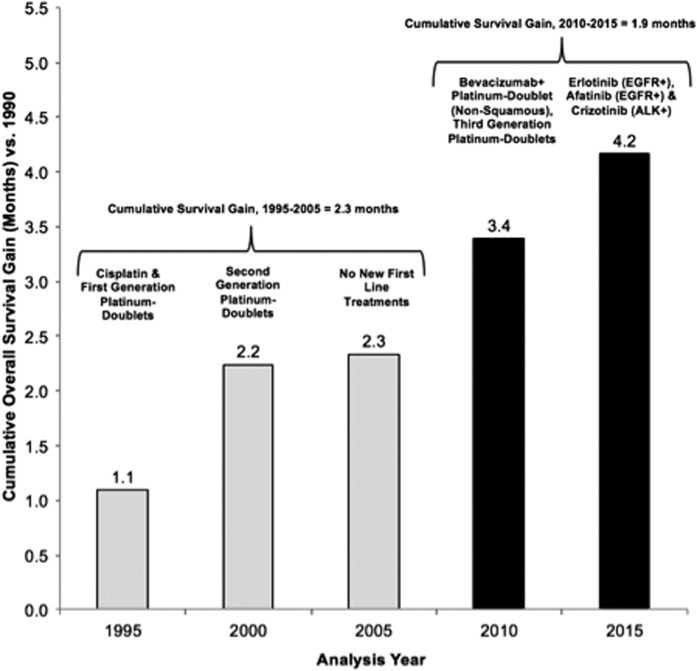
Considering the contribution of various agents and regimens, there has been an average of 4.2 months of OS improvement between 1990 and 2015. Approximately half of the survival (mean 2.3 months, 55%) between these analysis years can be attributed to the introduction of platinum‐doublet therapies, which were widely introduced in 2000 and are still the mainstay of initial treatment for most patients with NSCLC (Fig. 1). In the past 10 years since the introduction of erlotinib, the first targeted therapy option in 2005, targeted therapies have contributed approximately 1.9 months of mean survival and 45% to the overall additional life years gained.
Figure 1.
Cumulative mean overall survival gain (versus 1990) by analysis year and introduction of new first‐line systemic therapies. The new systemic therapy options in the given analysis year are listed above each bar. Of the 4.2‐month mean overall survival improvement achieved from 1990 to 2015, 1.9 months (45%) is estimated to be attributable to U.S. Food and Drug Administration approvals of targeted therapies in the past 10 years.
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor
Scenario Analyses: Increasing the Proportion of Patients Treated
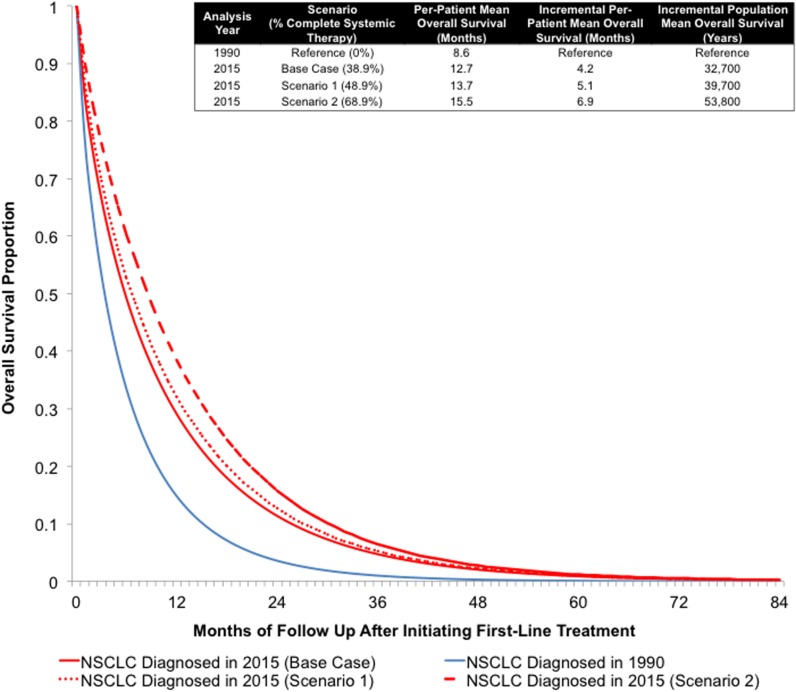
In general, the largest gains in survival for metastatic NCSLC patients can be achieved by moving patients who would otherwise only receive BSC to systemic therapy. Increasing the proportion of patients who receive at least one course of systemic therapy by 10% in 2015 is expected to increase the one‐year survival proportion from 28.5% to 31.9% and the mean OS per patient from 12.8 to 13.7 months. In terms of per‐patient and population‐level survival, the increased proportion receiving systemic therapy resulted in 0.9 additional months of survival and 7,200 additional life years, respectively (Fig. 2).
Figure 2.
Metastatic non‐small cell lung cancer estimated overall survival by year of diagnosis, 2015 versus 1990. When the proportion of patients receiving systemic therapy in 2015 was increased by 10% (Scenario 1) or 30% (Scenario 2), the improvement in survival relative to 1990 increased by 5.1 months (39,700 population life years) and 6.9 months (53,800 population life years), respectively.
Abbreviations: NSCLC, non‐small cell lung cancer.
A 30% increase in the proportion of patients receiving any systemic therapy in 2015 would increase the one‐year survival proportion from 28.5% to 38.4% and the mean OS per patient from 12.8 to 15.5 months. In terms of per‐patient and population‐level survival, the increased proportion receiving systemic therapy resulted in 2.7 additional months of survival and 21,500 additional life years, respectively (Fig. 2).
Scenario Analyses: Introduction of a New Treatment
The introduction of a new therapy in 2015 with 20% market share and with overall and progression‐free hazard ratios of 0.6 versus second‐generation platinum‐doublet chemotherapy increased the one‐year survival proportion from 28.5% to 32.2%, and the mean OS per‐patient increased from 12.8 to 15.0 months. On a population level, this survival gain amounted to 17,600 additional life years.
Discussion
Using simulation modeling informed with data from nationally representative databases, we estimated gains in OS for cohorts of patients diagnosed with metastatic NSCLC between 1990 and 2015. We demonstrated that, although there have been modest patient‐level survival gains as measured by one‐ and three‐year survival rates in metastatic NSCLC over the past 25 years, given the high incidence of metastatic lung cancer in the U.S., these gains amount to a meaningful number of additional life years on a population level. To the extent that new targeted agents are better tolerated, even in the absence of new breakthroughs, increasing the proportion of appropriate candidates for these agents who are treated would increase overall population survival by approximately 1.5 months, representing a 7% improvement over current levels.
In the early 1990s, there were essentially no effective systemic therapies to treat metastatic NSCLC, and the majority of patients received palliative measures following diagnosis. Since the early 2000s, the mainstay of first‐line treatment for metastatic NSCLC has involved platinum‐doublet regimens (e.g., cisplatin or carboplatin + gemcitabine, vinorelbine, or paclitaxel) based on evidence of improvements in progression‐free survival and OS from multiple clinical trials [7], [31]. However, due in part to the toxicity of platinum‐doublet therapy, a substantial proportion of NSCLC patients—nearly 60%—still do not initiate or complete systemic therapy [21], [22], [28]. The full range of reasons for the low rates of treatment are poorly understood, but several studies suggest that older persons and those with poor performance status, non‐Asian race/ethnicity, and rural residence are less likely to either be offered or accept systemic treatment [22], [28].
Our findings highlight several important points about advances in systemic therapy for metastatic NSCLC in the past 25 years and may offer clues to increasing treatment rates in the future. First, for much of the 1990s and 2000s, gains in survival were quite modest, reflecting the slow pace of innovation in this therapeutic era. This may have created a negative perception about treatment among patients and clinicians, particularly when contrasted to well‐publicized survival gains for other common metastatic malignancies such as breast cancer. Second, our model suggests that about 55% of OS gains are attributable to first‐ and second‐generation platinum‐doublet chemotherapy versus more recent therapeutic developments (45% of gains). The lesser degree of benefit seen after the advent of EGFR‐, VEGF‐, and ALK‐targeted and biomarker‐driven therapies is a bit misleading, however, because these therapies apply to a narrower population of patients. Because patients eligible for targeted and biomarker‐driven strategies predominantly have tumors of non‐squamous histology, the majority of survival gains in metastatic NSCLC were realized by that subgroup (versus squamous histology). Survival gains from targeted therapies, on a person level, often meet or exceed those afforded by platinum‐doublet agents.
Our “future treatment” scenario analysis suggests that modest but realistic increases in the proportion of patients receiving systemic therapy (i.e., by 10% and 30%, resulting in 49% and 69% of all patients, respectively) could substantially improve survival by 0.9 and 2.8 months, respectively, compared to the 2015 results. There are a number of factors that, if applied, could realize such increases in the treated proportion in the coming years. Perhaps the most important are that a greater proportion of lung cancer patients may now have access to care through expanded insurance coverage as a result of the Affordable Care Act and increased awareness of targeted therapy as genomic testing increases in the clinical community and the costs of testing decrease. Nonetheless, increases in the treated proportion are not a foregone conclusion, and efforts are needed to educate patients and clinicians about the changing landscape of metastatic NSCLC treatment and the benefits and risks of new treatment options.
There is also opportunity to improve metastatic NSCLC survival outcomes through the development of more effective therapies—particularly those that can be used by a relatively large proportion of the patient population (e.g., versus targeted therapies). One prominent example of this type of change is the emergence of immune checkpoint inhibitors, such as nivolumab, pembrolizumab, and atezolizumab. The FDA recently approved these agents as first and second‐line treatments for metastatic NSCLC. To estimate the potential survival impacts of this change, we evaluated a scenario in which 20% of patients not eligible for ALK‐ or EGFR‐targeted therapy received this type of hypothetical first‐line treatment. The analysis demonstrated that an OS gain of 2.3 months could be achieved by shifting patients currently receiving standard therapy to the new therapy (i.e., without changing the proportion receiving BSC only). Although this only a hypothetical scenario, it does demonstrate another pathway by which metastatic NSCLC survival could be rapidly improved in the coming years.
Our study has several limitations that should be considered when interpreting the results. First, models are simplified representations of complex and interrelated biologic, clinical, and behavioral factors. The primary purpose of this model is to show trends in treatment over time and their impact on survival. For that reason, we restricted the analysis to the most commonly used regimens at each time point when, in reality, a greater range of on‐ and off‐label treatments were provided to patients—particularly in more recent analysis years (e.g., 2010 and 2015). Second, we did not model the survival impacts of maintenance or second‐/third‐line therapies. Including second‐ and third‐line treatment would have only modestly increased the survival estimates at the expense of greater complexity. Additionally, our survival estimates are based on outcomes from large clinical trials and may represent the upper limit of survival observed in community practice. Nonetheless, we demonstrate good alignment with SEER OS outcomes across the range of analysis years (see supplemental Figs. 2–6). Third, the past 25 years have brought advances in supportive care that could have small impacts on survival. We did not explicitly evaluate the impacts of changes in BSC because of limited data and relatively small potential for survival impact. Lastly, we report estimated survival outcomes for 2015 based on extrapolations of historic treatment patterns because there is currently limited data available to evaluate treatment uptake and survival.
To our knowledge, this is the most comprehensive quantitative evaluation of survival gains over time for patients with metastatic NSCLC. In a 2013 abstract, Ravelo and colleagues analyzed the SEER–Medicare database to examine NSCLC patients 65 and older diagnosed from 1991–1995 and from 2006–2009. They found that median OS had increased by approximately 1 month (from 4.03 to 4.98 months), and one‐year survival has increased by 10.4% (from 16.6% to 27.0%) [32]. These results are very similar to the 1.1‐month median survival change and 7.8% one‐year OS gain estimated by our model for cohorts diagnosed in 1995 and 2010. The Patient Access to Cancer care Excellence project has created a model to estimate progress across multiple cancers using an “E‐score” based on evidence and value, but, to our knowledge, results for lung cancer have not been published [33].
Conclusion
Survival remains poor in metastatic NSCLC relative to other common cancers, but the development of new first‐line therapies over the past 25 years has resulted in modest per‐patient survival gains and substantial population‐level life year gains. These advances can be improved even further by increasing use of systemic therapies in the substantial proportion of patients who are suitable for treatment yet currently receive BSC only.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgment
This study was supported by funding from Genentech, Inc. Joshua A. Roth is supported by a training grant from AHRQ (1K12HS022982‐01). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Author Contributions
Conception/Design: Joshua A. Roth, Bernardo H.L. Goulart, Arliene Ravelo, Holli Kolkey, Scott D. Ramsey
Provision of study material or patients: Joshua A. Roth, Bernardo H.L. Goulart, Arliene Ravelo, Holli Kolkey, Scott D. Ramsey
Collection and/or assembly of data: Joshua A. Roth, Bernardo H.L. Goulart, Arliene Ravelo, Holli Kolkey, Scott D. Ramsey
Data analysis and interpretation: Joshua A. Roth, Bernardo H.L. Goulart, Arliene Ravelo, Holli Kolkey, Scott D. Ramsey
Manuscript writing: Joshua A. Roth, Bernardo H.L. Goulart, Arliene Ravelo, Holli Kolkey, Scott D. Ramsey
Final approval of manuscript: Joshua A. Roth, Bernardo H.L. Goulart, Arliene Ravelo, Holli Kolkey, Scott D. Ramsey
Disclosures
Joshua A. Roth: Genentech (C/A); Arliene Ravelo: Genentech (E), Roche Holdings (OI); Holli Kolkey: Genentech (E, OI); Scott D. Ramsey: Bayer, Genentech (C/A). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supplementary Information
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N NA, Krapcho M, Neyman N. et al (eds). SEER Cancer Statistics Review, 1975–2009. 2012; Available at http://seer.cancer.gov/csr/1975_2009_pops09/sections.html. Accessed August 23, 2012. [Google Scholar]
- 3. Warren JL, Butler EN, Stevens J et al. Receipt of chemotherapy among medicare patients with cancer by type of supplemental insurance. J Clin Oncol. 2015;33(4):312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Owonikoko TK, Ragin C, Chen Z et al. Real‐world effectiveness of systemic agents approved for advanced non‐small cell lung cancer: a SEER‐Medicare analysis. The Oncologist. 2013;18(5):600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goulart BH, Reyes CM, Fedorenko CR et al. Referral and treatment patterns among patients with stages III and IV non‐small‐cell lung cancer. J Oncol Pract. 2013;9(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davidoff AJ, Tang M, Seal B et al. Chemotherapy and survival benefit in elderly patients with advanced non‐small‐cell lung cancer. J Clin Oncol. 2010;28(13):2191–2197. [DOI] [PubMed] [Google Scholar]
- 7. Schiller JH, Harrington D, Belani CP et al. Comparison of four chemotherapy regimens for advanced non‐small‐cell lung cancer. N Engl J Med. 2002;346(2):92–98. [DOI] [PubMed] [Google Scholar]
- 8. Belani CP, Lee JS, Socinski MA et al. Randomized phase III trial comparing cisplatin‐etoposide to carboplatin‐paclitaxel in advanced or metastatic non‐small cell lung cancer. Ann Oncol. 2005;16(7):1069–1075. [DOI] [PubMed] [Google Scholar]
- 9. Shepherd FA, Dancey J, Ramlau R et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non‐small‐cell lung cancer previously treated with platinum‐based chemotherapy. J Clin Oncol. 2000;18(10):2095–2103. [DOI] [PubMed] [Google Scholar]
- 10. Scagliotti GV, Parikh P, von Pawel J et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551. [DOI] [PubMed] [Google Scholar]
- 11. Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. [DOI] [PubMed] [Google Scholar]
- 12. Mok TS, Wu YL, Thongprasert S et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. [DOI] [PubMed] [Google Scholar]
- 13. Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. [DOI] [PubMed] [Google Scholar]
- 14. Kwak EL, Bang YJ, Camidge DR et al. Anaplastic lymphoma kinase inhibition in non‐small‐cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shaw AT, Kim DW, Nakagawa K et al. Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. N Engl J Med. 2013;368(25):2385–2394. [DOI] [PubMed] [Google Scholar]
- 16. Solomon BJ, Mok T, Kim DW et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. [DOI] [PubMed] [Google Scholar]
- 17. Sandler A, Gray R, Perry MC et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. [DOI] [PubMed] [Google Scholar]
- 18. Garon EB, Ciuleanu TE, Arrieta O et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second‐line treatment of stage IV non‐small‐cell lung cancer after disease progression on platinum‐based therapy (REVEL): a multicentre, double‐blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. [DOI] [PubMed] [Google Scholar]
- 19. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus Docetaxel in Advanced Squamous‐Cell Non‐Small‐Cell Lung Cancer. N Engl J Med. 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 21. Ramsey SD, Howlader N, Etzioni RD et al. Chemotherapy use, outcomes, and costs for older persons with advanced non‐small‐cell lung cancer: evidence from surveillance, epidemiology and end results‐Medicare. J Clin Oncol. 2004;22(24):4971–4978. [DOI] [PubMed] [Google Scholar]
- 22. Lang K, Marciniak MD, Faries D et al. Trends and predictors of first‐line chemotherapy use among elderly patients with advanced non‐small cell lung cancer in the United States. Lung Cancer. 2009;63(2):264–270. [DOI] [PubMed] [Google Scholar]
- 23.IPSOS Healthcare Global Oncology Monitor . 1990. −2015. Available at: http://www.ipsos-na.com/products-tools/marketing/health-pharmaceuticals-product/global-oncology-monitor.aspx. Accessed January 11, 2017.
- 24.National Comprehensive Cancer Network (NCCN) . Lung Cancer Clinical Practice Guidelines in Oncology. Version 1. 2016.
- 25.Chemotherapy in non‐small cell lung cancer: a meta‐analysis using updated data on individual patients from 52 randomised clinical trials. Non‐small Cell Lung Cancer Collaborative Group . BMJ. 1995;311(7010):899–909. [PMC free article] [PubMed] [Google Scholar]
- 26. Wozniak AJ, Crowley JJ, Balcerzak SP et al. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non‐small‐cell lung cancer: a Southwest Oncology Group study. J Clin Oncol. 1998;16(7):2459–2465. [DOI] [PubMed] [Google Scholar]
- 27. Sequist LV, Yang JC, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. [DOI] [PubMed] [Google Scholar]
- 28. Ho C, Ramsden K, Zhai Y et al. Less toxic chemotherapy improves uptake of all lines of chemotherapy in advanced non‐small‐cell lung cancer: a 10‐year retrospective population‐based review. J Thorac Oncol. 2014;9(8):1180–1186. [DOI] [PubMed] [Google Scholar]
- 29. Briggs AH, Claxton K, Sculpher MJ. Decision modeling for health economic evaluation. New York, NY: Oxford University Press, Inc, 2006. [Google Scholar]
- 30.American Cancer Society . Lung Cancer (Non‐Small Cell) Information. 2015. Available at: http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Accessed January 12, 2016. [Google Scholar]
- 31. Fossella FV, DeVore R, Kerr RN et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non‐small‐cell lung cancer previously treated with platinum‐containing chemotherapy regimens. The TAX 320 Non‐Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18(12):2354–2362. [DOI] [PubMed] [Google Scholar]
- 32. Ravelo A, Guerin A, Latremouille‐Viau D et al. Survival Advancements In Advanced Non‐Small Cell Lung Cancer In The Past 20 Years: A Story of Hope. J Thorac Oncol. 2013; 8: P2.22–007. [Google Scholar]
- 33. Paddock S, Brum L, Sorrow K et al. PACE Continuous Innovation Indicators—a novel tool to measure progress in cancer treatments. Ecancermedicalscience. 2015;9:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.