On November 23, 2015, the U.S. Food and Drug Administration approved nivolumab for patients with advanced renal cell carcinoma who have received prior anti‐angiogenic therapy. The overall benefit/risk profile demonstrated in trial CA209025 supported this approval, and the trial design and results are presented here. Patients' prognostic risk category may serve as a putative predictive biomarker for treatment selection.
Keywords: Advanced renal cell carcinoma, Nivolumab, Immunotherapy, Biomarker
Abstract
On November 23, 2015, the U.S. Food and Drug Administration approved nivolumab (OPDIVO, Bristol‐Myers Squibb Company) for patients with advanced renal cell carcinoma (RCC) who have received prior anti‐angiogenic therapy. The approval was based on efficacy and safety data demonstrated in an open‐label, randomized study of 821 patients with advanced RCC who progressed after at least one anti‐angiogenic therapy. Patients were randomized to nivolumab or everolimus and followed for disease progression. The primary end point was overall survival. Subsequent therapies, including everolimus for patients who developed progressive disease on the nivolumab arm, were allowed, but no cross‐over was permitted. The median overall survival was 25.0 months on the nivolumab arm and 19.6 months on everolimus arm (hazard ratio: 0.73; 95% confidence interval: 0.60–0.89). The confirmed response rates were 21.5% versus 3.9%; median durations of response were 23.0 versus 13.7 months, and median times to response were 3.0 versus 3.7 months in the nivolumab and everolimus arms, respectively. A statistically significant improvement in progression‐free survival was not observed in this trial. The safety profile of nivolumab in renal cell cancer was similar to that in other disease settings. However, the incidence of immune‐mediated nephritis appeared to be higher in patients with RCC.
Implications for Practice.
The overall benefit/risk profile demonstrated in trial CA209025 supported the approval of nivolumab as an additional treatment option for patients with advanced renal cell carcinoma after anti‐angiogenic therapy. The use of nivolumab in patients who had received vascular endothelial growth factor‐targeted therapy resulted in a 5.4 month improvement in median overall survival compared with the everolimus arm. This difference is statistically significant and clinically meaningful.
Introduction
Renal cell carcinoma (RCC) is a serious and life‐threatening condition. In 2016, it is estimated that there will be 62,700 cases and 14,240 deaths from RCC in the U.S. [1]. RCC comprises about 3.8% of new cancers, with a median age at diagnosis of 64 years. Approximately 80% of RCC is of clear cell histology, with the rest being papillary, chromophobe, translocation, and collecting duct tumors. The rate of RCC has increased by 1.6% per year over the last decade (2002–2011). Multiple agents that target vascular endothelial growth factor (VEGF) or mammalian target of rapamycin (mTOR) have been approved for treatment of RCC based on an improvement in progression‐free survival (PFS) when compared with placebo or an active control [2], [3], [4], [5]. Temsirolimus was the first drug approved in the U.S. for the treatment of patients with poor prognostic factors on the basis of an increase in overall survival (OS). Prior to the approval of nivolumab, a typical treatment paradigm for advanced RCC consisted of a VEGF inhibitor followed by mTOR‐targeted therapy.
Nivolumab (OPDIVO, Bristol‐Myers Squibb Company) is a monoclonal antibody directed against programmed death‐1 (PD‐1). PD‐1 was found to be involved in the regulation of immune function [6]. Binding of PD‐1 ligand (PD‐L1) to PD‐1 has been reported to be implicated in tumor immune evasion [7], [8], [9]. Clinical development of nivolumab began in 2006 and trial CA209025 was subsequently initiated as a registration trial for nivolumab in advanced RCC. Based on results from this trial, Bristol‐Myers Squibb submitted a supplemental biological license application (BLA) to FDA for nivolumab for the treatment of advanced RCC in patients who have received prior anti‐angiogenic therapy [10].
Trial Design
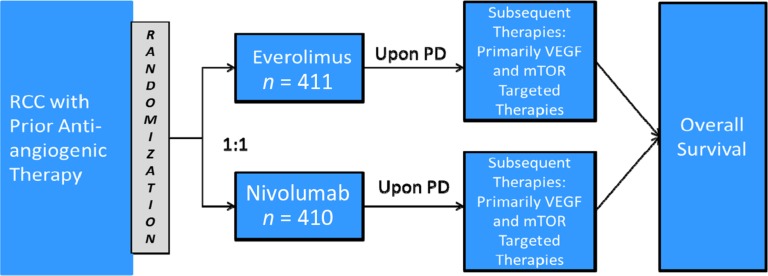
CA209025 was a randomized, active‐controlled, phase III trial to compare nivolumab and everolimus in patients with advanced or metastatic RCC who received prior anti‐angiogenic therapy (Fig. 1). Patients were treated with either nivolumab 3 mg/kg intravenously every 2 weeks or everolimus 10 mg orally daily. The primary objective was OS. Key secondary objectives included objective response rate (ORR), investigator‐determined PFS, and duration of response (DoR).
Figure 1.
CA209025 trial design.
Abbreviations: mTOR, mammalian target of rapamycin; PD, progressive disease; RCC, renal cell carcinoma; VEGF, vascular endothelial growth factor.
Patients with advanced or metastatic clear‐cell RCC who received prior anti‐angiogenic therapy were randomized 1:1 via an interactive voice response system (IVRS) to either nivolumab or everolimus. Patients were stratified by region (U.S./Canada, Western Europe, rest of the world), Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk group, and number of prior anti‐angiogenic regimens in the advanced or metastatic setting. Patients on both arms were allowed to continue treatment after initial investigator‐assessed Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1‐defined progression if they were assessed by the investigator to be deriving clinical benefit and tolerating study drug. Cross‐over was not permitted. However, because everolimus is approved for the treatment of RCC, patients with disease progression on nivolumab could receive everolimus as subsequent therapy. On July 18, 2015, the trial was stopped based on data‐monitoring committee review of the interim analysis of OS.
Key inclusion criteria were (a) advanced or metastatic, histologically confirmed clear‐cell RCC with measurable disease as defined by RECIST version 1.1; (b) at least one but no more than two prior anti‐angiogenic regimens; (c) no more than three total prior systemic regimens in the advanced or metastatic setting; and (d) prior cytokine therapy, vaccine therapy, and/or cytotoxic therapies were allowed. Key exclusion criteria were (a) any central nervous system (CNS) metastases; (b) prior treatment with an mTOR inhibitor; (c) autoimmune disease; and (d) requirement for systemic treatment with corticosteroids.
The trial planned to randomize 822 patients. Approximately 569 deaths were required to provide 90% power to detect a hazard ratio (HR) of 0.76 with an overall type 1 error of 5% (two‐sided). The HR of 0.76 corresponded to a 32% increase in the median OS, assuming a median OS of 14.8 months for everolimus and 19.5 months for nivolumab. One interim analysis was scheduled after 398 deaths (70% of total OS events needed for the final analysis).
OS was compared between the two arms at the interim and final analyses via a two‐sided, log‐rank test stratified by region, MSKCC risk group, and number of prior anti‐angiogenic regimens in the advanced or metastatic setting as entered into the IVRS. The HR and confidence interval (CI) were estimated using a stratified Cox proportional hazards model. OS curves for each randomized arm were estimated using the Kaplan‐Meier (KM) product limit method.
ORR was defined as the number of patients with a best response of complete response (CR) or partial response (PR) per RECIST 1.1, divided by the number of randomized patients. The comparison of ORR was carried out using a two‐sided Cochran‐Mantel‐Haenszel test stratified by the above stratification factors. ORRs and their corresponding 95% exact CIs were calculated by the Clopper‐Pearson method for each randomized arm. DoR was defined as the time from first response to the date of the first documented tumor progression as determined by the investigator (per RECIST 1.1 or clinical criteria) or death due to any cause, whichever occurred first, and estimated using the KM product‐limit method for patients who achieved a PR or CR. Median values along with two‐sided 95% CIs were to be calculated using the Brookmeyer and Crowley method.
PFS was defined as the time from randomization to the date of the first documented tumor progression as determined by the investigator (per RECIST 1.1 or clinical criteria) or death due to any cause, whichever occurred first. PFS was compared between the two randomized arms using a two‐sided, log‐rank test stratified by the factors used in the analysis of OS. HRs and corresponding two‐sided 95% CIs were estimated in a stratified Cox proportional hazards model. PFS curves for each treatment group were estimated using the KM product‐limit method.
Results
The trial randomized 821 patients, and 803 received the study drug. Key demographic and disease characteristics for all randomized patients in the two arms are summarized in Table 1. The majority of patients (77%) were treated with one prior anti‐angiogenic therapy. Patient distribution by MSKCC risk groups was 34% favorable, 47% intermediate, and 19% poor.
Table 1. Demographic parameters in Study CA209025.

Including anti‐CTLA4 agents, interferons, interleukins, and investigational immunotherapy.
Abbreviations: MSKCC, Memorial Sloan Kettering Cancer Center; PD‐1, programmed death‐1; PD‐L1, PD‐1 ligand; SD, standard deviation.
Efficacy
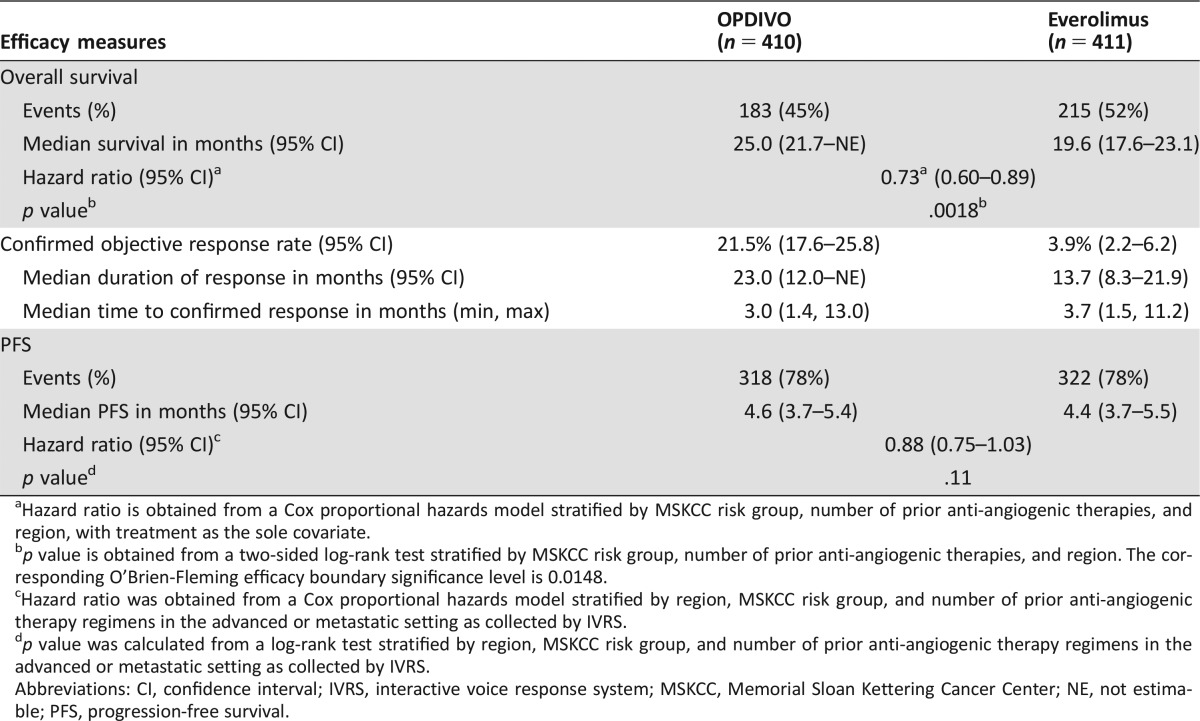
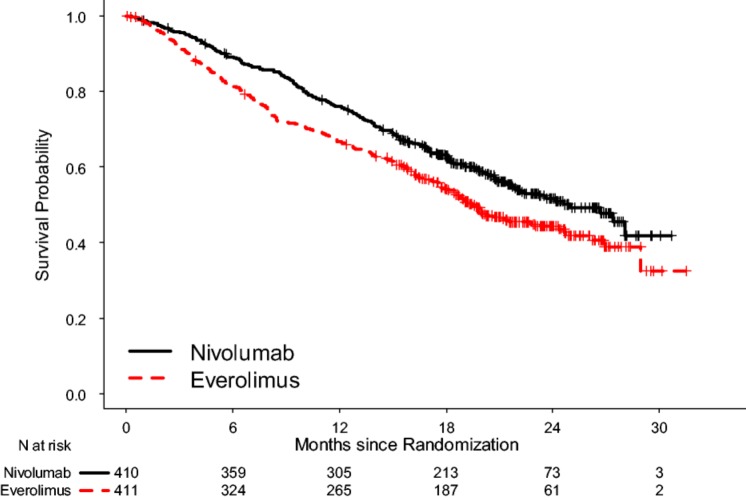
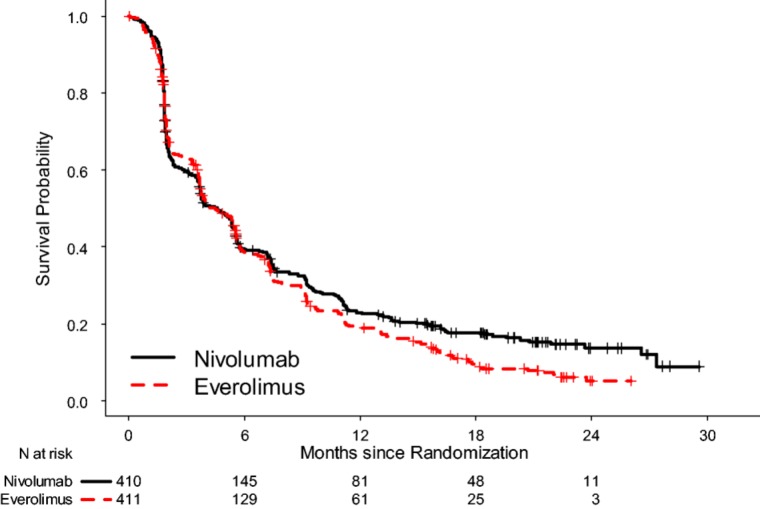
The prespecified interim OS analysis was conducted when 398 deaths occurred (70% of the planned number of events for final analysis) as of the trial cutoff date of June 18, 2015. Per the O'Brien‐Fleming boundary, the two‐sided significance level for the interim OS analysis with 398 deaths was 0.0148. There was a statistically significant improvement in OS for patients in the nivolumab arm compared with patients in the everolimus arm, with a 5.4‐month difference in median OS and a HR of 0.73 (95% CI: 0.60–0.89; two‐sided log rank p value = .0018; Table 2, Fig. 2). A hierarchical testing approach was applied to test key secondary end points at an alpha of 5% (two‐sided) following analysis of the primary end point of OS with ORR followed by PFS. Per investigator assessment by RECIST 1.1, the confirmed ORR was 21.5% and 3.9% with median response durations of 23.0 months and 13.7 months in the nivolumab and the everolimus arm, respectively. Median time to response appeared similar between the two arms. At the time of the OS analysis, PFS per investigator assessment was also examined. No statistically significant difference in PFS between the two treatment arms was observed. The median PFS was 4.6 months in the nivolumab arm and 4.4 months in the everolimus arm. The stratified HR was 0.88 (95% CI: 0.75–1.03) with a two‐sided log‐rank p value of .11 (Table 2, Fig. 3).
Table 2. Efficacy results of Trial CA209025.
Hazard ratio is obtained from a Cox proportional hazards model stratified by MSKCC risk group, number of prior anti‐angiogenic therapies, and region, with treatment as the sole covariate.
p value is obtained from a two‐sided log‐rank test stratified by MSKCC risk group, number of prior anti‐angiogenic therapies, and region. The corresponding O'Brien‐Fleming efficacy boundary significance level is 0.0148.
Hazard ratio was obtained from a Cox proportional hazards model stratified by region, MSKCC risk group, and number of prior anti‐angiogenic therapy regimens in the advanced or metastatic setting as collected by IVRS.
p value was calculated from a log‐rank test stratified by region, MSKCC risk group, and number of prior anti‐angiogenic therapy regimens in the advanced or metastatic setting as collected by IVRS.
Abbreviations: CI, confidence interval; IVRS, interactive voice response system; MSKCC, Memorial Sloan Kettering Cancer Center; NE, not estimable; PFS, progression‐free survival.
Figure 2.
Kaplan‐Meier curves for overall survival, in the intent‐to‐treat (ITT) population.
Figure 3.
Kaplan‐Meier curves for progression‐free survival, in the intent‐to‐treat (ITT) population.
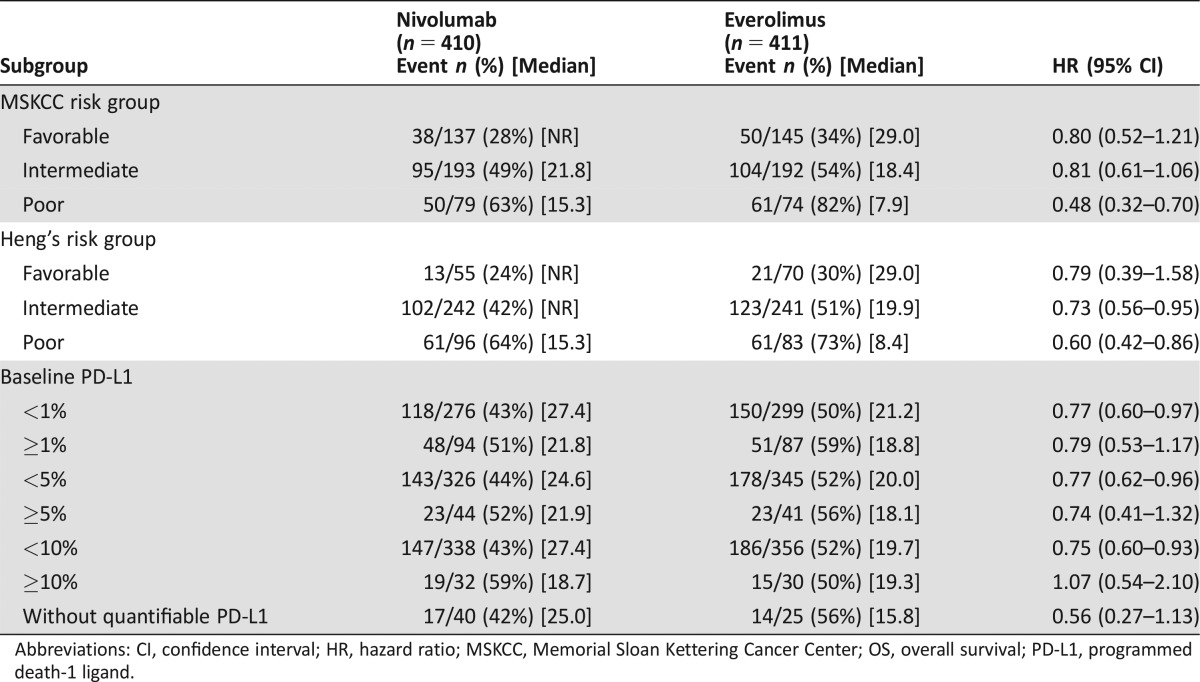
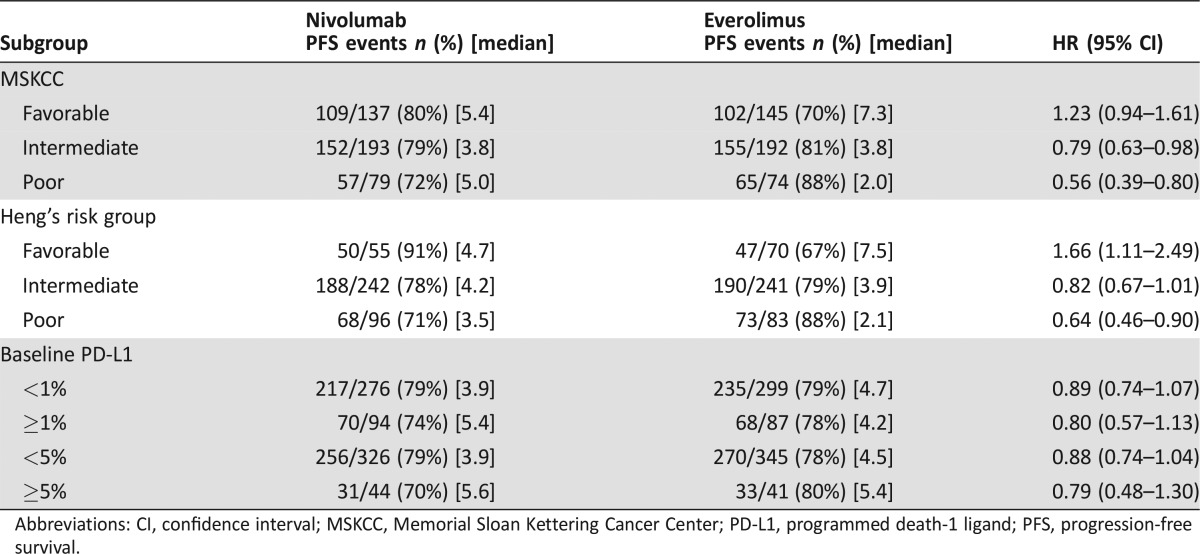
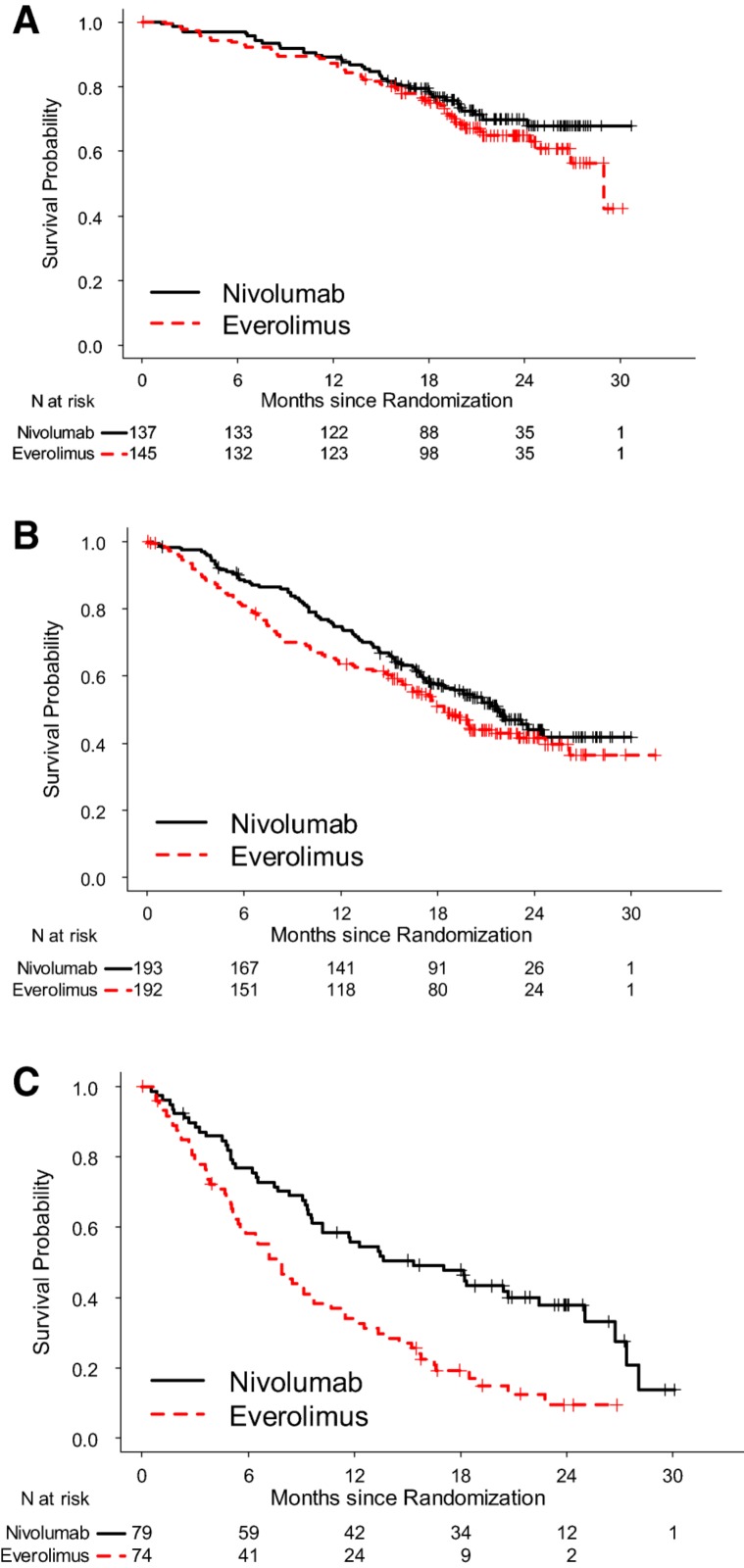
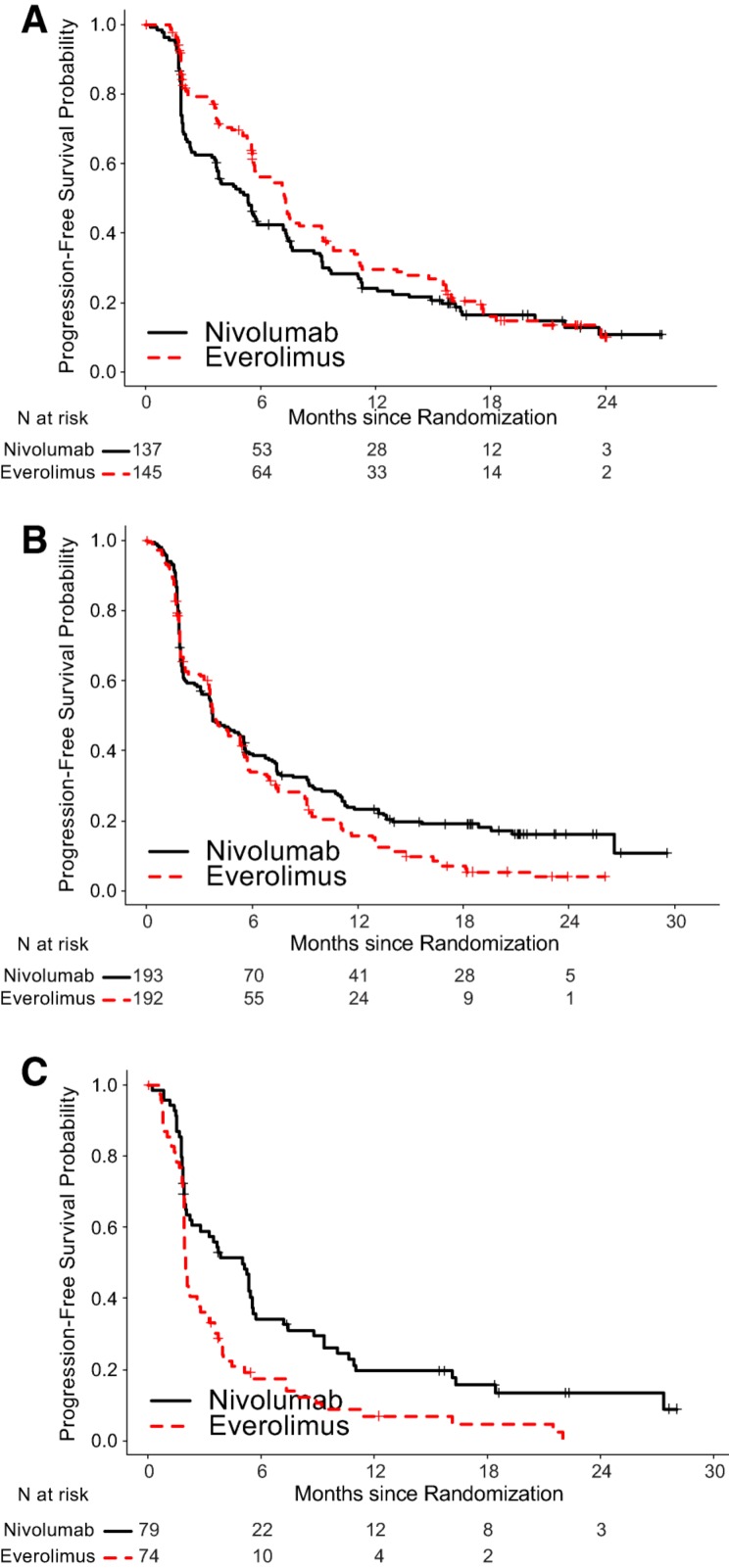
To assess potential predictive biomarkers, subgroup analyses for OS based on PD‐L‐1 status as determined by an immunohistochemistry assay and prognostic risk categories as defined by MSKCC and Heng's model were performed (Table 3) [11], [12]. Because PFS has been used for regulatory approval for anti‐neoplastic therapies in RCC, similar exploratory PFS subgroup analyses were performed (Table 4). There was little predictive value in primary tumor PD L‐1 status. Among patients in the MSKCC poor risk category at baseline, the HR for OS was 0.48 and 0.56 for PFS, whereas, among patients in the MSKCC favorable risk category, the HR for OS was 0.80 and 1.23 for PFS (Figs. 4, 5). These exploratory subgroup analyses suggest that patients in the poor prognostic risk category may derive the most benefit from nivolumab.
Table 3. OS subgroup analysis by MSKCC and Heng's risk categories as well as PD‐L1 status.
Abbreviations: CI, confidence interval; HR, hazard ratio; MSKCC, Memorial Sloan Kettering Cancer Center; OS, overall survival; PD‐L1, programmed death‐1 ligand.
Table 4. PFS subgroup analysis by MSKCC and Heng's risk categories as well as PD L‐1 status.
Abbreviations: CI, confidence interval; MSKCC, Memorial Sloan Kettering Cancer Center; PD‐L1, programmed death‐1 ligand; PFS, progression‐free survival.
Figure 4.
Kaplan‐Meier curves of OS based on MSKCC risk categories. (A): OS, MSKCC favorable; (B): OS, MSKCC intermediate; (C): OS, MSKCC poor.
Abbreviations: MSKCC, Memorial Sloan Kettering Cancer Center; OS, overall survival.
Figure 5.
Kaplan‐Meier curves of PFS based on MSKCC risk categories. (A): PFS, MSKCC favorable; (B): PFS, MSKCC intermediate; (C): PFS, MSKCC poor.
Abbreviations: MSKCC, Memorial Sloan Kettering Cancer Center; PFS, progression‐free survival.
Toxicity
The primary source of safety data comes from 406 patients who received nivolumab and 397 patients who received everolimus. Adverse events were assessed during the treatment period and for 100 days after the last dose of study drug. The median duration of treatment was 5.5 months (range: 1 day to 29.6+ months) in nivolumab‐treated patients and 3.7 months (range: 6 days to 25.7+ months) in everolimus‐treated patients. Study therapy was discontinued for adverse reactions in 16% of nivolumab‐treated patients and 19% of everolimus‐treated patients. Forty‐four percent of patients receiving nivolumab had a drug delay for an adverse reaction. Rate of death on treatment or within 30 days of the last dose of study drug was 4.7% on the nivolumab arm versus 8.6% on the everolimus arm. Serious adverse reactions occurred in 47% of patients receiving nivolumab. The most frequent serious adverse reactions reported in at least 2% of patients were acute kidney injury, pleural effusion, pneumonia, diarrhea, and hypercalcemia. The most common adverse reactions (reported in at least 20% of patients) were asthenic conditions, cough, nausea, rash, dyspnea, diarrhea, constipation, decreased appetite, back pain, and arthralgia. The most common laboratory abnormalities that worsened compared with baseline in ≥30% of patients included increased creatinine, lymphopenia, anemia, aspartate aminotransferase (AST), alkaline phosphatase, hyponatremia, elevated triglycerides, and hyperkalemia. Among patients with thyroid stimulating hormone (TSH) less than the upper limit of normal (ULN) at baseline, a greater proportion of patients experienced a treatment‐emergent elevation of TSH greater than the ULN in the nivolumab group compared with the everolimus group (26% and 14%, respectively).
Renal injury occurred in 7% (27/406) of patients on nivolumab and 3.0% (12/397) of patients on everolimus. This did not include elevations in laboratory creatinine. Immune‐mediated nephritis requiring corticosteroids occurred in 3.2% (13/406) of patients receiving nivolumab (one with grade 5, one with grade 4, five with grade 3, and six with grade 2). This 3.2% incidence of patients receiving steroids for renal dysfunction was approximately three times the incidence on previous nivolumab monotherapy studies. It is possible that the underlying renal abnormalities or reactions to renal antigens increased the incidence of nivolumab‐related renal dysfunction in this trial. Four patients were rechallenged, and two were able to continue treatment with nivolumab.
Pneumonitis, including interstitial lung disease, occurred in 5% (21/406) of patients receiving nivolumab and 18% (73/397) of patients receiving everolimus. In two patients, pneumonitis occurred after they had received nivolumab followed by everolimus. Seven patients had complete resolution. Among the six patients who resumed nivolumab, three did not have recurrence of pneumonitis. The incidence of other immune‐mediated adverse events in patients with renal cell cancer was consistent with the reported incidence in melanoma and non‐small cell lung cancer.
Discussion
The FDA's review and analyses of the clinical data from CA209025 found that the use of nivolumab in patients who had received VEGF‐targeted therapy resulted in a significant improvement in OS when compared with everolimus. Everolimus, an mTOR targeted therapy, is an appropriate comparator in this setting and is approved for the treatment of RCC in patients who have received prior sunitinib or sorafenib. The nivolumab arm demonstrated a 5.4‐month improvement in median OS compared with the everolimus arm. This difference is statistically significant and clinically meaningful.
Because further survival upon progressive disease frequently hinges upon the activity of subsequent therapy, the design of CA209025 did not answer the question of optimal treatment sequence for mRCC. This is especially because a statistically significant difference in PFS, which is a regulatory end point for approval in mRCC, was not seen in this study. Two recently approved therapies, lenvatinib plus everolimus and cabozantinib, both demonstrated PFS benefits, with cabozantinib also showing an OS benefit over everolimus alone. Furthermore, our retrospective exploratory subgroup analysis suggests that patients with favorable prognostic risk categories as defined by MSKCC or Heng's model might derive more PFS benefit from everolimus than from nivolumab. These data suggest that treatment selection post‐VEGF kinase inhibitor may depend on the patients’ prognostic risk category, allowing the prognostic risk category to serve as a putative predictive biomarker. This hypothesis may be worthy of further investigation. Within the framework of the current mRCC treatment paradigm, a plausible approach may consist of trials comparing targeted therapies with immune therapy in the second‐line setting in a patient population enriched by prognostic risk categories, allowing double cross‐over and using PFS2 as a possible end point. If prospectively validated, this may lead to a biomarker‐driven paradigm for RCC treatment selection in the era of precision medicine.
The toxicity profile of nivolumab is satisfactory compared with that of everolimus, an agent with a toxicity profile acceptable to patients with RCC. Except for higher incidence of immune‐mediated nephritis, the safety profile is not substantially changed from the original nivolumab BLA and other supplements. Thus, nivolumab has shown an acceptable safety profile with improved efficacy over that of everolimus.
Although uncertainties exist regarding the optimum treatment sequence due to the absence of a statistically significant difference in PFS between everolimus and nivolumab, the trial demonstrated the activity of nivolumab as a component in the paradigm of sequential therapies for RCC. With a favorable benefit/risk profile, nivolumab distinguished itself as a new class of treatment available to patients with advanced RCC.
Conclusion
Nivolumab demonstrated a clinically significant OS improvement as a new therapeutic option for advanced RCC in the post‐anti‐angiogenic setting with an acceptable toxicity profile. The evidence demonstrated in trial CA209025 met the statutory evidential standard, with the overall benefit/risk profile of nivolumab supporting regular approval. However, uncertainties exist as to optimum treatment sequence in the post‐VEGF setting. This is due to the absence of a statistically significant difference in PFS between everolimus and nivolumab and the lack of predictive biomarker(s). Further prospective studies are needed to characterize predictive biomarker(s) for treatment selection.
Acknowledgments
We acknowledge Ms. Kirsten Goldberg's editorial assistance in the preparation of our manuscript. This is a U.S. Government work. There are no restrictions on its use.
Author Contributions
Conception/Design: James Xunhai Xu, V. Ellen Maher, Lijun Zhang, Shenghui Tang, Rajeshwari Sridhara, Amna Ibrahim, Geoffrey Kim, Richard Pazdur
Provision of study material or patients: James Xunhai Xu, V. Ellen Maher, Lijun Zhang, Shenghui Tang, Rajeshwari Sridhara, Amna Ibrahim, Geoffrey Kim
Collection and/or assembly of data: James Xunhai Xu, V. Ellen Maher, Lijun Zhang, Shenghui Tang, Rajeshwari Sridhara, Amna Ibrahim, Geoffrey Kim
Data analysis and interpretation: James Xunhai Xu, Lijun Zhang, Rajeshwari Sridhara, Amna Ibrahim, Geoffrey Kim
Manuscript writing: James Xunhai Xu, V. Ellen Maher, Lijun Zhang, Shenghui Tang, Rajeshwari Sridhara, Amna Ibrahim, Geoffrey Kim, Richard Pazdur
Final approval of manuscript: James Xunhai Xu, V. Ellen Maher, Lijun Zhang, Shenghui Tang, Rajeshwari Sridhara, Amna Ibrahim, Geoffrey Kim, Richard Pazdur
Disclosures
The authors indicated no financial relationships.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Rini BI, Escudier B, Tomczak P et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet 2011;378:1931–1939. [DOI] [PubMed] [Google Scholar]
- 3. Motzer RJ, Escudier B, Oudard S et al. Efficacy of everolimus in advanced renal cell carcinoma: A double‐blind, randomised, placebo‐controlled phase III trial. Lancet 2008;372:449–456. [DOI] [PubMed] [Google Scholar]
- 4. Motzer RJ, Hutson TE, Glen H et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open‐label, multicentre trial. Lancet Oncol 2015;16:1473–1482. [DOI] [PubMed] [Google Scholar]
- 5. Choueiri TK, Escudier B, Powles T et al. Cabozantinib versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ishida Y, Agata Y, Shibahara K et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dong H, Zhu G, Tamada K et al. B7‐H1, a third member of the B7 family, co‐stimulates T‐cell proliferation and interleukin‐10 secretion. Nat Med 1999;5:1365–1369. [DOI] [PubMed] [Google Scholar]
- 8. Dong H, Strome SE, Salomao DR et al. Tumor‐associated B7‐H1 promotes T‐cell apoptosis: A potential mechanism of immune evasion. Nat Med 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- 9. Thompson RH, Gillett MD, Cheville JC et al. Costimulatory B7‐H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA 2004;101:17174–17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Motzer RJ, Bacik J, Murphy BA et al. Interferon‐alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289–296. [DOI] [PubMed] [Google Scholar]
- 12. Heng DY, Xie W, Regan MM et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor‐targeted agents: Results from a large, multicenter study. J Clin Oncol 2009;27:5794–5799. [DOI] [PubMed] [Google Scholar]