Abstract
The NF-κB pathway plays a pivotal role in proliferation, differentiation, apoptosis, and immune responses in mammals. The NF-κB inhibitor, IκB, has classically been characterized for its ability to sequester NF-κB transcription factors in the cytoplasm. Nevertheless, a nuclear fraction of IκBα has consistently been detected and associated with repression of nuclear NF-κB. Now we show that IκBα physically associates with different repression elements such as nuclear corepressors and histone acetyltransferases and deacetylases (HDACs). More remarkably, chromatin immunoprecipitation experiments demonstrate that IκBα is recruited to the promoter regions of the Notch-target gene, hes1, together with HDAC1 and -5, whereas we did not detect IκBα associated with classical NF-κB target genes such as IL6 and RANTES. TNF-α treatment results in a temporary release of IκBα from the hes1 promoter that correlates with increased histone acetylation and transcriptional activation. In addition, we demonstrate that both IκB kinase-α and -β are simultaneously recruited to the hes1 promoter in response to TNF-α, coinciding with a maximum of IκBα release and gene activation. Moreover, TNF-α-dependent histone H3 acetylation, release of IκBα from the hes1 promoter, and hes1 mRNA synthesis are affected in IKK-α–/– mouse embryonic fibroblasts. We propose that IκBα plays a previously undescribed role in regulating the recruitment of repression elements to specific promoters. Recruitment of IKKs to the nucleus in response to TNF-α may induce chromatin-associated IκBα release and gene activation. These findings provide additional insight in the cross-talk between NF-κB and other signaling pathways.
Keywords: IκB kinase, NF-κB, chromatin, TNF-α
The role of histone acetyltransferases and deacetylases (HDACs) in the control of individual gene expression has clearly been established. A diversity of complexes containing different combinations of corepressors and HDACs mediate specific gene silencing, thus playing critical roles in the regulation of biological processes, including cell proliferation, development, and tissue homeostasis (1–3). Nuclear corepressor (N-CoR) and silence mediator of retinoic acid and thyroid hormone (SMRT) are components of these multiprotein repression complexes and interact with other repression elements such as mSin3, Sharp (4), or different HDACs class I (5, 6) and class II (7). In addition, they also associate with several transcription factors (7, 8), including nuclear hormone receptors (9), MyoD (10), p65-NF-κB (11), or RBP-Jκ (12), which are thought to drive the repression complexes to specific gene promoters.
The IκB proteins are classically considered as the specific inhibitors of the NF-κB transcription factors (13). In the canonical NF-κB pathway, the NF-κB inhibitor IκB binds to different NF-κB homo- or heterodimers, thus masking their nuclear localization sequences and causing their cytoplasmic retention. Multiple NF-κB stimuli, including TNF-α, activate the IκB kinase complex that in turn binds and phosphorylates cytoplasmic IκB, leading to its proteasomal degradation (14, 15). This allows the NF-κB transcription factors to translocate into the nucleus and activate gene transcription (reviewed in refs. 15 and 16). Nevertheless, several reports have demonstrated that IκBα can also shuttle between the nucleus and the cytoplasm (17, 18), thus adding further complexity to the system. Although nucleocytoplasmic shuttling of IκBα is important for regulating NF-κB signaling termination (19, 20), its physiological relevance is not completely understood, thus pointing to the possibility that IκBα can play additional nuclear functions (21). Recently, a new nuclear function for IκB kinase (IKK-α) in phosphorylating histone H3 has been reported. IKK-α is specifically recruited to the chromatin of NF-κB-dependent promoters in response to TNF-α and participates in the activation of these genes. Nevertheless, not only IKK-α, but also IKK-β, is recruited to the chromatin, and they are both required for the appropriate transcriptional activation of NF-κB targets (22, 23).
We have shown that p65-NF-κB and IκBα participate in the regulation of the Notch-target gene, hes1, which is not a classical NF-κB target gene (11, 24), by modifying subcellular distribution of repression elements such as N-CoR. Besides, recent work showed the association of cytoplasmic IκBα with HDACs class I (25). Now, we present evidence that IκBα regulates transcription through its recruitment to the DNA regulatory regions of hes1 together with HDAC1 and -5. Release of IκBα in response to TNF-α is associated with acetylation of the hes1 promoter and increased transcriptional activation of the gene. In addition, IKK-α and -β are recruited to the hes1 promoter in response to TNF-α and release of hes1-associated IκBα together with TNF-α-dependent hes1 activation is affected in IKK-α-deficient mouse embryonic fibroblasts (MEFs). Surprisingly, this regulatory mechanism is not operating on two classical NF-κB-dependent genes such as IL-6 and RANTES.
Materials and Methods
Plasmids. Expression vectors for pCMV-HA-IκBα32–36, myc-SMRTc, f lag-N-CoR, f lag-HDAC4, f lag-HDAC5, f lag-HDAC1, and flag-HDAC2 have been described (8, 26–28). Myc-N-CoRc and GFP-N-CoR details are available upon request.
Antibodies. α-IκBα (sc-1643 and sc-371), α-HDAC5 (sc-11419), α-p65 (sc-109), α-HDAC1 (sc-7872), and α-IKK-β (sc-7330) were from Santa Cruz Biotechnology; α-Flag (clone M2) and α-β-actin were from Sigma; α-hemagglutinin (HA) was from Babco (Richmond, CA); α-SMRTe (06-891), α-IκBα (06-494), and α-acetyl-histone-H3 (06-599) were from Upstate Biotechnology (Lake Placid, NY); and α-IKK-α was from Oncogene (OP133). Irrelevant immunoglobulins from mouse, rabbit, and goat were from Sigma.
Cell Culture and Transfection. 3T3, 293T, L929sA, IκBα–/–, WT MEF, IKK-α–/–, and IKK-β–/– MEF were cultured in DMEM and 10% FBS. Cells were plated at subconfluence and transfected with Lipofectamine Reagent (Invitrogen). Murine and human TNF-α were purchased from PeproTech (Rocky Hill, NJ) and Upstate Biotechnology and were used at 25–40 ng/ml, respectively.
Coimmunoprecipitation Assays. Cells were lysed for 30 min at 4°C in 500 μl of immunoprecipitation (IP) buffer containing 0.5% Triton X-100, 1 mM EDTA, 100 μM Na-ortovanadate, 0.25 mM PMSF, and protease inhibitor mixture (Roche Diagnostics) in PBS. After centrifugation, supernatants were incubated for 3 h at 4°C with 1 μg of the indicated antibody coupled to Protein A-Sepharose beads.
Immunofluorescence. 3T3, 293T, and IκBα–/– MEF or IκBα WT MEF were seeded on slides and transfected with the different plasmids. After 48 h, cells were fixed in 3% paraformaldehyde and permeabilized in 0.3% Triton X-100/10% FBS/5% nonfat dry milk in PBS and incubated with the primary and secondary antibodies. Slides were visualized in an Olympus BX-60 (Melville, NY) microscope or in a Leica (Deerfield, IL) TCS-NT laser-scanning confocal microscope with the ×63 Leitz Plan-Apo objective (numerical aperture, 1.4).
Northern Blot Analysis. Total RNA was extracted by using the TRIzol Reagent (Invitrogen) following the manufacturer's instructions. Hybridization was analyzed in a PhosphoImager (Molecular Imager FX, Bio-Rad), and RNA levels were quantified with quantity one software (Bio-Rad). To evaluate the statistical significance, the area under the curve (AUC) was calculated for the increase from baseline between 0 and 60 min (AUC0–60min) by using the standard trapezoidal method.
Chromatin IP (ChIP) Assay. Chromatin from crosslinked cells was sonicated, incubated with primary antibodies in RIPA buffer, and precipitated with protein G/A–Sepharose. Crosslinkage of DNA–protein complexes was reversed, and DNA was used as a template for PCR. For second ChIP experiments, complexes from the first ChIP were eluted by incubation in 25 μl of 10 mM DTT for 30 min at 37°C. After centrifugation, the supernatant was diluted with RIPA buffer and subjected to the ChIP procedure. PCR primers are available upon request.
Results
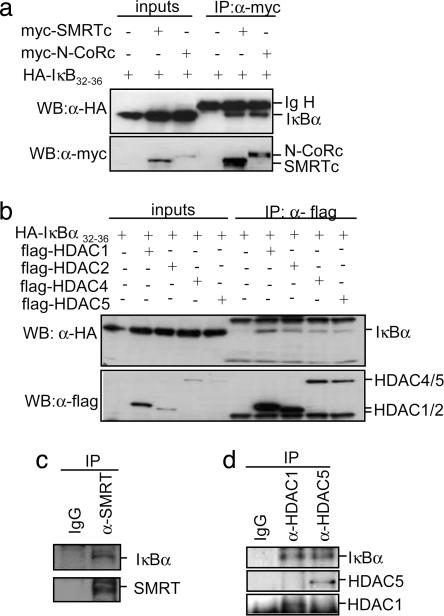
We previously described (11, 24) that p65 and IκBα can interact with the nuclear corepressor SMRT, thus modulating the transcriptional activation of NF-κB-independent promoters, and we hypothesized that IκBα together with p65 could play a direct role in transcriptional repression. Coprecipitation experiments demonstrated that the nondegradable IκBα32–36 mutant (29) physically interacts with N-CoRs (myc-N-CoR and myc-SMRT) (Fig. 1a), HDACs class I (flag-HDAC1 and -2) [as recently reported (25)] and HDACs class II (flag-HDAC4 and -5) (Fig. 1b). As a control, we did not detect IκBα32–36 in the precipitates from other myc-tagged proteins such as dishevelled1 (dvl1) or the nuclear form of Notch1 (N1-IC) (Fig. 7, which is published as supporting information on the PNAS web site). We next investigated whether these interactions occur under physiological conditions. Using 293T cell extracts, we demonstrated that endogenous IκBα coprecipitates with α-SMRT (Fig. 1c) and α-HDAC1 and -5 antibodies, whereas no IκBα was detected in the precipitates with nonrelevant IgG (Fig. 1d) or with α-p300 (Fig. 7). Moreover, by pull-down and coprecipitation experiments, we determined that N-CoRs and HDACs bind to different IκBα regions (AA151-179 for N-CoRs and AA180-317 HDACs), thus suggesting that both proteins directly bind to IκBα (see Supporting Text, which is published as supporting information on the PNAS web site, and Fig. 7).
Fig. 1.
IκBα binds to multiple repressor proteins including N-CoRs and HDACs. (a) Whole-cell lysates from 293T cotransfected with the indicated plasmids were immunoprecipitated with the α-myc followed by immunoblotting with α-HA; 1/50 of the input is shown. (Lower) Immunoblotting with α-myc to detect N-CoR and SMRT. (b) Lysates from 293T cells transfected with HA-IκBα alone or cotransfected with flag-HDAC4, -5, -1, or -2 were immunoprecipitated with α-flag. Coprecipitated IκBα was detected with α-HA; 1/50 of the input is shown (Left). Western blot with the α-flag is shown as a control for precipitation. (c) 293T cell lysates were precipitated with control IgG or α-SMRTe. (Upper) Coprecipitated IκBα. (d) Endogenous HDAC1 and -5 were precipitated from 293T cells with specific antibodies. (Upper) Coprecipitated IκBα was detected with the α-IκBα by Western blot.
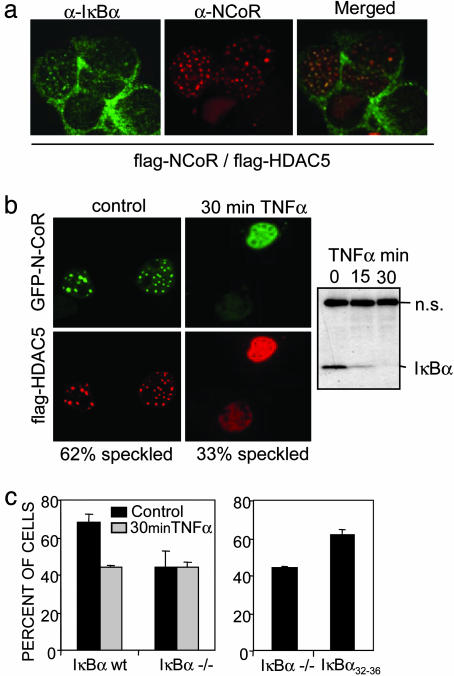
To address whether these interactions were taking place in the nucleus or in the cytoplasm, we determined the subcellular distribution of IκBα and the different repression elements. Consistent with previous reports (17), confocal microscopy revealed that endogenous IκBα is mainly distributed in the cytoplasm, although the presence of a nuclear fraction displaying a punctuate pattern was consistently detected. Because the different repression elements displayed a predominantly homogenous nuclear distribution (data not shown), colocalization experiments of the endogenous proteins were not informative. Taking advantage of the fact that coexpression of ectopic HDAC5 with N-CoR induced relocalization of both repression elements into nuclear speckles, we now performed colocalization experiments in these conditions. We detected endogenous IκBα colocalizing with some, but not all, N-CoR/HDAC5-containing nuclear speckles (Fig. 2a), suggesting that IκBα might be a component of specific repression complexes. Because different cytokines such as TNF-α lead to IκB phosphorylation and subsequent degradation (14), we tested whether IκB was involved in regulating repression complexes by incubating the N-CoR/HDAC5-transfected cells with TNF-α for 30 min. We observed a 30–40% reduction in the number of cells displaying N-CoR/HDAC5-containing speckles in these conditions (Fig. 2b). A similar reduction in the number of nuclear speckles was observed when N-CoR and HDAC5 were transfected into IκBα–/– MEF. Moreover, these complexes were not responsive to TNF-α treatment (Fig. 2c). When IκBα–/– MEF were reconstituted with IκBα32–36, the number of cells displaying the nuclear speckles increased up to 65%, similar to that observed in the IκBα WT MEF (Fig. 2c). Nevertheless, chronic TNF-α treatment plays a double role in regulating the levels of IκBα; first, triggering its degradation and next, activating its synthesis to terminate NF-κB signaling (30). Consistent with this, we observed that N-CoR/HDAC5-containing speckles increased after 2 h of TNF-α treatment (data not shown), coinciding with the increase in the levels of IκBα protein. Together, these observations strongly suggested a role for IκBα in the formation or stabilization of specific repression complexes.
Fig. 2.
Endogenous IκBα colocalizes with N-CoR/HDAC5 nuclear speckles. (a) Colocalization of endogenous IκBα with ectopic N-CoR/HDAC-5-containing nuclear speckles [confocal image (×630)]. (b) Subcellular localization of GFP-N-CoR and flag-HDAC5 in cotransfected 293T cells before (Left) and after 30 min of TNF-α treatment (Right). A minimum of 200 cells were counted for each condition in an Olympus BX-60 microscope [confocal image (×630)]. The α-IκBα immunoblot shows TNF-α-induced IκBα degradation. (c) Percentage of cells displaying N-CoR/HDAC5 speckles in IκBα+/+ and IκBα–/– MEFs cotransfected with GFP-N-CoR and flag-HDAC5 in the control or after 30 min of TNF-α treatment (Left) or in the IκBα–/– MEFs transiently transfected with IκBα32–36 (Right). A minimum of 200 cells were counted for each condition in an Olympus BX-60 microscope.
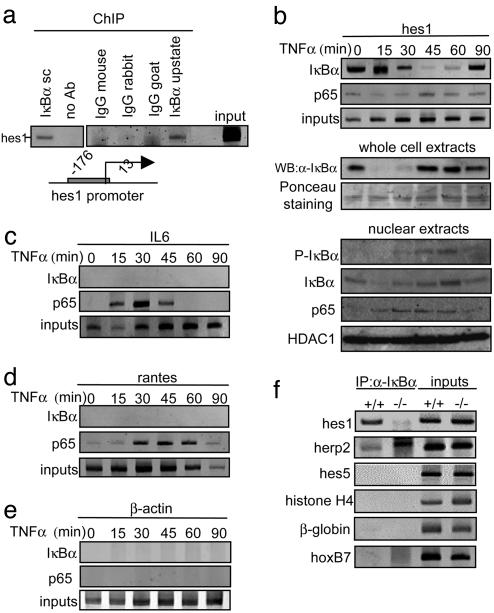
Because we previously reported that IκBα modulates the expression of different Notch-target genes such as hes1 (11), we now hypothesized that the IκBα fraction that interacts with repressor elements was directly responsible for hes1 repression. To test this, we performed ChIP assays to investigate whether IκBα protein was recruited to the promoter region of this gene. We consistently detected the hes1 promoter from chromatin precipitated with two different α-IκBα antibodies (Fig. 3a), thus indicating that this promoter is indeed occupied by IκBα. As a control, we could not amplify hes1 from precipitates with different nonrelevant immunoglobulins (Fig. 3a). We next investigated whether the recruitment of IκBα to the hes1 promoter was fine-tuned in response to TNF-α. For these studies, we performed ChIP assays from 3T3 cells treated with TNF-α at various times after stimulation. We detected a strong association of IκBα with the hes1 promoter in control conditions, that progressively decreased after 30 min of TNF-α treatment, was minimal at 45–60 min, and reappeared after 90 min of treatment (Figs. 3b and 4a). Of note is that IκBα dissociation kinetics was delayed compared with the kinetics of cytoplasmic IκBα degradation, suggesting the presence of a specific mechanism that regulates chromatin-associated IκBα (Fig. 3b). We next tested whether phosphorylation and degradation of IκBα by TNF-α were occurring in the nuclear fraction. Western blot from 3T3 nuclear extracts showed that nuclear levels of IκBα were moderately affected after TNF-α treatment, as described (31). In contrast, phosphorylation at serine 32–36 of IκBα consistently increased after 30–60 min of treatment, coinciding with the release of IκBα from the hes1 promoter, thus suggesting that the interaction between IκBα and the chromatin may be regulated by phosphorylation. As a control, p65 is recruited to the nucleus after TNF-α treatment, whereas HDAC1 is constitutively detected in the nuclear fraction (Fig. 3b).
Fig. 3.
IκBα is recruited to the hes1 gene regulatory sequences. (a) Chromatin obtained from 3T3 cells was immunoprecipitated with two different α-IκBα antibodies (Santa Cruz sc-1643 and Upstate Biotechnology 06-494) or different irrelevant immunoglobulins. Recruitment of IκBα to the hes1 promoter was analyzed by PCR. (b) Effect of TNF-α treatment in the recruitment of IκBα and p65 to the hes1 promoter was analyzed by ChIP assay followed by PCR (Top). Western blot analysis showing the kinetics of total IκBα levels after TNF-α treatment and Ponceau staining as a loading control (Middle). Western blot showing the levels of nuclear IκBα or phospho-IκBα after TNF-α treatment. Nuclear levels of p65 and HDAC1 are shown as a control (Bottom). (c–e) Recruitment of IκBα and p65 to the IL6 (c), RANTES (d), and β-actin (e) promoters was determined by ChIP assay followed by PCR analysis. All results are representative of at least two independent ChIP experiments. (f) Recruitment of IκBα to the indicated promoters analyzed by ChIP assay from IκBα+/+ and IκBα–/– MEF.
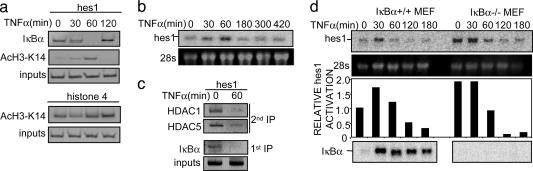
Fig. 4.
Recruitment of α-IκBα correlates with histone deacetylation and hes1 repression. (a) Chromatin prepared from TNF-α-treated 3T3 cells was immunoprecipitated with α-IκBα (Upper) and α-AcH3-K14 (Lower) antibodies. Coprecipitated DNA was analyzed by PCR with specific primers for hes1 or histone H4. Results are representative of two independent experiments. (b) TNF-α treatment induces a temporary activation of the endogenous hes1 transcription. Northern blot from 293T cells treated with TNF-α at different time points showing hes1 mRNA levels. 28s rRNA is shown as a loading control. (c) Chromatin from control or 60 min TNF-α-treated 3T3 cells was first precipitated with α-IκBα (Lower). Chromatin was then eluted and a second precipitation with α-HDAC1 or -5 antibodies was performed (Upper). The presence of hes1 promoter in the precipitates was determined by PCR analysis. (d) Northern blot showing relative hes1 transcriptional activation in IκBα+/+ (Left) and IκBα–/– (Right) MEF treated with TNF-α at the indicated time points. 28s ribosomal RNA is shown as a loading control. RNA levels were quantified, and the ratio between hes1 and 28s is represented (Lower). Induction of IκBα transcription in the IκBα+/+ is shown as a control of TNF-α activation (Lower).
Because we previously reported that not only IκBα but also p65 plays a role in regulating hes1 transcription (11), and because p65 can bind to a very broad spectrum of NF-κB-dependent and -independent genes (32), we tested whether the hes1 promoter was also occupied by p65. We observed that p65 was only marginally, but constitutively, associated with the hes1 promoter in a TNF-α-independent manner (Fig. 3b), suggesting that hes1 is not regulated by the recruitment of NF-κB transcription factors to its promoter. Further studies were performed to define whether IκBα was also associated with classical NF-κB-dependent promoters such as IL6 and RANTES. We detected IκBα recruitment to these promoters neither in control conditions nor after TNF-α stimulation up to 90 min (Fig. 3 c and d). As expected, p65 was recruited to these promoters in a TNF-α-dependent manner (Fig. 3 c and d). In contrast, we did not detect association of IκBα or p65 to the β-actin gene (Fig. 3e). Altogether, these results demonstrate that IκBα binds to the hes1 promoter, and that this association is regulated by TNF-α. Because of the kinetics observed for the association of IκBα and p65 to the hes1 promoter, the possibility that p65 may be involved in recruiting IκBα to the chromatin instead of IκBα binding itself might be considered. We next extended our study to different Notch targets and other unrelated genes, and we detected IκBα associated not only with hes1 but also with the Notch-target gene herp-2, but not with hes5 or the Notch-unrelated genes histone H4, β-globin, or hoxB7. As a control, we used IκBα–/– MEF and, as expected, we could not amplify any of these promoters from the IκBα precipitates (Fig. 3f).
Because we speculated that chromatin-associated IκBα is playing a role in gene repression, we investigated whether IκBα recruitment inversely correlates with acetylation of histone H3 and transcriptional activation of the hes1 promoter in response to TNF-α. Our results demonstrate that acetylation of Lys-14 of histone H3 in the hes1 promoter progressively increased after 30 min of TNF-α treatment, being maximal at 60 min (Fig. 4a). This kinetics nicely correlates with the release of IκBα from the hes1 promoter (Fig. 4a) and with the increased transcriptional activation measured by hes1 mRNA levels (Fig. 4b). In contrast, we did not observe any changes in the acetylation levels of the histone H4 promoter (Fig. 4a).
Based on these results, together with the observed interaction of IκBα with different repression elements, we hypothesized that nuclear IκBα could be recruiting deacetylase activities to specific promoters. To test this possibility, we precipitated chromatin from untreated or 60-min TNF-α-treated 3T3 cells with the α-IκBα, followed by a second precipitation with α-HDAC1 or -5 antibodies (Fig. 4c). We consistently detected hes1 in both α-HDAC1 and -5 precipitates from untreated cells, indicating that IκBα and HDACs coincide on this promoter in the absence of stimuli. As a control, we could not detect hes1 promoter in the precipitates from TNF-α-treated cells when IκBα is not present in this promoter (Fig. 4c). To further confirm the role of IκBα in repressing the hes1 gene, we performed Northern blot assays and determined hes1 mRNA levels in the IκBα–/– MEF compared with WT after TNF-α treatment. In the absence of stimuli, we detected higher levels of the hes1 mRNA in the IκBα–/– MEF compared with WT (Fig. 4d). Surprisingly, hes1 transcription was consistently down-regulated after 60-min TNF-α treatment in both knockout and WT cells (Fig. 4d), suggesting that IκBα is not required for the late repression of this gene or that in the absence of IκBα, other compensatory mechanisms are operational. These observations strongly support that chromatin-associated IκBα is involved in the recruitment of specific repression complexes in the noninduced state, and that TNF-α regulates hes1 transcriptional activation by modulating the association of IκBα with the hes1 promoter.
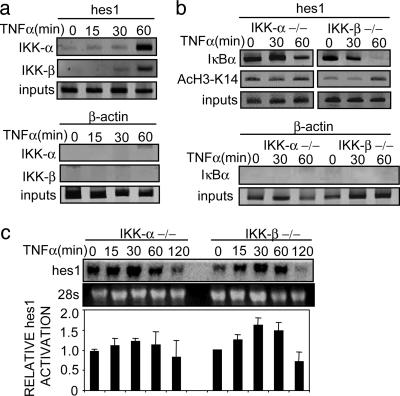
While this work was in progress, a new mechanism for TNF-α-dependent gene activation involving a nuclear function of IKKs, the upstream regulators of the NF-κB signaling, was reported (22, 23). Both IKK-α and -β are shown to be recruited to NF-κB-dependent promoters after TNF-α treatment, where IKK-α is responsible for phosphorylating Ser-10 of histone H3 and activation of gene transcription. Nuclear function of IKK-β is still unknown. Because IKKs could also play a role in NF-κB-independent gene transcription (33), we next investigated whether these kinases are involved in hes1 transcription in response to TNF-α stimulation. ChIP assay with α-IKK-α and -β antibodies demonstrated that both kinases are recruited to the hes1 promoter in response to TNF-α with a maximum at 60 min of treatment (Fig. 5a), whereas, as a control, we did not detect IKK-α or -β bound to the β-actin gene. The simultaneous presence of both IKKs on the hes1 promoter coincides with its higher transcriptional activity and with the release of IκBα from the chromatin (Fig. 4 a and b). Because IκBα is the best-characterized IKK substrate, and we observed that phosphorylation of nuclear IκBα is maximal at 30–60 min of TNF-α treatment (Fig. 3b), it is tempting to speculate that recruitment of IKKs to the promoters could induce phosphorylation and subsequent degradation of chromatin-associated IκBα. To further determine the role of IKKs in the regulation of chromatin associated IκBα, we performed ChIP assays with α-IκBα from IKK-α–/– and -β–/– MEFs. Release of IκBα from the hes1 promoter was affected in IKK-α–/– cells, because we detected only a slight decrease after 60 min of TNF-α treatment (Fig. 5b) compared with 3T3 cells (Fig. 4a). In contrast, in IKK-β–/– MEFs, TNF-α stimulation results in the release of hes1-associated IκBα after 60 min (Fig. 5b). We next tested the acetylation kinetics of the hes1 promoter in response to TNF-α in the absence of IKK-α or -β. Consistent with the stabilization of hes1-associated IκBα in the IKK-α–/– cells, TNF-α treatment results in minor changes in the acetylation of Lys-14 of histone H3 in this promoter (Fig. 5b), concomitant with a reduced transcriptional activation of the gene (Fig. 5c). In contrast, in the IKK-β–/– cells, Lys-14 of histone H3 on the hes1 promoter is acetylated in response to TNF-α coinciding with the transcriptional activation of the hes1 gene (Fig. 5 b and c). Altogether, these results suggest that IKK-α plays a predominant role in the release of IκBα from the hes1 gene, although both IKK-α and -β are recruited to the promoter in response to TNF-α. Additional work should be done to better characterize the role of hes1-associated IKK-α and -β.
Fig. 5.
IKK-α and -β are recruited to the hes1 promoter and correlate with IκBα release. (a) Chromatin prepared from TNF-α-treated 3T3 cells was immunoprecipitated with α-IKK-α or -β antibodies. Association of IKKs to the hes1 (Upper) or β-actin (Lower) genes was analyzed by PCR analysis. (b) Chromatins from TNF-α-treated IKK-α–/– and -β–/– MEFs were precipitated with α-IκBα and α-AcH3-K14 antibodies. (Upper) Recruitment of IκBα and acetylation of histone H3 on the hes1 promoter was determined by PCR analysis. (Lower) IκBα is not recruited to the β-actin gene in these cells. (c) Northern blot showing hes1 transcriptional activation in IKK-α–/– (lanes 1–5) and IKK-β–/– (lanes 6–10) MEF treated with TNF-α at the indicated time points. 28s ribosomal RNA is shown as a loading control. RNA levels were quantified and the ratio between hes1 and 28s is represented (Lower). Differences between IKK-α–/– and -β–/– were statistically significant, being the median area under the curve (AUC0min-60min) of 3.43 and 4.18, respectively (P = 0.032). One representative of three independent experiments is shown.
In summary, our results are consistent with a previously undescribed role for IκBα in recruiting HDAC activity to repress the hes1 gene. TNF-α treatment results in the release of hes1-associated IκBα, coinciding with the transcriptional activation of this gene. In addition, both IKK-α and -β proteins are simultaneously recruited to the hes1 promoter, strongly suggesting a role for these proteins in regulating hes1 transcription. In agreement with this, IκBα release, histone acetylation, and hes1 activation are impaired in IKK-α–/– cells.
Discussion
We previously demonstrated that IκBα binds to the SMRT corepressor, leading to changes in its subcellular localization and affecting the transcription of NF-κB-independent promoters (11). Although IκB proteins were first identified as cytoplasmic inhibitors of NF-κB, it is now clear that they shuttle between the nucleus and the cytoplasm (17). An important nuclear function postulated for IκBα is the repression of the NF-κB signal by inducing the nuclear export of the transcription factor (34). In this work, we investigate the putative role of nuclear IκBα in transcriptional repression on non-NF-κB target genes. We here describe that IκBα interacts with N-CoRs and HDACs and is recruited to the regulatory sequences of the Notch-target promoters hes1 and herp2. Chromatin-associated IκBα is released from the hes1 promoter in response to TNF-α correlating with increased levels of histone H3 acetylation, strongly suggesting that deacetylase activity is associated with the presence of IκBα. Consistent with this, we demonstrate that HDAC1 and -5 are associated with the hes1 promoter together with IκBα. Moreover, IκBa–/– cells showed increased basal hes1 transcription and failed to up-regulate hes1 in response to TNF-α. Nevertheless, late TNF-α-dependent repression is still operating in these cells. Although this repression could be explained by IκBα-independent mechanisms, we consider most likely that other IκB members could compensate IκBα deficiency. In agreement with this, there is strong evidence for IκBβ compensation in IκBα–/– cells (20, 35).
Although this is a completely unexpected function for IκBα, a new mechanism for TNF-α-dependent gene activation has recently been reported involving a nuclear function of another upstream regulator of the NF-κB signaling, IKK-α (22, 23). IKK-α is recruited to NF-κB-dependent promoters after TNF-α-treatment to phosphorylate Ser-10 of histone H3 and to activate gene transcription. In fact, both IKK-α and -β are recruited to NF-κB-dependent promoters, although the function of chromatin-associated IKK-β is not known. Recently, the possibility that IKKs are associated with NF-κB-independent promoters has been suggested (33). We have demonstrated that both IKK-α and -β are recruited to the hes1 promoter, and that the release of IκBα is affected in IKK-deficient cells. Because IκBα is a well known IKK substrate, and based in the kinetics of nuclear IκBα phosphorylation, it is reasonable to speculate that recruitment of IKKs to NF-κB-independent promoters upon TNF-α treatment could induce phosphorylation and subsequent degradation of chromatin-associated IκBα. Because we cannot exclude that most of the nuclear IκBα is phosphorylated in the cytoplasm, future work should address whether IκBα bound to specific promoters is phosphorylated and requires the recruitment of IKK-α and/or -β. Because maximal recruitment of IKKs to the hes1 promoter is observed after 60 min of TNF-α induction, coinciding with the maximal activation of this gene, it is possible that hes1-associated IKK-α is also phosphorylating histone H3, as reported for NF-κB-dependent genes (22, 23).
Current experiments are directed toward investigating the role of IκBα in different promoters as well as the mechanism that dictates the specificity of the IκBα-containing complexes that respond to TNF-α. That we could not detect IκBα in IL6 or RANTES promoters suggests this regulatory mechanism does not generally operate on NF-κB target genes. Nevertheless, numerous genes are regulated by NF-κB and, to completely exclude this possibility, a broader spectrum of early- and late-responding NF-κB promoters should be analyzed in more detail.
The physiological significance of the TNF-α-dependent hes1 up-regulation is extremely intriguing. Hes1 is a basic helix–loop–helix protein with a well characterized transcriptional repression function. Most of the Hes1 target genes are tissue-specific transcriptional activators involved in cell differentiation such as MASH1 or neurogenin (36). Based on our results, it is tempting to speculate on a putative role of Hes1 protein in the postactivation repression or delayed activation of NF-κB-dependent genes after TNF-α treatment. Indeed, Hes1 has already been reported to repress an NF-κB target gene (37). This hypothesis is currently under investigation.
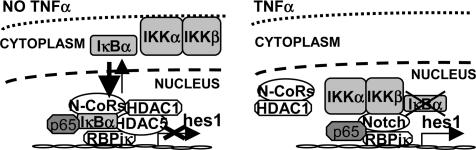
Together, our results support the idea that IκBα binds to the chromatin in specific promoters (hes1 and herp2) to facilitate HDAC recruitment. After TNF-α treatment, IKK-α and -β associate with the same promoters, leading to the release of IκBα and permitting gene activation (see model in Fig. 6).
Fig. 6.
Model illustrates that nuclear IκBα is recruited to the hes1 promoter together with repressor elements under basal conditions. Upon TNF-α induction, IKKs are recruited to this promoter resulting in IκBα release and transcriptional activation.
Supplementary Material
Acknowledgments
We thank M. Karin (University of California at San Diego, Los Angeles), R. Evans (The Salk Institute, San Diego), A. Israel (Pasteur Institute, Paris), and S. Schreiber (Harvard University, Cambridge, MA) for kindly providing plasmids; A. Hoffmann and D. Baltimore (California Institute of Technology, Los Angeles) for IκBα–/– MEF; and M. Karin for IKK-α–/– and -β–/– MEF. We thank F. Torres for statistical analysis; J. Inglés-Esteve, all of the members of the lab, and W. Vanden Berghe for helpful discussions; and Serveis Cientifico-Tècnics, Universitat de Barcelona Bellvitge, for confocal microscopy technical support. L.E. is an investigator from the Carlos III program (ISCIII/02/3027). C.A. is a recipient of a MCyT predoctoral fellowship (BES-2002-0028). This work was supported by the Comisión Interministerial de Ciencia y Tecnología, Plan Nacional de Salud (Grant SAF2001-1191), and the Fundació La Marató TV3 (Grant 030730).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HDAC, histone acetyltransferase and deacetylase; N-CoR, nuclear corepressor; SMRT, silence mediator of retinoic acid and thyroid hormone; MEF, mouse embryonic fibroblast; IP, immunoprecipitation; ChIP, chromatin IP; IκB, NF-κB inhibitor; HA, hemagglutinin; IKK, IκB kinase.
References
- 1.Hermanson, O., Jepsen, K. & Rosenfeld, M. G. (2002) Nature 419, 934–939. [DOI] [PubMed] [Google Scholar]
- 2.Jepsen, K., Hermanson, O., Onami, T. M., Gleiberman, A. S., Lunyak, V., McEvilly, R. J., Kurokawa, R., Kumar, V., Liu, F., Seto, E., et al. (2000) Cell 102, 753–763. [DOI] [PubMed] [Google Scholar]
- 3.Jepsen, K. & Rosenfeld, M. G. (2002) J. Cell Sci. 115, 689–698. [DOI] [PubMed] [Google Scholar]
- 4.Shi, Y., Downes, M., Xie, W., Kao, H. Y., Ordentlich, P., Tsai, C. C., Hon, M. & Evans, R. M. (2001) Genes Dev. 15, 1140–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagy, L., Kao, H. Y., Chakravarti, D., Lin, R. J., Hassig, C. A., Ayer, D. E., Schreiber, S. L. & Evans, R. M. (1997) Cell 89, 373–380. [DOI] [PubMed] [Google Scholar]
- 6.Heinzel, T., Lavinsky, R. M., Mullen, T. M., Soderstrom, M., Laherty, C. D., Torchia, J., Yang, W. M., Brard, G., Ngo, S. D., Davie, J. R., et al. (1997) Nature 387, 43–48. [DOI] [PubMed] [Google Scholar]
- 7.Kao, H. Y., Downes, M., Ordentlich, P. & Evans, R. M. (2000) Genes Dev. 14, 55–66. [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, S. K., Kim, J. H., Lee, Y. C., Cheong, J. & Lee, J. W. (2000) J. Biol. Chem. 275, 12470–12474. [DOI] [PubMed] [Google Scholar]
- 9.Zamir, I., Harding, H. P., Atkins, G. B., Horlein, A., Glass, C. K., Rosenfeld, M. G. & Lazar, M. A. (1996) Mol. Cell. Biol. 16, 5458–5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey, P., Downes, M., Lau, P., Harris, J., Chen, S. L., Hamamori, Y., Sartorelli, V. & Muscat, G. E. (1999) Mol. Endocrinol. 13, 1155–1168. [DOI] [PubMed] [Google Scholar]
- 11.Espinosa, L., Ingles-Esteve, J., Robert-Moreno, A. & Bigas, A. (2003) Mol. Biol. Cell 14, 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao, H. Y., Ordentlich, P., Koyano-Nakagawa, N., Tang, Z., Downes, M., Kintner, C. R., Evans, R. M. & Kadesch, T. (1998) Genes Dev. 12, 2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteside, S. T. & Israel, A. (1997) Semin. Cancer Biol. 8, 75–82. [DOI] [PubMed] [Google Scholar]
- 14.Brown, K., Gerstberger, S., Carlson, L., Franzoso, G. & Siebenlist, U. (1995) Science 267, 1485–1488. [DOI] [PubMed] [Google Scholar]
- 15.Karin, M. & Ben-Neriah, Y. (2000) Annu. Rev. Immunol. 18, 621–663. [DOI] [PubMed] [Google Scholar]
- 16.Li, Q. & Verma, I. M. (2002) Nat. Rev. Immunol. 2, 725–734. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, C., Van Antwerp, D. & Hope, T. J. (1999) EMBO J. 18, 6682–6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, T. T., Kudo, N., Yoshida, M. & Miyamoto, S. (2000) Proc. Natl. Acad. Sci. USA 97, 1014–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renard, P., Percherancier, Y., Kroll, M., Thomas, D., Virelizier, J. L., Arenzana-Seisdedos, F. & Bachelerie, F. (2000) J. Biol. Chem. 275, 15193–151999. [DOI] [PubMed] [Google Scholar]
- 20.Huang, T. T. & Miyamoto, S. (2001) Mol. Cell. Biol. 21, 4737–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh, S. & Karin, M. (2002) Cell 109, S81–S96. [DOI] [PubMed] [Google Scholar]
- 22.Anest, V., Hanson, J. L., Cogswell, P. C., Steinbrecher, K. A., Strahl, B. D. & Baldwin, A. S. (2003) Nature 423, 659–663. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto, Y., Verma, U. N., Prajapati, S., Kwak, Y. T. & Gaynor, R. B. (2003) Nature 423, 655–659. [DOI] [PubMed] [Google Scholar]
- 24.Espinosa, L., Santos, S., Ingles-Esteve, J., Munoz-Canoves, P. & Bigas, A. (2002) J. Cell Sci. 115, 1295–1303. [DOI] [PubMed] [Google Scholar]
- 25.Viatour, P., Legrand-Poels, S., van Lint, C., Warnier, M., Merville, M. P., Gielen, J., Piette, J., Bours, V. & Chariot, A. (2003) J. Biol. Chem. 278, 46541–46548. [DOI] [PubMed] [Google Scholar]
- 26.Grozinger, C. M. & Schreiber, S. L. (2000) Proc. Natl. Acad. Sci. USA 97, 7835–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie, J. & Black, D. L. (2001) Nature 410, 936–939. [DOI] [PubMed] [Google Scholar]
- 28.Yang, W. M., Yao, Y. L., Sun, J. M., Davie, J. R. & Seto, E. (1997) J. Biol. Chem. 272, 28001–28007. [DOI] [PubMed] [Google Scholar]
- 29.DiDonato, J., Mercurio, F., Rosette, C., Wu-Li, J., Suyang, H., Ghosh, S. & Karin, M. (1996) Mol. Cell. Biol. 16, 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann, A., Levchenko, A., Scott, M. L. & Baltimore, D. (2002) Science 298, 1241–1245. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez, M. S., Thompson, J., Hay, R. T. & Dargemont, C. (1999) J. Biol. Chem. 274, 9108–9115. [DOI] [PubMed] [Google Scholar]
- 32.Martone, R., Euskirchen, G., Bertone, P., Hartman, S., Royce, T. E., Luscombe, N. M., Rinn, J. L., Nelson, F. K., Miller, P., Gerstein, M., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 12247–12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anest, V., Cogswell, P. C. & Baldwin, A. S. (2004) J. Biol. Chem. 279, 31183–31189. [DOI] [PubMed] [Google Scholar]
- 34.Arenzana-Seisdedos, F., Thompson, J., Rodriguez, M. S., Bachelerie, F., Thomas, D. & Hay, R. T. (1995) Mol. Cell. Biol. 15, 2689–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng, J. D., Ryseck, R. P., Attar, R. M., Dambach, D. & Bravo, R. (1998) J. Exp. Med. 188, 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kageyama, R. & Nakanishi, S. (1997) Curr. Opin. Genet. Dev. 7, 659–665. [DOI] [PubMed] [Google Scholar]
- 37.Fujimori, K., Fujitani, Y., Kadoyama, K., Kumanogoh, H., Ishikawa, K. & Urade, Y. (2003) J. Biol. Chem. 278, 6018–6026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.