Abstract
Spermatogonial stem cells (SSCs) self-renew and produce large numbers of committed progenitors that are destined to differentiate into spermatozoa throughout life. However, the growth factors essential for self-renewal of SSCs remain unclear. In this study, a serum-free culture system and a transplantation assay for SSCs were used to identify exogenous soluble factors that promote proliferation of SSCs. Mouse pup testis cells were enriched for SSCs by selection with an anti-Thy-1 antibody and cultured on STO (SIM mouse embryo-derived thioguanine and ouabain resistant) feeders in a serum-free defined medium. In the presence of glial cell line-derived neurotrophic factor (GDNF), SSCs from DBA/2J strain mice formed densely packed clumps of cells and continuously proliferated. However, other strains of mice required the addition of soluble GDNF-family receptor α-1 and basic fibroblast growth factor to support replication. The functional transplantation assay proved that the clump-forming cells are indeed SSCs. Thus, GDNF-induced cell signaling plays a central role in SSC self-renewal. The number of SSCs in culture doubled every 5.6 days, and the clump-forming cells strongly expressed Oct-4. Under these conditions, SSCs proliferated over 6 months, reconstituted long-term spermatogenesis after transplantation into recipient testes, and restored fertility to infertile recipients. The identification of exogenous factors that allow continuous proliferation of SSCs in vitro establishes the foundation to study the basic biology of SSCs and makes possible germ-line modification by sophisticated technologies. Moreover, the ability to recover, culture indefinitely, and transplant SSCs will make the germ-line of individual males available for periods extending beyond a normal lifetime.
The spermatogenic system depends on stem cells, which have the ability to self-renew and generate a large number of differentiated germ cells in most species. In mammals, millions of spermatozoa are produced every day from spermatogonial stem cells (SSCs), the germ-line stem cells in the testis (1). To maintain normal spermatogenesis, the processes of self-renewal and differentiation of SSCs must be precisely regulated by intrinsic gene expression in the stem cells and extrinsic signals, including soluble factors or adhesion molecules from the surrounding microenvironment, the stem cell niche (2). Although SSCs are infrequent in the testis, presumably ≈1 in 3,000–4,000 cells in adult mouse testis (3), SSCs can be identified unequivocally by a functional transplantation assay (4, 5). Fluorescence-activated cell sorting (FACS) in conjunction with the transplantation assay for SSCs has identified the antigenic profile of SSCs as αv-integrin–/dim α6-integrin+ Thy-1lo/+ throughout postnatal life in the mouse (6, 7). Although the surface phenotype and functional properties of SSCs have been characterized by using the transplantation assay, regulatory mechanisms for SSC self-renewal remain elusive.
Glial cell line-derived neurotrophic factor (GDNF) is a member of the transforming growth factor-β superfamily and originally was identified as a survival factor for midbrain dopaminergic neurons (8). Although it was shown that GDNF is a potent trophic factor for several types of neurons and has a critical role in kidney morphogenesis (9), GDNF also has been found to be a key factor in fate determination of SSCs (10). Although GDNF is secreted normally from Sertoli cells in the seminiferous tubules, overexpression of GDNF in mouse testes appeared to stimulate self-renewal of stem cells and block spermatogonial differentiation (10, 11). Conversely, in the testes of mice with one GDNF-null allele, undifferentiated spermatogonia disappeared in older males and resulted in Sertoli cell-only seminiferous tubules (10). These in vivo studies suggest that GDNF has a crucial role in spermatogenesis by acting in a paracrine manner. However, the role of this factor relative to other constituents of the in vivo microenvironment is unclear. It was shown that undifferentiated spermatogonia express the receptor for GDNF, which consists of the GDNF-family receptor α1 (GFRα1) and c-Ret receptor tyrosine kinase (10, 11); however, because the rare SSCs cannot be distinguished from the large population of undifferentiated spermatogonia, it remains to be determined whether the stem cells express the receptor.
Studies on the maintenance and proliferation of SSCs in culture are enormously valuable in understanding their biology and in identifying crucial growth factors for self-renewal. Long- and short-term culture of SSCs have been attempted in several laboratories (12–14), and in our previous studies, GDNF appeared to have a beneficial effect on maintenance during a 7-day culture period (6, 14). In a recent report, SSCs derived from DBA/2 background mouse gonocytes, precursors of SSCs in neonate testis, proliferated in culture using a complex, undefined medium containing leukemia inhibitory factor (LIF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), GDNF, and 1% FBS (13). However, SSCs from other strains (e.g., C57BL/6or129/Sv) did not proliferate in the same culture condition (13). Thus, growth requirements for mouse SSCs remain unclear. Although these in vitro studies also suggest that GDNF may be important for replication of SSCs, the complex and undefined parameters introduced by serum in the medium and contaminating testis somatic cells have made the role of GDNF and other molecules in self-renewal of SSCs impossible to evaluate.
To address this problem, we developed a serum-free culture system for SSCs that permitted an evaluation of environmental factors for SSC maintenance and replication (6). By using the serum-free culture system optimized for SSCs, we found GDNF-induced cell signaling plays a central role in SSC self-renewal.
Materials and Methods
Donor and Recipient Mice. Two transgenic mouse lines expressing reporter genes, hybrid Fn progeny of B6.129S7-Gtrosa26 (designated ROSA; The Jackson Laboratory) and inbred C57BL/6-TgN(ACTbEGFP)1Osb (designated C57GFP; The Jackson Laboratory) were used to distinguish donor cells from recipient cells after transplantation. ROSA mice express the Escherichia coli lacZ gene that encodes a β-galactosidase (β-gal) protein in virtually all cell types, including all stages of spermatogenesis (15). Donor ROSA cells are identified by staining with the β-gal substrate 5-bromo-4-choloro-3-indolyl β-d-galactoside (X-gal). C57GFP mice express a GFP reporter gene in most cells of this mouse (16). Wild-type mouse lines used were DBA/2J, C57BL/6, SJL, and 129/SvCP (all from The Jackson Laboratory). Pup testis cells (4.5–7.5 days postpartum, dpp; day of birth is 0.5 dpp) were collected from the hemizygous transgenic mice, DBA/2J × ROSA, C57BL/6 × ROSA, or C57GFP × ROSA and from inbred nontransgenic mice, including C57BL/6, 129/SvCP, and SJL. NCr nude male mice (nu/nu, Taconic Farms), immunologically compatible C57BL/6 × 129/SvCP F1 hybrid male mice and W54/Wv male pup mice were used for recipients (4, 5, 17). For details of testis cell transplantation and recipient analysis, see Supporting Text, which is published as supporting information on the PNAS web site.
Cell Culture. The culture system for SSCs consisted of serum-free medium and mitotically inactivated STO (SIM mouse embryo-derived thioguanine and ouabain resistant) cell feeders (≈5 × 104 cells per cm2) as described (6). STO cells (STO SNL76/7 cells) were obtained from A. Bradley (The Wellcome Trust Sanger Institute, London). The serum-free medium for SSCs consisted of minimum essential medium α (Invitrogen catalog no. 12561), 0.2% BSA (ICN catalog no. 810661), and a supplement (6). The source, type, and lot of BSA are important. ICN catalog no. 810661, lot no. 2943C, MP Biochemicals (formerly ICN) catalog no. 194774, lot no. R14550, and Sigma catalog no. A3803, lot no. 064K0720 support SSC proliferation. Testis cells enriched for SSCs were prepared from pups by magnetic-activated cell sorting (MACS) with magnetic microbeads conjugated to anti-Thy-1 antibody (Miltenyi Biotec) as described (6). The enriched SSCs were cultured on STO feeders in wells of a 12-well plate at densities of 6–10 × 104 cells per well in 1.5 ml of the serum-free medium with or without growth factors as indicated. Human GDNF (R & D Systems), rat GFRα1-Fc fusion protein (GFRα1-Fc, R & D Systems), and human bFGF (BD Biosciences) were used at a final concentration of 40 ng/ml, 300 ng/ml, and 1 ng/ml, respectively. Cells were subcultured with trypsin-EDTA (Invitrogen catalog no. 25200) at 5- to 7-day intervals at a 1:2–4 dilution. All cultures were maintained at 37°C in a humidified 5% CO2/95% air atmosphere. The medium was changed every 2–3 days. For further details about culture experiments and characterization of cultured germ cells, see Supporting Text.
Results
SSCs Derived from DBA/2J-Background Mice Can Be Cultured in Serum-Free Medium Supplemented with GDNF. SSCs were enriched by MACS with anti-Thy-1 antibody (MACS Thy-1 cells) from pups, which were obtained by mating ROSA mice to DBA/2J or C57BL/6 mice (DBA × ROSA or C57 × ROSA, respectively). Enriched SSCs isolated from pups of each strain were placed onto STO feeders in a serum-free defined medium with or without GDNF. In the presence of GDNF, some MACS Thy-1 cells divided and formed clumps with tight intercellular contacts resulting in a ball or mass of cells. Every 5–7 days, the clumps were dissociated into single cells with trypsin-EDTA and sub-cultured to fresh STO feeders. DBA × ROSA-derived MACS Thy-1 cells continued dividing and regenerated clumps in the subculture (Fig. 1A), whereas C57 × ROSA MACS Thy-1 cells formed small clumps after the first subculture, which gradually disappeared with subsequent subculturing. DBA × ROSA pup cells without GDNF also gradually disappeared. The clump-forming cells were stained with an antibody against germ cell nuclear antigen (GCNA1) (Fig. 1B), which is a germ-cell-specific marker (18), indicating that the clumps consisted of germ cells. When cultured cells were incubated with X-gal, expression of β-gal was seen specifically in clumps (Fig. 1C).
Fig. 1.
Expansion of DBA × ROSA SSCs in serum-free medium supplemented with GDNF. (A) DBA × ROSA pup testis cells formed clumps with tight intercellular contacts in culture. (B) Cultured cells in clumps express GCNA1 (dark blue), a marker for germ cells. (C) Expression of β-gal was detected only in germ cell clumps. β-gal expressing cells stain blue with X-gal. (D) Macroscopic appearance of recipient testis 2 months after transplantation with DBA × ROSA MACS Thy-1 cells cultured for 10 weeks in the presence of GDNF. Each blue-stained area indicates donor-derived spermatogenesis. Testis was stained with X-gal. (E) Freshly isolated MACS Thy-1 cells and cultured cells were transplanted into recipient testes. The number of donor-derived spermatogenic colonies per 105 MACS Thy-1 cells originally seeded in culture is shown. The transplantation assay demonstrated expansion of DBA × ROSA SSCs in culture with GDNF. DBA × ROSA SSCs cultured without GDNF and C57 × ROSA SSCs cultured with or without GDNF were not maintained. Data are shown as means ± SEM for six recipient testes per time point. Most error bars do not extend beyond the symbol. (Scale bar, 100 μmin A–C and 2 mm in D.)
During subculturing, a continuous increase in cell clump number was observed, suggesting that they contained SSCs. Therefore, after 1, 3, 6, and 10 weeks in vitro, cultured cells were transplanted into the seminiferous tubules of busulfan-treated nude mouse testes to determine the ability of the expanding germ cells to form colonies of spermatogenesis. Only SSCs are able to generate colonies of complete spermatogenesis (4, 5). In addition, at the beginning of each experiment, freshly isolated MACS Thy-1 cells were transplanted to determine the stem cell activity of the original cell population. Two months after transplantation, donor-derived colonies of spermatogenesis in recipient testes were counted (Fig. 1D). The number of donor-derived spermatogenic colonies produced from each experimental group, at the individual time points, per 105 cells of MACS Thy-1 cells originally placed in culture (day 0), is shown in Fig. 1E. Only germ cell clumps derived from DBA × ROSA pups in culture with GDNF continued to generate spermatogenic colonies during the culture period (Fig. 1E). β-gal-expressing cells were found only in clumps (Fig. 1C); therefore, the clumps from DBA × ROSA pups indeed contained SSCs. After 10 weeks of culture, 105 MACS Thy-1 cells collected from DBA × ROSA pups generated 7.6 ± 1.4 × 105 (mean ± SEM, n = 6, Fig. 1E) colonies in the presence of GDNF, but no colonization was observed in cultures without addition of the growth factor (Fig. 1E). Cells from C57 × ROSA pups had no stem cell activity after 10 weeks of culture with or without GDNF (Fig. 1E). These results demonstrate that SSCs of DBA × ROSA pups are able to proliferate in serum-free medium and that GDNF is an essential growth factor. However, GDNF alone did not support in vitro expansion of SSCs derived from C57 × ROSA mice.
SSCs Derived from C57BL/6-Background Mice Require GDNF, Soluble GFRα1, and bFGF for in Vitro Proliferation. Subsequently, we searched for additional factors to support stem cells of C57 × ROSA pups. Cellular responses to GDNF are mediated by a multicomponent receptor complex consisting of c-Ret receptor tyrosine kinase and a glycosyl phosphatidylinositol-anchored ligand-binding subunit, GFRα1, in many cell types (9). Although it has been shown that mouse spermatogonia express GFRα1 (10, 11), we reasoned that adding soluble GFRα1 molecules might potentiate the stimulatory signal through the c-Ret receptor or modulate the signaling pathways (19). In addition, bFGF, a critical growth factor for primordial germ cells (PGCs) in vitro (20, 21), was examined. MACS Thy-1 cells from C57 × ROSA pups were cultured with GDNF alone or in the combination with soluble GFRα1 (GFRα1-Fc), bFGF, or both. The cultured cells were transplanted into recipient mouse testes at specific time intervals as described above. Again, GDNF alone did not support expansion of SSCs from C57 × ROSA; however, addition of soluble GFRα1 along with GDNF improved the maintenance of germ cell clumps and showed modest expansion of SSCs in culture (Fig. 2A). A similar but better response was seen when bFGF was added with GDNF, and the degree of SSC proliferation in the culture with GDNF and bFGF was greater than that in the combination of GDNF and soluble GFRα1. When all three factors, GDNF, soluble GFRα1, and bFGF, were added to the culture medium, expansion of the germ cell clumps was dramatically enhanced. Transplantation of the cultures at each time point confirmed that the observed increase in germ cell clumps reflected an increase in SSCs (Fig. 2 A).
Fig. 2.
Expansion of SSCs in serum-free medium supplemented with GDNF, soluble GFRα1, and bFGF. (A) MACS Thy-1 cells from C57 × ROSA were cultured in the conditions indicated. Fresh MACS Thy-1 cells and cultured cells were transplanted into recipient testes. The number of donor-derived spermatogenic colonies per 105 MACS Thy-1 cells originally seeded in culture is shown. The transplantation assay demonstrated a synergistic effect of soluble GFRα1 and bFGF on expansion of C57 × ROSA SSCs cultured with GDNF. Data are shown as means ± SEM for six recipient testes per time point. Most error bars do not extend beyond symbol. (B–E) Development and growth of germ cell clumps from 129/SvCP MACS Thy-1 pup testis cells. (Scale bar, 50 μm.) MACS Thy-1 cells on STO feeders after 5 h in culture (B), initiation of cell clump formation at 2 days (C), growth of germ cell clumps at 5 days (D), and continuous expansion of germ cell clumps at 5 months (E) are shown.
After 10 weeks (72 days) of culture with the three growth factors, 105 MACS Thy-1 cells generated 1.04 ± 0.23 × 106 (mean ± SEM, n = 6, Fig. 2 A) colonies (similar to the value for DBA × ROSA SSCs, see above). Before culture, 105 MACS Thy-1 cells generated 205 ± 26 (mean ± SEM, n = 6, Fig. 2 A) colonies. Therefore, the stem cell activity increased 5,073-fold (1.04 × 106/205); a single stem cell produced >5,000 copies in 72 days. In this experiment, the number of SSCs in culture doubled every 5.8 days (72/log25,073). Results from three separate experiments indicated an average doubling rate of 5.6 ± 0.2 days (mean ± SEM, n = 3). Furthermore, three factors, GDNF, soluble GFRα1, and bFGF, supported expansion of germ cell clumps from all mouse strains examined, including three inbred strains, 129/SvCP (Fig. 2 B–E), C57BL/6, and SJL, and germ cell clumps could be generated from neonates (0.5–1.5 days postpartum) and pups as well as cryptorchid or wild-type adult mice (data not shown). Pups appeared to provide the best source of cells for in vitro proliferation.
Constant Stem Cell Activity Is Maintained in Cultured Thy-1+ Germ Cells. The surface antigenic phenotype of SSCs in the mouse testis is αv-integrin–/dim α6-integrin+ Thy-1lo/+ (6, 7). Although the antigenic phenotype of SSCs is consistent throughout postnatal life, stem cell activity in the αv-integrin–/dim α6-integrin+ Thy-1lo/+ population changes depending on age or physiological condition (6). We investigated the surface phenotype of continuously cultured germ cells and evaluated the stem cell activity by phenotype. Results of transplantation assays in conjunction with FACS analysis demonstrated that the culture conditions exclusively supported proliferation of αv-integrin–/dim α6-integrin+ Thy-1lo/+ germ cells (Supporting Text and Fig. 5, which are published as supporting information on the PNAS web site). In addition, stem cell activity calculated by colony number per 105 αv-integrin–/dim α6-integrin+ Thy-1lo/+ cells transplanted was relatively constant during a 13-week culture period (488 ± 41, mean ± SEM, n = 30, 15–92 days in culture) and similar to the value for freshly isolated MACS Thy-1 cells (478 ± 46, mean ± SEM, n = 6) that was assessed by transplantation before culture (Table 1, which is published as supporting information on the PNAS web site).
Phenotypic Characteristics of Cultured SSCs. Because in vitro expansion of SSCs and formation of germ cell clumps depended on GDNF, we examined the presence of molecules important in signal transduction by this growth factor. Immunocytochemistry for c-Ret receptor tyrosine kinase demonstrated that all cells in germ cell clumps expressed c-Ret (Fig. 3A). In addition, FACS analysis detected expression of GFRα1 and neural cell adhesion molecule (NCAM), which has been identified recently as an alternative GDNF receptor (22), by clump-forming germ cells (Fig. 3B).
Fig. 3.
Phenotypic characteristics of cultured 129/SvCP SSCs. (A) Immunocytochemistry of c-Ret receptor tyrosine kinase. All cells in germ cell clumps express the c-Ret receptor (green). (B) FACS analyses for GFRα1 and NCAM expression on clump-forming germ cells. Filled histograms represent stained germ cells with the antibodies indicated. Open histograms indicate isotype control antibody-stained cells. Cultured SSCs expressed GFRα1 and NCAM. (x axis, Log fluorescence intensity.) (C) AP activity on SSCs and ES cells. Cultured germ cell clumps have lower AP activity (red) than ES cells. Staining time and protocol were identical for the two cell types. (D) Immunocytochemistry of Oct-4 on SSCs and ES cells. Germ cell clumps and ES cells express a high level of Oct-4 (green). (Scale bar, 50 μm in A and 100 μmin C and D.)
Developmentally, SSCs originate from PGCs, which differentiate from epiblast cells at ≈7 days postcoitum in mouse embryos (2, 23). Embryonic stem (ES) cells are thought to arise from epiblast cells when mouse embryos are cultured, and these cells remain pluripotent, similar to epiblast cells, when maintained in vitro under appropriate conditions (24). Thus, SSCs, PGCs, and ES cells are closely related stem cells, and all contribute to the germ line. It is well established that ES cells and PGCs express a high level of alkaline phosphatase (AP) and Oct-4, a member of the POU transcription factors (23, 25). After induction of differentiation, the expression of both of these molecules is reduced and subsequently lost. In addition, expression of Oct-4 is critical for self-renewal and pluripotency of ES cells (24, 25). We found that cultured SSCs, particularly small germ cell clumps, had clearly lower AP activity than ES cells (Fig. 3C), but the expression of Oct-4 in cultured SSCs was high and similar to that of ES cells (Fig. 3D).
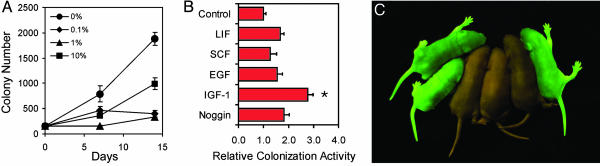
FBS Is Detrimental to Expansion of Spermatogonial Stem Cells. Although ES cells and PGCs are generally cultured with relatively high concentrations of FBS (20, 21, 24), the effect of serum on SSC survival varied depending on medium and feeder cell type in our previous study (6). Therefore, we investigated the effect of FBS on SSCs when added to the serum-free culture system that was adequate for long-term proliferation of SSCs. C57 × ROSA-derived SSCs that had been maintained with GDNF, soluble GFRα1, and bFGF for several weeks were then cultured in the same media to which was added FBS at concentrations of 0%, 0.1%, 1%, and 10% for 2 weeks. All concentrations of FBS inhibited germ cell clump development, and the effect was greatest at 0.1% and 1% serum. Cells were harvested and transplanted to recipient testes after 7 and 14 days of culture, and the number of colonies formed per 105 cells placed in culture was determined (Fig. 4A). After a 2-week exposure to serum, the stem cell activity was reduced significantly to 20–60% of that in serum-free conditions (P < 0.001).
Fig. 4.
Biological characteristics of cultured SSCs. (A) Effect of FBS on proliferation of SSCs. C57 × ROSA SSCs were exposed to FBS at the concentration indicated for 2 weeks. Cells were transplanted after 7 and 14 days of culture. At each time point, the number of spermatogenic colonies formed per 105 cells placed in culture is shown. All values are means ± SEM, and five to six recipient testes were analyzed per time point. Proliferation of SSCs at 14 days was decreased significantly in all concentrations of FBS compared to serum-free medium (Bonferroni-adjusted P value < 0.001). (B) Effect of soluble factors on proliferation of SSCs. Soluble factors indicated were added individually in the culture of C57 × ROSA SSCs. Control culture contained GDNF, soluble GFRα1, and bFGF with no additional factors. After 6 weeks of culture with additional factors, cultured SSCs were transplanted into recipient testes to evaluate stem cell activity. The data are shown as relative colonization activity, the number of colonies per 105 donor cells originally placed in culture relative to that obtained with the control culture (means ± SEM, n = 10–12). A significant effect (asterisk) was observed in culture with IGF-1 (2.77 ± 0.68-fold increase, Bonferroni-adjusted P value < 0.001). (C) Restoration of fertility in infertile recipients by transplantation of cultured SSCs. Progeny from W54/Wv mice transplanted with C57GFP × ROSA-derived germ cell clumps that were cultured for 11 weeks in vitro. Because the transplanted SSCs are haploid for the GFP transgene, 50% of progeny should express GFP. EGF, epidermal growth factor.
Effects of Various Growth Factors on Proliferating SSCs. Expression of gp130, the shared signal transducing receptor component for the IL-6 family of cytokines (e.g., IL-6 or LIF), and a low level of c-Kit receptor tyrosine kinase expression was detected on the cell surface of clump-forming germ cells (Fig. 6, which is published as supporting information on the PNAS web site), suggesting that LIF and stem cell factor (SCF) may affect proliferation of SSCs in vitro. These growth factors also have been shown to have crucial roles in the self-renewal and differentiation of ES cells and PGCs (24, 26). In addition to LIF and SCF, we also investigated the effect of epidermal growth factor, insulin-like growth factor-1 (IGF-1), and Noggin, an antagonist for bone morphogenetic proteins, on proliferating SSCs because these soluble factors have been suggested to influence self-renewal of stem cells (27–29). The factors were added individually in cultures of C57 × ROSA-derived SSCs maintained with GDNF, soluble GFRα1, and bFGF for several weeks. SSCs were cultured with these factors for 6 weeks and transplanted into recipient testes to evaluate the stem cell activity (Fig. 4B). Only IGF-1 significantly increased stem cell activity (≈2.8-fold, P < 0.001).
Cultured SSCs Transplanted into Infertile Recipients Restore Fertility. Finally, we examined whether cultured SSCs could restore fertility when transplanted into infertile recipients. We used W54/Wv mutant mice as recipients for transplantation; these mice are congenitally infertile because of mutations in the c-Kit receptor. In addition, it has been shown that the W54/Wv pup testis environment enhances efficient colonization by transplanted stem cells (17). SSCs carrying a GFP reporter gene were cultured for 11 weeks and then transplanted into pup testes of W54/Wv mutant mice. Recipients were mated to C57BL/6 × SJL female mice, and progeny were produced 110 days after transplantation (Fig. 4C). Similar results were obtained when SSCs carrying the lacZ reporter gene were used (data not shown). There were no abnormalities in the progeny, and they were fertile.
Discussion
Specific growth requirements for in vitro expansion of mouse SSCs were determined by culturing testis cells and transplanting the resulting germ cells to seminiferous tubules of recipient mice, where they generated donor-derived spermatogenesis. When pup testis cells enriched for SSCs by Thy-1 antibody selection were cultured on STO feeder cells in serum-free defined medium with GDNF, GFRα1, and bFGF, SSCs continuously proliferated for >6 months without loss of function. Cultured germ cell clumps were generated from neonate, pup, and adult testes of several mouse strains and could reconstitute normal spermatogenesis after transplantation into seminiferous tubules. Cultured SSCs restored fertility when transplanted into infertile W54/Wv mice, indicating that fully functional spermatozoa were produced from in vitro proliferating SSCs. In the experiments with C57 × ROSA mice, the number of SSCs in culture doubled every ≈5.6 days. This rate of doubling is similar to that estimated for adult SSCs after transplantation into busulfan-treated testes (30), suggesting that factor-dependent proliferation of SSCs in vitro closely resembles the process of stem cell replication in vivo after transplantation.
To identify the essential growth factors, several characteristics of the culture system were critical, including a testis cell population highly enriched for SSCs, a serum-free defined medium, and STO feeders. This system avoids the use of contaminating testis somatic cells and serum, which contribute unknown factors that may stimulate apoptosis or differentiation of stem cells (6, 14). STO feeders provide an environment that is known to support several types of stem cells (20, 21, 31). The importance of establishing a minimum culture system has been demonstrated in experiments by examining effects of serum, basal medium, and feeder cells on populations of testis cells enriched for SSC by various techniques (6). The minimal condition described in this report allowed for the evaluation of individual growth factors for their effect on SSC proliferation. This approach will serve as a paradigm to establish conditions for culture of SSC from other species, allowing identification of universal or conserved factors necessary for proliferation of SSCs.
In this study, SSCs from DBA/2J strain mice crossed to ROSA mice required only GDNF for in vitro expansion, which indicates that GDNF is an essential growth factor for SSCs in vitro. The complementary role of soluble GFRα1 in mouse strains other than DBA/2J supports the critical role of this signaling pathway. In fact, the expression of c-Ret, a receptor for GDNF, was confirmed on germ cell clumps growing in vitro. In addition, the expression of NCAM on these germ cells provides a second possible signaling pathway for GDNF, because p140NCAM associated with GRFα1 is an alternative receptor for GDNF (22). Expression of p140NCAM is present on type A spermatogonia and gonocytes (32), suggesting that SSCs likely express this isoform as well. Thus, at least two possible receptor pathways exist for the GDNF effect on stem cells. Several signal transduction cascades are activated by c-Ret and p140NCAM, including phosphoinositide 3-OH kinase/Akt, the Src family kinases, and the mitogen-activated protein kinases (9). Elucidation of the signaling cascades after activation of GDNF receptors in SSCs will allow a critical assessment of the cellular and molecular mechanisms required for self-renewal of SSCs. The serum-free in vitro system provides a powerful approach to understand signaling events involved in self-renewal of SSCs and to compare these mechanisms to other stem cells.
In addition to GDNF, soluble GFRα1 and bFGF are required and act synergistically to support in vitro expansion of SSCs derived from several mouse strains other than DBA/2J, which suggests an inherent genetic difference between DBA/2J and other genetic backgrounds. In a previous report, only SSCs from DBA/2 mice could be successfully cultured, possibly reflecting the less stringent requirements of this strain (13). Expression of higher levels of the GDNF receptor components or related signaling molecules in DBA/2J SSCs could result in the difference observed in vitro. Because the effect is seen in DBA/2J heterozygotes, it likely results from a dominant allele(s). In some neural cells, c-Ret stimulation by soluble GFRα1 potentiates and modulates downstream signaling (19), which may account for the essential nature of soluble GFRα1 for proliferation of SSCs in most mouse strains. bFGF is a crucial factor for in vitro proliferation of PGCs (20, 21), and the requirement for this factor apparently continues when SSCs form germ cell clumps in vitro. In neural cells, bFGF can induce production of GDNF and GFRα1, which acts in an autocrine manner to decrease apoptotic signaling in the cells (33). Similar mechanisms may be active in SSCs. In addition, we cannot exclude the possibility that factors other than GDNF, soluble GFRα1, and bFGF are produced from feeders and act on SSCs.
Adding IGF-1 to the cultures containing GDNF, soluble GFRα1, and bFGF increased the number of SSCs during a 6-week incubation, suggesting that proliferation of SSCs can be modulated by IGF-1. Because IGF-1 binding to the IGF-1 receptor tyrosine kinase has been demonstrated to stimulate the phosphoinositide 3-OH kinase/Akt and mitogen-activated protein kinase pathways that mediate cell proliferation or cell survival (34), the IGF system may cooperate with the GDNF signaling pathway to support self-renewal of SSCs. Because gp130 expression was detected on proliferating SSCs, the gp130/IL6-related cytokines system also may be important for SSC self-renewal. It has been established that signal transducer and activator of transcription 3 (STAT3) activation via gp130 is essential and sufficient for self-renewal of mouse ES cells (24). Moreover, survival and proliferation of PGCs in vitro require gp130-mediated activation (35). Conservation of signaling pathways among closely related stem cells, like ES cells, PGCs, and SSCs, is not unexpected. In this context, expression of Oct-4 in cultured SSCs might also be expected, because high-level Oct-4 expression is seen in ES cells and PGCs. Because the level of Oct-4 influences the decision for self-renewal or differentiation in mouse ES cells (24, 25), it may also regulate fate determination of SSCs.
Although SSCs and ES cells share some characteristics, significant differences exist. First, although FBS supports ES cells in culture, the constituents of FBS are detrimental to SSC proliferation. No serum concentration evaluated was able to enhance stem cell proliferation above that obtained in serum-free medium. The balance of negative and positive factors in serum does not favor SSC self-renewal in culture. Second, expression of AP is high in ES cells but low in SSC clumps and cannot be identified in gonocytes and undifferentiated spermatogonia of postnatal mouse testes (23). Third, SSCs do not generate tumors when transplanted to nude mice, whereas ES cells produce highly invasive teratocarcinomas when injected into mice (36, 37). In older GDNF-overexpressing transgenic mice, nonmetastatic tumors resembling human seminomas, but not teratocarcinomas, developed (10). However, germ cell clumps exposed to GDNF in vitro for 3 months have not formed tumors when injected into seminiferous tubules or s.c. (data not shown). Therefore, fundamental differences must exist between these two related stem cells in their differentiation potential.
In adults, SSCs are the only stem cells that are able to transmit genetic information to subsequent generations. Therefore, SSCs provide an alternative method to modify the germ line of animals, and in vitro proliferation of SSCs will make possible sophisticated genetic manipulation of these cells, including targeted modification. In addition, because a large number of these cells can be generated in culture, they represent a powerful resource for gene analysis and functional genomics. Furthermore, modulating culture conditions that control the self-renewal vs. differentiation decision of SSCs to produce functional gametes in vitro will create a valuable model for studying the molecular and cellular biology of male germ cell differentiation, and may allow development of new therapeutic strategies for infertility. Finally, because SSCs of many mammalian species are known to proliferate on the basement membrane of mouse seminiferous tubules (2), identical or similar signaling mechanisms and culture requirements are likely to be applicable to other species.
Supplementary Material
Acknowledgments
We thank Drs. R. Behringer, J. Oatley, B.-Y. Ryu, and E. Sandgren for critical evaluation of the manuscript and helpful comments, C. Brensinger for statistical analysis, J. Hayden for photography, C. Freeman and R. Naroznowski for assistance with animal maintenance and experimentation, and Drs. A. Bradley (The Wellcome Trust Sanger Institute) and G. Enders (University of Kansas Medical School, Kansas City) for materials. This work was supported by National Institute of Child Health and Human Development Grant 044445, the Common-wealth and General Assembly of Pennsylvania, and the Robert J. Kleberg, Jr., and Helen C. Kleberg Foundation.
Author contributions: H.K. and R.L.B. designed research; H.K. and M.R.A. performed research; H.K. contributed new reagents/analytic tools; H.K. analyzed data; H.K. wrote the paper; and R.L.B. edited and revised the paper.
Abbreviations: SSC, spermatogonial stem cell; FACS, fluorescence-activated cell sorting; GDNF, glial cell line-derived neurotrophic factor; GFRα1; GDNF-family receptor α1; LIF, leukemia inhibitory factor; bFGF, basic fibroblast growth factor; β-gal, β-galactosidase; X-gal, 5-bromo-4-choloro-3-indolyl β-d-galactoside; MACS, magnetic-activated cell sorting; PGC, primordial germ cell; ES, embryonic stem; NCAM, neural cell adhesion molecule; AP, alkaline phosphatase; IGF-1, insulin-like growth factor 1.
See Commentary on page 16395.
References
- 1.Meistrich, M. L. & van Beek, M. E. A. B. (1993) in Cell and Molecular Biology of the Testis, eds. Desjardins, C. & Ewing, L. L. (Oxford Univ. Press, New York), pp. 266–295.
- 2.Brinster, R. L. (2002) Science 296, 2174–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tegelenbosch, R. A. & de Rooij, D. G. (1993) Mutat. Res. 290, 193–200. [DOI] [PubMed] [Google Scholar]
- 4.Brinster, R. L. & Zimmermann, J. W. (1994) Proc. Natl. Acad. Sci. USA 91, 11298–11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinster, R. L. & Avarbock, M. R. (1994) Proc. Natl. Acad. Sci. USA 91, 11303–11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubota, H., Avarbock, M. R. & Brinster, R. L. (2004) Biol. Reprod. 71, 722–731. [DOI] [PubMed] [Google Scholar]
- 7.Kubota, H., Avarbock, M. R. & Brinster, R. L. (2003) Proc. Natl. Acad. Sci. USA 100, 6487–6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin, L. F., Doherty, D. H., Lile, J. D., Bektesh, S. & Collins, F. (1993) Science 260, 1130–1132. [DOI] [PubMed] [Google Scholar]
- 9.Sariola, H. & Saarma, M. (2003) J. Cell Sci. 116, 3855–3862. [DOI] [PubMed] [Google Scholar]
- 10.Meng, X., Lindahl, M., Hyvonen, M. E., Parvinen, M., de Rooij, D. G., Hess, M. W., Raatikainen-Ahokas, A., Sainio, K., Rauvala, H., Lakso, M., et al. (2000) Science 287, 1489–1493. [DOI] [PubMed] [Google Scholar]
- 11.Yomogida, K., Yagura, Y., Tadokoro, Y. & Nishimune, Y. (2003) Biol. Reprod. 69, 1303–1307. [DOI] [PubMed] [Google Scholar]
- 12.Jeong, D., Mclean, D. J. & Griswold, M. D. (2003) J. Androl. 24, 661–669. [DOI] [PubMed] [Google Scholar]
- 13.Kanatsu-Shinohara, M., Ogonuki, N., Inoue, K., Miki, H., Ogura, A., Toyokuni, S. & Shinohara, T. (2003) Biol. Reprod. 69, 612–616. [DOI] [PubMed] [Google Scholar]
- 14.Nagano, M., Ryu, B. Y., Brinster, C. J., Avarbock, M. R. & Brinster, R. L. (2003) Biol. Reprod. 68, 2207–2214. [DOI] [PubMed] [Google Scholar]
- 15.Nagano, M. & Brinster, R. L. (1998) APMIS 106, 47–55. [DOI] [PubMed] [Google Scholar]
- 16.Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. (1997) FEBS Lett. 407, 313–319. [DOI] [PubMed] [Google Scholar]
- 17.Shinohara, T., Orwig, K. E., Avarbock, M. R. & Brinster, R. L. (2001) Proc. Natl. Acad. Sci. USA 98, 6186–6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enders, G. C. & May, I. I. (1994) Dev. Biol. 163, 331–340. [DOI] [PubMed] [Google Scholar]
- 19.Paratcha, G., Ledda, F., Baars, L., Coulpier, M., Besset, V., Anders, J., Scott, R. & Ibanez, C. F. (2001) Neuron 29, 171–184. [DOI] [PubMed] [Google Scholar]
- 20.Matsui, Y., Zsebo, K. & Hogan, B. L. (1992) Cell 70, 841–847. [DOI] [PubMed] [Google Scholar]
- 21.Resnick, J. L., Bixler, L. S., Cheng, L. & Donovan, P. J. (1992) Nature 359, 550–551. [DOI] [PubMed] [Google Scholar]
- 22.Paratcha, G., Ledda, F. & Ibanez, C. F. (2003) Cell 113, 867–879. [DOI] [PubMed] [Google Scholar]
- 23.Cooke, J. E., Godin, I., Ffrench-Constant, C., Heasman, J. & Wylie, C. C. (1993) Methods Enzymol. 225, 37–58. [DOI] [PubMed] [Google Scholar]
- 24.Smith, A. G. (2001) Annu. Rev. Cell Dev. Biol. 17, 435–462. [DOI] [PubMed] [Google Scholar]
- 25.Pesce, M. & Scholer, H. R. (2001) Stem Cells 19, 271–278. [DOI] [PubMed] [Google Scholar]
- 26.Donovan, P. J. (1994) Curr. Topics Dev. Biol. 29, 189–225. [DOI] [PubMed] [Google Scholar]
- 27.Jamora, C., DasGupta, R., Kocieniewski, P. & Fuchs, E. (2003) Nature 422, 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musaro, A., Giacinti, C., Borsellino, G., Dobrowolny, G., Pelosi, L., Cairns, L., Ottolenghi, S., Cossu, G., Bernardi, G., Battistini, L., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 1206–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds, B. A. & Weiss, S. (1996) Dev. Biol. 175, 1–13. [DOI] [PubMed] [Google Scholar]
- 30.Nagano, M. C. (2003) Biol. Reprod. 69, 701–707. [DOI] [PubMed] [Google Scholar]
- 31.Kubota, H. & Reid, L. M. (2000) Proc. Natl. Acad. Sci. USA 97, 12132–12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, L. H., Jester, W. F., Jr., & Orth, J. M. (1998) J. Androl. 19, 365–373. [PubMed] [Google Scholar]
- 33.Lenhard, T., Schober, A., Suter-Crazzolara, C. & Unsicker, K. (2002) Mol. Cell Neurosci. 20, 181–197. [DOI] [PubMed] [Google Scholar]
- 34.LeRoith, D. & Roberts, J. (2003) Cancer Lett. 195, 127–137. [DOI] [PubMed] [Google Scholar]
- 35.Koshimizu, U., Taga, T., Watanabe, M., Saito, M., Shirayoshi, Y., Kishimoto, T. & Nakatsuji, N. (1996) Development (Cambridge, U.K.) 122, 1235–1242. [DOI] [PubMed] [Google Scholar]
- 36.Evans, M. J. & Kaufman, M. H. (1981) Nature 292, 154–156. [DOI] [PubMed] [Google Scholar]
- 37.Martin, G. R. (1981) Proc. Natl. Acad. Sci. USA 78, 7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.