Abstract
Although glycolysis is highly conserved, it is remarkable that several unique isozymes in this central metabolic pathway are found in mammalian sperm. Glyceraldehyde 3-phosphate dehydrogenase-S (GAPDS) is the product of a mouse gene expressed only during spermatogenesis and, like its human ortholog (GAPD2), is the sole GAPDH isozyme in sperm. It is tightly bound to the fibrous sheath, a cytoskeletal structure that extends most of the length of the sperm flagellum. We disrupted Gapds expression by gene targeting to selectively block sperm glycolysis and assess its relative importance for in vivo sperm function. Gapds–/– males were infertile and had profound defects in sperm motility, exhibiting sluggish movement without forward progression. Although mitochondrial oxygen consumption was unchanged, sperm from Gapds–/– mice had ATP levels that were only 10.4% of those in sperm from WT mice. These results imply that most of the energy required for sperm motility is generated by glycolysis rather than oxidative phosphorylation. Furthermore, the critical role of glycolysis in sperm and its dependence on this sperm-specific enzyme suggest that GAPDS is a potential contraceptive target, and that mutations or environmental agents that disrupt its activity could lead to male infertility.
Keywords: glycolysis, gene targeting, infertility
Sperm motility is essential for normal fertilization, and asthenozoospermia, or low sperm motility, is common in infertile men. In a recent study of 1,085 sperm samples from infertile men, 81% had defects in motility, and 19% had asthenozoospermia without other defects in sperm number or morphology (1). Motility is generated by the extremely long flagellum that comprises >90% of the length of a mammalian sperm. This process requires substantial ATP to support coordinated movement of the central axoneme and surrounding flagellar structures (2). ATP is hydrolyzed by dynein ATPases, which function as force-generating molecular motors along the axoneme. Although quiescent in the epididymis, mammalian sperm display vigorous forward movement, termed activated or progressive motility, immediately upon ejaculation or collection into physiological medium. The motility waveform changes in the female reproductive tract, with increases in both the amplitude and asymmetry of flagellar bending. These changes result in a whiplash-like motion, termed hyperactivated motility, which facilitates sperm transport in the oviduct and penetration of the zona pellucida surrounding the oocyte (3).
Potential sources of ATP to support sperm motility are compartmentalized in distinct regions along the length of the flagellum. Oxidative phosphorylation is confined to the proximal segment of the flagellum where the mitochondria are localized (middle piece). In contrast, glycolysis appears to be restricted to the principal piece, which is distal to the middle piece and is the longest segment of the sperm flagellum (4–8). Several glycolytic enzymes in mammalian sperm are distinct from the isozymes present in somatic tissues. Three isozymes, glyceraldehyde 3-phosphate dehydrogenase-S (GAPDS), phosphoglycerate kinase-2, and lactate dehydrogenase-C4, are encoded by paralogous genes expressed only during spermatogenesis (5, 9–13). At least five other enzymes in the glycolytic pathway have unique structural or functional properties in spermatogenic cells (6, 14–18). GAPDS, one of the germ cell-specific isozymes, is tightly bound to the fibrous sheath, a cytoskeletal structure that defines the limits of the principal piece (4). This localization of respiration and glycolysis in distinct compartments led us to question whether both metabolic pathways are needed to provide sufficient ATP along the entire length of the flagellum to support activated and hyperactivated sperm motility.
Although oxidative phosphorylation is more efficient than glycolysis for ATP production, it is unclear whether diffusion of ATP from the middle-piece mitochondria could adequately supply all of the energy needs in the distal regions of the flagellum (19). There appear to be some species differences in sperm metabolism (2), but previous in vitro studies reported that glycolysis is required for mouse (20–22), rat (23), and human sperm (24, 25) to achieve hyperactivated motility and penetrate the zona pellucida during fertilization. The tyrosine phosphorylation of sperm proteins that occurs during acquisition of hyperactivated motility in vitro also depends upon glycolysis (26, 27). Based on these in vitro results, we hypothesized that elimination of a sperm-specific glycolytic enzyme would block hyperactivated motility in vivo, resulting in male infertility.
Materials and Methods
Generation of Gapds–/– Mice. Gapds was isolated from a P1 phage library of 129/OlaHsd mouse genomic DNA (Incyte Genomics, Palo Alto, CA). Nucleotide numbers in the Gapds genomic sequence refer to GenBank accession no. U09964. After digestion with Tth111I and SspI, the DNA restriction fragment beginning in exon 1 and ending in intron 4 (nucleotides 1887–7193) was cloned into the SrfI site of the cloning vector pUCBM21/KO (28). This fragment, along with thymidine kinase (tk) and neomycin resistance (neo) genes, was ligated into the NotI–XhoI site of pMC1TKbpA (29), which contains another tk cassette. The Gapds DNA fragment beginning in exon 6 and ending in exon 9 (nucleotides 8108–9181) was amplified by PCR using the P1 clone as template and then ligated into the SpeI–XbaI site of pMC1TKbpA to produce the gene-targeting construct pL3KaSTK2 (Fig. 5, which is published as supporting information on the PNAS web site). Transfection of pL3KaSTK2 DNA, screening of targeted TC-1 embryonic stem (ES) cells (gift of Philip Leder, Harvard Medical School, Boston), and blastocyst injections were performed as described (28). The PCR primers used to detect homologous recombination were forward 5′-AGCGTTGGCTACCCGTGATA-3′ and reverse 5′-CGTGATAGCCGAGTAAGAAGCAGG-3′, corresponding to sequences in the neo gene and exon 9, respectively. Genotypes were confirmed by Southern blotting (Fig. 6, which is published as supporting information on the PNAS web site) after digestion with DraI, using a probe (nucleotides 1080–1830) outside the targeting construct (Fig. 5). Chimeric males generated from correctly targeted ES cells were mated with C57BL/6N females to obtain germ-line transmission. Heterozygous animals were mated to produce Gapds–/– and Gapds+/+ males for the analysis of fertility and sperm function. Animal maintenance and experimental protocols were approved by the University of North Carolina and National Institute on Environmental Health Sciences Institutional Animal Care and Use Committees.
Western Blotting and Immunocytochemistry. Sperm were collected from the cauda epididymis, washed in PBS (140 mM NaCl/10 mM phosphate buffer, pH 7.4), and lysed in SDS sample buffer. Testis lysates were prepared by homogenization in lysis buffer (1 ml per testis) containing 140 mM NaCl, 0.1% Triton X-100, Complete protease inhibitor mixture (Roche Applied Science, Indianapolis), and 20 mM Hepes buffer, pH 7.4. Lysates corresponding to 2 × 104 sperm or 0.5 mg of testis (wet weight) were analyzed by Western blotting using anti-mouse GAPDS antibody B1 (4), as described (30). For the detection of GAPDS by indirect immunofluorescence, sperm were fixed on glass slides and stained with the same anti-mouse GAPDS antibody (30).
GAPDS Enzyme Activity. Cauda epididymal sperm were collected in M16 or M2 medium (Specialty Media, Lavellette, NJ), washed with PBS, suspended in enzyme assay buffer (5), and sonicated for 7 sec on ice. The GAPDS/GAPDH enzyme assay was carried out as described (31). To monitor GAPDS substrate accumulation, glyceraldehyde 3-phosphate was assayed (32) immediately after collection of cauda epididymal sperm.
Histology and Electron Microscopy. Testes were fixed in Bouin's solution, dehydrated, and embedded in paraffin (4). Sections were stained with hematoxylin/eosin for histological analysis. Sperm ultrastructure was examined by transmission and scanning electron microscopy as described (30), except that sperm analyzed by scanning electron microscopy were demembranated by treating with 1% Triton X-100 in PBS for 15 min at room temperature before fixation with 5% glutaraldehyde in 0.2 M sodium cacodylate buffer.
Fertility and Sperm Motility. To monitor mating behavior, individual Gapds–/– males were housed with two superovulated females for 2 h, and females were then examined for copulatory plugs. To determine fertility, individual Gapds–/– males were mated continuously with two WT females for 1 month, followed by a second month with two different females. All females were maintained for 1 month after the mating period. Individual Gapds–/– females were mated with one WT male for 2 months. To assess motility, sperm were collected from the cauda epididymis of 4- to 6-month-old mice in M16 medium and incubated at 37°C in 5% CO2 and air. At the indicated time points, sperm were diluted 10-fold with M2 medium, and motility was scored by phase–contrast microscopy. More than 200 sperm were examined from each animal at each time point. Videos were recorded at real-time rate by using Nomarski differential interference contrast optics.
Quantitative parameters of sperm motility were determined by computer-assisted sperm analysis (CASA), as described for rat sperm with adjustments for tracking smaller mouse sperm (33). Cauda epididymides were removed and transported in M16 medium on ice, and sperm were collected within 60 min. Sperm were collected in M16 (37°C, 5% CO2 and air), and CASA parameters were determined immediately after collection (time 0) and after incubation for 1 h. Sperm tracks (1.5 sec, 90 frames) were captured at 60 Hz by using an htm-ivos sperm analysis system (Hamilton Thorne Research, Beverly, MA, software version 12). Statistical analyses comparing WT and Gapds–/– CASA parameters were conducted by using the General Linear Models procedure in sas (SAS Institute, Cary, NC). Differences were considered significant when P < 0.05.
Sperm ATP Levels. After incubation in M16 medium at 37°C in 5% CO2 and air, sperm were centrifuged at 1,000 × g for 3 min. Excess medium was removed, and sperm in the remaining 50 μl were resuspended, transferred to a tube containing 450 μl of boiling extraction buffer (4 mM EDTA/0.1 M Tris·HCl, pH 7.8), and incubated at 100°C for 2 min. The supernatant was collected after centrifugation at 20,000 × g for 5 min and diluted 10-fold with H2O. ATP was measured in duplicate 50-μl aliquots of the supernatant by using a luciferase bioluminescence assay according to the manufacturer's protocol (ATP Bioluminescence Assay kit CLS II; Roche Applied Science).
Oxygen Consumption. Sperm were collected from the cauda epididymis and vas deferens in glucose-free M2 medium. Oxygen consumption of the sperm suspension was determined by using an oxygen probe (YSI 5531, YSI, Yellow Springs, OH) calibrated to air-saturated medium. Samples (4–6 × 107 sperm in glucose-free M2 medium) were stirred vigorously in the reaction chamber (1.8 ml) at room temperature. The reaction was terminated by adding 1 mM KCN, and the baseline was obtained by additional monitoring. Consumed oxygen was calculated by using the solubility factor of 0.237 μmol/ml.
Results and Discussion
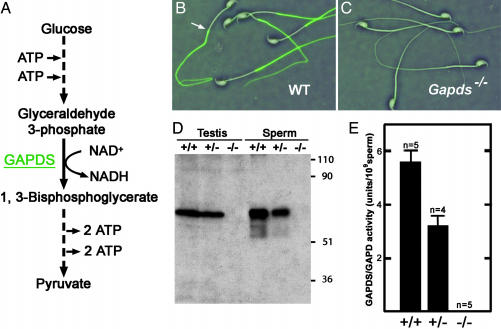
Sperm Glycolysis Is Blocked by the Targeted Deletion of GAPDS. Previous studies detected only the germ cell-specific GAPDS/GAPD2 isozyme in both mouse and human sperm (4, 5). Because this enzyme precedes both ATP-generating steps in glycolysis, elimination of GAPDS activity should block all glycolytic ATP production in sperm (Fig. 1A). ATP levels should be further depleted under these conditions by the ATP-consuming steps that precede GAPDS in the glycolytic pathway. We deleted exon 5 and part of exon 6 of Gapds by gene targeting (Fig. 5) to eliminate the downstream catalytic cysteine and six of eight amino acids essential for NAD+ cofactor binding (9, 34). Disruption of the gene was confirmed by Southern blot analysis (Fig. 6). GAPDS protein was not detected in sperm or testis from homozygous mutant (Gapds–/–) males by immunocytochemistry (compare Fig. 1 B and C) or Western blotting (Fig. 1D) with antibodies specific for the sperm isozyme. GAPDS enzyme activity was absent in sperm from Gapds–/– males and was reduced by ≈50% in sperm from heterozygous (Gapds+/–) animals (Fig. 1E). The lack of enzymatic activity in sperm from Gapds–/– males verified previous immunocytochemical results indicating that the somatic GAPDH isozyme is not present in sperm (4, 5). The block in glycolysis at the reaction catalyzed by GAPDS resulted in a 4-fold accumulation of the substrate, glyceraldehyde 3-phosphate, in sperm from Gapds–/– mice (1.22 ± 0.02 μmol per 107 sperm, n = 3) compared with the levels of substrate in sperm from WT mice (0.30 ± 0.01 μmol per 107 sperm, n = 3).
Fig. 1.
Targeted disruption of Gapds. (A) Abbreviated diagram of sperm glycolysis showing that GAPDS is required for all ATP production via this pathway. (B and C) Indirect immunofluorescence shows that GAPDS is localized in the principal piece of the flagellum in WT sperm (B) but is absent in sperm from Gapds–/– mice (C). Sperm morphology is indistinguishable in these merged immunofluorescence and phase-contrast images. The arrow in B denotes the junction between the middle piece and principal piece of the flagellum. (D) Testis and sperm proteins from WT (+/+), heterozygous (+/–), and homozygous mutant (–/–) males were analyzed by Western blotting with an antibody specific for GAPDS. Similar results were obtained with antibodies that recognize peptide sequences either upstream (data not shown) or downstream of the deletion. The relative molecular weights (×10–3) of protein standards are shown at right. (E) GAPDS/GAPDH enzyme activity was measured for sperm isolated from +/+, +/–, and –/– males. Values show mean activity ± SEM.
GAPDS Is Required for Male Fertility. Gapds–/– males were infertile, although they produced comparable numbers of sperm to those of WT males and had normal testis weights, body weights (Table 1), and testis histology (Fig. 7, which is published as supporting information on the PNAS web site). Mating behavior also appeared normal when assessed by housing individual Gapds–/– males with two superovulated females for 2 h. Six of seven Gapds–/– males mated with at least one female within the 2-h period, as confirmed by the presence of copulatory plugs. However, Gapds–/– males sired no pups when mated continuously with two WT females for 1 month, followed by a second month with two different females (n = 7 males, n = 28 females). Heterozygous (Gapds+/–) males, as well as Gapds–/– females, had normal fertility. Gapds–/– mice of both sexes were produced in the expected proportions from heterozygous matings (64 homozygous mutant, 140 heterozygous, 59 WT) and were not visibly different from their WT littermates.
Table 1. Characteristics of Gapds-/- and WT males.
| Genotype | Sperm count, × 107 | Testes weight, g | Body weight, g |
|---|---|---|---|
| WT (n = 6) | 2.6 ± 0.31 | 0.22 ± 0.01 | 34.8 ± 2.3 |
| Gapds-/- (n = 6) | 2.6 ± 0.22 | 0.22 ± 0.01 | 31.8 ± 2.1 |
Values are means ± SEM. For each animal, the sperm count was the number collected from both cauda epididymides, and the testes weight was the combined weight of both testes.
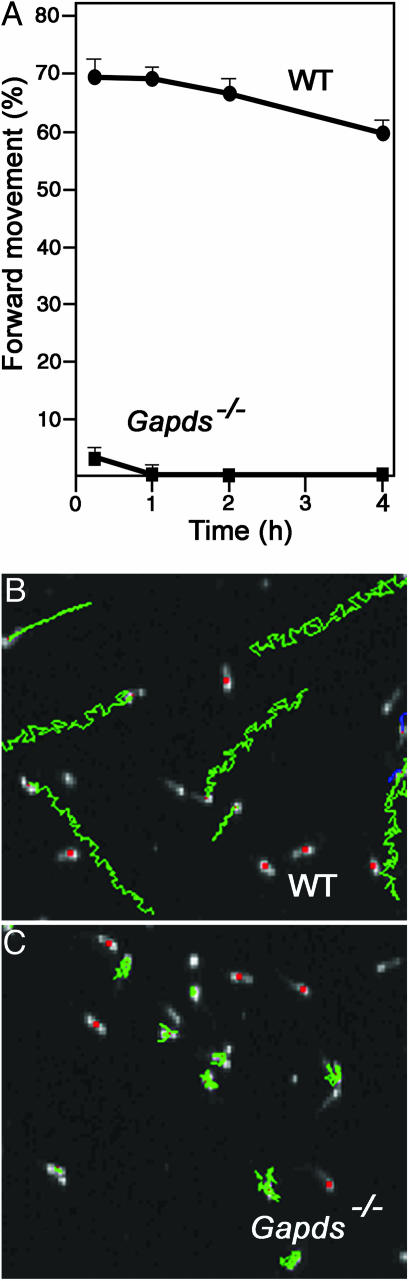
Sperm Motility Is Severely Impaired in Gapds–/– Mice. Sperm from Gapds–/– males exhibited normal morphology by phase-contrast microscopy (compare Fig. 1 B and C) but had profound defects in motility that were more severe than predicted. For both WT and Gapds–/– males, >50% of sperm typically displayed some movement immediately after isolation from the cauda epididymis in M16 medium at 37°C in 5% CO2 and air. However, the flagellar motion of sperm lacking GAPDS was quite sluggish and rarely resulted in forward movement (Movie 1, which is published as supporting information on the PNAS web site) compared with the vigorous progressive motility of WT sperm (Movie 2, which is published as supporting information on the PNAS web site). Only 3.2 ± 1.5% of sperm from Gapds–/– males (n = 6) showed some forward movement immediately after collection from the cauda epididymis in M16 medium, whereas 69.5 ± 2.6% of sperm from WT males (n = 6) showed rapid progressive motility (Fig. 2A). The motility of WT sperm remained high over a 4-h incubation period. Sperm from Gapds–/– mice showed no forward movement after 2 h, although 50–60% of sperm showed weak nonprogressive movement throughout the 4-h incubation period.
Fig. 2.
GAPDS is required for sperm motility. (A) Sperm were collected from WT (n = 6) and Gapds–/– mice (n = 6) and incubated in M16 medium at 37°C in 5% CO2 and air. Forward movement was scored by phase-contrast microscopy for >200 sperm per animal at each time point. Mean percentages of forward movement ± SEM are shown for WT (circles) and Gapds–/– (squares) males. (B and C) Sperm tracks generated by CASA. Green tracks illustrate the movement of individual sperm in 1.5 sec and red dots indicate immotile or dead sperm. WT sperm (B) displayed vigorous progressive motility, whereas sperm from Gapds–/– males (C) were motile but nonprogressive.
CASA was used for quantitative comparisons of the motility parameters of sperm from Gapds–/– (n = 4) and WT (n = 3) males. Only 0.1% of motile sperm from Gapds–/– mice were progressive (average path velocity of >50 μm/sec and straightness >50%), compared with 60.8% of motile WT sperm (P < 0.0001). Mean straight-line velocities of sperm lacking GAPDS were only 8.2 μm/sec, compared with 63 μm/sec for WT sperm (P < 0.0001). All other CASA parameters measured were also significantly lower for sperm from Gapds–/– mice immediately after collection and after incubation in M16 for 1 h (Table 2, which is published as supporting information on the PNAS web site). CASA-generated sperm tracks showed clear differences between the highly progressive motility of WT sperm (Fig. 2B) and the nonprogressive back-and-forth movement of sperm lacking GAPDS (Fig. 2C). In some cases, video analysis of sperm from Gapds–/– mice detected flagellar bending in the middle piece that was not propagated effectively along the principal piece of the sperm tail (Movie 3, which is published as supporting information on the PNAS web site).
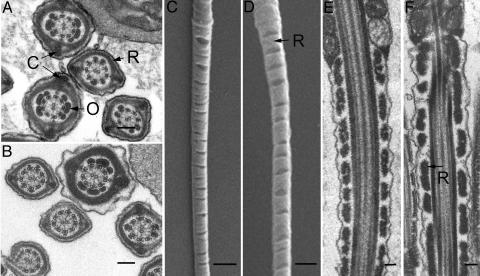
GAPDS Is Not Required for Formation of the Fibrous Sheath. Because GAPDS is tightly bound to the fibrous sheath (4), we examined sperm ultrastructure by scanning and transmission electron microscopy to determine whether the structure of the flagellum was altered in Gapds–/– mice. The fibrous sheath is composed of two longitudinal columns (C, Fig. 3A) connected by circumferential ribs (R, Fig. 3A). Because the flagellum tapers in a proximal to distal direction, the diameter of the fibrous sheath gradually decreases, and the outer dense fibers (O, in Fig. 3A) terminate along the length of the principal piece. The fibrous sheaths were indistinguishable in appearance in flagellar cross sections of sperm from WT (Fig. 3A) and Gapds–/– (Fig. 3B) mice, both in proximal and more distal regions of the principal piece. Because the longitudinal columns and ribs were correctly positioned around the axoneme throughout the entire length of the principal piece in sperm from Gapds–/– mice, GAPDS is not required for formation of the fibrous sheath. Although we observed substantial variation in rib spacing in sperm from both genotypes, gaps between the ribs of the fibrous sheath sometimes appeared wider in sperm from Gapds–/– mice (Fig. 3 D and F) compared with sperm from WT mice (Fig. 3 C and E). However, it appears unlikely that these subtle structural changes cause the profound differences observed in sperm motility.
Fig. 3.
Fibrous sheath formation does not require GAPDS. Sperm ultrastructure was compared by transmission (A, B, E, and F) and scanning (C and D) electron microscopy. The ribs (R) and columns (C) of the fibrous sheath appear similar in cross sections of sperm from WT (A) and Gapds–/– (B) mice. The outer dense fibers (O) and axonemes are also indistinguishable in these flagellar cross sections. Longitudinal sections of sperm from WT (C and E) and Gapds–/– (D and F) mice show some variation in the spacing between the ribs of the fibrous sheath. (Bar: 0.1 μmin A, B, E, and F;1 μmin C and D.)
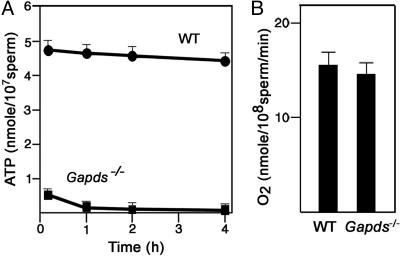
Sperm ATP Levels Are Very Low When GAPDS Is Absent. A more striking defect was found when sperm ATP levels were measured. Immediately after collection from the cauda epididymis in M16 medium, sperm from Gapds–/– mice had ATP levels that were only 10.4% of the levels in sperm from WT mice (Fig. 4A). ATP concentrations declined even further when sperm from Gapds–/– mice were incubated at 37°C for 4 h in 5% CO2 and air, reaching values that were 1.9% of WT sperm levels. Sperm from WT mice showed no significant change in ATP levels when incubated for 4 h under identical conditions. It is likely that the extremely low ATP levels in sperm from Gapds–/– mice are responsible for the observed lack of progressive motility.
Fig. 4.
Sperm from Gapds–/– mice have extremely low ATP levels. (A) Sperm ATP was extracted and measured by using a luciferase bioluminescence assay after incubation in M16 medium at 37°C in 5% CO2 and air. Mean ATP levels ± SEM were ≈10-fold higher for sperm from WT mice (n = 6) compared with sperm from Gapds–/– males (n = 6). (B) Oxygen consumption was determined with an oxygen probe. Sperm from WT (n = 4) and Gapds–/– (n = 4) mice consumed similar amounts of oxygen, indicating comparable mitochondrial activity.
Loss of GAPDS Does Not Alter Sperm Mitochondrial Oxygen Consumption. Because pyruvate produced in the glycolytic pathway (Fig. 1 A) is a major substrate for mitochondrial metabolism, a defect in sperm glycolysis could alter mitochondrial ATP production. However, sperm were collected and assayed in M16 medium, which contains physiological concentrations of both pyruvate (0.33 mM) and lactate (23.28 mM). A monocarboxylate transporter (MCT2) is localized in the sperm flagellum (35, 36) and should enable sperm to metabolize these exogenous substrates via the mitochondrial tricarboxylic acid cycle. We also tested the effects of increasing the pyruvate concentration in the medium up to 20 mM and found that these additions did not enhance the motility of sperm lacking GAPDS. Furthermore, sperm from WT and Gapds–/– mice had similar oxygen consumption levels (Fig. 4B), providing evidence that the low ATP levels observed in sperm lacking GAPDS were not due to mitochondrial defects.
We initially predicted that Gapds–/– males would be infertile because of defects in hyperactivated motility. However, the observed defects were substantially more severe, with sperm exhibiting very minimal movement and extremely low ATP levels. These results imply that most of the energy required for sperm motility is generated by glycolysis. This conclusion is supported by the finding that mice lacking the testis-specific cytochrome cT, the only cytochrome c in sperm mitochondria, are fertile (37). In addition, in vitro studies indicate that inhibitors of oxidative metabolism do not block fertilization (20) or sperm motility (38) in the presence of glucose. Taken together, these results indicate that glycolysis, but not oxidative phosphorylation, is essential for sperm motility and fertility.
Distinctive features of GAPDS and other germ cell-specific glycolytic enzymes may be critical for ensuring that there is a sufficient localized supply of ATP along the length of the sperm flagellum for sperm motility. For example, GAPDS has a novel proline-rich N-terminal extension and is tightly bound to the fibrous sheath, which extends throughout most of the length of the sperm tail (4, 9). It remains to be determined whether the germ cell-specific glycolytic enzymes have lower Km values for substrates and/or less sensitivity to feedback regulation by ATP, leading to higher local ATP concentrations for the sperm dynein ATPases. Another difference between the GAPDH isozymes is that GAPDS appears to be more sensitive than the somatic isozyme to substrate inhibition by the active metabolite of α-chlorohydrin and related toxicants (39–41). Based on its unique features and restricted expression during spermatogenesis, as well as the infertility, lack of progressive sperm motility and low sperm ATP levels of Gapds–/– males, GAPDS may be an excellent and highly specific target for male contraceptive strategies. Because GAPD2, the ortholog of GAPDS, is the only GAPDH isozyme in human sperm (5), specific inhibition of GAPD2 is likely to have similar effects on sperm motility and fertility in men.
Supplementary Material
Acknowledgments
We thank P. L. Magyar and C. Rogers for excellent technical assistance. This research was supported by the National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement U54 HD35041 as part of the Specialized Cooperative Centers Program in Reproductive Research and by National Institutes of Health Fogarty International Center Grant TW/HD00627. The information in this document has been funded in part by the U.S. Environmental Protection Agency, subjected to review by the National Health and Environmental Effects Research Laboratory, and approved for publication.
Abbreviations: GAPDS, glyceraldehyde 3-phosphate dehydrogenase-S; CASA, computer-assisted sperm analysis.
References
- 1.Curi, S. M., Ariagno, J. I., Chenlo, P. H., Mendeluk, G. R., Pugliese, M. N., Sardi Segovia, L. M., Repetto, H. E. & Blanco, A. M. (2003) Arch. Androl. 49, 343–349. [DOI] [PubMed] [Google Scholar]
- 2.Mann, T. & Lutwak-Mann, C. (1981) in Male Reproductive Function and Semen (Springer, New York), pp. 198–268.
- 3.Ho, H. C. & Suarez, S. S. (2001) Reproduction 122, 519–526. [DOI] [PubMed] [Google Scholar]
- 4.Bunch, D. O., Welch, J. E., Magyar, P. L., Eddy, E. M. & O'Brien, D. A. (1998) Biol. Reprod. 58, 834–841. [DOI] [PubMed] [Google Scholar]
- 5.Welch, J. E., Brown, P. L., O'Brien, D. A., Magyar, P. L., Bunch, D. O., Mori, C. & Eddy, E. M. (2000) (2000) J. Androl. 21, 328–338. [PubMed] [Google Scholar]
- 6.Mori, C., Nakamura, N., Welch, J. E., Gotoh, H., Goulding, E. H., Fujioka, M. & Eddy, E. M. (1998) Mol. Reprod. Dev. 49, 374–385. [DOI] [PubMed] [Google Scholar]
- 7.Beyler, S. A., Wheat, T. E. & Goldberg, E. (1985) Biol. Reprod. 32, 1201–1210. [DOI] [PubMed] [Google Scholar]
- 8.Westhoff, D. & Kamp G. (1997) J. Cell Sci. 110, 1821–1829. [DOI] [PubMed] [Google Scholar]
- 9.Welch, J. E., Schatte, E. C., O'Brien, D. A. & Eddy, E. M. (1992) Biol. Reprod. 46, 869–878. [DOI] [PubMed] [Google Scholar]
- 10.McCarrey, J. R. & Thomas, K. (1987) Nature 326, 501–505. [DOI] [PubMed] [Google Scholar]
- 11.Boer, P. H., Adra, C. N., Lau, Y. F. & McBurney, M. W. (1987) Mol. Cell. Biol. 7, 3107–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakai, I., Sharief, F. S. & Li, S. S. (1987) Biochem. J. 242, 619–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millan, J. L., Driscoll, C. E., LeVan, K. M. & Goldberg, E. (1987) Proc. Natl. Acad. Sci. USA 84, 5311–5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori, C., Welch, J. E., Fulcher, K. D., O'Brien, D. A. & Eddy, E. M. (1993) Biol. Reprod. 49, 191–203. [DOI] [PubMed] [Google Scholar]
- 15.Buehr, M. & McLaren, A. (1981) J. Reprod. Fertil. 63, 169–173. [DOI] [PubMed] [Google Scholar]
- 16.Gillis, B. A. & Tamblyn, T. M. (1984) Biol. Reprod. 31, 25–35. [DOI] [PubMed] [Google Scholar]
- 17.Russell, D. L. & Kim, K. H. (1996) Biol. Reprod. 55, 11–18. [DOI] [PubMed] [Google Scholar]
- 18.Edwards, Y. H. & Grootegoed, J. A. (1983) J. Reprod. Fertil. 68, 305–310. [DOI] [PubMed] [Google Scholar]
- 19.Turner, R. M. (2003) J. Androl. 24, 790–803. [DOI] [PubMed] [Google Scholar]
- 20.Fraser, L. R. & Quinn, P. J. (1981) J. Reprod. Fertil. 61, 25–35. [DOI] [PubMed] [Google Scholar]
- 21.Cooper, T. G. (1981) Gamete Res. 9, 55–74. [Google Scholar]
- 22.Urner, F. & Sakkas, D. (1996) Int. J. Androl. 19, 91–96. [DOI] [PubMed] [Google Scholar]
- 23.Bone, W., Jones, N. G., Kamp, G. & Cooper, T. G. (2000) J. Reprod. Fertil. 118, 127–135. [DOI] [PubMed] [Google Scholar]
- 24.Hoshi, K., Tsukikawa, S. & Sato, A. (1991) Tohoku J. Exp. Med. 165, 99–104. [DOI] [PubMed] [Google Scholar]
- 25.Williams, A. C. & Ford, W. C. (2001) J. Androl. 22, 680–695. [PubMed] [Google Scholar]
- 26.Travis, A. J., Jorgez, C. J., Merdiushev, T., Jones, B. H., Dess, D. M., Diaz-Cueto, L., Storey, B. T., Kopf, G. S. & Moss, S. B. (2001) J. Biol. Chem. 276, 7630–7636. [DOI] [PubMed] [Google Scholar]
- 27.Urner, F., Leppens-Luisier, G. & Sakkas, D. (2001) Biol. Reprod. 64, 1350–1357. [DOI] [PubMed] [Google Scholar]
- 28.Dix, D. J., Allen, J. W., Collins, B. W., Mori, C., Nakamura, N., Poorman-Allen, P., Goulding, E. H. & Eddy, E. M. (1996) Proc. Natl. Acad. Sci. USA 93, 3264–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soriano, P., Montgomery, C., Geske, R. & Bradley, A. (1991) Cell 64, 693–702. [DOI] [PubMed] [Google Scholar]
- 30.Miki, K., Willis, W. D., Brown, P. R., Goulding, E. H., Fulcher, K. D. & Eddy, E. M. (2002) Dev. Biol. 248, 331–342. [DOI] [PubMed] [Google Scholar]
- 31.Velick, S. (1955) Methods Enzymol. 1, 401–403. [Google Scholar]
- 32.Racker, E. (1984) in Methods of Enzymatic Analysis, eds. Bergmeyer, J. & Grabl, M. (Verlag Chemie, Basel), 3rd Ed., Vol. 6, pp. 561–565. [Google Scholar]
- 33.Cancel, A. M., Lobdell, D., Mendola, P. & Perreault, S. D. (2000) Hum. Reprod. 15, 1322–1328. [DOI] [PubMed] [Google Scholar]
- 34.Welch, J. E., Brown, P. R., O'Brien, D. A. & Eddy, E. M. (1995) Dev. Genet. 16, 179–189. [DOI] [PubMed] [Google Scholar]
- 35.Garcia, C. K., Brown, M. S., Pathakm, R. K. & Goldstein, J. L. (1995) J. Biol. Chem. 270, 1843–1849. [DOI] [PubMed] [Google Scholar]
- 36.Boussouar, F., Mauduit, C., Tabone, E., Pellerin, L., Magistretti, P. J. & Benahmed, M. (2003) Biol. Reprod. 69, 1069–1078. [DOI] [PubMed] [Google Scholar]
- 37.Narisawa, S., Hecht, N. B., Goldberg, E., Boatright, K. M., Reed, J. C. & Millan, J. L. (2002) Mol. Cell. Biol. 22, 5554–5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukai, C. & Okuno, M. (2004) Biol. Reprod. 71, 540–547. [DOI] [PubMed] [Google Scholar]
- 39.Brown-Woodman, P. D. C. & White, I. G. (1975) Contraception 11, 69–78.1116362 [Google Scholar]
- 40.Brown-Woodman, P. D., Mohri, H., Mohri, T., Suter, D. & White, I. G. (1978) Biochem. J. 170, 23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones, A. R. & Cooper, T. G. (1999) Int. J. Androl. 22, 130–138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.