Abstract
A recent study suggests that coherence of 20–40 Hz brain oscillations in the hippocampus and upstream lateral entorhinal cortex may support encoding of task-relevant information during associative learning. Coordination of local hippocampal circuits in this frequency range could be important for encoding new information.
It has long been thought that brain rhythms are important markers for the temporal coordination of spiking activity in organized neural circuits. Brain oscillations are dynamic, with neural networks often exhibiting unique combinations of oscillatory frequencies during different behavioral states. Within a given circuit, the timing of rhythmic spiking activity can be heavily influenced by the intrinsic properties of the neurons, the anatomical connections within neural networks, or a change in inputs that may modulate the effectiveness of communication within the system. It has been hypothesized that changes in the frequency of the rhythms reflect the dynamics of neuronal interactions within a local circuit during the sending, receiving, and internal processing of information. Igarashi et al. [1] have now reported evidence that rhythmic changes in the hippocampus and a major afferent cortical area during learning may reflect temporal coordination in the network that supports the coding of new information in the firing patterns of ensembles of single neurons.
The hippocampus is a brain structure within the medial temporal lobe known for its important role in the formation of episodic memories. The exact mechanisms through which the hippocampus encodes associations between elements of an experience to form an episodic memory are not clearly understood. It is known that, over the course of learning, neurons in the hippocampus develop selective firing patterns that reflect relevant associations between events and the places where they occur [2], as well as associations between objects and spatially directed responses [3]. Furthermore, object and spatial response associations depend upon the hippocampal system in monkeys and rats [4,5].
Igarashi et al. [1] investigated how rhythmic coordination between the hippocampus and the lateral entorhinal cortex (LEC) may support the encoding of these associations. They trained rats to associate distinct odors with different reward locations. As these associations were learned, they observed coherence in the 20–40 Hz frequency range in the CA1 subregion of the hippocampus and the LEC during the odor sampling period, suggesting that the regions may be communicating more effectively. The onset of this coherence was highly correlated with the development of distinct CA1 and LEC ensemble representations for the different odor cues. Thus, this finding introduces an important rhythmic dynamic that could be an integral contributor to associative learning.
The increases in power and the development of coherence between CA1 and LEC in the 20–40 Hz frequency range could signal a change in the processing state of local CA1 circuits during odor sampling. Notably, the increases in 20–40 Hz power in CA1 and LEC were highly time-locked to odor sampling intervals, with greatly reduced power in this frequency range before or after odor sampling. This observation suggests that there is a cue-instigated change in the processing state within these regions. The transition could have been the result of behavior-mediated changes in the inputs that drive local CA1 circuit activity, which may in turn respond differently to task information as a consequence. Notably, it is only the distal (dCA1, bordering the subiculum), and not the proximal (pCA1, bordering CA3), region of the CA1 that demonstrates coherence with LEC (Figure 1). The anatomical segregation of entorhinal projections to the dCA1 and pCA1 regions suggests that this coherence may reflect direct input into dCA1 from the LEC [6]. Specifically, the medial entorhinal cortex (MEC) projects primarily to pCA1 while the LEC projects primarily to dCA1. Moreover, while increases in 20–40 Hz power were observed during odor sampling in CA1 and LEC, MEC did not demonstrate a similar power increase in that frequency range. Thus, it is possible that dCA1–LEC coupling is mediated by the direct driving of dCA1 activity by LEC at the onset of learning.
Figure 1. Anatomical connections between entorhinal cortex and the hippocampus.
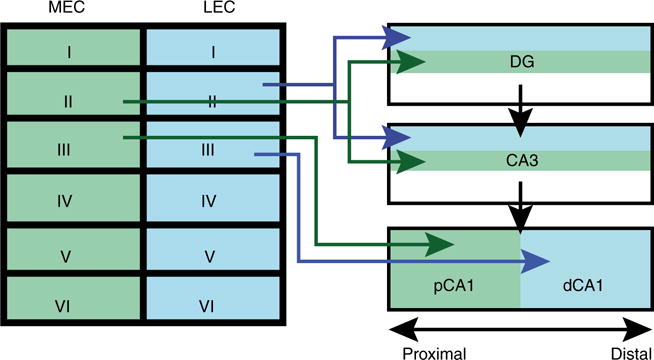
A diagram of inputs from the MEC (blue) and LEC (green) projections to the dentate gyrus (DG), CA3, and CA1 subregions. Note that inputs from MEC and LEC converge onto the same neurons in DG and CA3, but are separately projected onto different neurons in the distal part of CA1 (dCA1) and the proximal part of CA1 (pCA1).
Although pCA1, dCA1, and LEC demonstrate increases in 20–40 Hz power during odor sampling even before learning, it is only dCA1 and LEC that exhibit coupling correlated with learning and the formation of distinct ensemble representations. Above and beyond the correlative increases in power and LFP coherence between the two regions, this 20–40 Hz coherence occurs primarily during correct trials, suggesting that 20–40 Hz rhythmic mechanisms might be necessary for successful performance of the task. Future studies will need to assess what the individual cells driven at a 20–40 Hz frequency range are encoding, to address how the ensemble relates to the rhythm and why their coordination in this frequency range may be important for hippocampal function. For example, it would be interesting to know whether cells with 20–40 Hz coherence are the most selective to a given odor cue.
The 20–40 Hz coherence observed specifically between dCA1 and LEC likely reflects the type of association formed during learning this particular task. The LEC is often traditionally thought of as a high-order associative cortex that supports object and event processing [7,8]. Because the MEC, which is commonly viewed as critical to spatial processing, is not participating in this rhythm, the 20–40 Hz coupling may support associations between specific odors and the particular behavioral responses the animal makes to obtain rewards, rather than associations between the odors and places where rewards are found. Clearly, though, the results suggest that the odor–response association also involves the hippocampus, even though the entorhinal cortex can support object and spatial response associations in the absence of hippocampal function [4]. These findings do not clarify the functional role of coupling between LEC and the interconnected dCA1 observed here. Also, future studies will be needed to determine whether 20–40 Hz coupling is a common feature of hippocampal–cortical interactions in different types of associations, or whether the coherence in the dCA1–LEC circuit is unique within the medial temporal lobe.
How might coordination in the 20–40 Hz frequency range be distinctly important for associative learning more so than other frequency ranges? While Igarashi et al. [1] suggest coherence may be important “…because coupling provides sufficient coincidence of pre- and postsynaptic activity for synaptic strengthening to take place and because coincident firing among afferent neurons facilitates such strengthening”, this reasoning is not specific to a given frequency range. The authors’ work adds to a growing body of research in which increases in 20–40 Hz power and coherence are seen during the presentation of utilized task cues [9–12]. It is possible that the 20–40 Hz frequency range is optimal for a coordinated ‘handshaking’ across multiple brain structures processing this cue information [13–15]. Computational models have also shown that the underlying physiology of the 20–40 Hz frequency range in associational cortex can produce mechanisms of cell assembly formation and manipulation that are distinct from higher gamma rhythms [16]. In particular, individual gamma rhythmic cell assemblies that normally compete with one another through feedback inhibition can co-exist without competition when nested inside a beta (15–30 Hz) rhythm, thus facilitating development of a larger coordinated cell assembly. It could be the case that coherence in the 20–40 Hz range in the medial temporal lobe similarly facilitates the formation of new assemblies. Overall, this new study [1] emphasizes how a network oscillatory state influences information processing, and that the emergence of coordinated rhythmic activity may contribute to the formation of memory.
References
- 1.Igarashi KM, Lu L, Colgin LL, Moser MB, Moser EI. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature. 2014;510:143–147. doi: 10.1038/nature13162. [DOI] [PubMed] [Google Scholar]
- 2.Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA. Single neurons in the monkey hippocampus and learning of new associations. Science. 2003;300:1578–1581. doi: 10.1126/science.1084324. [DOI] [PubMed] [Google Scholar]
- 4.Yang T, Bavley RL, Fomalont K, Blomstrom KJ, Mitz AR, Turchi J, Rudebeck PH, Murray EA. Contributions of the hippocampus and entorhinal cortex to rapid visuomotor learning in rhesus monkeys. Hippocampus. 2014 Apr 20; doi: 10.1002/hipo.22294. http://dx.doi.org/10.1002/hipo.22294. (epub ahead of print) [DOI] [PMC free article] [PubMed]
- 5.Eichenbaum H, Fagan A, Mathews P, Cohen NJ. Hippocampal system dysfunction and odor discrimination learning in rats: impairment or facilitation depending on representational demands. Behav Neurosci. 1988;102:331–339. doi: 10.1037//0735-7044.102.3.331. [DOI] [PubMed] [Google Scholar]
- 6.Van Strien NM, Cappaert NLM, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- 7.Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. J Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- 9.Buschman TJ, Denovellis EL, Diogo C, Bullock D, Miller EK. Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron. 2012;76:838–846. doi: 10.1016/j.neuron.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howe MW, Atallah HE, McCool A, Gibson DJ, Graybiel AM. Habit learning is associated with major shifts in frequencies of oscillatory activity and synchronized spike firing in striatum. Proc Natl Acad Sci USA. 2011;108:16801–16806. doi: 10.1073/pnas.1113158108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinn LK, Nitz DA, Chiba AA. Learning-dependent dynamics of beta-frequency oscillations in the basal forebrain of rats. Eur J Neurosci. 2010;32:1507–1515. doi: 10.1111/j.1460-9568.2010.07422.x. [DOI] [PubMed] [Google Scholar]
- 12.Leventhal DK, Gage GJ, Schmidt R, Pettibone JR, Case AC, Berke JD. Basal ganglia beta oscillations accompany cue utilization. Neuron. 2012;73:523–536. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bibbig A, Traub RD, Whittington MA. Long-range synchronization of gamma and beta oscillations and the plasticity of excitatory and inhibitory synapses: a network model. J Neurophysiol. 2002;88:1634–1654. doi: 10.1152/jn.2002.88.4.1634. [DOI] [PubMed] [Google Scholar]
- 15.Pinto DJ, Jones SR, Kaper TJ, Kopell N. Analysis of state-dependent transitions in frequency and long-distance coordination in a model oscillatory cortical circuit. J Comput Neurosci. 2003;15:283–298. doi: 10.1023/a:1025825102620. [DOI] [PubMed] [Google Scholar]
- 16.Kopell N, Whittington MA, Kramer MA. Neuronal assembly dynamics in the beta1 frequency range permits short-term memory. Proc Natl Acad Sci USA. 2011;108:3779–3784. doi: 10.1073/pnas.1019676108. [DOI] [PMC free article] [PubMed] [Google Scholar]


