Abstract
CD38 is an ectoenzyme that catalyzes the conversion of β-nicotinamide adenine dinucleotide (β-NAD) to cyclic adenosine diphosphoribose (cADPR) and adenosine diphosphoribose (ADPR) and NADP to nicotinic acid adenine dinucleotide phosphate (NAADP) and adenosine diphosphoribose-2’-phosphate (ADPR-P). The metabolites of NAD and NADP have roles in calcium signaling in different cell types including airway smooth muscle (ASM) cells. In ASM cells, inflammatory cytokines augment CD38 expression and to a greater magnitude in cells from asthmatics, indicating a greater capacity for the generation of cADPR and ADPR in ASM from asthmatics. CD38 deficient mice develop attenuated airway responsiveness to inhaled methacholine following allergen sensitization and challenge compared to wild-type mice indicating its potential role in asthma. Regulation of CD38 expression in ASM cells is achieved by mitogen activated protein kinases, specific isoforms of PI3 kinases, the transcription factors NF-κB and AP-1, and post-transcriptionally by microRNAs. This review will focus on the role of CD38 in intracellular calcium regulation in ASM, contribution to airway inflammation and airway hyperresponsiveness in mouse models of allergic airway inflammation, the transcriptional and post-transcriptional mechanisms of regulation of expression, and outline approaches to inhibit its expression and activity.
Keywords: Airway smooth muscle, asthma, CD38, microRNAs, calcium regulation
1. Introduction
Asthma is a chronic inflammatory disease of the lung affecting over 300 million people globally. Despite several decades of research on asthma pathophysiology and pharmacology, efficacious anti-asthma medications continue to be an unmet medical need as more than half of asthmatics are inadequately controlled. Fulfilling this need involves, in part, identifying novel therapeutic targets for asthma. Allergen exposure in susceptible individuals leads to airway inflammation characterized by release of mediators such as cytokines and chemokines. These molecules regulate the expression of a variety of genes in the resident airway cells including airway smooth muscle (ASM) cells leading to important phenotypic changes, including airway remodeling and heightened bronchoconstriction.
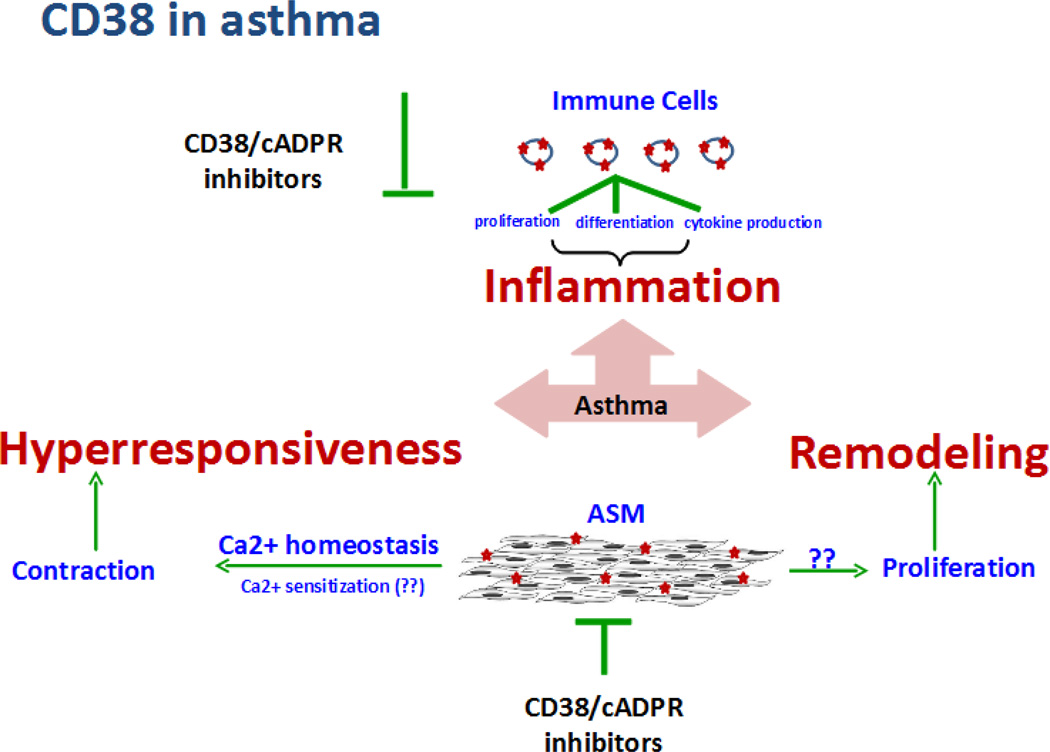
Different cell types mediate the structural and functional changes underlying the asthmatic phenotype. For example, immune cells account for inflammatory response to allergen and smooth muscle cells contribute towards hyperresponsiveness and bronchoconstriction. The ASM is also capable of modulating local inflammatory response via production and release of chemokines and cytokines. Interestingly, we and others have demonstrated that CD38, a plasma membrane bound multifunctional enzyme, is expressed on immune and ASM cells, and plays roles in the orchestration of immune responses, regulation of ASM contraction and airway hyperresponsiveness. Therefore, CD38 is an attractive therapeutic target in asthma (Figure 1). This review summarizes the data highlighting the role of CD38 in asthma pathogenesis with an emphasis on approaches to therapeutically target CD38 in asthma.
Figure 1. CD38-cADPR in asthma pathogenesis.
Allergen-induced inflammation, remodeling, and hyperresponsiveness are the major components of asthma. Resident airway cells such as ASM cells and migrated immune cells contribute to asthma pathogenesis. CD38 is expressed on immune cells and smooth muscle cells. CD38 on immune cells functions as cell surface marker and contributes to inflammatory response in asthma. ASM is the principal contractile component of the airways and CD38 via production of cADPR, a calcium elevating second messenger, contributes to smooth muscle contractility and airway hyperresponsiveness. Regulating CD38 expression or its enzyme activity or cADPR effect in effector cells may effectively mitigate multiple features of asthma.
2. Receptor and Enzymatic functions of mammalian CD38
The protein encoded by CD38 is a type II transmembrane glycoprotein with a molecular weight of ∼45kDa (Ferrero, et al., 2000; Lee, 2006; Zocchi, et al., 1993). CD38 expression is ubiquitous and has been described in smooth muscle cells (Deshpande, et al., 2003; White, et al., 2000), pancreatic cells (Okamoto, et al., 1997), astrocytes (Banerjee, et al., 2008; Kou, et al., 2009; Mamik, et al., 2011), and in B lymphocytes (Deaglio, et al., 2003; Malavasi, et al., 2011; Morabito, et al., 2002). As a functional molecule, CD38 is a dimer and the catalytic site is contained within the central part of the molecule (Munshi, et al., 2000; Zhao, et al., 2012; Zhao, et al., 2015). In addition, CD38 appears to be localized within lipid microdomains of the plasma membrane (Lund, et al., 2006) where it associates with several other proteins to form a complex. Among the proteins that CD38 forms a complex in B lymphocytes are CD19/CD81 (Song, et al., 2016), CXCR4, a chemokine receptor (Majid, et al., 2011), and CD49d, an adhesion molecule (Zucchetto, et al., 2012).
It is interesting to note that the mammalian CD38 has similarity (∼35% amino acid identity) to a soluble form of the enzyme adenosine diphospho-ribosyl cyclase (ADP-ribosyl cyclase) in the ovotestis of the mollusk Aplysia californica (Lee & Aarhus, 1991; States, et al., 1992). ADP-ribosyl cyclase is capable of cyclizing NAD+ to form cyclic ADP ribose (cADPR) (Lee & Aarhus, 1991). cADPR has been shown to release calcium from intracellular stores that are distinct from that released by IP3 in sea urchin eggs (Dargie, et al., 1990), porcine airway smooth muscle cells (Prakash, et al., 1998) and Ascidian oocytes (Albrieux, et al., 1998). The mammalian CD38 possesses ADP ribosyl cyclase activity that converts NAD+ to cADPR as well as cADPR hydrolase activity that converts cADPR to ADPR (Howard, et al., 1993; Zocchi, et al., 1993). It is important to note that CD38 is predominantly a NAD glycohydrolase enzyme and generates ADPR directly from NAD (Schuber & Lund, 2004). Therefore, ADPR production largely comes from direct conversion of NAD by CD38. Nevertheless, cADPR has been described as a second messenger molecule capable of releasing calcium from the intracellular stores in a variety of cells (Lee, 2011). cADPR hydrolase activity of CD38 coverts cADPR to ADPR thereby controlling the calcium-mobilizing activity of cADPR. Other reports have also identified an enzymatic activity of CD38 that is capable of converting NADP+ to NAADP+ via a base-exchange reaction in a pH-dependent fashion (Aarhus, et al., 1995; Chini, et al., 2002; Lee, 1999). In airway smooth muscle cells, cADPR releases calcium from the sarcoplasmic reticulum via activation of the ryanodine receptor channels (Prakash, et al., 1998; White, et al., 2003). NAADP signaling has also been shown to play a role in muscarinic receptor-induced contraction of guinea pig trachea (Aley, et al., 2013) (Figure 2). A byproduct of NAD+ metabolism by CD38 is nicotinamide and CD38 is a critical enzyme involved in NAD+ homeostasis and cellular energy metabolism (Al-Abady, et al., 2013; Barbosa, et al., 2007; Camacho-Pereira, et al., 2016; Chini, 2009; Young, et al., 2006). The receptor functions of CD38 appear to be distinct from the enzymatic functions that generate calcium-mobilizing second messenger molecules (Lund, 2006).
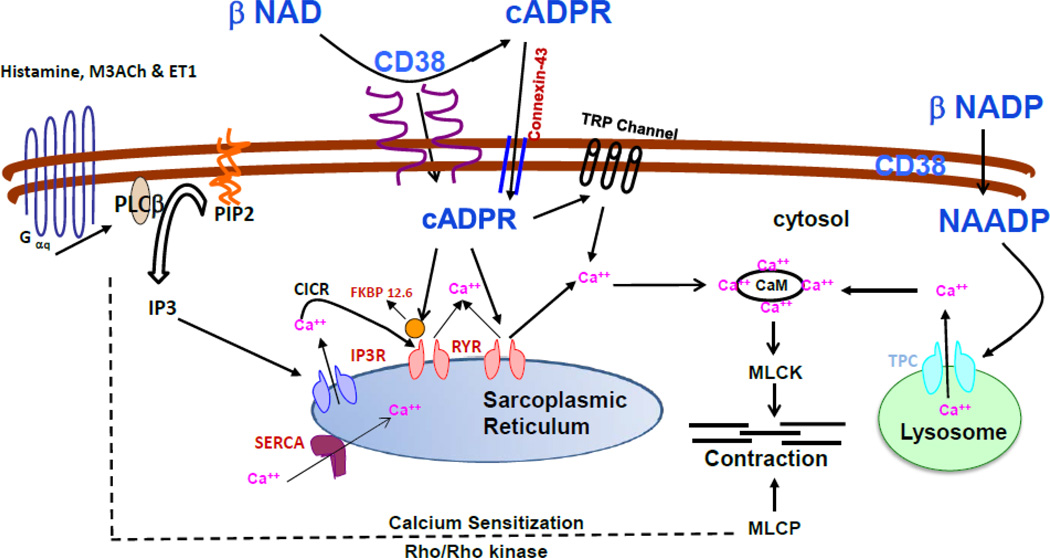
Figure 2. Model depicting CD38/cADPR-mediated calcium release and functional effect in ASM.
Stimulation of ASM cells with Gq-coupled GPCR agonists results in the production of inositol 1,4,5 triphosphate (IP3) which in turn binds to its receptors on sarcoplasmic reticulum (SR). cADPR produced by the catalytic action of ADP-ribosyl cyclase (part of membrane bound protein CD38) acts as a calcium releasing second messenger presumably via ryanodine receptors (RyR) on the SR membrane. Extracellularly produced cADPR is believed to enter cell via membrane channel formed by connexin-43. Accessory proteins such as FKBP12.6 and calmodulin (CaM) are known to be involved in the cADPR-mediated calcium release. Recent studies have shown that cADPR sensitizes store-operated calcium entry via TRP channels on the plasma membrane to open and ADPR actually gates the channels. cADPR-mediated calcium release is involved in the regulation of global as well as local/compartmentalized (e.g. oscillations) calcium homeostasis in ASM cells. In vivo studies using CD38 null mice have confirmed the functional role of CD38/cADPR-mediated calcium release in the regulation of bronchomotor tone. Calcium sensitization via RhoA/ROCK contributes to ASM contraction. However, the role of cADPR in mediating calcium sensitization mechanism is not established (dotted lines). CD38 converts NADP into NAADP which is known to release calcium from intracellular stores such as lysosomes (shown on the right side of the figure).
The best-characterized mammalian ADP-ribosyl cyclase enzyme is CD38. Thus, CD38 is considered to be critical for calcium-dependent biological processes in vivo. Early reports identified CD38 in the activation and proliferation of T lymphocytes (Lund, 2006). Prior investigations in neutrophils revealed that cADPR produced by CD38 is required for neutrophil chemotaxis and bacterial clearance, providing a critical role for this molecule in inflammation and innate immunity (Lund, 2006). In this review, we will outline the contribution of CD38 to intracellular calcium regulation of airway smooth muscle, its role in airway inflammation and airway hyperresponsiveness in the context of allergic airway disease, the regulation of CD38 expression and specifically the role of MAP kinases and transcription factors involved, and post-transcriptional regulation of expression by microRNAs (miRNAs). We will identify therapeutic targets that can result in modulation of CD38 expression in an attempt to provide a potential translational relevance to the small molecule inhibitors of CD38 and cADPR antagonists in the mitigation of airway hyperresponsiveness in diseases such as asthma.
3. CD38/cADPR in the regulation of intracellular calcium in airway smooth muscle
Our laboratory undertook studies to delineate the contribution of the two major pathways of SR calcium release, namely that mediated by activation of the IP3-receptor channels and that mediated by activation of the ryanodine receptor channels (Deshpande, et al., 2003; Kannan, et al., 1997; Kotlikoff, et al., 2004; Prakash, et al., 1998). The involvement of CD38-derived cADPR in the regulation of intracellular calcium responses to agonists has been supported by studies in our laboratory (Figure 2). In earlier studies, we showed that exposure of ASM cells to acetylcholine elicited oscillations in intracellular calcium (Pabelick, et al., 1999; Prakash, Kannan, et al., 1997a, 1997b; Prakash, et al., 2000; Prakash, van der Heijden, et al., 1997). While the peak to trough amplitude of the intracellular calcium oscillations was relatively constant in any region of the cell as measured by real-time confocal microscopy, the frequency of the oscillations increased with increasing concentration of the agonist. Furthermore, the velocity of propagation of the oscillations from one region to another region of the cell also increased with increasing agonist concentration. The spatial and temporal integration of the intracellular calcium oscillations resulted in a mean calcium response to an agonist whose magnitude increased with the concentration of the agonist. During ongoing intracellular calcium oscillations, addition of 8-amino-cADPR, a cADPR antagonist (Walseth & Lee, 1993) resulted in slowing of the frequency of oscillations, whereas addition of cADPR (to permeabilized cells) caused an increase in the frequency of the oscillations (Prakash, et al., 1998). This slowing and the increase in the frequency of intracellular calcium oscillations would be reflected respectively in a reduction and increase of the mean calcium response. In permeabilized ASM cells, addition of cADPR causes a calcium transient. This calcium transient is absent in cells preincubated with ryanodine or ruthenium red which inhibit calcium release from the sarcoplasmic reticulum through ryanodine receptor channels, but present in the presence of heparin which inhibits calcium release through IP3-receptor channels (Prakash, et al., 1998). Studies from other investigators showed that addition of cADPR increased the amplitude and frequency of spontaneous transient inward currents, providing evidence for spontaneous calcium release by cADPR (Y. X. Wang, et al., 2004). While these results indicate that cADPR mediates calcium release and regulates the frequency of intracellular calcium oscillations, the definitive proof that CD38-derived cADPR contributes to regulation of intracellular calcium came from other studies in our laboratory. The magnitude of the intracellular calcium responses to agonists in airway smooth muscle cells obtained from CD38 knockout mice were significantly attenuated compared to responses in cells obtained from wild-type mice (Deshpande, et al., 2005).
The mechanisms by which cADPR causes calcium release in ASM cells have been explored. In airway myocytes obtained from CD38 knockout mice, the calcium response elicited by the addition of acetylcholine or endothelin-1 is significantly attenuated as compared to responses elicited in myocytes obtained from wild-type mice (Deshpande, et al., 2005). Furthermore, pre-incubation of myocytes from wild-type mice with ryanodine causes significant attenuation of calcium responses to acetylcholine, while having no significant effect on the calcium response in myocytes obtained from CD38 knockout mice (Deshpande, et al., 2005). It is also interesting to note that in porcine ASM cells, the calcium response elicited by acetylcholine or endothelin-1, but not by histamine, is significantly attenuated in the presence of 8-Br-cADPR, a competitive membrane-permeant antagonist of cADPR (White, et al., 2003). Histamine-induced calcium response is not sensitive to inhibition by ryanodine as the response to acetylcholine or endothelin-1. These observations strongly indicate that the agonist-selectivity of cADPR-mediated calcium release is due to recruitment of ryanodine-receptor mediated calcium release by some agonists but not by all agonists in ASM (White, et al., 2003). During ongoing intracellular calcium oscillations initiated by the addition of acetylcholine, exposure to cADPR increases the frequency of these oscillations. Other studies have shown that cADPR-mediated calcium release from the sarcoplasmic reticulum may involve binding to accessory proteins such as FK506-binding protein (Y. X. Wang, et al., 2004; X. Zhang, et al., 2009). In the pancreatic acinar cells, addition of cADPR causes the dissociation of FK506-binding protein from the ryanodine receptors (Ozawa, 2004, 2006). A similar mechanism of binding of cADPR to FKBP-12.6 leading to its dissociation from ryanodine receptor channels has been proposed for arterial and tracheal smooth muscle cells (Tang, et al., 2002; Y. X. Wang, et al., 2004). Further evidence that cADPR-mediated calcium response involves binding to FK506-binding protein is supported by a lack of calcium response in ASM cells obtained from FKBP12.6 knockout mice (Y. X. Wang, et al., 2004). It should be emphasized that dissociation of FKBP-12.6 from ryanodine receptor channels by cADPR as the mechanism underlying the activation of the channels has been challenged by other investigations (Copello, et al., 2001). Alternately, cADPR/FKBP-12.6 complex can bind to ryanodine receptors to cause its activation. It should also be noted that other accessory proteins have been suggested in the actions of cADPR in different cell types (Venturi, et al., 2012). In summary, experimental evidence strongly indicate that cADPR-mediated calcium release from the intracellular stores involves interaction with the ryanodine receptor channels directly or indirectly via involving accessory proteins such as FK506-binding protein (Figure 2).
4. Calcium Regulation in Airway Smooth Muscle by Inflammatory Cytokines
Airflow limitation that occurs in inflammatory diseases such as asthma arises from heightened contraction of ASM. Contraction of ASM is mediated by spasmogens that act via G-protein-coupled receptors and include acetylcholine released from post-ganglionic parasympathetic nerve terminals within the airways, and histamine, leukotrienes and prostaglandins that are released from mast cells. Regulation of smooth muscle contraction is achieved by phosphorylation of myosin light chain (MLC) by the MLC kinase (MLCK) (Chiba, et al., 2010; Kitazawa, Gaylinn, et al., 1991; Kitazawa, Masuo, et al., 1991; Shirazi, et al., 1994). Dephosphorylation of MLC is brought forth by myosin phosphatase that terminates the contractile process.
Phosphorylation of myosin phosphatase by Rho-kinase, a serine/threonine kinase, inactivates myosin phosphatase, resulting in contraction of smooth muscle (Somlyo & Somlyo, 2003). An increase in the intracellular calcium concentration activates MLCK and results in a calcium-dependent smooth muscle contraction (Figure 2). On the other hand, the inactivation of myosin phosphatase by Rho-Kinase results from an increase in sensitivity to calcium and this process is termed calcium sensitization. A variety of mechanisms have been proposed to account for the rise in intracellular calcium following stimulation of ASM by agonists that act via G-protein-coupled receptors. In ASM, an important and significant source of rise in intracellular calcium concentration by spasmogens is calcium release from intracellular stores such as the sarcoplasmic reticulum (SR). Thus, mechanisms that regulate calcium release from the SR can have a significant impact on contractility of airway smooth muscle.
We investigated the regulation of CD38/cADPR signaling in the intracellular calcium responses to agonists by inflammatory cytokines (Deshpande, et al., 2004; Deshpande, et al., 2003). Human ASM cells were isolated from lungs of healthy subjects and asthmatics (de-identified lungs from donors and with Institutional approval). Cells were grown to confluence and growth-arrested in serum-free medium for 24–48 hr. Exposure of human airway smooth muscle cells to inflammatory cytokines such as IL-13 and TNF-α for 24–48 hr caused an increased expression of CD38 and NAD+-catalyzed cADPR generation. The intracellular calcium responses to agonists such as acetylcholine, bradykinin and ET-1 were significantly augmented in cells treated with the cytokines compared to responses in vehicle-treated cells. Furthermore, pre-incubation of cells with 8-bromo-cADPR, a cell permeant competitive cADPR antagonist (Sethi, et al., 1997; Walseth & Lee, 1993), resulted in a significant reduction in the intracellular calcium responses to agonists and the responses seen in the cells were comparable to those seen in cells treated with the vehicle. Knockdown of CD38 expression by siRNA or following transfection with anti-sense oligonucleotides also caused a reduction in the intracellular responses to agonists in cytokine-treated cells (Kang, et al., 2005). These results clearly showed that the augmented intracellular calcium responses to agonists following cytokine exposure can be attributed to the increased CD38 expression and cADPR-mediated calcium release, and CD38 expression and cADPR-mediated calcium responses to agonists are expected to be greater in an inflammatory milieu such as in the lungs of asthmatics. In this context, we compared the induction of CD38 expression by TNF-α in ASM cells obtained from asthmatics and non-asthmatics (Jude, et al., 2010). Exposure to TNF-α resulted in a concentration-dependent induction of CD38 expression and ADP-ribosyl cyclase activity in ASM cells from both asthmatics and non-asthmatics. However, the magnitude of CD38 induction was significantly greater in cells from asthmatics than in cells from non-asthmatics, suggesting a greater capacity for cADPR-mediated calcium response to agonists. This differential induction of CD38 expression is due to increased transcriptional regulation involving ERK and p38 MAPK activation and is independent of changes in NF-κB or AP-1 activation. The findings suggest a potential role for CD38 in the pathophysiology of asthma (Table 1).
Table 1.
Regulation of CD38 expression in smooth muscle.
| Cytokines | |
| IL-1β | Up |
| UpTNF-α | Up |
| IFN-γ | Up |
| IL-13 | Up |
| Hormones | |
| Glucocorticoids | Down |
| Estrogen | Up |
| Progesterone | Down |
| MicroRNAs | |
| miR-140-3p | Down |
| miR-708-5p | Down |
| miR-499-5p | Unknown |
| miR-155 | Unknown |
| Transcription Factors | |
| NF-κB | Up |
| AP-1 | Up |
Airway inflammatory diseases such as asthma are characterized by structural changes in the lungs collectively called as “airway remodeling” and phenotype modulation of airway cells (Prakash, 2013). Airway remodeling includes excessive accumulation of smooth muscle mass, sub-epithelial fibrosis, deposition of extracellular matrix including collagen and fibronectin, mucus cell hyperplasia and thickening of airway wall. Airway cells including ASM cells undergo phenotypic changes due to inflammation. ASM cells are “contractile” in nature and assume a “synthetic or proliferative” phenotype during airway inflammation thereby contributing to ASM remodeling. Considering the up-regulation of CD38-cADPR in ASM by inflammatory cytokines, it is plausible that this pathway plays a critical role in the regulation of ASM growth, a hallmark feature of airway remodeling. Future studies are needed to establish the role of CD38-cADPR pathway in ASM cell proliferation. Interestingly, lack of CD38 in coronary arterial smooth muscle cells induced a proliferative phenotype (Xu, et al., 2015; Y. Zhang, et al., 2014). This phenotype switch is accompanied by increased expression of vimentin in smooth muscle cells, collagen deposition around the arterial wall, and decreased expression of contractile phenotype markers such as calponin, SM22α and α-Smooth muscle actin. Stimuli that enhance atherosclerosis in arteries enhance phenotype switch induced by CD38 deficiency suggesting a potential role of CD38-cADPR in the regulation of smooth muscle proliferation and development of atherosclerosis. Furthermore, CD38 or cADPR mediated signaling pathway has been implicated in the regulation of differentiation of stem cell or development of tumors (Nipp, et al., 2014; Wei, et al., 2012). Chronic lymphocytic leukemia is a good example to demonstrate the role of CD38 in human proliferative disease (Vaisitti, et al., 2015). Airway remodeling in asthma comprises a similar cellular phenotype modulation, and based on the findings described above it is reasonable to hypothesize that CD38-cADPR signaling pathway plays a role in airway remodeling.
Studies have demonstrated synthetic functions of ASM cells in which ASM cells secrete inflammatory cytokines and chemokines and play an immunomodulatory role in asthma (Tliba & Panettieri, 2009). Tliba et al., demonstrated that CD38-cADPR-mediated signaling contributes to transcriptional regulation of inflammatory genes in ASM cells (Jain, et al., 2008; Tliba, et al., 2004). Further, interferon (IFN)-β is an additional signaling molecule that mediates TNFα-induced up-regulation of CD38 expression and thereby contributing to immunomodulatory function of ASM cells.
5. Contribution of CD38 to Airway Hyperresponsiveness in Mouse Models of Allergic Airway Disease
Our initial studies were directed at determining whether the CD38 knockout mice have an airway phenotype that is distinct from that of the wild-type mice. The changes in resistance to airflow and dynamic compliance following inhaled methacholine challenge were significantly attenuated in the CD38 knockout mice compared to wild-type mice (Deshpande, et al., 2005). We then investigated the methacholine responsiveness of CD38 knockout and wild-type mice exposed to the asthma-relevant cytokines TNF-α and IL-13. CD38 knockout mice developed significantly lower airway responsiveness to inhaled methacholine following IL-13 challenge than wild-type control mice. Airway inflammation in response to IL-13 was similarly robust in both mice, but airway reactivity to inhaled methacholine was significantly lower in the CD38 knockout mice than in wild-type controls. Using tracheal ring preparations from these mice, it was evident that CD38 within ASM mediated the IL-13-associated increase in isometric force generation to carbachol stimulation. Airway smooth muscle relaxation to the β-adrenergic receptor agonist isoproterenol was similar between the two mice (Guedes, et al., 2006). A single intranasal challenge with TNF-α caused airway hyperresponsiveness to methacholine in wild-type but not in CD38 knockout mice without major changes in airway inflammation. More prolonged exposure to TNF-α caused similarly robust airway inflammation in the mice, but the differences in airway phenotype were no longer present (Guedes, et al., 2008). Together, these results provide evidence that CD38 expressed in ASM cells plays a significant role in airway hyperresponsiveness to two asthma-relevant cytokines.
Earlier studies from Lund’s laboratory indicated that CD38 knockout mice developed attenuated T cell-dependent humoral immune response to protein antigens (Cockayne, et al., 1998). The underlying mechanisms for this attenuated allergen-induced antibody response were complex and include defective priming of T cells by Cd38−/− dendritic cells and inefficient migration of Cd38−/− dendritic cells from sites of inflammation to the draining lymph nodes (Partida-Sanchez, et al., 2007). Therefore, we hypothesized that allergen sensitization and challenge would result in an attenuated asthmatic phenotype in the CD38 knockout mice (Guedes, Jude, et al., 2015). We compared the airway responsiveness of CD38 knockout and wild-type mice following intranasal sensitization and intranasal challenge with fungal antigens as well as following intraperitoneal sensitization followed by intranasal challenge with ovalbumin antigen. Regardless of the route of allergen sensitization, the CD38 knockout mice developed significantly attenuated methacholine responsiveness compared to wild-type mice. However, the airway inflammatory response characterized by the number and types of inflammatory cells in the bronchoalveolar lavage (BAL) fluid was comparable in the two groups of mice. The changes in BAL fluid content of IL-5 and IL-13 were similar between CD38 knockout and wild-type mice in both allergen models. On the other hand, BAL fluid eotaxin-2 concentration increased following challenge with either allergen but the increase was lower in the CD38 knockout mice following fungal allergen challenge but not following ovalbumin. These findings indicated that CD38 expression is critical for the development of heightened airway hyperresponsiveness following allergen sensitization and challenge, although the magnitude of airway inflammation may not be dependent on CD38 expression. To explore this mechanism further, we developed bone marrow chimeras to assess if reciprocal transfer of Cd38+/+ or Cd38−/− bone marrow-derived cells would restore the airway phenotype in the hosts under naïve conditions and following ovalbumin sensitization and challenge. Reciprocal bone marrow transfer did not affect the airway phenotype under naïve conditions. Following ovalbumin challenge, bone marrow transfer from either source partially reversed the airway phenotype in CD38 knockout mice whereas the airway constriction observed in WT hosts was unaffected regardless of whether the bone marrow cells were derived from WT or CD38 deficient animals (Guedes, Jude, et al., 2015). These results thus confirm that loss of CD38 in hematopoietic cells is not sufficient to prevent airway hyperresponsiveness to allergen sensitization and challenge and that airway inflammation is not the main determinant of airway hyperresponsiveness in these models. Jain et al. used Aspergillus fumigatus sensitization and challenge model and demonstrated that both wild-type and CD38 knockout mice developed a robust inflammatory response, although AHR to methacholine was attenuated in CD38 knockout mice compared to that in wild-type mice (Jain, et al., 2008). Furthermore, tracheal rings obtained from wild-type mice treated with TNF-α demonstrated enhanced contractile responses to carbachol and bardykinin suggesting airway hypercontractility. Cytokine-induced airway hyperresponsiveness was completely abrogated in tracheal rings obtained from CD38 knockout mice.
Altogether, the above findings in the cytokine and allergen models are consistent with the role of CD38 in ASM calcium responses and contractility to agonists and provide evidence that this mechanism is the main driver for CD38-mediated airway hyperresponsiveness.
6. Regulation of CD38 Expression in Human Airway Smooth Muscle
The cd38 gene is localized on chromosome 4 in human and chromosome 5 in the mouse (Ferrero, et al., 2000). The >80 kb length gene encoding CD38 has 8 exons and binding sites for several transcription factors. In human myeloid cells, CD38 expression is induced by retinoic acid binding to the retinoic acid response element located within the first intron (Mehta & Cheema, 1999). Response elements for transcription factors AP-1 and NF-κB in the cd38 gene have been described in erythropoietic cells (Sun, et al., 2006). In addition, glucocorticoid response elements (GREs) localized within the promoter region of the cd38 gene have been identified (Tirumurugaan, et al., 2008). In human ASM cells transfected with the 3 kb human cd38 promoter, exposure to TNF-α resulted in a two-fold activation of the promoter. However, there was no induction of promoter activity in TNF-α stimulated human ASM cells transfected with a 1.8 kb cd38 promoter that lacked the NF-κB binding site, or promoter constructs lacking NF-κB and/or AP-1 sites, or in the presence of dexamethasone. These findings therefore confirmed the functional importance of NF-κB, AP-1 and GREs in the cd38 promoter in the transcriptional regulation of CD38 expression (Tirumurugaan, et al., 2008).
We and other investigators have reported the regulation of CD38 expression by cytokines involved in asthma pathogenesis (Table 1). The cytokines such as TNF-α and IL-1β signal via NF-κB and regulate CD38 expression. Glucocorticoids are most commonly used as anti-asthma medications and Tliba et al. investigated the effect of glucocorticoids on cytokine-induced up-regulation of CD38 expression (Tliba, et al., 2006; Tliba, et al., 2004). Glucocorticoid treatment inhibits CD38 expression induced by TNF-α alone or in combination with IL-1β or IL-13. Interestingly, CD38 expression in human ASM cells in response to a combination of TNF-α and IFN-γ was insensitive to glucocorticoid. Glucocorticoid effect on CD38 expression potentially involves binding of glucocorticoid to GRE in the promoter region of cd38 gene. TNF-α and IFN-γ together inhibit glucocorticoid-induced glucocorticoid receptor-α-DNA binding activity. These studies collectively demonstrate that the regulation of CD38 expression in airways during inflammation is complex and involves a combination of positive and negative regulators (Table 1).
Recent investigations have centered on post-transcriptional mechanisms of regulation of CD38 expression in human ASM. In this context, we have investigated the role of microRNAs (miRNAs) in such regulation (Deshpande, et al., 2015; Dileepan, et al., 2014; Guedes, Deshpande, et al., 2015; Jude, et al., 2012). miRNAs are small non-coding RNAs that are involved in the regulation of gene expression by binding to the 3’-Untranslated Region (3’UTR) of target mRNAs (Bartel, 2004; Ha & Kim, 2014; He & Hannon, 2004). This binding results in the degradation of mRNA and/or translational repression. Furthermore, a single miRNA is capable of regulating several genes. Altered miRNA expression has been implicated in airway inflammation (Chiba, et al., 2009; Dileepan, et al., 2014; Jude, et al., 2012; Kuhn, et al., 2010). We have identified miR-140-3p and miR-708 in the regulation of CD38 expression as well as many chemokine genes in ASM (Dileepan, et al., 2014). Target prediction algorithms have identified multiple binding sites for several miRNAs in CD38 3’UTR (Table 1). However, expression levels measured by qRT-PCR revealed miR-140-3p and miR-708 as likely candidates for post-transcriptional regulation of expression. We investigated the mechanisms by which these miRNAs regulate CD38 expression in human ASM cells. Over-expression of miR-708 by transfection increased the expression of PTEN, a phosphatase that terminates PI3K signaling (Dileepan, et al., 2014). This increased PTEN expression was also associated with a decrease in AKT phosphorylation. miR-708 transfection of cells also resulted in enhanced expression of MKP-1, a phosphatase that terminates signaling through MAPKs. This increased MKP-1 expression was associated with decreased JNK MAPK phosphorylation. Prior studies have demonstrated that the PI3K/AKT signaling pathway has a major role in the proliferation of ASM cells from asthmatics (Burgess, et al., 2008). Dual luciferase reporter assays in a heterologous cell line also revealed binding of miR-708 to cd38 3’UTR. These results indicated that miR-708 binds to 3’UTR to regulate CD38 expression and indirectly regulates airway inflammation, ASM contractility and cell proliferation by regulating JNK MAP kinase activation and PI3K/AKT signaling.
Over-expression of miR-140-3p by transfection in human ASM cells also decreased CD38 expression and the CD38-associated enzyme activities with no changes in CD38 mRNA stability (Jude, et al., 2012). Dual luciferase reporter assays revealed binding of miR-140-3p to 3’UTR to regulate CD38 expression. Transfection of cells with the miR-140-3p mimic resulted in decreased activation of p38 MAPK as well as NF-κB. It should be emphasized that MAPK and PI3K signaling as well as NF-κB activation are required for expression of several pro-inflammatory genes. These findings indicated that there may be common as well as distinct mechanisms by which the two miRNAs regulate CD38 expression in human ASM cells. Inhibition of JNK and p38 MAPKs along with decreased PI3K/AKT signaling and NF-κB activation should have profound anti-inflammatory effects. We propose that targeted delivery of these miRNAs into the lungs should result in significant anti-inflammatory effects. In this context, in our recent studies we examined the effect of transfection of human ASM cells with miR-140-3p and miR-708 on candidate inflammation-associated gene expression and validated the expression of some of these genes using qRT-PCR and release of some of the chemokines by ELISA (Dileepan, et al., 2016). Among the most significant biologic functions for the differentially expressed gene set following miRNA transfection in cells exposed to TNF-α were decreased inflammatory response, cytokine expression and signaling. In miR-708 transfected cells, qRT-PCR revealed inhibition of expression of the following chemokine genes: CCL11, CXCL10, CCL2 and CXCL8, with inhibition of CCL11 release. In cells transfected with miR-140-3p on the other hand, there was inhibition of expression of CCL11, CXCL12, CXCL10, CCL5 and CXCL8, with inhibition of CXCL12 release. Furthermore, miR-708 transfection also resulted in inhibition of expression of genes known to contribute to the pathogenesis of asthma such as RARRES2, CD44 and ADAM33. These results showed that there are genes targeted by both miRNAs, while exerting distinct effects on inflammation-associated gene expression in human ASM cells (Dileepan, et al., 2016). Many of the chemokines whose expression is modulated by the miRNAs are involved in the recruitment of inflammatory cells into the airways such as eosinophils, basophils, mast cells and T lymphocytes in allergic airway disease. Furthermore, signaling through PI3K/AKT is also involved in ASM cell proliferation in asthma (Burgess, et al., 2008). Down-regulation of PTEN and DUSP-1, and p38 and JNK MAP kinases as well as the transcription factor NF-κB is involved in mediating inflammation. Several reports have demonstrated that airway smooth muscle has both contractile and immunomodulatory functions (Alrashdan, et al., 2012; Ammit, et al., 2000; Ammit, et al., 2002; Damera & Panettieri, 2011; Dekkers, et al., 2009; Ghaffar, et al., 1999; Hershenson, et al., 2008; John, et al., 2009; Knox, et al., 2001; Ozier, et al., 2011; Panettieri, et al., 1995; Tliba & Panettieri, 2009; K. Zhang, et al., 2007). During persistent airway inflammation, chemokines and cytokines elaborated by inflammatory cells and ASM cells can lead to cell proliferation and airway remodeling. miRNAs such as miR-140-3p and miR-708 play a critical role in the regulation of multiple of features of asthma pathogenesis. Most importantly, miR-140-3p and miR-708 regulate CD38 expression and cADPR production. Therefore, we propose that targeting these two miRNA networks may offer a novel therapeutic mechanism to regulate airway inflammation, ASM proliferation and airway remodeling via target proteins such as CD38 in diseases such as asthma.
Several recent reports provide evidence for altered expression of miRNAs in the induction of allergic airway inflammation. In a mouse model of allergic airway inflammation induced by house dust mite allergen, there was evidence for induction of expression of miR-126 with airway hyperresponsiveness to inhaled methacholine (Mattes, et al., 2009). In mice exposed to an antagomir specific for miR-126 followed by allergen sensitization and challenge, there was decreased release of IL-5 and IL-13 into the lungs, with decreased mucus hypersecretion, recruitment of eosinophils and attenuated methacholine responsiveness. In a chronic allergen challenge model, the authors noticed that anti-miR-126 had no significant effects on eosinophil recruitment into the lungs as well as the inflammatory response in the airway wall. In this chronic model of allergen challenge, miR-126 expression was elevated in the airway wall for a short time period and returned to baseline values, although the asthmatic phenotype of the mice was preserved beyond this period. These results suggest other mechanisms, such as multiple miRNAs may be involved in the switch from a normal to an asthmatic phenotype. In a study involving miR-155 knockout mice, there was indication for reduced Th2 cell numbers and chemokine expression in the lungs (Malmhall, et al., 2014). T cells deficient in miR-155 also produced significantly reduced quantities of IL-4. miR-155 knockout mice also exhibited reduced eosinophil numbers and mucus secretion compared with wild-type mice. miR-155 expression is higher in airway smooth muscle cells exposed to inflammatory cytokines than in cells from non-asthmatic subjects, with elevated COX-2 expression and PGE2 secretion. Exposure of cells to anti-miR-155 resulted in decreased PGE2 secretion. These results suggest that miR-155 may have a role in hyporesponsiveness to (β2-adrenoceptor agonists and targeting this miRNA may have a beneficial effect in restoring responsiveness to this class of bronchodilators. Other studies have shown specific effects of miR-21 in priming the lungs toward a Th2 and IL-13 skewed phenotype (Lu, et al., 2009). Our recent studies on miR-140-3p and miR-708 reveal differential expression in asthmatic vs. non-asthmatic ASM and significant down-regulation of expression following exposure to TNF-α (Dileepan, et al., 2014; Jude, et al., 2012). We believe that up-regulation of expression of these miRNAs should result in significant attenuation of airway hyperresponsiveness, a hallmark feature of asthma. This could stem from the direct inhibitory effect of miR-140-3p and miR-708 on CD38 expression, cADPR-mediated calcium regulation, and airway responsiveness. However, the challenge in this approach is to deliver miRNAs into the lungs in sufficient quantities and over a long period of time to be effective in reversing the asthmatic response to allergen exposure.
7. Design and characterization of CD38 inhibitors
CD38 is an attractive drug target due to its involvement in airway inflammation and airway hyperresponsiveness and many other physiological processes (Figure 1). Several laboratories have developed small molecule CD38 inhibitors (Becherer, et al., 2015; Blacher, et al., 2015; Dong, et al., 2011; Escande, et al., 2013; Haffner, et al., 2015; Hara-Yokoyama, et al., 1996; Jiang, et al., 2009; Kellenberger, et al., 2011; Kwong, et al., 2012; Moreau, et al., 2013; Sauve & Schramm, 2002; Wall, et al., 1998; S. Wang, et al., 2014; Wu, et al., 2013; Zhou, et al., 2012). Currently over 200 compounds that inhibit CD38 have been reported (S. Zhang, et al., 2015). Chemically, these compounds are quite diverse and include NAD analogs, heterocyclic compounds and flavonoids (Table 2). The availability of the crystal structure of CD38 liganded with various substrates or products has aided in the rational design of CD38 inhibitors and in the identification of the key amino acid residues necessary for inhibitor interaction (Graeff, et al., 2006; Q. Liu, et al., 2005, 2006). The small molecule inhibitors can be categorized into two groups, covalent and non-covalent inhibitors (also known as irreversible and reversible inhibitors, respectively (Z. Liu, et al., 2013). The covalent group of inhibitors (also known as mechanism based inhibitors) take advantage of the catalytic mechanism of CD38 to form a covalent bond with the active site glutamic acid at position 226 of human CD38 (Sauve & Schramm, 2002). This group of inhibitors are NAD analogs with the 2’-position of the nicotinamide ribose modified with a fluoro-modified arabinose or with 2’-deoxyribose. These modifications can form the covalent reaction intermediate with CD38 but are hydrolyzed at a very slow rate, effectively inhibiting the enzyme activities of CD38 (Sauve & Schramm, 2002). The non-covalent inhibitors bind to the active site of CD38 in a reversible fashion. The covalent inhibitors in general are more potent than the non-covalent inhibitors described thus far. However, considering issues with stability and membrane permeability of the covalent inhibitors, it is thought that the non-covalent inhibitors will be better for future development as therapeutics and research tools (Z. Liu, et al., 2013; S. Zhang, et al., 2015).
Table 2.
Drugs targeting CD38 or CD38 regulated signaling pathways.
| Classification | Examples | Comments |
|---|---|---|
| CD38 inhibitors | Thiazoloquin(az)olin(on)es 4-amino-8-quinoline carboxamides Kuromanin (Flavinoid) K-rhein (Anthranoid) |
IC50=7nM against human CD38 (Becherer et al.) IC50=76nM against human CD38 (Haffner et al.) Inhibits CLL chemotaxis, adhesion and in vivo homing (Kellenberger et al.) Inhibition of glioma progress in a mouse model (Blacher et al.) |
| Monoclonal antibody |
Daratumumab | FDA approved for multiple myeloma. Stimulates complement-dependent cytoxicity and antibody-dependent cellular cytotoxicity (de Weers et al., and Laubach et al.) |
| cADPR antagonist |
8-bromo-cADPR | Attenuation of sepsis-induced organ damage in rats (Peng et al.) |
| NAADP antagonist |
NED-19 | Inhibition of muscarinic receptor-induced contractions of guinea pig tracheal contraction (Naylor et al.) |
Despite the growing number of small molecule CD38 inhibitors that have been reported, most of these compounds have low potency (IC50 of 10 µM or greater) and there has been very little data on the selectivity and biological activity of these drugs. This section will focus on CD38 inhibitors whose activity has been examined in biological systems.
Dong et al. synthesized a series of membrane permeant N-substituted nicotinamide derivatives and found several to have moderate ability to inhibit CD38 (Dong, et al., 2011). Two of these compounds were tested for their ability to relax agonist induced muscle contraction in rat or guinea pig models. Compound 4 was able to relax rat aortic rings previously contracted by phenylephrine (Dong, et al., 2011). A structurally similar compound that did not inhibit CD38 was ineffective in this system. Compounds 4 and 7 were able to relax guinea pig tracheal strips previously contracted with acetylcholine (Dong, et al., 2011). In both these systems, the potency of the compounds was higher in the in vivo systems than in the in vitro CD38 inhibition assays. This is most like due to the membrane permeability of the drugs, which allow them to concentrate within the cells (Dong, et al., 2011). Deng et al. tested compound 4 from this study in an ozone-induced airway hyperresponiveness mouse model and found that it was not effective (Deng, et al., 2013).
Two studies employing high throughput screening of large chemical libraries resulted in the identification of lead compounds that led to the synthesis of potent CD38 inhibitors with nanomolar potencies (Becherer, et al., 2015; Haffner, et al., 2015). Chemically these compounds were thiazoloquin(az)olin(on)es and 4-amino-8-quinoline carboxamides, respectively (Becherer, et al., 2015; Haffner, et al., 2015). Both studies chose compounds that effectively inhibited CD38 and also had promising pharmacokinetic data in mice (compound 78c, IC50 = 7.3 nM from Haffner et al. (Haffner, et al., 2015) and compound 1ah, IC50 = 115 nM and compound 1ai, IC50 = 46 nM from Becherer et al. (Becherer, et al., 2015)) to test for their ability to raise tissue NAD levels in a diet induced obesity mouse model, as an indication of CD38 inhibition. Compound 78c was able to increase NAD levels by 536% in liver after two hours (Haffner, et al., 2015). Compounds 1ai and 1ah were also effective in elevating NAD levels in liver (1483% and 515% after two hours by 1ai and 1ah, respectively (Becherer, et al., 2015). The compounds from these studies should be useful in studying their potential as therapeutics in a number of disease models, including asthma.
The usefulness of small molecule CD38 inhibitors has been tested in two other systems. Vaisitti et al. showed that kuromanin, a flavonoid CD38 inhibitor (Kellenberger, et al., 2011), effectively blocks the chemotaxis, adhesion and in vivo homing of chronic lymphocytic leukemia (CLL) cells (Vaisitti, et al., 2015). Blacher et al. recently identified the natural anthranoid, 4,5-dihydoxyanthraquinone-2-carboxylic acid (rhein) and its water soluble potassium salt (K-rhein) potent CD38 inhibitors (IC50s = 1.2 and 0.8 µM, respectively) (Blacher, et al., 2015). A glioma progression mouse model was used to show that K-rhein administration significantly inhibited glioma progression (Blacher, et al., 2015). This effect was observed in WT but not CD38 knockout mice, suggesting that the effect of K-rhein was due to inhibition of CD38 activity (Blacher, et al., 2015). These two studies provide further evidence that drugs that inhibit CD38 will be useful therapeutically.
A humanized monoclonal antibody against CD38, daratumumab, is FDA-approved for treatment of multiple myeloma (de Weers, et al., 2011; Laubach, et al., 2014). Multiple myeloma plasma cells express high levels of CD38. Daratumumab induces antibody dependent cell cytotoxicity and complement dependent cytotoxicity (de Weers, et al., 2011). The cells which mediate these responses to daratumumab are unknown.
It may also be possible to inhibit CD38 action by blocking its downstream signaling events. CD38 generates two signaling molecules, cADPR and NAADP, both of which are involved in calcium signaling. 8-bromo-cADPR is a cell permeant, competitive antagonist of cADPR action (Sethi, et al., 1997; Walseth & Lee, 1993). It has been used primarily in in vitro studies to dissect cADPR signaling pathways. Recently, Peng et al. demonstrated that the CD38/cADPR pathway was activated in sepsis using a cecal ligation and puncture rat model (Peng, Ai, et al., 2016). They also demonstrated that administration of 8-bromo-cADPR protected organs (heart, liver, kidneys) from sepsis-induced damage (Peng, Ai, et al., 2016; Peng, Zou, et al., 2016). These studies demonstrate that blocking cADPR action and/or CD38 activity may be useful in treating sepsis. NAADP signaling has been shown to be involved in muscarinic receptor-induced contraction of guinea pig trachea (Aley, et al., 2013), NED-19, a NAADP antagonist (Naylor, et al., 2009) blocked carbachol-induced contraction of guinea pig trachea. To date, there are no studies that describe the use of any of the CD38 inhibitors and/or antibodies in animal models of allergic airway disease. CD38 and the signaling pathways it generates (cADPR and NAADP) play a role in the pathophysiology of airway diseases and are therefore attractive drug targets. Drugs that target the enzyme activities and/or the actions (cADPR and NAADP antagonists) should prove useful in the mitigation of airway hyperresponsiveness in diseases such as asthma.
8. Summary and Future Directions
CD38, a bifunctional membrane protein, is expressed on immune cells as well as on airway mesenchymal cells such as ASM cells. Experimental data thus far suggest multiple roles of CD38-cADPR pathway in the pathogenesis of asthma including in the regulation of allergen-induced airway inflammation and airway hyperresponsiveness. Additional studies are needed to determine if CD38-cADPR plays a role in the regulation of features of airway remodeling such as ASM hyperplasia. Expression and enzyme activities of CD38 and genetic variations in the cd38 gene in healthy and asthmatic human subjects need to be established. Our studies on CD38-cADPR are restricted to immune cells and ASM cells. Functional role of CD38 in other airway cell types such as fibroblasts, mucus producing cells and epithelial cells needs to be investigated. The fact that these cells contribute significantly to asthma pathogenesis underscores the importance of studying CD38 in other airway cells. Multiple small molecule inhibitors described above have been developed for inhibiting CD38 function. These inhibitors can be exploited as novel therapeutics in asthma considering the multiple roles of CD38 in the asthma pathogenesis. In addition, the roles of microRNAs, specifically miR-140-3p and miR-708-5p, in the development and/or reversal of airway inflammation, airway hyperresponsiveness and airway remodeling following allergen sensitization and challenge is another area of future investigation.
Acknowledgments
Funding source:
The work presented in this review has been supported through grants from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors declare that there are no conflicts of interest.
References
- Aarhus R, Graeff RM, Dickey DM, Walseth TF, Lee HC. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J Biol Chem. 1995;270:30327–30333. doi: 10.1074/jbc.270.51.30327. [DOI] [PubMed] [Google Scholar]
- Al-Abady ZN, Durante B, John Moody A, Billington RA. Large changes in NAD levels associated with CD38 expression during HL-60 cell differentiation. Biochemical and Biophysical Research Communications. 2013;442:51–55. doi: 10.1016/j.bbrc.2013.10.170. [DOI] [PubMed] [Google Scholar]
- Albrieux M, Lee HC, Villaz M. Calcium signaling by cyclic ADP-ribose, NAADP, and inositol trisphosphate are involved in distinct functions in ascidian oocytes. J Biol Chem. 1998;273:14566–14574. doi: 10.1074/jbc.273.23.14566. [DOI] [PubMed] [Google Scholar]
- Aley PK, Singh N, Brailoiu GC, Brailoiu E, Churchill GC. Nicotinic acid adenine dinucleotide phosphate (NAADP) is a second messenger in muscarinic receptor-induced contraction of guinea pig trachea. J Biol Chem. 2013;288:10986–10993. doi: 10.1074/jbc.M113.458620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrashdan YA, Alkhouri H, Chen E, Lalor DJ, Poniris M, Henness S, Brightling CE, Burgess JK, Armour CL, Ammit AJ, Hughes JM. Asthmatic airway smooth muscle CXCL10 production: mitogen-activated protein kinase JNK involvement. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1118–L1127. doi: 10.1152/ajplung.00232.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammit AJ, Hoffman RK, Amrani Y, Lazaar AL, Hay DW, Torphy TJ, Penn RB, Panettieri RA., Jr Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth-muscle cells. Modulation by cyclic adenosine monophosphate. Am J Respir Cell Mol Biol. 2000;23:794–802. doi: 10.1165/ajrcmb.23.6.4184. [DOI] [PubMed] [Google Scholar]
- Ammit AJ, Lazaar AL, Irani C, O’Neill GM, Gordon ND, Amrani Y, Penn RB, Panettieri RA., Jr Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth muscle cells: modulation by glucocorticoids and beta-agonists. Am J Respir Cell Mol Biol. 2002;26:465–474. doi: 10.1165/ajrcmb.26.4.4681. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Walseth TF, Borgmann K, Wu L, Bidasee KR, Kannan MS, Ghorpade A. CD38/cyclic ADP-ribose regulates astrocyte calcium signaling: implications for neuroinflammation and HIV-1-associated dementia. J Neuroimmune Pharmacol. 2008;3:154–164. doi: 10.1007/s11481-008-9105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa MT, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P, Chini EN. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. Faseb j. 2007;21:3629–3639. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Becherer JD, Boros EE, Carpenter TY, Cowan DJ, Deaton DN, Haffner CD, Jeune MR, Kaldor IW, Poole JC, Preugschat F, Rheault TR, Schulte CA, Shearer BG, Shearer TW, Shewchuk LM, Smalley TL, Jr, Stewart EL, Stuart JD, Ulrich JC. Discovery of 4-Amino-8-quinoline Carboxamides as Novel, Submicromolar Inhibitors of NAD-Hydrolyzing Enzyme CD38. J Med Chem. 2015;58:7021–7056. doi: 10.1021/acs.jmedchem.5b00992. [DOI] [PubMed] [Google Scholar]
- Blacher E, Ben Baruch B, Levy A, Geva N, Green KD, Garneau-Tsodikova S, Fridman M, Stein R. Inhibition of glioma progression by a newly discovered CD38 inhibitor. Int J Cancer. 2015;136:1422–1433. doi: 10.1002/ijc.29095. [DOI] [PubMed] [Google Scholar]
- Burgess JK, Lee JH, Ge Q, Ramsay EE, Poniris MH, Parmentier J, Roth M, Johnson PR, Hunt NH, Black JL, Ammit AJ. Dual ERK and phosphatidylinositol 3-kinase pathways control airway smooth muscle proliferation: differences in asthma. J Cell Physiol. 2008;216:673–679. doi: 10.1002/jcp.21450. [DOI] [PubMed] [Google Scholar]
- Camacho-Pereira J, Tarrago MG, Chini CC, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, Chini EN. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016;23:1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Matsusue K, Misawa M. RhoA, a possible target for treatment of airway hyperresponsiveness in bronchial asthma. J Pharmacol Sci. 2010;114:239–247. doi: 10.1254/jphs.10r03cr. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Tanabe M, Goto K, Sakai H, Misawa M. Down-regulation of miR-133a contributes to up-regulation of Rhoa in bronchial smooth muscle cells. Am J Respir Crit Care Med. 2009;180:713–719. doi: 10.1164/rccm.200903-0325OC. [DOI] [PubMed] [Google Scholar]
- Chini EN. CD38 as a regulator of cellular NAD: a novel potential pharmacological target for metabolic conditions. Curr Pharm Des. 2009;15:57–63. doi: 10.2174/138161209787185788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini EN, Chini CC, Kato I, Takasawa S, Okamoto H. CD38 is the major enzyme responsible for synthesis of nicotinic acid-adenine dinucleotide phosphate in mammalian tissues. Biochem J. 2002;362:125–130. doi: 10.1042/0264-6021:3620125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Muchamuel T, Grimaldi JC, Muller-Steffner H, Randall TD, Lund FE, Murray R, Schuber F, Howard MC. Mice deficient for the ecto-nicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses. Blood. 1998;92:1324–1333. [PubMed] [Google Scholar]
- Copello JA, Qi Y, Jeyakumar LH, Ogunbunmi E, Fleischer S. Lack of effect of cADP-ribose and NAADP on the activity of skeletal muscle and heart ryanodine receptors. Cell Calcium. 2001;30:269–284. doi: 10.1054/ceca.2001.0235. [DOI] [PubMed] [Google Scholar]
- Damera G, Panettieri RA., Jr Does airway smooth muscle express an inflammatory phenotype in asthma? Br J Pharmacol. 2011;163:68–80. doi: 10.1111/j.1476-5381.2010.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargie PJ, Agre MC, Lee HC. Comparison of Ca2+ mobilizing activities of cyclic ADP-ribose and inositol trisphosphate. Cell Regul. 1990;1:279–290. doi: 10.1091/mbc.1.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, Peipp M, Valerius T, Slootstra JW, Mutis T, Bleeker WK, Anderson KC, Lokhorst HM, van de Winkel JG, Parren PW. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- Deaglio S, Capobianco A, Bergui L, Durig J, Morabito F, Duhrsen U, Malavasi F. CD38 is a signaling molecule in B-cell chronic lymphocytic leukemia cells. Blood. 2003;102:2146–2155. doi: 10.1182/blood-2003-03-0989. [DOI] [PubMed] [Google Scholar]
- Dekkers BG, Maarsingh H, Meurs H, Gosens R. Airway structural components drive airway smooth muscle remodeling in asthma. Proc Am Thorac Soc. 2009;6:683–692. doi: 10.1513/pats.200907-056DP. [DOI] [PubMed] [Google Scholar]
- Deng Z, Gao ZC, Ge HQ, Zhang LR, Zhou JJ, Zhu ZP, Wu DY, Sun SY, Chen L, Pu XP. Treatment responses of procaterol and CD38 inhibitors in an ozone-induced airway hyperresponsiveness mice model. Biol Pharm Bull. 2013;36:1348–1355. doi: 10.1248/bpb.b13-00290. [DOI] [PubMed] [Google Scholar]
- Deshpande DA, Dileepan M, Walseth TF, Subramanian S, Kannan MS. MicroRNA Regulation of Airway Inflammation and Airway Smooth Muscle Function: Relevance to Asthma. Drug Dev Res. 2015;76:286–295. doi: 10.1002/ddr.21267. [DOI] [PubMed] [Google Scholar]
- Deshpande DA, Dogan S, Walseth TF, Miller SM, Amrani Y, Panettieri RA, Kannan MS. Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: role of CD38/cyclic adenosine diphosphate ribose pathway. Am J Respir Cell Mol Biol. 2004;31:36–42. doi: 10.1165/rcmb.2003-0313OC. [DOI] [PubMed] [Google Scholar]
- Deshpande DA, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose-mediated Ca2+ signaling contributes to airway smooth muscle hyper-responsiveness. Faseb j. 2003;17:452–454. doi: 10.1096/fj.02-0450fje. [DOI] [PubMed] [Google Scholar]
- Deshpande DA, White TA, Guedes AG, Milla C, Walseth TF, Lund FE, Kannan MS. Altered airway responsiveness in CD38-deficient mice. Am J Respir Cell Mol Biol. 2005;32:149–156. doi: 10.1165/rcmb.2004-0243OC. [DOI] [PubMed] [Google Scholar]
- Dileepan M, Jude JA, Rao SP, Walseth TF, Panettieri RA, Subramanian S, Kannan MS. MicroRNA-708 regulates CD38 expression through signaling pathways JNK MAP kinase and PTEN/AKT in human airway smooth muscle cells. Respir Res. 2014;15:107. doi: 10.1186/s12931-014-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dileepan M, Sarver AE, Rao SP, Panettieri RA, Jr, Subramanian S, Kannan MS. MicroRNA Mediated Chemokine Responses in Human Airway Smooth Muscle Cells. PLoS One. 2016;11:e0150842. doi: 10.1371/journal.pone.0150842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Si YQ, Sun SY, Pu XP, Yang ZJ, Zhang LR, Zhang LH, Leung FP, Lam CM, Kwong AK, Yue J, Zhou Y, Kriksunov IA, Hao Q, Lee HC. Design, synthesis and biological characterization of novel inhibitors of CD38. Org Biomol Chem. 2011;9:3246–3257. doi: 10.1039/c0ob00768d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, O’Neil L, White TA, Sinclair DA, Chini EN. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes. 2013;62:1084–1093. doi: 10.2337/db12-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero E, Saccucci F, Malavasi F. The making of a leukocyte receptor: origin, genes and regulation of human CD38 and related molecules. Chem Immunol. 2000;75:1–19. doi: 10.1159/000058763. [DOI] [PubMed] [Google Scholar]
- Ghaffar O, Hamid Q, Renzi PM, Allakhverdi Z, Molet S, Hogg JC, Shore SA, Luster AD, Lamkhioued B. Constitutive and cytokine-stimulated expression of eotaxin by human airway smooth muscle cells. Am J Respir Crit Care Med. 1999;159:1933–1942. doi: 10.1164/ajrccm.159.6.9805039. [DOI] [PubMed] [Google Scholar]
- Graeff R, Liu Q, Kriksunov IA, Hao Q, Lee HC. Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine nicotinic acid adenine dinucleotide phosphate (NAADP) synthesis and hydrolysis activities. J Biol Chem. 2006;281:28951–28957. doi: 10.1074/jbc.M604370200. [DOI] [PubMed] [Google Scholar]
- Guedes AG, Deshpande DA, Dileepan M, Walseth TF, Panettieri RA, Jr, Subramanian S, Kannan MS. CD38 and airway hyper-responsiveness: studies on human airway smooth muscle cells and mouse models. Can J Physiol Pharmacol. 2015;93:145–153. doi: 10.1139/cjpp-2014-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes AG, Jude JA, Paulin J, Kita H, Lund FE, Kannan MS. Role of CD38 in TNF-alpha-induced airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol. 2008;294:L290–L299. doi: 10.1152/ajplung.00367.2007. [DOI] [PubMed] [Google Scholar]
- Guedes AG, Jude JA, Paulin J, Rivero-Nava L, Kita H, Lund FE, Kannan MS. Airway responsiveness in CD38-deficient mice in allergic airway disease: studies with bone marrow chimeras. Am J Physiol Lung Cell Mol Physiol. 2015;308:L485–L493. doi: 10.1152/ajplung.00227.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes AG, Paulin J, Rivero-Nava L, Kita H, Lund FE, Kannan MS. CD38-deficient mice have reduced airway hyperresponsiveness following IL-13 challenge. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1286–L1293. doi: 10.1152/ajplung.00187.2006. [DOI] [PubMed] [Google Scholar]
- Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- Haffner CD, Becherer JD, Boros EE, Cadilla R, Carpenter T, Cowan D, Deaton DN, Guo Y, Harrington W, Henke BR, Jeune MR, Kaldor I, Milliken N, Petrov KG, Preugschat F, Schulte C, Shearer BG, Shearer T, Smalley TL, Jr, Stewart EL, Stuart JD, Ulrich JC. Discovery, Synthesis, and Biological Evaluation of Thiazoloquin(az)olin(on)es as Potent CD38 Inhibitors. J Med Chem. 2015;58:3548–3571. doi: 10.1021/jm502009h. [DOI] [PubMed] [Google Scholar]
- Hara-Yokoyama M, Kukimoto I, Nishina H, Kontani K, Hirabayashi Y, Irie F, Sugiya H, Furuyama S, Katada T. Inhibition of NAD+ glycohydrolase and ADP-ribosyl cyclase activities of leukocyte cell surface antigen CD38 by gangliosides. J Biol Chem. 1996;271:12951–12955. doi: 10.1074/jbc.271.22.12951. [DOI] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Hershenson MB, Brown M, Camoretti-Mercado B, Solway J. Airway smooth muscle in asthma. Annu Rev Pathol. 2008;3:523–555. doi: 10.1146/annurev.pathmechdis.1.110304.100213. [DOI] [PubMed] [Google Scholar]
- Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- Jain D, Keslacy S, Tliba O, Cao Y, Kierstein S, Amin K, Panettieri RA, Jr, Haczku A, Amrani Y. Essential role of IFNbeta and CD38 in TNFalpha-induced airway smooth muscle hyper-responsiveness. Immunobiology. 2008;213:499–509. doi: 10.1016/j.imbio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Congleton J, Liu Q, Merchant P, Malavasi F, Lee HC, Hao Q, Yen A, Lin H. Mechanism-based small molecule probes for labeling CD38 on live cells. J Am Chem Soc. 2009;131:1658–1659. doi: 10.1021/ja808387g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John AE, Zhu YM, Brightling CE, Pang L, Knox AJ. Human airway smooth muscle cells from asthmatic individuals have CXCL8 hypersecretion due to increased NF-kappa B p65, C/EBP beta, and RNA polymerase II binding to the CXCL8 promoter. J Immunol. 2009;183:4682–4692. doi: 10.4049/jimmunol.0803832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jude JA, Dileepan M, Subramanian S, Solway J, Panettieri RA, Jr, Walseth TF, Kannan MS. miR-140-3p regulation of TNF-alpha-induced CD38 expression in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2012;303:L460–L468. doi: 10.1152/ajplung.00041.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jude JA, Solway J, Panettieri RA, Jr, Walseth TF, Kannan MS. Differential induction of CD38 expression by TNF-{alpha} in asthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2010;299:L879–L890. doi: 10.1152/ajplung.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BN, Deshpande DA, Tirumurugaan KG, Panettieri RA, Walseth TF, Kannan MS. Adenoviral mediated anti-sense CD38 attenuates TNF-alpha-induced changes in calcium homeostasis of human airway smooth muscle cells. Can J Physiol Pharmacol. 2005;83:799–804. doi: 10.1139/y05-081. [DOI] [PubMed] [Google Scholar]
- Kannan MS, Prakash YS, Brenner T, Mickelson JR, Sieck GC. Role of ryanodine receptor channels in Ca2+ oscillations of porcine tracheal smooth muscle. Am J Physiol. 1997;272:L659–L664. doi: 10.1152/ajplung.1997.272.4.L659. [DOI] [PubMed] [Google Scholar]
- Kellenberger E, Kuhn I, Schuber F, Muller-Steffner H. Flavonoids as inhibitors of human CD38. Bioorg Med Chem Lett. 2011;21:3939–3942. doi: 10.1016/j.bmcl.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Gaylinn BD, Denney GH, Somlyo AP. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1991;266:1708–1715. [PubMed] [Google Scholar]
- Kitazawa T, Masuo M, Somlyo AP. G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proc Natl Acad Sci U S A. 1991;88:9307–9310. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox AJ, Corbett L, Stocks J, Holland E, Zhu YM, Pang L. Human airway smooth muscle cells secrete vascular endothelial growth factor: up-regulation by bradykinin via a protein kinase C and prostanoid-dependent mechanism. Faseb j. 2001;15:2480–2488. doi: 10.1096/fj.01-0256com. [DOI] [PubMed] [Google Scholar]
- Kotlikoff MI, Kannan MS, Solway J, Deng KY, Deshpande DA, Dowell M, Feldman M, Green KS, Ji G, Johnston R, Lakser O, Lee J, Lund FE, Milla C, Mitchell RW, Nakai J, Rishniw M, Walseth TF, White TA, Wilson J, Xin HB, Woodruff PG. Methodologic advancements in the study of airway smooth muscle. J Allergy Clin Immunol. 2004;114:S18–S31. doi: 10.1016/j.jaci.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Kou W, Banerjee S, Eudy J, Smith LM, Persidsky R, Borgmann K, Wu L, Sakhuja N, Deshpande MS, Walseth TF, Ghorpade A. CD38 regulation in activated astrocytes: implications for neuroinflammation and HIV-1 brain infection. J Neurosci Res. 2009;87:2326–2339. doi: 10.1002/jnr.22060. [DOI] [PubMed] [Google Scholar]
- Kuhn AR, Schlauch K, Lao R, Halayko AJ, Gerthoffer WT, Singer CA. MicroRNA expression in human airway smooth muscle cells: role of miR-25 in regulation of airway smooth muscle phenotype. Am J Respir Cell Mol Biol. 2010;42:506–513. doi: 10.1165/rcmb.2009-0123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong AK, Chen Z, Zhang H, Leung FP, Lam CM, Ting KY, Zhang L, Hao Q, Zhang LH, Lee HC. Catalysis-based inhibitors of the calcium signaling function of CD38. Biochemistry. 2012;51:555–564. doi: 10.1021/bi201509f. [DOI] [PubMed] [Google Scholar]
- Laubach JP, Tai YT, Richardson PG, Anderson KC. Daratumumab granted breakthrough drug status. Expert Opin Investig Drugs. 2014;23:445–452. doi: 10.1517/13543784.2014.889681. [DOI] [PubMed] [Google Scholar]
- Lee HC. A unified mechanism of enzymatic synthesis of two calcium messengers: cyclic ADP-ribose and NAADP. Biol Chem. 1999;380:785–793. doi: 10.1515/BC.1999.098. [DOI] [PubMed] [Google Scholar]
- Lee HC. Structure and enzymatic functions of human CD38. Mol Med. 2006;12:317–323. doi: 10.2119/2006-00086.Lee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC. Cyclic ADP-ribose and NAADP: fraternal twin messengers for calcium signaling. Sci China Life Sci. 2011;54:699–711. doi: 10.1007/s11427-011-4197-3. [DOI] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 1991;2:203–209. doi: 10.1091/mbc.2.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kriksunov IA, Graeff R, Munshi C, Lee HC, Hao Q. Crystal structure of human CD38 extracellular domain. Structure. 2005;13:1331–1339. doi: 10.1016/j.str.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Liu Q, Kriksunov IA, Graeff R, Munshi C, Lee HC, Hao Q. Structural basis for the mechanistic understanding of human CD38-controlled multiple catalysis. J Biol Chem. 2006;281:32861–32869. doi: 10.1074/jbc.M606365200. [DOI] [PubMed] [Google Scholar]
- Liu Z, Graeff RM, Jin H, Zhang L, Zhang L. Studies on CD38 Inhibitors and Their Application to cADPR-Mediated Ca2 + Signaling. Messenger. 2013;2:19–32. [Google Scholar]
- Lu TX, Munitz A, Rothenberg ME. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J Immunol. 2009;182:4994–5002. doi: 10.4049/jimmunol.0803560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund FE. Signaling properties of CD38 in the mouse immune system: enzyme-dependent and -independent roles in immunity. Mol Med. 2006;12:328–333. doi: 10.2119/2006-00099.Lund. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund FE, Muller-Steffner H, Romero-Ramirez H, Moreno-Garcia ME, Partida-Sanchez S, Makris M, Oppenheimer NJ, Santos-Argumedo L, Schuber F. CD38 induces apoptosis of a murine pro-B leukemic cell line by a tyrosine kinase-dependent but ADP-ribosyl cyclase- and NAD glycohydrolase-independent mechanism. Int Immunol. 2006;18:1029–1042. doi: 10.1093/intimm/dxl037. [DOI] [PubMed] [Google Scholar]
- Majid A, Lin TT, Best G, Fishlock K, Hewamana S, Pratt G, Yallop D, Buggins AG, Wagner S, Kennedy BJ, Miall F, Hills R, Devereux S, Oscier DG, Dyer MJ, Fegan C, Pepper C. CD49d is an independent prognostic marker that is associated with CXCR4 expression in CLL. Leuk Res. 2011;35:750–756. doi: 10.1016/j.leukres.2010.10.022. [DOI] [PubMed] [Google Scholar]
- Malavasi F, Deaglio S, Damle R, Cutrona G, Ferrarini M, Chiorazzi N. CD38 and chronic lymphocytic leukemia: a decade later. Blood. 2011;118:3470–3478. doi: 10.1182/blood-2011-06-275610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmhall C, Alawieh S, Lu Y, Sjostrand M, Bossios A, Eldh M, Radinger M. MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung. J Allergy Clin Immunol. 2014;133:1429–1438. doi: 10.1016/j.jaci.2013.11.008. 1438.e1421-1427. [DOI] [PubMed] [Google Scholar]
- Mamik MK, Banerjee S, Walseth TF, Hirte R, Tang L, Borgmann K, Ghorpade A. HIV-1 and IL-1beta regulate astrocytic CD38 through mitogen-activated protein kinases and nuclear factor-kappaB signaling mechanisms. J Neuroinflammation. 2011;8:145. doi: 10.1186/1742-2094-8-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes J, Collison A, Plank M, Phipps S, Foster PS. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci U S A. 2009;106:18704–18709. doi: 10.1073/pnas.0905063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta K, Cheema S. Retinoid-mediated signaling pathways in CD38 antigen expression in myeloid leukemia cells. Leuk Lymphoma. 1999;32:441–449. doi: 10.3109/10428199909058401. [DOI] [PubMed] [Google Scholar]
- Morabito F, Mangiola M, Stelitano C, Deaglio S, Callea V, Malavasi F. Peripheral blood CD38 expression predicts time to progression in B-cell chronic lymphocytic leukemia after first-line therapy with high-dose chlorambucil. Haematologica. 2002;87:217–218. [PubMed] [Google Scholar]
- Moreau C, Liu Q, Graeff R, Wagner GK, Thomas MP, Swarbrick JM, Shuto S, Lee HC, Hao Q, Potter BV. CD38 Structure-Based Inhibitor Design Using the 1-Cyclic Inosine 5’-Diphosphate Ribose Template. PLoS One. 2013;8:e66247. doi: 10.1371/journal.pone.0066247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi C, Aarhus R, Graeff R, Walseth TF, Levitt D, Lee HC. Identification of the enzymatic active site of CD38 by site-directed mutagenesis. J Biol Chem. 2000;275:21566–21571. doi: 10.1074/jbc.M909365199. [DOI] [PubMed] [Google Scholar]
- Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, Galione A, Churchill GC. Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nipp RD, Volkheimer AD, Davis ED, Chen Y, Weinberg JB, Friedman DR. CD38 variation as a prognostic factor in chronic lymphocytic leukemia. Leuk Lymphoma. 2014;55:191–194. doi: 10.3109/10428194.2013.786070. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Takasawa S, Nata K. The CD38-cyclic ADP-ribose signaling system in insulin secretion: molecular basis and clinical implications. Diabetologia. 1997;40:1485–1491. doi: 10.1007/s001250050854. [DOI] [PubMed] [Google Scholar]
- Ozawa T. Elucidation of the ryanodine-sensitive Ca2+ release mechanism of rat pancreatic acinar cells: modulation by cyclic ADP-ribose and FK506. Biochim Biophys Acta. 2004;1693:159–166. doi: 10.1016/j.bbamcr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Ozawa T. FK506 induces biphasic Ca2+ release from microsomal vesicles of rat pancreatic acinar cells. Int J Mol Med. 2006;18:187–191. [PubMed] [Google Scholar]
- Ozier A, Allard B, Bara I, Girodet PO, Trian T, Marthan R, Berger P. The pivotal role of airway smooth muscle in asthma pathophysiology. J Allergy (Cairo) 2011;2011:742710. doi: 10.1155/2011/742710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabelick CM, Prakash YS, Kannan MS, Jones KA, Warner DO, Sieck GC. Effect of halothane on intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol. 1999;276:L81–L89. doi: 10.1152/ajplung.1999.276.1.L81. [DOI] [PubMed] [Google Scholar]
- Panettieri RA, Jr, Lazaar AL, Pure E, Albelda SM. Activation of cAMP-dependent pathways in human airway smooth muscle cells inhibits TNF-alpha-induced ICAM-1 and VCAM-1 expression and T lymphocyte adhesion. J Immunol. 1995;154:2358–2365. [PubMed] [Google Scholar]
- Partida-Sanchez S, Rivero-Nava L, Shi G, Lund FE. CD38: an ecto-enzyme at the crossroads of innate and adaptive immune responses. Adv Exp Med Biol. 2007;590:171–183. doi: 10.1007/978-0-387-34814-8_12. [DOI] [PubMed] [Google Scholar]
- Peng QY, Ai ML, Zhang LN, Zou Y, Ma XH, Ai YH. Blocking NAD(+)/CD38/cADPR/Ca(2+) pathway in sepsis prevents organ damage. J Surg Res. 2016;201:480–489. doi: 10.1016/j.jss.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Peng QY, Zou Y, Zhang LN, Ai ML, Liu W, Ai YH. Blocking Cyclic Adenosine Diphosphate Ribose-mediated Calcium Overload Attenuates Sepsis-induced Acute Lung Injury in Rats. Chin Med J (Engl) 2016;129:1725–1730. doi: 10.4103/0366-6999.185854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol. 2013;305:L912–L933. doi: 10.1152/ajplung.00259.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Kannan MS, Sieck GC. Nitric oxide inhibits ACh-induced intracellular calcium oscillations in porcine tracheal smooth muscle. Am J Physiol. 1997a;272:L588–L596. doi: 10.1152/ajplung.1997.272.4.L588. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Kannan MS, Sieck GC. Regulation of intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol. 1997b;272:C966–C975. doi: 10.1152/ajpcell.1997.272.3.C966. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Kannan MS, Walseth TF, Sieck GC. Role of cyclic ADP-ribose in the regulation of [Ca2+]i in porcine tracheal smooth muscle. Am J Physiol. 1998;274:C1653–C1660. doi: 10.1152/ajpcell.1998.274.6.C1653. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Pabelick CM, Kannan MS, Sieck GC. Spatial and temporal aspects of ACh-induced [Ca2+]i oscillations in porcine tracheal smooth muscle. Cell Calcium. 2000;27:153–162. doi: 10.1054/ceca.1999.0106. [DOI] [PubMed] [Google Scholar]
- Prakash YS, van der Heijden HF, Kannan MS, Sieck GC. Effects of salbutamol on intracellular calcium oscillations in porcine airway smooth muscle. J Appl Physiol (1985) 1997;82:1836–1843. doi: 10.1152/jappl.1997.82.6.1836. [DOI] [PubMed] [Google Scholar]
- Sauve AA, Schramm VL. Mechanism-based inhibitors of CD38: a mammalian cyclic ADP-ribose synthetase. Biochemistry. 2002;41:8455–8463. doi: 10.1021/bi0258795. [DOI] [PubMed] [Google Scholar]
- Schuber F, Lund FE. Structure and enzymology of ADP-ribosyl cyclases: conserved enzymes that produce multiple calcium mobilizing metabolites. Curr Mol Med. 2004;4:249–261. doi: 10.2174/1566524043360708. [DOI] [PubMed] [Google Scholar]
- Sethi JK, Empson RM, Bailey VC, Potter BV, Galione A. 7-Deaza-8-bromo-cyclic ADP-ribose, the first membrane-permeant, hydrolysis-resistant cyclic ADP-ribose antagonist. J Biol Chem. 1997;272:16358–16363. doi: 10.1074/jbc.272.26.16358. [DOI] [PubMed] [Google Scholar]
- Shirazi A, Iizuka K, Fadden P, Mosse C, Somlyo AP, Somlyo AV, Haystead TA. Purification and characterization of the mammalian myosin light chain phosphatase holoenzyme. The differential effects of the holoenzyme and its subunits on smooth muscle. J Biol Chem. 1994;269:31598–31606. [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Song H, Xi J, Li GG, Xu S, Wang C, Cheng T, Li H, Zhang Y, Liu X, Bai J. Upregulation of CD19(+)CD24(hi)CD38(hi) regulatory B cells is associated with a reduced risk of acute lung injury in elderly pneumonia patients. Intern Emerg Med. 2016;11:415–423. doi: 10.1007/s11739-015-1377-3. [DOI] [PubMed] [Google Scholar]
- States DJ, Walseth TF, Lee HC. Similarities in amino acid sequences of Aplysia ADP-ribosyl cyclase and human lymphocyte antigen CD38. Trends Biochem Sci. 1992;17:495. doi: 10.1016/0968-0004(92)90337-9. [DOI] [PubMed] [Google Scholar]
- Sun L, Iqbal J, Zaidi S, Zhu LL, Zhang X, Peng Y, Moonga BS, Zaidi M. Structure and functional regulation of the CD38 promoter. Biochem Biophys Res Commun. 2006;341:804–809. doi: 10.1016/j.bbrc.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Tang WX, Chen YF, Zou AP, Campbell WB, Li PL. Role of FKBP12.6 in cADPR-induced activation of reconstituted ryanodine receptors from arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2002;282:H1304–H1310. doi: 10.1152/ajpheart.00843.2001. [DOI] [PubMed] [Google Scholar]
- Tirumurugaan KG, Kang BN, Panettieri RA, Foster DN, Walseth TF, Kannan MS. Regulation of the cd38 promoter in human airway smooth muscle cells by TNF-alpha and dexamethasone. Respir Res. 2008;9:26. doi: 10.1186/1465-9921-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tliba O, Cidlowski JA, Amrani Y. CD38 expression is insensitive to steroid action in cells treated with tumor necrosis factor-alpha and interferon-gamma by a mechanism involving the up-regulation of the glucocorticoid receptor beta isoform. Mol Pharmacol. 2006;69:588–596. doi: 10.1124/mol.105.019679. [DOI] [PubMed] [Google Scholar]
- Tliba O, Panettieri RA., Jr Noncontractile functions of airway smooth muscle cells in asthma. Annu Rev Physiol. 2009;71:509–535. doi: 10.1146/annurev.physiol.010908.163227. [DOI] [PubMed] [Google Scholar]
- Tliba O, Panettieri RA, Jr, Tliba S, Walseth TF, Amrani Y. Tumor necrosis factor-alpha differentially regulates the expression of proinflammatory genes in human airway smooth muscle cells by activation of interferon-beta-dependent CD38 pathway. Mol Pharmacol. 2004;66:322–329. doi: 10.1124/mol.104.001040. [DOI] [PubMed] [Google Scholar]
- Vaisitti T, Audrito V, Serra S, Buonincontri R, Sociali G, Mannino E, Pagnani A, Zucchetto A, Tissino E, Vitale C, Coscia M, Usai C, Pepper C, Gattei V, Bruzzone S, Deaglio S. The enzymatic activities of CD38 enhance CLL growth and trafficking: implications for therapeutic targeting. Leukemia. 2015;29:356–368. doi: 10.1038/leu.2014.207. [DOI] [PubMed] [Google Scholar]
- Venturi E, Pitt S, Galfre E, Sitsapesan R. From eggs to hearts: what is the link between cyclic ADP-ribose and ryanodine receptors? Cardiovasc Ther. 2012;30:109–116. doi: 10.1111/j.1755-5922.2010.00236.x. [DOI] [PubMed] [Google Scholar]
- Wall KA, Klis M, Kornet J, Coyle D, Ame JC, Jacobson MK, Slama JT. Inhibition of the intrinsic NAD+ glycohydrolase activity of CD38 by carbocyclic NAD analogues. Biochem J. 1998;335(Pt 3)):631–636. doi: 10.1042/bj3350631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth TF, Lee HC. Synthesis and characterization of antagonists of cyclic-ADP-ribose-induced Ca2+ release. Biochim Biophys Acta. 1993;1178:235–242. doi: 10.1016/0167-4889(93)90199-y. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhu W, Wang X, Li J, Zhang K, Zhang L, Zhao YJ, Lee HC, Zhang L. Design, synthesis and SAR studies of NAD analogues as potent inhibitors towards CD38 NADase. Molecules. 2014;19:15754–15767. doi: 10.3390/molecules191015754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Zheng YM, Mei QB, Wang QS, Collier ML, Fleischer S, Xin HB, Kotlikoff MI. FKBP12.6 and cADPR regulation of Ca2+ release in smooth muscle cells. Am J Physiol Cell Physiol. 2004;286:C538–C546. doi: 10.1152/ajpcell.00106.2003. [DOI] [PubMed] [Google Scholar]
- Wei WJ, Sun HY, Ting KY, Zhang LH, Lee HC, Li GR, Yue J. Inhibition of cardiomyocytes differentiation of mouse embryonic stem cells by CD38/cADPR/Ca2+ signaling pathway. J Biol Chem. 2012;287:35599–35611. doi: 10.1074/jbc.M112.392530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TA, Johnson S, Walseth TF, Lee HC, Graeff RM, Munshi CB, Prakash YS, Sieck GC, Kannan MS. Subcellular localization of cyclic ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities in porcine airway smooth muscle. Biochim Biophys Acta. 2000;1498:64–71. doi: 10.1016/s0167-4889(00)00077-x. [DOI] [PubMed] [Google Scholar]
- White TA, Kannan MS, Walseth TF. Intracellular calcium signaling through the cADPR pathway is agonist specific in porcine airway smooth muscle. Faseb j. 2003;17:482–484. doi: 10.1096/fj.02-0622fje. [DOI] [PubMed] [Google Scholar]
- Wu D, Ting K, Duan Y, Li N, Li J, Zhang L, Lee H, Zhang L. Synthesis and activity of novel indole derivatives as inhibitors of CD38. Acta Pharmaceutica Sinica B. 2013;3:245–253. [Google Scholar]
- Xu M, Li XX, Wang L, Wang M, Zhang Y, Li PL. Contribution of Nrf2 to Atherogenic Phenotype Switching of Coronary Arterial Smooth Muscle Cells Lacking CD38 Gene. Cell Physiol Biochem. 2015;37:432–444. doi: 10.1159/000430366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GS, Choleris E, Lund FE, Kirkland JB. Decreased cADPR and increased NAD+ in the Cd38−/− mouse. Biochem Biophys Res Commun. 2006;346:188–192. doi: 10.1016/j.bbrc.2006.05.100. [DOI] [PubMed] [Google Scholar]
- Zhang K, Shan L, Rahman MS, Unruh H, Halayko AJ, Gounni AS. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: role in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L375–L382. doi: 10.1152/ajplung.00045.2007. [DOI] [PubMed] [Google Scholar]
- Zhang S, Xue X, Zhang L, Zhang L, Liu Z. Comparative Analysis of Pharmacophore Features and Quantitative Structure-Activity Relationships for CD38 Covalent and Non-covalent Inhibitors. Chem Biol Drug Des. 2015;86:1411–1424. doi: 10.1111/cbdd.12606. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tallini YN, Chen Z, Gan L, Wei B, Doran R, Miao L, Xin HB, Kotlikoff MI, Ji G. Dissociation of FKBP12.6 from ryanodine receptor type 2 is regulated by cyclic ADP-ribose but not beta-adrenergic stimulation in mouse cardiomyocytes. Cardiovasc Res. 2009;84:253–262. doi: 10.1093/cvr/cvp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu M, Xia M, Li X, Boini KM, Wang M, Gulbins E, Ratz PH, Li PL. Defective autophagosome trafficking contributes to impaired autophagic flux in coronary arterial myocytes lacking CD38 gene. Cardiovasc Res. 2014;102:68–78. doi: 10.1093/cvr/cvu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YJ, Lam CM, Lee HC. The membrane-bound enzyme CD38 exists in two opposing orientations. Sci Signal. 2012;5:ra67. doi: 10.1126/scisignal.2002700. [DOI] [PubMed] [Google Scholar]
- Zhao YJ, Zhu WJ, Wang XW, Zhang LH, Lee HC. Determinants of the membrane orientation of a calcium signaling enzyme CD38. Biochim Biophys Acta. 2015;1853:2095–2103. doi: 10.1016/j.bbamcr.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ting KY, Lam CM, Kwong AK, Xia J, Jin H, Liu Z, Zhang L, Cheung Lee H, Zhang L. Design, synthesis and biological evaluation of noncovalent inhibitors of human CD38 NADase. ChemMedChem. 2012;7:223–228. doi: 10.1002/cmdc.201100487. [DOI] [PubMed] [Google Scholar]
- Zocchi E, Franco L, Guida L, Benatti U, Bargellesi A, Malavasi F, Lee HC, De Flora A. A single protein immunologically identified as CD38 displays NAD+ glycohydrolase, ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities at the outer surface of human erythrocytes. Biochem Biophys Res Commun. 1993;196:1459–1465. doi: 10.1006/bbrc.1993.2416. [DOI] [PubMed] [Google Scholar]
- Zucchetto A, Vaisitti T, Benedetti D, Tissino E, Bertagnolo V, Rossi D, Bomben R, Dal Bo M, Del Principe MI, Gorgone A, Pozzato G, Gaidano G, Del Poeta G, Malavasi F, Deaglio S, Gattei V. The CD49d/CD29 complex is physically and functionally associated with CD38 in B-cell chronic lymphocytic leukemia cells. Leukemia. 2012;26:1301–1312. doi: 10.1038/leu.2011.369. [DOI] [PubMed] [Google Scholar]