Abstract
Erythema nodosum leprosum (ENL) is a painful inflammatory complication of leprosy occurring in 50% of lepromatous leprosy patients and 5–10% of borderline lepromatous patients. It is a significant cause of economic hardship, morbidity and mortality in leprosy patients. Our understanding of the causes of ENL is limited. We performed a systematic review of the published literature and critically evaluated the evidence for the role of neutrophils, immune complexes (ICs), T-cells, cytokines, and other immunological factors that could contribute to the development of ENL. Searches of the literature were performed in PubMed. Studies, independent of published date, using samples from patients with ENL were included. The search revealed more than 20,000 articles of which 146 eligible studies were included in this systematic review. The studies demonstrate that ENL may be associated with a neutrophilic infiltrate, but it is not clear whether it is an IC-mediated process or that the presence of ICs is an epiphenomenon. Increased levels of tumor necrosis factor-α and other pro-inflammatory cytokines support the role of this cytokine in the inflammatory phase of ENL but not necessarily the initiation. T-cell subsets appear to be important in ENL since multiple studies report an increased CD4+/CD8+ ratio in both skin and peripheral blood of patients with ENL. Microarray data have identified new molecules and whole pathophysiological pathways associated with ENL and provides new insights into the pathogenesis of ENL. Studies of ENL are often difficult to compare due to a lack of case definitions, treatment status, and timing of sampling as well as the use of different laboratory techniques. A standardized approach to some of these issues would be useful. ENL appears to be a complex interaction of various aspects of the immune system. Rigorous clinical descriptions of well-defined cohorts of patients and a systems biology approach using available technologies such as genomics, epigenomics, transcriptomics, and proteomics could yield greater understanding of the condition.
Keywords: erythema nodosum leprosum, leprosy, type 2 reaction, immunology, systematic review, TNF-α, neutrophils, immune complexes
Introduction
Leprosy is an infectious disease predominantly of skin and peripheral nerves, caused by the obligate, intracellular, acid-fast bacillus Mycobacterium leprae. The organism shows tropism for macrophages and Schwann cells (1). The pathology and clinical phenotype of leprosy is determined by the host immune response to M. leprae (2). Patients develop leprosy on a clinical spectrum ranging from tuberculoid leprosy through borderline forms to lepromatous leprosy (LL) of the Ridley–Jopling classification (2). Patients with tuberculoid leprosy have a strong cell-mediated immune response to M. leprae limiting the disease to a few well-defined skin lesions and/or peripheral nerves (3). Patients with LL have absent cellular immunity and high titers of antibodies against M. leprae, which are not effective in controlling the bacilli (4).
Multi-drug therapy (MDT) is highly effective for treating the infection (1). However, despite this, 30–40% of patients with leprosy undergo immune-mediated inflammatory episodes such as Type 1 reactions (T1R) and erythema nodosum leprosum (ENL or Type 2 reactions) (5).
ENL is a painful inflammatory complication occurring in 50% of LL patients and 5–10% of borderline lepromatous leprosy (BL) patients particularly those with a bacterial index above 4 (6), whereas T1R predominantly affect those with borderline tuberculoid leprosy (BT), mid-borderline, and BL leprosy. Individuals with ENL present crops of painful, erythematous skin nodules with systemic symptoms of fever and malaise (6). ENL is a multisystem disorder and other organ involvement includes iritis, arthritis, lymphadenitis, orchitis, and neuritis (6). The histology of ENL skin lesions often shows an intense perivascular infiltrate of neutrophils throughout the dermis and subcutis (7) and vasculitis with edema of the endothelium together with granulocyte infiltration of vessels walls (8–10). However, not all ENL skin biopsies show evidence of vasculitis (10–13).
ENL is usually treated with high-dose oral corticosteroids or thalidomide if it is available and affordable. High doses of clofazimine are also commonly used (6). Treatment often lasts for many months or years. Few patients experience a single episode of acute ENL with the majority experiencing recurrent or chronic disease (6, 14). Prolonged use of oral corticosteroids is associated with multiple adverse effects (6). Our group has demonstrated that ENL results in significant economic hardship, morbidity, and mortality in patients (15, 16).
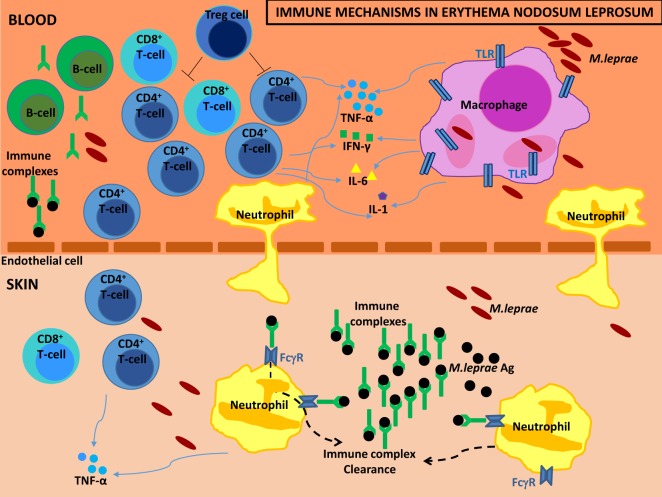
ENL is often described as a neutrophilic immune-complex-mediated condition, while there is evidence that T-cells further complicate the immunopathology. Elevated levels of certain cytokines such as tumor necrosis factor (TNF)-α and other immunological factors have been associated with episodes of ENL.
We performed a systematic review of the published literature and critically evaluated the current evidence for the role of immunological factors that have been associated with the ENL. We created a flowchart showing our search strategy by identifying the studies to be included in this systematic review (Figure 1). We divided the systematic review into sections according to the immune parameter under investigation including neutrophils, immune complexes/complement, T-cells, and cytokines. Furthermore, we sought to identify possible methodological issues that might account for discrepancies between studies and to make recommendations for future immunological studies of ENL. The studies that we considered to have the most important findings are discussed in detail, while all the studies included in the review are summarized in the comprehensive tables.
Figure 1.
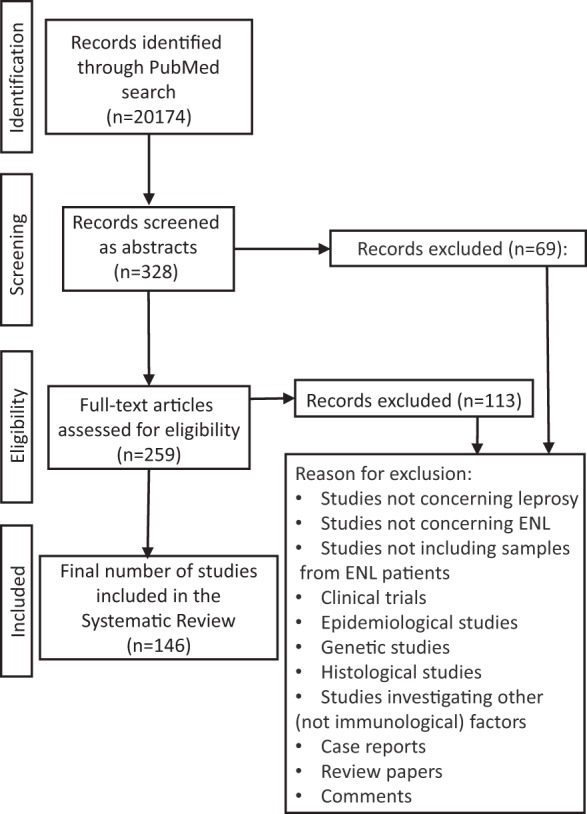
Flowchart. Flowchart of included studies.
Methodology
The Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) 2015 guideline was used to prepare this systematic review (17).
Searching
Searches of the literature were performed up to 31st October 2016 in PubMed by the first author. Keywords used were: Hansen* OR Type 2 OR Type II OR leprosy OR lepra*, AND reaction OR erythema nodosum leprosum OR ENL. The references included in each study were also checked for potentially relevant publications.
Inclusion Criteria
Immunological studies in PubMed, independent of published date, using samples from patients with ENL were included. Human samples including sera, peripheral blood mononuclear cells (PBMC), skin biopsies, or any other tissue were eligible for inclusion. Publications in languages other than English were translated.
An immunological study was defined as any study of the molecular and cellular components that comprise the immune system, including their function and interaction.
Results of Search
The search in PubMed revealed 95,771 records, which were narrowed down by using restrictions, species (humans), and search fields (title/abstracts), leading to 20,174 records (Figure 1). A total of 19,846 studies were excluded by title because they did not address leprosy or ENL. Others were excluded because they did not include samples from ENL patients or they were clinical trials, epidemiological studies, case reports, review papers, commentaries, histological studies, genetic studies, and investigations of non-immunological factors. The abstracts of the remaining 328 titles were reviewed and a further 69 studies were excluded due to the same considerations.
The 259 papers were obtained full text of which 113 were excluded for the reasons described above. When there was doubt about studies, the first and second author agreed on whether they should be included in the systematic review. Data were extracted from the 146 eligible studies. Of these 146 eligible studies, 5 studies investigated the role of neutrophils in ENL, 28 studies investigated the role of immune complexes and complement in ENL, 44 studies investigated the role of T-cells in ENL, and 49 studies investigated the role of cytokines in ENL, of which 30 investigated the role of TNF-α in ENL. Sixty-four studies investigated the role of other immunological factors in ENL.
Data Synthesis and Analysis
Data extraction from each study was conducted by the first author. Structured forms were designed for each of the five main sections of the systematic review: neutrophils, immune complexes and complement, T-cellular immunity, cytokines, and other immunological molecules or factors involved in the pathophysiology of ENL. Data were collected on the setting (study location and country of affiliation of the authors), study design and characteristics of the subjects (ENL case definition, study population included, number of patients with ENL, control subjects, timing of sampling, treatment for ENL and leprosy treatment), study measures and main findings reported by the study authors. A study could include multiple measures and therefore be part of more than one section of the systematic review.
What is the Role of Neutrophils in ENL?
Neutrophils are the predominant immune cell population in human blood and provide protection through phagocytosis, generation of neutrophil extracellular traps (NETs), and secretion of antimicrobial peptides (18). Recent evidence supports a role for neutrophils in the orchestration of adaptive immunity, engaged with lymphocytes and antigen-presenting cells (APCs) (19).
Neutrophils are considered to be the histological hallmark of ENL (7, 13). The histology of ENL skin lesions shows an intense perivascular infiltrate of neutrophils throughout the dermis and subcutis (7, 13). However, not all ENL lesions are characterized by the presence of neutrophils (12, 20–22) and the timing of biopsies appears crucial in detecting neutrophil infiltration (7, 23). A study of skin biopsies of ENL lesions within 72 h of onset showed a predominance of neutrophils in 30.4% of biopsies. Skin biopsies performed between 9 and 12 days showed neutrophils in 1.6% of specimens and increasing numbers of lymphocytes, plasma cells, and histiocytes (7). Neutrophils may precede the chemotaxis of lymphocytes into ENL lesions, but it is unclear why neutrophils are not always present in the initial stage of ENL.
The study by Lee et al. used DNA microarray and bioinformatic pathway analysis of gene expression profiles in skin biopsies obtained from six patients with ENL compared to seven LL controls (24). They identified 57 functional groups and 17 canonical pathways characteristic of ENL. Their striking finding was the “cell movement” functional pathway composed of 188 genes. From the list of genes of the “cell movement” pathway, 25 were identified to be involved specifically in neutrophil recruitment including the genes for P-selectin, E-selectin, and its ligands (24). Using immunohistochemistry, they showed that E-selectin was expressed in a vascular pattern and at higher levels in ENL skin lesions than in LL, although this was not quantified (24). They described an integrated pathway of TLR2/Fc Receptor activation triggering induction of interleukin (IL)-1β, which together with interferon (IFN)-γ, induced E-selectin expression on endothelial cells and neutrophil migration and adhesion to endothelial cells (24). Interestingly, thalidomide inhibited this neutrophil recruitment pathway (24).
A recent Brazilian study reported that surface CD64 (FcγRI) expression on circulating neutrophils increased significantly during ENL, while BL/LL patients without ENL had lower levels of CD64 (25). In addition, CD64 expression on neutrophils decreased after thalidomide treatment (25). Moreover, the higher levels of CD64 on circulating neutrophils were correlated with disease severity (25). This study demonstrated the potential of CD64 as an early biomarker for ENL and as a marker of severity (25). CD64 (FcγRI) is the high-affinity receptor for monomeric IgG1 and IgG3 (26). While resting neutrophils express low levels of CD64 (26), an increase of neutrophil CD64 surface expression is observed in certain Gram negative bacterial infections (27) and has been associated with the prognosis of disseminated intravascular coagulation during sepsis (28). The authors suggested that CD64 upregulation during ENL could be due to the presence of inflammatory cytokines such as IFN-γ and GM-CSF (29) or certain intracellular components of fragmented M. leprae bacilli following treatment with MDT (25). This was further supported by clinical studies showing that although ENL may also occur before initiation of treatment with MDT, the incidence of ENL is higher during treatment with MDT (5, 30).
Studies in the 70s tried to assess the polymorphonuclear leukocyte (PMN) functions in different forms of leprosy and ENL, investigating whether ENL is associated with PMN activation (31, 32). The nitro blue tetrazolium (NBT) test that measures PMN activation was increased in six patients described as LL with leprosy reactions compared with non-reactional leprosy patients (from across the leprosy spectrum) and healthy controls (31). In addition, LL patients with reactions had lower PMN activation when treated with steroids or thalidomide, although this was not significant (31). Another study found the resting NBT levels in different leprosy groups (tuberculoid, lepromatous, and patients with ENL) to be within normal limits (32). However, the sera from patients with ENL produced significantly increased levels of PMN activation as measured by the NBT test when incubated with PMN cells from healthy controls and patients with ENL (32). This finding suggested that sera from ENL patients may lead to activation of neutrophils. However, when cell motility was studied as a marker of PMN activation using random migration, chemotaxis, and chemokinesis, all three were defective in lepromatous patients with or without complicating ENL (32).
Oliveira et al. reported the apoptotic rate of neutrophils to be greatly accelerated in ENL patients compared to BL/LL patients and healthy volunteers (33). Neutrophils isolated from leprosy patients (ENL and BL/LL) released TNF-α and IL-8, after stimulation with lipopolysaccharide (LPS) or M. leprae (33). Interestingly, in vitro TNF-α production by neutrophils was inhibited by thalidomide at both 3 and 6 h post-stimulation with LPS (33). This supports the role of neutrophils as effector cells actively producing pro-inflammatory cytokines and not only as migratory cells following chemoattractants.
There is little direct evidence of the actual role of neutrophils in ENL, despite the cell being the histological hallmark of ENL. There are multiple histological studies showing the presence of neutrophils in ENL lesions; however, only five studies investigated whether neutrophils actively take part in ENL as effector cells (Table 1). It remains unclear whether the neutrophil initiates ENL or is recruited to the site of the affected skin lesion under the action of chemokines such as IL-8 secreted by other cell types.
Table 1.
Studies of neutrophils in ENL.
| Reference; study site(s) | Study population | Timing of screening | MDT status | ENL treatment | Type of samples | Measures | Findings |
|---|---|---|---|---|---|---|---|
| Goihman-Yahr et al. (31); Venezuela | 6 ENL, 32 BL/LL, 6 treated ENL, 9 indeterminate, 11 tuberculoid, 14 HC | ND | ND | Excluded patients on steroids except treated ENL | Peripheral blood neutrophils | Reduction of nitro blue tetrazolium (NBT) | Increased neutrophil activation in ENL |
| Serum | Neutrophil response to endotoxin | Lower neutrophil activation after ENL treatment | |||||
| Plasma | Effect of adding sera and plasma from ENL to neutrophils of HC | Sera from ENL did not activate neutrophils from HC | |||||
| Sher et al. (32); South Africa | 8 ENL, 17 BT, 11 lepromatous, HC | ND | ND | ENL not receiving steroids or other anti-inflammatory drugs | Peripheral blood neutrophils | PMN leukocyte motility | Defect in random migration, chomotaxis, and chemokinesis in both ENL and lepromatous patients |
| Serum | Reduction of nitro blue tetrazolium (NBT) | Reconstitution of PMN leukocytes from HC and ENL with sera from ENL led to increased neutrophil activation | |||||
| Oliveira et al. (33); Brazil δ | 10 BL/LL:6 ENL, 10 HC | ND | On MDT | ND | Peripheral blood neutrophils | Apoptosis | Increased apoptosis in ENL |
| DNA fragmentation extracted from neutrophils | Stimulated neutrophils secrete IL-8 and tumor necrosis factor (TNF)-α | ||||||
| TNF-α and IL-8 | |||||||
| Lee et al. (24); USA ε | 6 ENL, 7 LL | ND | ND | ND | Skin | Microarrays and gene expression analysis | Genes involved in neutrophil recruitment identified |
| Ability of HUVEC to bind neutrophils from HC | Thalidomide diminished neutrophil binding to HUVECs stimulated with cytokines | ||||||
| Schmitz et al. (25); Brazil ε | 62 leprosy: 22 ENL, 16 HC | ENL: before and 7 days after thalidomide | Patients before and after MDT | ENL: before and after thalidomide | Peripheral blood neutrophils | CD64 expression | CD64 upregulated on neutrophils during ENL |
| Higher CD64 on neutrophils from severe ENL | |||||||
| CD64 decreased after thalidomide | |||||||
β, also in Table 2; γ, also in Table 3; δ, also in Table 4; ε, also in Table 5.
BB, mid-borderline leprosy; BL, borderline lepromatous leprosy; BT, borderline tuberculoid leprosy; ENL, erythema nodosum leprosum; HC, healthy controls; HUVEC, human umbilical vein endothelial cells; ICs, immune complexes; LL, lepromatous leprosy polar; ND, not described; PMN, polymorphonuclear; SLE, systemic lupus erythematosus; TB, tuberculosis; TT, tuberculoid leprosy polar.
What is the Role of Immune Complexes in ENL?
An IC or antigen-antibody complex is the result of binding of one or more antibody molecules with one or more antigen molecules (34). The ability of ICs to activate the complement system and to interact with a number of cells determines their biological properties (35). ICs activate complement pathways that opsonize or coat antigen–antibody complexes with large numbers of C3 molecules (36). Opsonization facilitates the clearance of ICs by the macrophage system (36). By maintaining complexes in solution, the complement allows clearance of ICs from their site of formation, minimizing local inflammatory consequences (36).
It was hypothesized that ENL is an IC-mediated disorder because it has some clinical features in common with the Arthus reaction, a type III hypersensitivity reaction that involves the deposition of ICs mainly in the vascular walls, serosa, and glomeruli and is characterized histologically by vasculitis with a polymorphonuclear cell infiltrate (37). The multisystem involvement of ENL resembling autoimmune diseases associated with ICs such as systemic lupus erythematosus (SLE), also lends credence to this theory.
Multiple studies have been performed investigating ICs in ENL. The widely cited study of Wemambu et al included 17 patients with ENL and six uncomplicated LL controls (37). Direct immunofluorescence demonstrated granular deposits of immunoglobulin and complement in a perivascular distribution in association with a polymorph infiltrate in the dermis of 10 out of 17 ENL lesions but not in any lesions of uncomplicated LL (37). However, such deposition is not conclusive evidence of ICs. The presence of soluble mycobacterial antigen was seen in ICs in only 3 out of 17 ENL lesions (37). The authors hypothesized that ENL results from the deposition of ICs in and around venules of the connective tissue septa of subcutaneous fat (37). The study was repeated using 38 patients with ENL and 13 LL controls and demonstrated the presence of immunoglobulin, complement, and mycobacterial antigen in less than half of the skin biopsies from patients with ENL and none of the LL control biopsies (22). Non-specific granular deposits of IgG were demonstrated along the collagen and elastic fibers in the dermis of all 25 patients with ENL in another study, not in any of the 10 LL patient controls (38). However, the deposits were not consistently seen in and around the blood vessels (38). Later studies in ENL suggest that these ICs are extravascular and hence ENL differs from the Arthus reaction (39, 40). These studies taken together provide evidence of an association of ICs and ENL but they do not necessarily support that ICs are the trigger leading to ENL.
Circulating ICs have been demonstrated in patients across the leprosy spectrum (41). The level of circulating ICs in the sera of leprosy patients have been measured in many studies using different immunological techniques (42–54) of which the most commonly used are C1q immunoassays (42, 43, 51). This highlights the fact that the use of different immunoassays to detect circulating ICs in studies may explain the contradictory results. The first study measuring ICs in sera of leprosy patients performed C1q immunoassays in samples from LL patients, tuberculoid leprosy patients, and healthy volunteers and showed that more than 70% of LL patients had demonstrable ICs (43). A subsequent study demonstrated increased occurrence of ICs in both the sera of ENL patients (80%) and uncomplicated LL patients (82%), indicating that the presence of circulating ICs is not a characteristic feature of ENL per se (46). Wager et al. analyzed sera from 135 leprosy patients using the platelet aggregation test (PAT) which had been previously suggested to be a sensitive detector of IgG complexes in other immunological and infectious diseases (55, 56) and concluded that PAT is a sensitive detector of IgG complexes peculiar to LL (44). No ICs were detected in the sera of leprosy patients using the C1q immunoassay (44).
Specific mycobacterial antigens (41) or antibodies against M. leprae antigens (50, 57) have been identified in the ICs derived from sera of lepromatous patients with or without ENL. Rojas et al. precipitated ICs from sera and detected antibodies against phenolic glycolipid-1 (PGL-1) (50) and major cytosolic protein of M. leprae (MCP-I). The finding that ICs are composed of anti-PGL-I and anti-MCP-I antibodies supports the concept that ENL is an IC-mediated disorder (50). However, the composition of circulating ICs of leprosy controls (combined BT and BL/LL) also showed high levels of anti-PGL-I antibodies (50) again suggesting that ICs are not specific to ENL.
Dupnik et al. used DNA microarrays to examine gene expression in PBMC isolated from patients with ENL and matched leprosy controls (58). Several components of the classical complement pathway showed increased expression in PBMC from patients with ENL: C1qA, B, and C and the complement receptors C3AR1 and C5AR1 (58). Increased intensity of fluorescent staining for C1q in skin lesions of ENL compared to BT and BL/LL controls was demonstrated (58). The finding of increased C1q deposition in the skin of ENL does not necessarily mean IC deposition has occurred (35). However, these data do support activation of the classical complement pathway in ENL, which may result from antigen–antibody formation.
Earlier studies in leprosy looked at the role of free complement in the sera of lepromatous patients (59). The serum C3 levels were decreased in patients with ENL, whereas they were elevated in LL controls (60). The low levels of C3 supported the concept that ENL is mediated by an antigen-antibody reaction and may be due to its utilization during the course of such antigen-antibody reactions. Similar decreased serum complement levels have been reported in other IC disorders such as acute glomerulonephritis (61–63) and acute systemic lupus erythematosus (SLE) (64, 65). It has been suggested that ENL is characterized by complement hypercatabolism because the level of the C3 breakdown product C3d in the sera was increased in 70% of the patients with ENL but in only 18% of patients with uncomplicated LL (46).
In other IC-associated diseases such as SLE, systemic vasculitides, and nephritis, defective complement-mediated solubilization of immune precipitates have been observed (48, 49, 66). Similarly, leprosy patients with ENL were shown to have markedly reduced solubilization levels that remained low for 3 months, whereas the C3d and circulating IC levels returned to baseline levels (48). Circulating ICs isolated from sera across the leprosy spectrum as PEG precipitates were shown to be efficient activators of the alternative complement pathway. In addition, PEG precipitates from BL/LL leprosy patients including those with ENL were shown to activate the classical complement pathway as well (52).
A Brazilian study of 46 patients with ENL investigated the association between the MHC class III complement proteins C2, BF, C4A, and C4B and leprosy (67). All patients who were homozygous for the silent C4B allele (C4B*Q0) and thus C4B-deficient had ENL (67). Increased frequency of ENL was also associated with those who were hemizygous for the C4B*Q0 allele. The relative risk of patients suffering from ENL carrying the C4B*Q0 allele was 5.3 compared with LL patients without C4B*Q0 (67). Interestingly, their findings suggested that C4B deficiency could play an important role in the abnormal immune response to M. leprae and to the lack of IC clearance, leading to ENL reactions (67). Hemizygous C4 deficiencies are associated with immune complex diseases such as SLE (68).
There is lack of evidence to support a causative role of ICs in ENL, which requires the deposition of ICs in tissues, the presence of bacterial antigens in these ICs, and the interaction of the ICs with the complement cascade and with phagocytic cells (35). Although there are 28 studies investigating the presence of ICs in the skin or circulating ICs in the sera of patients with ENL (Table 2), their role remains uncertain. It is unclear whether they are involved in the pathogenesis of ENL or simply an epiphenomenon.
Table 2.
Human studies on ENL investigating immune complexes and complement.
| Reference; study site(s) | Study population | Timing of screening | MDT status | ENL treatment | Type of samples | Measures | Findings |
|---|---|---|---|---|---|---|---|
| de Azevedo and de Melo (59); Brazil | 37 lepromatous, 33 tuberculoid, 18 “lepra reaction” | ND | ND | ND | Serum | Complement unit (K) | Reduced complement activity in the reactional group |
| Angular inclination (1/n) | |||||||
| Wemambu et al. (37); United Kingdom and Malaysia | 17 ENL, 6 lepromatous | ND | ND | ND | Skin | Immunoglobulin | Perivascular deposits of immunoglobulin and complement |
| Serum | Complement | Mycobacterial antigen in some ENL skin lesions | |||||
| Waters et al. (22); United Kingdom and Malaysia ε | 38 lepromatous with ENL, 13 lepromatous | ND | ND | ND | Skin | Immunoglobulin and complement | Immunoglobulin and complement perivascular in some ENL skin lesions |
| Serum | Detection of mycobacterial antigen in the ICs | Mycobacterial antigen present in ICs | |||||
| Gelber et al. (69); Taiwan and USA | 15 LL with ENL, 47 BT-LL | 3 or more specimens time-span up to 4 months in BT and LL/ENL and up to 6 months to LL without ENL | ND | ND | Serum | C1q precipitin activity | Association of C1q precipitin activity with ENL |
| Complement levels C3 | |||||||
| Cryoglobulins | |||||||
| Bjorvatn et al. (46); Ethiopia and Switzerland | 13 ENL, 7 LL, 6 tuberculoid, pulmonary TB, 30 HC | ND | All on dapsone or clofazimine | ENL patients received treatment for ENL | Serum | ICs with 125I-C1q binding assay | ICs increased in ENL and LL but also in tuberculoid leprosy |
| Complement | Increased C3d level in most patients with ENL | ||||||
| Tung et al. (51); Ethiopia | 22 BL/LL with ENL, 23TT-LL, 17 SLE | ND | 19/23 non-ENL on dapsone | Untreated ENL | Serum | C1q ICs | Circulating ICs in 67% of leprosy by C1q test |
| Raji test ICs | Only 7% of this 67% showed ICs by the Raji test | ||||||
| Anthony et al. (60); India ε | 25 LL with ENL, 10 LL without ENL | Active ENL lesions at the time of the biopsy | ND | ND | Skin | Immunoglobulin deposits in skin | Immunoglobulin deposits in ENL skin but not in LL |
| Decreased serum complement in ENL | |||||||
| Serum | Complement in sera | Elevated levels in LL | |||||
| Wager et al. (44); Finland, Brazil, and Ethiopia | 11 ENL, 112 leprosy, 61 LL, 7 tuberculoid, 28 SLE, 42 RA, 374 HC | ND | ND | ND | Serum | ICs with Platelet aggregation test (PAT) | Higher PAT titers toward the lepromatous end of the spectrum |
| Other sero-immunological parameters | No significant differences for ENL patients | ||||||
| Izumi et al. (70); Japan γ | 12 ENL, 49 active lepromatous, 24 inactive lepromatous, 7 borderline, 6 tuberculoid, 9 HC | ND | ND | ND | Serum | C4, C3c, C3 activator | C3 activator and C3c concentrations higher in ENL compared with active lepromatous |
| Harikrishan et al. (71); India ε | 20 active LL, 15 ENL active and subsided, 20 HC | ENL: during the active and the subsided phase | ND | ND | Serum | Complement factor C3 | Increased levels of C3 in LL and ENL |
| Decrease in C3 during the “subsided phase” of reaction | |||||||
| Saha et al. (72); India | 20 ENL, 15 HC | Initial sample first visit, subsequent on ENL clinical remission 4 weeks later | ND | Second sample: on antireactional treatment | Serum | Complement C1q, C3, C4 | C3 level decreased during ENL, while increased after remission C3d increased during ENL, and remained elevated after clinical remission in most patients |
| No significant difference in C1q or C4 during ENL | |||||||
| Valentijn et al. (54); Netherlands and Surinam | 70 leprosy throughout the whole spectrum, 11 HC | ND | ND | ENL patients possibly on thalidomide treatment? | Serum | ICs | Elevated C3d significantly associated with ENL |
| Complement C1q, C3, C4 | |||||||
| Mshana et al. (73); Ethiopia | 26 ENL, 20 BL/LL | Skin biopsies of ENL less than 12 h old | ND | ND | Skin | Immunoglobulin deposits Complement deposits |
No ICs around blood vessels in ENL lesions IC formation common feature in LL |
| Mycobacterial antigens | Absence of immunoglobulin or C3 deposits in early ENL | ||||||
| Extracellular antigens not seen | |||||||
| Ridley and Ridley (39); Malaysia, PNG, Ethiopia, and UK | 20 ENL, 10 non-reactional leprosy | ND | ND | ND | Skin | Immunoglobulins IgG, IgM, IgA, IgE Complement C3, C4, C1q, C3d |
ENL lesions had disintegration of macrophages and release of bacterial antigen combined first with IgM, later with IgG, present together with complement components of the classical pathway |
| ICs were both extracellular and in neutrophils and macrophages | |||||||
| ICs were extravascular | |||||||
| Ramanathan et al. (74); India | 10 BT, 10 LL, 10 BT reactional, 30 LL reactional | ND | All patients on dapsone | Sampling before antireactional treatment | Serum | C3 and C4 | Increased C3d in both BT reactional (T1R) and LL reactional |
| ICs Isolated ICs for IgG, IgA, IgM, C3, C4 and antimycobacterial antibody |
Circulating ICs in all reactional patients No antimycobacterial antibody in ICs from LL reactional patients | ||||||
| Saha et al. (75); India | 20 ENL, 15 HC | Before and 4 weeks after starting treatment for ENL | ND | Second sample: on antireactional treatment | Serum | Quantitative analysis of composition of PEG precipitates (immunoglobulins, complement components, autoantibodies and acute phase proteins) and anticomplementary activity of PEG precipitates | Anticomplementary activity of PEG precipitates more in the lepromatous than in normal sera, independent of the presence of ENL |
| Ramanathan et al. (48); India | 32 LL in reaction, 10 BT, 10 BT in reaction, 10 LL uncomplicated, 15 HC | ND | BT and LL without reaction treatment for at least 2 years; all patients were on dapsone | ND | Serum | ICs by fluid phase 125I-labeled conglutinin binding assay; serum C3d Complement-mediated solubilization of immune precipitates |
Reduced solubilization of in vitro formed immune precipitates by the sera of ENL patients C3d, ICs, and solubilization levels correlated with the clinical course of reaction ICs and C3d decline after clinical subsidence of ENL |
| Sehgal et al. (76); India ε | 21 T1R or ENL | ND | ND | ND | Serum | Complement C3 | Lower level of C3 during ENL |
| Chakrabarty et al. (77); India | 27 BB-BL-LL: 7 ENL, 4 T1R | Initial blood collected at the onset of reaction and subsequent 4 weeks after ENL remission | Patients on MDT | Second blood sample on antireactional treatment | Serum | Solubilization of preformed ICs (125I-BSA-anti-BSA complexes) | The mean solubilizing capacity of the reaction patients’ sera during the reaction was not significantly different from LL without ENL |
| After clinical remission of the reaction, most patients showed no increase in the ICs solubilization | |||||||
| Rao and Rao (78); India ε | 44 ENL, 39 BL/LL, 22 post-ENL | ENL: before starting treatment with anti-inflammatory drugs/steroids | 20 BL/LL untreated and 19 BL/LL treated with dapsone less than a year | Untreated first sample and second post-ENL sample after discontinuation of antireactional treatment | Serum | C3 and C4 levels IgG, IgA, IgM, C3, and C4 levels in the ICs ICs by PEG method |
C3 and C4 levels were not significantly different in ENL compared to BL/LL and post-ENL C3 and C4 levels in the ICs reduced insignificantly in ENL than BL/LL and post-ENL IgG, IgA, and IgM in ICs showed no significant differences from LL to ENL and post-ENL |
| Post-ENL: ensuring that the patient had not taken anti-inflammatory drugs/steroids for at least 3 or 7 days | |||||||
| Sehgal et al. (79); India | 17 ENL | Before antireactional treatment and 1 week after the clinical subsidence of ENL | On MDT | First sample untreated and second sample on prednisolone in 10 patients | Serum | Complement components: classic pathway: C1q and C4 Alternative pathway: C3, C3d, Factor B |
No significant change in classical pathway in ENL reaction C3 elevated, C3d decreased and increase of Factor B after ENL |
| Jayapal 1989 (47); India | 37 leprosy: 9 ENL, 6 bacterial endocarditis, syphilis, SLE, HC | ND | All leprosy patients on dapsone | ENL patients on clofazimine, prednisolone, antihistamine and chloroquine | Serum | ICs with PEG method | ICs higher in ENL than in LL |
| Sehgal et al. (81); India | 18 T1R, 17 ENL, non-reactional controls | During and after reaction | On MDT | ND | Serum | Complement components: classic pathway: C1q and C4 Alternative pathway: C3, C3d, Factor B |
Classic pathway: no significant change in C1q and C4 during ENL Alternative pathway: increase in C3d during ENL; decrease of C3 during ENL; reduction of Factor B during ENL; elevation of C3 and Factor B after ENL |
| Tyagi et al. (52); India | 20 BL/LL with ENL, 20 TT/BT, 20 BT with reaction, 20 BL/LL; 15 HC | ND | ND | ND | Serum | ICs by PEG precipitation Mycobacterial ICs in PEG precipitates; CH50 assay and AH50 assay (complement consumption) |
PEG ICs from BL/LL and ENL higher IgG and IgM antimycobacterial antibodies than TT/BT, BT reactional (T1R) and HC No significant functional differences between the PEG ICs from reactional and non-reactional leprosy |
| Ramanathan et al. (49); India ε | 26 BL/LL: 11 ENL, 24 HC | Before initiation of treatment and 2-monthly intervals | Untreated and then on MDT | Treated but after sampling | Serum | ICs by PEG method | High levels of ICs in both LL and ENL |
| C3d | Lower levels of complement-induced IC solubilization in ENL | ||||||
| Complement-induced IC solubilization | Highest levels of ICs and C3d at the time of ENL | ||||||
| Scollard et al. (82); Thailand ε | 4 cured leprosy, 10 non-reactional leprosy BT/BL/LL, 8 ENL patients (5 LL/3 BL), 3 T1R, 4 HC | ND | ND | ND | Blisters induced over representative skin lesion | ICs | ICs in ENL similar to that of active leprosy (either lepromatous or tuberculoid) Higher ICs in blisters than in matching sera |
| Serum | |||||||
| Rojas et al (50); Brazil ε | 19 ENL, 10 BL/LL, 13 family contacts; 15 healthy non-contacts | ND | Both untreated and patients on MDT for 1–72 months | ND | Serum | ICs; anti-PGL-I IgM in IC precipitated from sera; anti-10-kDa hsp IgG in IC precipitated from sera | ENL highest levels of ICs compared with all other groups IgM anti-PGL-I and IgG anti-MCP-1 heat shock protein antibodies constituents of ICs in ENL |
| Dupnik et al. (58); Brazil δ, ε | 11 ENL, 11 T1R, 19 non-reactional leprosy, additional 6 ENL, 11T1R, 11 HC | ND | 3 ENL pre-treatment, 2 ENL on treatment and 6 ENL post-treatment; Leprosy controls matched for stage of treatment | Excluded patients who had received corticosteroids within 7 days or thalidomide within 28 days of enrollment | PBMC | Microarray and qPCR for transcriptional profile of PBMC; IHC for C1q in skin lesions | Complement and coagulation pathway common in ENL and T1R |
| Skin | Transcripts uniquely increased in ENL included complement receptors C3AR1 and C5AR1 | ||||||
| C1q staining higher in both ENL and T1R compared with non-reactional leprosy | |||||||
α, also in Table 1; γ, also in Table 3; δ, also in Table 4; ε, also in Table 5.
BB, mid-borderline leprosy; BL, borderline lepromatous leprosy; BT, borderline tuberculoid leprosy; ENL, erythema nodosum leprosum; HC, healthy controls; ICs, immune complexes; LL, lepromatous leprosy polar; ND, not described; PEG, polyethylene glycol; PNG, Papua New Guinea; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TB, tuberculosis; TT, tuberculoid leprosy polar; WHO, World Health Organization.
What is the Role of T-Cells in ENL?
T-lymphocytes are part of the adaptive immune response which help to eliminate bacterial, viral, parasitic infections or malignant cells. The antigen specificity of the T-cell is based on recognition through the T-cell receptor (TCR) of unique antigenic peptides presented by major histocompatibility complex (MHC)-molecules on APCs: B cells, macrophages, and dendritic cells. There are two major T-cell lineages, defined by the presence of two surface co-receptor molecules, namely, CD4 and CD8. CD4+ cells when they are activated produce cytokines as effector T helper cells, whereas CD8+ lymphocytes form effector cytotoxic T lymphocytes (CTL). Furthermore, activated CD4+ T helper cells can be subdivided into Th1, Th2, Th17, and T regulatory (Treg) subsets based on the production of signature cytokines (83).
Early studies investigating T-cell biology in the pathophysiology of ENL reported that ENL patients had higher T-cell numbers in peripheral blood than uncomplicated LL patients, although both LL and ENL patients had a significantly lower percentage and absolute number of T-cells compared to healthy controls (84). In addition, the high numbers of T-cells observed during ENL remained high post-ENL treatment compared to the LL controls (85).
Patients with ENL had increased CD4+ T cell numbers and a simultaneous decrease in CD8+ T cell numbers and an increased CD4+/CD8+ ratio in the blood compared to LL controls (86, 87), while ENL patients had decreased CD4+/CD8+ ratio after successful treatment. This ratio increased in those patients who had an ENL recurrence (87). An increased CD4+/CD8+ ratio in ENL patients was reported by several subsequent studies (87–92). In acute SLE, it has been suggested that the failure of CD8+ T-cell activity could lead to increased IgG production and to the subsequent formation of ICs (93). However, there are studies in ENL reporting a decreased CD4+/CD8+ ratio compared to non-reactional LL controls (94) or a similar ratio (95, 96).
The first immunohistological studies of T-cell subsets in skin lesions included small numbers of ENL patients and assessed the percentage and ratio of CD4+ and CD8+ T cells by comparing them to non-ENL lepromatous specimens (89, 91, 97–103). ENL skin lesions, like peripheral blood, were characterized by an increased CD4+/CD8+ ratio in all but one of these studies (89, 91, 97–100, 102, 103).
CD4+ T cells differentiate according to the microenvironment into Th1, Th2 cells, or subsets of Th17 and Treg (104). Recent studies have reported the frequency of the newly described Th17 and Treg subsets in leprosy (105, 106). Using flow cytometry in ENL, the absolute numbers and proportion of Tregs were shown to be significantly lower during ENL although FoxP3 expression, a marker they used to define Tregs, was higher (107). Tregs suppress or downregulate induction and proliferation of effector T cells (108). Therefore, the observation of lower numbers of Tregs in ENL could account for the relatively higher proportion of T cells previously described in multiple ENL studies. Two more publications from the same group addressed the frequency of Tregs in ENL, defined as CD4+CD25high FoxP3+ cells and reported the ratio of Treg/Teffector cells to be low in ENL (109, 110). These results should be interpreted with caution since dichotomizing cells into CD25high and CD25low to identify Tregs is highly subjective. There is no consensus on the thresholds of CD25 expression to delineate Tregs within the CD25high population (111). Variations in FoxP3 expression within the CD25high population have been observed even in healthy individuals (112).
A recent study that used flow cytometry described a significant reduction in percentage of CD4+CD25+FoxP3+ Treg cells and mean fluorescence intensity of FoxP3 in PBMC in patients with ENL compared to LL controls (113). The observed reduction of Tregs in ENL patients could lower the inhibitory effects on effector T cells and therefore lead to enhanced Th17 activity, tipping the balance toward inflammation, as previously described in other conditions such as tuberculous pleural effusion (114). Interestingly, an increase of FoxP3 mRNA expression by PBMC in ENL patients compared to LL controls has also been reported (113). The conflicting results for FoxP3 could be due to variation in the flow cytometry gating or the fact that FoxP3 mRNA may not be translated to functional FoxP3. A previous study measured the expression of Foxp3 by qPCR in skin biopsies and PBMC of five patients with ENL and detected Foxp3 in all skin and PBMC samples. An upward trend of Foxp3 in PBMC was described during the first 21 days of thalidomide treatment (115). The authors suggested that thalidomide may boost Tregs by T-cell costimulation via CD28 and therefore augment the IL-2-dependent number and/or function of Tregs (115). However, the changes in Foxp3 expression did not reach statistical significance, while no IL-2 mRNA was detected in any samples (115). Another study addressed FoxP3 expression by immunohistochemistry in skin but there was no difference in patients with ENL compared to non-reactional leprosy controls across the spectrum (116). Recent research suggests that Tregs constitute a stable cell lineage whose committed state in a changing environment is ensured by DNA demethylation of the Foxp3 locus irrespective of ongoing Foxp3 expression (117). Further investigation is needed to better define the role of Tregs in the pathogenesis of ENL.
Patients with ENL do not exhibit the phenomenon of “anergy” of cell-mediated immune response observed in untreated LL patients (118). Patients with ENL had elevated mean proliferative responses to several mitogens compared to uncomplicated LL patients (86, 87), while an enhancement in T-cell-related functions during the acute phase of an ENL reaction has also been described (94).
The interpretation of the role of T cell subsets in ENL is hampered by small sample sizes and methodological issues. 63.6% of the 44 studies investigating the role of T-cells in ENL (Table 3) are cross-sectional and lack serial sampling before and after treatment for ENL. However, it appears that T cell subsets do play an important role in ENL because multiple studies report an increased CD4+/CD8+ ratio in ENL patients in both skin and peripheral blood.
Table 3.
Human studies on ENL investigating T-cell biology.
| Reference; study site(s) | Study population | Timing of sampling | MDT status | ENL treatment status | Type of samples | Measures | Findings |
|---|---|---|---|---|---|---|---|
| Lim et al. (84); USA and Korea (mixed ethnic background) | 7 LL ENL, 20 active LL, 9 inactive LL, 4 BB, 3 indeterminate leprosy | ND | All patients treated with Dapsone or Clofazimine or Rifampicin for varying durations | 5 had received various doses of steroids and 3 were treated with steroids at the time of the study | Blood | T lymphocyte numbers by the rosette assay | ENL showed T-lymphocyte numbers significantly higher than LL |
| LL had lower T-lymphocyte numbers than HC | |||||||
| Anders et al. (119); Papua New Guinea | 31 leprosy: 13 BL/LL with amyloidosis (11/13 frequent ENL), 9 BL/LL ENL without amyloidosis, 9 BL/LL with few or no ENL episodes | ND | Approximately half patients on clofazimine and other half on dapsone | 2 ENL at testing: 1 steroids and 1 stibophen | Blood | Lympohocyte transformation tests | Patients with a history of frequent ENL had greater cell-mediated responses to PHA than patients without ENL |
| Izumi et al. (70); Japan β | 12 ENL, 49 active lepromatous, 24 inactive lepromatous, 7 borderline, 6 tuberculoid, 9 HC | ND | ND | ND | PBMC | Percentage and number of Tμ (T cells with Fc receptor for IgG) and Tγ (T cells with Fc receptor for IgM) | No significant differences between different clinical groups |
| Bach et al. (86); France (multiple ethnic groups) | 9 BL/LL with no recent history of ENL, 9 BL/LL suffered from ENL less than 2 months prior to the investigation, 13 BT/TT, HC | ND | Some untreated and others on MDT | Certain ENL on antireactional treatment | Blood | T cell subsets; Proliferative responses to mitogens | Increased %age of helper T cells in ENL |
| Decreased %age of suppressor T cells in ENL | |||||||
| Elevated proliferative responses to mitogens in ENL | |||||||
| Con A-induced suppressive activity | Most ENL decrease of suppressive index, whereas none of the LL or TT patients had a diminished suppressive activity | ||||||
| Dubey et al. (120); India | 41 untreated cases of leprosy, 64 TT and LL taking antileprosy treatment, reactional (8 ENL and 10T1Rs), 11/41 follow-up from untreated leprosy patients | ND | 64 cases on antileprosy treatment | Untreated cases of ENL? | Blood | Lymphocytic culture: percentage of Blast transformation | Blast percentage in ENL slightly higher than T1R |
| Mshana et al. (90); Ethiopia | 21 BL/LL, 10 BT, 5 ENL | ND | All patients received MDT but unclear whether sampled prior to MDT | No patient on thalidomide | Blood | Lymphoproliferative responses to PPD or PHA T-cell subsets |
Higher responses to PPD or PHA in ENL Decreased number of suppressor cells prior to ENL, which increased with clinical recovery from ENL |
| Mshana et al. (88); Ethiopia | 69 leprosy patients: 26 ENL, 13 HC | Untreated samples | Untreated samples | Untreated samples | Blood | T lymphocyte subpopulations; lymphoproliferation using M. leprae, PHA and PPD | ENL patients had decrease in suppressor cells and an increase of CD4+/CD8+ ratio compared to LL ENL had higher responses to both PHA and PPD BL/LL patients with or without ENL lower proliferative responses to M. leprae than BT patients and HC |
| Wallach et al. (87); France (samples from multiple ethnic groups) | 9 recent ENL, 6 bacteriologically positive patients of which 1 ENL more than 5 years ago, 9 treated leprosy patients of which 3 had ENL | Described in detail each patient duration of disease | All treatment described in detail | Some on antireactional treatment | Blood | T cell subsets; Lymphocyte transformation tests: proliferative responses to mitogens | ENL patients have elevated Helper/Suppressor ratio |
| Mean proliferative responses elevated in ENL | |||||||
| Bach et al. (121); France | 8 treated lepromatous without recent ENL with BI < 1 +, 6 lepromatous with BI > 2+ (untreated or suffering a relapse, without recent ENL reaction), 12 lepromatous who underwent at least one ENL episode, 13 tuberculoid, 41 HC | ND | ND | ND | PBMC | T-cell subsets; Proliferative response to M. leprae and PPD of isolated T-cell subsets | ENL decreased CD8+ T cell percentages and increased CD4+/CD8+ ratios T-cell subset percentages returned to normal either when the bacterial load was reduced by treatment or when the ENL reaction resolved ENL episodes associated with improvement of T-cell unresponsiveness to various antigens or mitogens |
| Modlin et al. (97); USA | 15 non-reactional leprosy BT/BB/BL/LL, 17 reactional (6 T1R, 9 ENL, 2 Lucio’s reaction) | ND | Results did not differ between treated and untreated subjects | 3 ENL had no therapy | Skin | T lymphocyte subsets | The helper/suppressor ratio in ENL was significantly higher than in non-reactional lepromatous disease |
| Modlin et al. (98); USA | 14 leprosy patients (4 tuberculoid, 2 borderline in T1R, 1 BL, 7 lepromatous of which 5 ENL), 8 HC | ND | 6 treated patients | ND | Skin | T lymphocyte subsets | ENL lesions showed 2:1 predominance of helper cells whereas in the lesions without ENL the helper: suppressor ratio was 1:1 smaller |
| Sasiain et al. (122); Argentina | 16 ENL, 12 HC | First blood sample ND; 9 ENL 20-30 days after stopping thalidomide | All patients on MDT | Thalidomide in patients with ENL | PBMC | ConA-induced suppressor response | Suppressor T-cell function was reduced during ENL and after ENL than HC |
| Narayanan et al. (89); India ε | 7 LL ENL, 6 BT T1R, 5BL T1R, 18 BT-LL | ND | ND | ND | Skin | T cell phenotypes | Lesions of ENL showed increase in T cells with a predominance of the helper/inducer subset; CD4+/CD8+ ratio was higher in ENL and T1R than non-reactional lesions |
| Rea et al. (96); USA ε | 19 ENL, 24 LL non-reactional with treatment, 12 LL non-reactional no treatment, 18 LL with long-term treatment, 4 LL with Lucio’s, 13 BL, 13 T1R, 18 Tuberculoid, 13 Tuberculoid with long-term treatment | ND | Some patients on MDT | ENL before receiving thalidomide | PBMC | T cell subsets | Active LL patients have lymphopenia, a proportionate reduction in the numbers of each of the three T cell subsets |
| Insignificant changes in T cell subsets expressed as percentages and in the helper: suppressor ratio | |||||||
| Laal et al. (94); India ε | 15 ENL, 13 LL | During active ENL and 1 week to 4 months after stopping treatment | On MDT | First sample before initiation of antireactional treatment | Blood | Leukocyte migration inhibition test | ENL significant inhibition of antigen-induced leukocyte migration |
| Lymphoproliferation | Lymphoproliferation enhanced during the acute phase of ENL | ||||||
| Second sample 1 week to 4 months after stopping treatment | Suppressor cell activity; T cell subsets | Enhanced antigen-stimulated suppression of mitogen responses in ENL | |||||
| Leukocyte migration inhibition, lymphoproliferation, and suppressor cell activity were reduced in post-ENL to the unresponsive state seen in stable LL | |||||||
| Lower CD4+/CD8+ ratio in ENL compared to LL | |||||||
| Modlin et al. (99); USA | 12 ENL and 10 non-reactional leprosy; 19 ENL blood samples | ND | ENL biopsies: 8/12 treated with dapsone; ENL blood: 15/19 treated | Some ENL were treated | Blood | T lymphocyte subsets | ENL tissue more cells of the helper-inducer phenotype and fewer of the suppressor-cytotoxic phenotype, as compared with non-reactional LL |
| Skin | No correlation between tissue and blood helper-suppressor ratios | ||||||
| Wallach et al. (91); France | ND | ND | ND | ND | Blood | T cell helper-suppressor (HS) ratio | HS ratio higher in ENL lesions and blood than non-ENL leprosy controls |
| Skin | |||||||
| Modlin et al. (100); USA | Biopsies: 25 ENL, 23 tuberculoid, 23 non-reactional lepromatous; | ND | Some patients received treatment | Some patients on treatment? | Blood | Skin: number of T cells, T cell subsets; Blood: lepromin-induced suppression of the Con A stimulation | Increases in both CD4+/CD8+ ratio and the number of IL2-positive cells in ENL Suppressor activity decreased significantly in ENL Suppressor activity returned to normal after ENL subsided |
| Blood: 18 ENL | |||||||
| Skin | |||||||
| Rao and Rao (123); India ε | 44 ENL, 39 BL/LL, 22 post-ENL | ENL patients before starting ENL treatment, post-ENL after patient had not taken anti-inflammatory/steroids for at least 3 and 7 days | From 39 non-reactional cases: 20 untreated and 19 with dapsone for less than a year | Before starting treatment for ENL with steroids or anti-inflammatory drugs, post-ENL: ensuring that the patient had not taken anti-inflammatory drugs or steroids for at least 3 and 7 days, respectively | Blood | Sub-population of T cells with receptors for Fc portion of IgG (Tr) and Fc portion of IgM (Tμ) | Tμ/Tr ratio higher in ENL than lepromatous and post-ENL patients |
| Rao and Rao (85); India | 77 leprosy: 44 ENL | ENL: before starting anti-ENL treatment, post-ENL: After patient had noe taken anti-inflammatory drugs or steroids for at least 3 and 7 days | 19 patients treated with dapsone for less than 1 year | Before starting treatment for ENL with anti-inflammatory drugs or steroids | Blood | Leykocyte migration inhibition test (LMIT) | No significant difference in mean migratory index to PHA, PPD, sonicate M. leprae |
| Enumeration of early and total T lymphocytes | Whole M. leprae increased response in ENL compared to LL Lower migratory indices to whole M. leprae in post-ENL than LL |
||||||
| %age of early T lymphocytes increased in ENL compared to LL | |||||||
| %age of early T lymphocytes remained high in post-ENL compared to LL | |||||||
| Cell-mediated immune responses enhanced during ENL and return to LL levels once the episode is over | |||||||
| Shen et al. (101); USA | 10 ENL, 8TT/BT, 10 BL/LL, 10 T1R | ND | ND | ND | Skin | CD3, CD4, CD8 and Ta1 (memory) positive cells | CD3, CD4 and CD8 showed percentages of positive cells in lesions similar between patient groups |
| PBMC | No significant difference in%age of memory T-cells in ENL compared to LL | ||||||
| Bottasso et al. (124); Argentina | 8 LL/ENL, 17 LL, 9 TT, 11 HC | ND | Patients on MDT | Patients with ENL were not on thalidomide treatment but unknown whether they were on steroids | Blood | T-Lymphocytes count absolute and relative; Lymphocyte functional assay: capacity of rosetta formation | Active LL showed a decrease in T-lymhocytes |
| ENL showed a restoration of the levels of T-lymphocytes | |||||||
| Rasheed et al. (125); Zambia and Pakistan | 167 leprosy of which 21 LL/ENL, 12 BL/T1R, 24 BT/T1R, 46 endemic HC | ND | ND | ND | Serum Lymphocytes | Lymphocytotoxic activity | Lymphocytotoxic activity scores were significantly raised in patients with reactions |
| Sasiain et al. (126); Argentina | 53 leprosy patients TT/BT/BB/BL/LL and 9 LL/ENL, 23 HC | ND | Received MDT | Thalidomide for ENL | PBMC | Proportion of CD8+ cells | Proportion of CD8+ cells was low in LL patients and tended to normalize during ENL episodes |
| M. leprae-induced suppression of T-cell proliferation; Induction of IL-2R by culture with M. leprae | |||||||
| PHA- and ConA-induced proliferation | |||||||
| Bhoopat et al. (127); Thailand ε | 57 ENL (19 acute/38 chronic), 61 active LL, 33 cured leprosy | 26 BL/35 LL newly diagnosed and untreated | ND | If corticosteroid and/or thalidomide was initiated before or during the study, precise timing of medication was recorded with respect to the time of collection of laboratory specimens | Blisters induced over a representative skin lesion | T cell subsets in situ | The lesions of chronic ENL showed a decreased number of CD8+ cells and increased helper/suppressor ratio compared to those in acute ENL and non-reactional leprosy; Systemic administration of corticosteroids caused a reduction in the CD4+ cell population but did not change CD8+ cell population |
| Rea and Modlin (102); USA δ | ND | ND | ND | ND | Skin | T-cell phenotypes: CD4+ versus CD8+ cells, γ/δ and α/β receptor-bearing lymphocytes, T-memory and T-naïve cells | ENL lesions predominance of CD4+ cells similar to those in tuberculoid (TT/BT?) and T1R |
| LL patients showed an excess of CD8+cells | |||||||
| Tyagi et al. (53); India | 4 TT/BT, 5 BL/LL, 4 ENL | ND | ND | ND | Blood | Effect of isolated circulating ICs from BL/LL or ENL patients to lymphocyte transformation test on T cells of HC | PEG precipitates isolated from BL/LL or ENL subjects had a significant suppressive effect on lymphocyte proliferation in HC |
| Foss et al. (128); Brazil δ | 28 lepromatous: 11 ENL, 23 tuberculoid, 19 HC | ND | lepromatous patients 86% treated with dapsone | 11 ENL at time of blood collection no immunosuppressive drug | Blood | T lymphocyte response to concanavalin A | Marked reduction on concanavalin A-induced lymphoproliferation in patients with ENL |
| Santos et al. (129); Brazil ε | 59 LL/BL, 10 ENL, 4 T1R, 4 post-reactional | ND | On MDT | No specific treatment for reactions before blood collection | PBMC | Lymphocyte proliferation after ConA and M. leprae | T1R showed greater lymphocyte proliferation compared to all other groups |
| de la Barrera et al. (130); Argentina | 7 TT/BT, 20 BL/LL of which 3 ENL | ND | All patients on MDT | ND | PBMC | T-cell cytotoxic activity induced by M. leprae and M.tb heat shock protein (HSP) | M. leprae hsp65 induced cytotoxic responses only in those MB patients undergoing ENL |
| Vieira et al. (131); Brazil δ, ε | 95 MB leprosy (30LL/65BL) of which 51 ENL | At leprosy diagnosis and at onset of reactional episode | Time of MDT for each ENL | Sample before thalidomide and steroids? | PBMC | Lymphocyte transformation test (LTT) | Some patients showed lymphoproliferative response during ENL |
| Mahaisavariya et al. (103); Thailand | 17 non-reactional, 8 T1R, 12 ENL | Biopsy at the time of diagnosis and not the time of reaction | ND | ND | Skin | T-lymphocyte subsets | %age of CD8 infiltration reduced in ENL compared with non-reactional lepromatous |
| The CD4+/CD8+ ratio of ENL statistically significant higher than from the non-reactional lepromatous group | |||||||
| Tadesse et al. (132); Ethiopia δ | 33 leprosy: 14 BT, 11 BT T1R, 8 ENL, 11 HC | ND | Certain leprosy patients were treated on MDT | All ENL treated with steroids | PBMC | Lymphocyte blast transformation | Thalidomide treatment did not alter the lymphoproliferative response to the mycobacterial antigens during ENL |
| Mohanty et al. (133); India | 21 BL/LL ENL, 38 TT/BT/BL/LL, 29 BT/BL T1R, 19 HC | ND | ND | ND | PBMC | Immune responses against Stress proteins of M. leprae (lymphoproliferation) | ENL: no significant role of stress proteins except a heightened lymphoproliferative response to the 28 kDa antigen |
| Serum | |||||||
| Villahermosa et al. (134); Philippines δ, ε | 22 ENL | Before thalidomide and at study weeks 3 and 7 during thalidomide | MDT continued during the study | Samples untreated for antireactional drugs and during thalidomide treatment | Blood | Lymphocyte proliferation assays (LPA) to phytohemagglutinin and concanavalin A | Low LPA values pre-thalidomide in both PBMC and whole blood |
| PMBC | |||||||
| Attia et al. (107); Egypt | 38 leprosy: 6 ENL, 38 HC | Untreated samples | Untreated samples | Untreated samples; excluded patients on immunosuppressive drugs | Blood | Frequency of circulating Tregs; FoxP3 expression | Significantly lower frequency of Tregs but higher FoxP3 expression in ENL |
| Massone et al. (116); Brazil ε | 20 leprosy: 3 ENL | Biopsies at the time of diagnosis | 10, 12 and 13 months after beginning of MDT for LL | Untreated for antireactional treatment | Skin | Presence, frequency and distribution of Tregs | No statistical difference in FoxP3 expression between TT, BT, BL, and LL |
| Significant increase in FoxP3 expression in T1R compared to ENL | |||||||
| Rada et al. (135); Venezuela ε | ? ENL81 LL, 41 BL, 41 BB, 3% BT | ND | ND | ND | Blood | Cell-mediated immunological tests to mycobacterial proteins | T-lymphocyte proliferative response in reactional and non-reactional patients was negative |
| Saini et al. (136); India δ | 21 MB: 16 ENL, 5 T1R | ENL blood during reaction and at 0.5 and 1 year after the onset of reaction | Duration of MDT described | ENL patients received steroids | PBMC | Lymphoproliferation of PBMC stimulated with M. leprae, recombinant Lsr2 and 6 synthetic peptides spanning the Lsr2 | All patients with active ENL showed lymphoproliferation in response to peptides A and F |
| Abdallah et al. (109); Egypt δ | 43 leprosy: 6 ENL, 40 HC | Untreated patients | Untreated samples | Untreated | Blood | Circulating Tregs | Tregs/Teffs lowest in ENL |
| Attia et al. (110); Egypt δ | 43 leprosy: 6 ENL, 40 HC | Untreated patients | Untreated samples | Untreated | Blood | CD4(+) CD25(high)Foxp3 (+) regulatory cells | CD4(+)CD25(high)FoxP3(+) Treg levels lowest in ENL |
| Treg/Teffs lowest in ENL | |||||||
| Hussain et al. (92); India | 50 leprosy (28 without reactions, 11 T1R, 11 ENL), 50 HC, 50 pulmonary TB (25 HIV-TB co-infected and 25 without HIV infection), 50 HIV-positive | ND | Reactional episodes following antileprosy treatment | ND | Blood | CD3+, CD4+, CD8+ and CD4+/CD8+ ratio with flow cytometry | CD4+ counts raised during ENL compared to MB patients whereas CD8+ counts lower The CD4+/CD8+ ratio doubled during reactional episodes of T1R and ENL |
| Parente et al. (137); Brazil | 2 ENL, 103 leprosy TT/BT/BB/BL/LL 9 indeterminate, 8 T1R | 2 ENL: 12 and 10 months after initiation of MDT | 2 ENL after initiation of MDT | ND | Skin | Frequency and distribution of regulatory T cells | No significant differences in ENL |
| Saini et al. (113); India δ | 66 leprosy: 15 T1Rs, 15 ENL, 36 BT/LL | Newly diagnosed leprosy patients prior to institution of antireaction therapy | Freshly diagnosed patients: untreated subjects | Newly diagnosed leprosy patients prior to institution of antireaction therapy | PBMC | MLSA stimulated and unstimulated PBMC: gene expression with PCR array for 84 genes; T cell phenotypes | Increase in FOXP3 gene expression in ENL |
| Th17 cells with intracellular IL-17A, F are increased in ENL and CD4+IL-21+ cells are higher in ENL | |||||||
| Significant upregulation of CD4+CCR6+ cells in ENL | |||||||
| Tregs decreased in ENL | |||||||
α, also in Table 1; β, also in Table 2; δ, also in Table 4; ε, also in Table 5.
BB, mid-borderline leprosy; BL, borderline lepromatous leprosy; BT, borderline tuberculoid leprosy; ENL, erythema nodosum leprosum; HC, healthy controls; HS, helper-suppressor; HSP, heat shock protein; ICs, immune complexes; LL, lepromatous leprosy polar; LTT, lymphocyte transformation test; LPA, lymphocyte proliferation assay; MB, multibacillary; ND, not described; PEG, polyethylene glycol; PHA, purified phhytohaemagglutinin; PPD, RT23 tuberculin-purified protein derivative; SLE, systemic lupus erythematosus; TB, tuberculosis; TT, tuberculoid leprosy polar.
What is the Role of TNF-α or Other Cytokines in ENL?
A role for TNF-α in ENL was first suggested by a Brazilian study that included 18 ENL patients at various stages of treatment with steroids or thalidomide (138). Serum TNF-α levels varied widely: from undetectable to extremely high levels (138). There was no obvious correlation between severity of ENL and cytokine levels, while patients who had received treatment had lower levels of TNF-α (138). High serum TNF-α levels were subsequently shown to decrease significantly during thalidomide treatment (139). These findings have been reproduced in other populations measuring serum TNF-α levels (128, 131, 140–147), whereas two studies failed to show increased levels of serum TNF-α during ENL (148, 149). The high variability in serum TNF-α between studies might be due to patient differences. Although genetic differences between different ethnic groups cannot be ruled out, it still remains unclear why there is such a high variability in the TNF-α levels between individuals presenting ENL.
A study of the plasma levels of TNF-α reported increased levels during ENL (150) while other studies contradicted this finding (115, 134, 151). In fact, Haslett et al., which included 20 male ENL patients excluding patients with moderate or severe ENL–associated neuritis, reported circulating plasma TNF-α levels to be lower at time of ENL diagnosis than LL controls (115). There was an upward trend in plasma TNF-α levels during thalidomide treatment which returned to baseline levels after discontinuation of thalidomide (115). This is an indication that thalidomide may in fact stimulate paradoxical overproduction of TNF-α (115). The inhibition of TNF-α by thalidomide may be prominent when macrophage production of this cytokine is high but in mild disease plasma levels may not reflect lesional TNF-α production (115). Increased TNF-α levels after thalidomide treatment has been described in other conditions such as toxic epidermal necrolysis (152) and aphthous ulcers in patients with human immunodeficiency virus infection (153). It has been suggested that the mechanism of the paradoxical overproduction of TNF-α by thalidomide could be due to the propensity of thalidomide to costimulate T-cells to produce cytokines including TNF-α (154). All the patients in the study of Haslett et al. showed improvement in ENL after receiving thalidomide during the first 21 days of treatment (115).
Interestingly, the studies that measured the ex vivo PBMC production of TNF-α in response to lipopolysaccharide, BCG, or M. leprae in patients with ENL as compared to BL/LL patients showed consistently greater amounts of TNF-α secretion in patients with ENL (150, 155–157).
The successful use of the anti-TNF therapy with infliximab and etanercept in three patients with ENL, resulting reduction of inflammation and treatment of ENL, is additional evidence of the inflammatory role of TNF-α in ENL (158–160).
The results of studies on IFN-γ are more consistent than those on TNF-α suggesting an important role for IFN-γ in the pathophysiology and occurrence of ENL. A clinical trial administered recombinant IFN-γ to BL/LL patients as a replacement therapy because LL is characterized by anergy to antigens of M. leprae and inability to produce IFN-γ (150). Repeated intradermal injection of recombinant IFN-γ induced ENL in 6 out of 10 BL/LL patients within 7 months compared to an incidence of 15% per year in patients who received MDT alone (150). Elevated serum IFN-γ was found in patients with ENL who also had high TNF-α levels (139). Other studies have demonstrated an increase of serum IFN-γ (143, 144, 148) and an increase of IFN-γ mRNA in PBMC (161–163) and in skin biopsies (161, 164) during ENL. There is a study reporting serum IFN-γ to be significantly lower in patients at the onset of ENL, which increased after thalidomide treatment (142). However, IFN-γ has been identified by Ingenuity Pathway Analysis networks as the second most significant upstream regulator (after CCL5) of the expression changes in microarrays performed in PBMC derived from patients with ENL (58).
There are contradictory findings about the role of serum IL-1β levels. Most studies have reported that serum IL-1β levels may have a prognostic value for developing ENL (144, 148, 165, 166) and that there is a statistically significant correlation between TNF-α and IL-1β (140). However, studies failed to show any association of serum IL-1β or plasma IL-1β with ENL (138, 151). IL-1β mRNA in PBMC was upregulated at the onset of ENL (161) but not in skin lesions (167).
IL-2 has a key role in the immune system primarily by its direct effects on T-cells such as promoting differentiation of different T-cell subsets and contributing to the development of T-cell immunological memory. IL-2 signals through the IL-2 receptor (IL2R), which is essential for the signaling in T-cells. There were no differences in the serum IL-2 or IL2 mRNA in skin biopsies between ENL and patients with LL (115, 148, 151). However, four studies reported an increase in soluble IL-2 receptor (sIL2R) levels (115, 131, 165, 168) or IL2Rp55 mRNA in PBMC (161) in patients with ENL.
Serum IL-6 (147, 151, 169, 170) and IL-6 mRNA in PBMC and skin (161) have been reported to be elevated during ENL. IL-6 tag single-nucleotide polymorphisms have been reported to be a risk factor for ENL (170) and IL-6 plasma levels were correlated with the IL-6 genotypes (170). A study reported increased serum IL-6 receptor (sIL6R) levels in ENL, which declined significantly after the completion of a corticosteroid treatment (143). However, other studies did not show associations of IL-6 serum levels with ENL (134, 139, 143).
An ex vivo study in PBMC isolated from ENL patients and LL controls showed a correlation of raised levels of cytokines IL-17A and its isomers as well as other Th17-associated cytokines IL-21, IL-22, and IL-23 with ENL (113). However, other studies failed to detect an association of ENL with serum IL-17 (110, 151, 171).
There are 49 studies measuring cytokines in ENL (Table 4), and the majority of these studies show a significant increase of the pro-inflammatory cytokines during ENL. TNF-α appears to be a regulator of the condition while there is substantial evidence supporting a role for IFN-γ as well. There is also evidence that other cytokines such as IL-1β and IL-6 or cytokine receptors such as sIL2R and sIL6R are also involved. Therefore, inhibitors of these molecules may be useful in a clinical setting. It is possible that genetic differences could account for differences observed between studies but methodological differences are also likely factors.
Table 4.
Human studies on ENL investigating cytokines.
| Reference; study site(s) | Study population | Timing of sampling | MDT status | ENL treatment status | Type of samples | Measures | Findings |
|---|---|---|---|---|---|---|---|
| Filley et al. (168); India ε | 7 ENL | Before, during and after the episode | All patients on MDT | ENL treated with steroids and/or thalidomide | Serum | IL2R | IL2R increase during ENL |
| Rea and Modlin (102); USA γ | ND | ND | ND | ND | Skin | IL-2 positive and IFN-γ positive mRNA-bearing lymphocytes | IL2- positive lymphocytes prevalent in ENL and in tuberculoid lesions |
| Cells expressing IFN-γ mRNA in ENL lesions slightly increased compared to lepromatous | |||||||
| Sarno et al. (138); Brazil | 18 ENL, 39 BT/BL/BB/LL, 4 T1R | ND | 16/18 patients on various stages of MDT/2 untreated | 3 ENL on thalidomide and 7 ENL on prednisone; others untreated for reaction | Serum | Tumor necrosis factor (TNF)-α and IL-1 | TNF varied from undetectable to extremely high levels in ENL |
| No correlation between severity of ENL and cytokine level | |||||||
| Neither TNF nor IL-1 correlate with number or duration of ENL episodes | |||||||
| Treated patients with steroids or thalidomide lower TNF | |||||||
| Sehgal et al. (172); India | 11 ENL, 14 T1R, 20 leprosy non-reactional, 10 HC | Before starting antireactional treatment and when clinical signs of reaction had abated | On MDT | Samples before and after starting antireactional treatment | Serum | IL-2R | T1R upgrading group higher IL-2R than ENL |
| Sullivan et al. (173); USA ε | ND | ND | ND | ND | Skin | IFN-γ and TNF-α mRNA | IFN-γ mRNA in ENL similar to tuberculoid |
| In LL and ENL lesions about 0.2% of cells expressed TNF-α | |||||||
| Barnes et al. (155); USA | 12 active ENL, 14 inactive ENL, 6 T1R; 11 LL | ND | All patients had received less than 5 years chemotherapy | ND | PBMC | TNF-α | ENL: the levels of TNF-α release by PBMC were higher than any other leprosy |
| Thalidomide reduced TNF-α by more than 90% | |||||||
| Parida et al. (140); India | 12 ENL, 64 leprosy TT/BT/BB/BL/LL, 14 T1R | ND | Most patients before MDT treatment | ND | Serum | TNF and IL-1 | Patients undergoing T1R or ENL showed high TNF levels |
| Significant correlation between TNF and IL-1 in reaction | |||||||
| Sampaio et al. (150); Brazil and USA | 13 LL ENL, 15 LL, 9 HC | ND | All patients were receiving MDT during the study. | 7 ENL patient blood samples before starting treatment with thalidomide and 6 1-2 weeks after thalidomide | Plasma | TNF-α | ENL patients greater release of TNF-α from monocytes |
| PBMC | High plasma TNF-α in ENL | ||||||
| Monocytes | |||||||
| Bhattacharaya et al. (146); India | 11 ENL, 14 T1R, 20 leprosy without reactions, 20 HC | Before treatment and after clinical remission of reaction | on MDT | Before antireactional treatment with steroids | Serum | TNF | TNF levels in acute ENL were higher but not significant and rose to become significant following treatment and clinical remission than HC and MB controls |
| Foss et al. (128); Brazil γ | 28 lepromatous: 11 ENL, 23 tuberculoid, 19 HC | ND | 86% of lepromatous patients treated with dapsone | Time of blood collection no immunosuppressive drug | Serum | TNF-α | TNF was elevated in the serum of ENL patients |
| Sampaio et al. (139); Brazil ε | 49 BL/LL: 24 developed ENL | At the time of developing ENL, during thalidomide treatment, or after thalidomide treatment was discontinued; collected at 1-3, 6-7, and/or 13-21 days of thalidomide and 1-2 months after thalidomide | MDT was continued through the study | Thalidomide treatment for ENL | Sera | TNF-α, IL-6, IFN-γ | ENL highest TNF-α levels, which decreased significantly during thalidomide treatment Serum IFN-γ elevated in patients with high TNF-α levels |
| Santos et al. (156); Brazil | 14 ENL (4 BL/10 LL), 12 BL/LL, 11 HC, 4 ENL post-reactions | ND | Half untreated and the other half treated with MDT | ENL patients were treated with thalidomide? | PBMC | TNF-α: spontaneous and M. leprae stimulated | ENL patients showed significantly greater release of TNF-α both spontaneously and induced by M. leprae-induced release in ENL patients |
| Vieira et al. (131); Brazil γ, ε | 95 MB (30 LL/65 BL) of which 51 ENL | At leprosy diagnosis and at onset of reactional episode | Time of MDT for each ENL | Sample before thalidomide and steroids? | Serum | TNF-α, soluble IL-2R | TNF-α increased in 70.6% of ENL patients |
| Memon et al. (141); Pakistan | 12 ENL, 27 leprosy (TT/BT/BL/LL), 14 household contacts and 22 endemic HC with no known leprosy contact | At the onset of ENL before initiation of treatment for reaction and after the reaction had subsided | 10/12 ENL received previous MDT | Samples before antireactional treatment | Serum | TNF-α | TNF levels higher during acute phase of ENL and declined after clinical remission of the reaction |
| Moubasher et al. (148); Egypt | 35 reactional (19 ENL/16 T1R), 55 leprosy, 20 HC | ND | Untreated ENL? | Untreated ENL? | Serum | IFN-γ, IL-2, IL-2R, IL-10, TNF-α, IL-1β | Both T1R and ENL showed significantly higher serum IFN-γ, IL-2R and IL-1β compared to non-reactional leprosy ENL showed increased levels of IL-10 compared to T1R |
| Moubasher et al. (165); Egypt | 35 reactional (19 ENL), 36 non-reactional, 20 HC | PB patients assessed after 6 and 12 months of MDT/MB assessed after 12 months of MDT; Before and at the end of treatment with MDT | Before and after treatment with MDT | Corticosteroids were given to control the reactions | Serum | IL-2R, IL-10, IL-1β | IL-1β levels may have a prognostic marker for the development of reactions |
| Partida-Sanchez et al. (142); Mexico ε | 9 ENL, 10 non-ENL, 10 HC | Beginning of reaction and after 1 and 2 months of thalidomide | All patients on MDT | Untreated samples and after 1 and 2 months of thalidomide | Serum | TNF-α, IFN-γ | TNF-α was significantly higher in ENL compared to non-ENL |
| TNF levels decreased after ENL treatment | |||||||
| IFN-γ significantly lower in patients at the onset of ENL and increased after thalidomide | |||||||
| Sampaio et al. (147); Brazil | 18 MB with ENL (5BL/13LL) | Biopsies at diagnosis, at onset of reaction, and after 3 and/or 7 days of pentoxifylline; Serum: day 0 (during ENL), 3-7, 10-14, 30 and 60 days after pentoxifylline | 7 patients with ENL newly diagnosed; others on MDT | Pentoxyfylline, 2 ENL patients on thalidomide | PBMC | Serum TNF-α, IL-6, IL-10 | Elevated TNF-α in the sera of ENL |
| Serum | TNF-α, IL-6, IL-10 release by PBMC following M. leprae stimulation or LPS stimulation | Treatment with pentoxifylline reduced TNF-α | |||||
| Serum levels of IL-6 increased during ENL | |||||||
| High TNF-α mRNA expression in lesions during ENL which decreased following treatment with pentoxifylline | |||||||
| Skin | TNF-α, IL-6, IL-10 gene expression at skin | IL-6 mRNA reduced by up to 50-fold after treatment | |||||
| Moraes et al. (161); Brazil | 53 leprosy: 20 ENL, 11 T1R | At the time of leprosy diagnosis (unreactional) and at the onset of first reactional episode (reactional) | MDT was continued through the study | No anti-inflammatory drugs at the time of sample collection | PBMC | IL-1β, IL-6, IL-8, GM-CSF, IFN-γ, IL-2Rp55, perforin, TNFβ, TNF-α mRNA in PBMC; IL-4, IL-6, IL-8, IL10, IL-12, IFNγ, TNFα mRNA in skin | In 7 ENL higher incidence of IFN-γ, perforin, GM-CSF, IL2R mRNA in blood |
| Upregulation of IL-1β, IL-6, GM-CSF, IL-2R, IFN-γ mRNA in blood at onset of ENL at 3 ENL follow-up | |||||||
| 3 patients sequential sampling and after thalidomide | Skin | Skin lesions ENL: IFNγ and IL-4 differentially expressed | |||||
| Oliveira et al. (33);Brazil α | 10 BL/LL: 6 ENL, 10 HC | ND | On MDT | ND | Blood, P.B.Neutrophils | TNF-α, IL-8 | Stimulated neutrophils secrete IL-8 and TNFα |
| Increased TNF-α secretion from neutrophils after LPS stimulation | |||||||
| Thalidomide inhibited TNF-α by neutrophils | |||||||
| Goulart et al. (174); Brazil | 19 leprosy: 5 ENL/3 T1R, 9 HC | Untreated samples | Untreated samples | Untreated samples | PBMC | TGF-β1 in supernatants from adherent PBMC after stimulation with PGL-1, LPS or serum-free RPMI | Adherent PBMC from ENL secrete higher TGF-β1 |
| Moraes et al. (164); Brazil | 13 MB: 10 ENL, 3 T1R | Before and during pentoxyfilline or thalidomide | All patients on MDT | Before and during pentoxyfilline or thalidomide | Skin | mRNA expression: IFN-γ, IL-6, IL-10, IL-12 p40, TNF-α, IL-4 | Expression of IFN-γ, IL-6, IL-10, IL-12 p40, TNF-α at the onset of reactional episodes (T1R and ENL) but IL-4 rarely detected Follow-up: TNF-α mRNA and IFN-γ, IL-6 and IL12p40 mRNA decreased after thalidomide or pentoxyfylline |
| Nath et al. (162); India | 36 ENL, 105 TT/BL/LL 7T1R, 9 HC | ND | All patients on MDT | ENL patients before antireactional treatment | PMBC | IFN-γ, IL-4, IL-10, IL-12 | ENL: 58% demonstrated a polarized Th1 pattern with only 30% expressing both cytokines |
| Nath et al. (163); India | 1 BL/7 LL ENL, 2 BL/6 LL8 stable | ND | Most patients on MDT | ENL patients prior to antireactional therapy | PBMC | Real-time PCR forIFN-γ, IL4, IL10, p40 IL12 | IFN-γ detectable in all and IL12p40 in half of ENL IL12p40 mRNA higher in ENL compared to stable lepromatous |
| Sampaio et al. (157); Brazil | 15 leprosy: 10 ENL | ND | On MDT | ND | PBMC, monocytes, monocytes/T-lymphocytes cocultures | TNF-α after stimulation with M. leprae | Isolated monocytes from ENL released significantly more TNF-α in response to M. leprae than monocytes from non-reactional |
| Tadesse et al. (132); Ethiopia γ | 14 BT, 11 BT T1R, 8 ENL, 11 HC | ND | ND | All ENL treated with steroids | PBMC | TNF-α in culture supernatants | Thalidomide resulted in suppression of TNF-α production |
| Haslett et al. (115); Nepal | 20 ENL, 20 LL with no history of ENL within the preceding 30 days | Blood samples: days 0, 3, 7, 14, and 28 of thalidomide; ELISPOT: days 0, 7, 21, and 28 | All (except 1 patient) on MDT | Excluded patients who had received immunomodulating therapy within the preceding month | Plasma | Plasma levels of IFN-γ, TNF-α, soluble IL2R, IL-12, IL-12 p40 and IL-12 p70 | Circulating TNF-α levels lower at ENL diagnosis than controls |
| Flow cytometry: days 0, 7, and 21; qRT-PCR: PBMCs days 0, 7, 21 | T-cells | ELISPOT for IFN-γ; Flow cytometry for cytokine production by T cells | Upward trend during thalidomide ENL baseline plasma levels of IL-12 lower than control | ||||
| Skin | qPCR: IL-2 genes | Baseline levels of sIL2R higher in ENL than controls | |||||
| Thalidomide increased T cell subsets expressing both IL-2 and IFN-γ | |||||||
| Villahermosa et al. (134); Philippines γ, ε | 22 ENL | Before thalidomide and at study weeks 3 and 7 during thalidomide | MDT was continued | Samples untreated for antireactional drugs and during thalidomide | Plasma | TNF-α, IL-6 | TNF-α levels not detected |
| IL-6 unchanged or reduced following thalidomide from week 0 to week 3 | |||||||
| IL-6 undetectable at weeks 3 and 7 | |||||||
| Belgaumkar et al. (169); India | 71 BT/BB/BL, 11 pure neuritic, 6 T1R, 1 ENL, 30 HC | Untreated samples | Untreated samples | Patients on antileprosy treatment or steroids were excluded | Serum | IL-6, IFN-γ | The one patient with ENL had higher levels of IL-6 and IFN-γ in comparison to the BL/LL patients without reactions |
| Iyer et al. (143); Indonesia ε | 131 TT/BT/BB/BL/LL, 44 ENL, 5 T1R, 112 HC | ND | Patients on MDT | Prednisolone to treat reactions | Serum | IL-6, IFN-γ, TNF-α, IL-6R, IL-10, IL-4, sCD27 | IFN-γ and IL-6R increased in ENL compared to non-ENL |
| Completion of corticosteroid treatment: IFN-γ, TNF-α, sIL6R declined | |||||||
| Stefani et al. (151); Brazil | 10 ENL, 10 T1R, 29 non-reactional controls | Newly detected untreated patients | Untreated samples | Untreated samples | Plasma | TNF-α, IFN-γ, IL12p70, IL-2, IL-17, IL-1β, IL-6, IL-15, IL-5, IL-8, MIP-α, MIP-β, RANTES, MCPI, CCL11/eotaxin, CXCL10, IL-4, IL-10, IL13, IL-1Rα, IL-7, IL-9, G-CSF, PDGF BB, bFGF, VEGF | IL-6, IL-7 and PDGF BB elevated in ENL |
| Motta et al. (175); Brazil | 44 leprosy of which 15 ENL, 10 HC | Baseline and 7 days after therapy for oral infection | ND | ND | Serum | IL-1, TNF-α, IL-6, IFN-γ, IL-10 | No specific finding for ENL |
| Teles et al. (176); Brazil ε | 32 leprosy: 10 ENL, 8 T1R | 4 ENL patients before and during reaction | All patients on MDT | ND | Skin | TNF-α gene expression and levels in supernatants | PBMC stimulated with M. leprae: upregulation of gene expression of TNF-α and increase of TNF-α in supernatants after 1, 3, and 6 h |
| PBMC | |||||||
| Jadhav et al. (149); India ε | 303 MB: 5 ENL | Serum samples at the time of recruitment | Newly registered: no MDT | Untreated | Serum | TNF-α | No significant outcome for ENL |
| Madan et al. (144); India | 61 leprosy: 4 ENL and 2 ENL during study | Untreated samples, during reactional episodes and after completion of treatment | Untreated patients | Patients on steroids were excluded | Serum | TNF-α, IFN-γ, IL-1β, IL-10 | All cytokines were raised in reactional (both T1R and ENL) compared to non-reactional IFN-γ, IL-1β and IL-10 were higher in ENL but only IL-10 was statistically significant compared to T1R |
| Levels of all cytokines decreased after MDT | |||||||
| Rodrigues et al. (145); Brazil | 18 LL with ENL during treatment; 13 non-reactional BT, 37 non-reactional BL/LL, 25 BL with T1R during treatment; 21 HC | Beginning of leprosy treatment, at diagnosis of reactional episode and at 3-5 years post-treatment | Samples before and during MDT | Untreated samples and after treatment with prednisolone | Serum | TNF-α | TNF-α higher during ENL than prior to the reaction |
| Chaitanya et al. (177); India | 21 ENL, 80 T1R, 80 leprosy without reaction, 94 non-leprosy | Untreated samples | Untreated samples | Untreated samples | Serum | IL-17F | IL-17F elevated during T1R but no significant difference in ENL |
| Lockwood et al. (178); India ε | 303 MB leprosy: 13 ENL | Skin biopsies at enrollment | Before MDT | Before antireactional treatment | Skin | TNF-α and TGF-β immunostaining | TNF-α: similar levels ENL and non-ENL TGF-β: no difference in ENL and non-ENL |
| Martiniuk et al. (179); Nepal and USA ε | 7 ENL | Pre- and post- treatment with thalidomide | ND | Pre- and post- treatment with thalidomide | Skin biopsies | RT-PCR for hIL-17A, hIL-17B, hIL-17C, hIL-17D, hIL-17E, hIL17F | IL17A, was consistently seen before and after thalidomide |
| Reduction in IL17B, IL17E and increase of IL17C following thalidomide | |||||||
| Sousa et al. (170); Brazil | 33 ENL, 54 T1R, 16 reaction-free leprosy | ND | 63.8% presented ENL during MDT | ND | Plasma | IL-6 | Higher IL-6 in ENL and T1R compared to non-reactional |
| Abdallah et al. (171); Egypt | 43 leprosy: 6 ENL, 43 HC | Untreated samples | Untreated samples | Untreated samples | Serum | IL-17, IL-4 | Overproduction of IL-4 in LL patients |
| Saini et al. (136); India γ | 21 MB: 16 ENL, 5 T1R | ENL blood during reaction and at 0.5 and 1 year after the onset of reaction | Duration of MDT described | ENL patients received steroids | PBMC | PBMC stimulated with M. leprae, recombinant Lsr2 and 6 synthetic peptides spanning the Lsr2 sequence: IFN-γ | During ENL stimulated PBMC showed IFN-γ release |
| Abdallah et al. (109); Egypt γ | 43 leprosy: 6 ENL, 40 HC | Untreated patients | Untreated samples | Untreated samples | Serum | IL-1β, IL-4, IL12p70, IFN-γ | IL-4 highest among LL compared to ENL |
| Attia et al. (110); Egypt γ | 43 leprosy: 6 ENL, 40 HC | Untreated samples | Untreated samples | Untreated samples | Serum | IL-17, IL-22, IL-10, TGF-β | No statistically significant difference between groups |
| Berrington et al. (167); Nepal | 85 leprosy: 9 ENL, 35 BL/LL non-reactional | ND | ND | ND | Skin | RT-PCR for CCL1, CCL2, CCL17, CCL18, IFNA1, IFNA8, IFNB1, IFNG, IL10, IL12a, IL12b, IL13, IL17a, IL18, IL1b, IL1ra, IL21, IL22, IL23, IL27, IL29, IL4, IL6, TNF | CCL18, IL12b and CD14 elevated in lesions of ENL but failed to reach significance when adjusted for multiple comparisons |
| Sallam et al. (166); Egypt | 43 leprosy: 6 ENL, 43 HC | Untreated samples | Untreated samples | Untreated samples, excluded patients on corticosteroids | Serum | IL-1β, IL-12 | Higher IL-1β in ENL compared to non- reactional |
| No significant difference for IL-12 | |||||||
| Dupnik et al. (58); Brazil β, ε | 11 ENL, 11 T1R, 19 leprosy without reactions for microarray; 6 ENL, 11 T1R, 11 non-reactional for qPCR; 3 ENL for ICH | ND | 3/11 ENL pre-treatment, 2/11 ENL on treatment and 6/11 post-treatment; leprosy controls matched for length of treatment | Excluded patients on steroids within 7 days and thalidomide within 28 days of enrollment | PBMC | Microarrays followed by qPCR | Cytokine-cytokine receptor interaction has been in the top 3 KEGG pathways in ENL CCL5 followed by IFN-γ was the most significant upstream regulator of the expression changes in the array |
| Saini et al. (113); India γ | 66 leprosy: 15 T1R, 15 ENL, 36 stable leprosy without previous history or clinical evidence of reactions | Newly diagnosed leprosy patients prior to institution of antireaction therapy | Untreated samples | Untreated samples | PBMC | Antigen (MLSA) stimulated and unstimulated PBMC: gene expression with PCR array for 84 genes ELISA for cytokines IL-17A/F, IL-21, IL-22, IL-23A, IL-6, IL-1β, IFN-γ, TGF-β in supernatants |
IL-23A mRNA expression increased in ENL IL-23R expression increased in ENL High expression of CCL20 and CCL22 in ENL ENL significant fold increase in IFN-γ Culture supernatants: Higher IL-17A/F in ENL patients compared to LL IL23A increased compared to LL IL-1β increased in ENL |
| Dias et al. (80); Brazil ε | 30 ENL, 24 BL/LL, 31 HC | Upon diagnosis of reaction | BL/LL before MDT but most ENL patients on MDT | Before treatment with thalidomide or steroids | PBMC | TNF, IL-6 and IL-1β in response to TLR9 agonist | Higher production of TNF-α, IL-6, IL-1β in response to TLR9 agonist |
| TLR9 antagonist inhibited the secretion of cytokines in response to M. leprae lysate | |||||||
α, also in Table 1; β, also in Table 2; γ, also in Table 3; ε, also in Table 5.
BB, mid-borderline leprosy; BL, borderline lepromatous leprosy; BT, borderline tuberculoid leprosy; ENL, erythema nodosum leprosum; HC, healthy controls; ICs, immune complexes; LL, lepromatous leprosy polar; ND, not described; P.B.neutrophils, peripheral blood neutrophils; SLE, systemic lupus erythematosus; TB, tuberculosis; TT, tuberculoid leprosy polar.
What Other Immune Mechanisms are Implicated in ENL?
Sixty-four studies on other immunological factors in ENL have been performed (Table 5).
Table 5.
Human studies on ENL investigating other immunological factors.
| Reference; study site(s) | Study population | Timing of sampling | MDT status | ENL treatment status | Type of samples | Measures | Findings |
|---|---|---|---|---|---|---|---|
| Waters et al. (22); United Kingdom and Malaysia β | 38 lepromatous ENL | ND | ND | ND | Serum | Immunoglobulins | No differences in immunoglobulin levels |
| Reichlin et al. (180); Malaysia | 13 LL of which 7 ENL | ND | ND | ND | Blood | Euglobulin IgG | Levels of euglobulin IgG higher in the ENL-positive patients than in ENL-negative patients |
| Serum IgG | |||||||
| Anthony et al. (60); India β | 25 LL ENL, 10 LL without ENL | Active ENL lesions? | ND | ND | Serum | Immunoglobulins | High levels of immunoglobulins in both LL and ENL |
| Harikrishan et al. (71); India β | 20 active LL; 15 ENL during active and subsided phase; 20 HC | ENL: during the active and subsided phase | ND | ND | Plasma | Immunoglobulins IgG, IgM, IgA | Serum levels of IgG and IgM during subsidence of ENL were significantly lower compared to that during the active phase of ENL |
| Humphres et al. (181); USA | 14 LL ENL, 28 BL/LL, 21 HC | Multiple Serial sampling | 10/19 LL patients untreated, 9/19 LL patients Dapsone | Corticosteroids day prior to initial assay for NK activity and was continued through treatment | PBMC | Natural killer cell activity | Natural killer cell activity significantly depressed in ENL |
| Rea and Yoshida (182); USA | 108 leprosy (4 untreated ENL, 14 dapsone-treated active ENL, 10 dapsone-treated inactive ENL), 25 HC | ND | 54 untreated patients and others dapsone-treated | Untreated | Blood | Macrophage migration inhibition activity | Positive serum inhibitory activity strongly associated with reactional states (ENL or T1R or Lucio’s reaction) in both treated and untreated patients |
| Miller et al. (183); USA | 9 leprosy: 3 T1Rs and 2 ENL | Serial sampling from date of initiation of therapy until the first year of treatment | On MDT | Reactional episodes were treated with corticosteroids and 1 ENL received thalidomide | Plasma | Antibodies to Mycobacterial Arabinomannan | High levels of antibody to Arabinomannan in 2 ENL patients |
| Narayanan et al. (89); India γ | 35 leprosy patients: 7 LL with ENL, 6 BT, 6 BT with T1R, 4 BL, 5BL with T1R, 8 LL | ND | ND | ND | Skin | B cells | No increase of B cells in any of the lesions |
| Rea et al. (96); USA γ | 19 ENL, 67 BL/LL 4 LL with Lucio’s, 13 T1R, 18 Tuberculoid, 13 Tuberculoid long-term treatment | ND | Some patients on MDT | ENL before receiving thalidomide | PBMC | B-cells | B-cell percentage in the PBMC of ENL similar LL |
| Schwerer et al. (184); USA | 121 leprosy (including ENL), 28 contacts, 15 HC | ND | ND | ND | Serum | Anti-PGL I IgM levels | Serum anti-PGL I IgM levels lower in ENL compared to patients with comparable BI |
| Andreoli et al. (40); India | 12 ENL | ND | All patients on MDT with specified duration | Treated with prednisone and/or thalidomide | Serum | Circulatory IgM antibody levels to the PGL I; IgM, IgG, IgA antibody levels to M. leprae antigenic preparation | During ENL: decrease of circulatory IgM antibody levels to PGL I but no significant change to IgG, IgM or IgA antibody levels to the soluble antigens from M. leprae |
| Laal et al. (94); India γ | 15 ENL, 13 LL | During active ENL and 1 week to 4 months after stopping treatment | Treatment with combination antileprosy drugs was continued throughout | First sample before initiation of antireactional treatment; second sample 1 week to 4 months after treatment | PBMC | B cells | B-cell percentages in PBMC of ENL patients were similar to those of uncomplicated LL |
| Blavy et al. (185); Senegal | 34 ENL and 50 leprosy patients | ND | ND | ND | Lymphocytes | HLA phenotyping | Not significant findings of any HLA phenotype regarding ENL |
| Levis et al. (186); USA | ND | ND | ND | ND | Serum | IgM and IgG antibodies to PGL-I | ENL lower anti-PGL-I IgM than non-ENL of comparable BI |
| Rao and Rao (123); India γ | 44 ENL, 39 LL, 22 post-ENL | ENL cases before starting treatment for ENL, post-ENL after the patient had not taken anti-inflammatory drugs or steroids for at least 3 and 7 days | From 39 non-reactional: 20 untreated and 19 with dapsone for less than a year | ENL before starting ENL treatment, post-ENL after the patient had not taken anti-inflammatory drugs or steroids for at least 3 and 7 days | Blood | B lymphocytes in peripheral blood | B cells: no difference between groups |
| Sehgal et al. (76); India β | 21 patients with leprosy reactions either T1R or ENL | ND | ND | ND | B-cells | Percentage and absolute count of B-cells; Immunoglobulins IgG, IgA, IgM | During ENL a significant increase in the percentage and absolute count of B-lymphocytes Significantly elevated serum immunoglobulin values after subsidence of ENL |
| Serum | |||||||
| Levis et al. (187); USA | 40 ENL, 63 leprosy without ENL, HC | ND | ND | ND | Serum | IgM antibody to PGL-I; IgM and IgG Abs to M.tb and M. leprae LAM | No correlation between IgM or IgG Ab to LAM and bacillary index |
| Rao and Rao (85); India | 44 ENL, 39 lepromatous, 22 post-ENL | ENL blood before starting ENL treatment, post-ENL after patient does not take any anti-inflammatory drugs or steroids for the last 3 or 7 days | 20 patients no previous MDT and 19 treated with dapsone | Before starting treatment with anti-inflammatory drugs or steroids | Blood | Leukocyte migration inhibition test | Lower migratory indices to whole M. leprae during ENL |
| Rao and Rao (78); India β | 44 ENL, 39 BL/LL, 22 post-ENL | ENL before starting anti-ENL treatment, post-ENL ensuring that the patient had not taken anti-inflammatory drugs or steroids for at least 3 or 7 days | 20 BL/LL untreated and 19 BL/LL treated with dapsone | Before starting treatment with steroids or anti-inflammatory drugs | Serum | IgG, IgA, IgM | IgG and IgM decreased in ENL than lepromatous and post-ENL |
| Serum IgA elevated in ENL than lepromatous group and further increase post-ENL | |||||||
| Filley et al. (168); India δ | 7 ENL | Before, during and after the episode | All patients on MDT | ENL was treated with steroids and/or thalidomide | Serum | %GO | During ENL%GO transiently raised, and this rise parallels an increase in circulating IL2R |
| Bhoopat et al. (127); Thailand γ | 57 ENL (19 acute/38 chronic), 61 active LL, 33 control patients whose leprosy had been cured | 26 BL and 35 LL newly diagnosed and untreated | ND | When treatment with corticosteroids and/or thalidomide was initiated precise timing was recorded with respect to the time of collection of specimens | Blisters induced over a representative skin lesion | IgM antibody to PGL-I and Tac peptide | IgM antibody to PGL-I and Tac peptide levels were elevated in chronic ENL lesions |
| Corticosteroids reduced IgM antibody to PGL-I but did not change the levels of Tac peptide | |||||||
| Ramanathan et al. (49); India β | 26 BL/LL of which 11 ENL, 24 HC | Blood was taken before initiation of treatment and then to 2-month intervals up to 20 months | Untreated and then on MDT samples every 2 months | Treated but after blood sampling | Serum | IgG, IgA and IgM | ENL no significant relation with immunoglobulin levels |
| Sullivan et al. (173); USA δ | ND | ND | ND | ND | Skin | ICAM-1, ICAM-1 ligand LFA-1 | Prominent keratinocyte ICAM-1 expression |
| Scollard et al. (82); Thailand β | 4 cured leprosy, 10 leprosy (5BT, 3BL, 2LL), 8 ENL patients (5LL and 3BL), 3 T1R, 4 HC | ND | ND | ND | Blisters induced over representative skin lesion | Immunoglobulins (IgG, IgA, IgM) to whole M. leprae and to PGL-I | No statistically significant difference regarding immunoglobulins |
| Sera | |||||||
| Sehgal et al. (188); India | 25 leprosy with reactions (of which 11 ENL), 20 leprosy without reactions, 10 HC | ND | On MDT | Reactional patients on prednisolone | Lymphocytes | Lymphocyte adenosine deaminase activity (L-ADA) | The patients with leprosy reactions (both ENL and T1Rs) had higher enzyme l-ADA than controls (the enzyme has a role in activation, differentiation and proliferation of lymphocytes) |
| Sampaio et al. (139); Brazil δ | 49 BL/LL of which 24 ENL | ENL at the time of developing ENL, during thalidomide treatment, or after thalidomide treatment | MDT was continued through the study | Certain ENL during thalidomide treatment | Skin biopsies | MCH II and ICAM-1 in histology | MHC II and ICAM-1 on epidermal keratinocytes in ENL downregulated with thalidomide |
| Santos et al. (129); Brazil γ | 10 ENL, 59 LL/BL, 4 T1R, 4 post-reactional | ND | On MDT | No antireactional treatment before blood collection | PBMC, Monocytes | Monocyte activation by procoagulant activity, HLA-DR | No significant difference in monocyte activation between the different groups No significant differences in HLA-DR between groups |
| Singh et al. (189); India | 44 active ENL, 48 prior history of ENL, 125 stable lepromatous, 40 HC not endemic | ND | ND | Untreated samples | Serum | Antibodies against B cell epitopes of M. leprae recombinant protein LSR | Antibodies against a specific distinct peptide region only in patients undergoing ENL |
| Kifayet and Hussain (190); Pakistan | 67 BL/LL acute ENL, 83 non-reactional BL/LL, 77 endemic HC | ND | Most on MDT but 83 non-reactional less than 2 weeks of MDT | ND | Plasma | M. leprae-specific IgG subclasses | Lower concentrations of all IgG subclasses during ENL but lower IgG1 and IgG3 during ENL before treatment |
| Kifayet et al. (191); Pakistan | 13 ENL acute and post-remission of reaction, 16 non-reactional stable LL, 32 endemic HC | During acute ENL (n = 13) and after the reaction has subsided | ND | ND | Plasma | IgG subclasses M. leprae-specific antibodies; Detection and enumeration of antibody-secreting B cells by ELISPOT | Polyclonal IgG1 elevated in acute ENL compared LL controls and decreased when ENL subsided IgG2 antibodies lower during acute ENL and increased after reaction has subsided Discrepancy in serum concentrations and B cell frequency |
| B-cells | |||||||
| Vieira et al. (131); Brazil γ, δ | 95 MB leprosy (30 LL and 65 BL) of which 51 ENL | At leprosy diagnosis and at onset of reactional episode | Time of MDT for each ENL patients in study | Sample before thalidomide and steroids? | Serum | Circulating anti-neural and antimycobacterial antibodies | Detection of anti-neural (anti-ceramide and anti-galactocerebroside) antibodies in ENL sera |
| No difference between reactional and non-reactional lepromatous patients regarding IgM antibodies | |||||||
| Higher levels of anti-ceramide IgM and diminished levels of anti-galactocerebroside antibodies in reactional compared to non-reactional patients | |||||||
| Rojas et al. (50); Brazil β | 19 ENL, 10 BL/LL non-ENL patients, 13 family contacts; 15 healthy non-contacts | ND | Both untreated patients and patients on MDT for 1-72 months | ND | Serum | Anti-PGL-I IgM, IgG responses to recombinant 10-kDa heat shock protein | IgM anti-PGL-I and IgG anti-10-kDa heat shock protein antibodies were constituents of the immune complexes in patients with ENL while free antibody levels did not differentiate between ENL and non-ENL patients |
| Beuria et al. (192); India | 18 ENL, 44 BL/LL, 62 BT/TT, 17 HC | ND | Most patients on MDT | ND | Serum | IgG subclass levels to M. leprae sonicated antigens (MLSA) and PGL-I | ENL patients showed a significant fall in IgG3 antibody to MLSA and PGL-I compared to BL/LL leprosy controls |
| Freire et al. (193); Brazil | 59 leprosy (including 12 ENL), 60 HC | ND | 11/12 ENL were on MDT and 1/12 with dapsone | ND | Serum | Anti-neutrophil cytoplasmic antibodies (ANCA) | ANCA are present in 28.8% of leprosy patients but are not related to vasculitis in the ENL reaction and are not a marker of a specific clinical from |
| Partida-Sanchez et al. (142); Mexico δ | 9 ENL, 10 non-ENL leprosy, 10 HC | Beginning of reaction and after 1 and 2 months of thalidomide | All patients on MDT | Before thalidomide, second sample after 1 month of thalidomide and third after 2 months | Plasma | IgM and IgG antibody subclasses to M. leprae sonicated extract | ENL at the onset of reaction had slightly higher anti-M. leprae IgG1 and IgG2 antibodies compared to non-ENL but not statistically significant |
| Stefani et al. (194); Brazil | 600 leprosy: 31 ENL, 45 T1R, HC | Untreated | Before MDT treatment | Untreated | Serum | IgM and IgG anti-PGL-I | Patients presenting with T1R or ENL at leprosy diagnosis have same level of IgM anti-PGL-I antibody response as leprosy patients without reactions at diagnosis |
| Beuria et al. (195); India | 44 BL/LL, 62 TT/BT, 18 ENL, 15 T1R, 17 HC | ND | BL/LL: 90% on MDT | Steroids after collection of samples | Serum | IgG1, IgG2, IgG3 and IgG4 to LAM | Reduction in IgG3 in ENL compared to active BL/LL Higher IgG1 in ENL than T1R |
| TT/BT: mostly untreated | |||||||
| Hamerlinck et al. (196); Philippines, Netherlands | 13 ENL, 22 T1R, 26 leprosy unreactional, 10 HC | Serial samples during MDT: 2 ENL follow-up and received corticosteroids, 14 leprosy free of reactions, 4 T1R 6, 12, 18, 30 months during follow-up | 13 ENL before MDT, 2 ENL during MDT | During ENL before treatment | Serum | Neopterin | T1R and ENL higher neopterin levels compared to non-reactional individuals Corticosteroid treatment reduces levels of neopterin |
| Mahaisavariya et al. (197); Thailand | 95 leprosy patients: 63 non-reactional, 19 T1R, 13 ENL | A biopsy at time of diagnosis and an additional biopsy later, in some cases at the time of reaction | ND | Before antireactional treatment? | Skin | Mast cells | Reduction of mast cell counts in both T1R and ENL compared to non-reactional patients |
| Schon et al. (198); Ethiopia | 4 ENL, 5 T1R | ND | ENL cases: 2 MDT untreated and 2 MDT-treated | Steroids | Urine | Urinary levels of metabolites of NO | Urinary nitric oxide metabolites decreased significantly after steroid treatment |
| Antunes et al. (199); Brazil | 3 ENL, 3 T1R | First biopsy during reactional episode and second during remission | On MDT | Thalidomide for ENL | Skin | Neuropeptides; quantification of mast cells and their subsets | Increase mast cells in the inflammatory infiltrate of the reactional (both T1R and ENL) biopsies compared to non-reactional |
| Rada et al. (200); Venezuela | 29 ENL, 19 MB not reactional, 11 PB, 28 HC | Before treatment | Untreated samples | Untreated samples | Serum | Nitrite/Nitrate levels | Supernatants of PBMC from ENL patients significantly elevated levels of nitrite/nitrates compared to LL or tuberculoid leprosy |
| PBMC | |||||||
| Sunderkotter et al. (201); Brazil | Skin Biopsies: 41 non-reactional leprosy, 8 ENL, 10 T1R; Serum samples: 16ENL, 5RR, 7TT, 13BT/BB/LL, 19 HC | Untreated samples | Skin biopsies: 42 untreated non-reactional leprosy | Before treatment with steroids or thalidomide | Serum Skin |
MRP8, MRP14 | Increase of serum levels of MRP8 and MRP14 in ENL Higher percentage of MRP8+ and MRP14+ cells in ENL skin lesions than non-reactional |
| 8 ENL of which 5 MDT | |||||||
| Nigam et al. (202); India | 80 leprosy: 10 ENL and 10 T1R; 20 HC | ND | ND | ND | Serum | Deaminase | Deaminase levels were higher in patients with reaction |
| Villahermosa et al. (134); Philippines γ, δ | 22 ENL | Before thalidomide administration and at study weeks 3 and 7 during thalidomide treatment | MDT was continued during the study | Samples untreated for antireactional drugs and during thalidomide treatment | Urine | Neopterin | ENL higher neopterin values in urine than HC |
| Iyer et al. (143); Indonesia δ | 131 leprosy patients (44 ENL), 112 HC | ND | Patients were classified irrespective of MDT status | Prednisolone to treat reactions | Plasma | Neopterin | Neopterin no significant difference between ENL and non-ENL |
| Mohanty et al. (203); India | 14 ENL before and after resolution of ENL, 5 LL | Before commencing antireactional therapy and after resolution of ENL | All patients on MDT | Before commencing antireactional treatment | Urine | Urinary nitric oxide metabolites | Urinary nitric oxide metabolite higher in ENL compared to non-reactional LL |
| These levels were reduced with resolution of reaction following antireactional therapy | |||||||
| Santos et al. (204); Brazil | 8 leprosy: 3 ENL | ND | MDT during the study: length of MDT described | Thalidomide during the study | PBMC | B7-1 expression (flow cytometry and IHC) | Higher B7 expression in ENL and T1R patients than non-reactional in both PBMC and cutaneous lesions |
| Skin | |||||||
| Silva et al. (205); Brazil | 25 leprosy: 5 ENL and 8 T1R | 0, 2, 4, 6, and 12 months of MDT | All patients on MDT | Untreated | Plasma | PGL-I levels | Serum PGL-I levels did not differ significantly between ENL and non-ENL |
| Neopterin | No significant correlation of neopterin between ENL and non-ENL | ||||||
| Brito Mde et al. (206); Brazil | 104 reactions after completion of MDT (44 ENL), 104 with no post-treatment reactions (8 ENL) | ND | All patients were treated with MDT; half had finished MDT | ND | Plasma | ML flow (IgM anti-PGL-I positive serology) | The patients with positive serology after MDT presented a 10.4 fold greater chance of developing post-treatment reactions (ENL or T1R) |
| Iyer et al. (207); Indonesia | 78 leprosy (36 ENL and 3 T1R), 36 HC | ND | 30 untreated and 48 treated patients | Reactions were treated using prednisolone | Serum | Chitotriocidase | Serum chitotriosidase activity elevated in ENL compared with HC but not with non-ENL leprosy |
| Skin | Significant decline of serum chitotriosidase following corticosteroid treatment in ENL | ||||||
| Lee et al. (24); USA α | 6 ENL, 7 LL | ND | ND | ND | Skin | Microarrays and gene expression; IHC for E-selectin | Upregulation of gene expression: in ENL lesions of the selectin family of adhesion molecules |
| IHC: higher levels of E-selectin in ENL lesions | |||||||
| Massone et al. (116); Brazil γ | 20 leprosy biopsies (3 ENL) | Biopsies at the time of diagnosis | 10, 12 and 13 months after beginning of MDT for LL | Untreated | Skin | Presence, frequency and distribution of plasmacytoid dendritic cells | CD123 expression was observed in 2/3 ENL biopsies |
| Rada et al. (135); Venezuela γ | 81 LL, 41 BL, 41 BB, 3% BT | ND | ND | ND | Blood | Serological immunological tests to various mycobacterial proteins | Mean antibody values against complete mycobacterial proteins higher in non-reactional individuals |
| Teles et al. (176); Brazil δ | 32 leprosy: 10 ENL, 8 T1R | 4 ENL patients before and during reaction biopsy samples | All patients were receiving MDT | ND | Skin | MMP-2, MMP-9, TIMP-1 | RT-PCR for MMP-2 and MMP-9 versus TIMP-1 in ENL sequential samples in 4 ENL patients: TNF-α, MMP-2 and MMP-9 mRNA enhanced IHC and confocal microscopy: absence of MMP positivity in ENL epidermis ELISA in sera of ENL: elevated MMP-9 but not TIMP-1 compared to non-reactional patients |
| Serum | |||||||
| Jadhav et al. (149); India δ | 303 MB followed up for 2 years: 5 ENL | Serum samples at the time of recruitment | Newly registered MB patients: no MDT | Untreated | Serum | Antibodies to PGL-I, LAM, ceramide, S100 | No statistically significant outcome for ENL |
| Lockwood et al. (178); India δ | 303 new MB leprosy (13 ENL) | Skin biopsies at enrollment | Before MDT treatment started | Before antireactional treatment | Skin | Immunostaining for CD68 and iNOS | Reactional biopsies had significantly fewer CD68+ cells than non-reactional |
| Nearly all biopsies in the LL group had CD68+ cells present and these were not altered in ENL | |||||||
| Nerve | ENL showed some iNOS staining but not significant differences with non-ENL | ||||||
| Martiniuk et al. (179); Nepal and USA δ | 7 ENL | Pre- and post-treatment with thalidomide | ND | Pre- and post- treatment with thalidomide | Skin | RT-PCR for hRORγT, hCD70, hCD27, hPLZF-1, hCTLA4, hAHR, hiNOS2, hARNT, hIDO, hGARP, hCD46 | Reduction in CD70, GARP, IDO and increase of RORγT, ARNT following thalidomide treatment |
| Singh et al. (208); India | 240 leprosy: 19 ENL, 69 BL/LL | ND | ND | ND | Serum | IgG antibodies against keratin | No significant difference in ENL |
| Dupnik et al. (58); Brazil β, δ | 11 ENL, 11 T1R, 19 leprosy controls without reactions for microarray; additional 28 leprosy (6 ENL, 11 T1R, 11 non-reactional) for qPCR validation; 3 ENL for IHC | ND | 3/11 ENL pre-treatment, 2/11 ENL on treatment and 6/11 post-treatment; leprosy controls matched for stage of treatment | Excluded patients on steroids within 7 days and thalidomide within 28 days of enrollment | PBMC | Microarray and qPCR for transcriptional profile of PBMC; Flow cytometry for monocyte populations | Top 3 KEGG pathways in ENL were S.aureus infection, SLE, cytokine-cytokine receptor interaction |
| No significant difference in the proportion of circulating monocytes between reactional and non-reactional PBMC | |||||||
| Mandal et al. (209); India | 15 reactional (both ENL and T1R), 15 HC | ND | ND | ND | PBMC | Vitamin D receptor (VDR) mRNA | All the individuals with low VDR expression manifested ENL |
| Dias et al. (80); Brazil δ | 30 ENL, 24 BL/LL, 31 HC | Upon diagnosis of reaction | BL/LL before MDT but most ENL on MDT | Before treatment with thalidomide or steroids | PBMC (monocytes, B-cells, pDCs) | Expression of TLR9 | Skin lesions and PBMC of ENL express higher levels of TLR-9 |
| Skin | |||||||
| Schmitz et al. (25); Brazil α | 62 leprosy: 22 ENL, 16 HC | ENL: before and 7 days after thalidomide | Patients before and after MDT | Before and after thalidomide | Skin | CD64 expression by qPCR and IHC | CD64 mRNA and protein expressed in ENL lesions |
| Thalidomide reduced CD64 expression | |||||||
α, also in Table 1; β, also in Table 2; γ, also in Table 3; δ, also in Table 4.
BB, mid-borderline leprosy; BL, borderline lepromatous leprosy; BT, borderline tuberculoid leprosy; ENL, erythema nodosum leprosum; HC, healthy controls; ICAM-1, keratinocyte intracellular adhesion molecule 1; ICs, immune complexes; LAM, lipoarabinomannan; LL, lepromatous leprosy polar; MLSA, M. leprae sonicated antigens; ND, not described; PGL I, phenolic glycolipid I; SLE, systemic lupus erythematosus; TB, tuberculosis; TT, tuberculoid leprosy; polar,%GO, proportion of oligosaccharide chains on the Fc fragment of IgG which terminate with N-acetylglucosamine and not galactose.
Innate Immunity
Genetic studies have shown associations between several single-nucleotide polymorphisms (SNP) of innate immunity genes such as NOD2 (210), the natural resistance-associated macrophage protein (NRAMP1) (211), and TLR1 (212, 213) with ENL.
A recent study from Brazil, which investigated whether DNA sensing via TLR9, constitutes a major inflammatory pathway during ENL (80) showed that both the skin lesions and peripheral leukocytes (B-cells, monocytes, and plasmacytoid dendritic cells) of ENL patients express higher TLR9 levels than BL/LL controls (80). In addition, the levels of endogenous human and pathogen-derived TLR9 ligands (human and mycobacterial DNA-histone complexes) were also higher in the circulation of ENL patients than BL/LL controls (80). Furthermore, stimulation of PBMC isolated from ENL patients with TLR9 agonist led to higher levels of TNF-α, IL-6, and IL-1β, than those of non-reactional leprosy and healthy controls. Usage of a TLR9 synthetic antagonist was able to significantly inhibit the secretion of pro-inflammatory cytokines after stimulation with M. leprae lysate (80). This is the first study to support the potential of TLR signaling inhibitors as a therapeutic strategy for ENL (80).
B-Lymphocytes and Immunoglobulins
Early studies enumerated B lymphocytes in skin lesions (89) and in peripheral blood (76, 78, 94, 123) of patients with ENL, while most of these studies did not find any association between B-cells and the development of ENL. Other studies looked at the IgM PGL-I in sera as a marker for ENL (40, 184, 192, 206), but most of these studies did not show an association (50, 82, 149, 187, 194, 205). Significantly lower serum levels of IgG1 and IgG3 subclasses of M. leprae-specific antibodies have been demonstrated in ENL patients compared to the BL/LL controls (190). This decrease of M. leprae-specific IgG1 and IgG3 antibodies in sera has not been related to downregulation of B cell responses since ENL episodes were characterized by an increase of polyclonal IgG1 antibody synthesis by the B cells, declining after subsidence of the reaction (191). The authors suggested that activation of B-cells is restricted to IgG1-secreting B cells in the blood of patients with lepromatous disease (191), while the lower serum concentrations of M. leprae-specific IgG1 and IgG3 (190) could be due to antibody deposition in the tissues (191). Interestingly, surface CD64 (FcγRI), the high-affinity receptor for monomeric IgG1 and IgG3 is expressed at higher levels on circulating neutrophils derived from ENL patients compared to non-reactional leprosy controls (25). The higher CD64 neutrophil expression could explain the presence of lower serum IgG1 and IgG3 levels in ENL patients compared to BL/LL controls.
New Suggested Pathogenetic Mechanisms
Two recent studies of gene expression provide evidence of activation of novel molecular pathways in ENL.
Lee et al performed bioinformatic pathways analysis of gene expression profiles in leprosy skin lesions and found “cell movement” as the top biological pathway characterizing ENL (24). The study further described a neutrophil recruitment pathway including genes of key molecules that mediate neutrophil binding to endothelial cells (24). This neutrophil recruitment pathway characterizing ENL was inhibited by thalidomide (24). Consistent with these findings is a study of transcriptional profiles in PBMC of leprosy patients by Dupnik et al which identified “granulocyte adhesion and diapedesis” as one of the top canonical pathways characterizing ENL (58). Dupnik et al. identified 517 differentially expressed genes in patients with ENL (58). The pathway analysis revealed that the top three Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways that changed in ENL were Staphylococcus aureus infection, systemic lupus erythematosus (SLE), and cytokine-cytokine receptor interaction, while the complement and coagulation pathway was also associated with ENL (58). CCL5 was the most significant upstream regulator in the array followed by IFN-γ (58). Transcripts uniquely increased in ENL included the complement receptors C3AR1 and C5AR1 while uniquely decreased transcripts in ENL included IL-10 and cytotoxic T-lymphocyte associates protein 4 (CTLA-4), modulators of T-cell responses (58). Hepcidin, catholicidin, antimicrobial peptides, C1q, and defencins had also an increased expression in ENL, while CCL2, CCL3, and SOD2 could be potential biomarkers for ENL (58). Transcripts increased in PBMC from ENL patients also included FcγR1 (CD64), FPR1, and FPR2, which recognize formylated peptides produced by bacteria triggering receptor on myeloid cells 1 (TREM1) and the related molecule triggering receptor expressed on myeloid cells-like 1 (TREML-1) (58).
The microarray studies performed in skin lesions and PBMC have generated a long list of candidate genes that regulate immune function to be associated with ENL. These merit further research.
Limitations of the Systematic Review
PubMed was the only database used to identify eligible studies. This will have resulted in studies published in journals not listed in PubMed being omitted from our review. A search of gray literature may also have contributed data which may have influenced our conclusions. The high heterogeneity of included studies in terms of study questions and outcomes and the different methodologies used meant that a meta-analysis was not possible.
Methodological Considerations of the Studies Included in the Systematic Review
Many of the studies of immunological features of ENL contain significant limitations in both design and reporting. Most seriously 66% of the studies did not have a case definition of ENL.
More than 70% of studies sampled individuals at a single time point. Sampling at two time points was seen in 21.2% of studies, 3 time points in 2.7% of studies, whereas 4 or more time points was described in only 5.5% of studies. Some studies did not have appropriate controls- patients with uncomplicated BL and LL. Although 93.2% of studies used BL/LL patients as controls, the remaining 6.8% of studies used other control groups such as healthy volunteers or leprosy contacts or tuberculoid leprosy patients or patients with Type 1 reaction. Often the controls were not matched for age, sex or treatment status. Controls should be matched for age and sex since these factors may influence T cell and neutrophil numbers and functions (214–216) as well as TNF-α and other cytokine levels (217).
ENL is a condition that can be acute, recurrent, or chronic, and therefore, the timing of sample collection is crucial. No information on the timing of the sampling is described in 54.8% of all studies. The importance of timing for sample collection during ENL could explain the discrepancies observed in multiple studies as has been suggested in the studies addressing the role of neutrophils in ENL. Studies using serial sampling yield more meaningful data compared to cross-sectional studies. The interval between time points is important and needs to be kept as consistent as possible for all study subjects.
Only one study matched BL/LL controls and ENL cases for length of MDT. Patients may develop ENL prior to the diagnosis of leprosy, during MDT or after successful completion of MDT. MDT may affect the immune status of leprosy patients and thus the matching of cases and controls for this variable is important. Two of the components of MDT, dapsone and clofazimine, have been associated with alterations in neutrophil and lymhocyte function (218–220). Dapsone stimulates neutrophil migration (218) and inhibits production of Prostaglandin E2 by neutrophils (220). In addition, dapsone inhibits lymphocyte transformation (218). On the other hand, clofazimine enhances production of Prostaglandin E2 by neutrophils (220). Dapsone and anti-dapsone antibodies have been identified in circulating ICs of leprosy patients (221). Circulating cytokine and chemokine levels also change with MDT (165, 222, 223). In addition, gene expression studies could be affected by MDT since the MDT component rifampicin may modify the expression of certain housekeeping genes (224). A total of 30.8% of studies did not report the MDT status of their cases or controls, 12.3% collected untreated patient samples, whereas 56.2% collected patient samples at various stages of MDT.
The effect of immunosuppressive drugs used to treat ENL on the findings of studies is an important factor which should be considered. In 37.7% of studies, there was no reporting of whether participants were on ENL treatment when samples were collected. Treatment with corticosteroids affects T-cells and neutrophil function (225, 226) and also gene expression studies by influencing housekeeping genes (224). Treatment with thalidomide may increase the neutrophil numbers, at least partially through differentially modulating the surface expression of markers CD18 and CD44 by the neutrophils in the bone marrow and the spleen (227). Thalidomide treatment may also affect T-cell functions by suppressing CD4+ T-cell proliferation while increasing their conversion to CD4+FoxP3+ Tregs (228). Moreover, thalidomide treatment may reduce cytokine levels (229). Less than half (34.2%) of studies indicate that samples were obtained prior to the start of ENL treatment.
Only 17.8% of all studies collected samples from more than one system, while samples from both blood and skin were described in only 12.3% of all studies.
Future Study Design
Studies of ENL may be difficult to design and conduct. In addition, no animal model of ENL is available. Obtaining sufficient numbers of patients so that studies are adequately powered is difficult unless multicenter studies are performed which increase the logistical complexity and cost of the research. Patients are often on treatment (both MDT and immunosuppression) which may influence the study outcomes.
A large cohort study of newly diagnosed patients with BL and LL would be optimal in allowing matching of cases and controls. Some BL/LL patients who have not developed ENL at enrollment in the study should be recruited and followed until they develop the disorder. Detailed clinical information which includes demographic data, ENL severity using a robust measure, treatment status, in conjunction with well-timed and documented specimen collection (preferably of blood and skin), effective specimen storage, and transportation. ENL is a systemic disease and ideally samples from more than one system, i.e., both blood and skin should be obtained where appropriate. Well-designed laboratory experiments using a wide range of techniques should be used to interrogate such important specimens.
Conclusion
Figure 2 gives an overview of the immunology of ENL.
Figure 2.
Immune mechanisms in erythema nodosum leprosum (ENL). The diagram illustrates the different immune mechanisms which have been described in the literature of ENL. High volume of immune complexes (ICs) are formulated due to the increased antibody formation by the B cells and the increased mycobacterial antigens by fragmentation of the M. leprae bacilli. ICs are deposited in the skin. Neutrophils are drawn to the skin where they help in the IC clearance using their surface Fcγ receptors. An increase of CD4+/CD8+ T cell subset ratio in both peripheral blood and skin characterizes the disorder. Macrophages form the M. leprae intracellular niche and in concert with neutrophils and T-cells secret high levels of tumor necrosis factor (TNF)-α and other pro-inflammatory cytokines to further complicate the phenotype of ENL.
Our understanding of the causes of ENL is limited. The factors that initiate and/or sustain it might help to identify strategies to prevent or control the associated inflammation.
There is some evidence to support a role for neutrophils and ICs/complement in the inflammation associated with ENL; however, their role in the initiation of ENL remains unclear. The increase of TNF-α and other pro-inflammatory cytokines during ENL has been shown in multiple investigations, while suppression of TNF-α leads to clinical improvement. T-cell subsets appear to be important in ENL since multiple reports describe an increased CD4+/CD8+ ratio in ENL patients compared to BL/LL controls.
New technologies such as microarray studies pave the way and may lead to novel immunological pathways associated with ENL. Further research of the association of ENL with pathophysiological pathways such as the SLE pathway or the S. aureus infection pathway may improve our understanding of the disorder and potentially lead to novel therapeutic strategies. There are still large gaps in our understanding of this severe complication of leprosy despite the large number of studies examining the immunology of ENL. A systems biology approach may provide new insights.
This systematic review has highlighted the complex interactions at play in ENL and the difficulty in elucidating the various inflammatory pathways. We should rise to the challenge of understanding how these mechanisms operate and interact so that we can improve the treatment of patients with ENL.
Author Contributions
AP and SW were responsible for the study concept and design; made critical revision of the manuscript for important intellectual content. AP was responsible for acquisition, analysis, and interpretation of data and for drafting the manuscript. AP, SW, and DL edited the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Anna and Andreas Goulielmos for proof reading and grammatical error corrections.
Funding
This work was supported by Hospital and Homes of St. Giles.
References
- 1.Britton WJ, Lockwood DN. Leprosy. Lancet (2004) 363(9416):1209–19. 10.1016/S0140-6736(04)15952-7 [DOI] [PubMed] [Google Scholar]
- 2.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis (1966) 34(3):255–73. [PubMed] [Google Scholar]
- 3.Turk JL, Waters MF. Cell-mediated immunity in patients with leprosy. Lancet (1969) 2(7614):243–6. 10.1016/S0140-6736(69)90009-9 [DOI] [PubMed] [Google Scholar]
- 4.Moran CJ, Ryder G, Turk JL, Waters MF. Evidence for circulating immune complexes in lepromatous leprosy. Lancet (1972) 2(7777):572–3. 10.1016/S0140-6736(72)91962-9 [DOI] [PubMed] [Google Scholar]
- 5.Scollard DM, Smith T, Bhoopat L, Theetranont C, Rangdaeng S, Morens DM. Epidemiologic characteristics of leprosy reactions. Int J Lepr Other Mycobact Dis (1994) 62(4):559–67. [PubMed] [Google Scholar]
- 6.Walker SL, Balagon M, Darlong J, Doni SN, Hagge DA, Halwai V, et al. ENLIST 1: an international multi-centre cross-sectional study of the clinical features of erythema nodosum leprosum. PLoS Negl Trop Dis (2015) 9(9):e0004065. 10.1371/journal.pntd.0004065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mabalay MC, Helwig EB, Tolentino JG, Binford CH. The histopathology and histochemistry of erythema nodosum leprosum. Int J Lepr (1965) 33:28–49. [PubMed] [Google Scholar]
- 8.Anthony J, Vaidya MC, Dasgupta A. Ultrastructure of skin in erythema nodosum leprosum. Cytobios (1983) 36(141):17–23. [PubMed] [Google Scholar]
- 9.Murphy GF, Sanchez NP, Flynn TC, Sanchez JL, Mihm MC, Jr, Soter NA. Erythema nodosum leprosum: nature and extent of the cutaneous microvascular alterations. J Am Acad Dermatol (1986) 14(1):59–69. 10.1016/S0190-9622(86)70008-X [DOI] [PubMed] [Google Scholar]
- 10.Sehgal VN, Gautam RK, Koranne RV, Beohar PC. The histopathology of type I (lepra) and type II (ENL) reactions in leprosy. Indian J Lepr (1986) 58(2):240–3. [PubMed] [Google Scholar]
- 11.Adhe V, Dongre A, Khopkar U. A retrospective analysis of histopathology of 64 cases of lepra reactions. Indian J Dermatol (2012) 57(2):114–7. 10.4103/0019-5154.94278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarita S, Muhammed K, Najeeba R, Rajan GN, Anza K, Binitha MP, et al. A study on histological features of lepra reactions in patients attending the Dermatology Department of the Government Medical College, Calicut, Kerala, India. Lepr Rev (2013) 84(1):51–64. [PubMed] [Google Scholar]
- 13.Job CK, Gude S, Macaden VP. Erythema nodosum leprosum. A clinico-pathologic study. Int J Lepr (1964) 32:177–84. [PubMed] [Google Scholar]
- 14.Pocaterra L, Jain S, Reddy R, Muzaffarullah S, Torres O, Suneetha S, et al. Clinical course of erythema nodosum leprosum: an 11-year cohort study in Hyderabad, India. Am J Trop Med Hyg (2006) 74(5):868–79. [PubMed] [Google Scholar]
- 15.Chandler DJ, Hansen KS, Mahato B, Darlong J, John A, Lockwood DN. Household costs of leprosy reactions (ENL) in rural India. PLoS Negl Trop Dis (2015) 9(1):e0003431. 10.1371/journal.pntd.0003431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker SL, Lebas E, Doni SN, Lockwood DN, Lambert SM. The mortality associated with erythema nodosum leprosum in ethiopia: a retrospective hospital-based study. PLoS Negl Trop Dis (2014) 8(3):e2690. 10.1371/journal.pntd.0002690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (2015) 349:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 18.Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, et al. Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog (2015) 11(3):e1004651. 10.1371/journal.ppat.1004651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leliefeld PH, Koenderman L, Pillay J. How neutrophils shape adaptive immune responses. Front Immunol (2015) 6:471. 10.3389/fimmu.2015.00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abalos RM, Tolentino JG, Bustillo CC. Histochemical study of erythema nodosum leprosum (ENL) lesions. Int J Lepr Other Mycobact Dis (1974) 42(4):385–91. [PubMed] [Google Scholar]
- 21.Hussain R, Lucas SB, Kifayet A, Jamil S, Raynes J, Uqaili Z, et al. Clinical and histological discrepancies in diagnosis of ENL reactions classified by assessment of acute phase proteins SAA and CRP. Int J Lepr Other Mycobact Dis (1995) 63(2):222–30. [PubMed] [Google Scholar]
- 22.Waters MF, Turk JL, Wemambu SN. Mechanisms of reactions in leprosy. Int J Lepr Other Mycobact Dis (1971) 39(2):417–28. [PubMed] [Google Scholar]
- 23.Pepler WJ, Kooij R, Marshall J. The histopathology of acute panniculitis nodosa leprosa (erythema nodosum leprosum). Int J Lepr (1955) 23(1):53–60. [PubMed] [Google Scholar]
- 24.Lee DJ, Li H, Ochoa MT, Tanaka M, Carbone RJ, Damoiseaux R, et al. Integrated pathways for neutrophil recruitment and inflammation in leprosy. J Infect Dis (2010) 201(4):558–69. 10.1086/650318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitz V, Prata RB, Barbosa MG, Mendes MA, Brandao SS, Amadeu TP, et al. Expression of CD64 on circulating neutrophils favoring systemic inflammatory status in erythema nodosum leprosum. PLoS Negl Trop Dis (2016) 10(8):e0004955. 10.1371/journal.pntd.0004955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogg N. The structure and function of Fc receptors. Immunol Today (1988) 9(7–8):185–7. 10.1016/0167-5699(88)91206-6 [DOI] [PubMed] [Google Scholar]
- 27.Herra CM, Keane CT, Whelan A. Increased expression of Fc gamma receptors on neutrophils and monocytes may reflect ongoing bacterial infection. J Med Microbiol (1996) 44(2):135–40. 10.1099/00222615-44-2-135 [DOI] [PubMed] [Google Scholar]
- 28.Song SH, Kim HK, Park MH, Cho HI. Neutrophil CD64 expression is associated with severity and prognosis of disseminated intravascular coagulation. Thromb Res (2008) 121(4):499–507. 10.1016/j.thromres.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 29.Buckle AM, Hogg N. The effect of IFN-gamma and colony-stimulating factors on the expression of neutrophil cell membrane receptors. J Immunol (1989) 143(7):2295–301. [PubMed] [Google Scholar]
- 30.Voorend CG, Post EB. A systematic review on the epidemiological data of erythema nodosum leprosum, a type 2 leprosy reaction. PLoS Negl Trop Dis (2013) 7(10):e2440. 10.1371/journal.pntd.0002440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goihman-Yahr M, Rodriguez-Ochoa G, Aranzazu N, Convit J. Polymorphonuclear activation in leprosy. I. Spontaneous and endotoxin-stimulated reduction of nitroblue tetrazolium: effects of serum and plasma on endotoxin-induced activation. Clin Exp Immunol (1975) 20(2):257–64. [PMC free article] [PubMed] [Google Scholar]
- 32.Sher R, Anderson R, Glover A, Wadee AA. Polymorphonuclear cell function in the various polar types of leprosy and erythema nodosum leprosum. Infect Immun (1978) 21(3):959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliveira RB, Moraes MO, Oliveira EB, Sarno EN, Nery JA, Sampaio EP. Neutrophils isolated from leprosy patients release TNF-alpha and exhibit accelerated apoptosis in vitro. J Leukoc Biol (1999) 65(3):364–71. [DOI] [PubMed] [Google Scholar]
- 34.Wiggins RC, Cochrane CG. Immune-complex-mediated biologic effects. N Engl J Med (1981) 304(9):518–20. 10.1056/NEJM198102263040904 [DOI] [PubMed] [Google Scholar]
- 35.Hoiby N, Doring G, Schiotz PO. The role of immune complexes in the pathogenesis of bacterial infections. Annu Rev Microbiol (1986) 40:29–53. 10.1146/annurev.mi.40.100186.000333 [DOI] [PubMed] [Google Scholar]
- 36.Schifferli JA, Ng YC, Peters DK. The role of complement and its receptor in the elimination of immune complexes. N Engl J Med (1986) 315(8):488–95. 10.1056/NEJM198608213150805 [DOI] [PubMed] [Google Scholar]
- 37.Wemambu SN, Turk JL, Waters MF, Rees RJ. Erythema nodosum leprosum: a clinical manifestation of the arthus phenomenon. Lancet (1969) 2(7627):933–5. 10.1016/S0140-6736(69)90592-3 [DOI] [PubMed] [Google Scholar]
- 38.Anthony J, Vaidya MC, Dasgupta A. Immunological methods employed in an attempt to induce erythema nodosum leprosum (ENL) in mice. Lepr India (1978) 50(3):356–62. [PubMed] [Google Scholar]
- 39.Ridley MJ, Ridley DS. The immunopathology of erythema nodosum leprosum: the role of extravascular complexes. Lepr Rev (1983) 54(2):95–107. [DOI] [PubMed] [Google Scholar]
- 40.Andreoli A, Brett SJ, Draper P, Payne SN, Rook GA. Changes in circulating antibody levels to the major phenolic glycolipid during erythema nodosum leprosum in leprosy patients. Int J Lepr Other Mycobact Dis (1985) 53(2):211–7. [PubMed] [Google Scholar]
- 41.Chakrabarty AK, Maire M, Saha K, Lambert PH. Identification of components of IC purified from human sera. II. Demonstration of mycobacterial antigens in immune complexes isolated from sera of lepromatous patients. Clin Exp Immunol (1983) 51(2):225–31. [PMC free article] [PubMed] [Google Scholar]
- 42.Furukawa F, Ozaki M, Imamura S, Yoshida H, Pinrat A, Hamashima Y. Associations of circulating immune complexes, clinical activity, and bacterial index in Japanese patients with leprosy. Arch Dermatol Res (1982) 274(1–2):185–8. 10.1007/BF00510372 [DOI] [PubMed] [Google Scholar]
- 43.Rojas-Espinosa O, Mendez-Navarrete I, Estrada-Parra S. Presence of C1q-reactive immune complexes in patients with leprosy. Clin Exp Immunol (1972) 12(2):215–23. [PMC free article] [PubMed] [Google Scholar]
- 44.Wager O, Penttinen K, Almeida JD, Opromolla DV, Godal T, Kronvall G. Circulating complexes in leprosy studied by the platelet aggregation test. The platelet aggregation test and its relation to the Rubino test and other sero-immunological parameters in 135 patients with leprosy. Clin Exp Immunol (1978) 34(3):326–37. [PMC free article] [PubMed] [Google Scholar]
- 45.Geniteau M, Adam C, Verroust P, Pasticier A, Saimot G, Coulaud JP, et al. [Immune complexes and complement in leprosy (author’s transl)]. Nouv Presse Med (1981) 10(45):3697–700. [PubMed] [Google Scholar]
- 46.Bjorvatn B, Barnetson RS, Kronvall G, Zubler RH, Lambert PH. Immune complexes and complement hypercatabolism in patients with leprosy. Clin Exp Immunol (1976) 26(3):388–96. [PMC free article] [PubMed] [Google Scholar]
- 47.Jayapal N, Shanmugasundaram N, Thomas PA, Valli PR, Thyagarajan SP, Subramanian S. A simple method to quantitate circulating immune complexes in different diseases. Indian J Pathol Microbiol (1989) 32(1):33–9. [PubMed] [Google Scholar]
- 48.Ramanathan VD, Sharma P, Ramu G, Sengupta U. Reduced complement-mediated immune complex solubilization in leprosy patients. Clin Exp Immunol (1985) 60(3):553–8. [PMC free article] [PubMed] [Google Scholar]
- 49.Ramanathan VD, Tyagi P, Ramanathan U, Katoch K, Sengupta U, Ramu G. Persistent reduced solubilization of immune complexes in lepromatous leprosy patients with reactions. Int J Lepr Other Mycobact Dis (1991) 59(1):5–11. [PubMed] [Google Scholar]
- 50.Rojas RE, Demichelis SO, Sarno EN, Segal-Eiras A. IgM anti-phenolic glycolipid I and IgG anti-10-kDa heat shock protein antibodies in sera and immune complexes isolated from leprosy patients with or without erythema nodosum leprosum and contacts. FEMS Immunol Med Microbiol (1997) 19(1):65–74. 10.1111/j.1574-695X.1997.tb01073.x [DOI] [PubMed] [Google Scholar]
- 51.Tung KS, Kim B, Bjorvatn B, Kronvall G, McLaren LC, Williams RC, Jr. Discrepancy between Clq deviation and Raji cell tests in detection of circulating immune complexes in patients with leprosy. J Infect Dis (1977) 136(2):216–21. 10.1093/infdis/136.2.216 [DOI] [PubMed] [Google Scholar]
- 52.Tyagi P, Ramanathan VD, Girdhar BK, Katoch K, Bhatia AS, Sengupta U. Activation of complement by circulating immune complexes isolated from leprosy patients. Int J Lepr Other Mycobact Dis (1990) 58(1):31–8. [PubMed] [Google Scholar]
- 53.Tyagi P, Patil SA, Girdhar BK, Katoch K, Sengupta U. Suppressive effect of circulating immune complexes from leprosy patients on the lymphocyte proliferation induced by M. leprae antigens in healthy responders. Int J Lepr Other Mycobact Dis (1992) 60(4):562–9. [PubMed] [Google Scholar]
- 54.Valentijn RM, Faber WR, Lai AFRF, Chan Pin Jie JC, Daha MR, van Es LA. Immune complexes in leprosy patients from an endemic and a nonendemic area and a longitudinal study of the relationship between complement breakdown products and the clinical activity of erythema nodosum leprosum. Clin Immunol Immunopathol (1982) 22(2):194–202. 10.1016/0090-1229(82)90037-X [DOI] [PubMed] [Google Scholar]
- 55.Penttinen K, Myllyla G, Vaheri A, Vesikari T, Kaariainen L. The platelet aggregation test (PA) as an immunological method in virology. Prog Immunobiol Stand (1970) 4:672–5. [PubMed] [Google Scholar]
- 56.Yanase K, Imamura S. Detection of circulating immune complexes in some skin diseases by platelet aggregation test. Br J Dermatol (1979) 100(2):227–8. 10.1111/j.1365-2133.1979.tb05567.x [DOI] [PubMed] [Google Scholar]
- 57.Rojas RE, Segal-Eiras A. Characterization of circulating immune complexes in leprosy patients and their correlation with specific antibodies against Mycobacterium leprae. Clin Exp Dermatol (1997) 22(5):223–9. 10.1046/j.1365-2230.1997.2620675.x [DOI] [PubMed] [Google Scholar]
- 58.Dupnik KM, Bair TB, Maia AO, Amorim FM, Costa MR, Keesen TS, et al. Transcriptional changes that characterize the immune reactions of leprosy. J Infect Dis (2015) 211(10):1658–76. 10.1093/infdis/jiu612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Azevedo MP, de Melo PH. A comparative study of the complementary activity of serum in the polar forms of leprosy and in the leprosy reaction. Int J Lepr Other Mycobact Dis (1966) 34(1):34–8. [PubMed] [Google Scholar]
- 60.Anthony J, Vaidya MC, Dasgupta A. Immunoglobulin deposits in erythema nodosum leprosum (ENL). Hansen Int (1978) 3(1):12–7. [Google Scholar]
- 61.Lange K, Wasserman E, Slobody LB. The significance of serum complement levels for the diagnosis and prognosis of acute and subacute glomerulonephritis and lupus erythematosus disseminatus. Ann Intern Med (1960) 53:636–46. 10.7326/0003-4819-53-4-636 [DOI] [PubMed] [Google Scholar]
- 62.Lewis EJ, Carpenter CB, Schur PH. Serum complement component levels in human glomerulonephritis. Ann Intern Med (1971) 75(4):555–60. 10.7326/0003-4819-75-4-555 [DOI] [PubMed] [Google Scholar]
- 63.Ohi H, Tamano M. Decreased apolipoprotein levels are associated with decreased complement levels in acute glomerulonephritis. Nephron (2001) 88(4):389–90. 10.1159/000046028 [DOI] [PubMed] [Google Scholar]
- 64.Baatrup G, Petersen I, Kappelgaard E, Jepsen HH, Svehag SE. Complement-mediated solubilization of immune complexes. Solubilization inhibition and complement factor levels in SLE patients. Clin Exp Immunol (1984) 55(2):313–8. [PMC free article] [PubMed] [Google Scholar]
- 65.Grevink ME, Horst G, Limburg PC, Kallenberg CG, Bijl M. Levels of complement in sera from inactive SLE patients, although decreased, do not influence in vitro uptake of apoptotic cells. J Autoimmun (2005) 24(4):329–36. 10.1016/j.jaut.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 66.Schifferli JA, Morris SM, Dash A, Peters DK. Complement-mediated solubilization in patients with systemic lupus erythematosus, nephritis or vasculitis. Clin Exp Immunol (1981) 46(3):557–64. [PMC free article] [PubMed] [Google Scholar]
- 67.de Messias IJ, Santamaria J, Brenden M, Reis A, Mauff G. Association of C4B deficiency (C4B*Q0) with erythema nodosum in leprosy. Clin Exp Immunol (1993) 92(2):284–7. 10.1111/j.1365-2249.1993.tb03393.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hauptmann G, Tappeiner G, Schifferli JA. Inherited deficiency of the fourth component of human complement. Immunodefic Rev (1988) 1(1):3–22. [PubMed] [Google Scholar]
- 69.Gelber RH, Drutz DJ, Epstein WV, Fasal P. Clinical correlates of C1Q-precipitating substances in the sera of patients with leprosy. Am J Trop Med Hyg (1974) 23(3):471–5. [DOI] [PubMed] [Google Scholar]
- 70.Izumi S, Sugiyama K, Matsumoto Y, Nagai T. Numerical changes in T cell subsets (T gamma and T mu) in leprosy patients. Microbiol Immunol (1980) 24(8):733–40. 10.1111/j.1348-0421.1980.tb02874.x [DOI] [PubMed] [Google Scholar]
- 71.Harikrishan S, Balakrishnan S, Bhatia VN. Serum immunoglobulin profile and C3 level in lepromatous leprosy patients. Lepr India (1982) 54(3):454–60. [PubMed] [Google Scholar]
- 72.Saha K, Chakraborty AK, Sharma V, Sehgal VN. An appraisal of third complement component (C3) and breakdown product (C3d) in erythema nodosum leprosum (ENL). Lepr Rev (1982) 53(4):253–60. [DOI] [PubMed] [Google Scholar]
- 73.Mshana RN, Humber DP, Belehu A, Harboe M. Immunohistological studies of skin biopsies from patients with lepromatous leprosy. J Clin Immunol (1983) 3(1):22–9. 10.1007/BF00919135 [DOI] [PubMed] [Google Scholar]
- 74.Ramanathan VD, Parkash O, Ramu G, Parker D, Curtis J, Sengupta U, et al. Isolation and analysis of circulating immune complexes in leprosy. Clin Immunol Immunopathol (1984) 32(3):261–8. 10.1016/0090-1229(84)90270-8 [DOI] [PubMed] [Google Scholar]
- 75.Saha K, Chakrabarty AK, Sharma VK, Sehgal VN. Polyethylene glycol precipitates in serum during and after erythema nodosum leprosum – study of their composition and anticomplementary activity. Int J Lepr Other Mycobact Dis (1984) 52(1):44–8. [PubMed] [Google Scholar]
- 76.Sehgal VN, Gautam RK, Sharma VK. Immunoprofile of reactions in leprosy. Int J Dermatol (1986) 25(4):240–4. 10.1111/j.1365-4362.1986.tb02233.x [DOI] [PubMed] [Google Scholar]
- 77.Chakrabarty AK, Kashyap A, Sehgal VN, Saha K. Solubilization of preformed immune complexes in sera of patients with type 1 and type 2 lepra reactions. Int J Lepr Other Mycobact Dis (1988) 56(4):559–65. [PubMed] [Google Scholar]
- 78.Rao TD, Rao PR. Serum immune complexes in erythema nodosum leprosum reactions of leprosy. Indian J Lepr (1988) 60(2):189–95. [PubMed] [Google Scholar]
- 79.Sehgal VN, Sharma V, Sharma VK. The effect of anti-reactional drugs on complement components in the type II, erythema nodosum leprosum, reaction. Br J Dermatol (1988) 119(2):255–8. 10.1111/j.1365-2133.1988.tb03209.x [DOI] [PubMed] [Google Scholar]
- 80.Dias AA, Silva CO, Santos JP, Batista-Silva LR, Acosta CC, Fontes AN, et al. DNA sensing via TLR-9 constitutes a major innate immunity pathway activated during erythema nodosum leprosum. J Immunol (2016) 197(5):1905–13. 10.4049/jimmunol.1600042 [DOI] [PubMed] [Google Scholar]
- 81.Sehgal VN, Sharma V, Sharma VK. Comprehensive evaluation of complement components in the course of type I (Lepra) and type II (ENL) reactions. Int J Dermatol (1989) 28(1):32–5. 10.1111/j.1365-4362.1989.tb01306.x [DOI] [PubMed] [Google Scholar]
- 82.Scollard DM, Bhoopat L, Kestens L, Vanham G, Douglas JT, Moad J. Immune complexes and antibody levels in blisters over human leprosy skin lesions with or without erythema nodosum leprosum. Clin Immunol Immunopathol (1992) 63(3):230–6. 10.1016/0090-1229(92)90227-F [DOI] [PubMed] [Google Scholar]
- 83.Kim HJ, Cantor H. CD4 T-cell subsets and tumor immunity: the helpful and the not-so-helpful. Cancer Immunol Res (2014) 2(2):91–8. 10.1158/2326-6066.CIR-13-0216 [DOI] [PubMed] [Google Scholar]
- 84.Lim SD, Kiszkiss DF, Jacobson RR, Choi YS, Good RA. Thymus-dependent lymphocytes of peripheral blood in leprosy patients. Infect Immun (1974) 9(2):394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rao TD, Rao PR. Enhanced cell-mediated immune responses in erythema nodosum leprosum reactions of leprosy. Int J Lepr Other Mycobact Dis (1987) 55(1):36–41. [PubMed] [Google Scholar]
- 86.Bach MA, Chatenoud L, Wallach D, Phan Dinh Tuy F, Cottenot F. Studies on T cell subsets and functions in leprosy. Clin Exp Immunol (1981) 44(3):491–500. [PMC free article] [PubMed] [Google Scholar]
- 87.Wallach D, Cottenot F, Bach MA. Imbalances in T cell subpopulations in lepromatous leprosy. Int J Lepr Other Mycobact Dis (1982) 50(3):282–90. [PubMed] [Google Scholar]
- 88.Mshana RN, Haregewoin A, Harboe M, Belehu A. Thymus dependent lymphocytes in leprosy. I. T lymphocyte subpopulations defined by monoclonal antibodies. Int J Lepr Other Mycobact Dis (1982) 50(3):291–6. [PubMed] [Google Scholar]
- 89.Narayanan RB, Laal S, Sharma AK, Bhutani LK, Nath I. Differences in predominant T cell phenotypes and distribution pattern in reactional lesions of tuberculoid and lepromatous leprosy. Clin Exp Immunol (1984) 55(3):623–8. [PMC free article] [PubMed] [Google Scholar]
- 90.Mshana RN, Haregewoin A, Belehu A. Thymus-dependent lymphocytes in leprosy. II. Effect of chemotherapy on T-lymphocyte subpopulations. J Clin Immunol (1982) 2(2):69–74. 10.1007/BF00916889 [DOI] [PubMed] [Google Scholar]
- 91.Wallach D, Flageul B, Cottenot F, Bach MA. Patients with erythema nodosum leprosum lack T-suppressor cells. Arch Dermatol (1985) 121(11):1379. 10.1001/archderm.1985.01660110027002 [DOI] [PubMed] [Google Scholar]
- 92.Hussain T, Kulshreshtha KK, Yadav VS, Katoch K. CD4+, CD8+, CD3+ cell counts and CD4+/CD8+ ratio among patients with mycobacterial diseases (leprosy, tuberculosis), HIV infections, and normal healthy adults: a comparative analysis of studies in different regions of India. J Immunoassay Immunochem (2015) 36(4):420–43. 10.1080/15321819.2014.978082 [DOI] [PubMed] [Google Scholar]
- 93.Sakane T, Steinberg AD, Green I. Studies of immune functions of patients with systemic lupus erythematosus. I. Dysfunction of suppressor T-cell activity related to impaired generation of, rather than response to, suppressor cells. Arthritis Rheum (1978) 21(6):657–64. 10.1002/art.1780210608 [DOI] [PubMed] [Google Scholar]
- 94.Laal S, Bhutani LK, Nath I. Natural emergence of antigen-reactive T cells in lepromatous leprosy patients during erythema nodosum leprosum. Infect Immun (1985) 50(3):887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bullock WE. Leprosy: a model of immunological perturbation in chronic infection. J Infect Dis (1978) 137(3):341–54. 10.1093/infdis/137.3.341 [DOI] [PubMed] [Google Scholar]
- 96.Rea TH, Bakke AC, Parker JW, Modlin RL, Horwitz DA. Peripheral blood T lymphocyte subsets in leprosy. Int J Lepr Other Mycobact Dis (1984) 52(3):311–7. [PubMed] [Google Scholar]
- 97.Modlin RL, Gebhard JF, Taylor CR, Rea TH. In situ characterization of T lymphocyte subsets in the reactional states of leprosy. Clin Exp Immunol (1983) 53(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- 98.Modlin RL, Hofman FM, Taylor CR, Rea TH. T lymphocyte subsets in the skin lesions of patients with leprosy. J Am Acad Dermatol (1983) 8(2):182–9. 10.1016/S0190-9622(83)70021-6 [DOI] [PubMed] [Google Scholar]
- 99.Modlin RL, Bakke AC, Vaccaro SA, Horwitz DA, Taylor CR, Rea TH. Tissue and blood T-lymphocyte subpopulations in erythema nodosum leprosum. Arch Dermatol (1985) 121(2):216–9. 10.1001/archderm.121.2.216 [DOI] [PubMed] [Google Scholar]
- 100.Modlin RL, Mehra V, Jordan R, Bloom BR, Rea TH. In situ and in vitro characterization of the cellular immune response in erythema nodosum leprosum. J Immunol (1986) 136(3):883–6. [PubMed] [Google Scholar]
- 101.Shen JY, Hofman FM, Gunter JR, Modlin RL, Rea TH. In situ identification of activated Ta1+ T lymphocytes in human leprosy skin lesions. Int J Lepr Other Mycobact Dis (1987) 55(3):494–8. [PubMed] [Google Scholar]
- 102.Rea TH, Modlin RL. Immunopathology of leprosy skin lesions. Semin Dermatol (1991) 10(3):188–93. [PubMed] [Google Scholar]
- 103.Mahaisavariya P, Kulthanan K, Khemngern S, Pinkaew S. Lesional T-cell subset in leprosy and leprosy reaction. Int J Dermatol (1999) 38(5):345–7. 10.1046/j.1365-4362.1999.00621.x [DOI] [PubMed] [Google Scholar]
- 104.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest (2004) 114(9):1209–17. 10.1172/JCI23395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saini C, Prasad HK, Rani R, Murtaza A, Misra N, Narayan NP, et al. Lsr 2 of Mycobacterium leprae and its synthetic peptides elicit restitution of in vitro T cell responses in erythema nodosum leprosum and reversal reactions in lepromatous leprosy patients. Clin Vaccine Immunol (2013) 20(5):673–82. 10.1128/CVI.00762-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saini C, Ramesh V, Nath I. CD4+ Th17 cells discriminate clinical types and constitute a third subset of non Th1, Non Th2 T cells in human leprosy. PLoS Negl Trop Dis (2013) 7(7):e2338. 10.1371/journal.pntd.0002338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Attia EA, Abdallah M, Saad AA, Afifi A, El Tabbakh A, El-Shennawy D, et al. Circulating CD4+ CD25 high FoxP3+ T cells vary in different clinical forms of leprosy. Int J Dermatol (2010) 49(10):1152–8. 10.1111/j.1365-4632.2010.04535.x [DOI] [PubMed] [Google Scholar]
- 108.Boer MC, Joosten SA, Ottenhoff TH. Regulatory T-cells at the interface between human host and pathogens in infectious diseases and vaccination. Front Immunol (2015) 6:217. 10.3389/fimmu.2015.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abdallah M, Attia EA, Saad AA, El-Khateeb EA, Lotfi RA, Abdallah M, et al. Serum Th1/Th2 and macrophage lineage cytokines in leprosy; correlation with circulating CD4(+) CD25(high) FoxP3(+) T-regs cells. Exp Dermatol (2014) 23(10):742–7. 10.1111/exd.12529 [DOI] [PubMed] [Google Scholar]
- 110.Attia EA, Abdallah M, El-Khateeb E, Saad AA, Lotfi RA, Abdallah M, et al. Serum Th17 cytokines in leprosy: correlation with circulating CD4(+) CD25 (high)FoxP3 (+) T-regs cells, as well as down regulatory cytokines. Arch Dermatol Res (2014) 306(9):793–801. 10.1007/s00403-014-1486-2 [DOI] [PubMed] [Google Scholar]
- 111.Santegoets SJ, Dijkgraaf EM, Battaglia A, Beckhove P, Britten CM, Gallimore A, et al. Monitoring regulatory T cells in clinical samples: consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol Immunother (2015) 64(10):1271–86. 10.1007/s00262-015-1729-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brusko T, Wasserfall C, McGrail K, Schatz R, Viener HL, Schatz D, et al. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes (2007) 56(3):604–12. 10.2337/db06-1248 [DOI] [PubMed] [Google Scholar]
- 113.Saini C, Siddiqui A, Ramesh V, Nath I. Leprosy reactions show increased Th17 cell activity and reduced FOXP3+ Tregs with concomitant decrease in TGF-beta and increase in IL-6. PLoS Negl Trop Dis (2016) 10(4):e0004592. 10.1371/journal.pntd.0004592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ye ZJ, Zhou Q, Du RH, Li X, Huang B, Shi HZ. Imbalance of Th17 cells and regulatory T cells in tuberculous pleural effusion. Clin Vaccine Immunol (2011) 18(10):1608–15. 10.1128/CVI.05214-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haslett PA, Roche P, Butlin CR, Macdonald M, Shrestha N, Manandhar R, et al. Effective treatment of erythema nodosum leprosum with thalidomide is associated with immune stimulation. J Infect Dis (2005) 192(12):2045–53. 10.1086/498216 [DOI] [PubMed] [Google Scholar]
- 116.Massone C, Nunzi E, Ribeiro-Rodrigues R, Talhari C, Talhari S, Schettini AP, et al. T regulatory cells and plasmocytoid dentritic cells in hansen disease: a new insight into pathogenesis? Am J Dermatopathol (2010) 32(3):251–6. 10.1097/DAD.0b013e3181b7fc56 [DOI] [PubMed] [Google Scholar]
- 117.Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity (2012) 36(2):262–75. 10.1016/j.immuni.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 118.Rea TH, Levan NE. Variations in dinitrochlorobenzene responsivity in untreated leprosy: evidence of a beneficial role for anergy. Int J Lepr Other Mycobact Dis (1980) 48(2):120–5. [PubMed] [Google Scholar]
- 119.Anders EM, McAdam KP, Anders RF. Cell-mediated immunity in amyloidosis secondary to lepromatous leprosy. Clin Exp Immunol (1977) 27(1):111–7. [PMC free article] [PubMed] [Google Scholar]
- 120.Dubey GK, Joglekar VK, Hardas UD, Chaubey BS. A study of cell mediated immunity in leprosy. Lepr India (1981) 53(2):197–203. [PubMed] [Google Scholar]
- 121.Bach MA, Hoffenbach A, Lagrange PH, Wallach D, Cottenot F. Mechanisms of T-cell unresponsiveness in leprosy. Ann Immunol (1983) 134D(1):75–84. [PubMed] [Google Scholar]
- 122.Sasiain MC, Ruibal Ares B, Balina LM, Valdez R, Bachmann AE. ConA-induced suppressor cells in lepromatous leprosy patients during and after erythema nodosum leprosum. Int J Lepr Other Mycobact Dis (1983) 51(3):321–7. [PubMed] [Google Scholar]
- 123.Rao TD, Rao PR. Tr, T mu and B lymphocytes in erythema nodosum leprosum reactions of leprosy. Indian J Lepr (1986) 58(4):601–8. [PubMed] [Google Scholar]
- 124.Bottasso O, Puig N, Amerio N, Morini JC. [Study of T lymphocyte subpopulations in patients with leprosy, using incubation with theophylline]. Med Cutan Ibero Lat Am (1988) 16(5):397–401. [PubMed] [Google Scholar]
- 125.Rasheed FN, Locniskar M, McCloskey DJ, Hasan RS, Chiang TJ, Rose P, et al. Serum lymphocytotoxic activity in leprosy. Clin Exp Immunol (1989) 76(3):391–7. [PMC free article] [PubMed] [Google Scholar]
- 126.Sasiain MD, de la Barrera S, Valdez R, Balina LM. Reduced suppressor cell response to Mycobacterium leprae in lepromatous leprosy. Infect Immun (1989) 57(3):951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bhoopat L, Scollard DM, Theetranont C, Chiewchanvit S, Nelson DL, Utaipat U. Studies of human leprosy lesions in situ using suction-induced blisters: cell changes with IgM antibody to PGL-1 and interleukin-2 receptor in clinical subgroups of erythema nodosum leprosum. Asian Pac J Allergy Immunol (1991) 9(2):107–19. [PubMed] [Google Scholar]
- 128.Foss NT, de Oliveira EB, Silva CL. Correlation between TNF production, increase of plasma C-reactive protein level and suppression of T lymphocyte response to concanavalin A during erythema nodosum leprosum. Int J Lepr Other Mycobact Dis (1993) 61(2):218–26. [PubMed] [Google Scholar]
- 129.Santos DO, Suffys PN, Moreira AL, Bonifacio K, Salgado JL, Esquenazi D, et al. Evaluation of chemiluminescence, procoagulant activity and antigen presentation by monocytes from lepromatous leprosy patients with or without reactional episodes. Lepr Rev (1994) 65(2):88–99. [DOI] [PubMed] [Google Scholar]
- 130.de la Barrera S, Fink S, Finiasz M, Minnucci F, Valdez R, Balina LM, et al. Lack of cytotoxic activity against Mycobacterium leprae 65-kD heat shock protein (hsp) in multibacillary leprosy patients. Clin Exp Immunol (1995) 99(1):90–7. 10.1111/j.1365-2249.1995.tb03477.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vieira LM, Sampaio EP, Nery JA, Duppre NC, Albuquerque EC, Scheinberg MA, et al. Immunological status of ENL (erythema nodosum leprosum) patients: its relationship to bacterial load and levels of circulating IL-2R. Rev Inst Med Trop Sao Paulo (1996) 38(2):103–11. 10.1590/S0036-46651996000200004 [DOI] [PubMed] [Google Scholar]
- 132.Tadesse A, Taye E, Sandoval F, Shannon EJ. Thalidomide does not modify the ability of cells in leprosy patients to incorporate [3H]-thymidine when incubated with M. leprae antigens. Lepr Rev (2003) 74(3):206–14. [PubMed] [Google Scholar]
- 133.Mohanty KK, Joshi B, Katoch K, Sengupta U. Leprosy reactions: humoral and cellular immune responses to M. leprae, 65kDa, 28kDa, and 18 kDa antigens. Int J Lepr Other Mycobact Dis (2004) 72(2):149–58. [DOI] [PubMed] [Google Scholar]
- 134.Villahermosa LG, Fajardo TT, Jr, Abalos RM, Balagon MV, Tan EV, Cellona RV, et al. A randomized, double-blind, double-dummy, controlled dose comparison of thalidomide for treatment of erythema nodosum leprosum. Am J Trop Med Hyg (2005) 72(5):518–26. [PubMed] [Google Scholar]
- 135.Rada E, Aranzazu N, Rodriguez V, Borges R, Convit J. [Serological and cellular reactivity to mycobacterial proteins in Hansen’s disease]. Invest Clin (2010) 51(3):325–40. [PubMed] [Google Scholar]
- 136.Saini C, Prasad HK, Rani R, Murtaza A, Misra N, Shanker Narayan NP, et al. Lsr2 of Mycobacterium leprae and its synthetic peptides elicit restitution of T cell responses in erythema nodosum leprosum and reversal reactions in patients with lepromatous leprosy. Clin Vaccine Immunol (2013) 20(5):673–82. 10.1128/CVI.00762-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parente JN, Talhari C, Schettini AP, Massone C. T regulatory cells (TREG)(TCD4+CD25+FOXP3+) distribution in the different clinical forms of leprosy and reactional states. An Bras Dermatol (2015) 90(1):41–7. 10.1590/abd1806-4841.20153311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sarno EN, Grau GE, Vieira LM, Nery JA. Serum levels of tumour necrosis factor-alpha and interleukin-1 beta during leprosy reactional states. Clin Exp Immunol (1991) 84(1):103–8. [PMC free article] [PubMed] [Google Scholar]
- 139.Sampaio EP, Kaplan G, Miranda A, Nery JA, Miguel CP, Viana SM, et al. The influence of thalidomide on the clinical and immunologic manifestation of erythema nodosum leprosum. J Infect Dis (1993) 168(2):408–14. 10.1093/infdis/168.2.408 [DOI] [PubMed] [Google Scholar]
- 140.Parida SK, Grau GE, Zaheer SA, Mukherjee R. Serum tumor necrosis factor and interleukin 1 in leprosy and during lepra reactions. Clin Immunol Immunopathol (1992) 63(1):23–7. 10.1016/0090-1229(92)90088-6 [DOI] [PubMed] [Google Scholar]
- 141.Memon RA, Kifayet A, Shahid F, Lateef A, Chiang J, Hussain R. Low serum HDL-cholesterol is associated with raised tumor necrosis factor-alpha during ENL reactions. Int J Lepr Other Mycobact Dis (1997) 65(1):1–11. [PubMed] [Google Scholar]
- 142.Partida-Sanchez S, Favila-Castillo L, Pedraza-Sanchez S, Gomez-Melgar M, Saul A, Estrada-Parra S, et al. IgG antibody subclasses, tumor necrosis factor and IFN-gamma levels in patients with type II lepra reaction on thalidomide treatment. Int Arch Allergy Immunol (1998) 116(1):60–6. 10.1159/000023926 [DOI] [PubMed] [Google Scholar]
- 143.Iyer A, Hatta M, Usman R, Luiten S, Oskam L, Faber W, et al. Serum levels of interferon-gamma, tumour necrosis factor-alpha, soluble interleukin-6R and soluble cell activation markers for monitoring response to treatment of leprosy reactions. Clin Exp Immunol (2007) 150(2):210–6. 10.1111/j.1365-2249.2007.03485.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Madan NK, Agarwal K, Chander R. Serum cytokine profile in leprosy and its correlation with clinico-histopathological profile. Lepr Rev (2011) 82(4):371–82. [PubMed] [Google Scholar]
- 145.Rodrigues LS, Hacker MA, Illarramendi X, Pinheiro MF, Nery JA, Sarno EN, et al. Circulating levels of insulin-like growth factor-I (IGF-I) correlate with disease status in leprosy. BMC Infect Dis (2011) 11:339. 10.1186/1471-2334-11-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bhattacharya SN, Chattopadhaya D, Saha K. Tumor necrosis factor: status in reactions in leprosy before and after treatment. Int J Dermatol (1993) 32(6):436–9. 10.1111/j.1365-4362.1993.tb02816.x [DOI] [PubMed] [Google Scholar]
- 147.Sampaio EP, Moraes MO, Nery JA, Santos AR, Matos HC, Sarno EN. Pentoxifylline decreases in vivo and in vitro tumour necrosis factor-alpha (TNF-alpha) production in lepromatous leprosy patients with erythema nodosum leprosum (ENL). Clin Exp Immunol (1998) 111(2):300–8. 10.1046/j.1365-2249.1998.00510.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moubasher AD, Kamel NA, Zedan H, Raheem DD. Cytokines in leprosy, I. Serum cytokine profile in leprosy. Int J Dermatol (1998) 37(10):733–40. 10.1046/j.1365-4362.1998.00381.x [DOI] [PubMed] [Google Scholar]
- 149.Jadhav R, Suneetha L, Kamble R, Shinde V, Devi K, Chaduvula MV, et al. Analysis of antibody and cytokine markers for leprosy nerve damage and reactions in the INFIR cohort in India. PLoS Negl Trop Dis (2011) 5(3):e977. 10.1371/journal.pntd.0000977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sampaio EP, Moreira AL, Sarno EN, Malta AM, Kaplan G. Prolonged treatment with recombinant interferon gamma induces erythema nodosum leprosum in lepromatous leprosy patients. J Exp Med (1992) 175(6):1729–37. 10.1084/jem.175.6.1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stefani MM, Guerra JG, Sousa AL, Costa MB, Oliveira ML, Martelli CT, et al. Potential plasma markers of Type 1 and Type 2 leprosy reactions: a preliminary report. BMC Infect Dis (2009) 9:75. 10.1186/1471-2334-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wolkenstein P, Latarjet J, Roujeau JC, Duguet C, Boudeau S, Vaillant L, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet (1998) 352(9140):1586–9. 10.1016/S0140-6736(98)02197-7 [DOI] [PubMed] [Google Scholar]
- 153.Jacobson JM, Greenspan JS, Spritzler J, Ketter N, Fahey JL, Jackson JB, et al. Thalidomide for the treatment of oral aphthous ulcers in patients with human immunodeficiency virus infection. National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group. N Engl J Med (1997) 336(21):1487–93. 10.1056/NEJM199705223362103 [DOI] [PubMed] [Google Scholar]
- 154.Haslett PA, Hanekom WA, Muller G, Kaplan G. Thalidomide and a thalidomide analogue drug costimulate virus-specific CD8+ T cells in vitro. J Infect Dis (2003) 187(6):946–55. 10.1086/368126 [DOI] [PubMed] [Google Scholar]
- 155.Barnes PF, Chatterjee D, Brennan PJ, Rea TH, Modlin RL. Tumor necrosis factor production in patients with leprosy. Infect Immun (1992) 60(4):1441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Santos DO, Suffys PN, Bonifacio K, Marques MA, Sarno EN. In vitro tumor necrosis factor production by mononuclear cells from lepromatous leprosy patients and from patients with erythema nodosum leprosum. Clin Immunol Immunopathol (1993) 67(3 Pt 1):199–203. 10.1006/clin.1993.1065 [DOI] [PubMed] [Google Scholar]
- 157.Sampaio EP, Oliveira RB, Warwick-Davies J, Neto RB, Griffin GE, Shattock RJ. T cell-monocyte contact enhances tumor necrosis factor-alpha production in response to Mycobacterium leprae. J Infect Dis (2000) 182(5):1463–72. 10.1086/315902 [DOI] [PubMed] [Google Scholar]
- 158.Faber WR, Jensema AJ, Goldschmidt WF. Treatment of recurrent erythema nodosum leprosum with infliximab. N Engl J Med (2006) 355(7):739. 10.1056/NEJMc052955 [DOI] [PubMed] [Google Scholar]
- 159.Ramien ML, Wong A, Keystone JS. Severe refractory erythema nodosum leprosum successfully treated with the tumor necrosis factor inhibitor etanercept. Clin Infect Dis (2011) 52(5):e133–5. 10.1093/cid/ciq213 [DOI] [PubMed] [Google Scholar]
- 160.Chowdhry S, Shukla A, D’Souza P, Dhali T, Jaiswal P. Treatment of severe refractory erythema nodosum leprosum with tumor necrosis factor inhibitor etanercept. Int J Mycobacteriol (2016) 5(2):223–5. 10.1016/j.ijmyco.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 161.Moraes MO, Sarno EN, Almeida AS, Saraiva BC, Nery JA, Martins RC, et al. Cytokine mRNA expression in leprosy: a possible role for interferon-gamma and interleukin-12 in reactions (RR and ENL). Scand J Immunol (1999) 50(5):541–9. 10.1046/j.1365-3083.1999.00622.x [DOI] [PubMed] [Google Scholar]
- 162.Nath I, Vemuri N, Reddi AL, Bharadwaj M, Brooks P, Colston MJ, et al. Dysregulation of IL-4 expression in lepromatous leprosy patients with and without erythema nodosum leprosum. Lepr Rev (2000) 71(Suppl):S130–7. [DOI] [PubMed] [Google Scholar]
- 163.Nath I, Vemuri N, Reddi AL, Jain S, Brooks P, Colston MJ, et al. The effect of antigen presenting cells on the cytokine profiles of stable and reactional lepromatous leprosy patients. Immunol Lett (2000) 75(1):69–76. 10.1016/S0165-2478(00)00271-6 [DOI] [PubMed] [Google Scholar]
- 164.Moraes MO, Sarno EN, Teles RM, Almeida AS, Saraiva BC, Nery JA, et al. Anti-inflammatory drugs block cytokine mRNA accumulation in the skin and improve the clinical condition of reactional leprosy patients. J Invest Dermatol (2000) 115(6):935–41. 10.1046/j.1523-1747.2000.00158.x [DOI] [PubMed] [Google Scholar]
- 165.Moubasher AD, Kamel NA, Zedan H, Raheem DD. Cytokines in leprosy, II. Effect of treatment on serum cytokines in leprosy. Int J Dermatol (1998) 37(10):741–6. 10.1046/j.1365-4362.1998.00381.x [DOI] [PubMed] [Google Scholar]
- 166.Sallam MA, Attia EA, Soliman MS. Assessment of serum level of interleukin-1b and interleukin-12 in leprosy: impact of previous bacillus calmitte guerin vaccination. Arch Dermatol Res (2014) 306(2):189–95. 10.1007/s00403-013-1411-0 [DOI] [PubMed] [Google Scholar]
- 167.Berrington WR, Kunwar CB, Neupane K, van den Eeden SJ, Vary JC, Jr, Peterson GJ, et al. Differential dermal expression of CCL17 and CCL18 in tuberculoid and lepromatous leprosy. PLoS Negl Trop Dis (2014) 8(11):e3263. 10.1371/journal.pntd.0003263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Filley E, Andreoli A, Steele J, Waters M, Wagner D, Nelson D, et al. A transient rise in agalactosyl IgG correlating with free interleukin 2 receptors, during episodes of erythema nodosum leprosum. Clin Exp Immunol (1989) 76(3):343–7. [PMC free article] [PubMed] [Google Scholar]
- 169.Belgaumkar VA, Gokhale NR, Mahajan PM, Bharadwaj R, Pandit DP, Deshpande S. Circulating cytokine profiles in leprosy patients. Lepr Rev (2007) 78(3):223–30. [PubMed] [Google Scholar]
- 170.Sousa AL, Fava VM, Sampaio LH, Martelli CM, Costa MB, Mira MT, et al. Genetic and immunological evidence implicates interleukin 6 as a susceptibility gene for leprosy type 2 reaction. J Infect Dis (2012) 205(9):1417–24. 10.1093/infdis/jis208 [DOI] [PubMed] [Google Scholar]
- 171.Abdallah M, Emam H, Attia E, Hussein J, Mohamed N. Estimation of serum level of interleukin-17 and interleukin-4 in leprosy, towards more understanding of leprosy immunopathogenesis. Indian J Dermatol Venereol Leprol (2013) 79(6):772–6. 10.4103/0378-6323.120723 [DOI] [PubMed] [Google Scholar]
- 172.Sehgal VN, Bhattacharya SN, Shah Y, Sharma VK, Gupta CK. Soluble interleukin-2 receptors: levels in leprosy, and during and after type 1 (lepra) and type 2 (ENL) reactions. Lepr Rev (1991) 62(3):262–8. [DOI] [PubMed] [Google Scholar]
- 173.Sullivan L, Sano S, Pirmez C, Salgame P, Mueller C, Hofman F, et al. Expression of adhesion molecules in leprosy lesions. Infect Immun (1991) 59(11):4154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Goulart IM, Mineo JR, Foss NT. Production of transforming growth factor-beta 1 (TGF-beta1) by blood monocytes from patients with different clinical forms of leprosy. Clin Exp Immunol (2000) 122(3):330–4. 10.1046/j.1365-2249.2000.01376.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Motta AC, Furini RB, Simao JC, Ferreira MA, Komesu MC, Foss NT. The recurrence of leprosy reactional episodes could be associated with oral chronic infections and expression of serum IL-1, TNF-alpha, IL-6, IFN-gamma and IL-10. Braz Dent J (2010) 21(2):158–64. 10.1590/S0103-64402010000200012 [DOI] [PubMed] [Google Scholar]
- 176.Teles RM, Teles RB, Amadeu TP, Moura DF, Mendonca-Lima L, Ferreira H, et al. High matrix metalloproteinase production correlates with immune activation and leukocyte migration in leprosy reactional lesions. Infect Immun (2010) 78(3):1012–21. 10.1128/IAI.00896-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chaitanya S, Lavania M, Turankar RP, Karri SR, Sengupta U. Increased serum circulatory levels of interleukin 17F in type 1 reactions of leprosy. J Clin Immunol (2012) 32(6):1415–20. 10.1007/s10875-012-9747-3 [DOI] [PubMed] [Google Scholar]
- 178.Lockwood DN, Nicholls P, Smith WC, Das L, Barkataki P, van Brakel W, et al. Comparing the clinical and histological diagnosis of leprosy and leprosy reactions in the INFIR cohort of Indian patients with multibacillary leprosy. PLoS Negl Trop Dis (2012) 6(6):e1702. 10.1371/journal.pntd.0001702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Martiniuk F, Giovinazzo J, Tan AU, Shahidullah R, Haslett P, Kaplan G, et al. Lessons of leprosy: the emergence of TH17 cytokines during type II reactions (ENL) is teaching us about T-cell plasticity. J Drugs Dermatol (2012) 11(5):626–30. [PMC free article] [PubMed] [Google Scholar]
- 180.Reichlin M, Pranis RA, Gelber RH, Rees RJ, Taverne J, Turk JL. Correlation of euglobulin immunoglobulin G levels with erythema nodosum leprosum in lepromatous leprosy. Clin Immunol Immunopathol (1977) 8(2):335–44. 10.1016/0090-1229(77)90123-4 [DOI] [PubMed] [Google Scholar]
- 181.Humphres RC, Gelber RH, Krahenbuhl JL. Suppressed natural killer cell activity during episodes of erythema nodosum leprosum in lepromatous leprosy. Clin Exp Immunol (1982) 49(3):500–8. [PMC free article] [PubMed] [Google Scholar]
- 182.Rea TH, Yoshida T. Serum macrophage migration inhibition activity in patients with leprosy. J Invest Dermatol (1982) 79(5):336–9. 10.1111/1523-1747.ep12500088 [DOI] [PubMed] [Google Scholar]
- 183.Miller RA, Harnisch JP, Buchanan TM. Antibodies to mycobacterial arabinomannan in leprosy: correlation with reactional states and variation during treatment. Int J Lepr Other Mycobact Dis (1984) 52(2):133–9. [PubMed] [Google Scholar]
- 184.Schwerer B, Meeker HC, Sersen G, Levis WR. IgM antibodies against phenolic glycolipid I from Mycobacterium leprae in leprosy sera: relationship to bacterial index and erythema nodosum leprosum. Acta Leprol (1984) 2(2–4):394–402. [PubMed] [Google Scholar]
- 185.Blavy G, Thiam D, Ndoye B, Diakhate L, Millan J. [HLA and leprosy in Dakar: distribution of histocompatibility antigens in leprous patients and their relationship to ENL reactions]. Acta Leprol (1986) 4(1):93–9. [PubMed] [Google Scholar]
- 186.Levis WR, Meeker HC, Schuller-Levis G, Sersen E, Schwerer B. IgM and IgG antibodies to phenolic glycolipid I from Mycobacterium leprae in leprosy: insight into patient monitoring, erythema nodosum leprosum, and bacillary persistence. J Invest Dermatol (1986) 86(5):529–34. 10.1111/1523-1747.ep12354963 [DOI] [PubMed] [Google Scholar]
- 187.Levis WR, Meeker HC, Schuller-Levis G, Sersen E, Brennan PJ, Fried P. Mycobacterial carbohydrate antigens for serological testing of patients with leprosy. J Infect Dis (1987) 156(5):763–9. 10.1093/infdis/156.5.763 [DOI] [PubMed] [Google Scholar]
- 188.Sehgal VN, Bhattacharya SN, Shah Y, Rao YN, Gupta CK. Lymphocyte adenosine deaminase activity (L-ADA) in leprosy, during and after treatment of reactions. Clin Exp Dermatol (1992) 17(1):20–3. 10.1111/j.1365-2230.1992.tb02526.x [DOI] [PubMed] [Google Scholar]
- 189.Singh S, Jenner PJ, Narayan NP, Ramu G, Colston MJ, Prasad HK, et al. Critical residues of the Mycobacterium leprae LSR recombinant protein discriminate clinical activity in erythema nodosum leprosum reactions. Infect Immun (1994) 62(12):5702–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kifayet A, Hussain R. Selective decrease of M. leprae-specific IgG1 and IgG3 antibodies in leprosy patients associated with ENL. Int J Lepr Other Mycobact Dis (1996) 64(2):105–14. [PubMed] [Google Scholar]
- 191.Kifayet A, Shahid F, Lucas S, Hussain R. Erythema nodosum leprosum is associated with up-regulation of polyclonal IgG1 antibody synthesis. Clin Exp Immunol (1996) 106(3):447–53. 10.1046/j.1365-2249.1996.d01-860.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Beuria MK, Parkash O, Joshi B, Mohanty KK, Katoch K, Sengupta U. Levels of IgG subclasses in active and inactive cases in the disease spectrum of leprosy. Int Arch Allergy Immunol (1998) 115(1):61–6. 10.1159/000023831 [DOI] [PubMed] [Google Scholar]
- 193.Freire BF, Ferraz AA, Nakayama E, Ura S, Queluz TT. Anti-neutrophil cytoplasmic antibodies (ANCA) in the clinical forms of leprosy. Int J Lepr Other Mycobact Dis (1998) 66(4):475–82. [PubMed] [Google Scholar]
- 194.Stefani MM, Martelli CM, Morais-Neto OL, Martelli P, Costa MB, de Andrade AL. Assessment of anti-PGL-I as a prognostic marker of leprosy reaction. Int J Lepr Other Mycobact Dis (1998) 66(3):356–64. [PubMed] [Google Scholar]
- 195.Beuria MK, Mohanty KK, Katoch K, Sengupta U. Determination of circulating IgG subclasses against lipoarabinomannan in the leprosy spectrum and reactions. Int J Lepr Other Mycobact Dis (1999) 67(4):422–8. [PubMed] [Google Scholar]
- 196.Hamerlinck FF, Klatser PR, Walsh DS, Bos JD, Walsh GP, Faber WR. Serum neopterin as a marker for reactional states in leprosy. FEMS Immunol Med Microbiol (1999) 24(4):405–9. 10.1111/j.1574-695X.1999.tb01312.x [DOI] [PubMed] [Google Scholar]
- 197.Mahaisavariya P, Jiamton S, Manonukul J, Khemngern S. Mast cells in leprosy and leprosy reaction. Int J Dermatol (2000) 39(4):274–7. 10.1046/j.1365-4362.2000.00908.x [DOI] [PubMed] [Google Scholar]
- 198.Schon T, Leekassa R, Gebre N, Sundqvist T, Bizuneh E, Britton S. High dose prednisolone treatment of leprosy patients undergoing reactions is associated with a rapid decrease in urinary nitric oxide metabolites and clinical improvement. Lepr Rev (2000) 71(3):355–62. [DOI] [PubMed] [Google Scholar]
- 199.Antunes SL, Liang Y, Neri JA, Sarno EN, Haak-Frendscho M, Johansson O. Mast cell subsets and neuropeptides in leprosy reactions. Arq Neuropsiquiatr (2003) 61(2A):208–19. 10.1590/S0004-282X2003000200010 [DOI] [PubMed] [Google Scholar]
- 200.Rada E, Marzal M, Aranzazu N, Convit J. [Increase in nitric oxide concentrations in serum and mononuclear cell cultures from patients with Type II reaction state of Hansen’s disease]. Invest Clin (2003) 44(2):129–36. [PubMed] [Google Scholar]
- 201.Sunderkotter CH, Tomimori-Yamashita J, Nix V, Maeda SM, Sindrilaru A, Mariano M, et al. High expression of myeloid-related proteins 8 and 14 characterizes an inflammatorily active but ineffective response of macrophages during leprosy. Immunology (2004) 111(4):472–80. 10.1111/j.0019-2805.2004.01836.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nigam PK, Srivastava P, Patra PK. Serum adenosine deaminase levels in reactional and non-reactional leprosy. Indian J Dermatol Venereol Leprol (2005) 71(1):20–2. 10.4103/0378-6323.13780 [DOI] [PubMed] [Google Scholar]
- 203.Mohanty KK, Gupta M, Girdhar BK, Girdhar A, Chakma JK, Sengupta U. Increased level of urinary nitric oxide metabolites in leprosy patients during type 2 reactions and decreased after antireactional therapy. Lepr Rev (2007) 78(4):386–90. [PubMed] [Google Scholar]
- 204.Santos DO, Castro HC, Bourguignon SC, Bastos OM, Rodrigues CR, Van Heuverswyn H, et al. Expression of B7-1 costimulatory molecules in patients with multibacillary leprosy and reactional states. Clin Exp Dermatol (2007) 32(1):75–80. 10.1111/j.1365-2230.2006.02291.x [DOI] [PubMed] [Google Scholar]
- 205.Silva EA, Iyer A, Ura S, Lauris JR, Naafs B, Das PK, et al. Utility of measuring serum levels of anti-PGL-I antibody, neopterin and C-reactive protein in monitoring leprosy patients during multi-drug treatment and reactions. Trop Med Int Health (2007) 12(12):1450–8. 10.1111/j.1365-3156.2007.01951.x [DOI] [PubMed] [Google Scholar]
- 206.Brito Mde F, Ximenes RA, Gallo ME, Buhrer-Sekula S. Association between leprosy reactions after treatment and bacterial load evaluated using anti PGL-I serology and bacilloscopy. Rev Soc Bras Med Trop (2008) 41(Suppl 2):67–72. [DOI] [PubMed] [Google Scholar]
- 207.Iyer A, van Eijk M, Silva E, Hatta M, Faber W, Aerts JM, et al. Increased chitotriosidase activity in serum of leprosy patients: association with bacillary leprosy. Clin Immunol (2009) 131(3):501–9. 10.1016/j.clim.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 208.Singh I, Yadav AR, Mohanty KK, Katoch K, Bisht D, Sharma P, et al. Molecular mimicry between HSP 65 of Mycobacterium leprae and cytokeratin 10 of the host keratin; role in pathogenesis of leprosy. Cell Immunol (2012) 278(1–2):63–75. 10.1016/j.cellimm.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 209.Mandal D, Reja AH, Biswas N, Bhattacharyya P, Patra PK, Bhattacharya B. Vitamin D receptor expression levels determine the severity and complexity of disease progression among leprosy reaction patients. New Microbes New Infect (2015) 6:35–9. 10.1016/j.nmni.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Berrington WR, Macdonald M, Khadge S, Sapkota BR, Janer M, Hagge DA, et al. Common polymorphisms in the NOD2 gene region are associated with leprosy and its reactive states. J Infect Dis (2010) 201(9):1422–35. 10.1086/651559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Teixeira MA, Silva NL, Ramos Ade L, Hatagima A, Magalhaes V. [NRAMP1 gene polymorphisms in individuals with leprosy reactions attended at two reference centers in Recife, northeastern Brazil]. Rev Soc Bras Med Trop (2010) 43(3):281–6. 10.1590/S0037-86822010000300014 [DOI] [PubMed] [Google Scholar]
- 212.de Sales Marques C, Brito-de-Souza VN, Albuquerque Guerreiro LT, Martins JH, Amaral EP, Cardoso CC, et al. Toll-like receptor 1 (TLR1) N248S single nucleotide polymorphism is associated with leprosy risk and regulates immune activation during mycobacterial infection. J Infect Dis (2013) 208(1):120–9. 10.1093/infdis/jit133 [DOI] [PubMed] [Google Scholar]
- 213.Schuring RP, Hamann L, Faber WR, Pahan D, Richardus JH, Schumann RR, et al. Polymorphism N248S in the human toll-like receptor 1 gene is related to leprosy and leprosy reactions. J Infect Dis (2009) 199(12):1816–9. 10.1086/599121 [DOI] [PubMed] [Google Scholar]
- 214.Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood (2011) 118(22):5918–27. 10.1182/blood-2011-03-340281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tan Trao V, Huong PL, Thuan AT, Long HT, Trach DD, Wright EP. Responses to Mycobacterium leprae by lymphocytes from new and old leprosy patients: role of exogenous lymphokines. Ann Inst Pasteur Immunol (1988) 139(2):121–33. 10.1016/0769-2625(88)90034-7 [DOI] [PubMed] [Google Scholar]
- 216.Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol (2012) 2(11):120134. 10.1098/rsob.120134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fatima N, Faisal SM, Zubair S, Ajmal M, Siddiqui SS, Moin S, et al. Role of pro-inflammatory cytokines and biochemical markers in the pathogenesis of type 1 diabetes: correlation with age and glycemic condition in diabetic human subjects. PLoS One (2016) 11(8):e0161548. 10.1371/journal.pone.0161548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Anderson R, Gatner EM, van Rensburg CE, Grabow G, Imkamp FM, Kok SK, et al. In vitro and in vivo effects of dapsone on neutrophil and lymphocyte functions in normal individuals and patients with lepromatous leprosy. Antimicrob Agents Chemother (1981) 19(4):495–503. 10.1128/AAC.19.4.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Anderson R, Gatner EM. Changes in neutrophil motility accompanying dapsone and rifampicin therapy. Lepr Rev (1981) 52(1):19–22. [DOI] [PubMed] [Google Scholar]
- 220.Anderson R. Enhancement by clofazimine and inhibition by dapsone of production of prostaglandin E2 by human polymorphonuclear leukocytes in vitro. Antimicrob Agents Chemother (1985) 27(2):257–62. 10.1128/AAC.27.2.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Das PK, Klatser PR, Pondman KW, Huikeshoven H, Landheer JE, Leiker DL, et al. Dapsone and anti-dapsone antibody in circulating immune complexes in leprosy patients. Lancet (1980) 1(8181):1309–11. 10.1016/S0140-6736(80)91772-9 [DOI] [PubMed] [Google Scholar]
- 222.Trao VT, Huong PL, Thuan AT, Anh DD, Trach DD, Rook GA, et al. Changes in cellular response to mycobacterial antigens and cytokine production patterns in leprosy patients during multiple drug therapy. Immunology (1998) 94(2):197–206. 10.1046/j.1365-2567.1998.00485.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mendonca VA, Costa RD, Lyon S, Penido RA, Borges VO, Bretas TL, et al. Plasma levels of chemokines during leprosy specific treatment. Acta Trop (2010) 113(2):151–4. 10.1016/j.actatropica.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 224.Nishimura M, Koeda A, Suzuki E, Shimizu T, Kawano Y, Nakayama M, et al. Effects of prototypical drug-metabolizing enzyme inducers on mRNA expression of housekeeping genes in primary cultures of human and rat hepatocytes. Biochem Biophys Res Commun (2006) 346(3):1033–9. 10.1016/j.bbrc.2006.06.012 [DOI] [PubMed] [Google Scholar]
- 225.Libert C, Dejager L. How steroids steer T cells. Cell Rep (2014) 7(4):938–9. 10.1016/j.celrep.2014.04.041 [DOI] [PubMed] [Google Scholar]
- 226.Langereis JD, Oudijk EJ, Schweizer RC, Lammers JW, Koenderman L, Ulfman LH. Steroids induce a disequilibrium of secreted interleukin-1 receptor antagonist and interleukin-1beta synthesis by human neutrophils. Eur Respir J (2011) 37(2):406–15. 10.1183/09031936.00170409 [DOI] [PubMed] [Google Scholar]
- 227.Auttachoat W, Zheng JF, Chi RP, Meng A, Guo TL. Differential surface expression of CD18 and CD44 by neutrophils in bone marrow and spleen contributed to the neutrophilia in thalidomide-treated female B6C3F1 mice. Toxicol Appl Pharmacol (2007) 218(3):227–37. 10.1016/j.taap.2006.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kim BS, Kim JY, Lee JG, Cho Y, Huh KH, Kim MS, et al. Immune modulatory effect of thalidomide on T cells. Transplant Proc (2015) 47(3):787–90. 10.1016/j.transproceed.2014.12.038 [DOI] [PubMed] [Google Scholar]
- 229.Hernandez Mde O, Fulco Tde O, Pinheiro RO, Pereira Rde M, Redner P, Sarno EN, et al. Thalidomide modulates Mycobacterium leprae-induced NF-kappaB pathway and lower cytokine response. Eur J Pharmacol (2011) 670(1):272–9. 10.1016/j.ejphar.2011.08.046 [DOI] [PubMed] [Google Scholar]