Abstract
Strong experimental evidence confirms that HDL directly alleviates atherosclerosis. HDL particles display diverse atheroprotective functions in reverse cholesterol transport (RCT), antioxidant, anti-inflammatory, and antiapoptotic processes. In certain inflammatory disease states, however, HDL particles may become dysfunctional and proatherogenic. Flavonoids show the potential to improve HDL function through their well-documented effects on cellular antioxidant status and inflammation. The aim of this review is to summarize the basic science and clinical research examining the effects of dietary flavonoids on RCT and HDL function. Based on preclinical studies that used cell culture and rodent models, it appears that many flavonoids (e.g., anthocyanidins, flavonols, and flavone subclasses) influence RCT and HDL function beyond simple HDL cholesterol concentration by regulating cellular cholesterol efflux from macrophages and hepatic paraoxonase 1 expression and activity. In clinical studies, dietary anthocyanin intake is associated with beneficial changes in serum biomarkers related to HDL function in a variety of human populations (e.g., in those who are hyperlipidemic, hypertensive, or diabetic), including increased HDL cholesterol concentration, as well as HDL antioxidant and cholesterol efflux capacities. However, clinical research on HDL functionality is lacking for some flavonoid subclasses (e.g., flavanols, flavones, flavanones, and isoflavones). Although there has been a tremendous effort to develop HDL-targeted drug therapies, more research is warranted on how the intake of foods or specific nutrients affects HDL function.
Keywords: flavonoids, HDL, polyphenols, anthocyanins, atherosclerosis
Introduction
Even with the widespread use of statin-class drugs, coronary heart disease (CHD)3 continues to be the largest cause of death in the United States, contributing to >600,000 deaths/y (1). Atherosclerosis is a key contributor to CHD, and is caused by an accumulation of lipid in the arterial wall. Furthermore, disturbances in lipid metabolism and inflammation are thought to be fundamental to these disease processes (2). Despite aggressive lowering of LDL cholesterol, there still exists a residual risk of cardiovascular events in patients with established CHD (3). Strong experimental evidence confirms that atherosclerosis is directly alleviated by HDL. This is shown by infusions of HDLs into humans and animal models, which acutely increase the number of HDL particles and reduce the atherosclerotic plaque burden (4–9). Data from the Framingham Heart Study, initiated in 1948, first demonstrated an inverse relation between HDL cholesterol and the development of cardiovascular disease (CVD) (10, 11). Since that time, a majority of epidemiologic studies have supported this relation (12). Based on evidence from large prospective cohort studies, it can be estimated that for every 1 mg/dL (0.0259 mmol/L) increase in HDL cholesterol, there is a 2–3% reduction in CVD risk (12). Therapies aimed at increasing HDL cholesterol in those with low concentrations are under intense investigation in the hope of preventing future cardiovascular events (13–15). However, more recently, this association has been challenged by studies that suggest that plasma HDL cholesterol is not always an accurate predictor of CVD risk (16–21). One aspect concerning HDL that remains consistent is its important role in reverse cholesterol transport (RCT) (22). This pathway, as classically defined, is the movement of cholesterol from peripheral cells to circulating lipoproteins and its subsequent disposal to the liver for catabolism and excretion (23).
The atheroprotective effect of HDL is mainly attributed to its role in RCT; removing excess cholesterol from macrophages in the arterial wall, preventing foam cell formation, and the initial stages of atherosclerotic plaque development (23). HDL-mediated RCT can be loosely grouped into 3 main processes: building HDL particles through cellular cholesterol mobilization, remodeling of HDL particles by plasma proteins, and delivery of HDL-derived cholesterol to the liver. RCT is thought to be the major mechanism for cholesterol excretion in mammals, with a key mediator of this process being apoA-I, the major protein constituent of HDL. The first step of RCT, which involves cellular cholesterol mobilization, relies on apoA-I and HDL particle interactions to promote free cholesterol efflux by a variety of passive and active mechanisms (24). Although it is well known that HDLs remove cholesterol from cells, mechanistic details are still being resolved. However, important contributors to cellular cholesterol efflux appear to include ATP-binding cassette transporter A1 (ABCA1) and ATP-binding cassette transporter G1 (ABCG1) (25). In cholesterol-loaded macrophages, ABCA1 and ABCG1 are responsible for roughly 50% and 20% of total cholesterol efflux from cells, respectively (25). Accordingly, deficiency of these ATP-binding cassette transporters in macrophages has been shown to reduce RCT in mice (26). HDL formation, or biogenesis, involves the efflux of phospholipids and free cholesterol by ABCA1 to lipid-free apoA-I in the extracellular space, generating nascent HDL particles (27). After ABCA1 facilitates HDL biogenesis, these nascent HDLs become efficient substrates for further lipidation by other cellular pathways (e.g., ABCG1) and esterification of free cholesterol to cholesteryl esters (CEs) by lecithin-cholesterol acyltransferase (LCAT) (28, 29). Esterification via LCAT converts the amphipathic cholesterol molecule at the surface of the particle to a nonpolar compound that partitions into the particle’s core (28). The second step of RCT involves HDL remodeling by TG lipases (e.g., hepatic lipase) and various plasma lipid transfer proteins, such as cholesteryl ester transfer protein (CETP) (30). CETP is a circulating glycoprotein that promotes VLDL transition to an LDL particle by facilitating bidirectional transfer of CEs, TGs, and, to a lesser extent, phospholipids, from HDL particles to apoB-containing particles (30). The final step of RCT involves delivery of HDL-derived cholesterol to the liver by selective HDL CE uptake by scavenger receptor class B type I (SR-BI) and/or holoparticle internalization by other receptors (31, 32). SR-BI in hepatocytes mediates the selective uptake of CEs from the core of HDLs, without HDL protein catabolism. This selective uptake of CEs likely occurs at the plasma membrane and/or through endocytosis, permitting HDL particle recycling (33). Cholesterol delivery can also occur indirectly after CE transfer from HDL particles to apoB-containing lipoproteins, which allows for uptake via the hepatic LDL receptor (34, 35). This indirect pathway is particularly important for RCT in humans (34), but less so in rodents, which lack CETP activity (36). The importance of the RCT pathway has been validated through extensive studies in humans and by a vast number of animal models of atherosclerosis (37). Serum HDL cholesterol is the primary clinical measurement used as a surrogate metric of RCT. Although HDL cholesterol is a consistent indicator of CHD risk in epidemiologic studies, the use of HDL cholesterol as a therapeutic target has recently been questioned because trials of several HDL cholesterol–raising drugs showed a lack of efficacy in decreasing the risk of CHD (17, 38). Therefore, functional measures beyond HDL cholesterol (e.g., antioxidant and efflux capacity) may be more appropriate in the atheroprotective assessment of HDLs.
In addition to their role in RCT, HDL particles have shown diverse biological activities with antioxidant (39), anti-inflammatory (40), antiapoptotic (41), antithrombotic (42), and vasorelaxant properties (43). Much of the biological activity of HDL is due to its protein content, with it carrying >50 distinct proteins with known functions in inflammation, oxidation, protease inhibition, complement regulation, and innate immunity (44). Paraoxonase 1 (PON1), an HDL-associated lipolactonase, contributes to HDL antioxidant and anti-inflammatory functions by preventing both LDL and HDL particles from oxidation (45). PON1-deficient mice are more sensitive to lipoprotein oxidation and the development of atherosclerosis (39, 46). In certain inflammatory disease states, antiatherogenic HDL particles may become proatherogenic, and this may potentially explain some residual CVD risk in individuals with elevated HDL cholesterol (47). HDL particles may be viewed as shuttles that can be either anti-inflammatory or proinflammatory, depending on their cargo of proteins, enzymes, and lipids. As mentioned, atherosclerosis is an inflammatory disease, and low serum HDL cholesterol, along with impairments in cholesterol efflux (19), antioxidant (48), and anti-inflammatory properties (49), have been shown in patients with heart disease. Furthermore, the antioxidant and anti-inflammatory properties of HDLs are also impaired in obesity and other metabolic diseases (50–52). These inflammatory conditions can remodel HDLs to a proinflammatory particle with impaired antioxidant activity and compositional changes consisting of elevated serum amyloid A (SAA), an acute-phase protein, and reduced PON1 (53, 54). The identification of dysfunctional HDL particles provides a greater appreciation for HDL metabolism and a potential therapeutic target beyond simple HDL cholesterol content. For example, scavenger receptor class B type I knockout (Scarb1−/−) mice have markedly elevated HDL cholesterol (a >50% increase), but have reduced HDL PON1 activity and increased atherosclerosis (20, 55). Numerous HDL functional assays have been developed to examine the ability of HDL particles to mobilize cholesterol and stimulate NO production from cells, as well as inhibit oxidation, coagulation, and monocyte adhesion processes (56). HDL functional assays that were shown to be independent predictors of CHD status and coronary events include cholesterol efflux assays (19) and PON1 activity (57), respectively. The cholesterol efflux capacity of HDL was shown to be a significant predictor of CHD status even after plasma HDL cholesterol and apoA-I were adjusted for (19). Importantly, differences in cholesterol efflux capacity have been reported in humans with similar HDL cholesterol concentrations (58), supporting the need to examine metrics of HDL function beyond HDL cholesterol. Other quantitative measures of HDL, such as HDL particle number, may explain some of this variation (59). Furthermore, in light of the failures of HDL cholesterol–raising drugs (e.g., high-dose niacin and CETP inhibitors) to reduce CHD (17, 38), lifestyle changes or drug treatments that affect HDL functionality may prove to be more effective approaches. Thiazolidinedione administration in metabolic syndrome patients with low HDL cholesterol has been reported to improve the cholesterol efflux capacity of HDL (19). It is now clear that HDL cholesterol concentrations do not fully capture the functional variation in HDLs, especially under inflammatory conditions. Because many of the HDL-targeted drugs cause various side effects or do not reduce CVD risk, alternatives are needed. Therefore, it is important to consider how dietary components, such as flavonoid-rich foods, influence HDL functionality. Dietary flavonoids could potentially be used as adjunct therapies alongside drug treatments to enhance outcomes. The aim of this review is to summarize the basic science and clinical research examining dietary flavonoid intake on RCT, HDL metabolism, and HDL function.
Flavonoids as Atheroprotective Components of the Diet
Only a few dietary factors are known to strongly increase HDL cholesterol, including alcohol (60), saturated fats (61), and, to a lesser extent, dietary cholesterol (62). Consumption of these components in copious amounts is typically not recommended for CHD prevention. However, it is also important to consider the effects of foods and nutrients on HDL function, even if they are not shown to alter HDL cholesterol content, especially if the consumption of such foods and nutrients may confer antioxidant or anti-inflammatory benefits. Plants supply a substantial amount of polyphenols to the human diet, which are thought to contribute to the inverse relation between fruit and vegetable intake and chronic disease (63). Flavonoids, the most common type of polyphenol, consist of >5000 subclass members and are abundant in plant-based food and beverages, such as fruits, tea, berries, wine, and cocoa (64). Flavonoids share a common 3-ring nucleus structure, but are further subdivided based on variations in ring structure. Flavonoids are primarily found in the human diet as 7 major subclasses: anthocyanidins (e.g., cyanidin), flavanols (e.g., epicatechin), flavanones (e.g., naringenin), flavones (e.g., luteolin), flavonols (e.g., quercetin), isoflavones (e.g., genistein), and proanthocyanidins (oligomeric and polymeric flavonoids). Total dietary intake of flavonoids has been estimated to be in the range of 20–1000 mg/d (65–67), depending on the population studied and analytic methods used. Flavonoids may positively influence health through their well-documented effects on cellular antioxidant status and inflammation (68). Flavonoids may augment endogenous antioxidant defenses through nuclear factor, erythroid 2–like 2 (Nrf2) activation of antioxidant response elements of genes encoding antioxidant enzymes in vitro (69, 70). Anthocyanin intake has been shown to reduce systemic inflammation markers in humans while also suppressing LPS activation of NF-κB in human monocytes (71). The antioxidant defense boosting and anti-inflammatory properties of flavonoids show great potential for improving HDL function and cardiovascular health. However, much of the evidence comes from in vitro studies, which may not reflect in vivo scenarios, because cell studies do not account for bioavailability or biotransformation. Dietary flavonoid intake has been reported to be protective against CVD in a number of cross-sectional and prospective cohort studies (72–75). A recent meta-analysis examined the association between specific classes of flavonoid intake and CVD in prospective cohort studies (76). The dietary intake of anthocyanidins (RR: 0.89; 95% CI: 0.83, 0.96), flavanols (RR: 0.87; 95% CI: 0.80, 0.95), flavanones (RR: 0.88; 95% CI: 0.82, 0.96), flavones (RR: 0.88; 95% CI: 0.82, 0.96), flavonols (RR: 0.89; 95% CI: 0.84, 0.94), and proanthocyanidins (RR: 0.90; 95% CI: 0.82, 0.98) was inversely associated with the risk of CVD (76). Prospective cohort studies are useful for studying the effects of specific dietary components on long-term disease risk. However, because of the large size of cohorts, they can suffer from measurement error because of inaccuracy of dietary intake estimation, which may cause attenuation toward the null value (77). Furthermore, other differences in lifestyle patterns in low compared with high flavonoid consumers may confound analyses, such that it is difficult to discern whether associations with flavonoid intake are not merely reflecting other healthful diet or lifestyle patterns that prevent disease. Nevertheless, meta-analyses of cohort studies are useful because they can provide a weighted summary of available evidence in regard to dietary flavonoid intake and risk of CVD.
Current Status of Knowledge: Effects of Specific Flavonoids on HDL Metabolism and Function
The protective associations of dietary flavonoid intake with respect to CVD have been ascribed to their bioactivity as antioxidants and anti-inflammatory compounds. These properties may increase HDL cholesterol or RCT, or provide protection against HDL dysfunction in the context of inflammatory disease states, such as atherosclerosis or obesity.
Anthocyanidins
Anthocyanins (Greek “Anthos,” meaning “flower,” and “kyanos,” meaning “blue”) are pigments found in plant structures such as leaves, seedlings, petals, and fruits. Anthocyanins are glycosides that consist of an anthocyanidin (aglycone) attached to sugar moieties. Cyanidin, peonidin, pelargonidin, malvidin, delphinidin, and petunidin are the 6 major anthocyanidins that are commonly found in fruits and vegetables (78). Anthocyanins are highly concentrated in many berries, including black elderberry (1316 mg/100 g), black chokeberry (878 mg/100 g), and black currant (595 mg/100 g) (79). Estimated daily intake of anthocyanins in the United States is roughly 3.1 mg/d (67). Many preclinical and clinical studies have been conducted to evaluate the bioactivity of this flavonoid subclass on HDL metabolism.
Anthocyanins and their metabolites have been shown to prevent atherosclerosis in animal models, at least in part through anti-inflammatory properties and the stimulation of RCT. Cyanidin-3-glucoside (C3G) and a gut microbiota-derived metabolite, protocatechuic acid (PCA), have been demonstrated to positively affect HDL RCT in mice (80). In vitro, C3G and PCA appear to promote cholesterol efflux and HDL formation via the activation of liver X receptor (LXR) and/or the regulation of lipid transporters, including ABCA1 and ABCG1 (80–82). Cyanidin may act as an LXRα agonist without effects on hepatocyte TG accumulation (82), which may be due to additional activity as a PPARα ligand (83). C3G at high concentrations (50 μM) increased cholesterol efflux from the renal (HK-2) (84) and endothelial cells (81) in vitro. In both models, these effects were associated with an increase in LXRα expression, protein, or activation. Macrophage studies have shown similar results. Mouse peritoneal macrophages treated with C3G and peonidin-3-glucoside (1–100 μM) demonstrated a dose-dependent increase in cholesterol efflux to apoA-I, which was attributed to an induction of the PPARγ-LXRα-ABCA1 pathway (85). Although the aforementioned cell studies showed positive effects of anthocyanins, extrapolation of these findings to in vivo situations is limited, because these studies used very high concentrations (>50 μM) that are likely not achievable through diet. However, at much lower concentrations (up to 1 μM), the anthocyanin metabolite, PCA, was also shown to increase macrophage cholesterol efflux to apoA-I (80). Wang et al. (80) reported that C3G administration by oral gavage (50 mg ⋅ kg−1 body weight ⋅ d−1) to apoE−/− mice for 4 wk strongly increased serum HDL cholesterol and apoA-I concentrations and reduced atherosclerosis. Antiatherosclerotic effects were also reported with PCA (5 mg/kg body weight), a C3G metabolite, which notably did not influence HDL cholesterol or apoA-I, but improved in vivo RCT (80). In addition, gut microbiota appear to be important for such effects, because oral administration of broad-spectrum antibiotics abolished the atheroprotective effects of C3G, but not that of PCA (80). Wang et al. (80) further described how PCA improves cellular cholesterol efflux by suppressing the expression of microRNA 10b. This suppression in microRNA 10b leads to a subsequent increase in ABCA1/ABCG1 protein expression to regulate RCT and improve atherosclerosis. Anthocyanins also appear to influence other aspects of HDL function, such as PON1 activity. Supplementation of an anthocyanin-rich black elderberry extract (200 mg anthocyanins/kg body weight) for 6 wk was shown to increase hepatic LCAT mRNA, PON1 mRNA, and serum PON1 activity in apoE−/− mice (86). This effect of black elderberry on increasing serum PON1 activity has also been observed in longer studies in apoE−/− mice (24 wk) (C Millar and C Blesso, unpublished results, 2016). In addition, supplementation with black chokeberry extract for 4 wk increased plasma PON1 activity toward organophosphates in apoE−/− mice fed a diet high in saturated fat and cholesterol (15% total fat, 9% saturated fat, and 0.2% cholesterol by weight) (87). Black chokeberry contains considerable amounts of phenolic acids and proanthocyanidins, which may have also contributed to this effect (87).
A number of placebo-controlled trials reported that adults with dyslipidemia experience significant increases in serum HDL cholesterol of ∼10–20% after supplementation with a purified mixture of anthocyanins from bilberry and black currant (320 mg/d) (88–91). Not only did Zhu et al. (88) report an increase in HDL cholesterol (+11%) with anthocyanin supplementation, they also reported other benefits on HDL functionality in hypercholesterolemic adults. Anthocyanin supplementation increased HDL PON1 activity (+22%), HDL antioxidant capacity (+21%) via inhibition of dihydrorhodamine oxidation, and HDL cholesterol efflux capacity (+20%), whereas HDL lipid hydroperoxides were reduced (−24%) (88). Qin et al. (91) also reported a moderate decrease in serum CETP mass (−10%) and activity (−6%), and increased serum cholesterol efflux capacity (+20%) with anthocyanin supplementation (320 mg/d), which may explain the increases in HDL cholesterol (+14%) observed. Other populations have been studied as well. After 24 wk of purified anthocyanin supplementation (320 mg/d), middle-aged adults with type 2 diabetes mellitus had an increase in serum HDL cholesterol (+19%), although it did not alter plasma apoA-I (92). Although these studies used relatively high doses compared with the mean reported US intake of 3.1 mg/d (67), selectively consuming foods rich in anthocyanins could result in comparable intake amounts (e.g., 1 cup or 125 g blueberries contains ∼190 mg anthocyanins) (93). Nevertheless, supplemental forms of anthocyanins are commercially available and could also be consumed to achieve higher intake amounts. A higher dose of purified anthocyanins (640 mg/d) over a shorter duration (4 wk) resulted in a similar increase in serum HDL cholesterol in men with hypertension (94). Overall, there is strong evidence in preclinical and clinical studies to suggest that the anthocyanidin subclass of flavonoids improves HDL function and RCT.
Flavanols
The flavanol, or flavan-3-ol, subclass is mainly composed of epicatechin and catechin compounds. This group, which is found in cocoa, wine, grape juice, and teas, has been closely studied for its relation with heart disease (95). Flavanols, with an estimated intake in the United States of ∼157 mg/d, supply >80% of all dietary flavonoids in this population (67).
A major contributor of flavanols to the US diet is tea (67), with green tea being a rich source of epigallocatechin and epigallocatechin gallate (93). Green tea administration in tap water (2% wt:vol) for 6 wk was shown to increase serum PON1 activity and reduce apoB lipoprotein oxidation in diabetic rats, despite significantly reducing serum HDL cholesterol (96). In apoE−/− mice, feeding green tea polyphenol–enriched extra virgin olive oil for 2 mo resulted in a greater cholesterol efflux from peritoneal macrophages ex vivo than did feeding extra virgin olive oil (97). In end-stage renal disease patients, a single oral dose of a green tea extract (455 mg total catechins) significantly attenuated the reduction in serum PON1 activity and increases in oxidative stress and inflammation markers that are observed with a hemodialysis session (98).
Another rich source of flavanols in the human diet is cocoa (67). A human study in healthy and mildly hypercholesterolemic adults reported that supplementation with cocoa powder (133 mg flavanols/d) for 12 wk increased plasma HDL cholesterol (+24%) and reduced the susceptibility of LDL to oxidation compared with control (99). However, a crossover study in healthy and moderately hypercholesterolemic adults (200–240 mg/dL serum cholesterol) reported that ingestion of a soluble cocoa product in milk (45 mg flavanols/d) for 4 wk did not significantly alter serum HDL cholesterol and proinflammatory markers compared with plain milk (100). Furthermore, plasma HDL cholesterol in postmenopausal diabetic women supplemented with flavonoid-enriched chocolate (850 mg/d of flavanols) was not significantly altered, although the total cholesterol to HDL cholesterol ratio and HOMA-IR were decreased (−5% and −12%, respectively) (101). Although several studies suggest benefits of flavanols from green tea and cocoa on HDL cholesterol and PON1 activity, some report no change. Thus, more clinical research is warranted.
Flavanones
The identification of specific flavanones has grown dramatically over the past 20 y, from a handful of compounds to >400 naturally occurring isolates (102). Interestingly, flavanones are precursor molecules to the other flavonoids (102). Of all the subclasses, flavanones are the second largest contributor of flavonoids in US diets at ∼14.4 mg of daily intake/d (67). The major flavanones, naringenin and hesperetin, are commonly found in tomatoes and citrus fruits, and are at their highest concentrations in the peels (102).
Flavanones such as naringenin and hesperetin have been reported to beneficially alter lipid metabolism and reduce atherosclerosis in animal models (103). Both of these flavanones have been reported to increase HDL cholesterol concentrations in some (104, 105) but not all (106, 107) animal studies. However, little research has been done to evaluate the effect of dietary flavanones on HDL function. In hypercholesterolemic hamsters, a 12-wk supplementation with hesperetin (0.02% of diet by weight) was actually shown to decrease plasma PON1 paraoxonase activity, although one of its metabolites, ferulic acid, significantly increased PON1 activity (106). In hyperuricemic rats, daily gavage with orange juice (5 mL/kg) or hesperetin (5 mg/kg) for 2 wk increased both serum paraoxonase and arylesterase activities of PON1, whereas only orange juice increased HDL cholesterol (107). Hesperetin (5–15 μM) increased cellular cholesterol efflux from THP-1 macrophages to apoA-I (108). Data suggested that this increase in HDL formation was related to an induction in ABCA1 expression, possibly downstream to enhanced LXRα and PPARγ activities (108).
In clinical studies, there has been little investigation of flavanones on HDL function beyond HDL cholesterol. Consuming 750 mL orange juice for 4 wk (35 mg/d of hesperidin), but not at a lower intake, significantly increased plasma HDL cholesterol by 21% without affecting apoA-I concentrations in hypercholesterolemic adults (109). Supplementation with bergamot polyphenol extract (rich in flavanones) (∼135–270 mg flavanones/d) for 30 d was reported to increase plasma HDL cholesterol (+20–40% from baseline) relative to placebo in several dyslipidemic patient populations (110). However, other long-term human studies that used higher doses of flavanones (400–800 mg/d) did not show similar increases in HDL cholesterol in hyperlipidemic populations (111, 112). Similar to flavanols, the benefits of flavanones on the metabolism and function of HDL requires further investigation.
Flavones
Flavones are found at lower concentrations in fruits and vegetables than are other flavonoids (113). The few compounds that are commonly found in foods include luteolin and apigenin (114), with daily intake of flavones averaging ∼1.6 mg/d in the United States (67). The foods richest in flavones include celery, parsley, and thyme (113).
Luteolin at high concentrations (10–50 μM) was reported to inhibit the activation of LXRα/β by the agonist T0901317, and the expression of its target gene, ABCA1, in macrophages (115). Despite this apparent inhibitory effect on LXR activation observed in vitro, luteolin supplementation has been shown to increase HDL cholesterol in both ovariectomized mice (50 mg/kg daily for 12 wk) (116) and streptozotocin-induced type 1 diabetic rats (200 mg/kg for 8 wk) (117). Chrysin and wogonin are 2 flavones that have also been studied in macrophage cell lines. The addition of chrysin (10 μM) to RAW264.7 macrophages increased cellular cholesterol efflux to HDL, but not apoA-I (118). Furthermore, chrysin treatment of macrophages induced PPARγ transcriptional activity and increased the expression of PPARγ, LXR, ABCA1, and ABCG1 transcripts (118). Wogonin (40 μM) treatment increased cholesterol efflux from J774 macrophages by increasing ABCA1 protein stability (119). Because the high concentrations of flavones used in cell studies may be difficult to achieve by diet, it will be important to determine whether the effects on RCT shown in cell models are translatable in animal models and humans.
Flavonols
Major flavonol compounds include quercetin and kaempferol (113). Onions, broccoli, apples, green tea, and black grapes are all possible sources of such flavonols (120). Daily intake of flavonols in the United States is estimated to be ∼13 mg/d (67).
Most research on flavonols related to HDL function is focused on PON1 activity and cellular cholesterol efflux. Increased hepatic PON1 gene expression was seen in apoE3 transgenic (121) and C57BL/6 mice (122) fed a quercetin-enriched diet (0.2% wt:wt) for 6 wk. Quercetin supplementation in liquid ethanol–containing diets has been shown to increase hepatic PON1 expression and serum paraoxonase activity in both Ldlr−/− mice (12.5–25 mg/dL) (123) and Wistar rats (10 mg/L diet) (124). However, a human study showed no dose-dependent effect of quercetin ingestion (50, 100, and 150 mg/d) for 2 wk on plasma PON1 paraoxonase or arylesterase activities in healthy adults (122), possibly because of the short duration of the study or use of lower doses. In the previously described mouse studies, the dosages of quercetin used would be the equivalent of consuming >500 mg/d for a 70 kg person (125). Therefore, longer studies at increased dosages in humans should be conducted.
Mechanistic studies with quercetin demonstrate the activation of the PON1 gene promoter in hepatocytes and the induction of ABCA1 expression in macrophages. Huh7 human hepatoma cells incubated with quercetin (20–25 μM) showed increases in PON1 gene promoter activity, possibly via nuclear sterol regulatory element binding protein 2 (SREBP2) transactivation, resulting in inductions in cellular PON1 mRNA, protein, and activity (122, 126). The methylated metabolite of quercetin, isorhamnetin, was shown to have an even stronger effect on PON1 promoter activation in hepatoma cells (122). Quercetin also reportedly affects cholesterol efflux from cells and HDL formation. In THP-1 macrophages, quercetin (0.3 μM) effectively increased cellular cholesterol efflux to apoA-I and mature HDL, possibly related to the induction of ABCA1 expression caused by increased PPARγ and LXRα protein concentrations (127). A higher concentration of quercetin (20 μM) was also found to be effective, both by itself and synergistically with the endogenous antioxidant glutathione, in stimulating cholesterol efflux to apoA-I and HDL from J774 macrophages (128). These effects in J774 cells were related to a stimulation of the PPARα-ABCA1 pathway, but did not appear to involve the protein kinase A or Janus kinase 2 pathways (128). Furthermore, in RAW264.7 macrophages, quercetin (40–200 μM) increased cholesterol efflux to apoA-I in a dose-dependent manner via an increase in ABCA1 expression, which relied on activation of the p38 pathway and subsequent binding of Sp1 and LXR transcription factors to the promoter region of ABCA1 (129). However, these high concentrations may not be physiologically achievable, because the reported peak plasma concentrations with quercetin supplementation are well below this, at ∼3 μM in mice (0.2% of diet as quercetin) and 0.4 μM in humans (150 mg quercetin/d) (122). Kaempferol (2.5–10 μg/mL), another flavonol, dose-dependently increased cholesterol efflux from THP-1 macrophages by regulating ABCA1, ABCG1, CD36, and SR-BI expression (130). Although positive effects of flavonols have been observed in preclinical studies, it is important to point out that, in humans, much of the bioactivity will depend on the absorption and metabolism of these compounds. Thus, more clinical studies are warranted to determine the bioactivity and biotransformation of flavonols toward HDL function in humans.
Isoflavones
Isoflavones are distinct in their chemical structure, because they resemble the hormone estrogen and display affinity toward the estrogen receptor (ER) (131). Therefore, they can exhibit weak estrogenic effects. Genistein and daidzein are the major dietary isoflavones and are commonly found in soy products, such as tofu or soy milk (114). Adults in the United States consume on average ∼1.2 mg isoflavones/d (67).
The effects of isoflavones on HDL-PON1 activity have been evaluated in several animal studies. Estrogen has been shown to enhance PON1 activity via the ER in vitro, but without altering PON1 mRNA levels or protein concentrations (132). Daily oral administration of genistein (10 mg/kg body weight) for 8 wk did not alter HDL cholesterol or serum paraoxonase activity in ovariectomized Sprague-Dawley rats (133). In contrast, in female Sprague-Dawley rats with collagen-induced arthritis, daily oral administration of genistein (20 mg/kg body weight) or daidzein (20 mg/kg body weight) for 50 d significantly improved serum PON1 paraoxonase and arylesterase activities, but did not alter HDL cholesterol (134). When evaluating a mixture of soy isoflavones (21 mg genistein, 47 mg daidzein, and 34 mg glycitin/kg diet) fed for 8 wk under both normal and methionine choline–deficient diet conditions, rats experienced increases in plasma HDL cholesterol, PON1 paraoxonase, and arylesterase activities (135).
In clinical studies, the intake of soy protein containing isoflavones has been associated with a modest increase in plasma HDL cholesterol (136, 137). This effect appears to be due to the replacement of animal proteins with soy protein, and not specifically due to the isoflavone content (136, 138). However, certain subgroups may observe improvements in HDL cholesterol (+13%) with isoflavone supplementation, as was shown in postmenopausal women with a particular ERβ polymorphism (139). In postmenopausal women, consuming a mixture of isoflavones (66 mg glycitin/d, 41 mg daidzein/d, or 7 mg genistein/d) for 12 wk did not increase serum efflux capacity from rat Fu5AH hepatoma cells (primarily SR-BI–mediated) (140) or cAMP-stimulated J774 macrophages (primarily ABCA1-mediated) (141), although serum pre-β HDL concentrations did increase by 18% in the latter study (141). Based on animal studies, it appears that isoflavones may beneficially alter HDL PON1 activity in conditions of elevated oxidative stress and inflammation. In clinical studies, long-term isoflavone intake does not appear to alter the cholesterol efflux capacity of HDL from SR-BI– or ABCA1-dependent pathways. More research is necessary in humans to confirm the increases in HDL PON1 activity with isoflavone intake seen in animal studies.
Conclusions
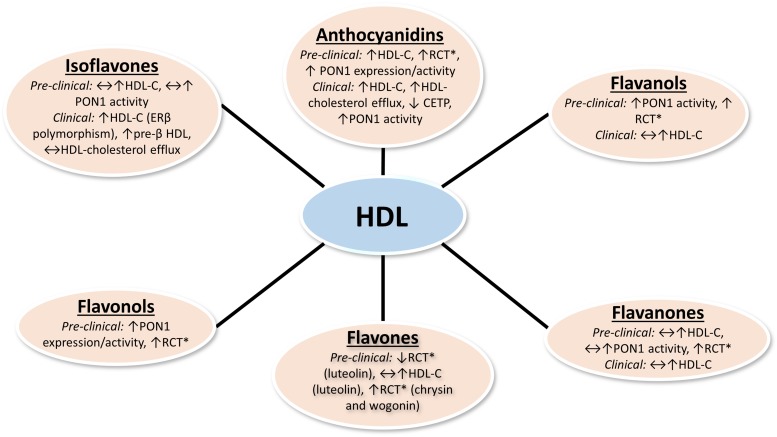
A summary of the reported effects of dietary flavonoids on RCT, HDL metabolism, and HDL function is shown in Figure 1. Given the recent paradigm shift toward the therapeutic targeting of HDL function rather than HDL cholesterol, it will be necessary to consider the effects of foods and nutrients on HDL function in order to determine the optimal diet for reducing the risk of heart disease. Flavonoids are major contributors to overall dietary polyphenol intake in the human diet. Based on preclinical studies that used cell culture and rodent models, it appears that dietary flavonoids influence multiple HDL functions beyond simple HDL cholesterol content, such as cholesterol efflux and antioxidant capacities (Table 1). However, the doses and concentrations of flavonoids used in preclinical studies may be difficult to acquire from diet alone in humans. Therefore, it may be necessary to consume flavonoids as nutraceuticals to achieve doses that show benefits, yet more clinical trials are warranted to test the efficacy of supplemental forms of flavonoids. Cell studies examining the effects of flavonoids on HDL metabolism often use very high concentrations, which may not be physiologically relevant. Furthermore, in vitro studies do not account for the bioavailability and biotransformation of these compounds. Thus, the benefits seen in cell models may not translate in vivo. In clinical studies, dietary anthocyanin intake is associated with beneficial changes in serum biomarkers related to HDL function (Table 2). However, more clinical research is warranted to examine HDL functionality in response to the intake of other flavonoids. Although there have been remarkable efforts to develop HDL-targeted drug therapies, research on how dietary components affect HDL function is still in its early stages.
FIGURE 1.
Reported effects of dietary flavonoids on RCT, HDL metabolism, and HDL function in preclinical and clinical studies. *RCT in preclinical studies measured by in vitro macrophage cholesterol efflux or in vivo macrophages-to-feces RCT assays. Changes in serum HDL cholesterol are not necessarily reflective of improvements in RCT. CETP, cholesteryl ester transfer protein; ERβ, estrogen receptor β HDL-C, HDL cholesterol concentration; PON1, paraoxonase 1; RCT, reverse cholesterol transport; ↑, increase; ↓ decrease; ↔, no change.
TABLE 1.
Summary of preclinical studies examining the effects of flavonoids on RCT, HDL metabolism, and HDL function1
| Studies and flavonoids |
| Anthocyanidins |
| ApoE−/− mice |
| C3G (50 mg/kg), PCA (5 mg/kg), or the 2 in combination for 14 d |
| ↑ In vivo RCT (80) |
| Black elderberry extract (200 mg anthocyanins/kg) for 6 wk |
| ↑ Serum PON1 activity, ↑ hepatic LCAT and PON1 mRNA, ↑ intestinal apoA-I mRNA, ↓ hepatic SAA1 mRNA (86) |
| Macrophages |
| C3G or peonidin-3-glucoside (1–100 μM) |
| Mouse peritoneal: ↑ cholesterol efflux, ↑ ABCA1, ↑ binding of apoA-I to ABCA1 (85) |
| PCA (0.25–1 μM) |
| Mouse peritoneal and THP-1: ↑ cholesterol efflux and ABCA1 and ABCG1 (80) |
| Human aortic endothelial cells |
| C3G (0.5–50 μM) |
| ↑ Cholesterol efflux, ↑ ABCG1 via LXRα (81) |
| HK-2 kidney cells |
| C3G or cyanidin (50 μM) |
| ↑ Cholesterol efflux via PPARα-LXRα-ABCA1 pathway (84) |
| HepG2 cells |
| C3G (10–100 μM) |
| ↓ CETP activity in cell media (91) |
| Flavanols |
| ApoE−/− mice |
| Green tea polyphenol–enriched extra virgin olive oil [7 μL/(mouse ⋅ d)] for 2 mo |
| ↔ PON1 activity (97) |
| ↑ Ex vivo cholesterol efflux from peritoneal macrophages (97) |
| Sprague-Dawley rats |
| Green tea (2% wt:vol) in drinking water for 6 wk in diabetic rats |
| ↓ HDL cholesterol, but ↑ PON-1 activity (96) |
| Flavanones |
| Rats |
| Sprague-Dawley: High cholesterol diet with naringenin (0.02%) or its metabolites, PHPP (0.012%), and PHB (0.012%), for 5 wk |
| ↑ HDL cholesterol (105) |
| Wistar: High cholesterol diet with flavanone-rich bergamot polyphenol extract (10 and 20 mg/kg) for 30 d |
| ↔ HDL cholesterol (110) |
| Wistar: Naringenin (50 mg/kg) with or without ethanol (6 g/kg) for 60 d |
| ↑ HDL cholesterol, LCAT, and LPL activities (104) |
| Hyperuricemic Wistar: Orange juice (5 mL/kg) or hesperetin (5 mg/kg) for 2 wk |
| Orange juice, ↑ HDL cholesterol; hesperetin, ↑ serum PON1 paraoxonase and arylesterase activities (107) |
| Golden Syrian hamsters |
| Atherogenic diets for 12 wk with or without hesperetin (0.02%) or ferulic acid (0.013%) |
| Ferulic acid, ↑ serum PON1 paraoxonase activity; no effects of hesperetin (106) |
| THP-1 macrophages |
| Hesperetin (5–15 μM) |
| ↑ Cholesterol efflux, ↑ ABCA1 mRNA and protein, ↑ LXR enhancer activity and ABCA1 promoter activity (108) |
| Flavones |
| Diabetic Sprague-Dawley rats |
| Luteolin (200 mg/kg) for 8 wk |
| ↑ HDL cholesterol (117) |
| Ovariectomized mice |
| Luteolin (50 mg/kg) for 84 d |
| ↑ HDL cholesterol (116) |
| Macrophages |
| Wogonin (20–40 μM) |
| J774A.1: ↑ cholesterol efflux and ABCA1 stability (119) |
| Chrysin (10 μM) |
| RAW264.7: ↑ cholesterol efflux, ABCA1 and ABCG1 mRNA (possibly through PPARγ-LXR pathway) (118) |
| Luteolin (10–50 μM) and LXR agonist for 3 h |
| RAW264.7: LXRα/β activation and ABCA1 induction by LXR agonist was fully ↓ with 50 μM luteolin; other doses partially ↓ LXR activation (115) |
| Flavonols |
| Rats |
| Wistar: Pair-fed Lieber-Decarli liquid diet or quercetin (10 mg/L) liquid diet for 4 wk |
| ↑ Hepatic PON1 expression and serum PON1 paraoxonase activity (124) |
| Mice |
| Ldlr−/−: On atherogenic diet, given quercetin (0–25 mg/dL) in drinking water for 8 wk |
| Quercetin (12.5 and 25 mg/dL), ↑ hepatic PON1 expression and serum PON1 paraoxonase activity (123) |
| ApoE3/ApoE4 transgenic: Control diet or quercetin-added (2 mg/g) diet, both containing 21% butter fat |
| ↑ Hepatic PON1 mRNA in ApoE3 transgenic mice (121) |
| C57BL/6: Quercetin (0.05–2 mg/g of diet) for 6 wk |
| Quercetin (≥0.1 mg/g of diet), ↑ PON1 expression (122) |
| Huh7 hepatoma cells |
| Quercetin (20 μM) |
| ↑ PON1 paraoxonase activity, protein, and gene expression, possibly via SREBP2 activation (126) |
| Quercetin (25 μM) and transfected with PON1 gene |
| ↑ PON1 promoter activity (122) |
| Macrophages |
| Quercetin (0.3–30 μM) |
| THP-1: ↑ cholesterol efflux to apoA-I and HDL via PPARγ-LXRα-ABCA1 pathway (127) |
| Quercetin (20 μM) alone, or with glutathione (200 μM), or PON1 (10 μg/mL) |
| J774A.1: ↑ cholesterol efflux alone and with glutathione, ↑ ABCA1 and PPARα expressions, was not via PKA or JAK2 pathways (128) |
| Quercetin (100–200 μM) |
| RAW264.7: ↑ cholesterol efflux and ↑ ABCA1 gene/protein expression via p38-dependent binding of Sp1 and LXR to ABCA1 promoter (129) |
| Kaempferol (35 μM) |
| THP-1: ↑ cholesterol efflux, ↓ CD36, ↑ ABCA1, ↑ ABCG1, ↑ SR-BI mRNA expression/protein (130) |
| Isoflavones |
| Rats |
| Arthritic Sprague-Dawley: Genistein or daidzein (20 mg/kg) for 50 d |
| ↑ Serum PON1 paraoxonase and arylesterase activities (134) |
| Ovariectomized Sprague-Dawley: Genistein (10 mg/kg) for 8 wk |
| ↔ HDL cholesterol or PON1 activity (133) |
| 100 mg/kg Isoflavones (genistein, daidzein, glycitin), with or without MCD diet for 8 wk |
| ↑ HDL cholesterol, PON1 paraoxonase, and arylesterase activities (135) |
ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1; CD36, cluster of differentiation 36; CETP, cholesteryl ester transfer protein; C3G, cyanidin-3-glucoside; C57BL/6, C57 black 6; JAK2, Janus kinase 2; LCAT, lecithin-cholesterol acyltransferase; LPL, lipoprotein lipase; LXR, liver X receptor; MCD, methionine choline–deficient; PCA, protocatechuic acid; PHB, ρ-hydroxybenzoic acid; PHPP, ρ-hyproxyphenylpropionic acid; PKA, protein kinase A; PON1, paraoxonase 1; RCT, reverse cholesterol transport; SAA1, serum amyloid A-1; Sp1, Sp1 transcription factor; SR-BI, scavenger receptor class B type I; SREBP2, sterol regulatory element binding protein 2; ↑, increase; ↓ decrease; ↔, no change.
TABLE 2.
Clinical studies examining the effects of flavonoids on HDL metabolism and HDL function1
| Author | Year | Flavonoids | Model | Study design | Results |
| Kurowska et al. (109) | 2000 | Flavanones | Hypercholesterolemic adults (n = 25) | 750 mL orange juice/d for 4 wk | ↑ HDL cholesterol (+21%) |
| Jung et al. (111) | 2003 | Flavanones | Hypercholesterolemic adults (n = 30) | Naringin (400 mg/d) for 8 wk | ↔ HDL cholesterol, but ↑ HDL cholesterol:total cholesterol (+22%) |
| Weggemans and Trautwein (136) | 2003 | Isoflavones | Adults (n = 959) | Meta-analysis of studies supplementing soy protein with isoflavones ≥14 d | Soy protein with isoflavones, ↑ HDL cholesterol, but not related to isoflavones |
| Zhan and Ho (137) | 2005 | Isoflavones | Adults (n = 3906) | Meta-analysis of studies supplementing soy protein with isoflavones or isoflavone extract | Soy proteins with isoflavones, ↑ HDL cholesterol only if ≥12 wk |
| Tormala et al. (140) | 2006 | Isoflavones | Postmenopausal women (n = 30) | Isoflavones (114 mg/d) for 3 mo | ↔ Serum lipids or serum cholesterol efflux capacity from Fu5AH cells |
| Hall et al. (139) | 2006 | Isoflavones | Postmenopausal women (n = 117) | Genistein and daidzein (50 mg/d) for 8 wk | ↑ HDL cholesterol (+13%) in estrogen receptor gene polymorphic subgroup |
| Badeau et al. (141) | 2007 | Isoflavones | Postmenopausal women (n = 56) | Isoflavones (114 mg/d) for 3 mo | ↔ Serum lipids or serum cholesterol efflux capacity from J774 macrophages |
| Hsu et al. (98) | 2007 | Flavanols | Hemodialysis patients (n = 10) | Catechins (455 mg/d or 910 mg/d) during dialysis | ↑ PON1 activity |
| Baba et al. (99) | 2007 | Flavanols | Healthy adults (n = 25) | Test drinks containing 26 g cocoa/d for 12 wk | ↑ HDL cholesterol (+24%) |
| Taku et al. (138) | 2007 | Isoflavones | Adults (n = 799) | Meta-analysis of studies supplementing soy protein or isoflavones ≥1 mo | Soy protein enriched with isoflavones, ↑ HDL cholesterol; soy isoflavones alone, ↔ HDL cholesterol |
| Qin et al. (91) | 2009 | Anthocyanins | Dyslipidemic adults (n = 120) | Anthocyanins (320 mg/d) for 12 wk | ↑ HDL cholesterol (+13.7%), ↑ serum cholesterol efflux capacity (+20%), and ↓ both plasma CETP mass (−10.4%) and activity (−6.3%) |
| Boesch-Saadatmandi et al. (122) | 2010 | Flavonols | Healthy adults (n = 35) | Quercetin (50, 100, or 150 mg/d) for 2 wk | ↔ PON1 activity |
| Demonty et al. (112) | 2010 | Flavanones | Hypercholesterolemic adults (n = 216) | Naringin (500 mg/d) or hesperidin (800 mg/d) for 4 wk | ↔ HDL cholesterol |
| Zhu et al. (89) | 2011 | Anthocyanins | Hypercholesterolemic adults (n = 150) | Anthocyanins (320 mg/d) for 12 wk | ↑ HDL cholesterol (+12%) |
| Mollace et al. (110) | 2011 | Flavanones | Hyperlipidemic and hyperglycemic patients (n = 237) | Bergamot polyphenol extract (500 or 1000 mg/d) for 30 d | ↑ HDL cholesterol (+20–40%) |
| Curtis et al. (101) | 2012 | Flavanols | Postmenopausal women with type 2 diabetes (n = 93) | Flavonoid-enriched chocolate (850 mg/d flavanols) for 1 y | ↔ HDL cholesterol, ↓ total cholesterol/HDL cholesterol (−5%) |
| Zhu et al. (90) | 2013 | Anthocyanins | Hypercholesterolemic adults (n = 150) | Anthocyanins (320 mg/d) for 24 wk | ↑ HDL cholesterol (+14%) |
| Hassellund et al. (94) | 2013 | Anthocyanins | Prehypertensive men (n = 31) | Anthocyanins (640 mg/d) for 4 wk | ↑ HDL cholesterol |
| Zhu et al. (88) | 2014 | Anthocyanins | Hypercholesterolemic adults (n = 122) | Anthocyanins (320 mg/d) for 24 wk | ↑ HDL cholesterol (+11.4%), PON1 activity (+18.7%), HDL antioxidant capacity (+20.8%), and HDL cholesterol efflux capacity (+17.7%) from J774 macrophages; ↓ HDL lipid hydroperoxides |
| Martinez-Lopez et al. (100) | 2014 | Flavanols | Normocholesterolemic (n = 24) and hypercholesterolemic (n = 20) adults | Cocoa flavanols (45 mg/d) with milk for 4 wk | ↔ HDL cholesterol vs. plain milk |
| Li et al. (92) | 2015 | Anthocyanins | Type 2 diabetic patients (n = 58) | Anthocyanins (320 mg/d) for 24 wk | ↑ HDL cholesterol (+19.4%) and markers of antioxidant capacity |
An increase in serum HDL cholesterol concentrations is not necessarily indicative of an improvement in reverse cholesterol transport. CETP, cholesteryl ester transfer protein; PON1, paraoxonase 1; ↑, increase; ↓ decrease; ↔, no change.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1; CE, cholesteryl ester; CETP, cholesteryl ester transfer protein; CHD, coronary heart disease; CVD, cardiovascular disease; C3G, cyanidin-3-glucoside; ER, estrogen receptor; LCAT, lecithin-cholesterol acyltransferase; LXR, liver X receptor; PCA, protocatechuic acid; PON1, paraoxonase 1; RCT, reverse cholesterol transport; SR-BI, scavenger receptor class B type.
References
- 1.Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep 2016;64:1–119. [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95. [DOI] [PubMed] [Google Scholar]
- 3.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007;357:1301–10. [DOI] [PubMed] [Google Scholar]
- 4.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest 1990;85:1234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyazaki A, Sakuma S, Morikawa W, Takiue T, Miake F, Terano T, Sakai M, Hakamata H, Sakamoto Y, Natio M, et al. Intravenous injection of rabbit apolipoprotein A-I inhibits the progression of atherosclerosis in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol 1995;15:1882–8. [DOI] [PubMed] [Google Scholar]
- 6.Shah PK, Yano J, Reyes O, Chyu KY, Kaul S, Bisgaier CL, Drake S, Cercek B. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation 2001;103:3047–50. [DOI] [PubMed] [Google Scholar]
- 7.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 2003;290:2292–300. [DOI] [PubMed] [Google Scholar]
- 8.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res 2008;103:1084–91. [DOI] [PubMed] [Google Scholar]
- 9.Tardy C, Goffinet M, Boubekeur N, Ackermann R, Sy G, Bluteau A, Cholez G, Keyserling C, Lalwani N, Paolini JF, et al. CER-001, a HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis 2014;232:110–8. [DOI] [PubMed] [Google Scholar]
- 10.Castelli WP, Doyle JT, Gordon T, Hames CG, Hjortland MC, Hulley SB, Kagan A, Zukel WJ. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation 1977;55:767–72. [DOI] [PubMed] [Google Scholar]
- 11.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977;62:707–14. [DOI] [PubMed] [Google Scholar]
- 12.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989;79:8–15. [DOI] [PubMed] [Google Scholar]
- 13.Linsel-Nitschke P, Tall AR. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat Rev Drug Discov 2005;4:193–205. [DOI] [PubMed] [Google Scholar]
- 14.Chapman MJ, Assmann G, Fruchart JC, Shepherd J, Sirtori C. Raising high-density lipoprotein cholesterol with reduction of cardiovascular risk: the role of nicotinic acid–a position paper developed by the European Consensus Panel on HDL-C. Curr Med Res Opin 2004;20:1253–68. [DOI] [PubMed] [Google Scholar]
- 15.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999;341:410–8. [DOI] [PubMed] [Google Scholar]
- 16.Kastelein JJ, van Leuven SI, Burgess L, Evans GW, Kuivenhoven JA, Barter PJ, Revkin JH, Grobbee DE, Riley WA, Shear CL, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med 2007;356:1620–30. [DOI] [PubMed] [Google Scholar]
- 17.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–22. [DOI] [PubMed] [Google Scholar]
- 18.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet 2012;380:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Eck M, Twisk J, Hoekstra M, Van Rij BT, Van der Lans CA, Bos IS, Kruijt JK, Kuipers F, Van Berkel TJ. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J Biol Chem 2003;278:23699–705. [DOI] [PubMed] [Google Scholar]
- 21.Pollard RD, Blesso CN, Zabalawi M, Fulp B, Gerelus M, Zhu X, Lyons EW, Nuradin N, Francone OL, Li XA, et al. Procollagen C-endopeptidase enhancer protein 2 (PCPE2) reduces atherosclerosis in mice by enhancing scavenger receptor class B1 (SR-BI)-mediated high-density lipoprotein (HDL)-cholesteryl ester uptake. J Biol Chem 2015;290:15496–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenson RS, Brewer HB Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012;125:1905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res 1995;36:211–28. [PubMed] [Google Scholar]
- 24.Cavelier C, Lorenzi I, Rohrer L, von Eckardstein A. Lipid efflux by the ATP-binding cassette transporters ABCA1 and ABCG1. Biochim Biophys Acta 2006;1761:655–66. [DOI] [PubMed] [Google Scholar]
- 25.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res 2007;48:2453–62. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest 2007;117:2216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulya A, Lee JY, Gebre AK, Thomas MJ, Colvin PL, Parks JS. Minimal lipidation of pre-beta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler Thromb Vasc Biol 2007;27:1828–36. [DOI] [PubMed] [Google Scholar]
- 28.Czarnecka H, Yokoyama S. Regulation of cellular cholesterol efflux by lecithin:cholesterol acyltransferase reaction through nonspecific lipid exchange. J Biol Chem 1996;271:2023–8. [DOI] [PubMed] [Google Scholar]
- 29.Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L, Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol 2006;26:534–40. [DOI] [PubMed] [Google Scholar]
- 30.Collet X, Tall AR, Serajuddin H, Guendouzi K, Royer L, Oliveira H, Barbaras R, Jiang XC, Francone OL. Remodeling of HDL by CETP in vivo and by CETP and hepatic lipase in vitro results in enhanced uptake of HDL CE by cells expressing scavenger receptor B-I. J Lipid Res 1999;40:1185–93. [PubMed] [Google Scholar]
- 31.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996;271:518–20. [DOI] [PubMed] [Google Scholar]
- 32.Röhrl C, Stangl H. HDL endocytosis and resecretion. Biochim Biophys Acta 2013;1831:1626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silver DL, Tall AR. The cellular biology of scavenger receptor class B type I. Curr Opin Lipidol 2001;12:497–504. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz CC, VandenBroek JM, Cooper PS. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. J Lipid Res 2004;45:1594–607. [DOI] [PubMed] [Google Scholar]
- 35.Tanigawa H, Billheimer JT, Tohyama J, Zhang Y, Rothblat G, Rader DJ. Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation 2007;116:1267–73. [DOI] [PubMed] [Google Scholar]
- 36.Guyard-Dangremont V, Desrumaux C, Gambert P, Lallemant C, Lagrost L. Phospholipid and cholesteryl ester transfer activities in plasma from 14 vertebrate species. Relation to atherogenesis susceptibility. Comp Biochem Physiol B Biochem Mol Biol 1998;120:517–25. [DOI] [PubMed] [Google Scholar]
- 37.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res 2009;50 Suppl:S189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67. [DOI] [PubMed] [Google Scholar]
- 39.Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 1998;394:284–7. [DOI] [PubMed] [Google Scholar]
- 40.Puranik R, Bao S, Nobecourt E, Nicholls SJ, Dusting GJ, Barter PJ, Celermajer DS, Rye KA. Low dose apolipoprotein A-I rescues carotid arteries from inflammation in vivo. Atherosclerosis 2008;196:240–7. [DOI] [PubMed] [Google Scholar]
- 41.Nofer JR, Levkau B, Wolinska I, Junker R, Fobker M, von Eckardstein A, Seedorf U, Assmann G. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J Biol Chem 2001;276:34480–5. [DOI] [PubMed] [Google Scholar]
- 42.Nofer JR, Walter M, Kehrel B, Wierwille S, Tepel M, Seedorf U, Assmann G. HDL3-mediated inhibition of thrombin-induced platelet aggregation and fibrinogen binding occurs via decreased production of phosphoinositide-derived second messengers 1,2-diacylglycerol and inositol 1,4,5-tris-phosphate. Arterioscler Thromb Vasc Biol 1998;18:861–9. [DOI] [PubMed] [Google Scholar]
- 43.Nofer JR, van der Giet M, Tolle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Godecke A, Ishii I, Kleuser B, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest 2004;113:569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 2007;117:746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackness M, Mackness B. Human paraoxonase-1 (PON1): gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2015;567:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, Subbanagounder G, Cheroutre H, Faull KF, Berliner JA, Witztum JL, et al. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem 2000;275:17527–35. [DOI] [PubMed] [Google Scholar]
- 47.Navab M, Reddy ST, Van Lenten BJ, Anantharamaiah GM, Fogelman AM. The role of dysfunctional HDL in atherosclerosis. J Lipid Res 2009;50 Suppl:S145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackness B, Davies GK, Turkie W, Lee E, Roberts DH, Hill E, Roberts C, Durrington PN, Mackness MI. Paraoxonase status in coronary heart disease: are activity and concentration more important than genotype? Arterioscler Thromb Vasc Biol 2001;21:1451–7. [DOI] [PubMed] [Google Scholar]
- 49.Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 2011;121:2693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferretti G, Bacchetti T, Masciangelo S, Bicchiega V. HDL-paraoxonase and membrane lipid peroxidation: a comparison between healthy and obese subjects. Obesity (Silver Spring) 2010;18:1079–84. [DOI] [PubMed] [Google Scholar]
- 51.Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, Snitker S, Horenstein RB, Hull K, Goldberg NH, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med 2006;3:e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sentí M, Tomás M, Fitó M, Weinbrenner T, Covas MI, Sala J, Masiá R, Marrugat J. Antioxidant paraoxonase 1 activity in the metabolic syndrome. J Clin Endocrinol Metab 2003;88:5422–6. [DOI] [PubMed] [Google Scholar]
- 53.Kappelle PJ, Bijzet J, Hazenberg BP, Dullaart RP. Lower serum paraoxonase-1 activity is related to higher serum amyloid a levels in metabolic syndrome. Arch Med Res 2011;42:219–25. [DOI] [PubMed] [Google Scholar]
- 54.Han CY, Chiba T, Campbell JS, Fausto N, Chaisson M, Orasanu G, Plutzky J, Chait A. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol 2006;26:1806–13. [DOI] [PubMed] [Google Scholar]
- 55.Van Eck M, Hoekstra M, Hildebrand RB, Yaong Y, Stengel D, Kruijt JK, Sattler W, Tietge UJ, Ninio E, Van Berkel TJ, et al. Increased oxidative stress in scavenger receptor BI knockout mice with dysfunctional HDL. Arterioscler Thromb Vasc Biol 2007;27:2413–9. [DOI] [PubMed] [Google Scholar]
- 56.Hafiane A, Genest J. High density lipoproteins: measurement techniques and potential biomarkers of cardiovascular risk. BBA Clin 2015;3:175–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mackness B, Durrington P, McElduff P, Yarnell J, Azam N, Watt M, Mackness M. Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation 2003;107:2775–9. [DOI] [PubMed] [Google Scholar]
- 58.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol 2010;30:796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vergeer M, Boekholdt SM, Sandhu MS, Ricketts SL, Wareham NJ, Brown MJ, de Faire U, Leander K, Gigante B, Kavousi M, et al. Genetic variation at the phospholipid transfer protein locus affects its activity and high-density lipoprotein size and is a novel marker of cardiovascular disease susceptibility. Circulation 2010;122:470–7. [DOI] [PubMed] [Google Scholar]
- 60.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ 1999;319:1523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146–55. [DOI] [PubMed] [Google Scholar]
- 62.Howell WH, McNamara DJ, Tosca MA, Smith BT, Gaines JA. Plasma lipid and lipoprotein responses to dietary fat and cholesterol: a meta-analysis. Am J Clin Nutr 1997;65:1747–64. [DOI] [PubMed] [Google Scholar]
- 63.Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 2005;45:287–306. [DOI] [PubMed] [Google Scholar]
- 64.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr 2002;22:19–34. [DOI] [PubMed] [Google Scholar]
- 65.Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in the Netherlands. Nutr Cancer 1993;20:21–9. [DOI] [PubMed] [Google Scholar]
- 66.Kühnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet 1976;24:117–91. [PubMed] [Google Scholar]
- 67.Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr 2007;137:1244–52. [DOI] [PubMed] [Google Scholar]
- 68.Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 2000;52:673–751. [PubMed] [Google Scholar]
- 69.Myhrstad MC, Carlsen H, Nordstrom O, Blomhoff R, Moskaug JO. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med 2002;32:386–93. [DOI] [PubMed] [Google Scholar]
- 70.Sun GY, Chen Z, Jasmer KJ, Chuang DY, Gu Z, Hannink M, Simonyi A. Quercetin attenuates inflammatory responses in BV-2 microglial cells: role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PLoS One 2015;10:e0141509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karlsen A, Retterstol L, Laake P, Paur I, Bohn SK, Sandvik L, Blomhoff R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr 2007;137:1951–4. [DOI] [PubMed] [Google Scholar]
- 72.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 1993;342:1007–11. [DOI] [PubMed] [Google Scholar]
- 73.Mursu J, Nurmi T, Tuomainen TP, Ruusunen A, Salonen JT, Voutilainen S. The intake of flavonoids and carotid atherosclerosis: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr 2007;98:814–8. [DOI] [PubMed] [Google Scholar]
- 74.Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen AH, Rimm EB. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013;127:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr 2012;95:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X, Ouyang YY, Liu J, Zhao G. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr 2014;111:1–11. [DOI] [PubMed] [Google Scholar]
- 77.Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst 2011;103:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol 2010;1:163–87. [DOI] [PubMed] [Google Scholar]
- 79.Pérez-Jiménez J, Neveu V, Vos F, Scalbert A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: an application of the phenol-explorer database. J Agric Food Chem 2010;58:4959–69. [DOI] [PubMed] [Google Scholar]
- 80.Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, Jin T, Ling W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res 2012;111:967–81. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y, Zhang Y, Wang X, Liu Y, Xia M. Cyanidin-3-O-beta-glucoside induces oxysterol efflux from endothelial cells: role of liver X receptor alpha. Atherosclerosis 2012;223:299–305. [DOI] [PubMed] [Google Scholar]
- 82.Jia Y, Hoang MH, Jun HJ, Lee JH, Lee SJ. Cyanidin, a natural flavonoid, is an agonistic ligand for liver X receptor alpha and beta and reduces cellular lipid accumulation in macrophages and hepatocytes. Bioorg Med Chem Lett 2013;23:4185–90. [DOI] [PubMed] [Google Scholar]
- 83.Jia Y, Kim JY, Jun HJ, Kim SJ, Lee JH, Hoang MH, Kim HS, Chang HI, Hwang KY, Um SJ, et al. Cyanidin is an agonistic ligand for peroxisome proliferator-activated receptor-alpha reducing hepatic lipid. Biochim Biophys Acta 2013;1831:698–708. [DOI] [PubMed] [Google Scholar]
- 84.Du C, Shi Y, Ren Y, Wu H, Yao F, Wei J, Wu M, Hou Y, Duan H. Anthocyanins inhibit high-glucose-induced cholesterol accumulation and inflammation by activating LXRalpha pathway in HK-2 cells. Drug Des Devel Ther 2015;9:5099–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xia M, Hou M, Zhu H, Ma J, Tang Z, Wang Q, Li Y, Chi D, Yu X, Zhao T, et al. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proliferator-activated receptor {gamma}-liver X receptor {alpha}-ABCA1 pathway. J Biol Chem 2005;280:36792–801. [DOI] [PubMed] [Google Scholar]
- 86.Farrell N, Norris G, Lee SG, Chun OK, Blesso CN. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct 2015;6:1278–87. [DOI] [PubMed] [Google Scholar]
- 87.Kim B, Ku CS, Pham TX, Park Y, Martin DA, Xie L, Taheri R, Lee J, Bolling BW. Aronia melanocarpa (chokeberry) polyphenol-rich extract improves antioxidant function and reduces total plasma cholesterol in apolipoprotein E knockout mice. Nutr Res 2013;33:406–13. [DOI] [PubMed] [Google Scholar]
- 88.Zhu Y, Huang X, Zhang Y, Wang Y, Liu Y, Sun R, Xia M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J Clin Endocrinol Metab 2014;99:561–9. [DOI] [PubMed] [Google Scholar]
- 89.Zhu Y, Xia M, Yang Y, Liu F, Li Z, Hao Y, Mi M, Jin T, Ling W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin Chem 2011;57:1524–33. [DOI] [PubMed] [Google Scholar]
- 90.Zhu Y, Ling W, Guo H, Song F, Ye Q, Zou T, Li D, Zhang Y, Li G, Xiao Y, et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutrition, metabolism, and cardiovascular diseases. Nutr Metab Cardiovasc Dis 2013;23:843–9. [DOI] [PubMed] [Google Scholar]
- 91.Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, Cao L, Ling W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr 2009;90:485–92. [DOI] [PubMed] [Google Scholar]
- 92.Li D, Zhang Y, Liu Y, Sun R, Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr 2015;145:742–8. [DOI] [PubMed] [Google Scholar]
- 93.Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010;2010:bap024. [DOI] [PMC free article] [PubMed]
- 94.Hassellund SS, Flaa A, Kjeldsen SE, Seljeflot I, Karlsen A, Erlund I, Rostrup M. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: a double-blind randomized placebo-controlled crossover study. J Hum Hypertens 2013;27:100–6. [DOI] [PubMed] [Google Scholar]
- 95.Schroeter H, Heiss C, Spencer JP, Keen CL, Lupton JR, Schmitz HH. Recommending flavanols and procyanidins for cardiovascular health: current knowledge and future needs. Mol Aspects Med 2010;31:546–57. [DOI] [PubMed] [Google Scholar]
- 96.Tas S, Sarandol E, Ziyanok S, Dirican M. Effects of green tea on serum paraoxonase/arylesterase activities in streptozotocin-induced diabetic rats. Nutr Res 2005;25:1061–74. [Google Scholar]
- 97.Rosenblat M, Volkova N, Coleman R, Almagor Y, Aviram M. Antiatherogenicity of extra virgin olive oil and its enrichment with green tea polyphenols in the atherosclerotic apolipoprotein-E-deficient mice: enhanced macrophage cholesterol efflux. J Nutr Biochem 2008;19:514–23. [DOI] [PubMed] [Google Scholar]
- 98.Hsu SP, Wu MS, Yang CC, Huang KC, Liou SY, Hsu SM, Chien CT. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am J Clin Nutr 2007;86:1539–47. [DOI] [PubMed] [Google Scholar]
- 99.Baba S, Osakabe N, Kato Y, Natsume M, Yasuda A, Kido T, Fukuda K, Muto Y, Kondo K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am J Clin Nutr 2007;85:709–17. [DOI] [PubMed] [Google Scholar]
- 100.Martínez-López S, Sarriá B, Sierra-Cinos JL, Goya L, Mateos R, Bravo L. Realistic intake of a flavanol-rich soluble cocoa product increases HDL-cholesterol without inducing anthropometric changes in healthy and moderately hypercholesterolemic subjects. Food Funct 2014;5:364–74. [DOI] [PubMed] [Google Scholar]
- 101.Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012;35:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan MK, Huma ZE, Dangles O. A comprehensive review on flavanones, the major citrus polyphenols. J Food Compos Anal 2014;33:85–104. [Google Scholar]
- 103.Mulvihill EE, Burke AC, Huff MW. Citrus flavonoids as regulators of lipoprotein metabolism and atherosclerosis. Annu Rev Nutr 2016;36:275–99. [DOI] [PubMed] [Google Scholar]
- 104.Jayachitra J, Nalini N. Effect of naringenin (citrus flavanone) on lipid profile in ethanol-induced toxicity in rats. J Food Biochem 2011;36:502–11. [Google Scholar]
- 105.Jeon SM, Kim HK, Kim HJ, Do GM, Jeong TS, Park YB, Choi MS. Hypocholesterolemic and antioxidative effects of naringenin and its two metabolites in high-cholesterol fed rats. Transl Res 2007;149:15–21. [DOI] [PubMed] [Google Scholar]
- 106.Kim HJ, Jeon SM, Lee MK, Cho YY, Kwon EY, Lee JH, Choi MS. Comparison of hesperetin and its metabolites for cholesterol-lowering and antioxidative efficacy in hypercholesterolemic hamsters. J Med Food 2010;13:808–14. [DOI] [PubMed] [Google Scholar]
- 107.Haidari F, Rashidi MR, Mohammad-Shahi M. Effects of orange juice and hesperetin on serum paraoxonase activity and lipid profile in hyperuricemic rats. Bioimpacts 2012;2:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iio A, Ohguchi K, Iinuma M, Nozawa Y, Ito M. Hesperetin upregulates ABCA1 expression and promotes cholesterol efflux from THP-1 macrophages. J Nat Prod 2012;75:563–6. [DOI] [PubMed] [Google Scholar]
- 109.Kurowska EM, Spence JD, Jordan J, Wetmore S, Freeman DJ, Piche LA, Serratore P. HDL-cholesterol-raising effect of orange juice in subjects with hypercholesterolemia. Am J Clin Nutr 2000;72:1095–100. [DOI] [PubMed] [Google Scholar]
- 110.Mollace V, Sacco I, Janda E, Malara C, Ventrice D, Colica C, Visalli V, Muscoli S, Ragusa S, Muscoli C, et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: from animal models to human studies. Fitoterapia 2011;82:309–16. [DOI] [PubMed] [Google Scholar]
- 111.Jung UJ, Kim HJ, Lee JS, Lee MK, Kim HO, Park EJ, Kim HK, Jeong TS, Choi MS. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin Nutr 2003;22:561–8. [DOI] [PubMed] [Google Scholar]
- 112.Demonty I, Lin Y, Zebregs YE, Vermeer MA, van der Knaap HC, Jakel M, Trautwein EA. The citrus flavonoids hesperidin and naringin do not affect serum cholesterol in moderately hypercholesterolemic men and women. J Nutr 2010;140:1615–20. [DOI] [PubMed] [Google Scholar]
- 113.Xiao J, Kai G. A review of dietary polyphenol-plasma protein interactions: characterization, influence on the bioactivity, and structure-affinity relationship. Crit Rev Food Sci Nutr 2012;52:85–101. [DOI] [PubMed] [Google Scholar]
- 114.Graf BA, Milbury PE, Blumberg JB. Flavonols, flavones, flavanones, and human health: epidemiological evidence. J Med Food 2005;8:281–90. [DOI] [PubMed] [Google Scholar]
- 115.Francisco V, Figueirinha A, Costa G, Liberal J, Ferreira I, Lopes MC, Garcia-Rodriguez C, Cruz MT, Batista MT. The flavone luteolin inhibits liver X receptor activation. J Nat Prod 2016;79:1423–8. [DOI] [PubMed] [Google Scholar]
- 116.Li F, Wong TY, Lin SM, Chow S, Cheung WH, Chan FL, Chen S, Leung LK. Coadministrating luteolin minimizes the side effects of the aromatase inhibitor letrozole. J Pharmacol Exp Ther 2014;351:270–7. [DOI] [PubMed] [Google Scholar]
- 117.Wang GG, Lu XH, Li W, Zhao X, Zhang C. Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid Based Complement Alternat Med 2011;2011:323171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang S, Zhang X, Liu M, Luan H, Ji Y, Guo P, Wu C. Chrysin inhibits foam cell formation through promoting cholesterol efflux from RAW264.7 macrophages. Pharm Biol 2015;53:1481–7. [DOI] [PubMed] [Google Scholar]
- 119.Chen CY, Shyue SK, Ching LC, Su KH, Wu YL, Kou YR, Chiang AN, Pan CC, Lee TS. Wogonin promotes cholesterol efflux by increasing protein phosphatase 2B-dependent dephosphorylation at ATP-binding cassette transporter-A1 in macrophages. J Nutr Biochem 2011;22:1015–21. [DOI] [PubMed] [Google Scholar]
- 120.Aherne SA, O’Brien NM. Dietary flavonols: chemistry, food content, and metabolism. Nutrition 2002;18:75–81. [DOI] [PubMed] [Google Scholar]
- 121.Boesch-Saadatmandi C, Niering J, Minihane AM, Wiswedel I, Gardeman A, Wolffram S, Rimbach G. Impact of apolipoprotein E genotype and dietary quercetin on paraoxonase 1 status in apoE3 and apoE4 transgenic mice. Atherosclerosis 2010;211:110–3. [DOI] [PubMed] [Google Scholar]
- 122.Boesch-Saadatmandi C, Egert S, Schrader C, Coumoul X, Barouki R, Muller MJ, Wolffram S, Rimbach G. Effect of quercetin on paraoxonase 1 activity–studies in cultured cells, mice and humans. J Physiol Pharmacol 2010;61(1):99–105. [PubMed] [Google Scholar]
- 123.Leckey LC, Garige M, Varatharajalu R, Gong M, Nagata T, Spurney CF, Lakshman RM. Quercetin and ethanol attenuate the progression of atherosclerotic plaques with concomitant up regulation of paraoxonase1 (PON1) gene expression and PON1 activity in LDLR−/− mice. Alcohol Clin Exp Res 2010;34:1535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gong M, Garige M, Varatharajalu R, Marmillot P, Gottipatti C, Leckey LC, Lakshman RM. Quercetin up-regulates paraoxonase 1 gene expression with concomitant protection against LDL oxidation. Biochem Biophys Res Commun 2009;379:1001–4. [DOI] [PubMed] [Google Scholar]
- 125.Center for Drug Evaluation and Research. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Rockville (MD): US Department of Health and Human Services; 2005. [Google Scholar]
- 126.Garige M, Gong M, Varatharajalu R, Lakshman MR. Quercetin up-regulates paraoxonase 1 gene expression via sterol regulatory element binding protein 2 that translocates from the endoplasmic reticulum to the nucleus where it specifically interacts with sterol responsive element-like sequence in paraoxonase 1 promoter in HuH7 liver cells. Metabolism 2010;59:1372–8. [DOI] [PubMed] [Google Scholar]
- 127.Lee SM, Moon J, Cho Y, Chung JH, Shin MJ. Quercetin up-regulates expressions of peroxisome proliferator-activated receptor gamma, liver X receptor alpha, and ATP binding cassette transporter A1 genes and increases cholesterol efflux in human macrophage cell line. Nutr Res 2013;33:136–43. [DOI] [PubMed] [Google Scholar]
- 128.Rosenblat M, Volkova N, Khatib S, Mahmood S, Vaya J, Aviram M. Reduced glutathione increases quercetin stimulatory effects on HDL- or apoA1-mediated cholesterol efflux from J774A.1 macrophages. Free Radic Res 2014;48:1462–72. [DOI] [PubMed] [Google Scholar]
- 129.Chang YC, Lee TS, Chiang AN. Quercetin enhances ABCA1 expression and cholesterol efflux through a p38-dependent pathway in macrophages. J Lipid Res 2012;53:1840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li XY, Kong LX, Li J, He HX, Zhou YD. Kaempferol suppresses lipid accumulation in macrophages through the downregulation of cluster of differentiation 36 and the upregulation of scavenger receptor class B type I and ATP-binding cassette transporters A1 and G1. Int J Mol Med 2013;31:331–8. [DOI] [PubMed] [Google Scholar]
- 131.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr 1999;129:758S–67S. [DOI] [PubMed] [Google Scholar]
- 132.Ahmad S, Scott JE. Estradiol enhances cell-associated paraoxonase 1 (PON1) activity in vitro without altering PON1 expression. Biochem Biophys Res Commun 2010;397:441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Agacayak E, Basaranoglu S, Tunc SY, Icen MS, Findik FM, Kaplan I, Evliyaoglu O, Gul T. Oxidant/antioxidant status, paraoxonase activity, and lipid profile in plasma of ovariectomized rats under the influence of estrogen, estrogen combined with progesterone, and genistein. Drug Des Devel Ther 2015;9:2975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mohammadshahi M, Haidari F, Saei AA, Rashidi B, Mahboob S, Rashidi MR. Soy protein, genistein, and daidzein improve serum paraoxonase activity and lipid profiles in rheumatoid arthritis in rats. J Med Food 2013;16:147–54. [DOI] [PubMed] [Google Scholar]
- 135.Ustundag B, Bahcecioglu IH, Sahin K, Duzgun S, Koca S, Gulcu F, Ozercan IH. Protective effect of soy isoflavones and activity levels of plasma paraoxonase and arylesterase in the experimental nonalcoholic steatohepatitis model. Dig Dis Sci 2007;52:2006–14. [DOI] [PubMed] [Google Scholar]
- 136.Weggemans RM, Trautwein EA. Relation between soy-associated isoflavones and LDL and HDL cholesterol concentrations in humans: a meta-analysis. Eur J Clin Nutr 2003;57:940–6. [DOI] [PubMed] [Google Scholar]
- 137.Zhan S, Ho SC. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr 2005;81:397–408. [DOI] [PubMed] [Google Scholar]
- 138.Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr 2007;85:1148–56. [DOI] [PubMed] [Google Scholar]
- 139.Hall WL, Vafeiadou K, Hallund J, Bugel S, Reimann M, Koebnick C, Zunft HJ, Ferrari M, Branca F, Dadd T, et al. Soy-isoflavone-enriched foods and markers of lipid and glucose metabolism in postmenopausal women: interactions with genotype and equol production. Am J Clin Nutr 2006;83:592–600. [DOI] [PubMed] [Google Scholar]
- 140.Törmälä RM, Nikander E, Tiitinen A, Väisänen-Tommiska M, Ylikorkala O, Mikkola TS. Serum cholesterol efflux potential in postmenopausal women treated with isolated isoflavones. Menopause 2006;13:96–101. [DOI] [PubMed] [Google Scholar]
- 141.Badeau R, Jauhiainen M, Metso J, Nikander E, Tikkanen MJ, Ylikorkala O, Mikkola TS. Effect of isolated isoflavone supplementation on ABCA1-dependent cholesterol efflux potential in postmenopausal women. Menopause 2007;14:293–9. [DOI] [PubMed] [Google Scholar]