Abstract
Entry of the enzymatic components of anthrax toxin [lethal factor (LF) and edema factor] into the cytosol of mammalian cells depends on the ability of the activated protective antigen (PA63) component to form a channel (pore) in the membrane of an acidic intracellular compartment. To investigate the mechanism of translocation, we characterized N-terminally truncated forms of the PA63-binding domain of LF (LFN). Deleting 27 or 36 residues strongly inhibited acid-triggered translocation of LFN across the plasma membrane of CHO-K1 cells and ablated the protein's ability to block PA63 channels in planar lipid bilayers at a small positive voltage (+20 mV). Fusing a H6-tag to the N terminus of the truncated proteins restored both translocation and channel-blocking activities. At +20 mV, N-terminal H6 and biotin tags were accessible to Ni2+ and streptavidin, respectively, added to the trans compartment of a planar bilayer. On the basis of these findings, we propose that the N terminus of PA63-bound LF or edema factor enters the PA63-channel under the influence of acidic pH and a positive transmembrane potential and initiates translocation in an N- to C-terminal direction.
Many toxins produced by pathogenic bacteria are specialized enzymes that have evolved ways to gain access to the cytosolic compartment of mammalian cells. There, they catalyze reactions detrimental to the host. All such toxins must penetrate a cytosol-contiguous membrane, and some members of this class contain a domain, or a separate polypeptide, that forms an aqueous transmembrane channel (pore) to mediate translocation of the enzymatic part(s). How such channels are formed and how they mediate translocation of a catalytic moiety across the membrane are topics of current interest.
Anthrax toxin is an ensemble of three proteins: Two are intracellularly acting enzymes, and the third transports both of the enzymatic moieties to the cytosol (1, 2). The enzymatic components are an adenylate cyclase termed “edema factor” (EF, 89 kDa) and a Zn2+-protease termed “lethal factor” (LF, 90 kDa). The transport component is a receptor-binding, poreforming protein termed “protective antigen” (PA, 83 kDa). PA, EF, and LF, which are secreted from the anthrax bacillus as unassociated nontoxic monomers, combine at the surface of receptor-bearing cells to form a series of toxic cell-bound complexes (3). Assembly begins when PA binds to a cellular receptor and is activated by a member of the furin family of proteases (4). This action removes a 20-kDa fragment (PA20) from the N terminus, leaving a 63-kDa fragment (PA63) bound to the receptor. Once freed from steric constraints imposed by PA20, PA63 spontaneously oligomerizes, generating a ring-shaped heptamer, (PA63)7 (also called the prepore) (5). EF and LF bind to the heptamer, competitively and with nanomolar affinities, by means of a homologous N-terminal domain (EFN or LFN, respectively) (6). The footprint of this domain is such that a maximum of three molecules of EF or LF, or combinations thereof, can bind simultaneously per (PA63)7 (7). The assembled complexes are endocytosed and trafficked to an acidic compartment, believed to be the endosome. There, the acidic pH induces a conformational rearrangement in (PA63)7, allowing it to form an aqueous transmembrane channel that mediates translocation of EF and LF to the cytosol (8).
Electrophysiological data and crystallographic evidence support a model of channel formation in which the 2β2–2β3 loops of the seven monomers of (PA63)7 combine to form a 14-strand transmembrane β-barrel (9, 10). The limiting diameter of the PA63 channel has been estimated from studies with tetraalkyl ammonium ions to be ≈12 Å, implying that a protein would need to unfold partially or fully to pass through (11, 12). Consistent with this concept, translocation of LFN-DTA, a fusion protein containing LFN linked to the N terminus of the catalytic A-chain of diphtheria toxin (DTA), was blocked either by introducing an artificial disulfide into the DTA moiety or by liganding this moiety with adenine or NAD+ (13). If unfolding of EF and LF is necessary for them to cross the membrane, they may thread through the channel vectorally, with the N or C terminus first. Here, we report results suggesting that a flexible 27-residue stretch of the N terminus of LFN enters the (PA63)7 channel and initiates PA-dependent translocation across the endosomal membrane. These and ancillary findings suggest a model in which the N terminus of LF or EF initiates translocation in an N- to C-terminal direction. Further, our results suggest a role for transmembrane potential in the translocation process.
Materials and Methods
Preparation of LFN Constructs. The plasmid pET15b-LFN containing the N-terminal 1–263 residues of LF inserted between NdeI and BamHI sites was used for all cloning steps. LFN constructs were made either with the N-terminal H6-tag, in which the protein was inserted between the NdeI and BamHI sites, or without the N-terminal H6-tag, in which it was inserted between the NcoI and BamHI sites of pET15b. We used 5′-CTAGGATCCTTACCCGTTGATCTTTAAGTTCTTCC-3′ to introduce a BamHI site and act as the reverse primer. The forward primers, designed to permit appropriate truncations and also introduce either an NcoI or NdeI site, were used in the amplification of non-H6-tagged LFN constructs or the H6-tagged H6-LFN constructs. Constructs of H6-LFN, Δ27, Δ36, and Δ39 were kindly provided by D. Borden Lacy (Harvard Medical School). LFN-DTA containing the A1C mutation was kindly provided by Steve Juris (Harvard Medical School).
Oligonucleotide 5′-CAT GGA TCC TTA GCC GTG ATG ATG ATG ATG ATG CGC CCG TTG ATC-3′ was used as a reverse primer to fuse a H6-tag to the C terminus of LFN and to introduce a BamHI site. Primers for LFN and LFN Δ27 were used as forward primers in the construction of LFN-H6 and LFN Δ27-H6.
Sequences were amplified from the pET15b-H6-LFN template by PCR. The PCR products were purified and double digested with either NcoI/BamHI or NdeI/BamHI. The digested fragments and pET15b vector were gel-purified, ligated, and transformed into Escherichia coli XL1-Blue by electroporation. Transformants were screened by digestion and verified by sequencing.
Constructs of H6-LFN Δ22 and H6-LFN Δ23 were made by using the QuikChange method of site-directed mutagenesis (Stratagene) using H6-LFN Δ21 and Δ24, respectively, as template. The PCR products were transformed into E. coli XL1-Blue and sequenced.
Protein Preparation. Purification of wild-type PA from BL21(DE3) and formation of PA63 heptamer were performed as described in ref. 14. LFN constructs that contained a H6-tag at either their N or C terminus were expressed and purified according to the pET system manual from Novagen. Their N-terminal H6-tag, which was removed by thrombin cleavage, consisted of the following 21 residues: Met–Gly–Ser–Ser–His–His–His–His–His–His–Ser–Ser–Gly–Leu–Val–Pro–Arg–Gly–Ser–His–Met. For conductance experiments, we used purified constructs containing either this 21-residue sequence or the product obtained by removal of the first 17 of these residues by thrombin treatment (hence leaving the additional Gly–Ser–His–Met sequence added to the N terminus). For cell-based assays, the sequences were as shown in Fig. 1, but with an additional Met residue at the N terminus. Protein concentrations were determined by using the Bio-Rad protein assay reagent.
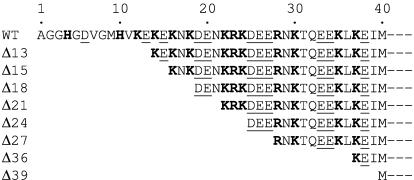
Fig. 1.
N-terminal sequence of LFN and deletion mutants. Shown are the sequences of the first 40 residues of wild-type (WT) LFN and that of each of the deletion mutants tested. Acidic residues are underlined; basic residues are in boldface type. Additional sequences present at the N terminus of various constructs, including the 21-residue H6-containing tag, are described in Materials and Methods.
Cell-Surface Binding and Translocation Assay. PA binding to the cell surface and translocation of 35S-LFN were assayed as described in refs. 13 and 15. Briefly, CHO–K1 cells in a 24-well plate were chilled on ice, washed, and incubated on ice for 2 h with trypsin-activated PA (saturating concentrations). The cells then were washed and incubated for an additional 2 h on ice with wild-type or mutant LFN that had been labeled with [35S]methionine in an in vitro transcription/translation system (Promega). The cells then were washed with ice-cold PBS at pH 5.0 or 8.0, incubated at 37°C for 1 min, and either treated with Pronase to digest residual untranslocated 35S at the cell surface or left untreated as controls. The cells then were lysed, and 35S liberated into the lysis buffer was assayed. The percent translocation was defined as dpm protected from Pronase/dpm bound to cells × 100.
Biotinylation of LFN and LFN-DTA. pET15b-H6-LFN was mutated to introduce a Cys residue either at its N terminus (A1C) or close to the C terminus (N242C) by using the QuikChange mutagenesis system (Stratagene). The N-terminal H6-tag was cleaved with thrombin before biotinylation. Biotinylation was performed basically according to Jakes et al. (16), and the extent of biotinylation was assessed by an SDS/PAGE streptavidin-binding assay as described in Qiu et al. (17). To ensure that all of the effect observed on planar lipid bilayers came from biotinylated LFN, the biotinylation products were purified through a monomeric avidin column (UltraLink, Pierce). Proteins purified on this column showed no evidence of residual nonbiotinylated LFN in the gel assay.
Planar Lipid Bilayer Experiments. Bilayers were formed at room temperature by using the brush technique of Mueller et al. (18) across a 200-μm-diameter hole in a Delrin cup separating 1-ml cis and trans compartments. Symmetric solutions of 100 mM KCl, 25 mM succinic acid, and 1 mM EDTA (pH 5.5) were used. EDTA was omitted in experiments in which Ni2+ was added. Bridges containing 3 M KCl in 3% agar connected solutions to Ag/AgCl electrodes in 3 M KCl baths. The membrane-forming solution was 30 mg/ml diphytanoylphosphatidyl choline in n-decane. PA63 heptamer (0.5–5 ng) was added to the cis compartment, which was held at +20 mV with respect to the trans compartment. The current was amplified through a BC-525C integrating bilayer clamp amplifier (Warner Instruments, Hamden, CT), filtered at a frequency of 0.1 kHz by a low-pass eight-pole Bessel filter, and computer-displayed through an analog/digital converter (Instrutech, Mineola, NY) with axograph 4.0 (Axon Instruments). LFN was added to the cis compartment after (PA63)7 channel formation stabilized. Ni2+ or streptavidin was added to either the cis or the trans compartment at a concentration of 500 μM or 6 μg/ml, respectively, at indicated time points. Each compartment was stirred continuously throughout the experiment with a small stir bar.
Results
Cell-Surface Binding and Translocation of Truncated Forms of LFN. To explore the manner in which EF and LF undergo PA63-dependent translocation, we deleted various numbers of residues from the N terminus of LFN and measured the PA63-dependent binding and translocation activities of the resulting constructs. The constructs were labeled with [35S]methionine in an in vitro transcription/translation system, and protection of cell-bound label against digestion with Pronase after an acid pulse was used as a measure of translocation. Earlier studies showed that the full-length LFN is protected under the conditions of the assay (13). As shown in Fig. 2A, deletion of the first 27, 36, or 39 residues strongly inhibited acid-triggered translocation of the protein across the plasma membrane of CHO-K1 cells. Only the 39-residue deletion (Δ39) inhibited binding of LFN to PA63 on cells (Fig. 2B) or, slightly, in solution (Fig. 2C). The solution studies were based on the ability of LFN to promote conversion of trypsin-activated PA to heptameric PA63. Together, these experiments showed that N-terminal truncations of 27 or 36 residues strongly impaired translocation without significantly affecting binding to PA63. These results suggested a pivotal role for this region of the protein in translocation.
Fig. 2.
The effects of various N-terminal deletions on the PA63-binding and translocation activities of LFN. H6-tagged constructs (dark gray bars) and non-H6-tagged constructs (light gray bars) were tested basically as described in ref. 13. (A) Translocation across the plasma membrane in response to low pH. (B) PA63-dependent binding of LFN to CHO-K1 cells. CHO-K1 cells were incubated with trypsin-nicked PA (2.4 × 10–8 M) for 2 h on ice, and the cells then were washed and incubated with various 35S-LFN truncations for another 2 h. The cells were treated with buffer (pH 5.0) at 37°C for 1 min to trigger translocation and then with Pronase to digest 35S-LFN remaining at the cell surface. Cell-associated 35S-LFN was determined by scintillation counting as a measure of translocation. PA63-dependent binding was measured in identical samples in which Pronase was omitted. The fraction translocated was defined as the amount of protected 35S-LFN divided by the amount bound. (Error bars, SEM.) The results shown in A and B are the average of three independent experiments. (C) LFN-induced oligomerization of PA63 in solution. Trypsin-activated PA (3 μg) was incubated with 1 μg of each LFN truncation mutant at room temperature for 1 h, and the samples then were electrophoresed on a 4–12% polyacrylamide gel. Y236A-LFN was included as a mutant form known to be deficient in PA binding.
Additional support for this hypothesis came from the finding that a H6-tag fused to the N terminus of the Δ27, Δ36, and Δ39 constructs restored translocation efficiency to wild-type levels, or higher (Fig. 2 A and B). The H6-tag dramatically enhanced translocation while slightly increasing the PA63-dependent binding of truncated or wild-type forms of LFN to CHO-K1 cells. These results suggested that the positively charged H6-tag interacts with the cation-selective channel, strongly enhancing translocation efficiency.
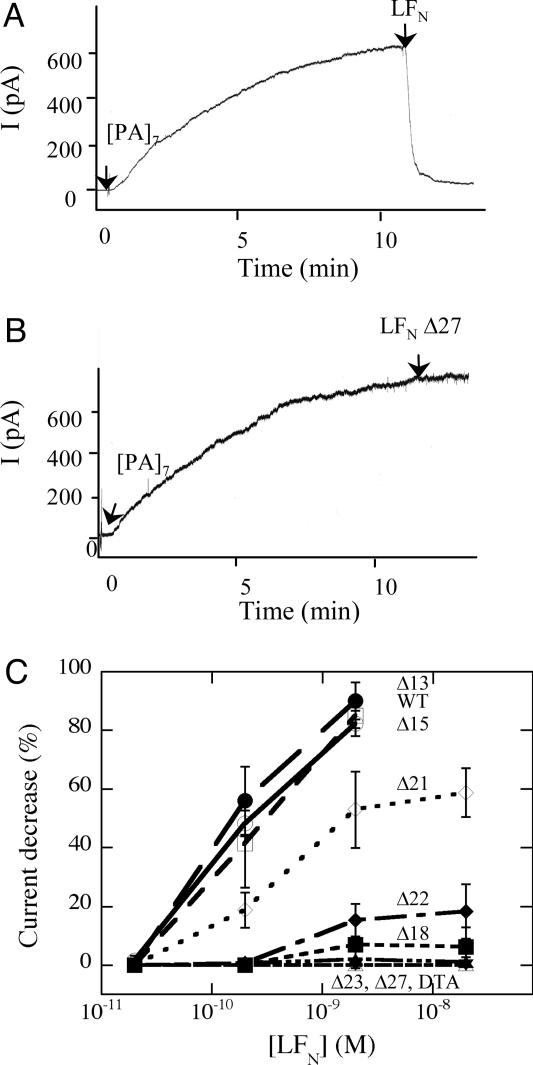
Inhibition of PA63-Mediated Ion Conductance in Planar Lipid Bilayers. Addition of EF, LF, or EFN to the cis compartment of a planar lipid bilayer system (the compartment to which PA heptamer is added) causes a voltage-dependent reduction in ion conductance of the cation-selective channels formed by PA63 (12). Also, LF or LFN inhibits PA63-dependent efflux of 86Rb+ from preloaded CHO-K1 cells (19). Consistent with these results, we found that with the voltage held at +20 mV, adding full-length LFN to the cis compartment caused a rapid 20- to 50-fold decrease of PA63-mediated conductance (Fig. 3A).
Fig. 3.
Effects of N-terminal deletions on the ability of LFN to inhibit PA63-dependent conductance in planar bilayers. Purified (PA63)7 (2 ng) was added to the cis compartment, containing 1 ml of buffer (100 mM KCl/25 mM succinate/1 mM EDTA, pH 5.5). (A and B) The cis compartment was held at +20 mV, and after channel formation stabilized, we added full-length LFN (A) or a truncated form, LFN Δ27 (B), to the cis compartment to give a final concentration of 20 nM. The traces in A and B are representative of 12 or 11 different trials, respectively. (C) Various concentrations of full-length or truncated forms of LFN were added to the cis compartment, and the percent current decrease was determined after 1 min. DTA, used as a negative control, did not interact with the channel. The curves in C are the average of three independent experiments, except for DTA, which was tested twice.
The effects of N-terminal truncations and H6-tags on the inhibition of ion conductance correlated well with their effects on translocation. The Δ27 construct caused little or no inhibition of ion conductance, whereas Δ13 and Δ15 were as potent inhibitors of conductance as full-length LFN (Fig. 3 B and C). Inhibitory activity tended to decrease as increasing numbers of residues were deleted, and constructs with deletions of 23 residues or more showed no inhibitory activity. A H6-tag fused to the N terminus of Δ27 restored the ability of this mutated form of LFN to block PA63 channels (Fig. 4A). In fact, fusing a H6-tag to the N terminus of any of the LFN truncation constructs, including Δ18, Δ24, Δ27, Δ36, and Δ39, restored channel-blocking activity (data not shown). In contrast, a H6-tag appended to the C terminus of either full-length LFN or the Δ27 construct had no effect (Fig. 4).
Fig. 4.
Effect of H6-tag on the channel-blocking activity of LFN truncations. Conditions were as in Fig. 3. (A) After channel formation stabilized, LFN Δ27 with a H6-tag at either its N or C terminus was added to the cis compartment as indicated. The trace is representative of three independent experiments, all with identical results. (B) The percentage decrease in current 1 min after LFN addition was plotted as a function of final LFN concentration. The curves are averaged from three independent experiments.
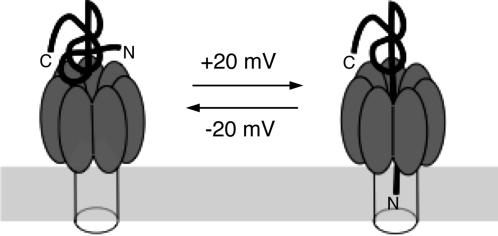
Interaction of Modified Forms of LFN with the PA63 Channel. The results described above suggest that the N-terminal region of PA63-bound LFN can enter the channel, blocking ion conductance in planar bilayers and initiating translocation in vivo (Fig. 5). In support of this model, N-terminally biotinylated LFN (A1C-biotin) blocked the PA63 channel as effectively as wild-type LFN, but preincubating it with streptavidin ablated blocking activity (Fig. 6A). Streptavidin did not inhibit binding of A1C-biotin to PA63, as shown by the PA63 oligomerization assay in solution (data not shown). Reduction of the disulfide bridge linking biotin to A1C released the streptavidin, allowing LFN to regain blocking activity (Fig. 6A). LFN biotinylated near the C terminus (N242C-biotin) retained channel-blocking activity in the presence or absence of streptavidin (Fig. 6B). A higher concentration of the derivatized LFN was needed to block channels in the presence of streptavidin, however, suggesting steric interference by streptavidin of binding of LFN to the PA63 channel. Consistent with this hypothesis, the N242C-biotin:streptavidin complex bound PA63 heptamer with a lower efficiency in solution (data not shown).
Fig. 5.
Illustration of the movement of N terminus of LFN into the PA63 channel under the influence of a transmembrane voltage of +20 mV.
Fig. 6.
Evidence that the N terminus of LFN enters the channel formed by (PA63)7.(A and B) LFN (0.6 μg) containing a biotin group disulfide-linked either to the N (A) or the C(B) terminus was incubated with 6 μg of streptavidin at room temperature for 10 min before being added to the cis compartment. The membrane-impermeant disulfide reducing agent Tris-carboxyethylphosphine (TCEP) (5 mM) was added as indicated. The voltage was held at +20 mV throughout the experiment. (C) Trans addition of Ni2+ slowed unblocking of N-terminal H6-tagged LFN. EDTA was omitted from the buffer. We added 500 μMNi2+ and 1 mM EDTA to the trans compartment at indicated time points. Although some of the channels became immediately unblocked at –20 mV, others took longer to unblock. This delay may be a consequence of the distribution of LFN penetration of channels at a given voltage. (D) Use of biotin-LFN-DTA to demonstrate penetration of the N terminus to the trans compartment. The buffer used was the same as in Fig. 3, except that 1 mM adenine was present. Eleven minutes before starting the recording, 2 ng of (PA63)7 was added to the cis compartment held at +20 mV. Then, biotin-LFN-DTA (4 nM) was added to the same compartment. At the break, the cis compartment was perfused with 10 ml of buffer, and 10 μg of streptavidin was added to the trans compartment, as indicated. The voltage was shifted to –20 mV, and TCEP (5 mM) was added to the trans compartment, as indicated. The small response at –20 mV before TCEP addition may reflect a small amount of protein not binding streptavidin. The traces shown in A, B, and C were representative of three, four, and four independent trials, respectively, all of which were essentially identical. The trace in D is representative of six trials, all but one of which were successful.
The hypothesis that the N terminus of LFN enters the PA63 channel received additional support from an experiment showing that an N-terminal H6 was accessible to Ni2+ added to the trans compartment. As shown in Fig. 6C, changing the voltage from +20 to –20 mV caused an immediate unblocking of channels. Because Ni2+ binds to the H6-tag, trans Ni2+ should affect the rate at which the N terminus of LFN returns to the cis side at negative voltages; that is, it should affect the rate of channel unblocking. This effect is precisely what we observed (Fig. 6C). When the voltage was stepped from +20 to –20 mV, the unblocking rate was slowed by Ni2+ added to the trans solution and was restored by the subsequent addition of EDTA to that solution. In contrast, trans Ni2+ had no effect on the unblocking rate if the H6-tag was attached to the C terminus of LFN instead of the N terminus (data not shown).
More definitive evidence that LFN enters the PA63 channel N terminus first came from a demonstration that the N terminus emerges into the trans solution while the C terminus is held in the cis compartment. For this experiment, we used LFN-DTA containing biotin disulfide-linked to A1C, and we added adenine to the cis solution. Adenine binds to DTA at its NAD site, stabilizing its native conformation and blocking translocation in the cell-surface translocation assay (13, 20). Thus, with adenine in the cis compartment, the C-terminal DTA moiety of biotin-LFN-DTA should be held on the cis side of the membrane. Under these circumstances, if the biotinylated N terminus enters channels and becomes accessible to streptavidin added to the trans side, unblocking of the channels at negative voltages should be prevented, and this is what we saw. After the addition of streptavidin to the trans solution, there was little unblocking at –20 mV (Fig. 6D). Subsequent addition of Tris-carboxyethylphosphine to the trans solution reduced the disulfide linking the biotin (with its attached streptavidin) to LFN, and this allowed the N terminus to return to the cis side at –20 mV, thereby unblocking the channels (Fig. 6D).
Discussion
We used LFN, the minimal PA63-binding translocation-competent domain of LF, to probe the way in which LF and EF are translocated. The crystal structure of LF shows that LFN contains a disordered stretch of 27 residues at the N terminus and that this stretch is connected to the compactly folded remainder of the domain (residues 40–263) by an outwardly projecting α-helix (helix 1α1; residues 28–39) (21). Our results suggest that this flexible N-terminal stretch fosters the initiation of translocation in an N- to C-terminal direction. Deleting the entire stretch strongly inhibited acid-induced translocation of LFN across the plasma membrane, whereas deleting only 13 residues had no significant effect on this activity. Consistent with this result, the flexible N-terminal stretch in EF is 10 residues shorter than in LF, but the two proteins translocate with similar efficiencies (13).
The effects of N-terminal truncations on translocation correlate with their effects on the ability of LFN to block the ion conductance of PA63 channels. Deleting more than ≈23 residues caused loss of channel-blocking activity, whereas deleting 15 or fewer had no effect. Intermediate-length deletions had variable effects that may depend in part on the charge of residues near the N terminus. Thus, the Δ18 mutation had less effect than the Δ21, perhaps because two acidic residues are exposed at the N terminus of the former (Fig. 1). The loss of activity seen with the longer deletions apparently does not result from alterations in the ability of LFN to bind to PA63, because truncations as long as 36 residues caused no significant loss of affinity. The hypothesis that channel blocking is a consequence of actual entry of the N terminus of LFN into the channel, as opposed to, for example, allosteric induction of channel closure, is supported strongly by the effect of trans streptavidin on biotin-tagged LFN-DTA. Thus, while the C terminus was held on the cis side of the membrane by adenine-liganded DTA, the N-terminal biotin tag became accessible to streptavidin in the trans compartment at +20 mV. This result indicates that the LFN moiety can span the entire breadth of the membrane under these conditions, with the C terminus held in the cis compartment and the N terminus extending into the trans compartment. We reported elsewhere (22) that when adenine is removed from the cis solution, translocation to the trans compartment can then occur (under higher positive membrane potential). Also, we found in the current study that the N-terminal H6-tag of H6-LFN is accessible to Ni2+ added to the trans compartment, indicating that the N terminus is either in the channel or in the trans compartment.
Which properties of the N terminus of LFN underlie its functionality in translocation and channel blocking? One may be its flexibility, presumably allowing movement into the channel. A second may be location. The structure of LF- or EF-liganded PA63 is not yet known, but LF and EF bind to sites on domain 1′ of PA63 at the mouth of the channel (14). A third feature is suggested by the striking effect of H6 fused to the N terminus of the truncation mutants. The H6-tag actually raised translocation and channel-blocking activities to levels above those of wild-type parents. The N-terminal H6-tag had a major effect even when the 21-residue sequence containing it was tethered directly to the ordered globular portion of the LFN structure (helix 1α1 in construct Δ27) (21). In contrast, a H6-tag at the C terminus had no effect. The fact that the translocation and channel-blocking activities occur in a pH range in which the side chain of His is expected to be protonated suggests a role for electrostatic potential. The lumen of the prepore is negatively charged, and presumably so is the cation-selective channel (8, 9). A positively charged N terminus might be attracted into the channel by electrostatics, even in the absence of an applied voltage, and indeed we have shown this (A.F., unpublished results). Applying a positive transmembrane voltage would be expected to foster this entry, and, as we saw, small positive voltage (+20 mV) caused channel blocking in the planar bilayer system. A negative voltage caused immediate unblocking, implying withdrawal of the N terminus into the cis compartment, and reapplying the positive voltage caused immediate reblocking. The latter result implies that, consistent with the high affinity of LF and EF for (PA63)7, LFN remains bound at the channel mouth even in the presence of a negative voltage.
There have been few studies of the transmembrane potential of the endosomal compartment, where translocation is believed to occur. There is consensus that the interior of the compartment is positive with respect to the cytosol: the same polarity that promotes channel blocking. Estimates of the magnitude of the potential vary widely, however, from +10 to +300 mV (23–25). Whereas the pore might discharge the transmembrane potential, the N terminus of LF or EF inserted into the prepore might block the pore before it opens. Recent evidence suggests that the pore may form within multivesicular endosomes, such that LF and EF initially are deposited into vesicles of cytosol contained within these endosomes (26). This reaction would be followed later by back fusion with the endosomal membrane, liberating LF and EF into the bulk cytosol. No estimates of the transmembrane potential of the internal vesicles have been made, to our knowledge.
The charge characteristics of the N-terminal regions of LF and EF are consistent with electrostatic potential drawing the N terminus of LF or EF into the PA63 channel (Fig. 1). There is a high density of ionizable residues, including a majority of cationic ones (at pH 5.5), extending through the first two-thirds of the sequence. Such reasoning also may explain the surprising finding that fusing short tracts of Lys, Arg, or His residues to the N terminus of DTA enabled this protein to enter cells in the presence of PA and inhibit protein synthesis (27). Entry promoted by polycationic tracts was inefficient relative to LFN-mediated entry; the most efficiently translocated of the constructs, Lys8-DTA, entered cells with ≈1/10th the efficiency of LFNDTA. Free LFN did not block entry of these constructs, suggesting that they interacted with sites on (PA63)7 distinct from the EF/LF sites. It was suggested that these constructs might bind weakly to anionic sites at the cell surface [perhaps on (PA63)7] and be codelivered with (PA63)7 to the endosome (27). Regardless of the route, once within the same compartment as (PA63)7, the polycationic N terminus could enter the anionic (PA63)7 channel and initiate translocation of the molten globular DTA by the same mechanism as EF and LF.
Two reports (28, 29) have described findings that might appear to challenge our model of translocation. These studies showed that LFN can mediate PA63-dependent entry of a fusion protein with DTA, regardless of the order of the two domains within the construct. One study (28) reported that DTA-LFN, in which the DTA moiety was N-terminal, inhibited protein synthesis in cells at ≈10% the efficiency of LFN-DTA, where it was C-terminal. The other study (29) reported that DTA-LFN with an N-terminal H6-tag inhibited protein synthesis with about the same efficiency as LFN-DTA. Comparison of the two studies suggests an increase in translocation efficiency by DTA-LFN, from 10% to equivalence with that of LFN-DTA, when an N-terminal H6-tag is present. This result is consistent with our model.
We are left, then, with the question of how the undecorated N terminus of DTA-LFN initiates translocation. First, we note that in our experiments LFN retained a significant level of translocation activity after we deleted 27, 36, or 39 residues from the N terminus (Fig. 2 A), indicating that the flexible 27-residue stretch at the N terminus is not absolutely required for translocation. Recent data show that acidic pH promotes unfolding of LFN in solution (30), and if (PA63)7-bound LFN is similarly perturbed, this might allow the truncated N terminus of LFN to enter the channel at low efficiency. Similar reasoning applies to DTA-LFN. There is evidence that the DTA chain must unfold at acidic pH to be translocated by the B-chain of this toxin (31), and it is believed to adopt a molten globule state under acidic conditions (32). If the DTA moiety of DTA-LFN assumes a similar state while the fusion protein is tethered by means of LFN to (PA63)7, this may provide sufficient flexibility for its N terminus to enter the channel and initiate translocation at the low efficiency observed.
In summary, the evidence presented suggests that the N terminus of bound LFN enters the (PA63)7 channel under the influence of a small positive voltage and initiates threading of unfolded (or progressively unfolding) forms of this protein through to the opposite face of the membrane. Elsewhere, we reported evidence that at higher positive voltages (≥40 mV), the entire LFN molecule translocates to the trans compartment, relieving the channel of blockage (22). The combined evidence from the two studies indicates that PA contains all of the molecular machinery needed to translocate LFN, and, hence, presumably full-length LF and EF, across a phospholipid bilayer. Thus, there is no absolute requirement of a receptor or any other proteinaceous factor for this process to occur. However, interaction of PA with one of its two known receptors, CMG2, regulates the pH at which (PA63)7 forms a channel (33, 34), and other proteins may influence the anthrax toxin translocation process in cells, as shown for diphtheria toxin (35).
Acknowledgments
We thank Steve Juris for providing LFN-DTA and Borden Lacy and Bryan Krantz (Harvard Medical School) for providing some of the constructs used. This work was supported by National Institutes of Health Grants AI 22021 and GM 29210.
Author contributions: S.Z., A.F., and R.J.C. designed research; S.Z. and A.F. performed research; S.Z., A.F., and R.J.C. analyzed data; and S.Z., A.F., and R.J.C. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: EF, edema factor; LF, lethal factor; PA, protective antigen; DTA, A-chain of diphtheria toxin.
References
- 1.Collier, R. J. & Young, J. A. (2003) Annu. Rev. Cell Dev. Biol. 19, 45–70. [DOI] [PubMed] [Google Scholar]
- 2.Leppla, S. H. (2000) in Bacterial Protein Toxins, eds. Aktories, K. & Just, I. (Springer, Berlin), pp. 445–472.
- 3.Pimental, R. A., Christensen, K. A., Krantz, B. A. & Collier, R. J. (2004) Biochem. Biophys. Res. Commun. 322, 258–262. [DOI] [PubMed] [Google Scholar]
- 4.Klimpel, K. R., Molloy, S. S., Thomas, G. & Leppla, S. H. (1992) Proc. Natl. Acad. Sci. USA 89, 10277–10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milne, J. C., Furlong, D., Hanna, P. C., Wall, J. S. & Collier, R. J. (1994) J. Biol. Chem. 269, 20607–20612. [PubMed] [Google Scholar]
- 6.Elliott, J. L., Mogridge, J. & Collier, R. J. (2000) Biochemistry 39, 6706–6713. [DOI] [PubMed] [Google Scholar]
- 7.Mogridge, J., Cunningham, K. & Collier, R. J. (2002) Biochemistry 41, 1079–1082. [DOI] [PubMed] [Google Scholar]
- 8.Blaustein, R. O., Koehler, T. M., Collier, R. J. & Finkelstein, A. (1989) Proc. Natl. Acad. Sci. USA 86, 2209–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petosa, C., Collier, R. J., Klimpel, K. R., Leppla, S. H. & Liddington, R. C. (1997) Nature 385, 833–838. [DOI] [PubMed] [Google Scholar]
- 10.Benson, E. L., Huynh, P. D., Finkelstein, A. & Collier, R. J. (1998) Biochemistry 37, 3941–3948. [DOI] [PubMed] [Google Scholar]
- 11.Blaustein, R. O. & Finkelstein, A. (1990) J. Gen. Physiol. 96, 905–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkelstein, A. (1994) Toxicology 87, 29–41. [DOI] [PubMed] [Google Scholar]
- 13.Wesche, J., Elliott, J. L., Falnes, P. O., Olsnes, S. & Collier, R. J. (1998) Biochemistry 37, 15737–15746. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham, K., Lacy, D. B., Mogridge, J. & Collier, R. J. (2002) Proc. Natl. Acad. Sci. USA 99, 7049–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sellman, B. R., Nassi, S. & Collier, R. J. (2001) J. Biol. Chem. 276, 8371–8376. [DOI] [PubMed] [Google Scholar]
- 16.Jakes, K. S., Kienker, P. K., Slatin, S. L. & Finkelstein, A. (1998) Proc. Natl. Acad. Sci. USA 95, 4321–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu, X. Q., Jakes, K. S., Kienker, P. K., Finkelstein, A. & Slatin, S. L. (1996) J. Gen. Physiol. 107, 313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller, P., Rudin, D. O., Tien, H. & Westcott, W. C. (1963) J. Phys. Chem. 67, 534–535. [Google Scholar]
- 19.Zhao, J., Milne, J. C. & Collier, R. J. (1995) J. Biol. Chem. 270, 18626–18630. [DOI] [PubMed] [Google Scholar]
- 20.Kandel, J., Collier, R. J. & Chung, D. W. (1974) J. Biol. Chem. 249, 2088–2097. [PubMed] [Google Scholar]
- 21.Pannifer, A. D., Wong, T. Y., Schwarzenbacher, R., Renatus, M., Petosa, C., Bienkowska, J., Lacy, D. B., Collier, R. J., Park, S., Leppla, S. H., et al. (2001) Nature 414, 229–233. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, S., Udho, E., Wu, Z., Collier, R. J. & Finkelstein, A. (2004) Biophys. J., 87, in press. [DOI] [PMC free article] [PubMed]
- 23.Van Dyke, R. W. & Belcher, J. D. (1994) Am. J. Physiol. 266, C81–C94. [DOI] [PubMed] [Google Scholar]
- 24.Rybak, S. L., Lanni, F. & Murphy, R. F. (1997) Biophys. J. 73, 674–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonawane, N. D., Thiagarajah, J. R. & Verkman, A. S. (2002) J. Biol. Chem. 277, 5506–5513. [DOI] [PubMed] [Google Scholar]
- 26.Abrami, L., Lindsay, M., Parton, R. G., Leppla, S. H. & van der Goot, F. G. (2004) J. Cell Biol. 166, 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanke, S. R., Milne, J. C., Benson, E. L. & Collier, R. J. (1996) Proc. Natl. Acad. Sci. USA 93, 8437–8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arora, N. & Leppla, S. H. (1994) Infect. Immun. 62, 4955–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milne, J. C., Blanke, S. R., Hanna, P. C. & Collier, R. J. (1995) Mol. Microbiol. 15, 661–666. [DOI] [PubMed] [Google Scholar]
- 30.Krantz, B. A., Trivedi, A. D., Cunningham, K., Christensen, K. A. & Collier, R. J. (2004) J. Mol. Biol. 344, 739–756. [DOI] [PubMed] [Google Scholar]
- 31.Falnes, P. O., Choe, S., Madshus, I. H., Wilson, B. A. & Olsnes, S. (1994) J. Biol. Chem. 269, 8402–8407. [PubMed] [Google Scholar]
- 32.Ren, J., Kachel, K., Kim, H., Malenbaum, S. E., Collier, R. J. & London, E. (1999) Science 284, 955–957. [DOI] [PubMed] [Google Scholar]
- 33.Lacy, D. B., Wigelsworth, D., Harrison, S. C. & Collier, R. J. (2004) Proc. Natl. Acad. Sci. USA 101, 13147–131511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santelli, E., Bankston, L. A., Leppla, S. H. & Liddington, R. C. (2004) Nature 430, 905–908. [DOI] [PubMed] [Google Scholar]
- 35.Ratts, R., Zeng, H., Berg, E. A., Blue, C., McComb, M. E., Costello, C. E., vanderSpek, J. C. & Murphy, J. R. (2003) J. Cell Biol. 160, 1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]