Abstract
Phase separation in the cytoplasm is emerging as a major principle in intracellular organization. In this process, sets of macromolecules assemble themselves into liquid compartments that are distinct from the surrounding medium but are not delimited by membrane boundaries. Here, we discuss how phase separation, where a component of one of the two phases is vesicles rather than macromolecules, could underlie the formation of synaptic vesicle (SV) clusters in proximity of presynaptic sites. The organization of SVs into a liquid phase could explain how SVs remain tightly clustered without being stably bound to a scaffold so that they can be efficiently recruited to release site by active zone components.
In Brief
Milovanovic and De Camilli discuss how clustering of synaptic vesicles at synapses could be accounted by their forming a distinct liquid phase within the cytoplasm. This organization may explain how synaptic vesicles are tightly packed, yet mobile, within clusters.
INTRODUCTION
A defining characteristic of neuronal chemical synapses is the presence of tightly packed clusters of synaptic vesicles (SVs) anchored to the presynaptic plasma membrane (Figure 1). These clusters ensure availability of SVs during strong and prolonged synaptic activity, when the speed of SV recycling becomes rate limiting. Several molecular components of the matrix that crosslinks SVs to each other have been identified (De Camilli et al., 1983; Denker et al., 2011; Fernández-Busnadiego et al., 2010; Gundelfinger et al., 2016; Huttner et al., 1983; Südhof, 2012; Takamori et al., 2006; Wilhelm et al., 2014). However, how these proteins cluster SVs and yet allow for their translocation to the sites of release remains elusive.
Figure 1.

Synaptic vesicles cluster in the presynaptic nerve terminal of a mouse brain; scale bar 200 nm (unpublished image courtesy of Dr. Yumei Wu, Yale School of Medicine).
The view of the cytosol as an aqueous solution where components are either anchored to the cytoskeleton or freely diffuse is being strongly challenged. Several recent studies have provided fresh insight into how macromolecules can self-organize within the cytoplasm to form highly dynamic organelle-like liquid compartments with no membrane boundaries (Brangwynne et al., 2009; Hyman et al., 2014; Li et al., 2012). Both proteins and RNA-protein complexes can reversibly form distinct liquid phases in the cytosol. While molecules within such phases are linked to each other by polyvalent weak interactions, the highly dynamic nature of such interactions confer them fluid-like properties. In principle, even membranous organelles could assemble into liquid phases if connected by appropriate linker proteins. In this perspective we will first provide an overview of the characteristics of known liquid sub-compartments within cells. Then, we will discuss how the properties of SV clusters raise the possibility that they represent an example of phase separation.
Liquid-liquid phase separation of cellular components into membrane-less organelles
A phase of matter is a portion of space with homogenous composition and in a given state (solid, liquid or gaseous). Change of conditions (i.e. temperature, pressure, osmolarity) can lead to a change of state, a process called phase transition. An obvious example of phase transition is the evaporation of water upon heating – the switch from a liquid to a gaseous state. Phase separation, in contrast, is a process in which one or multiple components in the same state segregate from each other into distinct but homogenous compartments. The segregation of hydrophobic substances from an aqueous solution to generate droplets (such as oil in water) represents such an example. In polymer chemistry, well-studied examples of liquid-liquid phase separation includes demixing of dextran and polyethylene glycol (Helfrich et al., 2002; Li et al., 2008; Long et al., 2005).
Distinct membrane-less liquid phases in cells
In cells, compartmentalization of the cytoplasm via the membrane boundaries that define membranous organelles allows for the concomitant occurrence of chemical reactions that require different environments. However, several subcellular compartments where specialized biochemical processes occur are not membrane bound. These include for example the nucleolus (Boisvert et al., 2007) and the “stress granules” involved in storage of mRNAs and translation factors (Anderson and Kedersha, 2008). As shown by a flurry of papers published over the last few years, many such compartments represent examples of phase separation. The concept that macromolecules can undergo phase separation in the cytoplasm arose more than two decades ago (Walter and Brooks, 1995). However, only recently data is emerging indicating that many of these membrane-less compartments arise by liquid-liquid phase separation (Bergeron-Sandoval et al., 2016; Brangwynne et al., 2009; Hyman et al., 2014; Li et al., 2012) and explaining the underlying mechanisms with studies in living cells and cell-free systems.
Liquid phases generated by protein/protein and protein/RNA interactions
Multivalency is the key property that enables macromolecules to undergo liquid-liquid phase separation (Li et al., 2012). It is entropically expensive to bring many molecules together in a cluster. Hence this is possible only if the enthalpy gain through ionic- and polar-interactions is large enough. However, if such interactions are too strong, the result is a solid phase, which would not allow the occurrence of biochemical reactions. The assembly of a liquid phase, in contrast, involves an ensemble of weak interactions that are strong enough to keep the molecules together and yet allow for their high mobility.
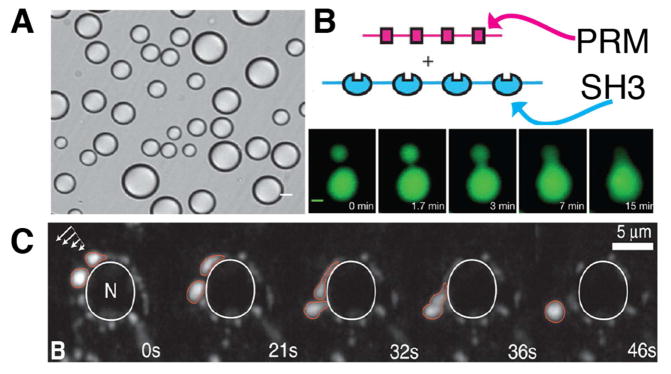
Many liquid phases are generated by interactions among proteins. Typically such phases involve folded modules (e.g. SH3, SH2, PDZ domains) and their ligands, i.e. proteins that contain short peptide sequences (such as proline-based motifs for SH3 domain) that fit the amino acid consensus for binding to such modules. Interactions, but not necessarily stoichiometric interactions, of these proteins with each other lead to dynamically cross-linked scaffolds that generate liquid droplets (Li et al., 2012). As in vitro experiments with purified proteins have shown, even just two multivalent proteins (for example, a short series of SH3 domains and of proline-based SH3 domain binding motifs) can self assemble into separate liquid droplets within an aqueous solution (Figure 2A,B). Multiple amino acid motifs with the potential to interact with protein modules are present in the cytoplasmic tails of a variety of plasma membrane proteins. This is a mechanism through which plasma membrane associated liquid-like phases can be assembled, such as in the case of actin polymerization in kidney podocytes (Banjade et al., 2015; Li et al., 2012) and of signaling scaffolds at the immunological synapse of T-cells (Su et al., 2016). Most recently, postsynaptic densities were also proposed to assemble at least in part via mechanisms typical of phase separation (Zeng et al., 2016).
Figure 2.
Liquid-liquid phase separation underlies the formation of membrane-less organelles. (A) In vitro reconstitutions of liquid-liquid phase separation of two engineered proteins (repeats of SH3 and cognate proline-rich motifs, PRM) that coalesce into droplets, as visualized by differential interference contrast microscopy. (B) Time-lapse imaging of two protein droplets that merge into a single mass; green: Oregon-green labeled SH3 repeats. (A and B are modified from Li et al., 2012). (C) Under shear street (white arrows: direction of induced shear flow) P granules (marked in red), which are RNA-protein complexes, drip along the nuclear envelope (marked in white) (modified from Brangwynne et al., 2009).
Presence of intrinsically disordered regions (IDRs), i.e. low amino acid complexity sequences that do not fold into any stable secondary or tertiary structure, is also a frequent characteristic of proteins responsible for the generation of liquid phases (Malinovska et al., 2013). Roughly a third of eukaryotic proteins contain IDR of different length (van der Lee et al., 2014). Amino acid consensus sequences for binding to protein-protein interaction modules are often found in these regions. In addition, IDRs can also interact with each other (Pak et al., 2016). For example, the sieve of the nuclear pores (Frey et al., 2006) and centrosomes were shown to be liquid phases (Zwicker et al., 2014) where IDRs are sufficient to induce the phase (Jiang et al., 2015). However, additional protein binding sites are necessary to recruit the components required for a functional centrosome and spindle pole assembly.
Liquid droplets can also be formed by protein-RNA interactions. In fact, the fluid nature of protein-RNA cohorts helped develop the concept of intracellular liquid droplets. Such liquid phases involve proteins with RNA-binding modules (e.g. RRM, KH domain, Zn-fingers) or basic amino acid patches (typically in IDRs) that bind RNA due to the negative charge of their diphosphate backbone (Elbaum-Garfinkle et al., 2015; Lin et al., 2015). RNA/protein droplets are involved in a range of processes such as embryonic development, response to stress and housekeeping RNA metabolism. For example, P granules, structures that appear during a single-cell stage of C. elegans development, are sparse throughout the cell. As development proceeds, P granules congregate and localize to the posterior size of the embryo, which helps to establish the asymmetric division and subsequent generation of germ cells (Schneider and Bowerman, 2003). Other membrane-less compartments resulting from protein/RNA interactions include nucleolus (Berry et al., 2015), the phase formed at the tail of RNA polymerase II during transcription (Kwon et al., 2013), stress granules (Wippich et al., 2013).
Liquid droplets can contain a variety of macromolecules (Fong et al., 2013). However, only some of them drive phase separation by their multivalent interactions (Lin et al., 2015). Other macromolecules can be transiently trapped within these droplets via specific interactions even though they are not necessary for their formation (Banani et al., 2016; Saha et al., 2016).
Dynamic nature of liquid phases
As shown by studies in living cells (for example RNA granules; Brangwynne et al., 2009; 2011) and cell-free systems (for example protein droplets; Li et al., 2012; Su et al., 2016), cytosolic phases generated by the mechanisms described above have the typical features expected for a liquid compartment. They acquire spherical shape as such shape minimizes the surface tension, unless hindered by adjacent structures or deformed by physical forces (i.e. they drip under sheer stress) (Figure 2A,C; Brangwynne et al., 2009). They can undergo fission and fusion. Upon fusion two liquid droplets will immediately collapse into each other to acquire a new larger spherical shape (Figure 2B). Macromolecules within the droplet are mobile and can exchange material with the surrounding cytosol (Brangwynne et al., 2009).
In some cases, mutations in proteins that can form liquid phases lead to the formation of amyloid-like aggregates that are the hallmark of some neurodegenerative diseases, such as amyotrophic lateral sclerosis, frontotemporal dementia, and inclusion body myopathy (Kim et al., 2013; Kwiatkowski et al., 2009; Neumann et al., 2006; Vance et al., 2009). Clearly, however, the molecular nature of membrane-less droplets and amyloid-like aggregates is fundamentally different as in amyloid-like aggregates the components are fibrillar and practically immobile (Lin et al., 2015; Molliex et al., 2015; Murakami et al., 2015; Patel et al., 2015). One potential explanation for the propensity of these protein to form aggregates is the high local concentration within droplets that may facilitate their re-organization into amyloid/fibril-like structures (Lee et al., 2016; Molliex et al., 2015).
Liquid-liquid phase separation undergoes regulation
Cellular liquid phases can be regulated by several mechanisms, such as posttranslational modifications and competition for binding between monovalent and multivalent macromolecules. Posttranslational modifications could either foster or inhibit liquid droplet formation and impact their stability once they are formed. For example, phosphorylation of tyrosine residue generates binding sites for SH2 domain, and thus trigger new interactions or increase the multivalency of a protein that acts as a key player in droplet formation (Li et al., 2012; Su et al., 2016). On the other hand, phosphorylation of serine/threonine residues within IDRs can disrupt interactions with other proteins or RNAs. Phosphorylation may decrease the overall positive charge of IDR, thus interfering with RNA binding or disrupt interactions of adjacent consensus sequences with for folded modules of partner proteins (e.g. SH3 domains) (Han et al., 2012; Kwon et al., 2013; 2014). Macromolecules that lack multivalency (i.e. contain only one binding site for a target molecule) could dissolve membrane-less droplet (Nott et al., 2015; Zhang et al., 2015). For instance, RNAs that contain a single protein binding sequence will deprive proteins from droplets: once the concentration of protein drops below a certain threshold, membrane-less droplets dissolve (Elbaum-Garfinkle et al., 2015).
SVs clusters fulfill criteria of phase separation
Clusters of SVs fit predicted properties of a liquid phase although they represent a phase in which one component of the phase are membrane vesicles rather than macromolecules.
SV clusters have homogeneous structures with sharp boundaries
SVs form very tight clusters, which have sharp boundaries despite the absence of a mechanic barrier that delimits them. Other organelles, including SV recycling intermediates such as clathrin coated pits and vesicles (Evergren et al., 2004; Ferguson et al., 2007; Milosevic et al., 2011) or endosome-like structures (Wu et al., 2014), tend to be segregated at the periphery of SV clusters. Only when the recycling process has generated a SV, the vesicle cluster will recapture this organelle.
SVs within clusters are mobile
In spite of being tightly clustered, SVs appear to be mobile within clusters, at least after stimulation. This speaks against their being anchored to a static scaffold. SV motility within clusters has been consistently suggested by the EM analysis of nerve terminals of the central and peripheral nervous system following endocytic labeling with extracellular tracers, such fluid phase markers and ligands of the luminal side of SV membranes. After both mild and strong stimuli, labeled SVs are always intermixed at random with unlabeled vesicles, i.e. vesicles that have not undergone fusion during the incubation with the tracer (Figure 4A–D; Ceccarelli et al., 1973; Harata et al., 2001; Heuser and Reese, 1973; Kraszewski et al., 1996; Rizzoli and Betz, 2004), while other labeled endocytic intermediates are localized at the edges of the cluster (Rizzoli and Betz, 2004; Wu et al., 2014). A random mobility of SVs within clusters was also observed during live imaging studies of SVs following labeling with FM1–43, a fluorescent endocytic tracer (Betz et al., 1992). To explain this finding, the occurrence of a cage surrounding SVs to prevent their dispersion had been hypothesized (Betz et al., 1992). SV clusters, however, disassemble slowly after cell lysis (De Camilli et al., 1983), speaking against the occurrence of a cage (whose disruption would result in rapid dispersion of vesicles) supporting the occurrence of a matrix that crosslinks them.
Figure 4.
Antibody-mediated perturbation of synapsin abolishes synaptic vesicle cluster.
(A and B) Synapses of the giant reticulospinal axon of the lamprey were microinjected with no antibody (A) or with anti-synapsin antibody (B), then stimulated at 1 Hz for 12 minutes, rested for 90 minutes and fixed. Scale bar 200 nm (image from Pieribone et al., 1995).
SVs within clusters can be exchanged with vesicles outside the clusters
Newly formed (or reformed) SVs are rapidly captured by the clusters. Likewise, SVs can escape a presynaptic cluster to be captured by another one, as exchange of SVs between neighboring synapses along the same axons has been reported (Darcy et al., 2006).
SV clusters can collapse into one another like two droplets
Live imaging of SVs labeled with fluorescent dyes revealed that coalescence of two SV clusters occurs with the pattern of liquid droplet fusion: two clusters merge rapidly into a single mass (Figure 1 and 4A), thus minimizing the interface area with the surrounding phase (Betz et al., 1992).
SVs are interconnected with each other by multivalent low affinity interactions of highly abundant proteins
As we discussed above, the potential for multivalent interactions is a critical feature of macromolecules that undergo phase separation. Several proteins with these properties are found in the matrix of SV clusters (Denker et al., 2011). These include proteins with low complexity amino acid regions, such as synuclein and synapsin, which are highly specific of synapses (Clayton and George, 1998; De Camilli et al., 1990; Südhof et al., 1989) and proteins with SH3 domains, such as intersectin, amphiphysin and endophilin (Evergren et al., 2004; Shupliakov, 2009; Winther et al., 2015). Some of these proteins are very abundant at synapses, implying that they may reach the critical concentration needed for phase separation. This is even more true if one considers that due to the abundance of SVs at presynaptic sites (Wilhelm et al., 2014), the effective concentration of macromolecules in this space is even higher than it would be in a cytoplasmic volume less densely populated by membranous organelles. For example, synapsin, a dimeric protein (Hosaka and Südhof, 1999) which is anchored to the lipid bilayer via its N-terminal region (Benfenati et al., 1989; Hosaka et al., 1999; Krabben et al., 2011) and binds a variety of pre-synaptically enriched SH3-domain proteins via SH3 binding motifs in its C-terminal region (domain D) (McPherson et al., 1994; Onofri et al., 2000; Winther et al., 2015), represents 9% of the total protein associated with SVs (De Camilli et al., 1990; Südhof et al., 1989). Domain D of synapsin 1, the most abundant of the three synapsins, is a low complexity, disordered region rich in Pro, Gly, Ser and Gln (Südhof et al., 1989). This region resembles other intrinsically disordered domains (such as FUS and hnRNPA1) (Molliex et al., 2015; Patel et al., 2015) that are seen as the hallmark of liquid-liquid phase separation. Thus, because of its molecular properties and of its abundance, synapsin 1 qualifies as a key player in SV phases.
In spite of its striking abundance on SVs, synapsin does not appear to be directly implicated in the docking or fusion of SVs with the presynaptic plasma membrane (Gitler et al., 2004; Pieribone et al., 1995; Rosahl et al., 1995), consistent with a role upstream of these events, such as a role in SV clustering. This role is further supported by several lines of evidence: First, nerve terminals of mice that lack all synapsins have a lower content of SVs and such vesicles are more disperse than at wild type synapses (Gitler et al., 2004). As clusters are not abolished, the action of synapsin is most likely overlapping with that of other proteins peripherally associated with their membranes, including synuclein (Vargas et al., 2017), Rab proteins and their interactors (Pavlos and Jahn, 2011). Second, anti-synapsin antibody injection in the giant axon of the lamprey results in dramatic loss of SV clusters (Figure 4A,B; Pieribone et al., 1995). Only the pool of vesicles more proximal to the active zones, most likely a pool cross-linked by other factors, remains. Third, synapsins are absent from the presynaptic sites of at least the majority of sensory cells, which lack axons and differ from bona-fide neurons in structural and functional properties (Kriegstein et al., 1999; Mandell et al., 1990). Presynaptic sites of these cells are not dispersed along multiple varicosities as in axons (an arrangement that requires a local SV clustering), but are localized either in cell somata (hair cells), or at the very end of short processes (retinal photoreceptors and bipolar cells) where SV accumulation can simply be the result of anterograde transport in the processes and where their further enrichment and docking at release sites is controlled by highly specialized structures, so-called ribbons (De Camilli, 1995; Matthews and Fuchs, 2010). Finally, synapsins are not present on SV membranes during the endocytic limb of the cycle, i.e. on the endocytic intermediates, such as clathrin coated vesicles, which are excluded from the clusters (Blondeau et al., 2004). How synapsin shedding and then re-binding is orchestrated during the SV cycle (perhaps through a change in the lipid composition of the bilayer) remains to be determined. It also remains unclear what is the physiological role of the ATPase module that represents the core of the synapsin molecule (Esser et al., 1998).
Assembly and disassembly of SVs within clusters can be controlled by phosphorylation/dephosphorylation of their components
As for liquid droplets described above, an important aspect of phase formation is the reversibility of this process. Blocking phosphatases by okadaic acid causes dispersion of synaptic vesicles clusters, both in cultured neurons and at the neuromuscular junction, whereas treatment with staurosporine, a protein kinase inhibitor, has the opposite effect (Henkel et al., 1996; Kraszewski et al., 1996). In fact, synapsin, because of its abundance in nerve terminals and prominent phosphorylation, was the first regulated synaptic phosphorprotein identified in brain (hence originally called Protein I; (De Camilli et al., 1979). Its phosphorylation/dephosphorylation at distinct sites impact its cross-linking properties (De Camilli et al., 1990; Hosaka et al., 1999). Conversely, when the SV cluster is exhausted, for example at dynamin deficient synapses, where the bulk of SV membranes is trapped in endocytotic intermediates that fail to undergo fission, synapsin is dispersed (Raimondi et al., 2011). The phosphorylation state of proteins in the clusters may also control the degree of motility (Chi et al., 2001), which may explain why SV clusters exhibit low motility at resting synapses (Gaffield et al., 2006; Kraszewski et al., 1996). Additionally, phosphorylation/dephosphorylation reactions may control the composition of the matrix connecting SVs. For example, it was reported that some endocytotic phosphoproteins are located within vesicle clusters at resting synapses, but are relocated to periactive zones to support endocytosis upon stimulation (e.g. amphiphysin, endophilin and intersectin) (Denker et al., 2011; Evergren et al., 2007; Shupliakov, 2009; Winther et al., 2015). Trapping of these proteins in the SV cluster phase may be a mechanism to ensure their high concentration near the sites of action.
Functional implications and relations of sv clusters to presynaptic active zones
The presence of a large reserve pool of SVs is critical for a reliable neurotransmission. While the basic operation of synapses can occur even upon dramatic reduction of SV clusters or of perturbation of the clusters, it is not surprising that the fidelity of synaptic transmission over a broad dynamic range requires a large reservoir of SVs (Pieribone et al., 1995; Raimondi et al., 2011). Having SV organized as a fluid phase rather than a rigid scaffold allows for their piecemeal recruitment at active zones without the need to dissociate them in bulk from the scaffold.
A fluid-like organization of SV clusters is not mutually exclusive with the occurrence of multiple pools of SVs. Vesicles more proximal to plasma membrane ‘active zones’ of secretion likely have additional interactions with the macromolecular complexes anchored to this membrane which structurally define this zones (Ackermann et al., 2015; Südhof, 2012). Several such proteins, for example piccolo and bassoon, have long, unfolded, filament-like domains that can extend for dozens of nm away from the plasma membrane (Gundelfinger et al., 2016). These proteins, which have binding sites for SVs or their associated proteins, may penetrate the SV phase and anchor subset of SVs to active zones. Supporting this scenario, even manipulations that drastically disrupt SV clusters do not completely deplete of SV the presynaptic active zone and some vesicles remain anchored to it (Pieribone et al., 1995). Conversely, clusters of SVs are still present at synapses upon deletion of active zone proteins, although SV docking is strongly reduced (Acuna et al., 2016; Wang et al., 2016). These observations support the existence of distinct but overlapping pools of SVs that are able to exchange vesicles between them.
The independence of SV clusters from a presynaptic active zone is illustrated by the occurrence of SV clusters in developing axons even before the formation of synapses. Small clusters of SVs which can be labeled by extracellular tracers, and thus undergo exo-endocytosis, can be observed in distal axonal regions even before contacts with a post-synaptic neuron is established (Kraszewski et al., 1995). These SV cohorts are highly motile and move in and out of the filopodia that explore the environment to encounter appropriate post-synaptic partners. Upon such encounter, large presynaptic SV clusters may originate from the coalescence of small clusters (Kraszewski et al., 1995). The occurrence of the early clusters may allow a small group of SVs to act as a functional unit and may facilitate the rapid assembly of synapses.
Finally, the independence of SV cluster formation from a presynaptic site is illustrated by the properties of SVs to organize themselves into clusters in cell bodies when the transport of synaptic vesicle precursors to axon endings is impaired both in C. elegans and in mammalian neurons (Hall and Hedgecock, 1991; Yonekawa et al., 1998).
Concluding remarks
We have discussed here how liquid phase separation principles could account for the known properties of SV clusters. As mentioned earlier in this perspective, a recent paper suggested that post-synaptic densities represent an example of phase separation in which a set of components is anchored to the postsynaptic plasma membrane (Zeng et al., 2016). Liquid phase properties of postsynaptic densities would explain how the stability of these structures is compatible with their dynamics. It would therefore appear that phase separation processes – to generate a signaling matrix postsynaptically and to cluster SVs presynaptically – are at the foundation of synapse formation and function. Even some aspects of presynaptic and postsynaptic plasma membrane protein clustering may be dependent on phase separation, as exemplified by the studies of plasma membrane proteins clustering at the immunological synapse (Su et al., 2016).
Much remains to be understood about SV clusters. What we discussed here allows formulation of testable hypothesis to further elucidate their structure and dynamics. In vitro reconstitution experiments with purified proteins and either SVs or artificial vesicles will be key to these investigations. For example, different outcome of these experiments are expected if formation of SV clusters occurs via phase separation rather than by binding to a scaffold. The independence of SV cluster formation from anchorage to a presynaptic site can be further tested in living neurons by manipulations that disrupt active zones and/or force ectopic accumulation of SVs and their associated proteins. Super-resolution microscopy of developing neurons and of intact or broken synapse preparations in wild type and genetically modified organisms will help assess the fluidity of vesicle clusters as expected for a liquid phase.
Beyond SV clusters, assembly into a fluid separated phase may apply to other membrane bound organelles and may underlie structures such as the cloud of Golgi vesicles, clusters of early endosomes and packages of viral particles, further generalizing the concept that fluid-fluid phase separation represents a major principle of intracellular organization.
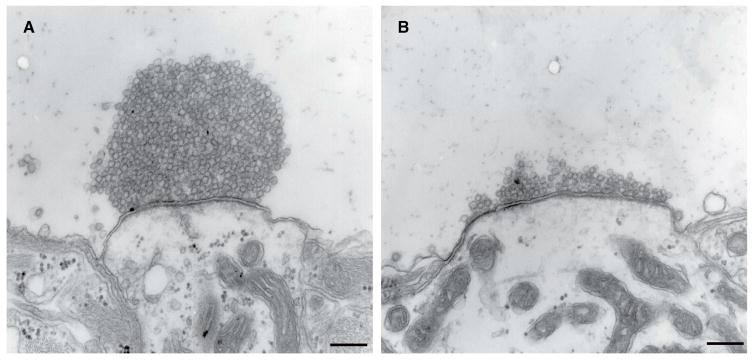
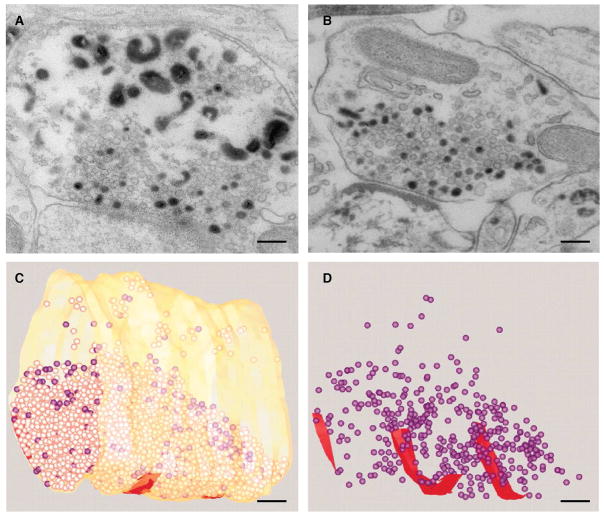
Figure 3.
Synaptic vesicles intermix within clusters and exclude other organelles.
(A and B) Mouse neurons in primary cultures were stimulated for 90 sec with high [K+] in the presence of extracellular horseradish peroxidase (HRP) and fixed after 3 (A) and 30 (B) minutes following HRP washout. Note in (A) the exclusion of large endocytic intermediates from SV clusters, and in both (A and B) the random intermixing of unlabeled SVs with recycled, HRP reaction product-positive vesicles. Scale bar 200 nm (images from Wu et al., 2014).
(C and D) 3D Reconstruction of a frog motor endplate stimulated at 30 Hz for 10 s in the presence of FM 1–43, then rested and fixed after 10 minutes. Synaptic vesicles positive for FM 1–43, as shown by photoconversion (purple) are randomly intermixed with unlabeled vesicles. Active zones are shown in red. Scale bar 200 nm (images from Rizzoli and Betz, 2004).
Acknowledgments
Authors have been supported by grants from the NIH (R37NS036251, DA018343) to P.D.C., and by the long-term postdoctoral fellowship from the Human Frontier Science Program to D.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann F, Waites CL, Garner CC. Presynaptic active zones in invertebrates and vertebrates. EMBO Rep. 2015;16:923–938. doi: 10.15252/embr.201540434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuna C, Liu X, Südhof TC. How to Make an Active Zone: Unexpected Universal Functional Redundancy between RIMs and RIM-BPs. Neuron. 2016;91:792–807. doi: 10.1016/j.neuron.2016.07.042. [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends in Biochem Sci. 2008;33:141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Banani SF, Rice AM, Peeples WB, Lin Y, Jain S, Parker R, Rosen MK. Compositional Control of Phase-Separated Cellular Bodies. Cell. 2016;166:651–663. doi: 10.1016/j.cell.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banjade S, Wu Q, Mittal A, Peeples WB, Pappu RV, Rosen MK. Conserved interdomain linker promotes phase separation of the multivalent adaptor protein Nck. Proc Nat Acad Sci USA. 2015;112:6426–35. doi: 10.1073/pnas.1508778112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati F, Greengard P, Brunner J, Bähler M. Electrostatic and hydrophobic interactions of synapsin I and synapsin I fragments with phospholipid bilayers. J Cell Biol. 1989;108:1851–1862. doi: 10.1083/jcb.108.5.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron-Sandoval LP, Safaee N, Michnick SW. Mechanisms and Consequences of Macromolecular Phase Separation. Cell. 2016;165:1067–1079. doi: 10.1016/j.cell.2016.05.026. [DOI] [PubMed] [Google Scholar]
- Berry J, Weber SC, Vaidya N, Haataja M, Brangwynne CP. RNA transcription modulates phase transition-driven nuclear body assembly. Proc Nat Acad Sci USA. 2015;112:5237–45. doi: 10.1073/pnas.1509317112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz WJ, Bewick GS, Ridge R. Intracellular Movements of Fluorescently Labeled Synaptic Vesicles in Frog Motor-Nerve Terminals During Nerve-Stimulation. Neuron. 1992;9:805–813. doi: 10.1016/0896-6273(92)90235-6. [DOI] [PubMed] [Google Scholar]
- Blondeau F, Ritter B, Allaire PD, Wasiak S, Girard M, Hussain NK, Angers A, Legendre-Guillemin V, Roy L, Boismenu D, Kearney RE, Bell AW, Bergeron JJM, McPherson PS. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc Natl Acad Sci USA. 2004;101:3833–3838. doi: 10.1073/pnas.0308186101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert FM, van Koningsbruggen S, Navascués J, Lamond AI. The multifunctional nucleolus. Nat Rev Mol Cell Biol. 2007;8:574–585. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Jülicher F, Hyman AA. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Mitchison TJ, Hyman AA. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Nat Acad Sci USA. 2011;108:4334–4339. doi: 10.1073/pnas.1017150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP, Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973;57:499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Greengard P, Ryan TA. Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci. 2001;4:1187–1193. doi: 10.1038/nn756. [DOI] [PubMed] [Google Scholar]
- Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249–254. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- Darcy KJ, Staras K, Collinson LM, Goda Y. Constitutive sharing of recycling synaptic vesicles between presynaptic boutons. Nat Neurosci. 2006;9:315–321. doi: 10.1038/nn1640. [DOI] [PubMed] [Google Scholar]
- De Camilli P. Neurotransmission. Keeping synapses up to speed Nature. 1995;375:450–451. doi: 10.1038/375450a0. [DOI] [PubMed] [Google Scholar]
- De Camilli P, Benfenati F, Valtorta F, Greengard P. The synapsins. Annu Rev Cell Biol. 1990;6:433–460. doi: 10.1146/annurev.cb.06.110190.002245. [DOI] [PubMed] [Google Scholar]
- De Camilli P, Harris SM, Huttner WB, Greengard P. Synapsin I (Protein I), a nerve terminal-specific phosphoprotein. II Its specific association with synaptic vesicles demonstrated by immunocytochemistry in agarose-embedded synaptosomes. J Cell Biol. 1983;96:1355–1373. doi: 10.1083/jcb.96.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P, Ueda T, Bloom FE, Battenberg E, Greengard P. Widespread distribution of protein I in the central and peripheral nervous systems. Proc Natl Acad Sci USA. 1979;76:5977–5981. doi: 10.1073/pnas.76.11.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker A, Kröhnert K, Bückers J, Neher E, Rizzoli SO. The reserve pool of synaptic vesicles acts as a buffer for proteins involved in synaptic vesicle recycling. Proc Nat Acad Sci USA. 2011;108:17183–17188. doi: 10.1073/pnas.1112690108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaum-Garfinkle S, Kim Y, Szczepaniak K, Chen CCH, Eckmann CR, Myong S, Brangwynne CP. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc Nat Acad Sci USA. 2015;112:7189–7194. doi: 10.1073/pnas.1504822112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser L, Wang CR, Hosaka M, Smagula CS, Südhof TC, Deisenhofer J. Synapsin I is structurally similar to ATP-utilizing enzymes. EMBO J. 1998;17:977–984. doi: 10.1093/emboj/17.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evergren E, Gad H, Walther K, Sundborger A, Tomilin N, Shupliakov O. Intersectin is a negative regulator of dynamin recruitment to the synaptic endocytic zone in the central synapse. J Neurosci. 2007;27:379–390. doi: 10.1523/JNEUROSCI.4683-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evergren E, Marcucci M, Tomilin N, Low P, Slepnev V, Andersson F, Gad H, Brodin L, De Camilli P, Shupliakov O. Amphiphysin is a component of clathrin coats formed during synaptic vesicle recycling at the lamprey giant synapse. Traffic. 2004;5:514–528. doi: 10.1111/j.1398-9219.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Brasnjo G, Hayashi M, Wölfel M, Collesi C, Giovedí S, Raimondi A, Gong LW, Ariel P, Paradise S, O’Toole E, Flavell R, Cremona O, Miesenböck G, Ryan TA, De Camilli P. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- Fernández-Busnadiego R, Zuber B, Maurer UE, Cyrklaff M, Baumeister W, Lucic V. Quantitative analysis of the native presynaptic cytomatrix by cryoelectron tomography. J Cell Biol. 2010;188:145–156. doi: 10.1083/jcb.200908082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong KW, Li Y, Wang W, Ma W, Li K, Qi RZ, Liu D, Songyang Z, Chen J. Whole-genome screening identifies proteins localized to distinct nuclear bodies. J Cell Biol. 2013;203:149–164. doi: 10.1083/jcb.201303145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Richter RP, Görlich D. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science. 2006;314:815–817. doi: 10.1126/science.1132516. [DOI] [PubMed] [Google Scholar]
- Gaffield MA, Rizzoli SO, Betz WJ. Mobility of synaptic vesicles in different pools in resting and stimulated frog motor nerve terminals. Neuron. 2006;51:317–325. doi: 10.1016/j.neuron.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Gitler D, Takagishi Y, Feng J, Ren Y, Rodriguiz RM, Wetsel WC, Greengard P, Augustine GJ. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J Neurosci. 2004;24:11368–11380. doi: 10.1523/JNEUROSCI.3795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundelfinger ED, Reissner C, Garner CC. Role of Bassoon and Piccolo in Assembly and Molecular Organization of the Active Zone. Front Synaptic Neurosci. 2016;7:19. doi: 10.3389/fnsyn.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DH, Hedgecock EM. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, Chen M, Xie Y, Allen J, Xiao G, McKnight SL. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell. 2012;149:768–779. doi: 10.1016/j.cell.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Harata N, Ryan TA, Smith SJ, Buchanan J, Tsien RW. Visualizing recycling synaptic vesicles in hippocampal neurons by FM 1–43 photoconversion. Proc Natl Acad Sci USA. 2001;98:12748–12753. doi: 10.1073/pnas.171442798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich MR, Mangeney-Slavin LK, Long MS, Djoko KY, Keating CD. Aqueous phase separation in giant vesicles. J Am Chem Soc. 2002;124:13374–13375. doi: 10.1021/ja028157+. [DOI] [PubMed] [Google Scholar]
- Henkel AW, Simpson LL, Ridge RM, Betz WJ. Synaptic vesicle movements monitored by fluorescence recovery after photobleaching in nerve terminals stained with FM1–43. J Neurosci. 1996;16:3960–3967. doi: 10.1523/JNEUROSCI.16-12-03960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka M, Hammer RE, Südhof TC. A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron. 1999;24:377–387. doi: 10.1016/s0896-6273(00)80851-x. [DOI] [PubMed] [Google Scholar]
- Hosaka M, Südhof TC. Homo- and heterodimerization of synapsins. J Biol Chem. 1999;274:16747–16753. doi: 10.1074/jbc.274.24.16747. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, Weber CA, Jülicher F. Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol. 2014;30:39–58. doi: 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang S, Huang Y, He X, Cui H, Zhu X, Zheng Y. Phase transition of spindle-associated protein regulate spindle apparatus assembly. Cell. 2015;163:108–122. doi: 10.1016/j.cell.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, Diaz Z, MacLea KS, Freibaum B, Li S, Molliex A, Kanagaraj AP, Carter R, Boylan KB, Wojtas AM, Rademakers R, Pinkus JL, Greenberg SA, Trojanowski JQ, Traynor BJ, Smith BN, Topp S, Gkazi AS, Miller J, Shaw CE, Kottlors M, Kirschner J, Pestronk A, Li YR, Ford AF, Gitler AD, Benatar M, King OD, Kimonis VE, Ross ED, Weihl CC, Shorter J, Taylor JP. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495:467–473. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabben L, Fassio A, Bhatia VK, Pechstein A, Onofri F, Fadda M, Messa M, Rao Y, Shupliakov O, Stamou D, Benfenati F, Haucke V. Synapsin I senses membrane curvature by an amphipathic lipid packing sensor motif. J Neurosci. 2011;31:18149–18154. doi: 10.1523/JNEUROSCI.4345-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraszewski K, Daniell L, Mundigl O, De Camilli P. Mobility of synaptic vesicles in nerve endings monitored by recovery from photobleaching of synaptic vesicle-associated fluorescence. J Neurosci. 1996;16:5905–5913. doi: 10.1523/JNEUROSCI.16-19-05905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraszewski K, Mundigl O, Daniell L, Verderio C, Matteoli M, De Camilli P. Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J Neurosci. 1995;15:4328–4342. doi: 10.1523/JNEUROSCI.15-06-04328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Kriegstein K, Schmitz F, Link E, Südhof TC. Distribution of synaptic vesicle proteins in the mammalian retina identifies obligatory and facultative components of ribbon synapses. Eur J Neurosci. 1999;11:1335–1348. doi: 10.1046/j.1460-9568.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Kwon I, Kato M, Xiang S, Wu L, Theodoropoulos P, Mirzaei H, Han T, Xie S, Corden JL, McKnight SL. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell. 2013;155:1049–1060. doi: 10.1016/j.cell.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon I, Xiang S, Kato M, Wu L, Theodoropoulos P, Wang T, Kim J, Yun J, Xie Y, McKnight SL. Poly-dipeptides encoded by the C9orf72 repeats bind nucleoli, impede RNA biogenesis, and kill cells. Science. 2014;345:1139–1145. doi: 10.1126/science.1254917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Zhang P, Kim HJ, Mitrea DM, Sarkar M, Freibaum BD, Cika J, Coughlin M, Messing J, Molliex A, Maxwell BA, Kim NC, Temirov J, Moore J, Kolaitis RM, Shaw TI, Bai B, Peng J, Kriwacki RW, Taylor JP. C9orf72 Dipeptide Repeats Impair the Assembly, Dynamics, and Function of Membrane-Less Organelles. Cell. 2016;167:774–788. e17. doi: 10.1016/j.cell.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Banjade S, Cheng HC, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, Russo PS, Jiang QX, Nixon BT, Rosen MK. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483:336–340. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lipowsky R, Dimova R. Transition from complete to partial wetting within membrane compartments. J Am Chem Soc. 2008;130:12252–12253. doi: 10.1021/ja8048496. [DOI] [PubMed] [Google Scholar]
- Lin Y, Protter DSW, Rosen MK, Parker R. Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol Cell. 2015;60:208–219. doi: 10.1016/j.molcel.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MS, Jones CD, Helfrich MR, Mangeney-Slavin LK, Keating CD. Dynamic microcompartmentation in synthetic cells. Proc Natl Acad Sci USA. 2005;102:5920–5925. doi: 10.1073/pnas.0409333102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinovska L, Kroschwald S, Alberti S. Protein disorder, prion propensities, and self-organizing macromolecular collectives. Biochim Biophys Acta. 2013;1834:918–931. doi: 10.1016/j.bbapap.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Mandell JW, Townes-Anderson E, Czernik AJ, Cameron R, Greengard P, De Camilli P. Synapsins in the vertebrate retina: absence from ribbon synapses and heterogeneous distribution among conventional synapses. Neuron. 1990;5:19–33. doi: 10.1016/0896-6273(90)90030-j. [DOI] [PubMed] [Google Scholar]
- Matthews G, Fuchs P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat Rev Neurosci. 2010;11:812–822. doi: 10.1038/nrn2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson PS, Czernik AJ, Chilcote TJ, Onofri F, Benfenati F, Greengard P, Schlessinger J, De Camilli P. Interaction of Grb2 via its Src homology 3 domains with synaptic proteins including synapsin I. Proc Natl Acad Sci USA. 1994;91:6486–6490. doi: 10.1073/pnas.91.14.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic I, Giovedí S, Lou X, Raimondi A, Collesi C, Shen H, Paradise S, O’Toole E, Ferguson S, Cremona O, De Camilli P. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron. 2011;72:587–601. doi: 10.1016/j.neuron.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, Taylor JP. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 2015;163:123–133. doi: 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Qamar S, Lin JQ, Schierle GSK, Rees E, Miyashita A, Costa AR, Dodd RB, Chan FTS, Michel CH, Kronenberg-Versteeg D, Li Y, Yang SP, Wakutani Y, Meadows W, Ferry RR, Dong L, Tartaglia GG, Favrin G, Lin WL, Dickson DW, Zhen M, Ron D, Schmitt-Ulms G, Fraser PE, Shneider NA, Holt C, Vendruscolo M, Kaminski CF, St George-Hyslop P. ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function. Neuron. 2015;88:678–690. doi: 10.1016/j.neuron.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VMY. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, Craggs TD, Bazett-Jones DP, Pawson T, Forman-Kay JD, Baldwin AJ. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol Cell. 2015;57:936–947. doi: 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofri F, Giovedi S, Kao HT, Valtorta F, Bongiorno Borbone L, De Camilli P, Greengard P, Benfenati F. Specificity of the binding of synapsin I to Src homology 3 domains. J Biol Chem. 2000;275:29857–29867. doi: 10.1074/jbc.M006018200. [DOI] [PubMed] [Google Scholar]
- Pak CW, Kosno M, Holehouse AS, Padrick SB, Mittal A, Ali R, Yunus AA, Liu DR, Pappu RV, Rosen MK. Sequence Determinants of Intracellular Phase Separation by Complex Coacervation of a Disordered Protein. Mol Cell. 2016;63:72–85. doi: 10.1016/j.molcel.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, Pozniakovski A, Poser I, Maghelli N, Royer LA, Weigert M, Myers EW, Grill S, Drechsel D, Hyman AA, Alberti S. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell. 2015;162:1066–1077. doi: 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Pavlos NJ, Jahn R. Distinct yet overlapping roles of Rab GTPases on synaptic vesicles. Small GTPases. 2011;2:77–81. doi: 10.4161/sgtp.2.2.15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernik AJ, Greengard P. Distinct pools of synaptic vesicles in neurotransmitter release. Nature. 1995;375:493–497. doi: 10.1038/375493a0. [DOI] [PubMed] [Google Scholar]
- Raimondi A, Ferguson SM, Lou X, Armbruster M, Paradise S, Giovedí S, Messa M, Kono N, Takasaki J, Cappello V, O’Toole E, Ryan TA, De Camilli P. Overlapping role of dynamin isoforms in synaptic vesicle endocytosis. Neuron. 2011;70:1100–1114. doi: 10.1016/j.neuron.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. The structural organization of the readily releasable pool of synaptic vesicles. Science. 2004;303:2037–2039. doi: 10.1126/science.1094682. [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Südhof TC. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Saha S, Weber CA, Nousch M, Adame-Arana O, Hoege C, Hein MY, Osborne-Nishimura E, Mahamid J, Jahnel M, Jawerth L, Pozniakovski A, Eckmann CR, Jülicher F, Hyman AA. Polar Positioning of Phase-Separated Liquid Compartments in Cells Regulated by an mRNA Competition Mechanism. Cell. 2016;166:1572–1584. e16. doi: 10.1016/j.cell.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SQ, Bowerman B. Cell polarity and the cytoskeleton in the Caenorhabditis elegans zygote. Annu Rev Genet. 2003;37:221–249. doi: 10.1146/annurev.genet.37.110801.142443. [DOI] [PubMed] [Google Scholar]
- Shupliakov O. The synaptic vesicle cluster: a source of endocytic proteins during neurotransmitter release. Neuroscience. 2009;158:204–210. doi: 10.1016/j.neuroscience.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Su X, Ditlev JA, Hui E, Xing W, Banjade S, Okrut J, King DS, Taunton J, Rosen MK, Vale RD. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science. 2016;352:595–599. doi: 10.1126/science.aad9964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC, Czernik AJ, Kao HT, Takei K, Johnston PA, Horiuchi A, Kanazir SD, Wagner MA, Perin MS, De Camilli P. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science. 1989;245:1474–1480. doi: 10.1126/science.2506642. [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- van der Lee R, Buljan M, Lang B, Weatheritt RJ, Daughdrill GW, Dunker AK, Fuxreiter M, Gough J, Gsponer J, Jones DT, Kim PM, Kriwacki RW, Oldfield CJ, Pappu RV, Tompa P, Uversky VN, Wright PE, Babu MM. Classification of intrinsically disordered regions and proteins. Chem Rev. 2014;114:6589–6631. doi: 10.1021/cr400525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, Miller CC, Shaw CE. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas KJ, Schrod N, Davis T, Fernández-Busnadiego R, Taguchi YV, Laugks U, Lucic V, Chandra SS. Synucleins Have Multiple Effects on Presynaptic Architecture. Cell Rep. 2017;18:161–173. doi: 10.1016/j.celrep.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Brooks DE. Phase separation in cytoplasm, due to macromolecular crowding, is the basis for microcompartmentation. FEBS L. 1995;361:135–139. doi: 10.1016/0014-5793(95)00159-7. [DOI] [PubMed] [Google Scholar]
- Wang SSH, Held RG, Wong MY, Liu C, Karakhanyan A, Kaeser PS. Fusion Competent Synaptic Vesicles Persist upon Active Zone Disruption and Loss of Vesicle Docking. Neuron. 2016;91:777–791. doi: 10.1016/j.neuron.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm BG, Mandad S, Truckenbrodt S, Kröhnert K, Schäfer C, Rammner B, Koo SJ, Claβen GA, Krauss M, Haucke V, Urlaub H, Rizzoli SO. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science. 2014;344:1023–1028. doi: 10.1126/science.1252884. [DOI] [PubMed] [Google Scholar]
- Winther ÅME, Vorontsova O, Rees KA, Näreoja T, Sopova E, Jiao W, Shupliakov O. An Endocytic Scaffolding Protein together with Synapsin Regulates Synaptic Vesicle Clustering in the Drosophila Neuromuscular Junction. J Neurosci. 2015;35:14756–14770. doi: 10.1523/JNEUROSCI.1675-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wippich F, Bodenmiller B, Trajkovska MG, Wanka S, Aebersold R, Pelkmans L. Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell. 2013;152:791–805. doi: 10.1016/j.cell.2013.01.033. [DOI] [PubMed] [Google Scholar]
- Wu Y, O’Toole ET, Girard M, Ritter B, Messa M, Liu X, McPherson PS, Ferguson SM, De Camilli P. A dynamin 1-, dynamin 3- and clathrin-independent pathway of synaptic vesicle recycling mediated by bulk endocytosis. Elife. 2014;3:e01621. doi: 10.7554/eLife.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekawa Y, Harada A, Okada Y, Funakoshi T, Kanai Y, Takei Y, Terada S, Noda T, Hirokawa N. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J Cell Biol. 1998;141:431–441. doi: 10.1083/jcb.141.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M, Shang Y, Araki Y, Guo T, Huganir RL, Zhang M. Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity. Cell. 2016;166:1163–1175. e12. doi: 10.1016/j.cell.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Elbaum-Garfinkle S, Langdon EM, Taylor N, Occhipinti P, Bridges AA, Brangwynne CP, Gladfelter AS. RNA Controls PolyQ Protein Phase Transitions. Mol Cell. 2015;60:220–230. doi: 10.1016/j.molcel.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker D, Decker M, Jaensch S, Hyman AA, Jülicher F. Centrosomes are autocatalytic droplets of pericentriolar material organized by centrioles. Proc Nat Acad Sci USA. 2014;111:2636–45. doi: 10.1073/pnas.1404855111. [DOI] [PMC free article] [PubMed] [Google Scholar]