Abstract
Saturated aza-heterocycles are highly privileged building blocks that are commonly encountered in bioactive compounds and approved therapeutic agents. These N-heterocycles are also incorporated as chiral auxiliaries and ligands in asymmetric synthesis. As such, development of methods to functionalize the α-methylene C–H bonds of these systems enantioselectively is of great importance, especially in drug discovery. Currently, enantioselective lithiation with (–)-sparteine followed by Pd(0) catalyzed cross coupling to prepare α-arylated amines is largely limited to pyrrolidines. Here we report a Pd(II)-catalyzed enantioselective α-C–H coupling of a wide range of amines, including ethyl amines, azetidines, pyrrolidines, piperidines, azepanes, indolines, and tetrahydroisoquinolines. Chiral phosphoric acids are demonstrated as effective anionic ligands for the enantioselective coupling of methylene C–H bonds with aryl boronic acids. This catalytic reaction not only affords high enantioselectivities, but also provides exclusive regioselectivity in the presence of two methylene groups in different steric environments.
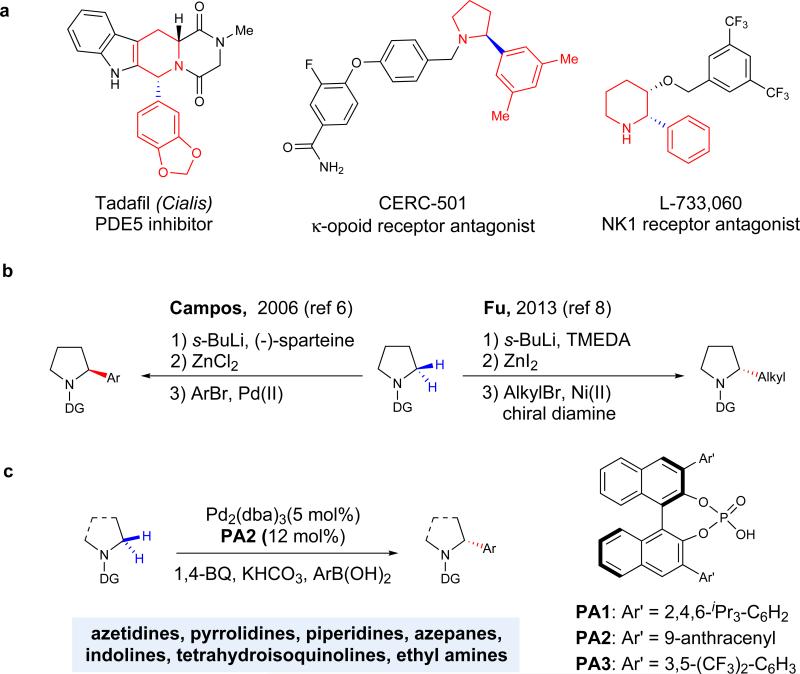
An aryl group at the α-position of an amine is frequently encountered in drug molecules (Fig. 1a) and auxiliaries for asymmetric transformations, with the stereochemistry at that position having a significant impact on the compound's bioactivity or ability to promote asymmetric induction. Forging a carbon–carbon bond asymmetrically at this position is a long-standing challenge in organometallic chemistry1-4. In his pioneering work, Beak reported the carbamate directed asymmetric α-lithiation of N-heterocycles using a chiral diamine, (–)-sparteine, and subsequent trapping with electrophiles5. In 2006, Campos et al. at Merck combined (–)-sparteine-mediated enantioselective α-lithiation with subsequent transmetallation with ZnCl2, followed by Negishi coupling with aryl bromides to provide the first protocol for an enantioselective α-arylation of pyrrolidines6. Catalytic dynamic resolution of the α-lithiated piperidine allowed for the extension of this methodology towards asymmetric synthesis of α-aryl piperidines7. In an interesting development, Fu and coworkers found that a combination of racemic α-lithiation with a Ni-catalyzed enantioconvergent alkyl cross coupling with alkyl halides gave access to chiral α-alkyl pyrrolidines8. Though significant progress in employing stoichiometric metallation at the α-position of amines to access enantioenriched α-arylated products has been made in the past decade, asymmetric arylation through these approaches is still limited to pyrrolidines and piperidines3-9.
Figure 1. Examples of important chiral α-arylated cyclic amines and approaches for the construction of α-stereocenters.
a, Numerous drug molecules, such as Cialis, CERC-501, and L-733,060, contain non-racemic α-arylated cyclic amines. These structures are ubiquitous throughout biological systems, where the orientation of the α –substituent can have a dramatic effect on the activity of the resulting product. b, Traditional approaches for asymmetric arylation and alkylation of pyrrolidines proceed via α-lithiation to install aryl6 and alkyl8 substituents. While effective for pyrrolidine substrates, the procedures are far less effective for larger ring systems or those with sensitive functional groups. c, In this work, enantioselective α-C(sp3)–H coupling of amines is carried out in the presence of a chiral phosphate anion, which permits the arylation of a diverse array of aliphatic amines through the use of a thioamide directing group.
Recently, we developed a Pd(II)-catalyzed coupling of the α-methylene C–H bonds of pyrrolidines, piperidines and azepanes with aryl boronic acids using a thioamide directing group derived from pivalic acid10. The importance of chiral aza-heterocycles prompted us to develop a methodology that could provide non-racemic products with the potential to extend this approach to a broad range of amines. While Pd-catalyzed enantioselective functionalizations of C(sp3)–H bonds via desymmetrization of two different carbon centers have recently gained momentum11-16, enantioselective α-arylation of aza-heterocycles would require the differentiation of methylene C–H bonds on the same carbon center. We began our investigations by extensively screening previously developed chiral mono-protected amino acid (MPAA) ligands11,12 in presence of Pd(OTFA)2 as the palladium source, but did not obtain any desired arylation products. This result is consistent with our previous unsuccessful attempts to utilize MPAA ligands to achieve enantioselective activation of methylene C–H bonds despite success of these ligands with desymmetrization of cyclobutyl C–H bonds at two different carbon centers.11 The superior reactivity of Pd(OTFA)2 compared to a lack of reactivity with Pd(OAc)2 or Pd(II)/MPAA indicates the significance of anionic ligands in this reaction. Thus, we directed our attention towards chiral anionic phosphates as ligands. Since the seminal work by Akiyama17 and Terada18, phosphoric acids (PA) derived from a 3,3’-biaryl-substituted binapthol core have found a wide variety of applications in asymmetric transformations as Lewis and Brønsted acid catalysts19. The combination of chiral phosphoric acids with transition metal catalysis has also been explored19-24. The concept of merging gold catalysis with chiral phosphates as counterions was established by Toste in an asymmetric hydroalkoxylation reaction of double bonds20. List demonstrated the use of palladium with chiral phosphate anion in Tsuji-Trost allylation and Overman rearrangement reactions to obtain products in a highly enantioselective manner21,22. Rainey and Gong have achieved enantioselective allylic C–H functionalizations via the formation of π-allylic complexes bound to chiral phosphoric acids23,24. However, Pd-catalyzed asymmetric insertion into prochiral C–H bonds using chiral phosphoric acids as anionic ligands has been met with limited success. In particular, prochiral sp2 C–H bonds (60:40 er) or benzylic C–H bonds (83:17 er) were effectively arylated when pyridine or a bidentate amide-quinoline are employed as directing groups25,26. In light of the significance of developing a catalytic, enantioselective method for functionalizing saturated aza-heterocycles, we set out to test whether chiral phosphoric acid ligands can influence the stereoselectivity of metal insertion into prochiral methylene C–H bonds in a cross-coupling reaction via Pd(II)/Pd(0) catalytic cycle.
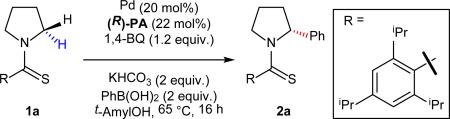
Preliminary attempts to achieve enantioinduction for α-C–H arylation of pyrrolidine commenced with an evaluation of a number of readily available chiral phosphoric acid ligands under our previously developed conditions for thioamide-directed α-arylation.10 These initial trials did not yield any rate enhancement or enantiocontrol, presumably due to the background reaction catalyzed by Pd(OTFA)2. We then turned to {PdCl(η3-C3H5)}2, as it is known to form a [Pd(allyl)-PA] complex27 which we hypothesized would enable higher reactivity than palladium allyl chloride, thus minimizing the contribution of the background reaction. After replacement of the Pd source, we found that, under these conditions, the thioamide-directed C–H coupling with aryl boronic acid via a Pd(II)/Pd(0) catalytic manifold is enabled by chiral phosphoric acid PA1 to give the desired product in 36% yield with noticeable enantioselectivity (55:45 er) (see Table 1 in the Supplementary Information). To improve the enantioselectivity, we proceeded to modify the directing group in an attempt to fine tune the steric environment of the substrate. It is important to note that a thioamide directing group is essential for this transformation to proceed, as the corresponding amides show no reactivity. Presumably both the electronic property and the size of sulfur atom are crucial for the formation of the highly strained fused ring system in the C–H activation step. A bulky triisopropylbenzothioamide on the pyrrolidine served to be the most suitable in terms of reactivity and enantiocontrol. This directing group can be easily installed in a single step by adding the amine to 2,4,6-triisopropylbenzothioyl chloride (see Supplementary Information for preparation). With this directing group on the substrate, BINOL phosphoric acids with various substitutions on 3,3’-positions were evaluated (Table 1). This study established PA2 as the most effective chiral ligand, affording the product in 52% yield and an encouraging 83:17 er. Interestingly, (R)-PA1 gave opposite isomer as the major product when compared to (R)-PA2 and (R)-PA3. Next, we decided to further investigate the role of the palladium source in an attempt to further increase the enantioselectivity (Table 1, entries 4-10). A significant effect on the reactivity and enantioselectivity was observed using different palladium sources with Pd2(dba)3 being the most efficient, giving the arylated pyrrolidine in 87% yield with 98:2 er. We currently hypothesize that the improvement in the er is due to the absence of an achiral anion in the system, as any Pd(II)X2 (X = Cl− or CF3CO2−) species may still provide some background reaction, thereby diminishing the er. The opposite isomer of the product (Table 2, 2a′) could be obtained with similar enantioselectivity when the (S)-isomer of PA2 was used. The need for the use of two equivalents of PhB(OH)2 is due to the observed homocoupling, which leads to biaryl formation.
Table 1.
Examination of ligands and palladium sources.
Yields were determined by 1H NMR analysis using CH2Br2 as an internal standard. Enantiomeric ratios (er) were determined by chiral high-performance liquid chromatography. The absolute configuration of 2a was determined by comparison with commercially available (R)-2-phenylpyrrolidine.
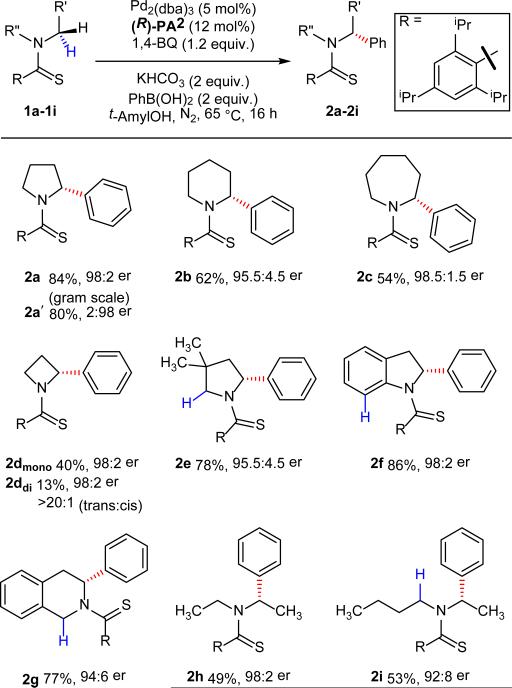
Table 2.
Scope of amines for palladium-catalyzed asymmetric α-C(sp3)–H coupling.
The values under each structure indicate isolated yields. Enantiomeric ratios (er) were determined by chiral high-performance liquid chromatography. 2a′ was obtained with (S)-PA2. Compound 2b was obtained using five equivalents of 1,4-BQ, three equivalents of phenyl boronic acid at 85 °C. Compounds 2c, 2h and 2i were obtained using two equivalents of 1,4-BQ.
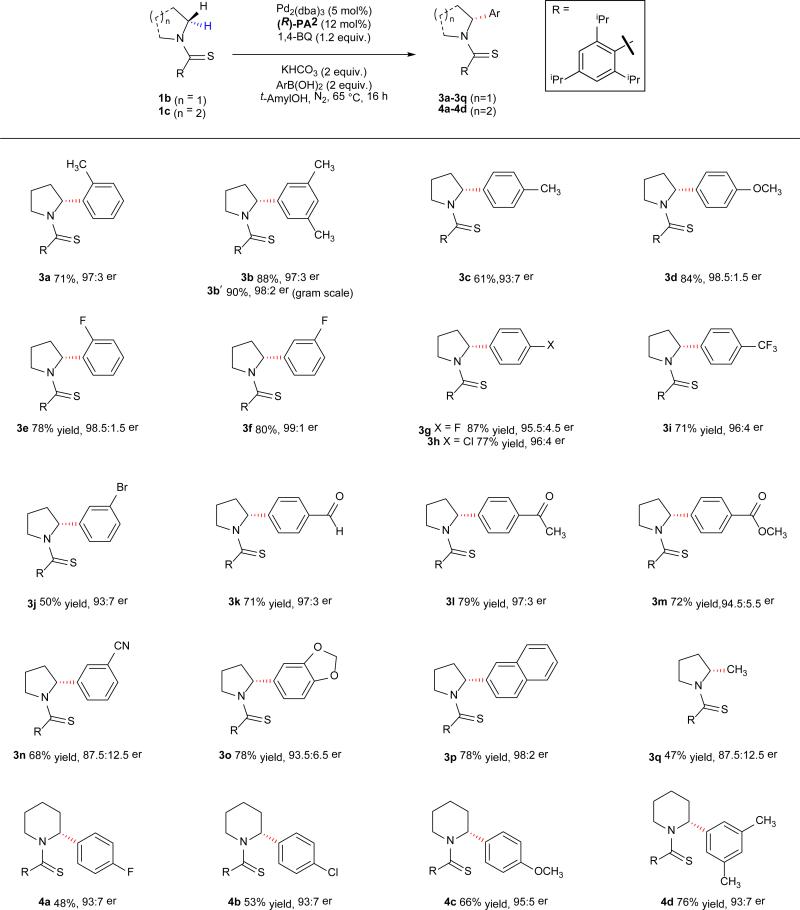
With the optimized protocol developed, we attempted to generate enantioenriched α-aryl piperidines9,28,29, which have thus far proven to be much more challenging substrates for α-lithiation when compared to pyrrolidine substrates9,29. Under the standard reaction conditions, the piperidine substrate gave only 24% conversion with 93:7 er. Increasing the equivalents of 1,4-benzoquinone (from one to five) and increasing the temperature (from 65 °C to 85 °C) improved the conversion to 70% with a slight loss in enantioselectivity (92:8 er). Finally, increasing the reaction concentration from 0.05 M to 0.2 M enabled the formation of the arylated products with 95.5:4.5 er (Table 2, 2b). Encouraged by the success of this methodology with pyrrolidine and piperidine substrates, we explored the potential of this catalytic system for the enantioselective arylation of other classes of amines. Arylation of the seven-membered ring (azepane) gave arylated product 2c in 54% yield and 98.5:1.5 er. Lithiation of azetidine and subsequent trapping with various electrophiles has been reported earlier with good enantioselectivity for methylation (91:9 er)30,31. However, C–H arylation of azetidine has not been demonstrated with either α-lithiation or our previous C–H activation protocol10. Importantly, the methodology disclosed herein allowed the first enantioselective synthesis of α-aryl azetidine starting from the parent azetidine derivative allowing for the synthesis of desired product 2dmono in excellent enantioselectivity (98:2 er). It was interesting to note that 1d also formed the transdiarylated product 2ddi with high enantio- and diastereocontrol (>20:1). Reaction with substituted pyrrolidines also proceeded smoothly, with C–H activation occurring exclusively on the carbon that was more distant from the substituents on the ring (2e). Submission of indoline as the substrate gave highly enantioenriched (98:2 er) arylated product 2f in 86% yield by selectively coupling at C-2 position. It is surprising that the sp2 C–H bond at the C-7 position remains intact. While tetrahydroquinoline gave low yield (20%), tetrahydroisoqinoline is arylated exclusively at the C-3 position in good yield and excellent enantioselectivity (2g). It is worth noting that under lithiation conditions, the functionalization will occur at the more acidic C-1 position32. Considering the broad utility of acyclic chiral amines in organic synthesis, we attempted asymmetric C–H arylation of N,N-diethyl- and N,N-ethylbutyl-amines. Both reactions proceeded to give synthetically useful yields and excellent enantioselectivity. Intriguingly, C–H arylation of 1i occurred regioselectively at the methylene C–H bond of the ethyl group, demonstrating the ability of this catalyst to distinguish C–H bonds in different steric environments.
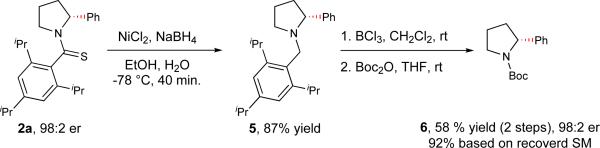
The scope of aryl coupling partners was also examined. Electron rich and electron withdrawing substituents at the ortho-, meta-, and para-position of the aryl ring were well-tolerated under the reaction conditions. Halogens on the aryl ring (-F, -Cl, -Br) were compatible under the reaction conditions (3e-3h, 3j), which could be of use in medicinal chemistry efforts where derivatization of the halogen may provide enantioenriched analogs. A variety of chemical functionalities on the aryl group were also compatible including a trifluoromethyl (3i), free aldehyde (3k), ketone (3l), ester (3m) and an ether (3o) functional group. Interestingly, the reaction worked efficiently even in the presence of a nitrile group on the coupling partner (3n), albeit with lower enantioselectivity (87.5:12.5 er). The significant erosion of enantioselectivity could be due to the undesired coordination of the nitrile with the Pd(II) catalyst. We were also able to carry out methylation in 47% yield with 87.5:12.5 er (3q), indicating the potential of this approach to provide a general method for the enantioselective alkylation of saturated N-heterocycles. The origin for the reduction of enantioselectivity in this alkylation reaction remains to be elucidated. However, pyridinylboronic acids as coupling partners showed no product formation under the reaction conditions. A few coupling partners were also tested on the piperidine substrate to obtain enantioenriched products 4a-4d in good yields and enantioselectivity. Next, we demonstrated the removal of the directing group by reduction of 2a in presence of NiCl2/NaBH4, followed by N-debenzylation with boron trichloride with complete preservation of the enantiomeric ratio (Figure 2). While finding milder conditions for removing the directing group is possible, we are currently engaged in the development of simpler amide directing groups for this enantioselective reaction instead. Meanwhile, we have also demonstrated the synthesis of 2a and 3b′ on the gram scale. Compound 3b′ was deprotected to furnish the free amine, which can be easily converted to CERC 501 (Figure 1, a) in one step33 (see page 44 in the Supplementary Information). Overall this development fills a major methodology gap by providing a uniform approach to the enantioselective C–H arylation of a wide range of saturated aza-heterocycles and alkyl amines. The efficiency of chiral anionic ligands in promoting Pd-catalyzed enantioselective activation of prochiral methylene C–H bonds on the same carbon is also fully demonstrated.
Figure 2. Removal of the thioamide directing group.
The directing group can be removed in two steps. The first step involves the reduction of 2a in presence of NiCl2 and NaBH4 to afford the dethiolated product 5. The second step involves the debenzylation of 5 in presence of BCl3 to afford the free amine, followed by Boc protection to give 6 in 58% yield and 98:2 er.
Methods
General procedure for the enantioselective arylation
To a reaction vial with a magnetic stir bar was added thioamide substrate (0.2 mmol), potassium bicarbonate (40 mg, 0.4 mmol,), arylboronic acid (0.4 mmol, unless otherwise noted), 1,4-benzoquinone (23.8 mg, 0.2 mmol, unless otherwise noted), PA2 (16.8 mg, 0.024 mmol), Pd2(dba)3 (9.15 mg, 0.01 mmol). The reaction tube was then connected to vacuum and backfilled with nitrogen three times. Then 1 ml of anhydrous 2-methyl-2-butanol was added and the mixture was stirred rapidly at 65 °C for 16 hours. The reaction was then cooled to room temperature and the mixture was passed through a pad of celite. After the celite was washed with ethyl acetate, the combined organic layers were concentrated under vacuum. The crude residue was purified by preparative TLC with ethyl acetate and hexanes (1:20) as solvent to obtain the pure product. Full experimental details and characterization of compounds can be found in the Supplementary Information.
Supplementary Material
Table 3.
Coupling partner scope with pyrrolidine and piperidine substrates.
The values under each structure indicate isolated yields. Enantiomeric ratios (er) were determined by chiral high-performance liquid chromatography. Compounds 3e-3i, 3k-n were obtained using two equivalents of 1,4-BQ. Compound 3q was obtained by using Na2CO3 as the base, twelve equivalents of methylboronic acid, at 80 °C. Compounds 4a-4d were obtained using five equivalents of 1,4-BQ, three equivalents of aryl boronic acid at 85 °C.
Acknowledgements
We gratefully acknowledge The Scripps Research Institute, the NIH (NIGMS, 2R01GM084019) for their financial support.
Footnotes
Author Contributions P.J. developed the enantioselective arylation reaction. P.V. and G.X. expanded the substrate scope. J.-Q.Y. conceived and supervised the project. J.-Q.Y. and P.J. wrote the manuscript.
Author Information The authors declare no competing financial interests. Readers are welcome to comment on the online version of this article.
Supplementary Information is available in the online version of the paper.
References
- 1.Royer J. Asymmetric Synthesis of Nitrogen Heterocycles. Wiley-VCH; Weinheim, Germany: 2009. [Google Scholar]
- 2.Nugent TC. Chiral Amine Synthesis: Methods, Developments and Applications. Wiley VCH; Weinheim, Germany: 2010. [Google Scholar]
- 3.Campos KR. Direct sp3 C–H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev. 2007;36:1069–1084. doi: 10.1039/b607547a. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BUW. Direct α-functionalization of saturated cyclic amines. Chem. Eur. J. 2012;18:10092–10142. doi: 10.1002/chem.201201539. [DOI] [PubMed] [Google Scholar]
- 5.Beak P, Kerrick ST, Wu S, Chu J. Complex induced proximity effects: enantioselective syntheses based on asymmetric deprotonations of N-Boc-pyrrolidines. J. Am. Chem. Soc. 1994;116:3231–3239. [Google Scholar]
- 6.Campos KR, Klapars A, Waldman JH, Dormer PG, Chen C-Y. Enantioselective, palladium-catalyzed α-arylation of N-Boc-pyrrolidine. J. Am. Chem. Soc. 2006;128:3538–3539. doi: 10.1021/ja0605265. [DOI] [PubMed] [Google Scholar]
- 7.Beng TK, Gawley RE. Application of catalytic dynamic resolution of N-Boc-2-lithiopiperidine to the asymmetric synthesis of 2-aryl and 2-vinyl piperidines. Org. Lett. 2011;13:394–397. doi: 10.1021/ol102682r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordier CJ, Lundgren RJ, Fu GC. Enantioconvergent cross-couplings of racemic alkylmetal reagents with unactivated secondary alkyl electrophiles: catalytic asymmetric Negishi α–alkylations of N-Boc-pyrrolidine. J. Am. Chem. Soc. 2013;135:10946–10949. doi: 10.1021/ja4054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stead D, et al. Asymmetric deprotonation of N-Boc piperidine: react IR monitoring and mechanistic aspects. J. Am. Chem. Soc. 2010;132:7260–7261. doi: 10.1021/ja102043e. [DOI] [PubMed] [Google Scholar]
- 10.Spangler JE, Kobayashi Y, Verma P, Wang D-H, Yu J-Q. α–Arylation of saturated azacycles and N-methylamines via palladium(II)-catalyzed C(sp3)–H coupling. J. Am. Chem. Soc. 2015;137:11876–11879. doi: 10.1021/jacs.5b06740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao K-J, Lin DW, Miura M, Zhu R-Y, Gong W, Wasa M, Yu J-Q. Palladium(II)-catalyzed enantioselective C(sp3)–H activation using a chiral hydroxamic acid ligand. J. Am. Chem. Soc. 2014;136:8138–8142. doi: 10.1021/ja504196j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan KSL, Fu H-Y, Yu J-Q. Palladium(II)-catalyzed highly enantioselective C–H arylation of cyclopropylmethylamines. J. Am. Chem. Soc. 2015;136:2042–2046. doi: 10.1021/ja512529e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakanishi M, Katayev D, Besnard C, Kündig EP. Fused indolines by palladium-catalyzed asymmetric C–C coupling involving an unactivated methylene group. Angew. Chem. Int. Ed. 2011;50:7438–7441. doi: 10.1002/anie.201102639. [DOI] [PubMed] [Google Scholar]
- 14.Anas S, Cordi A, Kagan HB. Enantioselective synthesis of 2-methyl indolines by palladium catalysed asymmetric C(sp3)–H activation/cyclisation. Chem. Comm. 2011;47:11483–11485. doi: 10.1039/c1cc14292e. [DOI] [PubMed] [Google Scholar]
- 15.Martin N, Pierre C, Davi M, Jazzar R, Baudoin O. Diastereo- and enantioselective intramolecular C(sp3)–H arylation for the synthesis of fused cyclopentanes. Chem. Eur. J. 2012;18:4480–4484. doi: 10.1002/chem.201200018. [DOI] [PubMed] [Google Scholar]
- 16.Saget T, Lemouzy S, Cramer N. Chiral monodentate phosphines and bulky carboxylic acids: cooperative effects in palladium-catalyzed enantioselective C(sp3)–H functionalization. Angew. Chem. Int. Ed. 2012;51:2238–2242. doi: 10.1002/anie.201108511. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama T, Itoh J, Yokota K, Fuchibe K. Enantioselective Mannich-type reaction catalyzed by a chiral Bronsted acid. Angew. Chem. Int. Ed. 2004;43:1566–1568. doi: 10.1002/anie.200353240. [DOI] [PubMed] [Google Scholar]
- 18.Uraguchi D, Terada M. Chiral Bronsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 2004;126:5356–5357. doi: 10.1021/ja0491533. [DOI] [PubMed] [Google Scholar]
- 19.Parmar D, Sugiono E, Raja S, Rueping M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 2014;114:9047–9153. doi: 10.1021/cr5001496. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton GL, Kang EJ, Mba M, Toste FD. A powerful chiral counterion strategy for asymmetric transition metal catalysis. Science. 2007;317:496–499. doi: 10.1126/science.1145229. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee S, List B. Chiral counteranions in asymmetric transition-metal catalysis: highly enantioselective Pd/Brønsted acid-catalyzed direct α-allylation of aldehydes. J. Am. Chem. Soc. 2007;129:11336–11337. doi: 10.1021/ja074678r. [DOI] [PubMed] [Google Scholar]
- 22.Jiang G, Halder R, Fang Y, List B. A highly enantioselective Overman rearrangement through asymmetric counteranion-directed palladium catalysis. Angew. Chem. Int. Ed. 2011;50:9752–9755. doi: 10.1002/anie.201103843. [DOI] [PubMed] [Google Scholar]
- 23.Chai Z, Rainey TJ. Pd(II)/Brønsted acid catalyzed enantioselective allylic C−H activation for the synthesis of spirocyclic rings. J. Am. Chem. Soc. 2012;134:3615–3618. doi: 10.1021/ja2102407. [DOI] [PubMed] [Google Scholar]
- 24.Wang P-S, Lin H-C, Zhai Y-J, Han Z-Y, Gong L-Z. Chiral counteranion strategy for asymmetric oxidative C(sp3)–H/C(sp3)–H coupling: enantioselective α-allylation of aldehydes with terminal alkenes. Angew. Chem. Int. Ed. 2014;53:11218–11221. doi: 10.1002/anie.201408199. [DOI] [PubMed] [Google Scholar]
- 25.Engle KM, Yu J-Q. Developing ligands for palladium(II)-catalyzed C–H functionalization: intimate dialogue between ligand and substrate. J. Org. Chem. 2013;78:8927–8955. doi: 10.1021/jo400159y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan S-B, Zhang S, Duan W-L. Palladium-catalyzed asymmetric arylation of C(sp3)–H bonds of aliphatic amides: controlling enantioselectivity using chiral phosphoric amides/acids. Org. Lett. 2015;17:2458–2461. doi: 10.1021/acs.orglett.5b00968. [DOI] [PubMed] [Google Scholar]
- 27.Zhang D, et al. Enantioselective palladium(II) phosphate catalyzed three-component reactions of pyrrole, diazoesters, and imines. Angew. Chem. Int. Ed. 2013;52:13356–13360. doi: 10.1002/anie.201306824. [DOI] [PubMed] [Google Scholar]
- 28.Ding J, Rybak T, Hall DG. Synthesis of chiral heterocycles by ligand-controlled regiodivergent and enantiospecific Suzuki Miyaura cross-coupling. Nat. Commun. 2014;5:5474. doi: 10.1038/ncomms6474. [DOI] [PubMed] [Google Scholar]
- 29.Bailey WF, Beak P, Kerrick ST, Ma S, Wiberg KB. An experimental and computational investigation of the enantioselective deprotonation of Boc-piperidine. J. Am. Chem. Soc. 2002;124:1889–1896. doi: 10.1021/ja012169y. [DOI] [PubMed] [Google Scholar]
- 30.Hodgson D, Kloesges J. Lithiation-electrophilic substitution of N-thiopivaloylazetidine. Angew. Chem. Int. Ed. 2010;49:2900–2903. doi: 10.1002/anie.201000058. [DOI] [PubMed] [Google Scholar]
- 31.Hodgson D, Mortimer CL, McKenna JM. Amine Protection/α-Activation with the tert-Butoxythiocarbonyl Group: Application to Azetidine Lithiation–Electrophilic Substitution. Org. Lett. 2015;17:330–333. doi: 10.1021/ol503441d. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Leonori D, Sheikh NS, Coldham I. Synthesis of 1-substituted tetrahydroisoquinolines by lithiation and electrophilic quenching guided by in situ IR and NMR spectroscopy and application to the synthesis of salsolidine, carnegine and laudanosine. Chem. Eur. J. 2013;19:7724–7730. doi: 10.1002/chem.201301096. [DOI] [PubMed] [Google Scholar]
- 33.Mitch C, et al H. Discovery of aminobenzyloxyarylamides as κ opioid receptor selective antagonists: application to preclinical development of a κ opioid receptor antagonist receptor occupancy tracer. J. Med. Chem. 2011;54:8000–8012. doi: 10.1021/jm200789r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.