Abstract
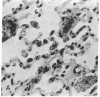
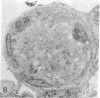
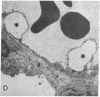
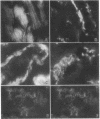
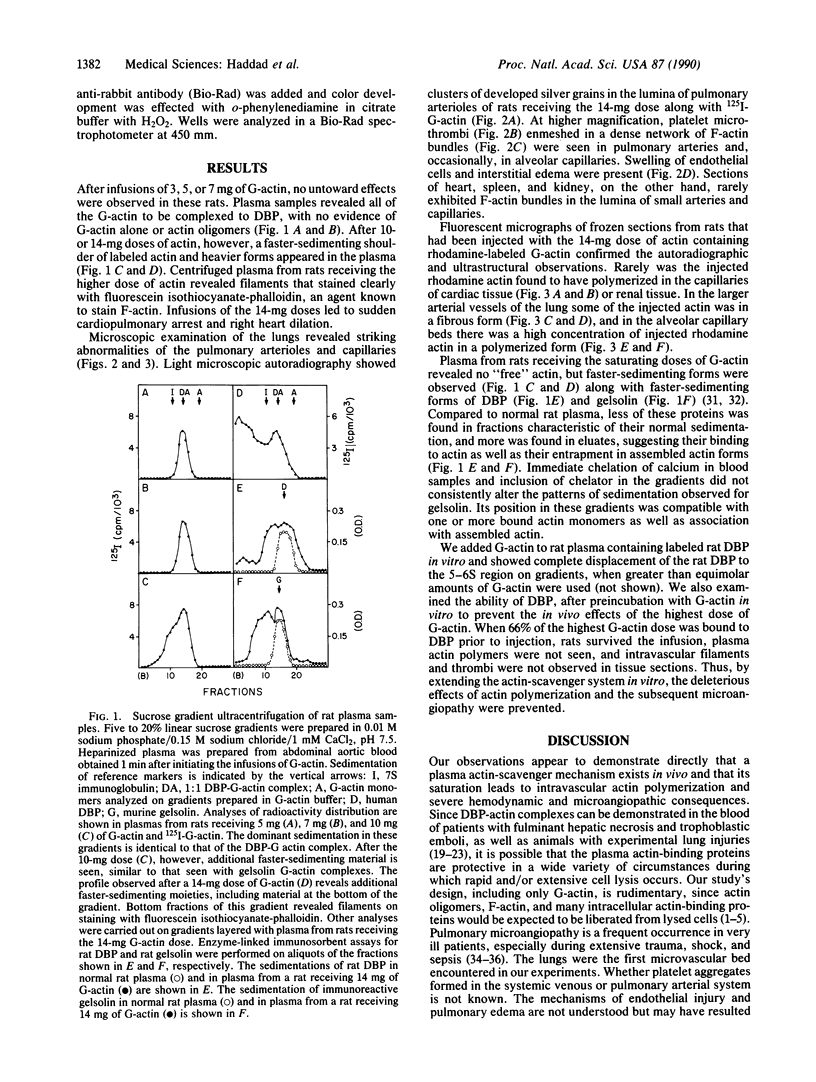
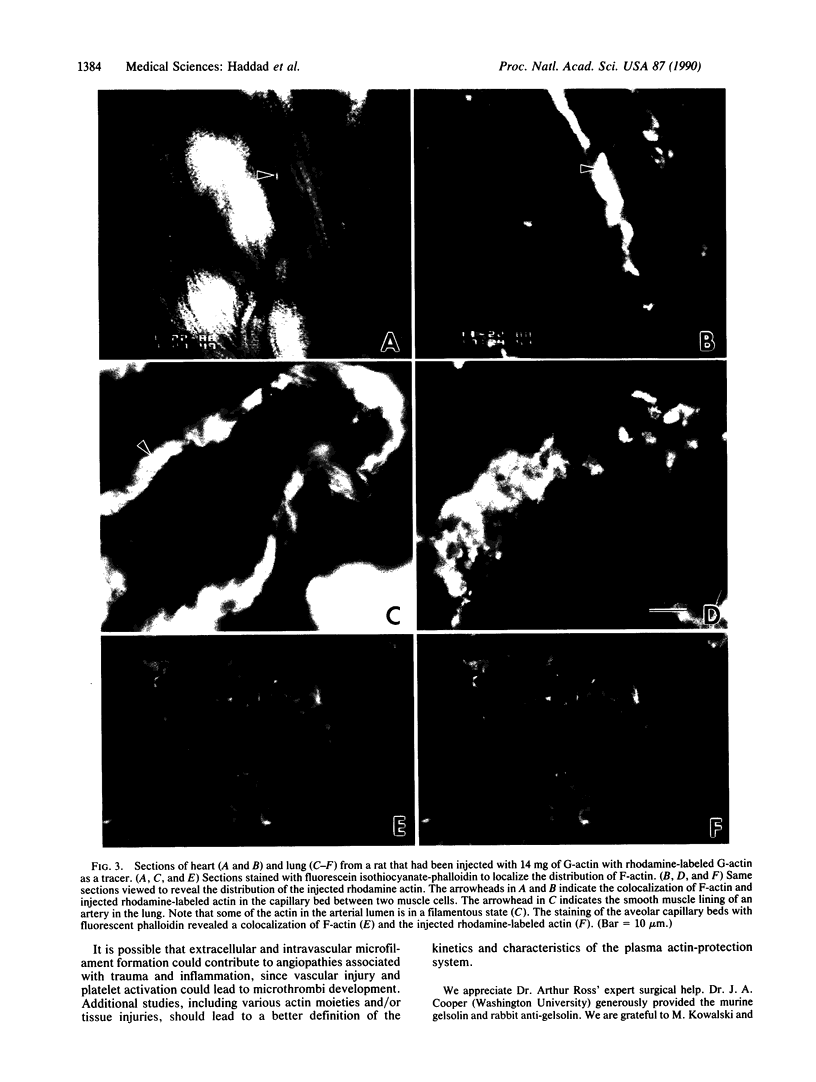
Two plasma proteins, vitamin D-binding protein (actin monomer sequestrant) and gelsolin (actin polymer severing), have been found in association with actin in plasma from ill humans and during experimental injury. In vitro, these are the only plasma proteins that display a high affinity for actin. We infused increasing amounts of globular actin intravenously to rats to evaluate its disposition in plasma and tissues. Intravascular filament formation, microthrombi, and endothelial injury were observed, especially in the pulmonary circulation. These pathological changes were not observed when the globular actin in the infusate had been preincubated with the vitamin D-binding protein in vitro. Complexes of actin with both proteins were found in the plasma, suggesting a saturable, plasma actin-binding system in vivo. Our findings suggest that in vivo saturation of these proteins' actin-binding capacities may serve as a paradigm for pulmonary vascular disorders seen during widespread tissue trauma and cell lysis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson J. F., Johns L. W., Pietra G. G. Initiation of lung cell proliferation by trypsin. Lab Invest. 1976 May;34(5):529–536. [PubMed] [Google Scholar]
- Brigham K. L. Metabolites of arachidonic acid in experimental lung vascular injury. Fed Proc. 1985 Jan;44(1 Pt 1):43–45. [PubMed] [Google Scholar]
- Bryan J. Gelsolin has three actin-binding sites. J Cell Biol. 1988 May;106(5):1553–1562. doi: 10.1083/jcb.106.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A. C., Quinn D. A., DeMarinis S. M., Beitz J. G., Zapol W. M. Platelet function in acute respiratory failure. Am J Hematol. 1987 Aug;25(4):377–388. doi: 10.1002/ajh.2830250404. [DOI] [PubMed] [Google Scholar]
- Chaponnier C., Borgia R., Rungger-Brändle E., Weil R., Gabbiani G. An actin-destabilizing factor is present in human plasma. Experientia. 1979 Aug 15;35(8):1039–1041. doi: 10.1007/BF01949928. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., David E. V. Serum vitamin D-binding protein is a third member of the albumin and alpha fetoprotein gene family. J Clin Invest. 1985 Dec;76(6):2420–2424. doi: 10.1172/JCI112256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke N. E., Walgate J., Haddad J. G., Jr Human serum binding protein for vitamin D and its metabolites. II. Specific, high affinity association with a protein in nucleated tissue. J Biol Chem. 1979 Jul 10;254(13):5965–5971. [PubMed] [Google Scholar]
- Cooper J. A., Bryan J., Schwab B., 3rd, Frieden C., Loftus D. J., Elson E. L. Microinjection of gelsolin into living cells. J Cell Biol. 1987 Mar;104(3):491–501. doi: 10.1083/jcb.104.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson D. L., Arnaud P., Galbraith R. M. Evidence of increased Gc:actin complexes in pregnant serum: a possible result of trophoblast embolism. Am J Reprod Immunol. 1983 Dec;4(4):185–189. doi: 10.1111/j.1600-0897.1983.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Van Alstyne E. L., Day J. R., Emerson D. L., Nel A. E., Lazarchick J., Galbraith R. M. Group-specific component (vitamin D binding protein) prevents the interaction between G-actin and profilin. Biochemistry. 1986 Oct 21;25(21):6467–6472. doi: 10.1021/bi00369a019. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Van Baelen H., Bouillon R., Shook T. E., Williams M. H., Nel A. E., Galbraith R. M. Role of group-specific component (vitamin D binding protein) in clearance of actin from the circulation in the rabbit. J Clin Invest. 1988 May;81(5):1519–1527. doi: 10.1172/JCI113484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad J. G., Fraser D. R., Lawson D. E. Vitamin D plasma binding protein. Turnover and fate in the rabbit. J Clin Invest. 1981 May;67(5):1550–1560. doi: 10.1172/JCI110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad J. G. Human serum binding protein for vitamin D and its metabolites (DBP): evidence that actin is the DBP binding component in human skeletal muscle. Arch Biochem Biophys. 1982 Feb;213(2):538–544. doi: 10.1016/0003-9861(82)90581-1. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Kowalski M. A., Sanger J. W. Actin affinity chromatography in the purification of human, avian and other mammalian plasma proteins binding vitamin D and its metabolites (Gc globulins). Biochem J. 1984 Mar 15;218(3):805–810. doi: 10.1042/bj2180805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper K. D., McLeod J. F., Kowalski M. A., Haddad J. G. Vitamin D binding protein sequesters monomeric actin in the circulation of the rat. J Clin Invest. 1987 May;79(5):1365–1370. doi: 10.1172/JCI112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. A., Schwartz J. H. Characterization of brevin, a serum protein that shortens actin filaments. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6798–6802. doi: 10.1073/pnas.78.11.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. E., Gooch J. An actin depolymerizing protein from pig plasma. FEBS Lett. 1981 Jan 12;123(1):49–53. doi: 10.1016/0014-5793(81)80017-8. [DOI] [PubMed] [Google Scholar]
- Harris H. E., Weeds A. G. Plasma actin depolymerizing factor has both calcium-dependent and calcium-independent effects on actin. Biochemistry. 1983 May 24;22(11):2728–2741. doi: 10.1021/bi00280a022. [DOI] [PubMed] [Google Scholar]
- Heffner J. E., Shoemaker S. A., Canham E. M., Patel M., McMurtry I. F., Morris H. G., Repine J. E. Acetyl glyceryl ether phosphorylcholine-stimulated human platelets cause pulmonary hypertension and edema in isolated rabbit lungs. Role of thromboxane A2. J Clin Invest. 1983 Feb;71(2):351–357. doi: 10.1172/JCI110776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P. A., Lind S. E. Capacity of human serum to depolymerize actin filaments. Blood. 1987 Aug;70(2):524–530. [PubMed] [Google Scholar]
- Janmey P. A., Lind S. E. Capacity of human serum to depolymerize actin filaments. Blood. 1987 Aug;70(2):524–530. [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P., Lind S. E. Sequential binding of actin monomers to plasma gelsolin and its inhibition by vitamin D-binding protein. Biochem Biophys Res Commun. 1986 Apr 14;136(1):72–79. doi: 10.1016/0006-291x(86)90878-8. [DOI] [PubMed] [Google Scholar]
- Jansson I., Lovén L., Rammer L., Lennquist S. Pulmonary trapping of platelets and fibrin after musculoskeletal trauma: an experimental model. J Trauma. 1985 Apr;25(4):288–298. doi: 10.1097/00005373-198504000-00002. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Janmey P. A., Mole J. E., Yin H. L. Isolation and properties of two actin-binding domains in gelsolin. J Biol Chem. 1985 Dec 5;260(28):15232–15238. [PubMed] [Google Scholar]
- Kwiatkowski D. J., Stossel T. P., Orkin S. H., Mole J. E., Colten H. R., Yin H. L. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature. 1986 Oct 2;323(6087):455–458. doi: 10.1038/323455a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laki K., Muszbek L. On the interaction of F-actin with fibrin. Biochim Biophys Acta. 1974 Dec 18;371(2):519–525. doi: 10.1016/0005-2795(74)90048-8. [DOI] [PubMed] [Google Scholar]
- Lee W. M., Emerson D. L., Werner P. A., Arnaud P., Goldschmidt-Clermont P., Galbraith R. M. Decreased serum group-specific component protein levels and complexes with actin in fulminant hepatic necrosis. Hepatology. 1985 Mar-Apr;5(2):271–275. doi: 10.1002/hep.1840050220. [DOI] [PubMed] [Google Scholar]
- Lees A., Haddad J. G., Lin S. Brevin and vitamin D binding protein: comparison of the effects of two serum proteins on actin assembly and disassembly. Biochemistry. 1984 Jun 19;23(13):3038–3047. doi: 10.1021/bi00308a030. [DOI] [PubMed] [Google Scholar]
- Lind S. E., Smith D. B., Janmey P. A., Stossel T. P. Depression of gelsolin levels and detection of gelsolin-actin complexes in plasma of patients with acute lung injury. Am Rev Respir Dis. 1988 Aug;138(2):429–434. doi: 10.1164/ajrccm/138.2.429. [DOI] [PubMed] [Google Scholar]
- Lind S. E., Smith D. B., Janmey P. A., Stossel T. P. Role of plasma gelsolin and the vitamin D-binding protein in clearing actin from the circulation. J Clin Invest. 1986 Sep;78(3):736–742. doi: 10.1172/JCI112634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Leod J. F., Kowalski M. A., Haddad J. G., Jr Interactions among serum vitamin D binding protein, monomeric actin, profilin, and profilactin. J Biol Chem. 1989 Jan 15;264(2):1260–1267. [PubMed] [Google Scholar]
- Miyachi Y., Vaitukaitis J. L., Nieschlag E., Lipsett M. B. Enzymatic radioiodination of gonadotropins. J Clin Endocrinol Metab. 1972 Jan;34(1):23–28. doi: 10.1210/jcem-34-1-23. [DOI] [PubMed] [Google Scholar]
- Norberg R., Thorstensson R., Utter G., Fagraeus A. F-Actin-depolymerizing activity of human serum. Eur J Biochem. 1979 Oct 15;100(2):575–583. doi: 10.1111/j.1432-1033.1979.tb04204.x. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Sanger J. W., Mittal B., Sanger J. M. Analysis of myofibrillar structure and assembly using fluorescently labeled contractile proteins. J Cell Biol. 1984 Mar;98(3):825–833. doi: 10.1083/jcb.98.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. C., Zapol W. M., Carvalho A. C. Platelet consumption and sequestration in severe acute respiratory failure. Am Rev Respir Dis. 1980 Sep;122(3):445–451. doi: 10.1164/arrd.1980.122.3.445. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Janmey P. A., Lind S. E. Circulating actin-gelsolin complexes following oleic acid-induced lung injury. Am J Pathol. 1988 Feb;130(2):261–267. [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Vaage J., Nicolaysen G., Waaler B. A. Aggregation fo blood platelets and increased hydraulic conductivity of pulmonary exchange vessels. Acta Physiol Scand. 1976 Oct;98(2):175–184. [PubMed] [Google Scholar]
- Van Baelen H., Bouillon R., De Moor P. Vitamin D-binding protein (Gc-globulin) binds actin. J Biol Chem. 1980 Mar 25;255(6):2270–2272. [PubMed] [Google Scholar]
- Vandekerckhove J. S., Sandoval I. V. Purification and characterization of a new mammalian serum protein with the ability to inhibit actin polymerization and promote depolymerization of actin filaments. Biochemistry. 1982 Aug 17;21(17):3983–3991. doi: 10.1021/bi00260a013. [DOI] [PubMed] [Google Scholar]
- Weeds A. Actin-binding proteins--regulators of cell architecture and motility. Nature. 1982 Apr 29;296(5860):811–816. doi: 10.1038/296811a0. [DOI] [PubMed] [Google Scholar]
- Yang F., Brune J. L., Naylor S. L., Cupples R. L., Naberhaus K. H., Bowman B. H. Human group-specific component (Gc) is a member of the albumin family. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7994–7998. doi: 10.1073/pnas.82.23.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Albrecht J. H., Fattoum A. Identification of gelsolin, a Ca2+-dependent regulatory protein of actin gel-sol transformation, and its intracellular distribution in a variety of cells and tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):901–906. doi: 10.1083/jcb.91.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. O., Goldschmidt-Clermont P. J., Emerson D. L., Lee W. M., Jollow D. J., Galbraith R. M. Correlation between extent of liver damage in fulminant hepatic necrosis and complexing of circulating group-specific component (vitamin D-binding protein). J Lab Clin Med. 1987 Jul;110(1):83–90. [PubMed] [Google Scholar]