Abstract
Retinal ganglion cell (RGC) death is the principal consequence of injury to the optic nerve. For several decades, we have understood that the RGC death process was executed by apoptosis, suggesting that there may be ways to therapeutically intervene in this cell death program and provide a more direct treatment to the cells and tissues affected in diseases like glaucoma. A major part of this endeavor has been to elucidate the molecular biological pathways active in RGCs from the point of axonal injury to the point of irreversible cell death. A major component of this process is the complex interaction of members of the BCL2 gene family. Three distinct family members of proteins orchestrate the most critical junction in the apoptotic program of RGCs, culminating in the activation of pro-apoptotic BAX. Once active, BAX causes irreparable damage to mitochondria, while precipitating downstream events that finish off a dying ganglion cell. This review is divided into two major parts. First, we summarize the extent of knowledge of how BCL2 gene family proteins interact to facilitate the activation and function of BAX. This area of investigation has rapidly changed over the last few years and has yielded a dramatically different mechanistic understanding of how the intrinsic apoptotic program is run in mammalian cells. Second, we provided a comprehensive analysis of nearly two decades of investigation of the role of BAX in the process of RGC death, much of which has provided many important insights into the overall pathophysiology of diseases like glaucoma.
Keywords: Glaucoma, Retinal ganglion cells, Intrinsic and extrinsic apoptosis, BCL2 gene family, BAX, BH3-only proteins, Mitochondrial outer membrane, Neuroinflammation, Secondary degeneration
1. Introduction
Retinal ganglion cells (RGCs) are long projection neurons that carry visual information from the retina to the brain. They are also the principal cell type affected in optic neuropathies, like glaucoma. Glaucoma is a major blinding disease, with an estimated 60 million people affected worldwide, or approximately 1 person in 40 over the age of 40 years old (Quigley, 2011; Quigley and Broman, 2006). The most relevant risk factor for this disease is an increase in intraocular pressure (IOP), which is suspected to increase strain on the optic nerve head, leading to changes that ultimately cause damage to the RGC axons as they exit through this structure. As a result, the only current treatment for glaucoma is to lower IOP, and while this is effective at slowing the progressive loss of RGCs, it is neither a cure, nor is it successful in a significant proportion of individuals. Research strategies in glaucoma have begun to focus on developing therapies that directly target the health of the affected RGCs. An effective RGC-directed therapy, combined with conventional IOP-lowering treatments, could provide substantially greater beneficial effects to preventing the progression of the disease. Two decades ago, several groups reported that RGCs die using a programmed cell death pathway called apoptosis, in response to both acute (i.e., axotomy) and chronic (experimental glaucoma) damage to the optic nerve (Berkelaar et al., 1994; Garcia-Valenzuela et al., 1994, 1995; Quigley et al., 1995). Subsequent to this, studies using genetically engineered mice demonstrated that this form of RGC apoptosis was executed principally using the intrinsic pathway, which involves mitochondrial dysfunction (Li et al., 2000). The realization that RGCs died using an intrinsic genetic program created the opportunity to directly target the biochemical pathways involved in the cell death process, and prevent RGC death (Almasieh et al., 2012). This initiated a flurry of studies aimed at providing protection to the RGCs (termed neuroprotection), which have ranged from blocking extracellular ion channels to the sustained application of neurotrophic factors (Galindo-Romero et al., 2013; Koeberle et al., 2010). While many of these studies have shown promise, the protective effects are universally transient. To date, only a single manipulation of RGCs, the deletion of the proapoptotic gene Bax in mice, has provided a virtually permanent blockade of the apoptotic pathway in RGCs after optic nerve damage (Li et al., 2000; Libby et al., 2005b; Semaan et al., 2010). The reason for this may stem from the role of the BAX protein acting as the principal regulator of mitochondrial involvement in the intrinsic apoptotic pathway, an event that is generally considered as the “point of no return” in the cell death process (Chang et al., 2002). As a consequence, the function of this protein acts as a bottle neck through which many different apoptotic pathways must pass through. Since it is likely that the activation of RGC death is a complex and redundant process in glaucoma, we have considered that therapeutic intervention targeting BAX function is the most provocative and effective strategy for neuroprotection.
Successfully targeting any biochemical process requires a detailed understanding of both the molecular mechanisms of the central protein, along with its upstream and downstream molecular players. BAX is a member of a larger gene family based on amino acid domain homologies found in the prototypical member, BCL2. In this review, the BCL2 gene family will be discussed in detail, including the history of its discovery, each of the three protein family members and how these members interact to regulate mitochondrial changes as part of the intrinsic apoptotic program. The mechanisms of BAX function will be discussed, including its activation, dimerization and oligomerization steps, and the consequences of this process on mitochondria and downstream apoptotic events. In this context, we will also discuss the pathophysiology of RGC death from time of injury until cellular phagocytosis. Mouse models used to study optic nerve damage will be used to highlight the BCL2 gene family functions in RGCs, including new data from our lab discussing BAX localization and timeline after optic nerve crush. Finally, we will discuss some of the quintessential findings from Bax-deficient mouse models, and their value as a tool to study RGC pathology.
2. The BCL2 gene family
A cell line derived from a patient with acute lymphoblastic leukemia was found to contain a t(14:18) chromosomal translocation (Pegoraro et al., 1984), which moved an immunoglobulin enhancer next to a gene located on chromosome 18 that was subsequently named BCL2 (B-cell lymphoma 2). This gene was quite unlike other oncogenes of the time because rather than promote cell proliferation (Tsujimoto et al., 1984), it promoted cell survival (Vaux et al., 1988). A series of studies showed that introduction of BCL2 into a wide variety of cells could block apoptotic-like forms of death (Korsmeyer, 1992). An important link between BCL2 protein function and apoptosis was further demonstrated by showing that the apoptosis-regulator ced-9 gene product from Caenorhabditis elegans could replace BCL2 function in mammalian cells (Hengartner and Horvitz, 1994). It wasn’t until 1993, when its first interacting partner was identified, BCL-2 associated X protein (BAX), that the true dynamic nature of the family began to unfold (Oltvai et al., 1993). Cell-free binding studies showed that these partners could form heterodimers, which helped propagate a theory supporting ratios of pro-death and pro-life proteins as a binary mechanism of apoptotic control (Oltvai et al., 1993). Other proapoptotic and anti-apoptotic proteins were discovered in the years to follow, but the family dynamic didn’t change again until the discovery of the BH3-only proteins, leading to the tripartite family we know today.
In the thirty years since the discovery of BCL2, many other family members have been described. All family members share one or more of 4 amino acid domains, known as BCL2 Homology (BH) domains. These proteins have been categorized into three distinct groups based on their function and how many BH domains they contain. Full length anti-apoptotic proteins, which contain all 4 BH domains, include BCL-2, BCL-XL, BCL-W, MCL-1, and A1. The full length pro-apoptotic proteins within the gene family include BAX, BAK and BOK. A third group, the BH3-only proteins, possessing varied levels of pro-apoptotic activity, contain only the BH3 domain, and includes BID, BIM, PUMA, NOXA, BAD, BMF, HRK, and BIK. BH3-only proteins are tasked as the first responders to signals of cellular stress, and attempt to initiate the intrinsic apoptotic pathway in one of two ways; by binding or neutralizing antiapoptotic BCL2 members or by directly activating BAX or BAK.
Every member of the gene family contains the BH3 domain, which is critical for intrafamilial protein interaction. A subset of the pro- and anti-apoptotic proteins (BAX (Suzuki et al., 2000), BAK (Moldoveanu et al., 2006), BCL-2 (Petros et al., 2001), BCL-X (Muchmore et al., 1996), BCL-W (Denisov et al., 2003)), along with BID (Chou et al., 1999; McDonnell et al., 1999), are made up of helical bundles that are more structurally similar than other members of the BCL2 family. These structural similarities among opposing family members may explain their ability to form dimers as well as interact with BH3-only proteins (Youle and Strasser, 2008). Predicted structures of the BH3-only proteins, with the exception of BID, show intrinsically unstructured proteins not resembling the other core BCL2 genes (Hinds et al., 2007).
As activators of the apoptotic ‘switch’ (pro-apoptotic proteins), BH3-only proteins each respond to specific distress signals from many different regions of the cell, whether it is cell detachment, extracellular death signals or DNA damage (Happo et al., 2012). BH3-only proteins can be transcriptionally or post-translationally regulated in order to become active in the apoptotic pathway. NOXA, PUMA, HRK and BIM are transcriptionally controlled after cellular stress (Puthalakath and Strasser, 2002). BIM, BAD and BMF are BH3-only proteins that are post-translationally regulated. Caspase 8 cleavage of BID creates the active form, truncated BID (tBID), which is also regulated by phosphorylation (Desagher et al., 2001; Li et al., 1998). BAD is also phosphorylated, which sequesters it in the cytosol and prevents its interaction with anti-apoptotic proteins. Dephosphorylation of BAD allows for interaction with BCL-X or BCL-2, and initiates its ‘effector’ state (Zha et al., 1996,1997). BMF is associated with dynein light chain and sequestered by myosin V, however with signals from cytoskeletal stress, BMF dissociates and binds anti-apoptotic proteins (Puthalakath et al., 2001).
2.1. BCL2 gene family protein interactions
The classic theory used to define BCL2 gene family protein interaction was the Rheostat Model, first described after heterodimerization between BAX and BCL-2 was discovered (Oltvai et al., 1993; Zamzami et al., 1998). This model describes a binary system where the ratio of BAX to BCL-2 determines cell fate. Living cells would have a higher concentration of BCL-2, which could bind and sequester free BAX molecules. An increase in the concentration of BAX would allow these molecules to form dimers and ‘tip the scale’ toward cell death. Although overexpression of BCL-2 does in fact prolong cell life (Vaux et al., 1988), simple overexpression of BAX does not accelerate cell death under steady state conditions (Semaan and Nickells, 2010). Evidence suggesting a more complex system than the Rheostat Model was first demonstrated in Richard Youle’s laboratory, which showed that heterodimers of anti- and pro-apoptotic proteins were likely an artifact of non-ionic detergents used in isolation studies (Hsu and Youle, 1997, 1998). Additionally, mutation studies on the mammalian BAX protein showed that it normally resides in a constitutively inactive state (George et al., 2007). Ultimately, the discovery of BH3-only proteins and other anti- and pro-apoptotic family members, revealed that the binary rheostat model for the BCL2 family no longer explained experimental outcomes, and a much more complex interaction model became evident. Nevertheless, some studies continue to provide experimental results in the context of the Rheostat Model.
Among the three BCL2 protein family members, each has the ability to interact with other members. The interaction of these family proteins likely evolved to create a system of checks and balances for the cell. Evolutionarily, a system that is not controlled simply by competing protein levels alone provides the opportunity for a cell to remedy the damaging stressors or to recover from an external insult, before it surrenders to the apoptotic pathway. It is only when these internal or external stressors fail to resolve, or the system is completely overwhelmed, that the pro-apoptotic family members persevere, and the cell initiates apoptosis.
BH3-only proteins possess the ability to interact with either of the other two members of the BCL2 subfamily, and upon interaction, function to sequester anti-apoptotic proteins or activate proapoptotic proteins (Gavathiotis et al., 2010; Willis et al., 2007). BH3-only proteins show preferential binding with their proteinprotein interaction partners (Kuwana et al., 2005). Some BH3-only proteins, like BIM, are direct activators of BAX, and have been shown to directly interact with BAX’s activation site (Gavathiotis et al., 2008). Other BH3-onlys, like BAD, are sometimes considered “sensitizers” for apoptosis progression because their direct interaction to BAX or BAK has not been documented, and instead they contribute by neutralizing anti-apoptotic proteins (Howells et al., 2011; Letai et al., 2002). BID, perhaps the most potent BH3-only protein, will both directly interact with BAX (Walensky et al., 2006), and also neutralize BCL-W (Wilson-Annan et al., 2003).
A caveat to the binding preferences of BCL-2 protein-protein interactions is that due to the homology among the BH3 domains within the family, BH3-only proteins have the ability to bind or interact with multiple partners. This allows non-preferred BH3-only proteins to compensate for others if stress signals derive from only one pathway. BIM and PUMA have a strong affinity for all anti-apoptotic proteins while BAD only strongly binds BCL-2, BCL-X and BCL-W, not MCL-1 or A1 (Chen et al., 2005). This phenomenon has also been demonstrated in BH3-only protein knockout cell lines or mice. Loss of one, two, or even three BH3-only proteins (Puma−/−Bid−/−/Bim−/− triple knockout mice) delayed the onset of apoptosis, but did not prevent BAX-dependent cell death (Harder and Libby, 2013). The same triple knockout mouse strain was shown to prevent apoptosis of thymocytes, suggesting that the BCL2 family proteins regulating apoptosis may be tissue specific (Ren et al., 2010). BH3-only proteins expressed in the retina and RGCs are discussed in section 4.4.
Anti-apoptotic proteins have multiple roles in the synergistic interplay of the BCL2 family genes, all which focus on cell survival. This family can bind and neutralize BH3-only proteins, but their principal function may be to antagonize BAX, acting as a chaperone to retro-translocate BAX from the mitochondrial outer membrane (MOM) (Edlich et al., 2011). Caveats to this function include the observation that only BCL-X, but not BCL-2, has been shown to retro-translocate BAX from the MOM (Edlich et al., 2011). Additionally, because BAK is already located at the MOM, only BAX has the potential to be retro-translocated. It may be no coincidence, then, that neurons, which only express a functional BAX protein (Jakobson et al., 2012; Uo et al., 2005) (see below), rely principally on the function of BCL-X (Deckwerth et al., 1996; Levin et al., 1997; Mosinger Ogilvie et al., 1998; White et al., 1998). Whether this retro-translocation occurs when BAX is inactive or active, remains to be determined. The concept of retro-translocation could have a significant impact on our understanding of BAX function. Currently, it is widely accepted that once BAX becomes active and forms dimers in the MOM, the cell cannot recover from these events. If the cell has an internal ability to retro-translocate BAX molecules in dimer form, understanding the mechanism of this process could be used to develop therapeutic strategies for mimicking BCL-X function.
Some autophagy related proteins not classically defined as BCL2 family members, have been reported to retain qualities of the BCL2 genes. Autophagy is a cell survival process that induces formation of autophagosomal compartments to engulf cellular debris and maintain cellular homeostasis. It is closely linked to the apoptotic pathway as an evolutionarily conserved mechanism to facilitate removal of the cell if it cannot clear excess debris. Anti-apoptotic proteins BCL-2 and BCL-X can bind and neutralize the autophagy regulator BECLIN-1 via its BH3 domain (Liang et al., 1998; Oberstein et al., 2007; Pattingre et al., 2005). The bound BECLIN-1-BCL-2 complex prevents BECLIN-1 dependent assembly of the preautophagosomal structure (Liang et al., 1998), and is considered the switch from autophagy to apoptosis (Marquez and Xu, 2012; Pattingre et al., 2005). Interestingly, the cleavage of BECLIN-1 or ATG5, (autophagy-related protein 5) by caspases can cause fragments of the proteins to become BH3-only apoptosis sensitizers (Kang et al., 2011; Yousefi et al., 2006). It is possible that other regulatory systems in the cell also contain proteins that can sensitize apoptosis through BCL2 gene family interaction.
Another candidate for cleavage-induced BH3-only sensitizers may be within the BCL2 gene family. In mouse neurons, the Bak gene is alternatively spliced to include a small 20 bp weak exon that normally resides in the 4th intron of the gene (Uo et al., 2005). This splice variant inserts a frame shift leading to a premature stop codon. Its confinement to neurons has led to the term N-Bak for the variant. Interestingly, the premature stop codon leads to a predicted protein that only contains the BAK BH3 domain. Whether or not N-BAK functions as a BH3-only protein is still in question. Forced expression of N-BAK in tissue culture cells promotes apoptosis (Sun et al., 2003; Uo et al., 2005), but endogenously, N-Bak transcripts are in low abundance, and may be translationally repressed through various mechanisms (Jakobson et al., 2012, 2013). Preliminary studies done by our group indicates that N-Bak/N-BAK transcripts are present in retinal neurons of mice and non-human primates.
Regulation of the intrinsic (mitochondrial) apoptotic program is controlled by the synergistic interaction of the three BCL2 family members discussed. This method of programmed cell death plays an important role in development, immunity, cancer, and neurodegenerative disease and can be initiated by cellular stressors like DNA damage, ER stress, viruses, or other cellular trauma. Cellular stress leads to activation of BH3-only proteins, which seek to interact and activate BAX or BAK at the MOM. Conversely, antiapoptotic family members work to counter this activation by binding and neutralizing the BH3-only proteins. Eventually, the anti-apoptotic proteins can no longer prevent BAX or BAK from initiating its function, and dimers and oligomers of pro-apoptotic proteins form at the MOM. This leads to mitochondrial outer membrane permeabilization (MOMP), disruption of the electron transport chain, and allows for cytochrome c and other apoptotic molecule release from the mitochondria. Cytochrome c initiates APAF-1 and apoptosome formation, leading to cleavage of caspases, initiation of the caspase cascade and ends in cellular breakdown (Tait and Green, 2010). Further detail of the process of BAX activation and function are provided in the next section.
3. The switch to cell death: BAX mechanisms
The initial steps of the intrinsic apoptotic pathway converge at BAX activity, whose function controls the cell’s commitment to the pathway by altering the physiology of resident mitochondria. BAX undergoes a series of ordered events leading to pore formation in the MOM, facilitating the release of signaling molecules such as cytochrome c. This definitive event is commonly considered the irreversible step in the apoptotic pathway, or the “switch” from life to death. As the central activator of apoptosis, the mechanism underlying BAX function has been intensely investigated for many years and has often been referred to the ‘holy grail’ of apoptotic research (Youle and Strasser, 2008). The mechanism can be organized into three main steps: BAX activation, BAX dimerization (nucleation of the reaction), and BAX oligomerization leading to pore formation. The events of each step of this process, as it is currently understood, are summarized below.
3.1. BAX activation
BAX activation is the first step in a series of events that control BAX function. BAX activation includes translocation to the MOM, a conformational change mediated by BH3-only proteins, and insertion into the MOM. BAX predominantly resides in the cytosol during steady-state conditions, and also cycles on and off the MOM (Fig. 1). It was first hypothesized that BAX also associated with the MOM under non-apoptotic conditions when levels of membrane-attached BAX protein in healthy cells were detected using alkali sensitivity assays (Eskes et al., 2000). Support for this hypothesis came years later, when the anti-apoptotic protein BCL-X was shown to regulate the cycling of BAX on and off the MOM during steady-state conditions (Edlich et al., 2011; Schellenberg et al., 2013). Therefore, BAX is in a constant state of flux, with a minority of protein associating with the MOM, while the majority is present in the cytosol. Only after apoptotic induction, does complete BAX redistribution to the MOM occur (Hsu et al., 1997; Wolter et al., 1997). This equilibrium of BAX distribution in normal cells is not necessarily a Sword of Damocles scenario for the cell. Pro-apoptotic proteins, such as BAK and BAX, in healthy cells, appear to play a role in mitochondrial dynamics. Cells lacking these proteins exhibit shortened mitochondria and reduced rates of fusion (Hoppins et al., 2011; Karbowski et al., 2006). Protein binding assays show that they interact with mitofusins (MFN1 and 2) (Cleland et al., 2011), GTPases that play an integral part in mitochondrial fusion. Studies suggest that BAX and BAK stabilize MFNs to regions of the MOM to facilitate the fusion process (Karbowski et al., 2006). As indicated below, this is a dramatic reversal of one of the predicted roles for BAX during apoptosis, where the BAX oligomer is implicated in regulating a burst of mitochondrial fission.
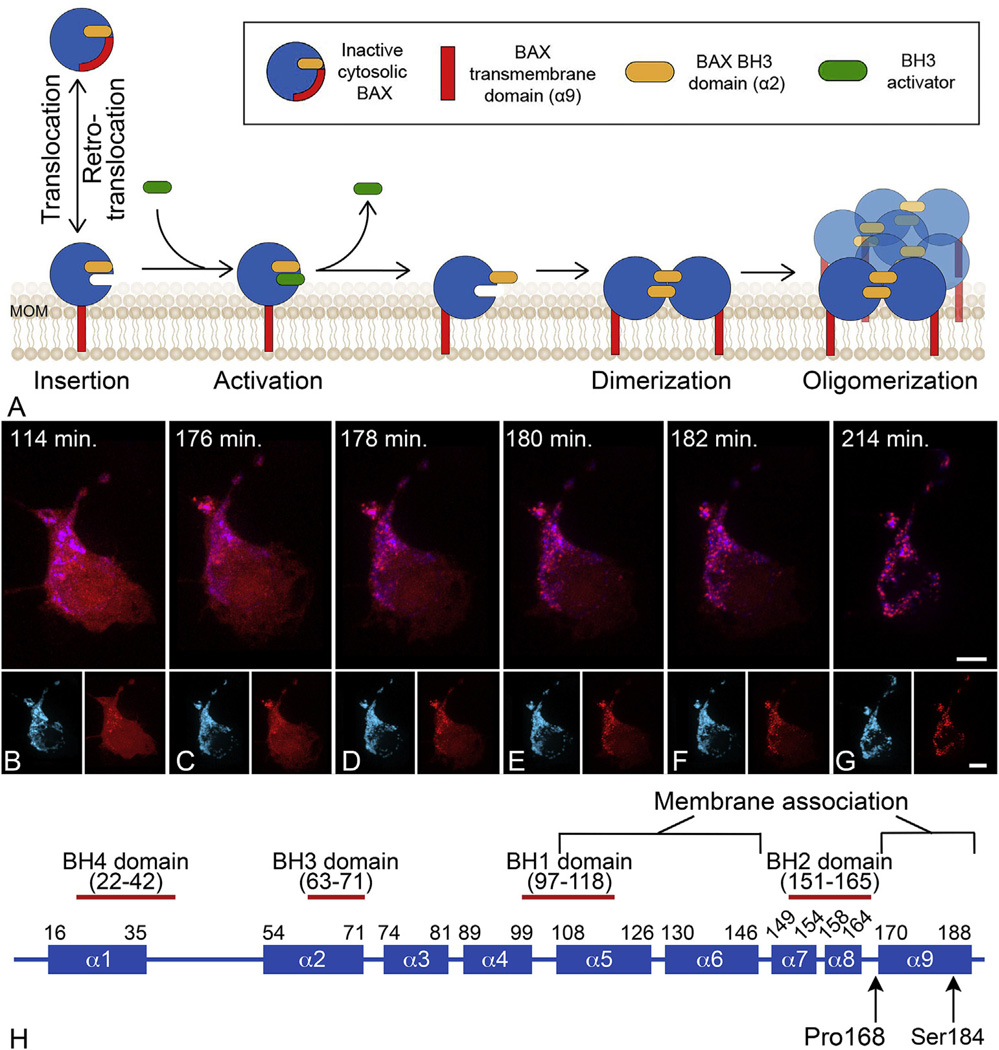
Fig. 1.
BAX protein activation at the mitochondrial outer membrane (MOM). (A) Schematic of the stages of BAX protein activation. Latent BAX proteins are globular and predominantly cytosolic. In healthy cells, globular BAX is in equilibrium with the MOM, and is retrotranslocated to the cytosol by BCL-X It is expected that the interaction with the MOM involves exposure of the α9 helical domain. Early during apoptosis, the cell expresses BH3-only proteins that have the function of sequestering anti-apoptotic proteins (not shown in diagram) and interacting with membrane associated BAX to cause further conformational changes to expose both a hydrophobic groove and the BH3 domain of BAX. This change allows homodimerization of BAX which then nucleates the formation of a large molecular weight oligomer. It is not clear at what stage activated BAX begins to form pores, but there is evidence that proteolipidic pores large enough to allow the release of cytochrome c can be formed by dimers, while oligomers can form supramolecular pores. Modified from (Czabotar et al., 2014). (B–G) Time lapse photography of a D407 tissue culture cell undergoing the process of BAX translocation and oligomerization to the mitochondria. Mitochondria are identified by blue fluorescent protein fused with a mitochondria localization peptide. BAX is identified by fusion to the mCherry fluorescent protein. The time stamp indicates the elapsed time since the addition of staurosporine to induce apoptosis. In these cells, BAX activation and completion to the large molecular weight oligomer occurs within 20 min. During this process, BAX moves from a diffuse cytoplasmic localization to a bright punctate appearance coincident with the mitochondria. The small insets for each frame show the individual channels for imaging. Size bars = 5 µm. (H) Domain map of mammalian BAX showing the 9 different α-helices. The numbering refers to the amino acid sequence. Two amino acids that affect BAX function are indicated (see text).
The BAX protein consists of 9 α-helices (Fig. 1). Cytosolic BAX is found as an inactive globular monomer in healthy cells. During apoptosis BAX becomes redistributed to the MOM, where it undergoes changes to enable it to insert into the membrane and assume an active conformation. The transmembrane domain (α9 helix) is critical for BAX translocation and association with the MOM (Nechushtan et al., 1999). Lack of, or mutations (P168A) in, the C-terminus prevent BAX from translocating to the MOM (Schinzel et al., 2004; Valentijn et al., 2008; Wolter et al., 1997). Other mutations in the α9 helix, like S184V, create a BAX mutant protein that autonomously targets the MOM and constitutively induces apoptosis without stimulation (Nechushtan et al., 1999). Other amino acid substitutions of S184, however, yield a highly protective phenotype. This contrast delineates translocation and apoptosis-inducing capabilities as two separate events (Kim et al., 2009). Interestingly, in the presence of wild type BAX, a mutant lacking the C-terminal portion of BAX can still participate in the recruitment at the MOM, possibly indicating an initial recruitment step using the α9 helix, and secondary recruitment using the α2 helix or BH3 domain (discussed below).
How globular BAX unfolds during activation has been the subject of numerous studies involving epitope exposure, X-ray crystallography, and NMR imaging. The α9 helix normally resides in a canonical hydrophobic groove of BAX (formed by helices α3-α5) when the protein is inactive. When α9 associates with the MOM, it will leave the canonical groove unoccupied (Suzuki et al., 2000). BH3-only proteins have been shown to induce BAX translocation to the MOM (Desagher et al., 1999) and to interact directly with BAX to cause activation (Czabotar et al., 2013; Walensky et al., 2006). The interaction of BAX and BH3 proteins likely occurs at the MOM, as demonstrated by studies using tBID (Lovell et al., 2008; Zhang et al., 2016). Some controversy has surrounded the exact location of the BAX activation site, whether it is at the canonical groove or at a rear site made up of α1 and α6 helices (Gavathiotis et al., 2008). Secondary studies have supported the canonical groove as the BAX activation site (Czabotar et al., 2013; Zhang et al., 2016), however it is also possible that different BH3-only proteins may interact at different sites of BAX.
The interaction with a BH3-only protein and the BAX activation site is the critical step that initiates a conformational change of BAX (Kim et al., 2009). Direct binding between these two proteins was originally undetectable, and therefore considered to be absent. It was later determined that the interaction between the BH3-only and the activation site of BAX occurs more like a ‘hit and run’ association, rather than direct binding (Kushnareva et al., 2012). Inactive versus active forms of BAX were first distinguished by the exposure of the N-terminal 6A7 epitope. The 6A7 epitope is only detectable when BAX is localized at the MOM, which suggests that during BAX redistribution and localization at the MOM, a conformational change must occur to allow for its exposure (Hsu and Youle, 1998). Further studies have identified the structural changes that take place during the conformational change (Suzuki et al., 2000). After a BH3-only protein interaction at the activation site of BAX, the α9 helix inserts into the MOM (Garcia-Saez et al., 2004). Then, the α6-α8 helices of BAX that make up the so-called latch domain, unhinge from a globular core domain (Czabotar et al., 2013), allowing the protein to lie closer to the MOM, where its α5 helices can associate with the lipid bilayer (Zhang et al., 2016). The dramatic rearrangement of helices also leads to exposure of BAX’s own BH3 domain (α2 helix) (Gavathiotis et al., 2010), which can interact with the exposed canonical groove of a second BAX molecule to form a homodimer (Fig. 1 - see below). The exposure of the BAX BH3 domain also confers a unique auto-activation property of this protein, as it can now function as a BH3-only protein, to activate other inactive BAX molecules at the MOM (Cartron et al., 2008). This auto-activation process does not appear to require the elaborate initial steps of MOM interaction regulated by the α9 helix, however, since activated wild type BAX is able to recruit P168A mutants in the formation of oligomers (Valentijn et al., 2008).
In summary, BAX activation initiates with BH3-only interaction at the canonical groove of the protein, inducing a conformational change, which can be described as a change from a globular protein to one that shows release of the α9 helix from the canonical groove. This is followed by α9 helix insertion into the MOM and unhinging of the α6-α8 helices from the core domain (α1-α5 helices), which also reveals the α2 helix or BH3 domain of BAX to allow for activation of other BAX molecules or BAX dimer formation.
3.2. BAX dimerization
Once active, BAX preferentially forms dimer units with other BAX or BAK molecules. The exposed BH3 domain of active BAX molecule is critical for formation of BAX hetero- or homodimers (Sundararajan et al., 2001; Wang et al., 1998). BAX dimers were first detected when BAX was introduced to nonionic detergents, and also in IL-3 deprived cells through crosslinking (Gross et al., 1998; Hsu and Youle, 1997). The proposed dimer structure described a joining of the exposed α2 helix from each BAX molecule, fit together in the opposite BAX molecules ‘core’ domain, in a mirrored (yin yang) fashion (Bleicken et al., 2010). This dimer structure has been confirmed using crystallography and double electron-electron resonance (Bleicken et al., 2014; Czabotar et al., 2013; Dewson et al., 2012).
The BAX dimer unit is considered the nucleation event for BAX function and facilitates the growth of BAX oligomers (Zhang et al., 2016), which is preferred in dimer-based units (Subburaj et al., 2015). The BAX dimer may also possess the ability to release apoptotic molecules and thus be the primary functional unit for MOMP. Work done by Volkmann and colleagues suggests that even single BAX molecules have the ability to create pores. Using nanodiscs of restricted size allowing only a single molecule to occupy the space, they demonstrated BAX-induced proteolipidic pores that measured 3.5 nm (35 angstroms) in diameter, which in theory would be large enough to release molecules like cytochrome c, which is 30 angstroms in diameter (Xu et al., 2013). These pores are not conventional channels in the membrane surrounded by BAX protein domains, but rather destabilization of the membrane induced by BAX (Gilbert et al., 2014). Experiments using BAX mutants that can only form dimers but not oligomers, supported this model by showing that cytochrome c was released, but not the larger SMAC molecule (Zhang et al., 2016). This work would suggest that oligomers are necessary to release molecules of greater size.
3.3. BAX oligomerization, MOMP, and mitochondrial fission
The requirement of BAX oligomers for MOMP has been directly linked for many years (Antonsson et al., 2000; Saito et al., 2000). Because BAX oligomers have never been detected without loss of cytochrome c and progression of apoptosis, we consider BAX oligomerization the quintessential event to identify committed, apoptotic cells. High molecular weight oligomers were first detected using gel filtration methods (Antonsson et al., 2001). The amino acid residues important for oligomer formation were dissected in a study of mutant BAX and gel filtration assays (George et al., 2007). Residues within the α5 helix were found to be required for further assembly of the oligomer in vitro.
As indicated above, BAX dimers are likely necessary for formation of BAX oligomers (Zhang et al., 2016). The mechanism of progression from BAX dimers to oligomer formation has been proposed to occur in two different ways. Conceptually, a transition from ‘nanopore’, made from a single dimer, to ‘supramolecular pores’ containing many dimers was described by (Xu et al., 2013). The ability of BAX dimers (‘nanopores’) to release cytochrome c supports the theory of a ‘nanopore’ (Zhang et al., 2016). The transition from ‘nanopore’ to ‘supramolecular pore’ is supported by work suggesting that BAX oligomers and pores grow in size (Gillies et al., 2015; Kuwana et al., 2002). BAX pores have also been suggested to be tunable in size depending on the concentration of available BAX (Bleicken et al., 2013). To address the nano- to supramolecular pore transition mechanistically, Zhang et al. describe a series of events where the dimerized BAX molecules initiate interaction and dimerization between the α9-α9 helices of a neighboring BAX dimer within the MOM. Mingling of multiple BAX dimers and their α9 helices create changes in membrane tension leading to membrane pore expansion. It was suggested that the BAX dimer is necessary for pore formation, and the α9-α9 interaction is critical for pore expansion. While this theory describes mechanisms for initial changes from a single dimer unit to a larger pore, the continued addition of BAX molecules to form large molecular weight oligomers has not been solved. Additionally, an essential interaction between the α9 helices does not resolve how P168A BAX mutants are able to participate in oligomer formation. An alternative theory for pore growth was suggested where single BAX monomers, once activated, will continue to associate and bind to the growing BAX oligomer (Kuwana et al., 2016).
The presence of pore forming capabilities have been known for nearly twenty years (Antonsson et al., 1997), but the structure and formation of the pore has been elusive. Unlike nuclear pores, the BAX oligomeric pore is not visible using conventional electron microscopic techniques, further confounding its existence. Many theories have been proposed, such as a daisy-chain organization of the dimers attached end to end to create a circle of dimers in the membrane (Reed, 2006). Studies examining bacterial toxins with similar membrane perforating capabilities (Gilbert et al., 2014) and BAX-derived peptides (Qian et al., 2008) suggested that the most likely pore classification was the proteolipidic pore. It is then conceivable that BAX pores could be made up of both BAX protein and lipids from the MOM, as studies have previously suggested (Basanez et al., 2002). Recently, a number of groups have been able to show, for the first time, ring-like organization of BAX at the MOM or in representative membranes. Stimulated emission depletion (STED) super-resolution nanoscopy revealed ring-like structures of endogenous BAX in the MOM of fixed cells, which was devoid of mitochondrial membrane proteins like TOM20 (Grosse et al., 2016). Single-molecule microscopy showed similar arc and ring-like structures with GFP-BAX at isolated mitochondria membranes. Additionally, using atomic force microscopy, topographical readings could detect deformities in the MOM in the middle of BAX ring and arc-like structures, strongly suggesting that BAX ring structures were indeed perforating the membrane (Salvador-Gallego et al., 2016). Finally using gold-labeled BAX or His-tagged BAX molecules, similar ring-like pore structures were demonstrated in liposomes (Kuwana et al., 2016). In these experiments, it is assumed that BAX rings and arcs are the product of BAX dimer and oligomer formation, but this has not yet been confirmed.
While MOMP is often considered the critical mitochondrial event precipitated by formation of the BAX oligomer, it may not be the only function of this structure. Some insight to an additional role has been revealed by evidence showing that the MOMP occurs early during the period of BAX redistribution to the MOM (Capano and Crompton, 2002; Lartigue et al., 2008). This temporal correlation with the onset of the BAX oligomer, rather than after oligomerization was complete, is consistent with studies mentioned above indicating that pores could possibly be formed by BAX monomers and/or dimers. Why then does the oligomer continue to grow? One explanation is to increase the pore size to facilitate release of larger molecules. An additional explanation is that the oligomer is a functional component of the mitochondrial fission machinery. Along with MOMP, it has long been recognized that apoptosis results in a dramatic increase in fission of damaged mitochondria (Karbowski and Youle, 2003), which follows the MOMP event (Arnoult et al., 2005). DRP1, an important protein that regulates the fission process, is translocated to the mitochondria after the onset of cell death (Frank et al., 2001). Protein-protein binding studies have suggested that DRP1 is a binding partner with BAX (Wang et al., 2015), with some going as far as suggesting that DRP1 promotes BAX oligomerization (Montessuit et al., 2010). Experiments utilizing dominant negative mutants of DRP1 to impair functional DRP1, showed inhibition of BAX oligomer formation, and cellular protection (Montessuit et al., 2010). Careful localization studies have also documented that BAX aggregates form at mitochondrial scission sites (Karbowski et al., 2002) and may help to destabilize the MOM, possibly by facilitating an increase in the concentration of cardiolipins in this region. Whether or not BAX actively participates in mitochondrial fission has not been directly tested. It is possible that scission sites of fissioning mitochondria simply provide a preferred environment for BAX oligomers to accumulate.
4. The role of BAX in mouse retinal ganglion cell pathophysiology
4.1. Mouse models of optic nerve damage
Virtually everything we know about Bcl2 gene family function in RGCs has been obtained using genetically modified mice. It is relevant to begin this section with a discussion of mouse models of optic nerve damage because Bax-deficient mice have helped inform the temporal sequence of events leading to both the overall pathology of glaucoma, and the more granular activation of the RGC apoptotic program. Retinal ganglion cell degeneration is a process characterized by a series of molecular events after the initial insult to the optic nerve. Optic nerve damage can be induced in one of two ways; either acutely, through direct damage to the optic nerve or chronically, by increasing intraocular pressure (IOP).
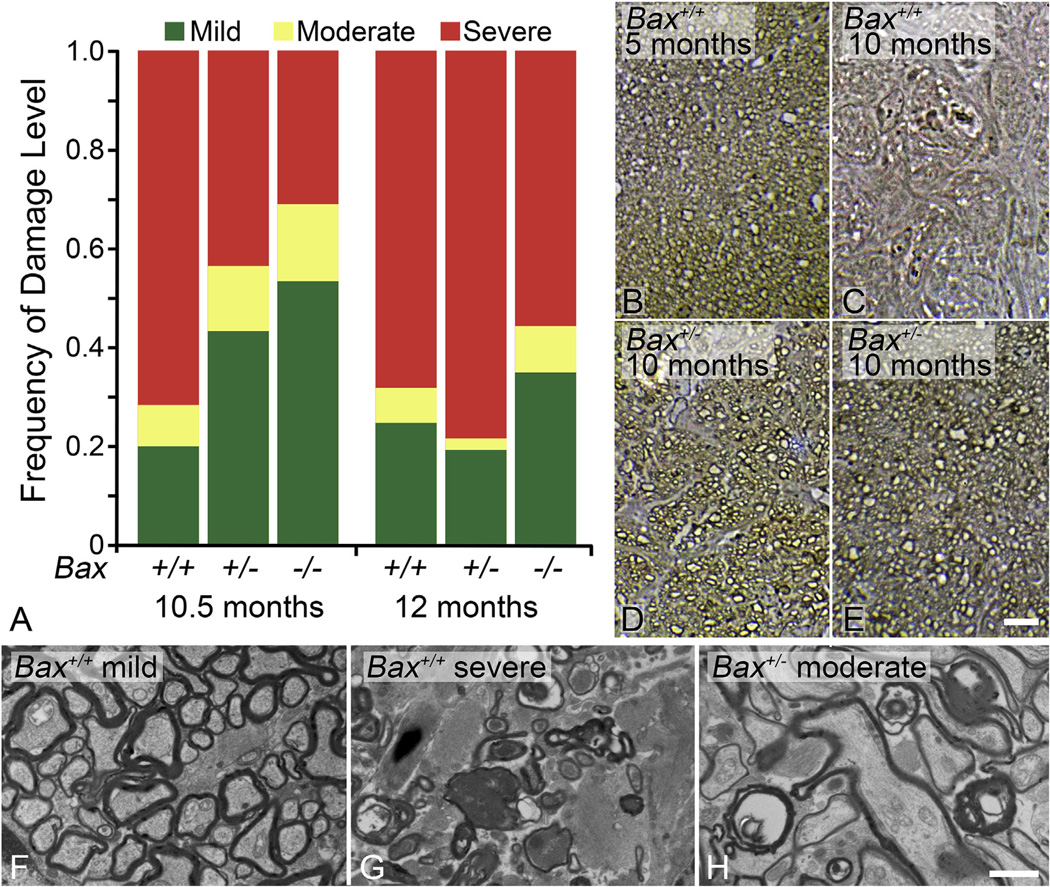
Increased IOP is a widely accepted risk factor for optic neuropathies like glaucoma (Heijl et al., 2002; Kass et al., 2002; Nickells et al., 2007; Quigley, 1993). The most widely studied mouse model for spontaneous development of increased IOP is the DBA/2J mouse. This strain develops high IOPs due to iris pigment dispersion and anterior chamber angle pathology, which includes the formation of synechiae in the region of the trabecular meshwork. The increase in IOP leads to numerous characteristics of traditional optic neuropathies (John et al., 1998). Optic nerve disease, including the loss of RGC somas, progresses beginning at 6–8 months of age, reaching end-stage disease in a majority of animals by 12 months of age (Libby et al., 2005a; Schlamp et al., 2006), and mimics the asynchronous damage to RGCs observed in human optic neuropathies. This model is advantageous because it does not require experimental intervention to induce elevated IOP, however there are limitations including the extended time-period to pathology, and asynchronous progression of disease in these animals. Additionally, the increase in IOP is not necessarily the only determining factor for glaucomatous pathology and its direct correlation to RGC loss has been controversial. IOPs in this model typically increase from an average of 8 mmHg at 4 months to 16 mmHg at 12 months. While this period of time is exemplified by the development of glaucomatous pathology, a partial longitudinal study of IOP in a small cohort of DBA/2J eyes was not able to find a significant correlation between ocular hypertension and axon loss (Scholz et al., 2008). Control DBA/2J-Rj mice in the same study, exhibited higher IOP levels (average 14 mmHg) throughout their lifetime without developing glaucomatous pathology or RGC loss. Collectively, these data suggested that inflammatory responses in the DBA/2J mice, or other unexplored factors, may be contributing to this pathology (Scholz et al., 2008). Conversely, other studies using methods to reduce IOP, including photodynamic therapy combined with Verteporfin (Matsubara et al., 2006) or anti-glaucoma medications (Schuettauf et al., 2002), showed that reduced IOPs resulted in reduced RGC loss later in life. IOP measurements (longitudinal vs. single time point) and end-stage age of mice (9–12 months) vary dramatically among studies, which further confounds the relationship between increased IOP and RGC loss.
Alternate models using experimental intervention to increase IOP include hypertonic saline injection of aqueous humour outflow pathways, limbal laser treatment, episceral vein cautery (reviewed in (Morrison et al., 2005); McKinnon et al., 2009)) and microbead anterior chamber injections (Sappington et al., 2010). All of these models work to disrupt the outflow pathway in some manner, either by clogging the trabecular meshwork or closing the primary angle. The duration and levels of increased IOP in these models are often variable, and the progression to optic nerve damage and RGC degeneration can range from 1 month to 9 months, again with varying levels of axon or RGC loss (Danias et al., 2006; Levkovitch-Verbin et al., 2002; Sappington et al., 2010). To date, only the DBA/2J mouse model has been used to study the contributions of Bax gene function on the pathology of an ocular hypertensive model of glaucoma. Studies in other models are warranted to help validate that intrinsic apoptosis is a common pathway activated in glaucoma, in general.
While models of IOP increase are relevant for studying IOP lowering mechanisms, the variability and asynchronous RGC degeneration and apoptosis makes it a less suitable model for studying molecular mechanisms in the RGCs. Optic nerve transection, or axotomy, and optic nerve crush are two mouse models that inflict direct damage to the optic nerve rather than pressure-induced damage. These methods provide a reproducible technique to induce more synchronous degeneration of the optic nerve and RGCs, which occurs on a much shorter time scale; RGC loss is detectable by 5–7 days and is essentially complete by 21 days (Li et al., 1999; Villegas-Perez et al., 1993). These methods combined have helped determine the series of molecular events that lead to RGC degeneration and apoptosis.
In addition to mouse models of optic nerve damage, experiments that introduce cytotoxic molecules by intravitreal injection have also been informative in elucidating the role of BAX and the apoptotic pathway in RGCs. These molecules include the non-hydrolysable glutamate analog, N-methyl-D-aspartate (NMDA) (Li et al., 1999, 2000; Libby et al., 2005a) and the inflammatory cytokine TNFα (Mac Nair et al., 2014; Nakazawa et al., 2006). NMDA elicits the rapid death of RGCs (and likely amacrine cells) after intravitreal injection, which is completed within 4 days depending on the dose (Li et al., 1999, 2002). TNFα, conversely, takes up to 8 weeks before RGC loss can be accurately quantified. For both of these molecules, the molecular pathways leading to RGC death do not require BAX function. This has created a paradox that is often casually overlooked in the vision community, because RGC death in all models of optic nerve damage, including DBA/2J glaucoma, exhibits complete dependence on BAX function. Yet compelling experimental evidence still indicates an important contribution for these cytotoxic molecules in the pathogenesis of glaucoma. A possible explanation that reconciles these two conflicting observations is discussed below in section 5.1.
4.2. BCL2 gene family proteins are essential for RGC death after optic nerve damage
The central role played by members of the Bcl2 gene family in the process of RGC death has been extensively studied using genetically modified mice. Evaluation of mRNA and protein levels of different BCL-2 family proteins indicated the importance of these proteins in retinal cell fate in general. Principally, quantitative analysis indicated that Bcl-X was the dominant anti-apoptotic gene expressed in the adult mammalian retina (Levin et al., 1997), implicating a role for a pro-apoptotic counterpart. Similarly, transgenic mice overexpressing BCL2 on the promoter for neuron-specific enolase, demonstrated significantly reduced levels of RGC death by programmed cell death during retinal development (Bonfanti et al., 1996; Martinou et al., 1994), as well as a dramatic protective effect on RGCs after acute optic nerve damage (Bonfanti et al., 1996; Cenni et al., 1996). Overexpression of BCL-X in an optic nerve transection model of P0 pups showed significant survival of RGCs 24 h after lesion (Liu et al., 2001), and overexpression using TAT-BCL-X protein showed RGC protection after optic nerve transection in adult mice (Liu et al., 2001). Later, a similar protective effect was observed in RGCs subjected to viral transfer and over-expression of an exogenous Bcl-X gene (Malik et al., 2005).
The role of BAX as the pro-apoptotic antagonist in RGCs was initially suggested by immunostaining studies showing an increase in BAX staining intensity in RGCs around 3–5 days after optic nerve damage (Isenmann et al., 1997). In this study, the increase in staining was interpreted as an increase in BAX protein accumulation, but this interpretation was made before it was understood that BAX translocated and oligomerized at the MOM (Wolter et al., 1997), thereby possibly giving the impression of increased accumulation by virtue of the change in signal-above background in these experiments. Evidence supporting a model in which RGCs rely on latent inactive BAX for cell death, include an attempt to reduce BAX levels, post injury, using siRNA, which failed to produce any significant effect (Lingor et al., 2005), while treatment with a peptide that prevented MOM localization was protective (Qin et al., 2004). Additionally, we have successfully preloaded RGCs with a functional BAX fusion protein without inducing widespread death (see below). Nevertheless, the concept that BAX protein increases in mammalian RGCs after optic nerve damage continues to persist in the field. In a general survey, we estimate that nearly 50% of publications exploring BAX regulation as part of their study demonstrate an increase in Bax expression, while 50% fail to find such an increase, including all the studies characterizing changes in the retinal transcriptome in response to optic nerve damage. Interestingly, the majority of studies showing an increase in BAX also convincingly show a decrease in BCL2, which is surprising since this protein is not prominently expressed in cells of the adult rodent retina (Levin et al., 1997), with the exception of Müller cells (Chen et al., 1994). It seems likely that there is a certain degree of dated understanding of the literature regarding the contemporary mechanism of action of Bcl2 gene family proteins, which has led to confusion surrounding the best practices to monitor BAX and its family partners in apoptosis within the retina.
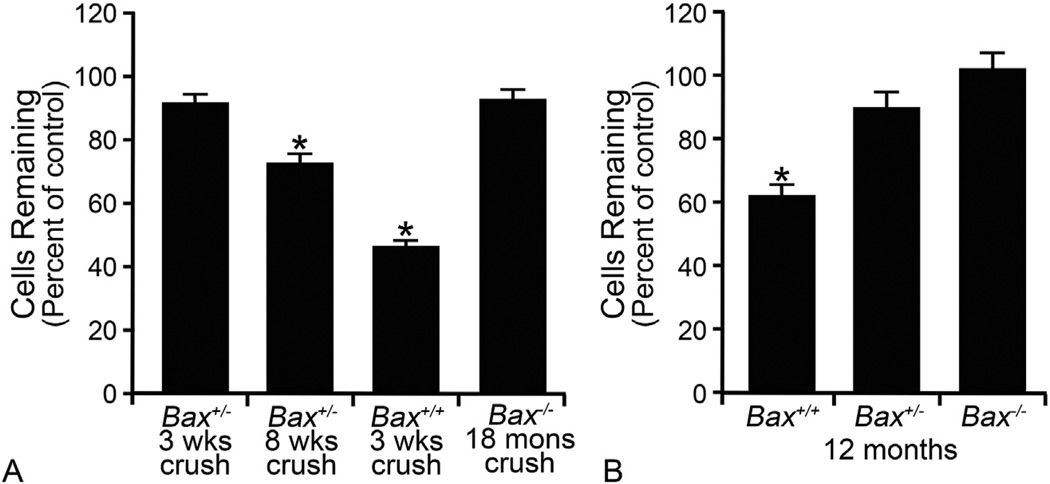
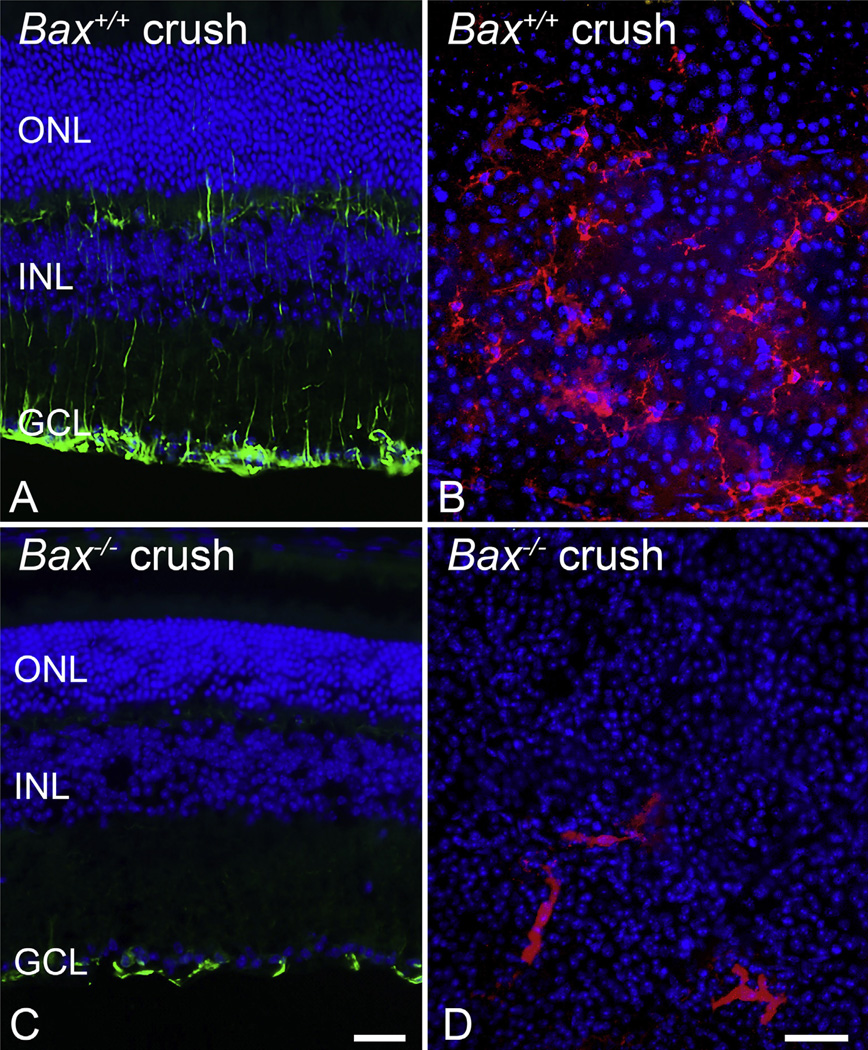
The functions of members of the Bcl2 gene family have typically been elucidated using knock-out mice (Table 1). The most dramatic evidence for a functional role for BAX in RGC death has been generated from studies using Bax-deficient mice. This knock out line was developed in the laboratory of the late Stanley Korsmeyer (Knudson et al., 1995) and subsequent studies evaluating the phenotypic effects of Bax deletion showed that multiple populations of neurons exhibited an absolute requirement for BAX to execute programmed cell death both during development and after stress (Deckwerth et al., 1996, 1998; Sun and Oppenheim, 2003; White et al., 1998). This included developmental pruning of RGCs (Mosinger Ogilvie et al., 1998; White et al., 1998), stress-induced RGC apoptosis in the mouse optic nerve crush model (Li et al., 2000) and in inherited glaucoma of the DBA/2J mouse (Howell et al., 2007; Libby et al., 2005b). A significant feature of RGC loss in these optic nerve damage models is that the blockade of apoptosis is essentially permanent. RGC numbers remain intact up to 18 months after acute optic nerve damage (Semaan et al., 2010) (Fig. 2) and at least 18 months of age in DBA/2J animals when a majority of mice exhibit end-stage disease (Libby et al., 2005a).
Table 1.
Bcl2 gene family knockout mouse phenotypes.
| Genotype | Systemic Phenotype | Retinal Phenotypea | Neuroprotective?b | References |
|---|---|---|---|---|
| Pro-Apoptotic | ||||
| Bax−/− | Viable, Male infertility | Supernumerary GCL, INL, Photoreceptors unaffected |
Yes | (Knudson et al., 1995; Li et al., 2000) |
| Bak−/− | Viable | None | Noc | (Lindsten et al., 2000) |
| Bax−/−/Bak−/− | High perinatal mortality, CNS/reproductive abnormalities, Increased myeloid and lymphoid cells |
Photoreceptors are protected from light damage |
Not testedd | (Hahn et al., 2004; Lindsten et al., 2000) |
| Bok−/− | Viable, Female has increased thymus and spleen weight |
None | Not tested | (Ke et al., 2012) |
| Anti-Apoptotic | ||||
| Bcl2−/− | Early postnatal mortality | n/a | n/a | (Veis et al., 1993) |
| Bcl-x−/− | Embryonic lethal | n/a | n/a | (Motoyama et al., 1995) |
| Bcl-w−/− | Viable, Male infertility | None | Not tested | (Print et al., 1998; Ross et al., 1998) |
| Mcl1−/− | Peri-implantation embryonic lethal | n/a | n/a | (Rinkenberger et al., 2000) |
| A1−/− | Viable, Increased neutrophil apoptosis | Unknown | Not tested | (Hamasaki et al., 1998) |
| BH3-only | ||||
| Bad−/− | Viable, Develop diffuse large B cell lymphoma | Unknown | Not tested | (Ranger et al., 2003) |
| Bid−/− | Viable, Resistant to Fas-induced hepatocellular apoptosis, Aged mice develop myeloid hyperplasia then malignancy |
None | No | (Harder and Libby, 2011; Harder and Libby, 2013; Yin et al., 1999; Zinkel et al., 2003) |
| Bik−/− | Viable | Unknown | Not tested | (Coultas et al., 2004) |
| Bim−/− | Viable, Accumulation of myeloid and lymphoid cells, Aged mice develop autoimmune kidney disease |
Increased retinal vasculature, Developmental abnormalities in optic nerve head |
Partial | (Bouillet et al., 1999; Harder et al., 2012b) |
| Bmf−/− | Viable, Develop B-cell restricted lymphadenopathy | Unknown | Not tested | (Labi et al., 2008) |
| Hrk(Dp5)−/− | Viable | Unknown | No | (Fernandes et al., 2013; Imaizumi et al., 2004) |
| Noxa−/− | Viable, Motor neurons protected after axotomy | Unknown | Not tested | (Kiryu-Seo et al., 2005; Villunger et al., 2003) |
| Puma(Bbc3)−/− | Viable | Supernumerary | No | (Harder and Libby, 2011; Villunger et al., 2003) |
| Bim−/−/Bid−/− | Viable | None | No | (Harder and Libby, 2013) |
|
Bim−/− Puma−/− |
Viable, enlarged lymph node and spleen, thymic hyperplasia |
Supernumerary | Partial | (Erlacher et al., 2006; Harder and Libby, 2013) |
| Bid−/−/Puma−/− | Viable | Supernumerary | No | (Harder and Libby, 2013) |
|
Bim−/−/Bid−/− Puma−/− |
Viable | Supernumerary | Partial | (Harder and Libby, 2013) |
| Bim−/−/Hrk−/− | Viable | None | No | (Fernandes et al., 2013) |
Designations of not/applicable (n/a) refer to mice that cannot be tested because the deletion is embryonic lethal. Designations of Unknown refer to no citations regarding a retinal phenotype in relevant mice.
Designations of n/a are the same as for the column on retinal phenotype. Designations of Not Tested refer to no neuroprotective study having been conducted using these mice.
Nickells et al., unpublished.
Neurons express a splice variant of Bak that disables the full-length protein (Uo et al., 2005). It is expected that neurons in Bax-deficient mice essentially have a Bax−/−/Bak−/− genotype.
Fig. 2.
Bax-deficient retinal ganglion cell (RGC) somas are resistant to optic nerve damage. The dependency on BAX protein for RGC apoptosis is demonstrated in mice with mutant Bax. (A) Total retinal neuron cell counts in the ganglion cell layer (GCL) of mice at times after optic nerve crush surgery. In this model, approximately 50% of the GCL neurons die by 3 weeks, which corresponds to the population of RGCs in this layer of the mouse retina (Schlamp et al., 2013). Bax+/− mice on certain genetic backgrounds (Semaan et al., 2010) exhibit complete resistance to crush insult by 3 weeks, but by 8 weeks, significant cell loss is evident Cell loss is completely ablated in Bax−/− mice, even 18 months after optic nerve insult. (B) RGCs die by BAX-dependent apoptosis in the DBA/2J model of chronic glaucoma. Graph of GCL neuron counts in mice aged 12 months exhibiting severe glaucomatous optic nerve damage. Cell counts are denoted as a function of the percentage cells counted in young mice with no disease. Only mice wild type for the Bax gene exhibit significant soma loss at this age. (*P < 0.001 in each graph). Fig. 2A was modified from (Semaan et al., 2010) and 2B was modified from (Libby et al., 2005b), with author permission.
4.3. The sequence of events leading up to BAX activation
Stress response signaling events occur during the early stages after optic nerve injury, and include the dual leucine zipper kinase (DLK) and the c-Jun N-terminal kinase (JNK) signaling pathways. DLK is likely responsible for modulating the initial stress response signaling after injury. DLK expression increases immediately after optic nerve damage in the axon, and later increases expression in the RGC soma (Huntwork-Rodriguez et al., 2013). Deletion of DLK expression in an optic nerve crush model provided significant RGC survival (Welsbie et al., 2013), and reduced expression of stress response, inflammatory and apoptotic genes, like ATF3, CHOP and Bim, but had little effect on regenerative properties of the axon after injury (Watkins et al., 2013). It was recently shown that Dlk-deficiency delayed somal RGC death after optic nerve injury, but axonal degeneration was unaffected (Fernandes et al., 2014). Downstream targets for DLK include MKK4/7 and JNK activation, the latter of which can participate in a feedback loop and stabilize DLK activity (Huntwork-Rodriguez et al., 2013). The JNK family consists of three isoforms, which are active when phosphorylated and will subsequently phosphorylate c-Jun, a transcription factor known to induce expression of neurodegenerative/apoptosis promoting genes. In RGCs after ONC, JNK activation (pJNK) occurs immediately (1hr) after insult and leads to phosphorylation of c-Jun. Lack of Jnk2 and Jnk3 provides protection to RGCs (Fernandes et al., 2012), as does inhibition of all JNKs using a broad spectrum JNK inhibitor, SP600125 (Liu et al., 2011). Notably, in neurons, c-Jun phosphorylation has been shown to affect levels of apoptotic proteins like BIM and HRK (Ma et al., 2007; Whitfield et al., 2001), and similar effects have been confirmed in RGCs using Jun-deficient mice (Fernandes et al., 2013; Harder et al., 2012b). An increase in ROS (reactive oxygen species) has been detected in retinas after increases in IOP, however, direct activation of signaling pathways have not been explored (Ko et al., 2005).
In addition to the activity of JNK/c-Jun, activated p53 has also been implicated in the transcription of the BAX activating BH3-only proteins BIM (Burns and El-Deiry, 2003), PUMA (Nakano and Vousden, 2001), and NOXA (Wong et al., 2005). Activity of JNKs stabilize p53 by phosphorylating sites that would normally target this transcription factor for ubiquitination and degradation (Fuchs et al., 1998). Additionally, increased HDAC activity, which is an early event in RGC death (see below), appears to mediate neuronal toxicity by deacetylating latent p53 allowing it to become transcriptionally active (Lebrun-Julien and Suter, 2015). The activity of p53 and its role in RGC death are well-documented. RGC death induced by ischemia and cytotoxicity (Li et al., 2002; Rosenbaum et al., 1998), and optic nerve axotomy (Wilson et al., 2013) is significantly attenuated in mice with induced mutant alleles of p53. In the axotomy model, p53-mediated gene transcription is regulated by apoptosis-stimulating proteins of p53 (ASPP) 1 and 2 (Wilson et al., 2013). RGC death can be partially protected by increased expression of iASPP, a regulatory inhibitor of these proteins (Wilson et al., 2014). It is important to note that neither deletion of p53 (Li et al., 2002; Wilson et al., 2013), nor JNKs 2 and 3 (Fernandes et al., 2012), is able to completely mimic the protective phenotype obtained by Bax-deletion. This underscores the probable redundancy of the BH3-only activation network, in which expression of some BH3-only genes are specific to the JNK signaling pathway and others are selective to p53 activation (Wong et al., 2005).
Several reports also indicate that BAX expression is p53-dependent. The human BAX gene promoter contains several consensus p53 binding sites. In experiments using a truncated version of this promoter to drive reporter gene expression, only cells expressing p53 were able to stimulate transcription (Miyashita and Reed, 1995). Further analysis, however, showed that p53-mediated responsiveness required an additional co-factor that bound to an adjacent site (Thornborrow and Manfredi, 2001). Conversely, studies using the murine Bax promoter have so far failed to show responsiveness to p53 (Schmidt et al., 1999; Semaan et al., 2010) even though this promoter contains a full p53 consensus binding site, and a second p53 consensus half site (Semaan et al., 2010). The concept that p53 activation may lead to BAX protein expression, suits a logical model explaining the involvement of p53 in the cell death process. Much of the work demonstrating this, however, was conducted before a complete understanding of the role of BH3-only proteins in the activation of BAX, and the structural and conformational changes that latent BAX proteins undergo during the activation process. While it is not unreasonable to speculate that dying cells enhance the apoptotic process by generating more BAX protein, this is likely not a primary mechanism in the activation response. Consistent with this, studies in RGCs showed that p53-mediated gene expression changes occur late in the death program of these cells (Fujita et al., 2015), and may actually succeed the period of maximal BAX activation in these cells (see below). The actual relationship between p53 and BAX protein expression is likely complicated and requires additional investigation.
4.4. The BH3-only proteins active in RGCs
Perhaps the most consequential changes affecting apoptotic commitment are the increase in expression of BH3-only proteins in RGCs, which are direct activators of BAX. Expression of all, but one, of the 8 known BH3-only proteins have been detected in the rodent retina, but what we know about each one varies. BIM expression is detected in the ganglion cell layer (GCL), and increases after ONC injury (Napankangas et al., 2003). This increase in expression is JUN-dependent (Fernandes et al., 2013; Harder et al., 2012b), while its apoptotic activity is regulated by JNK phosphorylation (Lei and Davis, 2003). Several studies utilizing Bim-deficient mice have also implicated this BH3-only protein as an important, but not essential, regulator of RGC death in mice after acute optic nerve damage (McKernan and Cotter, 2007). However, in the DBA/2J model of chronic optic nerve damage, Bim−/− mice were not protected from RGC loss in eyes with severe optic nerve disease, although there was an overall reduction in the number of eyes exhibiting end stage glaucomatous degeneration (Harder et al., 2012b). These results suggest that while BIM is perhaps a main activator of BAX in RGCs, it is not the only player. This precipitated a series of detailed experiments to serially delete multiple BH3-only proteins in an effort to recapitulate the protective effects seen in Bax−/− mice. Single knockouts of Bim−/−, Bid−/−, or Puma−/−, and double knockouts of Bim−/−/Bid−/−, or Puma−/−/Bid−/− mice did not show significantly prolonged RGC protection after ONC (Harder and Libby, 2011, 2013). Puma−/−/Bim−/− and triple-knockout Puma−/−/Bid−/−/Bim−/− mice did show moderate protection of RGCs after ONC, however not to the level observed in Bax−/− mice (Harder and Libby, 2013), suggesting that other BH3-only proteins are active and/or are compensating for the loss of PUMA, BID and/or BIM (see Table 2). HRK is present in RGCs and showed increased transcript abundance after axotomy in the rat retina (Wakabayashi et al., 2002), however Hrk−/− mice failed to protect RGCs after optic nerve crush injury. Even when combined with Bim, Hrk−/−/Bim−/− double knockout mice were not further protected when compared to Bim single knockouts (Fernandes et al., 2013). The remaining BH3-only proteins have been minimally explored in the retina. BAD is present in the GCL (McKernan and Cotter, 2007) and is dephos-phorylated after optic nerve injury (Huang et al., 2005). BIK is also present in the GCL, but little else is known about its function in RGCs (McKernan and Cotter, 2007). NOXA and BID have been detected in the retina, however localization in RGCs has not been determined (Donovan et al., 2006; Wilson et al., 2013). BMF expression in the mouse retina has not been reported.
Table 2.
Effect of BaCl2 treatment on nuclear shrinkage after optic nerve crush.
| Group | Nuclear Area (% of control) (Mean ± SD) | P1 value | P2 value |
|---|---|---|---|
| WT PBS | 63.36 ± 19.27 | 1.44 × 10−68 | |
| WT BaCl2 | 75.19 ± 20.35 | 5.24 × 10−40 | 7.49 × 10−21 |
| Bax−/− PBS | 74.44 ± 22.17 | 1.36 × 10−54 | |
| Bax−/− BaCl2 | 95.22 ± 24.41 | 0.003 | 1.02 × 10−60 |
Cell shrinkage, often referred to the apoptotic volume decrease, is a common hallmark of programmed cell death. The shrinkage has been attributed to a rapid efflux of K+ ions through delayed rectifier potassium channels (Bortner and Cidlowski, 2007; Pal et al., 2003). We assessed if the shrinkage of nuclei in retinal ganglion cells was mediated by a similar K+ efflux, by blocking potassium channels with BaCl2 (Bortner and Cidlowski, 2007). Left eyes of individual mice underwent optic nerve crush surgery and were then immediately injected with 1 µL of 1.5 mM BaCl2 into the vitreous. Nuclear area (µm2) of cells in experimental retinas, 5 days after crush surgery, was calculated as a percentage of the mean area of nuclei in cells in control fellow retinas. PI values reflect comparisons of the nuclear areas of cells in control vs experimental eyes for each group (ANOVA). P2 values reflect comparison between the percent shrinkage of PBS vs BaCl2-treated eyes for either wild type (WT) or Bax-deficient mice. At least 3 mice were evaluated in each group.
As discussed earlier, increased expression in BH3-only proteins is critical for interaction and activation of BAX, but it is not directly causal since the anti-apoptotic BCL2 genes can counteract BH3-only expression. The most highly expressed anti-apoptotic protein in the retina is BCL-X. BCL-X expression actually decreases after optic nerve crush (Levin et al., 1997), suggesting that RGCs are driving progression to apoptosis by both increasing ‘effector’ BH3-only signals, and decreasing pro-survival signals. Supporting evidence shows that Bcl-X deficiency accelerates the rate of RGC loss after optic nerve injury (Harder et al., 2012a), and as discussed above, experimentally increasing the levels of anti-apoptotic proteins provides protection. BCL-W is expressed in the developing retina, A1, and MCL-1 have not been examined in the murine retina.
4.5. Timing to the activation of BAX in RGCs in acute and chronic paradigms
Although we know that RGC death after optic nerve damage is BAX-dependent, little is known about the overall timing of events that occur in dying RGCs, including when BAX is actually activated in response to axonal damage. Since the point of BAX activation can be considered the point of no return in the apoptotic pathway, determining the interval before this occurs may be of therapeutic importance. Immunostaining for BAX in retinal sections provided some early insight into the timing of the response. Isenmann and colleagues (Isenmann et al., 1997) showed positive staining for BAX as early as 2 h after optic nerve crush damage, with a peak in labeling at 3 days (although no time points were evaluated between 3 and 6 days). A similar finding of increased BAX immunoreactivity was reported by a second group in a rat axotomy model, in which cells were stained positive at 4 days after damage (Napankangas et al., 2003).
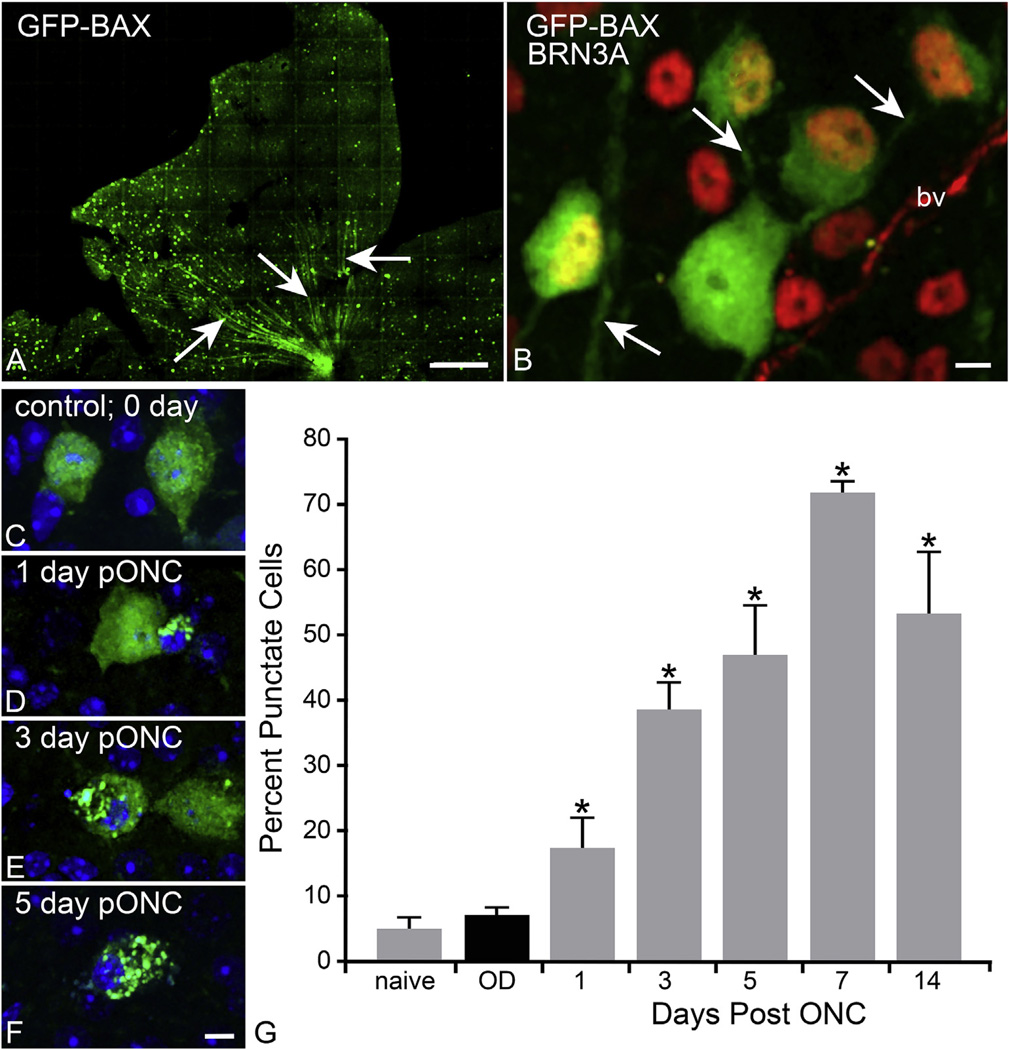
We investigated BAX activation in the retina after optic nerve damage using a different technology by first introducing a GFP-BAX fusion protein into RGCs at least 1 month prior to inducing cell death by optic nerve crush. The fusion protein construct was delivered to RGCs by AAV2-mediated transduction. AAV2/2 has been documented to efficiently and selectively transduce the ganglion cell layer (Hellstrom et al., 2009). C-terminal fusion proteins of BAX have been used to study the localization and function of BAX for nearly twenty years (Wolter et al., 1997) and function the same as wild type protein to induce cell death (Semaan and Nickells, 2010). In these experiments, CB6F1 mouse eyes received a single intravitreal injection of AAV2-GFP-BAX virus at a concentration expected to transduce approximately 25% of the RGCs in order to obtain good resolution of individual cells. After 1 month, eyes were examined for transduction of RGCs. Fig. 3 shows that fusion protein expression was stable for at least 7 months after transduction, and that a majority of labeled cells also co-localized with the RGC-specific marker BRN3A. Those that were BRN3A negative also exhibited long axon-like projections, suggesting that they were a subset of RGCs that do not exhibit BRN3A expression (Nadal-Nicolas et al., 2012; Schlamp et al., 2013).
Fig. 3.
Use of a GFP-BAX fusion protein to monitor BAX activation in retinal ganglion cells (RGCs) after optic nerve crush (ONC) in mice. In this experiment, mice were given an intravitreal injection of adeno-associated virus (AAV) serotype 2 carrying BAX fused to green fluorescent protein at the N-terminus (GFP-BAX). Such fusion proteins have been used for 2 decades to monitor BAX activation in tissue culture cells. Mice were housed for at least one month to allow for transgene expression before undergoing ONC surgery. AAV2 has a high affinity for RGCs, and can transduce upwards of 85% of these cells (Hellstrom et al., 2009). (A) Lobe of a mouse retina showing fluorescently labeled cells, 7 months after viral injection. A relatively low concentration of virus was injected (~108 particles) to purposely transduce about 25% of the RGCs. Arrows show labeled nerve fibers consistent with RGC expression of the transgene. Size bar = 200 µm. (B) Transduced cells were co-labeled with an antibody against BRN3A (red) to confirm transduction of RGCs. Most GFP-BAX positive cells are also BRN3A positive, although one cell is BRN3A negative. This may represent one of the 15% of the RGC population that does not express this transcription factor (Nadal-Nicolas et al., 2012). Arrows indicate the presence of GFP-BAX in long axon-like processes. Size bar = 10 µm (bv, blood vessel) (C–F) GFP-BAX localization in RGCs after ONC. Localization moves from diffuse cytoplasmic to a punctate pattern indicative of activated BAX. Size bar = 10 µm. (G) Quantification of GFP-BAX punctate localization after ONC. The number of punctate labeled cells is given as a percentage of transduced cells counted. A significant increase in punctate labeling was observed starting 1 day after crush, which peaked at day 7 (*P < 0.05). The total number of cells counted were: naïve – 287; contralateral control eyes (OD) – 2022; day 1 post ONC – 422; day 3–394; day 5–358; day 7–348; day 14–156.
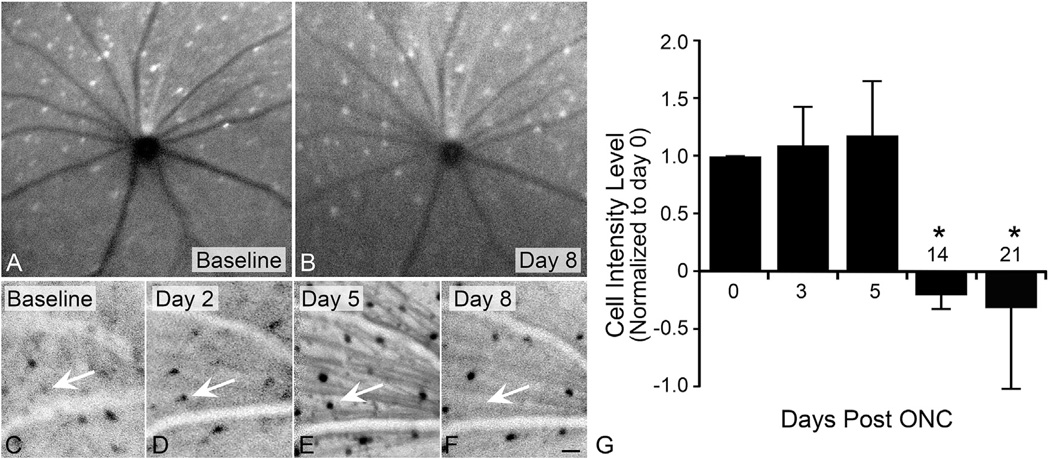
To assess the timing of BAX activation after acute optic nerve injury, mice were analyzed 1, 3, 5, 7, and 14 days after optic nerve crush surgery (n = 3–7 mice per timepoint). Cells exhibiting a punctate pattern, indicative of BAX activation and oligomer formation, were quantified as a percentage of the total transduced cells counted in the collected images (Fig. 3). In naïve and contralateral retinas, the majority of GFP-BAX expression in RGCs was distributed diffusely throughout the cytoplasm, similar to tissue culture cells before induction of apoptosis (See Fig. 1). Importantly, these data indicated that preloading cells with exogenous BAX did not elicit widespread RGC death. Retinas from crushed eyes, however, exhibited an increase in the percentage of BAX oligomerized cells as early as 1 day after damage, peaking at day 7, before declining at day 14. In this strain of mouse, cell loss becomes apparent at 7 days post crush injury (Li et al., 1999). To better understand the timeline of death and phagocytosis after an RGC has committed to apoptosis, we used a Heidelberg Spectralis confocal scanning laser ophthalmoscope (cSLO) to monitor single GFP-BAX expressing cells longitudinally over time after optic nerve injury. Using blue laser excitation from the cSLO, GFP-BAX expressing RGCs fluoresce and can be imaged each day after injury. The blood vessels within the retina create a unique landmark to use as a reference guide, which allowed us to monitor the same cell each day. A cohort of 12 mice were imaged prior to optic nerve injury to determine baseline readings, and identify prime candidates to follow after optic nerve injury. A subset of 5 mice were selected for imaging at 2, 5, 8, 14 and 21 days after optic nerve crush surgery. A number of cells were followed for the duration of the image timepoints and the fluorescence intensity was calculated. There was an overall increase in fluorescent intensity of cells in crushed retinas, peaking around 5 days after injury. By 8 days, some of these cells were no longer visible, which resulted in an overall decrease in the average intensity of the cells being followed (Fig. 4). While not statistically significant, the trending increase in intensity could be suggestive of peak BAX oligomer formation by day 5, which would align with the previous study analyzing retinal whole mounts (Fig. 3). The resolution of this technology, however, was not sufficient to distinguish between diffuse and punctate BAX. If our assumptions are correct, we could infer from this preliminary data that BAX activation (in a majority of cells) occurs within 3–7 days after axonal damage and that these cells can be phagocytosed as rapidly as 3 days after activation. These data help highlight an important timeframe for intervention if the cells can be saved.
Fig. 4.
Longitudinal imaging of GFP-BAX expressing cells in the retina after optic nerve crush surgery. Mice were first given an intravitreal injection of AAV2-GFP-BAX virus and housed for 4 weeks. After this time, all animals were imaged using a Heidelberg Spectralis HRA+OCT confocal scanning laser ophthalmoscope (cSLO), with an ultra-wide field angiography lens to allow non-contact imaging of both the central and mid-peripheral retinas of mouse eyes. Under blue laser excitation, GFP fluorescence can be visualized in the cells of the ganglion cell layer. Images were taken on mice dilated using Tropicamide, and under ketamine and xylazine anesthesia. Each eye was imaged at 7 different diopters to facilitate obtaining the best possible image of the inner retina. The best 3 images from each scanning session was used for quantitative analysis. After baseline scans were completed, the mice underwent optic nerve crush (ONC) surgery on the left eye, and were then subjected to scans every other day for 3 weeks. (A,B) Scans of a contralateral eye that did not undergo surgery, showing the baseline scan and a scan 8 days after surgery. The pattern of GFP-BAX expressing cells is unchanged. (C–F) Regions of a retina after ONC. Negative images are shown to enhance contrast of the fluorescent cells. Retinas of crushed eyes showed changes in the pattern of GFP-BAX labeled cells, with many of them apparently increasing in intensity by day 5. Some of these cells completely disappear by 8 days (arrow). Size bar = 30 µm. (G) Preliminary quantification of a random subset of cells (n = 5) in ONC-damaged retinas. Intensity was measured as the pixel density in a fixed box applied to the image (Image J). Data from each scan was corrected to background from the same scan. While intensity appears to increase slightly, this was not significantly different from baseline. It is possible that the appearance of an intensity increase may be an artifact from the redistribution and concentration of latent GFP-BAX to the MOM. This would be consistent with the widely accepted mechanism of intrinsic apoptosis activation involving the production of BH3-only proteins that act on latent BAX monomers. By 2 and 3 weeks, signal intensity significantly declines (*P < 0.05) as cells are either eliminated or phagocytosed (note that GFP fluorescence would be quenched in the acidic environment of lysosomes in microglia and/or dendritic cells that engulf fragmented RGCs (Mizushima et al., 2010)).
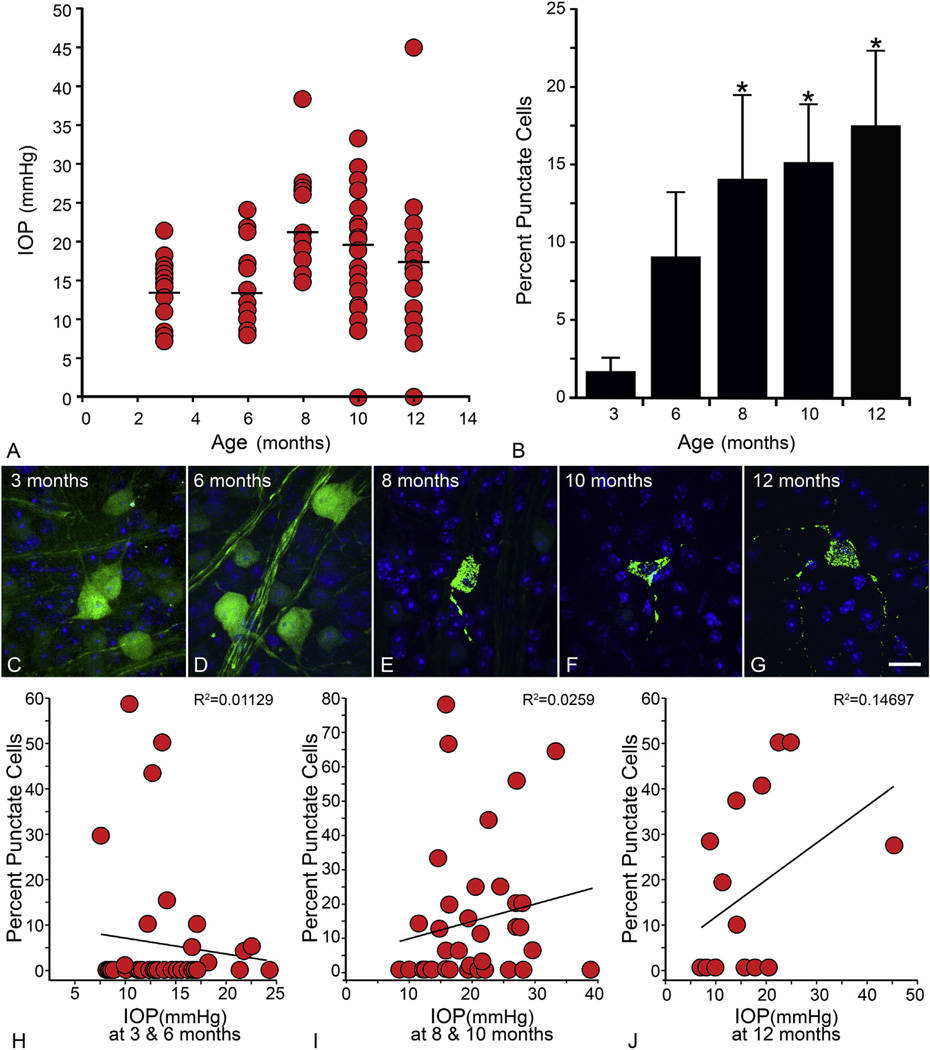
GFP-BAX localization in RGCs was also monitored in the DBA/2J chronic glaucoma model of optic nerve damage. For these experiments, AAV2-GFP-BAX intravitreal injections were administered one month prior to retina collection. The DBA/2J mouse begins to show an increase in intraocular pressure by 6–8 months and by 10 and 12 months, severe disease progression is visible, which is accompanied by significant loss of RGCs (Libby et al., 2005a). At ages 3, 6, 8,10 and 12 months, 16 or more eyes from at least 10 mice per group were quantified for percentage of BAX oligomerized cells. A similar trend was seen for this model when compared to the acute model. An increase in BAX oligomerized cells was seen at 6 months when compared to 3 month controls, however it was not significant. At 8, 10 and 12 months of age, the percentages of BAX oligomerized cells ranged from 15% to 20% and were statistically significant when each was compared to 3 month old controls (P < 0.05) (Fig. 5). Since we also measured IOP in these mice, just before euthanasia, we also examined if BAX localization was correlated to this metric. To minimize the variability associated with a typical reduction in IOP as a function of age (Libby et al., 2005a), we stratified the data into three different age groups (Fig. 5H – J). In each analysis, the R2 value remained nearly zero, indicating no association between an increase in IOP and percentage of punctate cells. As discussed in section 4.1, the development of glaucoma in these mice likely involves factors in addition to elevated IOP. Nevertheless, the temporal increase in the punctate localization of GFP-BAX in this study is consistent with the activation of this protein in lockstep with other indicators of disease progression in this model.
Fig. 5.
DBA/2J glaucoma is accompanied by the activation of GFP-BAX. In this experiment, DBA/2J mice were housed to different ages and then processed to determine the localization of GFP-BAX. One month prior to euthanasia, mice were injected with the AAV2-GFP-BAX virus. (A) Intraocular pressure (IOP) measurements of eyes at time of euthanasia. In this experiment, 19 eyes were evaluated at 3 months, 20 at 6 months, 17 at 8 months, 22 at 10 months, and 16 at 12 months. As the mice get older, IOP levels generally increase (mean IOP denoted by the straight line in each scatter plot). (B) Graph showing the change in GFP-BAX localization as a function of age. Young (3 month) mice show almost no punctate cells (punctate localization was detected in only 4 of 18 eyes). More punctate cells are detected at 6 months, when disease-related changes are first evident. This was highly variable, however, and not significantly greater than the 3 month cohort. A significant increase in the punctate localization was detected at all ages between 8 and 12 months (*P < 0.05). Overall, this pattern reflects other glaucoma-related pathology described in this strain (Libby et al., 2005b; Schlamp et al., 2006). The total number of cells counted were: 3 months–713; 6 months–560; 8 months–588; 10 months–254; 12 months–221. (C–G) Images of labeled cells showing the representative localization pattern of GFP-BAX as a function of age. Size bar = 10 µm (H–J) To assess the correlation between IOP at euthanasia and GFP-BAX localization, the data was stratified into younger (3 and 6 month old, H), mid-aged (8 and 10 months, I), and old mice (12 months, J) and the percent punctate labeled cells for each eye was graphed as a function of IOP at death. The correlation coefficients for each dataset show no significant association between the two metrics.
The experiments imaging GFP-BAX in RGCs after optic nerve crush suggest that this process becomes prevalent in cells beginning 3 days after damage. This aligns relatively closely to the known timing of other downstream apoptotic events in RGCs. Mitochondrial depolarization and permeabilization, along with release of cytochrome c, are key characteristics that define the formation of BAX oligomeric pores and the commitment to apoptosis (Tait and Green, 2010). In intrinsic apoptosis, after BAX permeabilizes the MOM, cytochrome c release will initiate formation of the apoptosome, which involves activation of caspase 9, followed by caspases 3/7 and others, leading to proteolytic cleavage within the cell, and eventual cell loss (Tait and Green, 2010). Along with the actual rate of BAX activation, the timing of MOMP and cytochrome c release in RGCs has not been documented, although it likely mirrors the activation response of BAX. In tissue culture cells, the process of BAX activation and oligomer formation occurs in about 20 min, and cytochrome c release is virtually simultaneous with the formation of the BAX oligomer (Capano and Crompton, 2002). Ultimately, it will be important to quantify the timing of these changes in RGCs in situ. Caspase 9 activity was shown to increase by 3 days post optic nerve lesion in the rat (Kermer et al., 2000), and Caspase 3 activation was first documented by 3–4 days after optic nerve injury, with a decline by 6 days (Kermer et al., 1999b). Others have since confirmed this trend of caspase 3 activity, peaking around 5 days after optic nerve crush in mice (Fernandes et al., 2012). After commitment to cell death and caspase activation, RGCs are phagocytosed by microglia and monocytes within the retina (Galindo-Romero et al., 2013). Based on reactive marker gene expression and FACS cell sorting studies, these cells appear to peak in activity by 7 days post injury (Lehmann et al., 2010; Mac Nair et al., 2014). Thus the timing of the downstream apoptotic events appear to fit well with the data showing peak BAX activation in RGCs.
MOMP is considered an irreversible step in apoptotic signaling, therefore intervention at the level of downstream events will likely not prevent cell death. Validation of this prediction has been documented in several different studies. Attempts to block APAF-1 function, a unit of the apoptosome, yielded moderate survival of RGCs compared to baseline (Lingor et al., 2005). Additionally, studies examining the inhibition of caspase activity traditionally provide only moderate and transient protection for RGCs after axotomy (Kermer et al., 1999a).
4.6. The role of BAX in mitochondrial fission in dying RGCs
An increase in mitochondrial fission is a characteristic of cells undergoing apoptosis (Martinou and Youle, 2011), and RGCs are no exception to this. Ju and colleagues have documented an increase in RGC mitochondrial fission in experimental glaucoma and IOP-induced ischemia-reperfusion injury (Ju et al., 2008), including a protective effect of the DRP1 inhibitor mdivi-1 (Park et al., 2011). A feature of mitochondrial dynamics in RGCs is the process of transcellular degradation, in which evulsions from the RGC axons passing through the optic nerve head and myelin transition zone, are engulfed by surrounding astrocytes. These evulsions contain fragmented mitochondria (Davis et al., 2014), indicating that this region is particularly important for normal mitochondrial dynamics of the RGCs. In the aging DBA/2J mouse, axonal mitochondria in this region are smaller and contain dystrophic cristae (Coughlin et al., 2015) and there is evidence that glaucoma is associated with an increase in mitochondrial fission and mitophagy (Coughlin et al., 2015; Kim et al., 2015). A similar phenotype of abnormal mitochondrial dynamics occurs in mice expressing a mutated form of the human Optineurin protein (E50K OPTN). The wild type Optineurin protein has a variety of functions, one of which appears to be trafficking of membranous organelles, such as mitochondrial fragments, through the process of macroautophagy (mitophagy). The E50K mutant is a rare variant that is associated with normal tension glaucoma, indicating that RGCs could be extremely sensitive to the disruption of normal mitochondrial dynamics. This sensitivity is best illustrated by mutations in the mitochondrial fusion protein optic atrophy 1 (OPA1), which is a causative allele for dominant optic atrophy, and also a susceptibility allele for normal tension glaucoma (Guo et al., 2012; Yu-Wai-Man et al., 2010). In Opa1-deficient mice, mitochondrial fragmentation is apparent, heterozygote animals exhibit early onset optic nerve degeneration, and RGC structure and synaptic connectivity are compromised (Davies et al., 2007; Williams et al., 2012). Similarly, RGCs in the OPTN E50K transgenic mice exhibit an increase in mitochondrial fission and degradation (Shim et al., 2016). This reportedly stimulates activation of the BAX-dependent apoptotic pathway, however this observation was based solely on western blot analysis of the change in BAX protein levels (Shim et al., 2016) and not on any observation of an increase in BAX activation and oligomerization in the optic nerves of these mice. Nevertheless, this study draws a compelling parallel with studies using tissue culture cells that correlate increased mitochondrial fission with the formation of BAX oligomers (Martinou and Youle, 2011) and underscores the importance of further studies that elucidate the function of BAX in normal and abnormal mitochondrial dynamics and RGC susceptibility.
4.7. The role of BAX in species with resistant RGCs
Unlike in mammals, the response of zebrafish RGCs to optic nerve damage is remarkably different. Zebrafish demonstrate a dramatic capacity to regenerate the ganglion cell layer and optic nerve after damage. This was originally attributed to a loss of existing RGCs, followed by repopulation of this cell layer from pluripotent precursor cells in the retina. More recent studies, however, show that neurogenesis is not a fundamental source of regenerating RGCs, and there is a significant level of RGC survival after optic nerve damage (Zou et al., 2013). The level of survival is dependent on the extent of optic nerve injury, with nearly 100% survival from a moderate crush injury, and 70–90% survival after axotomy. The reason for the increased resistance of zebrafish RGCs is not fully understood, but one posited explanation is that expression of the protective protein, Hsp70, is highly upregulated in RGCs in response to damage (Nagashima et al., 2011). Intrinsic apoptosis is similarly regulated by members of the bcl2 gene family in the zebrafish retina (Pant et al., 2013) and there is speculation that Hsp70 modulates cell death by inhibiting an increase in the expression of the pro-apoptotic Bax protein while facilitating an increase in Bcl-2 expression. To date, the data supporting this mechanism of RGC survival in the zebrafish is predominantly observational and has yet to be rigorously tested. Nevertheless, it does call attention to the potentially profound differences between the interaction of Bcl2 gene family proteins in the regulation of RGC death/survival across the spectrum of vertebrate species. Most pronounced is the potential difference in BAX/Bax protein regulation, which is highly controlled in mammalian cells, but appears to be a constitutively active protein in the zebrafish. This provides the fish RGCs with a very simple regulatory mechanism of increasing the level of Bax protein to activate the intrinsic apoptotic pathway. Conversely, for cells that are distinguished by their ability to survive optic nerve damage, this seems to be a very precarious molecular situation that they must protect against. Since it is in our long-term interest to better understand how zebrafish RGCs are able to both resist death and then regenerate their axons, in the hopes of applying those lessons to mammalian RGCs, it is clear that more fundamental research into the zebrafish RGC response is merited.
5. Discoveries made possible using Bax-deficient mice
5.1. Intrinsic vs. extrinsic apoptosis and the phenomenon of secondary degeneration
The involvement of mitochondria as a primary step in the activation of cell death is the principal distinguishing feature separating intrinsic from extrinsic apoptosis. Extrinsic apoptosis is distinguished by the activity of the tumor necrosis factor (TNF) family of ligands, such as TNFα, FasL, and the TNF-related apoptosis-inducing ligand (TRAIL). These ligands can bind to specialized receptors on cells containing “death domains”. Activated receptors then coalesce into death-inducing signaling complexes (DISCs) that function to activate caspase 8, which in turn stimulates the activation of other members of the caspase protease cascade. Several lines of evidence have implicated these ligands, particularly TNFα, in the pathophysiology of RGC death after optic nerve damage. RGCs are protected in a mouse model of experimental glaucoma in Tnf−/− animals (Nakazawa et al., 2006). Similarly, the TNFα receptor trap, Etanercept, is protective in a rat model of this disease (Roh et al., 2012). Careful studies of tissue from human glaucomatous eyes also show changes in the TNFα-signaling pathways, implicating them as contributors to the pathology of the human disease (Tezel, 2008; Yang et al., 2011).
While data relating TNFα-activity in the RGC death process is compelling, it creates a paradox in reconciling how mice lacking Bax, exhibit complete RGC survival in both acute optic nerve damage and chronic glaucoma models. Cells undergoing extrinsic apoptosis can be divided into two classes. Type I cells exhibit strong DISC formation and cannot be rescued by anti-apoptotic proteins of the BCL2 gene family (or deletion of pro-apoptotic members). Alternatively, type II cells exhibit inefficient DISC formation and are responsive to modulation of the BCL2 gene family proteins. In all cases, cells exhibiting caspase 8 activation can recruit the intrinsic pathway by the cleaving the potent BH3-only protein BID yielding active tBID. Activation of caspase 8, and the production of tBID, have been documented in models of experimental glaucoma (Huang et al., 2005; McKinnon et al., 2009). It may be possible that RGCs represent type II neurons, however with further evidence, it becomes unlikely that this is the case. Direct injection of TNFα into eyes promotes RGC degeneration, which is apparent over the course of 8 weeks (Kitaoka et al., 2006; Madigan et al., 1996; Nakazawa et al., 2006). This degenerative phenotype is not prevented in Bax-deficient mice (Mac Nair et al., 2014), suggesting that RGCs are type I responding cells. Alternatively, it is possible that TNFα signaling in the retina does not employ the activation of DISCs leading directly to the caspase cascade. TNFα is able to modulate the production of AMPA receptors in neurons, leading to receptors that have increased permeability to extracellular Ca2+. In a model of ocular hypertension, soluble TNFα is generated by Müller glia, which then stimulates an increase in Ca2+ sensitive AMPA receptors on RGCs (Cueva Vargas et al., 2015). This signaling pathway utilizing Müller cell generated TNFα is also stimulated by direct injection of the excitotoxin NMDA (Lebrun-Julien et al., 2009). Inhibitors of the AMPA receptors are able to block RGC death (Cueva Vargas et al., 2015), indicating that the influx of calcium elicits apoptosis. Similar to the results of directly injecting TNFα, Bax-deficiency in RGCs is also unable to protect them from a direct injection of NMDA (Li et al., 2000; Libby et al., 2005b).
A possible explanation for the protective effects observed in Bax-deficient mice may be rooted in the concept of secondary degeneration. Several independent studies have shown that damage to only a subset of RGCs by partial lesion of the optic nerve, leads to the death of undamaged RGCs in the retina (Fitzgerald et al., 2010; Levkovitch-Verbin et al., 2001, 2010; Payne et al., 2012; Yoles et al., 1997). The involvement of pro-inflammatory factors in this process is also well-studied, with the second wave of cell death being attenuated by small molecule inhibitors of excitotoxicity and inflammation (Levkovitch-Verbin et al., 2006, 2011; Yoles et al., 1997) or the specific deletion of the R1 receptor for TNFα (Tezel et al., 2004). The source of pro-inflammatory signaling molecules like TNFα is expected to be from retinal macro- and microglia (Almasieh et al., 2012; Tezel, 2008), cells which change their physiology and become “activated” in response to retinal damage. Studies suggest that inflammatory cytokine production by glia is most likely a characteristic of the activated phenotype (Mac Nair and Nickells, 2015; Soto and Howell, 2014; Suzuki et al., 2004). Interestingly, the process of activation of both classes of glia is dramatically muted in Bax-deficient retinas after optic nerve damage (Mac Nair et al., 2016) (Fig. 6), a phenomenon that was also reported for microglial activation in the neocortex after acute alcohol exposure (Ahlers et al., 2015). It is important to note that Bax-deficient glial cells still possess a functional copy of Bak, unlike neurons (Uo et al., 2005), and there is no evidence that Bax-deficient mice exhibit a similar block in developmental programmed cell death of glial populations (White et al., 1998), including those in the retina (Mac Nair et al., 2016). This is consistent with other observations that neither Bax deletion nor Bak deletion, individually, have any overt effect on the immune system (Opferman and Korsmeyer, 2003). Additionally, retinal glia in Bax-deficient mice are able to mount an activation response after NDMA exposure (Mac Nair et al., 2016). There is, however, a distinct difference in morphology of the microglial population in an uninjured retina. Bax-deficient retinas exhibit a greater number of microglial cells with amoeboid morphology in comparison to wild type littermates, which could be attributed to a subclass of microglia that may be enriched during development (Mac Nair et al., 2016). Despite these observations, the muted glial activation response in the Bax-deficient mice is likely linked to apoptotic processes ongoing in RGCs that are dependent on the activation of BAX. What these processes are, or what the signaling molecules may be, are currently unknown (Mac Nair et al., 2016). Nevertheless, the all-encompassing protective effect observed in Bax-deficient animals may include the prevention of secondary inflammatory responses that would otherwise lead to BAX-independent extrinsic apoptosis in RGCs.
Fig. 6.
Macroglial and microglial/macrophage activation is muted in Bax-deficient mice after optic nerve damage. Glial activation is a well-characterized effect in the retina in models of both acute optic nerve damage and glaucoma. Astrocytes and Müller cells upregulate a variety of markers including the intermediate filament protein glial fibrillar acidic protein (GFAP), while microglia/macrophages upregulate markers such as allograft inflammatory factor 1 (AIF1/IBA1). (A) GFAP staining (green) of a retinal section taken 7 days after crush injury in a wild type (Bax+/+) mouse showing labeled astrocytes in the ganglion cell layer (GCL) and Müller cell processes extending through the inner nuclear layer (INL) and into the outer nuclear layer (ONL). Typically, only astrocytes are labeled in uninjured retinas. (B) AIF1 staining (red) of a retinal wholemount, 7 days after crush. The ganglion cell layer is shown. A high density of labeled cells with ramified processes is evident (C) GFAP staining of a retina from a Bax−/− mouse at 7 days post-injury. The staining is restricted to astrocytes and no Müller cells appear labeled. (D) AIF1 staining of a retinal wholemount of a Bax−/− retina at 7 days after crush. Few positive cells are evident The overall staining patterns for these two markers in Bax-deficient animals is essentially the same for retinas from uninjured eyes. Size bars = 50 µm (A, C) and 100 µm (B, D). Cell nuclei are counterstained with 4′,6’-diamidino-2-phenylindole. Images modified from (Mac Nair et al., 2016) with author permission.
5.2. RGCs as zombies – deciphering the early changes in RGC apoptosis
Studies that have examined the timing of apoptotic events in Bax-deficient neurons suggest that the BAX dependent step is approximately mid-way through the cell death process (Deckwerth et al., 1998). Support for this statement is most evident in observations that Bax-deficient neurons exhibit pronounced atrophy after a damaging stimulus (Deckwerth et al., 1998; Sun and Oppenheim, 2003; White et al., 1998). This phenomenon has probably been most well-characterized in RGCs after optic nerve damage. Bax-deficient RGCs exhibit pronounced nuclear atrophy, which includes widespread histone deacetylation, nuclear shrinkage, and the silencing of RGC-specific gene expression (Janssen et al., 2013; Pelzel et al., 2012; Schlamp et al., 2001) (Fig. 7, Table 2), all features that are similarly observed early in the apoptotic process of wild type RGCs. Nuclei in Bax-deficient cells initiate heterochromatin formation, but this appears to arrest prematurely, leaving the process only partially complete. The classic exemplars of apoptotic cells, the formation of pyknotic nuclei (complete formation of heterochromatin), nuclear envelop breakdown, and nuclear fragmentation (Kerr et al., 1972), are not evident in Bax−/− RGCs (Janssen et al., 2013). This is probably not surprising, since pyknosis and nuclear breakdown have been attributed to the activity of caspases, which activate endonucleases (Kitazumi and Tsukahara, 2011) and facilitate lamin digestion in the nuclear envelope (Ruchaud et al., 2002), respectively.
Fig. 7.
Widespread deacetylation of histories in nuclei of cells in the ganglion cell layer after optic nerve crush occurs in both wild type and Bax-deficient mice. (A) Retinal section stained with an antibody recognizing pan-acetylated histone H4 (AcH4 – red) in nuclei of cells in the ganglion cell layer (GCL) of a wild type mouse. (B) Similar staining of cells in the GCL of a Bax−/− mouse retina. (C) In wild type animals, staining for AcH4 is absent in approximately 50% of the cells (arrows) by 5 days after optic nerve crush surgery. (D) A similar deacetylation event occurs in Bax−/− mice by 5 days (Arrows point to deacetylated nuclei). Cell nuclei are counterstained with 4′,6’-diamidino-2-phenylindole. Size bar = 10 µm.
Bax−/− mice can be used as an essential tool for studies focused on these early cellular changes in apoptosis because they provide experimental conditions lacking the complications of signaling and/or phagocytosis of dying cells. Bax-deficient mice have been particularly important in showing that silencing of normal gene expression precedes RGC loss in models of both acute optic nerve damage (Janssen et al., 2013; Schlamp et al., 2001) and glaucoma (Pelzel et al., 2012). The studies of nuclear atrophy have helped define and confirm a unique period of caspase-independent heterochromatin formation (Janssen et al., 2013), which is dependent on the activation of histone deacetylases (Pelzel et al., 2010; Schmitt et al., 2014). Preliminary experiments by our group have used these mice to interrogate the early role of potassium channels in the process of nuclear atrophy (Table 1).
After nuclear atrophy and gene silencing, very little is known about the signaling and health of Bax−/− RGC somas. Many of the signaling pathways prior to the BAX commitment stage, including BH3-only protein expression, have not been carefully evaluated in the Bax−/− mice. There is some evidence that they may be reversible, however, since JUN activation and phosphorylation appears to be reversed when Bax-deficient sympathetic neurons in culture have NGF restored to the media (Deckwerth et al., 1998). Determining the RGC expression levels, or lack of expression, of pro-apoptotic signals after what would normally be the time of complete RGC loss, is critical when considering therapeutic strategies. Further experimentation needs to be done to characterize the “steady-state” of injured Bax−/− RGCs. Since these cells survive well past 12 months, it seems likely that they continue to express genes needed at least for a minimal existence. Some of our preliminary studies, for example, indicate that one month after injury, Bax−/− RGCs can efficiently express from the promoters of glycolytic enzyme genes (data not shown). What they do not express, however, are genes that are required to define these cells as RGCs. It appears that loss of BAX has trapped RGCs in a state of limbo between being a functioning neuron and death. We affectionately refer to these cells as RGC zombies. What these latent RGCs are expressing, and how or if they can be manipulated to re-express RGC specific genes for regeneration of damaged axons, needs to be investigated further. It is relevant to note that in the context of therapeutics, it might be more manageable to reprogram a zombie RGC to regenerate an axon, than to create an entirely new cell using cell-replacement therapy.
5.3. The site of early damage in response to ocular hypertension
Observational studies conducted on human donor eyes and non-human primate models of experimental glaucoma provided compelling circumstantial evidence that the initial site of damage induced by elevated IOP was at the lamina cribrosa. Subsequently, many of these observations were later confirmed in rodent models of both chronic inherited and experimental glaucoma. A detailed review of laminar anatomy and changes can be found elsewhere (Nickells et al., 2012). These changes essentially consist of defects in both retrograde and anterograde axonal transport (Anderson and Hendrickson, 1974; Buckingham et al., 2008; Chidlow et al., 2011; Dandona et al., 1991; Pease et al., 2000; Radius and Bade, 1981). Ultrastructural studies also show early signs of axonal degeneration and accumulation of organelles in this region (Gaasterland et al., 1978; Howell et al., 2007). Ultimately, these focal defects correlate to regional patterns of degeneration of RGCs in the retina (Harwerth et al., 1999, 2002; Jakobs et al., 2005; Quigley, 2011; Quigley and Addicks, 1981; Schlamp et al., 2006; Soto et al., 2011). Bax-deficient DBA/2J mice have provided a direct test of the “lamina is the site of damage” hypothesis, by virtue of the phenomenon that the axon proximal to the site of injury in a Bax−/−neuron survives, while the distal axon degenerates (Sun and Oppenheim, 2003). The same phenomenon can be clearly observed in Bax−/− mice that have undergone optic nerve crush (Nickells et al., 2012), and thus provide a tool to identify the site of axonal damage in glaucoma. Evaluation of axonal integrity in glaucomatous Bax−/− DBA/2J mice showed that axons survived right up to the point of the glial lamina in these animals (Howell et al., 2007) (Fig. 8), thereby confirming that this is the site of axonal injury in mice, consistent with expectations in both humans and non-human primate models.
Fig. 8.
Proximal axons survive to the glial lamina in Bax-deficient DBA/2J mice with severe glaucoma. (A) Longitudinal section of the retina and optic nerve of a young DBA/2J mouse with no evidence of disease. Axons are labeled with an antibody to neurofilament (green) and are present in the retinal nerve fiber layer where they converge on the optic nerve head and pass through the laminar region into the optic nerve. (B) Longitudinal section of the retina and optic nerve of a 10 month old DBA/2J mouse with severe axon loss in the optic nerve. There is an absence of neurofilament staining both proximal and distal to the laminar region. (C) Longitudinal section of the retina and optic nerve of a 10 month old Bax−/− DBA/2J mouse with severe axon loss in the optic nerve. Neurofilament staining shows preservation of the proximal axons in the nerve fiber layer and up to the lamina. Cell nuclei are counterstained with 4′,6’-diamidino-2-phenylindole. Size bar = 75 µm. Modified from (Howell et al., 2007) with author permission.
5.4. Compartmentalized “self-destruct” pathways and the timing of pathology in glaucoma
RGCs, like other neurons, have distinct spatially isolated compartments that make up the cell, including the axon, cell soma, dendrite and synapse. The initial insult to RGCs after optic nerve damage is to the axons at the laminar region. Injury to the axon causes axonal degeneration, and cell soma death, two events that can occur independently and are thus likely to have molecularly distinct self-destruct pathways. In the theory of compartmentalized degeneration, classic Bax-dependent apoptotic signaling is responsible for RGC soma death (Howell et al., 2007; Li et al., 2000), while Bax-independent non-classical degeneration occurs in the RGC axon (Beirowski et al., 2005; Ikegami and Koike, 2003; Whitmore et al., 2005). Two important pieces of evidence support the theory of compartmentalized axon degeneration. First, in Bax−/− mice, although the RGC soma is protected from apoptosis (discussed in section 4.2), axonal degeneration still occurs in both acute and chronic models of optic nerve damage (Libby et al., 2005b; Whitmore et al., 2003). The second piece of evidence is that mice carrying the naturally occurring Wallerian degeneration slow (Wlds) mutation show significantly slower axonal degeneration, including preservation of neurites in vitro even after the cell soma has died (Deckwerth and Johnson, 1994). Comparison of DBA/2J mice with either Bax deletion, or carrying the Wlds allele, have yielded important information on the sequence of pathology in glaucoma in this strain. Whereas Bax-deficient DBA/2J mice exhibit axonal degeneration in glaucoma, even with soma survival, Wlds mice exhibit both axonal preservation and prolonged RGC survival (Howell et al., 2007). This provides compelling evidence that axonal damage and degeneration not only precedes RGC soma death, but also likely is the instigating signal for this process.
In contrast to the observations made in the DBA/2J mouse model, however, transgenic rats expressing the Wlds allele exhibit an apparent opposite phenotype. Induction of experimental ocular hypertension and glaucoma in these rats showed preservation of axonal integrity, but loss of RGC somas (Beirowski et al., 2008). The discrepancy between the rat and mouse models may be explained by the methodology used to quantify RGC loss. The Howell et al. group used total cell body counts, while Beirowski and colleagues used staining for RGC-selective marker gene expression. As discussed in section 5.3, early indicators of damage that occur prior to the BAX-dependent stage of apoptosis include silencing of normal gene expression. Thus, the studies using Wlds rats may be indicating that even in the absence of axonal degeneration, RGC somas are experiencing early damage signals in a model of glaucoma. This may mean that these signals are acting directly on the somas in the retina. Use of the Wlds allele, however, does not necessarily prevent the loss of axonal transport or other axonal degenerative properties resulting from ocular hypertension. Importantly, however, it is because these two events can be separated, that elucidating the mechanisms of each are imperative in order to develop protective therapies for RGCs in disease.
While these studies have helped promote an understanding on the sequence of pathology in glaucoma, they have typically been viewed in a binary fashion to help simplify interpretations. An interesting and often overlooked observation associated with these studies has been that prevention of soma death has a profound impact on slowing the rate of axonal degeneration. Bax heterozygous and null DBA/2J mice showed significant preservation of optic nerve integrity at 10.5 months of age, when wild type mice in this study showed a high percentage of severely degenerated nerves (Libby et al., 2005b). Light and electron microscopy of nerves from wild type and heterozygous mice show that axons are largely intact in 10 month old heterozygous mice, but that they do show areas of axonal dystrophy (Fig. 9). Since Bax+/− RGC somas are still protected at 10 months in these mice (Fig. 2), the delay in axonal degeneration may indicate that somas are able to provide some level of trophic support. This support would likely be sacrificed in wild type somas as they are executing the apoptotic program. Ultimately, the process of axonal degeneration exceeds any kind of support that the soma might be able to provide, since even Bax−/− DBA/2J mice exhibit severely degenerated optic nerves by 12 months of age (Libby et al., 2005b) (Fig. 9). Nevertheless, we anticipate that the development of therapies which are protective to the RGC somas, may be able to impact axonal degeneration long enough to have substantial benefit when combined with IOP lowering treatments that reduce the primary insult to the optic nerve.
Fig. 9.
Delay of axonal degeneration in Bax-deficient DBA/2J mice with glaucoma. (A) Frequency distribution graph of optic nerve damage of DBA/2J mice carrying different dosages of a functional Bax gene. In this experiment, a minimum of 49 optic nerves per group were sectioned, stained using paraphenylenediamine (PPD), and then scored for severity by 3 masked observers using a 3-stage scale. Mice with one or two mutant Bax alleles showed significantly less progressive disease than wild type counterparts at 10.5 months of age (P < 0.001 for Bax−/− and Bax−/− mice relative to wild type mice). By 12 months of age, however, all mice showed a similar level of disease progression (P > 0.10). Modified from (Libby et al., 2005b) with author permission. (B) Light micrograph of a PPD stained nerve of a Bax+/+ mouse with no signs of disease. PPD (along with osmium) stains myelinated axons. (C) Section of a Bax+/+ mouse at 10 months of age with severe disease. Only a glial scar is present (D, E) PPD stained nerves from 2 different Bax+/− mice, aged 10 months. These mice were both scored as having moderate damage, due to regions of scar tissue deposition. Size bar = 25 µm. (F) Transmission electron micrograph of a normal optic nerve from a young wild type mouse. (G) A diseased nerve from a 10 month old wild type mouse, showing extensive scarring and axonal remnants. (H) A Bax+/− mouse (10 months) with moderate damage. While most of the nerve appears normal, there are regions showing swollen and dystrophic axons next to axons that still exhibit good microtubule structure and the presence of organelles. Size bar (F–H) = 2 µm.
5.5. The influence of BAX intracellular concentration on the apoptotic program and its implications for neuroprotective strategies
In contrast to Bax deficiency, the effects of BAX overexpression have also been explored. When the rheostat model was still regarded as the model of BCL2 family interaction, higher levels of BAX expression were expected to induce apoptosis. However, mice overexpressing BAX under the neuron specific enolase promoter showed indistinguishable apoptotic profiles in the retina and brain when compared to wild type mice, in both steady state and apoptosis inducing conditions (Bernard et al., 1998). These results contradicted the rheostat model, but were supportive for the more complex model of BCL2 gene interaction.
Bax expression levels have also been monitored in Bax heterozygous (Bax+/−) animals that possess only one copy of the Bax gene and express about half the amount of Bax transcript and protein when compared to wild type controls (Semaan et al., 2010). After acute optic nerve damage in two different mouse strains, 129B6 and DBA/2J, wild type mice showed significant loss of RGCs at 3 weeks after optic nerve damage, while Bax−/− mice exhibited 100% survival, as expected. Interestingly, Bax+/− mice gave two opposing results. The 129B6Bax+/− mice showed significant RGC loss like the wild type mice, while DBA/2JBax+/− mice showed 100% survival mimicking the knockout phenotype. This result was explained by a single nucleotide polymorphism in the promoter region of Bax that affected the rate of transcription (Semaan et al., 2010). A single copy of the Bax allele with low transcription rates was sufficient to prolong the time to apoptotic commitment in RGCs. Importantly, although the DBA/2JBax+/− mice were protected at 3 weeks, by 8 weeks after optic nerve crush, RGC loss could be detected. This suggests that the amount of BAX present within a cell can drastically alter the time to death, but even at low expression levels, the cell will eventually succumb to BAX-dependent cell death.
From these experiments, a hypothesis was developed that a threshold of BAX was necessary to commit a cell to apoptosis. This hypothesis was tested in BAX-deficient HCT116 colorectal cancer cells, in which a doxycycline inducible BAX expression system was introduced, and the level of exogenous BAX protein was titrated to different concentrations. At low levels of BAX expression HCT116 cells were resistant to indomethacin-induced apoptosis at a time after induction when wild type cells had predominantly died. Consistent with the threshold hypothesis, apoptotic death was restored at a specific concentration of BAX. Importantly, the percentage of apoptosis did not change, even at very high levels of BAX expression (Semaan and Nickells, 2010) (Fig. 10). These experiments were conducted at a static timepoint, therefore it is possible that if given unlimited amount of time, even the low levels of BAX expression would commit a cell to apoptosis. The results from Bax+/− mice, and the BAX titration experiments, pose an interesting dilemma with respect to any strategy designed to impair BAX protein function. Further studies that assess how the BAX activation process is affected, as a function of BAX availability, are necessary to help design an effective therapy.
Fig. 10.
The concentration of BAX protein affects the dynamics of activating the intrinsic apoptotic pathway. Studies using Bax-deficient mice on different genetic backgrounds suggested that a threshold of BAX protein was required to activate the apoptotic program in RGCs. This hypothesis was tested in cell culture by titrating the level of a GFP-BAX fusion protein in BAX-deficient HCT116 cells before challenging them with indomethacin, which causes BAX-dependent apoptosis in these cells (Zhang et al., 2000). Titration of GFP-BAX was achieved using the Tet-on transcription system. A construct for GFP-BAX was cloned into a plasmid putting it under the control of the Tetracyline response element (pTRE). This plasmid, along with a plasmid containing the rtTA doxycycline (DOX)-responsive positive acting transcription factor (pTetON) was transfected into HCT116BAX−/− cells, which were then incubated with increasing levels of DOX (A) Increasing DOX produces a controlled increase in GFP-BAX that is generally linear, except at high doses. Cells lacking pTetON show no increase in GFP-BAX. (B) After 24 h being exposed to DOX, cells were challenged with Indomethacin for 48 h after which they were fixed and counted for the presence of apoptotic nuclei. Control cells in this experiment were transfected with pTetON and pTRE-GFP. Control cells expressing GFP and GFP-BAX exhibit low and statistically identical levels of apoptosis at concentrations of DOX up to 0.5 ng/mL At higher concentrations, GFP-BAX expressing cells showed a significant increase in apoptosis (*P < 0.0001). Importantly, there was no increase in the level of cell death no matter how high the concentration of latent GFP-BAX was in these cells. Modified from (Semaan and Nickells, 2010) with author permission. While these data support the concept of a threshold of BAX being required for cell death, more recent studies suggest that low BAX levels only create a delay in the onset of apoptosis. In mice, this delay occurs on the order of several months for retinal ganglion cells exposed to optic nerve damage (Semaan et al., 2010).
6. Perspectives and future directions
The study of BAX activation and function has become one of the most important areas of research in the field of apoptosis. While early theories suggested that the pro-apoptotic BAX protein and its anti-apoptotic counterparts were involved in a stoichiometric ballet for survival, two decades of study now show that activation of this protein is a more complicated process involving a third class of proteins carrying the all-important BH3 domain. In neurons, BAX is critically required to execute the intrinsic apoptotic pathway.
BAX function in the process of RGC death has been extensively studied. Eliminating the Bax gene in mice is the only completely effective protective strategy for RGCs in models of both acute optic nerve damage and glaucoma. Additionally, studies in mouse RGCs have helped (i) identify the site of damage to axons in glaucoma, (ii) enabled the hypothesis of compartmentalized self-destruct pathways in neurons, and (iii) clarified the mechanism of secondary degeneration and neuroinflammation in the retina, among other discoveries.
Can BAX be targeted for neuroprotective strategies? Studies suggest that BAX is activated, on average, within 3 days after axonal damage, providing an apparent window of opportunity for therapeutic intervention. A major focus of study should now be directed at an effective treatment to inhibit BAX, which mimics the effects of genetic ablation seen in mice. And what of RGC zombies? Even if the apoptotic program is arrested, these cells will not be functioning RGCs due to a lack of appropriate gene expression and an intact axon. These obstacles are already being addressed by several groups, who are trying not only to reset the zombie genome by reprogramming the RGC phenotype, but also to stimulate regeneration of the proximal axon stump. If successful, a strategy centered on BAX to prevent apoptosis could have significant repercussions not only on how optic neuropathies are treated, but also other neurodegenerative disorders as well.
Acknowledgments
This work was supported by grants from the National Eye Institute R01 EY012223, P30 EY016665, National Institutes of Health grants T32 GM081061 and S10OD018221. Some of this work was also supported by an unrestricted research support grant and a Senior Scientist Award from Research to Prevent Blindness, Inc. The authors would like to thank Mr. Joel Dietz for help with the Bax-deficient mouse colony, Dr. Gillian McLellan and Ms. Carol Rasmussen for help with the Spectralis OCT imaging, Drs. Aparna Lakkaraju and Kimberly Toops for assistance with live-cell imaging experiments and analysis, and Ms. Katherine Janssen for help with the BaCl2 experiments.
References
- Ahlers KE, Karacay B, Fuller L, Bonthius DJ, Dailey ME. Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration. Glia. 2015;63:1694–1713. doi: 10.1002/glia.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012;31:152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest. Ophthalmol. 1974;13:771–783. [PubMed] [Google Scholar]
- Antonsson B, Conti E, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale E, Sadoul R, Martinou JC. Inhibition of bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligo-merization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 2000;2:271–278. 345. [PMC free article] [PubMed] [Google Scholar]
- Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J. Biol. Chem. 2005;280:35742–35750. doi: 10.1074/jbc.M505970200. [DOI] [PubMed] [Google Scholar]
- Basanez G, Sharpe JC, Galanis J, Brandt TB, Hardwick JM, Zimmerberg J. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J. Biol. Chem. 2002;277:49360–49365. doi: 10.1074/jbc.M206069200. [DOI] [PubMed] [Google Scholar]
- Beirowski B, Adalbert R, Wagner D, Gramme DS, Addicks K, Ribchester RR, Coleman MP. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005;6:6. doi: 10.1186/1471-2202-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Coleman MP, Martin KR. The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur. J. Neurosci. 2008;28:1166–1179. doi: 10.1111/j.1460-9568.2008.06426.x. [DOI] [PubMed] [Google Scholar]
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J. Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard R, Dieni S, Rees S, Bernard O. Physiological and induced neuronal death are not affected in NSE-bax transgenic mice. J. Neurosci. Res. 1998;52:247–259. doi: 10.1002/(SICI)1097-4547(19980501)52:3<247::AID-JNR1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Bleicken S, Classen M, Padmavathi PV, Ishikawa T, Zeth K, Steinhoff HJ, Bordignon E. Molecular details of Bax activation, oligomerization, and membrane insertion. J. Biol. Chem. 2010;285:6636–6647. doi: 10.1074/jbc.M109.081539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicken S, Jeschke G, Stegmueller C, Salvador-Gallego R, Garcia-Saez AJ, Bordignon E. Structural model of active Bax at the membrane. Mol. Cell. 2014;56:496–505. doi: 10.1016/j.molcel.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicken S, Landeta O, Landajuela A, Basanez G, Garcia-Saez AJ. Proap-optotic Bax and Bak proteins form stable protein-permeable pores of tunable size. J. Biol. Chem. 2013;288:33241–33252. doi: 10.1074/jbc.M113.512087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L, Strettoi E, Chierzi S, Cenni MC, Liu XH, Martinou JC, Maffei L, Rabacchi SA. Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J. Neurosci. 1996;16:4186–4194. doi: 10.1523/JNEUROSCI.16-13-04186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. Cell shrinkage and monovalent cation fluxes: role in apoptosis. Arch. Biochem. Biophys. 2007;462:176–188. doi: 10.1016/j.abb.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoim-munity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ, Steele MR, Vetter ML, Marsh-Armstrong N, Horner PJ. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J. Neurosci. 2008;28:2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns TF, El-Deiry WS. Microarray analysis of p53 target gene expression patterns in the spleen and thymus in response to ionizing radiation. Cancer Biol. Ther. 2003;2:431–443. doi: 10.4161/cbt.2.4.478. [DOI] [PubMed] [Google Scholar]
- Capano M, Crompton M. Biphasic translocation of Bax to mitochondria. Biochem. J. 2002;367:169–178. doi: 10.1042/BJ20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron PF, Bellot G, Oliver L, Grandier-Vazeille X, Manon S, Vallette FM. Bax inserts into the mitochondrial outer membrane by different mechanisms. FEBS Lett. 2008;582:3045–3051. doi: 10.1016/j.febslet.2008.07.047. [DOI] [PubMed] [Google Scholar]
- Cenni MC, Bonfanti L, Martinou JC, Ratto GM, Strettoi E, Maffei L. Long-term survival of retinal ganglion cells following optic nerve section in adult bcl-2 transgenic mice. Eur. J. Neurosci. 1996;8:1735–1745. doi: 10.1111/j.1460-9568.1996.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Chang LK, Putcha GV, Deshmukh M, Johnson EM., Jr Mitochondrial involvement in the point of no return in neuronal apoptosis. Biochimie. 2002;84:223–231. doi: 10.1016/s0300-9084(02)01372-x. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Chen ST, Garey LJ, Jen LS. Bcl-2 proto-oncogene protein immunoreactivity in normally developing and axotomised rat retinas. Neurosci. Lett. 1994;172:11–14. doi: 10.1016/0304-3940(94)90650-5. [DOI] [PubMed] [Google Scholar]
- Chidlow G, Ebneter A, Wood JP, Casson RJ. The optic nerve head is the site of axonal transport disruption, axonal cytoskeleton damage and putative axonal regeneration failure in a rat model of glaucoma. Acta Neuropathol. 2011;121:737–751. doi: 10.1007/s00401-011-0807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JJ, Li H, Salvesen GS, Yuan J, Wagner G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell. 1999;96:615–624. doi: 10.1016/s0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- Cleland MM, Norris KL, Karbowski M, Wang C, Suen DF, Jiao S, George NM, Luo X, Li Z, Youle RJ. Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ. 2011;18:235–247. doi: 10.1038/cdd.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin L, Morrison RS, Horner PJ, Inman DM. Mitochondrial morphology differences and mitophagy deficit in murine glaucomatous optic nerve. Invest. Ophthalmol. Vis. Sci. 2015;56:1437–1446. doi: 10.1167/iovs.14-16126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Bouillet P, Stanley EG, Brodnicki TC, Adams JM, Strasser A. Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in he-mopoietic and endothelial cells but is redundant for their programmed death. Mol. Cell Biol. 2004;24:1570–1581. doi: 10.1128/MCB.24.4.1570-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva Vargas JL, Osswald IK, Unsain N, Aurousseau MR, Barker PA, Bowie D, Di Polo A. Soluble tumor necrosis factor alpha promotes retinal ganglion cell death in glaucoma via calcium-permeable AMPA receptor activation. J. Neurosci. 2015;35:12088–12102. doi: 10.1523/JNEUROSCI.1273-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, Lee EF, Yao S, Robin AY, Smith BJ, Huang DC, Kluck RM, Adams JM, Colman PM. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152:519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Dandona L, Hendrickson A, Quigley HA. Selective effects of experimental glaucoma on axonal transport by retinal ganglion cells to the dorsal lateral geniculate nucleus. Invest. Ophthalmol. Vis. Sci. 1991;32:1593–1599. [PubMed] [Google Scholar]
- Danias J, Shen F, Kavalarakis M, Chen B, Goldblum D, Lee K, Zamora MF, Su Y, Brodie SE, Podos SM, Mittag T. Characterization of retinal damage in the episcleral vein cauterization rat glaucoma model. Exp. Eye Res. 2006;82:219–228. doi: 10.1016/j.exer.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies VJ, Hollins AJ, Piechota MJ, Yip W, Davies JR, White KE, Nicols PP, Boulton ME, Votruba M. Opa1 deficiency in a mouse model of auto-somal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum. Mol. Genet. 2007;16:1307–1318. doi: 10.1093/hmg/ddm079. [DOI] [PubMed] [Google Scholar]
- Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, Kinebuchi M, Phan S, Zhou Y, Bihlmeyer NA, Nguyen JV, Jin Y, Ellisman MH, Marsh-Armstrong N. Transcellular degradation of axonal mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2014;111:9633–9638. doi: 10.1073/pnas.1404651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckwerth TL, Easton RM, Knudson CM, Korsmeyer SJ, Johnson EM., Jr Placement of the BCL2 family member BAX in the death pathway of sympathetic neurons activated by trophic factor deprivation. Exp. Neurol. 1998;152:150–162. doi: 10.1006/exnr.1998.6846. [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Elliott JL, Knudson CM, Johnson EM, Jr, Snider WD, Korsmeyer SJ. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron. 1996;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Johnson EM., Jr Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis) Dev. Biol. 1994;165:63–72. doi: 10.1006/dbio.1994.1234. [DOI] [PubMed] [Google Scholar]
- Denisov AY, Madiraju MS, Chen G, Khadir A, Beauparlant P, Attardo G, Shore GC, Gehring K. Solution structure of human BCL-w: modulation of ligand binding by the C-terminal helix. J. Biol. Chem. 2003;278:21124–21128. doi: 10.1074/jbc.M301798200. [DOI] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, Antonsson B, Martinou JC. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol. Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson G, Ma S, Frederick P, Hockings C, Tan I, Kratina T, Kluck RM. Bax dimerizes via a symmetric BH3:groove interface during apoptosis. Cell Death Differ. 2012;19:661–670. doi: 10.1038/cdd.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan M, Doonan F, Cotter TG. Decreased expression of pro-apoptotic Bcl-2 family members during retinal development and differential sensitivity to cell death. Dev. Biol. 2006;291:154–169. doi: 10.1016/j.ydbio.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, Neutzner A, Tjandra N, Youle RJ. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G, Michalak E, Strasser A, Villunger A. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J. Exp. Med. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligo-merization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KA, Harder JM, Fornarola LB, Freeman RS, Clark AF, Pang IH, John SW, Libby RT. JNK2 and JNK3 are major regulators of axonal injury-induced retinal ganglion cell death. Neurobiol. Dis. 2012;46:393–401. doi: 10.1016/j.nbd.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KA, Harder JM, John SW, Shrager P, Libby RT. DLK-dependent signaling is important for somal but not axonal degeneration of retinal ganglion cells following axonal injury. Neurobiol. Dis. 2014;69:108–116. doi: 10.1016/j.nbd.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KA, Harder JM, Kim J, Libby RT. JUN regulates early tran-scriptional responses to axonal injury in retinal ganglion cells. Exp. Eye Res. 2013;112:106–117. doi: 10.1016/j.exer.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, Bartlett CA, Harvey AR, Dunlop SA. Early events of secondary degeneration after partial optic nerve transection: an immunohisto-chemical study. J. Neurotrauma. 2010;27:439–452. doi: 10.1089/neu.2009.1112. [DOI] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Fuchs SY, Adler V, Buschmann T, Wu X, Ronai Z. Mdm2 association with p53 targets its ubiquitination. Oncogene. 1998;17:2543–2547. doi: 10.1038/sj.onc.1202200. [DOI] [PubMed] [Google Scholar]
- Fujita K, Nishiguchi KM, Yokoyama Y, Tomiyama Y, Tsuda S, Yasuda M, Maekawa S, Nakazawa T. In vivo cellular imaging of various stress/response pathways using AAV following axonal injury in mice. Sci. Rep. 2015;5:18141. doi: 10.1038/srep18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaasterland D, Tanishima T, Kuwabara T. Axoplasmic flow during chronic experimental glaucoma. 1. Light and electron microscopic studies of the monkey optic nervehead during development of glaucomatous cupping. Invest. Ophthalmol. Vis. Sci. 1978;17:838–846. [PubMed] [Google Scholar]
- Galindo-Romero C, Valiente-Soriano FJ, Jimenez-Lopez M, Garcia-Ayuso D, Villegas-Perez MP, Vidal-Sanz M, Agudo-Barriuso M. Effect of brain-derived neurotrophic factor on mouse axotomized retinal ganglion cells and phagocytic microglia. Invest. Ophthalmol. Vis. Sci. 2013;54:974–985. doi: 10.1167/iovs.12-11207. [DOI] [PubMed] [Google Scholar]
- Garcia-Saez AJ, Mingarro I, Perez-Paya E, Salgado J. Membrane-insertion fragments of bcl-xL, bax, and bid. Biochemistry. 2004;43:10930–10943. doi: 10.1021/bi036044c. [DOI] [PubMed] [Google Scholar]
- Garcia-Valenzuela E, Gorczyca W, Darzynkiewicz Z, Sharma SC. Apoptosis in adult retinal ganglion cells after axotomy. J. Neurobiol. 1994;25:431–438. doi: 10.1002/neu.480250408. [DOI] [PubMed] [Google Scholar]
- Garcia-Valenzuela E, Shareef S, Walsh J, Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp. Eye Res. 1995;61:33–44. doi: 10.1016/s0014-4835(95)80056-5. [DOI] [PubMed] [Google Scholar]
- Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol. Cell. 2010;40:481–492. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George NM, Evans JJ, Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes. Dev. 2007;21:1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RJ, Dalla Serra M, Froelich CJ, Wallace MI, Anderluh G. Membrane pore formation at protein-lipid interfaces. Trends Biochem. Sci. 2014;39:510–516. doi: 10.1016/j.tibs.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Gillies LA, Du H, Peters B, Knudson CM, Newmeyer DD, Kuwana T. Visual and functional demonstration of growing Bax-induced pores in mitochondrial outer membranes. Mol. Biol. Cell. 2015;26:339–349. doi: 10.1091/mbc.E13-11-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. Embo J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse L, Wurm CA, Bruser C, Neumann D, Jans DC, Jakobs S. Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. Embo J. 2016;35:402–413. doi: 10.15252/embj.201592789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Chen X, Zhang H, Li N, Yang X, Cheng W, Zhao K. Association of OPA1 polymorphisms with NTG and HTG: a meta-analysis. PLoS One. 2012;7:e42387. doi: 10.1371/journal.pone.0042387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn P, Lindsten T, Lyubarsky A, Ying GS, Pugh EN, Jr, Thompson CB, Dunaief JL. Deficiency of Bax and Bak protects photoreceptors from light damage in vivo. Cell Death Differ. 2004;11:1192–1197. doi: 10.1038/sj.cdd.4401486. [DOI] [PubMed] [Google Scholar]
- Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Nakayama K, Hatakeyama S. Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J. Exp. Med. 1998;188:1985–1992. doi: 10.1084/jem.188.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happo L, Strasser A, Cory S. BH3-only proteins in apoptosis at a glance. J. Cell Sci. 2012;125:1081–1087. doi: 10.1242/jcs.090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Ding Q, Fernandes KA, Cherry JD, Gan L, Libby RT. BCL2L1 (BCL-X) promotes survival of adult and developing retinal ganglion cells. Mol. Cell Neurosci. 2012a;51:53–59. doi: 10.1016/j.mcn.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Fernandes KA, Libby RT. The Bcl-2 family member BIM has multiple glaucoma-relevant functions in DBA/2J mice. Sci. Rep. 2012b;2:530. doi: 10.1038/srep00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Libby RT. BBC3 (PUMA) regulates developmental apoptosis but not axonal injury induced death in the retina. Mol. Neurodegener. 2011;6:50. doi: 10.1186/1750-1326-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Libby RT. Deficiency in Bim, Bid and Bbc3 (Puma) do not prevent axonal injury induced death. Cell Death Differ. 2013;20:182. doi: 10.1038/cdd.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwerth RS, Carter-Dawson L, Shen F, Smith EL, 3rd, Crawford ML. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 1999;40:2242–2250. [PubMed] [Google Scholar]
- Harwerth RS, Crawford ML, Frishman LJ, Viswanathan S, Smith EL, 3rd, Carter-Dawson L. Visual field defects and neural losses from experimental glaucoma. Prog. Retin Eye Res. 2002;21:91–125. doi: 10.1016/s1350-9462(01)00022-2. [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Ruitenberg MJ, Pollett MA, Ehlert EM, Twisk J, Verhaagen J, Harvey AR. Cellular tropism and transduction properties of seven adeno-associated viral vector serotypes in adult retina after intravitreal injection. Gene Ther. 2009;16:521–532. doi: 10.1038/gt.2008.178. [DOI] [PubMed] [Google Scholar]
- Hengartner MO, Horvitz HR. Activation of C. elegans cell death protein CED-9 by an amino-acid substitution in a domain conserved in Bcl-2. Nature. 1994;369:318–320. doi: 10.1038/369318a0. [DOI] [PubMed] [Google Scholar]
- Hinds MG, Smits C, Fredericks-Short R, Risk JM, Bailey M, Huang DC, Day CL. Bim, Bad and Bmf: intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell Death Differ. 2007;14:128–136. doi: 10.1038/sj.cdd.4401934. [DOI] [PubMed] [Google Scholar]
- Hoppins S, Edlich F, Cleland MM, Banerjee S, McCaffery JM, Youle RJ, Nunnari J. The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol. Cell. 2011;41:150–160. doi: 10.1016/j.molcel.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, Mahesh N, Porciatti V, Whitmore AV, Masland RH, John SW. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells CC, Baumann WT, Samuels DC, Finkielstein CV. The Bcl-2-associated death promoter (BAD) lowers the threshold at which the Bcl-2-interacting domain death agonist (BID) triggers mitochondria disintegration. J. Theor. Biol. 2011;271:114–123. doi: 10.1016/j.jtbi.2010.11.040. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of bax and bcl-x(L) during apoptosis. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- Huang W, Fileta JB, Dobberfuhl A, Filippopolous T, Guo Y, Kwon G, Grosskreutz CL. Calcineurin cleavage is triggered by elevated intraocular pressure, and calcineurin inhibition blocks retinal ganglion cell death in experimental glaucoma. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12242–12247. doi: 10.1073/pnas.0505138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntwork-Rodriguez S, Wang B, Watkins T, Ghosh AS, Pozniak CD, Bustos D, Newton K, Kirkpatrick DS, Lewcock JW. JNK-mediated phosphoryla-tion of DLK suppresses its ubiquitination to promote neuronal apoptosis. J. Cell Biol. 2013;202:747–763. doi: 10.1083/jcb.201303066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K, Koike T. Non-apoptotic neurite degeneration in apoptotic neuronal death: pivotal role of mitochondrial function in neurites. NeuroScience. 2003;122:617–626. doi: 10.1016/j.neuroscience.2003.08.057. [DOI] [PubMed] [Google Scholar]
- Imaizumi K, Benito A, Kiryu-Seo S, Gonzalez V, Inohara N, Lieberman AP, Kiyama H, Nunez G. Critical role for DP5/Harakiri, a Bcl-2 homology domain 3-only Bcl-2 family member, in axotomy-induced neuronal cell death. J. Neurosci. 2004;24:3721–3725. doi: 10.1523/JNEUROSCI.5101-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenmann S, Wahl C, Krajewski S, Reed JC, Bahr M. Up-regulation of Bax protein in degenerating retinal ganglion cells precedes apoptotic cell death after optic nerve lesion in the rat. Eur. J. Neurosci. 1997;9:1763–1772. doi: 10.1111/j.1460-9568.1997.tb01534.x. [DOI] [PubMed] [Google Scholar]
- Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J. Cell Biol. 2005;171:313–325. doi: 10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobson M, Jakobson M, Llano O, Palgi J, Arumae U. Multiple mechanisms repress N-Bak mRNA translation in the healthy and apoptotic neurons. Cell Death Dis. 2013;4:e777. doi: 10.1038/cddis.2013.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobson M, Lintulahti A, Arumae U. mRNA for N-Bak, a neuron-specific BH3-only splice isoform of Bak, escapes nonsense-mediated decay and is translationally repressed in the neurons. Cell Death Dis. 2012;3:e269. doi: 10.1038/cddis.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen KT, Mac Nair CE, Dietz JA, Schlamp CL, Nickells RW. Nuclear atrophy of retinal ganglion cells precedes the bax-dependent stage of apoptosis. Invest. Ophthalmol. Vis. Sci. 2013;54:1805–1815. doi: 10.1167/iovs.11-9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest. Ophthalmol. Vis. Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- Ju WK, Kim KY, Lindsey JD, Angert M, Duong-Polk KX, Scott RT, Kim JJ, Kukhmazov I, Ellisman MH, Perkins GA, Weinreb RN. Intraocular pressure elevation induces mitochondrial fission and triggers OPA1 release in glaucomatous optic nerve. Invest. Ophthalmol. Vis. Sci. 2008;49:4903–4911. doi: 10.1167/iovs.07-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of bax and bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10:870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829–730. [DOI] [PubMed] [Google Scholar]
- Ke F, Voss A, Kerr JB, O’Reilly LA, Tai L, Echeverry N, Bouillet P, Strasser A, Kaufmann T. BCL-2 family member BOK is widely expressed but its loss has only minimal impact in mice. Cell Death Differ. 2012;19:915–925. doi: 10.1038/cdd.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermer P, Ankerhold R, Klocker N, Krajewski S, Reed JC, Bahr M. Cas-pase-9: involvement in secondary death of axotomized rat retinal ganglion cells in vivo. Brain Res. Mol. Brain Res. 2000;85:144–150. doi: 10.1016/s0169-328x(00)00256-4. [DOI] [PubMed] [Google Scholar]
- Kermer P, Klocker N, Bahr M. Long-term effect of inhibition of ced 3-like caspases on the survival of axotomized retinal ganglion cells in vivo. Exp. Neurol. 1999a;158:202–205. doi: 10.1006/exnr.1999.7094. [DOI] [PubMed] [Google Scholar]
- Kermer P, Klocker N, Labes M, Thomsen S, Srinivasan A, Bahr M. Activation of caspase-3 in axotomized rat retinal ganglion cells in vivo. FEBS Lett. 1999b;453:361–364. doi: 10.1016/s0014-5793(99)00747-4. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol. Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Perkins GA, Shim MS, Bushong E, Alcasid N, Ju S, Ellisman MH, Weinreb RN, Ju WK. DRP1 inhibition rescues retinal ganglion cells and their axons by preserving mitochondrial integrity in a mouse model of glaucoma. Cell Death Dis. 2015;6:e1839. doi: 10.1038/cddis.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryu-Seo S, Hirayama T, Kato R, Kiyama H. Noxa is a critical mediator of p53-dependent motor neuron death after nerve injury in adult mouse. J. Neurosci. 2005;25:1442–1447. doi: 10.1523/JNEUROSCI.4041-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaoka Y, Kitaoka Y, Kwong JM, Ross-Cisneros FN, Wang J, Tsai RK, Sadun AA, Lam TT. TNF-alpha-induced optic nerve degeneration and nuclear factor-kappaB p65. Invest. Ophthalmol. Vis. Sci. 2006;47:1448–1457. doi: 10.1167/iovs.05-0299. [DOI] [PubMed] [Google Scholar]
- Kitazumi I, Tsukahara M. Regulation of DNA fragmentation: the role of caspases and phosphorylation. Febs J. 2011;278:427–441. doi: 10.1111/j.1742-4658.2010.07975.x. [DOI] [PubMed] [Google Scholar]
- Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Ko ML, Peng PH, Ma MC, Ritch R, Chen CF. Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic. Biol. Med. 2005;39:365–373. doi: 10.1016/j.freeradbiomed.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Koeberle PD, Wang Y, Schlichter LC. Kv1.1 and Kv1.3 channels contribute to the degeneration of retinal ganglion cells after optic nerve transection in vivo. Cell Death Differ. 2010;17:134–144. doi: 10.1038/cdd.2009.113. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ. Bcl-2: an antidote to programmed cell death. Cancer Surv. 1992;15:105–118. [PubMed] [Google Scholar]
- Kushnareva Y, Andreyev AY, Kuwana T, Newmeyer DD. Bax activation initiates the assembly of a multimeric catalyst that facilitates Bax pore formation in mitochondrial outer membranes. PLoS Biol. 2012;10:e1001394. doi: 10.1371/journal.pbio.1001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form su-pramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Olson NH, Kiosses WB, Peters B, Newmeyer DD. Pro-apoptotic Bax molecules densely populate the edges of membrane pores. Sci. Rep. 2016;6:27299. doi: 10.1038/srep27299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O’Reilly L, Strasser A, Villunger A. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J. Exp. Med. 2008;205:641–655. doi: 10.1084/jem.20071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lartigue L, Medina C, Schembri L, Chabert P, Zanese M, Tomasello F, Dalibart R, Thoraval D, Crouzet M, Ichas F, De Giorgi F. An intracellular wave of cytochrome c propagates and precedes Bax redistribution during apoptosis. J. Cell Sci. 2008;121:3515–3523. doi: 10.1242/jcs.029587. [DOI] [PubMed] [Google Scholar]
- Lebrun-Julien F, Suter U. Combined HDAC1 and HDAC2 depletion promotes retinal ganglion cell survival after injury through reduction of p53 target gene expression. ASN Neuro. 2015:7. doi: 10.1177/1759091415593066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun-Julien F, Duplan L, Pernet V, Osswald I, Sapieha P, Bourgeois P, Dickson K, Bowie D, Barker PA, Di Polo A. Excitotoxic death of retinal neurons in vivo occurs via a non-cell-autonomous mechanism. J. Neurosci. 2009;29:5536–5545. doi: 10.1523/JNEUROSCI.0831-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann U, Heuss ND, McPherson SW, Roehrich H, Gregerson DS. Dendritic cells are early responders to retinal injury. Neurobiol. Dis. 2010;40:17 7 e184. doi: 10.1016/j.nbd.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Levin LA, Schlamp CL, Spieldoch RL, Geszvain KM, Nickells RW. Identification of the bcl-2 family of genes in the rat retina. Invest. Ophthalmol. Vis. Sci. 1997;38:2545–2553. [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Dardik R, Vander S, Melamed S. Mechanism of retinal ganglion cells death in secondary degeneration of the optic nerve. Exp. Eye Res. 2010;91:127–134. doi: 10.1016/j.exer.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Kalev-Landoy M, Habot-Wilner Z, Melamed S. Minocycline delays death of retinal ganglion cells in experimental glaucoma and after optic nerve transection. Arch. Ophthalmol. 2006;124:520–526. doi: 10.1001/archopht.124.4.520. [DOI] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Quigley HA, Kerrigan-Baumrind LA, D’Anna SA, Kerrigan D, Pease ME. Optic nerve transection in monkeys may result in secondary degeneration of retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2001;42:975–982. [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Quigley HA, Martin KR, Valenta D, Baumrind LA, Pease ME. Translimbal laser photocoagulation to the trabecular mesh-work as a model of glaucoma in rats. Invest. Ophthalmol. Vis. Sci. 2002;43:402–410. [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Spierer O, Vander S, Dardik R. Similarities and differences between primary and secondary degeneration of the optic nerve and the effect of minocycline. Graefes Arch. Clin. Exp. Ophthalmol. 2011;249:849–857. doi: 10.1007/s00417-010-1608-2. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Schlamp CL, Nickells RW. Experimental induction of retinal ganglion cell death in adult mice. Invest. Ophthalmol. Vis. Sci. 1999;40:1004–1008. [PubMed] [Google Scholar]
- Li Y, Schlamp CL, Poulsen GL, Jackson MW, Griep AE, Nickells RW. p53 regulates apoptotic retinal ganglion cell death induced by N-methyl-D-aspartate. Mol. Vis. 2002;8:341–350. [PubMed] [Google Scholar]
- Li Y, Schlamp CL, Poulsen KP, Nickells RW. Bax-dependent and independent pathways of retinal ganglion cell death induced by different damaging stimuli. Exp. Eye Res. 2000;71:209–213. doi: 10.1006/exer.2000.0873. [DOI] [PubMed] [Google Scholar]
- Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J. Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Anderson MG, Pang IH, Robinson ZH, Savinova OV, Cosma IM, Snow A, Wilson LA, Smith RS, Clark AF, John SW. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neuro-degeneration. Vis. Neurosci. 2005a;22:637–648. doi: 10.1017/S0952523805225130. [DOI] [PubMed] [Google Scholar]
- Libby RT, Li Y, Savinova OV, Barter J, Smith RS, Nickells RW, John SW. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005b;1:17–26. doi: 10.1371/journal.pgen.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingor P, Koeberle P, Kugler S, Bahr M. Down-regulation of apoptosis mediators by RNAi inhibits axotomy-induced retinal ganglion cell death in vivo. Brain. 2005;128:550–558. doi: 10.1093/brain/awh382. [DOI] [PubMed] [Google Scholar]
- Liu H, Sun H, Liu C. Interference of the apoptotic signaling pathway in RGC stress response by SP600125 in moderate ocular hypertensive rats. Chin. J. Physiol. 2011;54:124–132. [PubMed] [Google Scholar]
- Liu XH, Collier RJ, Youle RJ. Inhibition of axotomy-induced neuronal apoptosis by extracellular delivery of a Bcl-XL fusion protein. J. Biol. Chem. 2001;276:46326–46332. doi: 10.1074/jbc.M108930200. [DOI] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Ma C, Ying C, Yuan Z, Song B, Li D, Liu Y, Lai B, Li W, Chen R, Ching YP, Li M. dp5/HRK is a c-Jun target gene and required for apoptosis induced by potassium deprivation in cerebellar granule neurons. J. Biol. Chem. 2007;282:30901–30909. doi: 10.1074/jbc.M608694200. [DOI] [PubMed] [Google Scholar]
- Mac Nair CE, Fernandes KA, Schlamp CL, Libby RT, Nickells RW. Tumor necrosis factor alpha has an early protective effect on retinal ganglion cells after optic nerve crush. J. Neuroinflammation. 2014;11:194. doi: 10.1186/s12974-014-0194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Nair CE, Nickells RW. Neuroinflammation in glaucoma and optic nerve damage. Prog. Mol. Biol. Transl. Sci. 2015;134:343–363. doi: 10.1016/bs.pmbts.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Mac Nair CE, Schlamp CL, Montgomery AD, Shestopalov VI, Nickells RW. Retinal glial responses to optic nerve crush are attenuated in Bax-deficient mice and modulated by purinergic signaling pathways. J. Neuroinflammation. 2016;13:93. doi: 10.1186/s12974-016-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan MC, Sadun AA, Rao NS, Dugel PU, Tenhula WN, Gill PS. Tumor necrosis factor-alpha (TNF-alpha)-induced optic neuropathy in rabbits. Neurol. Res. 1996;18:176–184. doi: 10.1080/01616412.1996.11740399. [DOI] [PubMed] [Google Scholar]
- Malik JM, Shevtsova Z, Bahr M, Kugler S. Long-term in vivo inhibition of CNS neurodegeneration by Bcl-XL gene transfer. Mol. Ther. 2005;11:373–381. doi: 10.1016/j.ymthe.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Marquez RT, Xu L. Bcl-2:Beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am. J. Cancer Res. 2012;2:214–221. [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, et al. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Youle RJ. Mitochondria in apoptosis: bcl-2 family members and mitochondrial dynamics. Dev. Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara A, Nakazawa T, Husain D, Iliaki E, Connolly E, Michaud NA, Gragoudas ES, Miller JW. Investigating the effect of ciliary body photodynamic therapy in a glaucoma mouse model. Invest. Ophthalmol. Vis. Sci. 2006;47:2498–2507. doi: 10.1167/iovs.05-0959. [DOI] [PubMed] [Google Scholar]
- McDonnell JM, Fushman D, Milliman CL, Korsmeyer SJ, Cowburn D. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell. 1999;96:625–634. doi: 10.1016/s0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- McKernan DP, Cotter TG. A Critical role for Bim in retinal ganglion cell death. J. Neurochem. 2007;102:922–930. doi: 10.1111/j.1471-4159.2007.04573.x. [DOI] [PubMed] [Google Scholar]
- McKinnon SJ, Schlamp CL, Nickells RW. Mouse models of retinal ganglion cell death and glaucoma. Exp. Eye Res. 2009;88:816–824. doi: 10.1016/j.exer.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu T, Liu Q, Tocilj A, Watson M, Shore G, Gehring K. The X-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol. Cell. 2006;24:677–688. doi: 10.1016/j.molcel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, Martinou JC. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JC, Johnson EC, Cepurna W, Jia L. Understanding mechanisms of pressure-induced optic nerve damage. Prog. Retin Eye Res. 2005;24:217–240. doi: 10.1016/j.preteyeres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Mosinger Ogilvie J, Deckwerth TL, Knudson CM, Korsmeyer SJ. Suppression of developmental retinal cell death but not of photoreceptor degeneration in Bax-deficient mice. Invest. Ophthalmol. Vis. Sci. 1998;39:1713–1720. [PubMed] [Google Scholar]
- Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al. Massive cell death of immature he-matopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SL, Fesik SW. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- Nadal-Nicolas FM, Jimenez-Lopez M, Salinas-Navarro M, Sobrado-Calvo P, Alburquerque-Bejar JJ, Vidal-Sanz M, Agudo-Barriuso M. Whole number, distribution and co-expression of brn3 transcription factors in retinal ganglion cells of adult albino and pigmented rats. PLoS One. 2012;7:e49830. doi: 10.1371/journal.pone.0049830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima M, Fujikawa C, Mawatari K, Mori Y, Kato S. HSP70, the earliest-induced gene in the zebrafish retina during optic nerve regeneration: its role in cell survival. Neurochem. Int. 2011;58:888–895. doi: 10.1016/j.neuint.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW, Benowitz LI. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J. Neurosci. 2006;26:12633–12641. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napankangas U, Lindqvist N, Lindholm D, Hallbook F. Rat retinal ganglion cells upregulate the pro-apoptotic BH3-only protein Bim after optic nerve transection. Brain Res. Mol. Brain Res. 2003;120:30–37. doi: 10.1016/j.molbrainres.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickells RW, Howell GR, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu. Rev. Neurosci. 2012;35:153–179. doi: 10.1146/annurev.neuro.051508.135728. [DOI] [PubMed] [Google Scholar]
- Nickells RW, Schlamp CL, Li Y, Kaufman PL, Heatley G, Peterson JC, Faha B, Ver Hoeve JN. Surgical lowering of elevated intraocular pressure in monkeys prevents progression of glaucomatous disease. Exp. Eye Res. 2007;84:729–736. doi: 10.1016/j.exer.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: beclin 1 is a novel BH3-only protein. J. Biol. Chem. 2007;282:13123–13132. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Opferman JT, Korsmeyer SJ. Apoptosis in the development and maintenance of the immune system. Nat. Immunol. 2003;4:410–415. doi: 10.1038/ni0503-410. [DOI] [PubMed] [Google Scholar]
- Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J. Neurosci. 2003;23:4798–4802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant SD, March LD, Famulski JK, French CR, Lehmann OJ, Waskiewicz AJ. Molecular mechanisms regulating ocular apoptosis in zebrafish gdf6a mutants. Invest. Ophthalmol. Vis. Sci. 2013;54:5871–5879. doi: 10.1167/iovs.12-11315. [DOI] [PubMed] [Google Scholar]
- Park SW, Kim KY, Lindsey JD, Dai Y, Heo H, Nguyen DH, Ellisman MH, Weinreb RN, Ju WK. A selective inhibitor of drp1, mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina. Invest. Oph-thalmol. Vis. Sci. 2011;52:2837–2843. doi: 10.1167/iovs.09-5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Payne SC, Bartlett CA, Harvey AR, Dunlop SA, Fitzgerald M. Myelin sheath decompaction, axon swelling, and functional loss during chronic secondary degeneration in rat optic nerve. Invest. Ophthalmol. Vis. Sci. 2012;53:6093–6101. doi: 10.1167/iovs.12-10080. [DOI] [PubMed] [Google Scholar]
- Pease ME, McKinnon SJ, Quigley HA, Kerrigan-Baumrind LA, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2000;41:764–774. [PubMed] [Google Scholar]
- Pegoraro L, Palumbo A, Erikson J, Falda M, Giovanazzo B, Emanuel BS, Rovera G, Nowell PC, Croce CM. A 14;18 and an 8;14 chromosome translocation in a cell line derived from an acute B-cell leukemia. Proc. Natl. Acad. Sci. U. S. A. 1984;81:7166–7170. doi: 10.1073/pnas.81.22.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzel HR, Schlamp CL, Nickells RW. Histone H4 deacetylation plays a critical role in early gene silencing during neuronal apoptosis. BMC Neurosci. 2010;11:62. doi: 10.1186/1471-2202-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzel HR, Schlamp CL, Waclawski M, Shaw MK, Nickells RW. Silencing of Fem1cR3 gene expression in the DBA/2J mouse precedes retinal ganglion cell death and is associated with histone deacetylase activity. Invest. Ophthalmol. Vis. Sci. 2012;53:1428–1435. doi: 10.1167/iovs.11-8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros AM, Medek A, Nettesheim DG, Kim DH, Yoon HS, Swift K, Matayoshi ED, Oltersdorf T, Fesik SW. Solution structure of the anti-apoptotic protein bcl-2. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3012–3017. doi: 10.1073/pnas.041619798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, de Kretser D, Metcalf D, Kontgen F, Adams JM, Cory S. Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12424–12431. doi: 10.1073/pnas.95.21.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Villunger A, O’Reilly LA, Beaumont JG, Coultas L, Cheney RE, Huang DC, Strasser A. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293:1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- Qian S, Wang W, Yang L, Huang HW. Structure of transmembrane pore induced by Bax-derived peptide: evidence for lipidic pores. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17379–17383. doi: 10.1073/pnas.0807764105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q, Patil K, Sharma SC. The role of Bax-inhibiting peptide in retinal ganglion cell apoptosis after optic nerve transection. Neurosci. Lett. 2004;372:17–21. doi: 10.1016/j.neulet.2004.08.075. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Open-angle glaucoma. N. Engl. J. Med. 1993;328:1097–1106. doi: 10.1056/NEJM199304153281507. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch. Oph-thalmol. 1981;99:137–143. doi: 10.1001/archopht.1981.03930010139020. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest. Ophthalmol. Vis. Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- Radius RL, Bade B. Pressure-induced optic nerve axonal transport interruption in cat eyes. Arch. Ophthalmol. 1981;99:2163–2165. doi: 10.1001/archopht.1981.03930021039011. [DOI] [PubMed] [Google Scholar]
- Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, Kutok JL, Le Beau MM, Greenberg ME, Korsmeyer SJ. Bad-deficient mice develop diffuse large B cell lymphoma. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes. Dev. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- Roh M, Zhang Y, Murakami Y, Thanos A, Lee SC, Vavvas DG, Benowitz LI, Miller JW. Etanercept, a widely used inhibitor of tumor necrosis factor-alpha (TNF-alpha), prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS One. 2012;7:e40065. doi: 10.1371/journal.pone.0040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rosenbaum PS, Gupta H, Singh M, Aggarwal A, Hall DH, Roth S, Kessler JA. The role of the p53 protein in the selective vulnerability of the inner retina to transient ischemia. Invest. Ophthalmol. Vis. Sci. 1998;39:2132–e2139. [PubMed] [Google Scholar]
- Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, MacGregor GR. Testicular degeneration in Bclw-deficient mice. Nat. Genet. 1998;18:251–256. doi: 10.1038/ng0398-251. [DOI] [PubMed] [Google Scholar]
- Ruchaud S, Korfali N, Villa P, Kottke TJ, Dingwall C, Kaufmann SH, Earnshaw WC. Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation. Embo J. 2002;21:1967–1977. doi: 10.1093/emboj/21.8.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Korsmeyer SJ, Schlesinger PH. BAX-dependent transport of cy-tochrome c reconstituted in pure liposomes. Nat. Cell Biol. 2000;2:553e555. doi: 10.1038/35019596. [DOI] [PubMed] [Google Scholar]
- Salvador-Gallego R, Mund M, Cosentino K, Schneider J, Unsay J, Schraermeyer U, Engelhardt J, Ries J, Garcia-Saez AJ. Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. Embo J. 2016;35:389–401. doi: 10.15252/embj.201593384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappington RM, Carlson BJ, Crish SD, Calkins DJ. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest. Ophthalmol. Vis. Sci. 2010;51:207–216. doi: 10.1167/iovs.09-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg B, Wang P, Keeble JA, Rodriguez-Enriquez R, Walker S, Owens TW, Foster F, Tanianis-Hughes J, Brennan K, Streuli CH, Gilmore AP. Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol. Cell. 2013;49:959–971. doi: 10.1016/j.molcel.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinzel A, Kaufmann T, Schuler M, Martinalbo J, Grubb D, Borner C. Conformational control of Bax localization and apoptotic activity by Pro168. J. Cell Biol. 2004;164:1021–1032. doi: 10.1083/jcb.200309013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlamp CL, Johnson EC, Li Y, Morrison JC, Nickells RW. Changes in Thy1 gene expression associated with damaged retinal ganglion cells. Mol. Vis. 2001;7:19 2 e201. [PubMed] [Google Scholar]
- Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006;7:66. doi: 10.1186/1471-2202-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlamp CL, Montgomery AD, Mac Nair CE, Schuart C, Willmer DJ, Nickells RW. Evaluation of the percentage of ganglion cells in the ganglion cell layer of the rodent retina. Mol. Vis. 2013;19:1387–1396. [PMC free article] [PubMed] [Google Scholar]
- Schmidt T, Korner K, Karsunky H, Korsmeyer S, Müller R, Moroy T. The activity of the murine Bax promoter is regulated by Sp1/3 and E-box binding proteins but not by p53. Cell Death Differ. 1999;6:873–882. doi: 10.1038/sj.cdd.4400562. [DOI] [PubMed] [Google Scholar]
- Schmitt HM, Pelzel HR, Schlamp CL, Nickells RW. Histone deacetylase 3 (HDAC3) plays an important role in retinal ganglion cell death after acute optic nerve injury. Mol. Neurodegener. 2014;9:39. doi: 10.1186/1750-1326-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz M, Buder T, Seeber S, Adamek E, Becker CM, Lutjen-Drecoll E. Dependency of intraocular pressure elevation and glaucomatous changes in DBA/2J and DBA/2J–Rj mice. Invest. Ophthalmol. Vis. Sci. 2008;49:613–621. doi: 10.1167/iovs.07-0745. [DOI] [PubMed] [Google Scholar]
- Schuettauf F, Quinto K, Naskar R, Zurakowski D. Effects of anti-glaucoma medications on ganglion cell survival: the DBA/2J mouse model. Vis. Res. 2002;42:2333–2337. doi: 10.1016/s0042-6989(02)00188-8. [DOI] [PubMed] [Google Scholar]
- Semaan SJ, Li Y, Nickells RW. A single nucleotide polymorphism in the Bax gene promoter affects transcription and influences retinal ganglion cell death. ASN neuro. 2010;2:e00032. doi: 10.1042/AN20100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaan SJ, Nickells RW. The apoptotic response in HCT116BAX−/− cancer cells becomes rapidly saturated with increasing expression of a GFP-BAX fusion protein. BMC Cancer. 2010;10:554. doi: 10.1186/1471-2407-10-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim MS, Takihara Y, Kim KY, Iwata T, Yue BY, Inatani M, Weinreb RN, Perkins GA, Ju WK. Mitochondrial pathogenic mechanism and degradation in optineurin E50K mutation-mediated retinal ganglion cell degeneration. Sci. Rep. 2016;6:33830. doi: 10.1038/srep33830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto I, Howell GR. The complex role of neuroinflammation in glaucoma. Cold Spring Harb. Perspect. Med. 2014:4. doi: 10.1101/cshperspect.a017269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto I, Pease ME, Son JL, Shi X, Quigley HA, Marsh-Armstrong N. Retinal ganglion cell loss in a rat ocular hypertension model is sectorial and involves early optic nerve axon loss. Invest. Ophthalmol. Vis. Sci. 2011;52:434–441. doi: 10.1167/iovs.10-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subburaj Y, Cosentino K, Axmann M, Pedrueza-Villalmanzo E, Hermann E, Bleicken S, Spatz J, Garcia-Saez AJ. Bax monomers form dimer units in the membrane that further self-assemble into multiple oligomeric species. Nat. Commun. 2015;6:8042. doi: 10.1038/ncomms9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Oppenheim RW. Response of motoneurons to neonatal sciatic nerve axotomy in Bax-knockout mice. Mol. Cell Neurosci. 2003;24:875–886. doi: 10.1016/s1044-7431(03)00219-7. [DOI] [PubMed] [Google Scholar]
- Sun YF, Yu LY, Saarma M, Arumae U. Mutational analysis of N-Bak reveals different structural requirements for antiapoptotic activity in neurons and proapoptotic activity in nonneuronal cells. Mol. Cell Neurosci. 2003;23:134–143. doi: 10.1016/s1044-7431(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Sundararajan R, Cuconati A, Nelson D, White E. Tumor necrosis factor-alpha induces Bax-Bak interaction and apoptosis, which is inhibited by adenovirus E1B 19K. J. Biol. Chem. 2001;276:45120–45127. doi: 10.1074/jbc.M106386200. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2×7 receptor-activated microglia. J. Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait SW, Green DR. Mitochondria and cell death: outer membrane per-meabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- Tezel G. TNF-alpha signaling in glaucomatous neurodegeneration. Prog. Brain Res. 2008;173:409–421. doi: 10.1016/S0079-6123(08)01128-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Yang J, Wax MB. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 2004;996:202–212. doi: 10.1016/j.brainres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Thornborrow EC, Manfredi JJ. The tumor suppressor protein p53 requires a cofactor to activate transcriptionally the human BAX promoter. J. Biol. Chem. 2001;276:15598–15608. doi: 10.1074/jbc.M011643200. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- Uo T, Kinoshita Y, Morrison RS. Neurons exclusively express N-Bak, a BH3 domain-only Bak isoform that promotes neuronal apoptosis. J. Biol. Chem. 2005;280:9065–9073. doi: 10.1074/jbc.M413030200. [DOI] [PubMed] [Google Scholar]
- Valentijn AJ, Upton JP, Bates N, Gilmore AP. Bax targeting to mitochondria occurs via both tail anchor-dependent and -independent mechanisms. Cell Death Differ. 2008;15:1243–1254. doi: 10.1038/cdd.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypo-pigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- Villegas-Perez MP, Vidal-Sanz M, Rasminsky M, Bray GM, Aguayo AJ. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J. Neurobiol. 1993;24:23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Kosaka J, Hommura S. Up-regulation of Hrk, a regulator of cell death, in retinal ganglion cells of axotomized rat retina. Neurosci. Lett. 2002;318:77–80. doi: 10.1016/s0304-3940(01)02487-9. [DOI] [PubMed] [Google Scholar]
- Walensky LD, Pitter K, Morash J, Oh KJ, Barbuto S, Fisher J, Smith E, Verdine GL, Korsmeyer SJ. A stapled BID BH3 helix directly binds and activates BAX. Mol. Cell. 2006;24:199–e210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Wang K, Gross A, Waksman G, Korsmeyer SJ. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol. Cell Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wang P, Liu B, Zhao J, Pang Q, Agrawal SG, Jia L, Liu FT. Dynamin-related protein Drp1 is required for Bax translocation to mitochondria in response to irradiation-induced apoptosis. Oncotarget. 2015;6:22598–22612. doi: 10.18632/oncotarget.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins TA, Wang B, Huntwork-Rodriguez S, Yang J, Jiang Z, Eastham-Anderson J, Modrusan Z, Kaminker JS, Tessier-Lavigne M, Lewcock JW. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4039–4044. doi: 10.1073/pnas.1211074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsbie DS, Yang Z, Ge Y, Mitchell KL, Zhou X, Martin SE, Berlinicke CA, Hackler L, Jr, Fuller J, Fu J, Cao LH, Han B, Auld D, Xue T, Hirai S, Germain L, Simard-Bisson C, Blouin R, Nguyen JV, Davis CH, Enke RA, Boye SL, Merbs SL, Marsh-Armstrong N, Hauswirth WW, DiAntonio A, Nickells RW, Inglese J, Hanes J, Yau KW, Quigley HA, Zack DJ. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4045–4050. doi: 10.1073/pnas.1211284110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J. Neurosci. 1998;18:1428–1439. doi: 10.1523/JNEUROSCI.18-04-01428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29:629–643. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- Whitmore AV, Libby RT, John SW. Glaucoma: thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes? Prog. Retin Eye Res. 2005;24:639–662. doi: 10.1016/j.preteyeres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Whitmore AV, Lindsten T, Raff MC, Thompson CB. The proapoptotic proteins Bax and Bak are not involved in Wallerian degeneration. Cell Death Differ. 2003;10:260–261. doi: 10.1038/sj.cdd.4401147. [DOI] [PubMed] [Google Scholar]
- Williams PA, Piechota M, von Ruhland C, Taylor E, Morgan JE, Votruba M. Opa1 is essential for retinal ganglion cell synaptic architecture and connectivity. Brain. 2012;135:493–505. doi: 10.1093/brain/awr330. [DOI] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Wilson AM, Chiodo VA, Boye SL, Brecha NC, Hauswirth WW, Di Polo A. Inhibitor of apoptosis-stimulating protein of p53 (iASPP) is required for neuronal survival after axonal injury. PLoS One. 2014;9:e94175. doi: 10.1371/journal.pone.0094175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AM, Morquette B, Abdouh M, Unsain N, Barker PA, Feinstein E, Bernier G, Di Polo A. ASPP1/2 regulate p53-dependent death of retinal ganglion cells through PUMA and Fas/CD95 activation in vivo. J. Neurosci. 2013;33:2205–2216. doi: 10.1523/JNEUROSCI.2635-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Annan J, O’Reilly LA, Crawford SA, Hausmann G, Beaumont JG, Parma LP, Chen L, Lackmann M, Lithgow T, Hinds MG, Day CL, Adams JM, Huang DC. Proapoptotic BH3-only proteins trigger membrane integration of prosurvival Bcl-w and neutralize its activity. J. Cell Biol. 2003;162:877–887. doi: 10.1083/jcb.200302144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HK, Fricker M, Wyttenbach A, Villunger A, Michalak EM, Strasser A, Tolkovsky AM. Mutually exclusive subsets of BH3-only proteins are activated by the p53 and c-Jun N-terminal kinase/c-Jun signaling pathways during cortical neuron apoptosis induced by arsenite. Mol. Cell Biol. 2005;25:8732–8747. doi: 10.1128/MCB.25.19.8732-8747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XP, Zhai D, Kim E, Swift M, Reed JC, Volkmann N, Hanein D. Three-dimensional structure of Bax-mediated pores in membrane bilayers. Cell Death Dis. 2013;4:e683. doi: 10.1038/cddis.2013.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Luo C, Cai J, Powell DW, Yu D, Kuehn MH, Tezel G. Neuro-degenerative and inflammatory pathway components linked to TNF-alpha/TNFR1 signaling in the glaucomatous human retina. Invest. Ophthalmol. Vis. Sci. 2011;52:8442–8454. doi: 10.1167/iovs.11-8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepato-cellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Yoles E, Müller S, Schwartz M. NMDA-receptor antagonist protects neurons from secondary degeneration after partial optic nerve crush. J. Neurotrauma. 1997;14:665–675. doi: 10.1089/neu.1997.14.665. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- Yu-Wai-Man P, Stewart JD, Hudson G, Andrews RM, Griffiths PG, Birch MK, Chinnery PF. OPA1 increases the risk of normal but not high tension glaucoma. J. Med. Genet. 2010;47:120–125. doi: 10.1136/jmg.2009.067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzami N, Brenner C, Marzo I, Susin SA, Kroemer G. Subcellular and submitochondrial mode of action of Bcl-2-like oncoproteins. Oncogene. 1998;16:2265–2282. doi: 10.1038/sj.onc.1201989. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Osipov K, Jockel J, Waksman G, Korsmeyer SJ. BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J. Biol. Chem. 1997;272:24101–24104. doi: 10.1074/jbc.272.39.24101. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Subramaniam S, Kale J, Liao C, Huang B, Brahmbhatt H, Condon SG, Lapolla SM, Hays FA, Ding J, He F, Zhang XC, Li J, Senes A, Andrews DW, Lin J. BH3-in-groove dimerization initiates and helix 9 dimerization expands Bax pore assembly in membranes. Embo J. 2016;35:208–236. doi: 10.15252/embj.201591552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkel SS, Ong CC, Ferguson DO, Iwasaki H, Akashi K, Bronson RT, Kutok JL, Alt FW, Korsmeyer SJ. Proapoptotic BID is required for myeloid ho-meostasis and tumor suppression. Genes. Dev. 2003;17:229–239. doi: 10.1101/gad.1045603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Tian C, Ge S, Hu B. Neurogenesis of retinal ganglion cells is not essential to visual functional recovery after optic nerve injury in adult zebrafish. PLoS One. 2013;8:e57280. doi: 10.1371/journal.pone.0057280. [DOI] [PMC free article] [PubMed] [Google Scholar]