Abstract
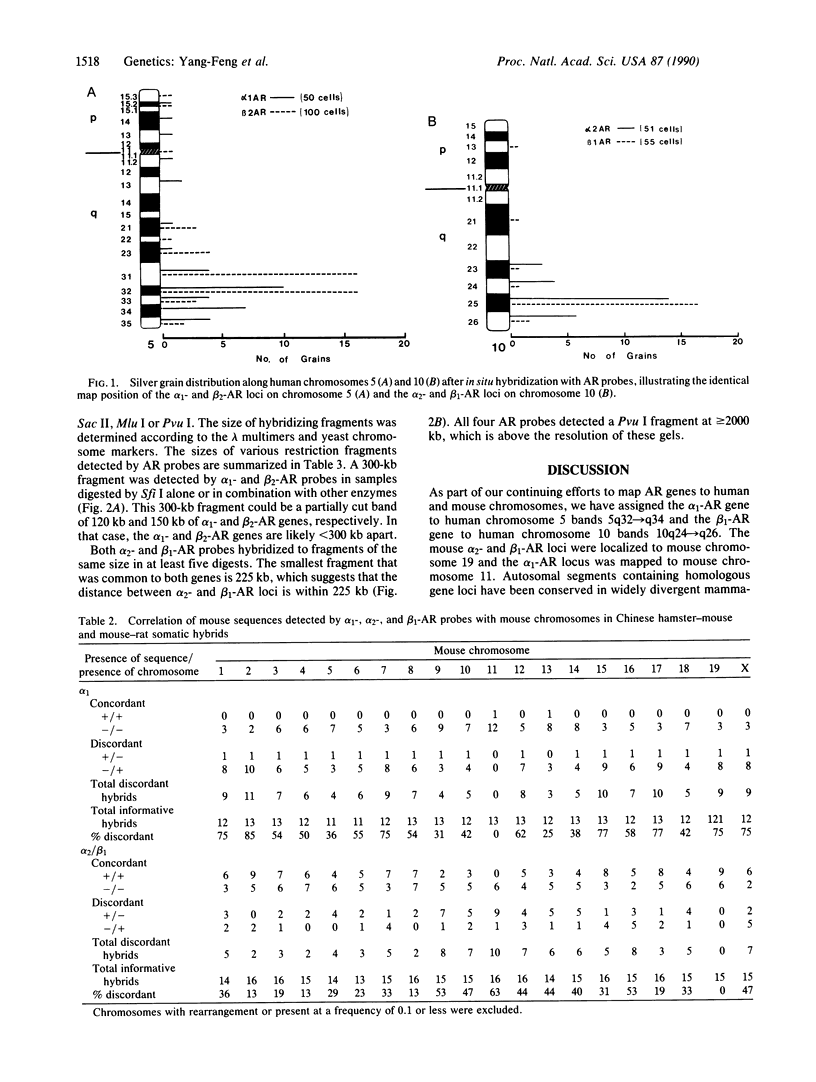
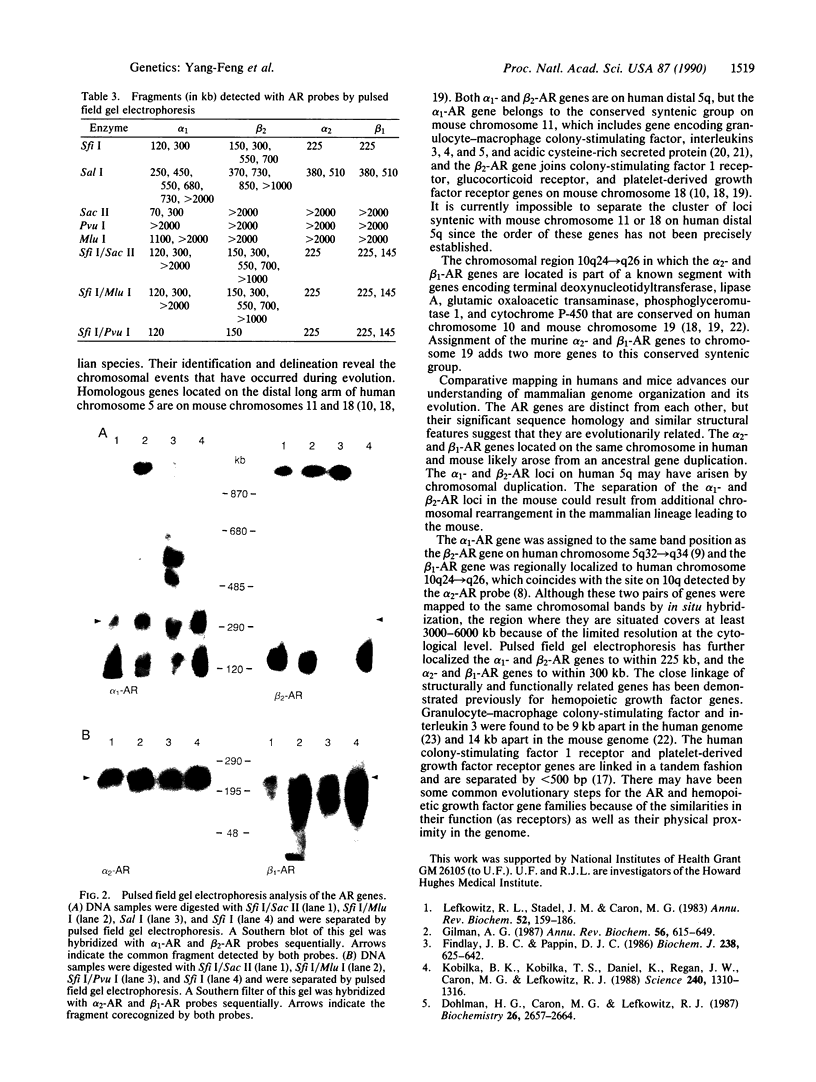
The adrenergic receptors (ARs) (subtypes alpha 1, alpha 2, beta 1, and beta 2) are a prototypic family of guanine nucleotide binding regulatory protein-coupled receptors that mediate the physiological effects of the hormone epinephrine and the neurotransmitter norepinephrine. We have previously assigned the genes for beta 2- and alpha 2-AR to human chromosomes 5 and 10, respectively. By Southern analysis of somatic cell hybrids and in situ chromosomal hybridization, we have now mapped the alpha 1-AR gene to chromosome 5q32----q34, the same position as beta 2-AR, and the beta 1-AR gene to chromosome 10q24----q26, the region where alpha 2-AR is located. In mouse, both alpha 2- and beta 1-AR genes were assigned to chromosome 19, and the alpha 1-AR locus was localized to chromosome 11. Pulsed field gel electrophoresis has shown that the alpha 1- and beta 2-AR genes in humans are within 300 kilobases (kb) and the distance between the alpha 2- and beta 1-AR genes is less than 225 kb. The proximity of these two pairs of AR genes and the sequence similarity that exists among all the ARs strongly suggest that they are evolutionarily related. Moreover, they likely arose from a common ancestral receptor gene and subsequently diverged through gene duplication and chromosomal duplication to perform their distinctive roles in mediating the physiological effects of catecholamines. The AR genes thus provide a paradigm for understanding the evolution of such structurally conserved yet functionally divergent families of receptor molecules.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow D. P., Bućan M., Lehrach H., Hogan B. L., Gough N. M. Close genetic and physical linkage between the murine haemopoietic growth factor genes GM-CSF and Multi-CSF (IL3). EMBO J. 1987 Mar;6(3):617–623. doi: 10.1002/j.1460-2075.1987.tb04799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Cotecchia S., Schwinn D. A., Randall R. R., Lefkowitz R. J., Caron M. G., Kobilka B. K. Molecular cloning and expression of the cDNA for the hamster alpha 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7159–7163. doi: 10.1073/pnas.85.19.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H. G., Caron M. G., Lefkowitz R. J. A family of receptors coupled to guanine nucleotide regulatory proteins. Biochemistry. 1987 May 19;26(10):2657–2664. doi: 10.1021/bi00384a001. [DOI] [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Lohse M. J., Kobilka B. K., Caron M. G., Lefkowitz R. J. The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature. 1988 Sep 22;335(6188):358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Findlay J. B., Pappin D. J. The opsin family of proteins. Biochem J. 1986 Sep 15;238(3):625–642. doi: 10.1042/bj2380625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frielle T., Collins S., Daniel K. W., Caron M. G., Lefkowitz R. J., Kobilka B. K. Cloning of the cDNA for the human beta 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Herrmann B. G., Barlow D. P., Lehrach H. A large inverted duplication allows homologous recombination between chromosomes heterozygous for the proximal t complex inversion. Cell. 1987 Mar 13;48(5):813–825. doi: 10.1016/0092-8674(87)90078-x. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Silver J., Kozak C. A. Genetic mapping of the mouse interleukin 3 gene to chromosome 11. J Immunol. 1987 May 1;138(9):3051–3054. [PubMed] [Google Scholar]
- Kobilka B. K., Dixon R. A., Frielle T., Dohlman H. G., Bolanowski M. A., Sigal I. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka B. K., Frielle T., Collins S., Yang-Feng T., Kobilka T. S., Francke U., Lefkowitz R. J., Caron M. G. An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature. 1987 Sep 3;329(6134):75–79. doi: 10.1038/329075a0. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Kobilka T. S., Daniel K., Regan J. W., Caron M. G., Lefkowitz R. J. Chimeric alpha 2-,beta 2-adrenergic receptors: delineation of domains involved in effector coupling and ligand binding specificity. Science. 1988 Jun 3;240(4857):1310–1316. doi: 10.1126/science.2836950. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Matsui H., Kobilka T. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J., Regan J. W. Cloning, sequencing, and expression of the gene coding for the human platelet alpha 2-adrenergic receptor. Science. 1987 Oct 30;238(4827):650–656. doi: 10.1126/science.2823383. [DOI] [PubMed] [Google Scholar]
- Lalley P. A., O'Brien S. J., Créau-Goldberg N., Davisson M. T., Roderick T. H., Echard G., Womack J. E., Graves J. M., Doolittle D. P., Guidi J. N. Report on the committee on comparative mapping. Cytogenet Cell Genet. 1987;46(1-4):367–389. doi: 10.1159/000132486. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Young I. G. Fine-structure mapping of the murine IL-3 and GM-CSF genes by pulsed-field gel electrophoresis and molecular cloning. Genomics. 1989 Aug;5(2):359–362. doi: 10.1016/0888-7543(89)90070-0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Stadel J. M., Caron M. G. Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem. 1983;52:159–186. doi: 10.1146/annurev.bi.52.070183.001111. [DOI] [PubMed] [Google Scholar]
- Roberts W. M., Look A. T., Roussel M. F., Sherr C. J. Tandem linkage of human CSF-1 receptor (c-fms) and PDGF receptor genes. Cell. 1988 Nov 18;55(4):655–661. doi: 10.1016/0092-8674(88)90224-3. [DOI] [PubMed] [Google Scholar]
- Searle A. G., Peters J., Lyon M. F., Hall J. G., Evans E. P., Edwards J. H., Buckle V. J. Chromosome maps of man and mouse. IV. Ann Hum Genet. 1989 May;53(Pt 2):89–140. doi: 10.1111/j.1469-1809.1989.tb01777.x. [DOI] [PubMed] [Google Scholar]
- Sundaresan S., Francke U. Genes for beta 2-adrenergic receptor and platelet-derived growth factor receptor map to mouse chromosome 18. Somat Cell Mol Genet. 1989 Jul;15(4):367–371. doi: 10.1007/BF01534975. [DOI] [PubMed] [Google Scholar]
- Yang-Feng T. L., Landau N. R., Baltimore D., Francke U. The terminal deoxynucleotidyltransferase gene is located on human chromosome 10 (10q23----q24) and on mouse chromosome 19. Cytogenet Cell Genet. 1986;43(3-4):121–126. doi: 10.1159/000132309. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Kovacic S., Kriz R., Wolf S., Clark S. C., Wellems T. E., Nienhuis A., Epstein N. The human genes for GM-CSF and IL 3 are closely linked in tandem on chromosome 5. Blood. 1988 Apr;71(4):958–961. [PubMed] [Google Scholar]