Abstract
Molecular and cellular mechanisms underlying the sustained metal tolerance of ectomycorrhizal fungi are largely unknown. Some of the main mechanisms involved in metal detoxification appear to involve the chelation of metal ions in the cytosol with thiol-containing compounds, such as glutathione, phytochelatins, or metallothioneins. We used an improved high-performance liquid chromatography method for the simultaneous measurement of thiol-containing compounds from cysteine and its derivatives (γ-glutamylcysteine, glutathione) to higher-molecular-mass compounds (phytochelatins). We found that glutathione and γ-glutamylcysteine contents increased when the ectomycorrhizal fungus Paxillus involutus was exposed to cadmium. An additional compound with a 3-kDa molecular mass, most probably related to a metallothionein, increased drastically in mycelia exposed to cadmium. The relative lack of phytochelatins and the presence of a putative metallothionein suggest that ectomycorrhizal fungi may use a different means to tolerate heavy metals, such as Cd, than do their plant hosts.
The response of ectomycorrhizal fungi to toxic metals is important, since these organisms are present at polluted sites, participate in crucial symbiotic relationships with trees that grow at these sites, and alleviate metal toxicity in the host plants (8, 13, 26). Paxillus involutus is an ectomycorrhizal fungus with a high tolerance to cadmium, one of the most toxic heavy metals, that can form symbioses with a broad range of host species. The uptake of metals by ectomycorrhizal fungi (2) and the effects of metals on biomass production (3) and nutrient uptake (1) in ectomycorrhizal fungi have been studied. The expression of enzymes involved in antioxidative response mechanisms is regulated by Cd in ectomycorrhizal fungi (12, 22) and in mycorrhizal roots of Pinus sylvestris associated with P. involutus (26).
Thiol compounds, including reduced glutathione (γ-glutamyl cysteinyl glycine), phytochelatins (PCs), and metallothioneins, are essential components of Cd detoxification pathways in various organisms (5-7, 11). Thiol composition of ectomycorrhizal fungi has not been studied in detail, although P. involutus and Laccaria laccata accumulate glutathione when exposed to Cd (7, 22). Reduced glutathione is the most abundant nonprotein thiol component of eukaryotic cells, acts as a free radical scavenger, and reacts with various oxidants to produce oxidized glutathione (17). Phytochelatins are a family of small cysteine-rich peptides capable of binding heavy metal ions via their SH group. The general structure of this set of peptides is [γ-GluCys]n-gly (n = 2 to 11). Phytochelatins are enzymatically synthesized from glutathione and have been found in some fungi, algae, and all plant species examined so far (for reviews, see references 4, 5, and 23). Phytochelatin synthase (γ-glutamylcysteine dipeptidyl transpeptidase; EC 2.3.2.15) catalyzes the addition of the γ-glutamylcysteine (γ-GluCys) moiety of glutathione onto another glutathione molecule (to produce PC2), or onto a preexisting PCn molecule to produce the corresponding PCn+1 derivative (9). This enzyme is activated by a broad range of heavy metals, including Cd, Ag, Pb, and Cu. Metallothioneins are cysteine-rich peptides that chelate metal ions by thiolate coordination. Metallothioneins were not produced by Saccharomyces cerevisiae in response to stress induced by high levels of Cd (21, 27), and the only fungus known to use both metallothioneins and phytochelatins for metal detoxification is Candida glabrata. C. glabrata produces metallothioneins when exposed to toxic concentrations of Cu but produces mainly phytochelatins in response to Cd stress (16).
The objective of this study was to evaluate the metabolic response of P. involutus to cadmium exposure by using a modified gradient reversed-phase high-performance liquid chromatography (HPLC) method (25). Our working hypothesis was that exposure of P. involutus cultures to cadmium would result in significant quantitative and qualitative changes in thiol compounds. Our results suggest that ectomycorrhizal fungi may use a different mechanism to tolerate heavy metals, such as Cd, than do their plant hosts.
MATERIALS AND METHODS
Chemicals, reagents, and standards.
All chemicals and solvents were of analytical or HPLC reagent grade and were used without further purification. All of the thiols in their reduced forms, 7-fluoro-2,1,3-benzoxadiazole-4-sulfonamide (ABD-F), and tri-n-butylphosphine, were purchased from Sigma (St. Louis, Mo.). Phytochelatin standards were produced in vitro (see below). Rabbit metallothionein (MT1 and MT2) standards were purchased from Sigma (catalog no. M7641). The stock solution of each thiol standard was prepared at a concentration of 1.0 mM in 10 mM HCl containing 4 mM EDTA and kept at −80°C for up to 2 months. Five-point calibration curves were produced daily with further dilution of stock solutions in a 5% (wt/vol) SSA (sulfosalicylic acid) solution containing 6.3 mM DTPA (diethylenetriaminepentaacetate) to concentrations ranging from 10 to 100, 10 to 100, and 1 to 100 μM for cysteine, γ-GluCys and glutathione, respectively.
Fungal culture and cadmium treatments.
P. involutus (ATCC 200175 Batsch Fries) was grown on cellophane-covered agar containing modified Melin-Norkrans (MMN) medium, as described previously (12). The MMN medium contained the following (in milligrams per liter): KH2PO4 (500), (NH4)2HPO4 (250), CaCl2 · 2H2O (50), NaCl (25), MgSO4 · 7H2O (150), thiamine HCl (0.1), and FeCl3 · 6H2O (1), plus glucose at a final concentration of 10 g/liter. The pH was adjusted to 5.5, and 10 g of granulated agar/liter was added before sterilization. After 7 days of growth on solid medium, fungal colonies were harvested without the cellophane and transferred individually to 140-mm petri dishes containing 50 ml of MMN liquid medium (pH 4.5) for 4 days as an adaptation period, with a daily change of the medium. Changes were made by transferring the colonies to a fresh petri dish containing 50 ml of MMN liquid medium. After adaptation, CdSO4 was added to a final concentration of 0.05 to 50 ppm. When necessary, 2 mM buthionine sulfoximine also was added to inhibit γ-GluCys synthase. Colonies were harvested 12 to 48 h after CdSO4 addition, frozen in liquid N2, and stored at −80°C for no more than 1 week before analysis.
Thiol extraction procedure.
All extraction and centrifugation steps were carried out at 4°C. Approximately 150 mg (wet weight) of mycelia was ground in a 2-ml microcentrifuge tube in liquid N2 by two 2-min strokes of a bead mixer-mill set at 30 pulses s−1 (Retsch MM 300; QIAGEN, Hilden, Germany). Three hundred microliters of a 5% (wt/vol) SSA solution containing 6.3 mM DTPA was then added. The resulting homogenate was centrifuged at 16,000 × g for 10 min, and the supernatant was stored at −80°C for up to 2 months.
Expression of AtPCS1 and production of phytochelatin standards.
Recombinant Arabidopsis thaliana phytochelatin synthase (AtPCS1) was expressed in yeast and in Escherichia coli as previously described (24). The untransformed and AtPCS1-pFL61-transformed S. cerevisiae cells were grown in 50 ml of synthetic dextrose medium without uracil until the optical density at 600 nm was 1. A 50 μM CdSO4 solution was added, and the culture was incubated at 30°C for 24 h. Cells were collected by centrifugation at 9,000 × g for 10 min and 4°C, and thiols were extracted as described above.
Expression of PiMT in yeast.
A fragment of a metallothionein gene was isolated from a P. involutus cDNA library (C. Jacob, unpublished data). The full-length open reading frame of PiMT1 was obtained by performing a 5′ rapid amplification of cDNA ends (RACE) reaction using the SMART RACE cDNA kit (Clontech Laboratories Inc., Palo Alto, Calif.) according to the manufacturer's instructions. The resulting full-length cDNA PiMT1 was cloned into the pGEM-T Easy vector (Promega, Madison, Wis.), sequenced, and submitted to the GenBank nucleotide database. The PiMT1 DNA was further subcloned from the pGEM-T Easy vector (Promega) into the pFL61 plasmid after NotI digestion (14). The pFL61-PiMT1 constructs and the empty vector pFL61 were used to transform the Δcup1 (strain DTY 113) as described previously (14). The pFL61 and pFL61-PiMT1-transformed S. cerevisiae cells were grown in 50 ml of synthetic dextrose medium without uracil until the optical density at 600 nm was 0.6. Cells were collected by centrifugation at 9,000 × g for 10 min and 4°C, and thiols were extracted as described above.
HPLC method.
The HPLC system consisted of a low-pressure-gradient solvent delivery pump (model L6200; Hitachi-Merck, Nogent-sur-Marne, France), an autosampler, a cooling sample device, a column oven (model AS-300; Thermo, Les Ullis, France), and a spectrofluorimetric detector (model FP-920; Jasco). The tray compartment containing sample vials was cooled to 8°C, and the HPLC system was operated overnight. A guard column (4 by 4 mm inner diameter) and an analytical column (250 by 4 mm inner diameter) packed with Nucleosil 100 C18 HD 5 μm (Macherey-Nagel GmbH & Co., Düren, Germany) were eluted with trifluoroacetic acid-H2O (0.1:100 [vol/vol]; mobile phase A) and acetonitrile-trifluoroacetic acid-H2O (50:0.1:50 [vol/vol/vol]; mobile phase B) at a column temperature of 40°C and a flow rate of 0.4 ml/min. Thiols were quantified with a glutathione calibration curve, corrected for the number of thiol groups present in each analyzed compound. Derivatization conditions were those described previously (25) with modifications. A 100-μl aliquot of fungal extract or standard solution was thawed at 20 ± 2°C and transferred to a 1.5-ml polypropylene tube kept in crushed ice, to which 50 μl of a 5% (vol/vol) tri-n-butylphosphine solution in dimethylformamide, 20 μl of 2 M NaOH, and 250 μl of 0.2 M borate buffer (pH 9.0) was added. After vortexing for 10 s using a vortex, a nitrogen stream was introduced for 10 s into the tube before capping it, in order to avoid air oxidation. After 5 min at 50°C, 30 μl of a 11.5 mM ABD-F solution in dimethylformamide was added. The resulting mixture was incubated at 50°C for 20 min, and the derivatization reaction was stopped by adding 70 μl of 1.0 M HCl. Acidified samples were cooled in ice until HPLC analysis (20 μl injected). The acetonitrile proportion during gradient elution was 10% for 5 min, 10 to 20% from 5 to 20 min, 20 to 50% from 20 to 50 min, and 50% from 50 to 55 min. Excitation and emission wavelengths were 385 and 515 nm, respectively.
Protein extraction and phytochelatin synthase assay.
All purification and centrifugation steps were carried out at 4°C. Approximately 100 mg of mycelia was ground in a chilled mortar and pestle with 10 volumes (wt/vol) of 50 mM HEPES-Na (pH 7.8) containing 10 mM 2-mercaptoethanol, 20% glycerol, 2 mM phenylmethylsulfonyl fluoride, 2% (wt/vol) polyvinyl pyrrolidone 40, and 10% (wt/vol) polyvinylpolypyrrolidone. The homogenate was centrifuged at 16,000 × g for 10 min, the supernatant was collected, and the centrifugation was repeated. Phytochelatin synthase reactions were performed at 35°C for 4 h with 180 μl of mycelia supernatant and 60 μl of phytochelatin synthase buffer (19), and reactions were stopped with 5% (wt/vol) SSA (final concentration). After vortexing, the mixture was centrifuged at 16,000 × g for 10 min, and the resulting supernatant was stored at −80°C until analyzed.
Nucleotide sequence accession number.
The full-length PiMT1 sequence was submitted to the GenBank nucleotide database and assigned accession number AY525379.
RESULTS
Detection of thiol-containing compounds.
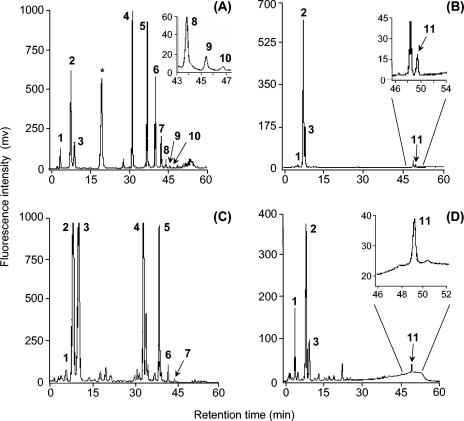
We separated thiol compounds, ranging from Cys and its derivatives, γ-GluCys and glutathione, to phytochelatins (up to polymerization degree of n = 8) on a reversed-phase column in a gradient mode (Fig. 1A). The ABD-F probe was more sensitive (ca. 10-fold) than Ellman's reagent (10) and generated fewer interfering by-products than monobromobimane (20), two thiol-derivatizing reagents commonly used in the post- and precolumn mode, respectively. Cys, glutathione, γ-GluCys, and an unidentified, late-eluting peak (compound no. 11; retention time = 49 min) were specifically detected following ABD-F derivatization in Cd-treated P. involutus mycelia (Fig. 1B). As a control we used S. cerevisiae, which normally lacks phytochelatin synthase, transformed with the Arabidopsis phytochelatin synthase gene (AtPCS1). Cys, glutathione, and γ-GluCys were detected in extracts of both AtPCS1-transformed (Fig. 1C) and untransformed S. cerevisiae cells exposed to Cd (50 μM; 24 h). In addition, phytochelatins ranging in size from n = 2 to 5 were present in transformed yeast cells (Fig. 1C) but not in untransformed S. cerevisiae cells (data not shown).
FIG. 1.
Chromatograms corresponding to thiol derivatized in a precolumn mode with ABD-F. (A) Standards, as follows: peak 1, cysteine; peak 2, glutathione; peak 3, γ-GluCys; *, dithiothreitol plus 2-mercaptoethanol; peak 4, PC2; peak 5, PC3; peak 6, PC4; peak 7, PC5; peak 8, PC6; peak 9, PC7; peak 10, PC8. (B) Extracts from colonies of P. involutus exposed to CdSO4 (50 ppm for 48 h) and derivatized with ABD-F. (C) Extract from S. cerevisiae cells (Δyap1) transformed with the A. thaliana phytochelatin synthase gene (AtPCS1), grown for 24 h with 50 μM CdSO4, and derivatized with ABD-F. (D) Extract from S. cerevisiae cells (Δcup1) transformed with the P. involutus metallothionein gene (PiMT1) and derivatized with ABD-F. Units are expressed in millivolts.
Preliminary identification of compound no. 11.
There was a linear relationship (y = 23.9x + 25.1; r2 = 0.99) between the volume of acetonitrile needed for elution of each phytochelatin and the logarithm of their degree of polymerization (n). Compound no. 11 (elution time = 49 min) could correspond to a PC11 of a 3-kDa molecular mass. However, there were no lower-order phytochelatins, i.e., phytochelatins with n values lower than 11, in our chromatograms. The overproduction of compound no. 11 following exposure to Cd also was insensitive to buthionine sulfoximine, a transition-state analog and specific inhibitor of γ-GluCys synthase that is required for glutathione, and thus for phytochelatin synthesis (10). In addition, no phytochelatin synthase activity was detected in P. involutus protein extracts, although it was detected in transformed yeast cells (data not shown). We further transformed S. cerevisiae cells with a metallothionein gene (PiMT1) isolated from P. involutus and analyzed the thiol content. Cys, glutathione, and γ-GluCys were detected in extracts of PiMT1-transformed S. cerevisiae cells (Fig. 1D). In addition, a late-eluting peak (compound no. 11; retention time = 49 min) was specifically detected in PiMT1-transformed yeast cells (Fig. 1D) but in neither pFL61-transformed S. cerevisiae cells (data not shown) nor AtPCS1-transformed S. cerevisiae cells (Fig. 1C).
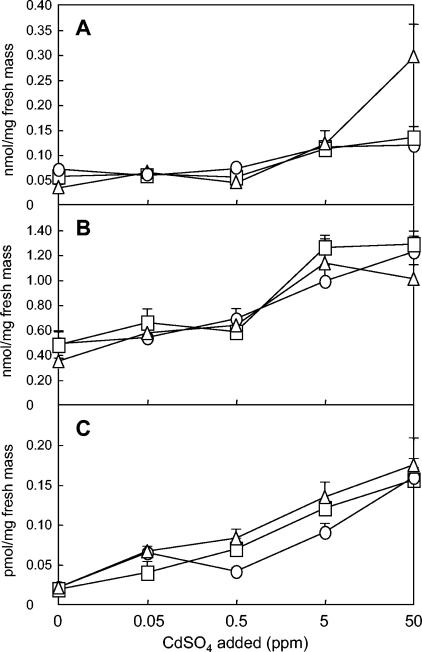
Quantitative variations of thiols in Cd-treated cultures of P. involutus.
The concentration of γ-GluCys (Fig. 2A) and glutathione (Fig. 2B) depended on Cd concentration and duration of exposure. Compound no. 11 (Fig. 1C) also increased drastically relative to controls lacking Cd (Fig. 2C). Cysteine remained unchanged in nearly all treatments (data not shown). We did not detect any phytochelatins in extracts of P. involutus cultures exposed to Cd when we used the same extraction procedure as that used for the AtPCS1-transformed S. cerevisiae cells.
FIG. 2.
Contents of γ-GluCys (A), glutathione (B), and a late-eluting peak (C) (expressed as glutathione equivalents) measured by HPLC with precolumn ABD-F derivatization and fluorescence detection in colonies of P. involutus after exposure to CdSO4 for 12 (○), 24 (□), or 48 (Δ) h. Results are the mean (± standard error) of five independent experiments.
DISCUSSION
By promoting nutrient exchange between the two partners, mycorrhizae exert a positive influence on plant survival under a variety of unfavorable environmental conditions. Mycorrhizae may be exploited to alleviate stress from metal diffusion and toxicity, but the molecular and cellular mechanisms underlying the sustained metal tolerance of mycorrhizal fungi are largely unknown. We used an improved reverse-phase HPLC analytical procedure based on an ABD-F precolumn derivatization to gain initial insight into the Cd response of the ectomycorrhizal fungus P. involutus. This procedure can simultaneously detect mono- and poly-thiols, including an as-yet-unidentified Cd-modulated compound (no. 11). In our HPLC system, homogeneously purified metallothioneins from rabbit liver have retention times of 48 to 52 min, which are similar to that of compound no. 11 (data not shown). Furthermore, by analyzing S. cerevisiae cells transformed with a metallothionein gene isolated from P. involutus, we detected this late-eluting peak (compound no. 11; retention time = 49 min). Therefore, compound no. 11 probably is a novel metallothionein. Further support of the hypothesis that compound no. 11 is an as-yet-unidentified metallothionein-like compound is provided by the following: (i) the fact that metallothioneins fractionated on a reversed-phase column under acetonitrile-methanol isocratic conditions also can be detected when derivatized on an ammonium 7-fluoro-2,1,3-benzoxadiazole-4-sulfonate (SBD-F) precolumn (18); (ii) the isolation of Cd-responsive metallothioneins in several fungi, including the metallothionein-like polypeptide, GmarMT1, from the arbuscular mycorrhizal fungus Gigaspora margarita (14). Neither metallothioneins nor phytochelatins were detected in the ectomycorrhizal fungus L. laccata following exposure to Cd (7); however, those investigators used an acidic (TCA) extraction method coupled with post-column DTNB [5,5′-dithiobis(2-nitrobenzoic acid)] derivatization. When we used these conditions in our system, we could no longer detect compound no. 11.
We found that levels of both glutathione and its biosynthetic precursor, γ-GluCys, increased in Cd-stressed P. involutus mycelia. Both of these compounds can form highly stable complexes with Cd and other thiophilic heavy metals, such as Hg, Cu, and Zn. The high Cd content of vacuoles in Cd-treated Paxillus mycelia (2) could occur if there were a specific permease, e.g., the Ycf1 membrane transporter of S. cerevisiae (15), involved in the translocation (and physical sequestration) of Cd-GS2 (or Cd-γ-GluCys2) complexes into P. involutus vacuoles. Another fairly important, and somewhat unexpected, result from this work is the complete lack of phytochelatins among the Cd-responsive thiols produced by P. involutus. Since phytochelatins are the major metal detoxification compounds in plants, it is even more remarkable that a completely different metal detoxification strategy is utilized by one of their fungal partners to help ensure that the toxic agent is excluded from the symbiosis. From this perspective, P. involutus is clearly closer to S. cerevisiae than it is to fission yeast or other phytochelatin producers, such as algae and plants (4, 21).
In conclusion, the results presented here demonstrate that an improved HPLC method can improve thiol detection significantly compared to more traditional approaches, thus providing new possibilities for studying the complex mechanisms of interactions between fungi and metals. This work provides new insights into the strategy adopted by ectomycorrhizal fungi to deal with toxic metals, thus improving our understanding of both the ecology and the agricultural applications of these useful fungal symbionts.
Acknowledgments
M. Courbot was supported by an ADEME doctoral fellowship.
We thank S. Ottonello (University of Parma) and B. Botton for helpful discussions and critical comments on the manuscript and C. Jacob for producing the pFL61-PiMT1 construct.
REFERENCES
- 1.Ahonen-Jonnarth, U., and R. D. Finlay. 2001. Effects of elevated nickel and cadmium concentrations on growth and nutrient uptake of mycorrhizal and non-mycorrhizal Pinus sylvestris seedlings. Plant Soil 236:129-138. [Google Scholar]
- 2.Blaudez, D., B. Botton, and M. Chalot. 2000. Cadmium uptake and subcellular compartmentation in the ectomycorrhizal fungus Paxillus involutus. Microbiology 146:1109-1117. [DOI] [PubMed] [Google Scholar]
- 3.Blaudez, D., C. Jacob, K. Turnau, J. V. Colpaert, U. Ahonen-Jonnarth, R. Finlay, B. Botton, and M. Chalot. 2000. Differential responses of ectomycorrhizal fungi to heavy metals in vitro. Mycol. Res. 104:1366-1371. [Google Scholar]
- 4.Cobbett, C., and P. Goldsbrough. 2002. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 53:159-182. [DOI] [PubMed] [Google Scholar]
- 5.Cobbett, C. S. 2000. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 123:825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gadd, G. M. 1993. Interactions of fungi with toxic metals. New Phytol. 124:25-60. [Google Scholar]
- 7.Galli, U., M. Meier, and C. Brunold. 1993. Effects of cadmium on nonmycorrhizal and mycorrhizal Norway Spruce seedlings Picea abies (L.) Karst and its ectomycorrhizal fungus Laccaria laccata (Scop. ex Fr.) Bk. & Br. Sulfate reduction, thiols and distribution of the heavy metal. New Phytol. 125:837-843. [DOI] [PubMed] [Google Scholar]
- 8.Godbold, D. L., G. Jentschke, S. Winter, and P. Marschner. 1998. Ectomycorrhizas and amelioration of metal stress in forest trees. Chemosphere 36:757-762. [Google Scholar]
- 9.Grill, E., S. Loffler, E. L. Winnacker, and M. H. Zenk. 1989. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 86:6838-6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grill, E., E. L. Winnacker, and M. H. Zenk. 1987. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc. Natl. Acad. Sci. USA 84:439-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall, J. L. 2002. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 53:1-11. [PubMed] [Google Scholar]
- 12.Jacob, C., M. Courbot, A. Brun, H. M. Steinman, J. P. Jacquot, B. Botton, and M. Chalot. 2001. Molecular cloning, characterization and regulation by cadmium of a superoxide dismutase from the ectomycorrhizal fungus Paxillus involutus. Eur. J. Biochem. 268:3223-3232. [DOI] [PubMed] [Google Scholar]
- 13.Jentschke, G., and D. L. Goldbold. 2000. Metal toxicity and ectomycorrhizas. Physiol. Plant 109:107-116. [Google Scholar]
- 14.Lanfranco, L., A. Bolchi, E. C. Ros, S. Ottonello, and P. Bonfante. 2002. Differential expression of a metallothionein gene during the presymbiotic versus the symbiotic phase of an arbuscular mycorrhizal fungus. Plant Physiol. 130:58-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, Z. S., Y. P. Lu, R. G. Zhen, M. Szczypka, D. J. Thiele, and P. A. Rea. 1997. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc. Natl. Acad. Sci. USA 94:42-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehra, R. K., and D. R. Winge. 1991. Metal-ion resistance in fungi: molecular mechanisms and their regulated expression. J. Cell. Biochem. 45:30-40. [DOI] [PubMed] [Google Scholar]
- 17.Meister, A., and M. E. Anderson. 1983. Glutathione. Annu. Rev. Biochem. 52:711-760. [DOI] [PubMed] [Google Scholar]
- 18.Miyairi, S., S. Shibata, and A. Naganuma. 1998. Determination of metallothionein by high-performance liquid chromatography with fluorescence detection using an isocratic solvent system. Anal. Biochem. 258:168-175. [DOI] [PubMed] [Google Scholar]
- 19.Nakazawa, R., H. Kato, Y. Kameda, and H. Takenaga. 2002. Optimum assay conditions of the activity of phytochelatin synthase from tobacco cells. Biol. Plant 45:311-313. [Google Scholar]
- 20.Newton, G. L., R. Dorian, and R. C. Fahey. 1981. Analysis of biological thiols: derivatization with monobromobimane and separation by reverse-phase high-performance liquid chromatography. Anal. Biochem. 114:383-387. [DOI] [PubMed] [Google Scholar]
- 21.Okuyama, M., Y. Kobayashi, M. Inouhe, H. Tohoyama, and M. Joho. 1999. Effect of some heavy metal ions on copper-induced metallothionein synthesis in the yeast Saccharomyces cerevisiae. Biometals 12:307-314. [DOI] [PubMed] [Google Scholar]
- 22.Ott, T., E. Fritz, A. Polle, and A. Schutzendubel. 2002. Characterization of antioxidative systems in the ectomycorrhiza-building basidiomycete Paxillus involutus (Bartsch) Fr. and its reaction to cadmium. FEMS Microbiol. Ecol. 42:359-366. [DOI] [PubMed] [Google Scholar]
- 23.Rauser, W. E. 1999. Structure and function of metal chelators produced by plants: the case for organic acids, amino acids, phytin, and metallothioneins. Cell. Biochem. Biophys. 31:19-48. [DOI] [PubMed] [Google Scholar]
- 24.Ruotolo, R., A. Peracchi, A. Bolchi, G. Infusini, A. Amoresano, and S. Ottonello. 2004. Domain organization of phytochelatin synthase: functional properties of truncated enzyme species identified by limited proteolysis. J. Biol. Chem. 279:14686-14693. [DOI] [PubMed] [Google Scholar]
- 25.Salazar, J. F., H. Schorr, W. Herrmann, B. Herbeth, G. Siest, and P. Leroy. 1999. Measurement of thiols in human plasma using liquid chromatography with precolumn derivatization and fluorescence detection. J. Chromatogr. Sci. 37:469-476. [DOI] [PubMed] [Google Scholar]
- 26.Schutzendubel, A., P. Nikolova, C. Rudolf, and A. Polle. 2002. Cadmium and H2O2-induced oxidative stress in Populus canescens roots. Plant Physiol. Biochem. 40:577-584. [Google Scholar]
- 27.Vido, K., D. Spector, G. Lagniel, S. Lopez, M. B. Toledano, and J. Labarre. 2001. A proteome analysis of the cadmium response in Saccharomyces cerevisiae. J. Biol. Chem. 276:8469-8474. [DOI] [PubMed] [Google Scholar]