Abstract
A chimeric enzyme associating feruloyl esterase A (FAEA) from Aspergillus niger and dockerin from Clostridium thermocellum was produced in A. niger. A completely truncated form was produced when the dockerin domain was located downstream of the FAEA (FAEA-Doc), whereas no chimeric protein was produced when the bacterial dockerin domain was located upstream of the FAEA (Doc-FAEA). Northern blot analysis showed similar transcript levels for the two constructs, indicating a posttranscriptional bottleneck for Doc-FAEA production. The sequence encoding the first 514 amino acids from A. niger glucoamylase and a dibasic proteolytic processing site (kex-2) were fused upstream of the Doc-FAEA sequence. By using this fusion strategy, the esterase activity found in the extracellular medium was 20-fold-higher than that of the wild-type reference strain, and the production yield was estimated to be about 100 mg of chimeric protein/liter. Intracellular and extracellular production was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, dockerin-cohesin interaction assays, and Western blotting. Labeled cohesins detected an intact extracellular Doc-FAEA of about 43 kDa and a cleaved-off dockerin domain of about 8 kDa. In addition, an intracellular 120-kDa protein was recognized by using labeled cohesins and antibodies raised against FAEA. This protein corresponded to the unprocessed Doc-FAEA form fused to glucoamylase. In conclusion, these results indicated that translational fusion to glucoamylase improved the secretion efficiency of a chimeric Doc-FAEA protein and allowed production of the first functional fungal enzyme joined to a bacterial dockerin.
Plant cell walls are composed of various polysaccharides and lignin, forming a rigid and complex matrix recalcitrant to microbial degradation. This structure is strengthened by cross-linkages such as diferulic acid bridges between adjacent hemicellulose chains (28) or between lignin and hemicellulose (24), increasing its resistance to microbial invasion. Aerobic and anaerobic microorganisms have developed two main efficient mechanisms of plant cell wall degradation. In the first system, microorganisms secrete free extracellular enzymes in contact with a substrate. The products of degradation constitute both nutrients for growth and regulators of the production of lignocellulolytic enzymes (9, 42, 43). Among these microorganisms, filamentous fungi such as Trichoderma spp. and Aspergillus spp. are especially good secretors of lignocellulolytic enzymes (1). Moreover, Aspergillus niger produces enzymes named feruloyl esterases (EC 3.1.1.73), a subclass of the carboxylic ester hydrolases, that are able to hydrolyze diferulate cross-links in plant cell walls, facilitating the access of main-chain-degrading enzymes to the polysaccharide backbone (8, 46). Furthermore, feruloyl esterases release cross-linked aromatic acids, such as ferulic acid, which is an attractive industrial compound by virtue of its antioxidant, photoprotectant properties (18) and its potential biotransformation to vanillin as a food flavor precursor (25).
A second system of degradation has evolved among anaerobic microorganisms that are subjected to more-drastic energetic constraints. Anaerobic fungi and bacteria produce high-molecular-mass complexes called cellulosomes, where different types of enzymes are bound to a scaffolding protein (for reviews, see references 38 and 39). Among bacteria, the cellulosomes from Clostridium spp. are the most studied complexes. In addition to a catalytic domain, all cellulosomal enzymes include a dockerin domain, usually in the C terminus, that allows their incorporation along the scaffoldin. The scaffoldin contains a cellulose-binding domain (11) and multiple copies of cohesin domains that interact with the dockerin on the cellulosomal enzymes. This binding is mediated by a high-affinity interaction (≥109 M−1) among complementary dockerin-cohesin domains (13, 29). The highly conserved dockerin domain is characterized by two Ca2+-binding sites with sequence similarity to the EF-hand motif (27). The multienzymatic complex facilitates a stronger synergistic effect among catalytic subunits than the free-enzyme system (5, 15, 22). Moreover, the fixation to the substrate mediated by the scaffoldin-borne carbohydrate binding module prevents large-scale diffusion of enzymes away from the cell, leading to a lower requirement of proteins for hydrolysis. In previous studies, chimeric bacterial cellulosomes were designed to evaluate the level of synergy when defined recombinant cellulases were incorporated onto scaffoldin proteins. Results confirmed an efficient synergistic effect by use of these chimeric cellulases with cellulose as the substrate (12, 14). Therefore, cellulosome hybrids with chimeric constructs could be used in interesting strategies to increase the synergistic effect between grafted enzymes (10). Chimeric cellulosomes should be a powerful method for improving enzymatic efficiency for degradation of plant cell walls and hence could have an important impact in biotechnology by valorizing lignocellulosic biomass (2, 3).
In the present work, our aim was to design and produce a chimeric protein composed of a noncellulolytic fungal enzyme fused to a bacterial dockerin domain. The feruloyl esterase A (FAEA) from A. niger was associated with the Cel48S dockerin from Clostridium thermocellum and produced in A. niger as a host of choice for large-scale production. Moreover, the chimeric enzyme was analyzed for its capacity to bind to the complementary cohesin domain of C. thermocellum.
MATERIALS AND METHODS
Strains and culture media.
Escherichia coli JM109 (Promega) was used for construction and propagation of vectors, and A. niger strain D15#26 (pyrG−) (16) was used for heterologous expression of the recombinant proteins. A. niger transformants were grown on selective solid minimal medium (without uridine) containing 70 mM NaNO3, 7 mM KCl, 11 mM KH2HPO4, 2 mM MgSO4, 1% (wt/vol) glucose, and trace elements (1,000× stock: 76 mM ZnSO4, 25 mM MnCl2, 18 mM FeSO4, 7.1 mM CoCl2, 6.4 mM CuSO4, 6.2 mM Na2MoO4, 174 mM EDTA).
Expression vectors.
For each of the expression vectors pFD, pDF, and pGDF, fusion of both the sequences (faeA [7] from A. niger and the dockerin sequence from C. thermocellum Cel48S) was performed by overlap extension PCR (19). The A. niger FAEA-encoding region was amplified by using the forward primer 5′-CCCTCATGAAGCAATTCTCTGC-3′ (with the BspHI site underlined) and the reverse primer 5′-AGGGCTTGTCTTGGCCCAAGTACAAGCTCC-3′ for plasmid pFD or the forward primer 5′-TTGCCGTACAAGAACGCCTCCACGAAGGC-3′ and the reverse primer 5′-ATTAAGCTTTTAGTGGTGGTGGTGGTGGTGCCAAGTTACAAGCTCCGC-3′ (with the HindIII site underlined) for plasmid pDF (the His tag is boldfaced in all sequences). The region coding for the dockerin domain from C. thermocellum Cel48S was amplified by using the forward primer 5′-GGAGCTTGTACTTGGGCCAAGACAAGCCCT-3′ and the reverse primer 5′-CCCAAGCTTTTAGTGGTGGTGGTGGTGGTGGTTCTTGTACGGCAATGT-3′ (HindIII site underlined) for plasmid pFD or the forward primer 5′-TTAGCGCGCCAAGACAAGCCCTAGCCCAT-3′ (BssHII site underlined) and the reverse primer 5′-GCCTTGCGTGGAGGCGTTCTTCTTGTACGGCAA-3′ for plasmid pDF. Both resultant overlapping fragments were mixed for each construct, and a fused fragment was synthesized by using only external primers. The fragments were cloned into BssHII-HindIII-linearized pAN52.4 for pDF and into NcoI-HindIII-linearized pAN52.3 for pFD. pGDF was constructed by using pDF as a template for PCR amplification of the DF sequence with the forward primer 5′-ACGGCGCCGGCCAATGTGATTTCCAAGCGC-3′ (NarI site underlined) containing the sequence encoding the kex-2 site (ANVISKR) and the reverse primer 5′-ATTAGATCTTTAGTGGTGGTGGTGGTGGTGCCAAGTACAAGCTCCGCT-3′ (BglII site underlined). The PCR product was cloned into NarI-BglII-linearized pAN56.1, generating pGDF. In these vectors, the Aspergillus nidulans glyceraldehyde-3-phosphate dehydrogenase gene (gpdA) promoter, the 5′ untranslated region of the gpdA mRNA, and the A. nidulans trpC terminator were used to drive the expression of recombinant sequences. Moreover, in the case of pDF, the 24-amino-acid glucoamylase (GLA) preprosequence from A. niger served to initiate secretion of the chimeric protein.
Aspergillus transformation and feruloyl esterase production.
Fungal cotransformation was carried out as described by Punt and van den Hondel (32) by using the expression vectors and pAB4.1 (41), containing the pyrG selection marker, in a 10:1 ratio. Transformants were selected for uridine prototrophy. Cotransformants containing expression vectors were selected as described in the following section.
Screening of the feruloyl esterase activity.
In order to screen the feruloyl esterase production in liquid medium for each expression vector, 30 individual transformants were cultivated in 100 ml of selective culture medium containing 70 mM NaNO3, 7 mM KCl, 200 mM Na2HPO4, 2 mM MgSO4, 5% (wt/vol) glucose, and trace elements and were inoculated with 106 spores/ml in a 500-ml baffled flask. The culture was monitored for 12 days at 30°C in a shaker incubator (130 rpm). The pH was adjusted to 5.5 daily with a 1 M citric acid solution. Each culture condition was performed in duplicate. From liquid culture medium, aliquots (1 ml) were collected and the mycelium was removed by filtration. Esterase activity was assayed as previously described (33) by using methyl ferulate (MFA) as a substrate. Activities were expressed in nanokatals; 1 nkat is defined as the amount of enzyme that catalyzes the release of 1 nmol of ferulic acid per s under established conditions. Each experiment was performed in duplicate and measured in triplicate, and the standard deviation was less than 1% of the mean.
Protein analysis.
Protein concentrations were determined by the method of Lowry et al. (26) with bovine serum albumin as the standard. Protein production was analyzed by using Coomassie blue-stained sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-12% PAGE) slab gels.
Preparation of the intracellular fraction.
Three grams (wet weight) of mycelium was ground in a mortar containing sterile sand at 4°C. A sterile solution of extraction buffer (100 mM morpholinepropanesulfonic acid [MOPS], 1 mM CaCl2 [pH 6]) was added to obtain a homogenous suspension, and the samples were centrifuged at 6,500 × g for 15 min. The supernatant was concentrated by using Amicon Ultra centrifugal filter devices (Millipore) to obtain a final volume of 250 μl.
Western blot analysis.
Extracellular proteins (corresponding to a supernatant volume of 70 μl) and intracellular proteins (6 μl) were electrophoresed in an SDS-12% polyacrylamide gel by the method of Laemmli (23) and electroblotted onto a BA85 nitrocellulose membrane (Schleicher and Schuell) at room temperature for 45 min. The membrane was incubated in blocking solution (50 mM Tris, 150 mM NaCl, and 2% [vol/vol] milk [pH 7.5]) overnight at 4°C. The membrane was then washed with Tris-buffered saline-0.2% Tween and treated with blocking solution containing an anti-FAEA serum at a dilution of 1:4,000. The membrane was incubated with goat anti-rabbit immunoglobulin G conjugated with horseradish peroxidase (Promega), and signals were detected with a chemiluminescence Western blotting kit (Roche).
Northern blot analysis.
Total RNA was isolated at various time points from biomass aliquots of A. niger as described by Wessels et al. (45). A 15-μg aliquot of total RNA was denatured at 65°C in a loading buffer mixture containing formamide and formaldehyde (37) and loaded onto a 1% Tris-acetate-EDTA agarose gel containing 6% formaldehyde. After electrophoresis, RNA was blotted onto a Hybond N+ membrane and UV cross-linked for 1 min (0.6 J · cm−1 · min−1) The blots were probed with a 32P-labeled probe consisting of the faeA and dockerin sequences; for the loading control, a PCR-amplified 18S DNA was used as a probe. Blotted membranes were hybridized overnight at 65°C in a buffer containing 0.5 M sodium phosphate buffer (pH 7.2) with 0.01 M EDTA, 7% (wt/vol) SDS, and 2% (wt/vol) blocking reagent (Roche Molecular Biochemicals). The most stringent posthybridization wash consisted of two 15-min washes in 0.2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate buffer [pH 7.0]) containing 1% (wt/vol) SDS at 65°C. The blots were exposed to X-ray film (Biomax MR; Eastman Kodak Company) overnight at room temperature.
Biotin labeling of protein and detection of biotin-labeled protein.
Biotinylation of C2CBD, containing a cohesin from C. thermocellum and the adjacent cellulose-binding domain (47), was performed with biotinyl N-hydroxysuccinimide ester as described in the biotin labeling kit instructions (Roche). Total extracellular proteins (corresponding to a supernatant volume of 200 μl) and total intracellular proteins (13 μl) were loaded onto an SDS-12% PAGE gel and transferred to a BA85 nitrocellulose membrane. Nonspecific binding was blocked by incubation with blocking solution. After overnight incubation, blocking solution containing 65 μg of biotinylated C2CBD was incubated, and labeled proteins were detected with the Western blotting kit according to the manufacturer's recommended procedures (Biotin/Streptavidin; Roche).
RESULTS
Design and production of chimeric proteins.
In order to produce a fungal enzyme that could be grafted onto a scaffolding protein, we designed two plasmids, pFD and pDF, containing the sequence encoding FAEA (Y09330) from A. niger fused upstream and downstream, respectively, to the dockerin domain of cellulase Cel48S from C. thermocellum. Both fusions were placed under the control of the gpdA promoter and the trpC terminator and contained the glucoamylase preprosequence of A. niger to target the secretion (Table 1, pFD and pDF). A. niger D15#26 protoplasts were cotransformed with a mixture of plasmid pAB4.1 and expression vectors. Transformants were selected for their abilities to grow on a minimal medium plate without uridine. For each construct, approximately 100 uridine prototrophic transformants were obtained per μg of expression vector. A fungal PCR colony was effected to control the integration of the expression cassette into the A. niger genome. Forty transformants were inoculated into a glucose-containing minimal medium, repressing endogenous faeA gene expression, and their extracellular medium was tested for esterase activity. Esterase activity was detected for transformants containing the FAEA-Doc expression cassette, whereas no activity was measured for any of the clones transformed by plasmid pDF or for the untransformed host. For FAEA-Doc transformants, esterase activity against MFA as the substrate ranged from 0.1 to 0.4 nkat/ml. Esterase activity was detected on day 3 and increased strongly until day 10 to reach a maximum of 0.4 nkat/ml for the clone exhibiting the highest activity (Fig. 1). This activity was estimated to be fivefold higher than that obtained with the wild-type strain BRFM281 under optimal conditions of production using maize bran as an inducer (25).
TABLE 1.
Expression vectors and strategy of constructions used in this work
| Construct | Vector used | Plasmid | Secretion system | Reference or source |
|---|---|---|---|---|
| FAEA | pAN52.3 | pF | ss (faeA) | 34 |
| FAEA-Doc | pAN52.3 | pFD | ss (faeA) | This study |
| Doc-FAEA | pAN52.4 | ss (glaA) | This study | |
| Doc-FAEA | pAN56.1 | pGDF | glaA carrier | This study |
FIG. 1.
Time course activity of the feruloyl esterase produced by the FAEA-Doc transformant. Activity was measured in the extracellular medium of the fungus by using MFA as the substrate.
Biochemical characterization of the chimeric proteins.
Total proteins in the supernatant were analyzed by SDS-PAGE, and a major band with a molecular mass close to 38 kDa was revealed for FAEA-Doc transformants (Fig. 2A). For Doc-FAEA transformants, no predominant band was observed, indicating that the lack of activity was due to a lack of Doc-FAEA protein production rather than to an inactivated chimeric protein. Immunodetection of the FAEA-Doc and Doc-FAEA proteins was performed using antibodies raised against FAEA (Fig. 2B). Western blot analysis showed a unique band corresponding to the 38-kDa protein, demonstrating that this protein was the chimeric FAEA-Doc fusion. The difference in molecular mass between purified FAEA (36 kDa) and the FAEA-Doc fusion (38 kDa) did not match with the expected size, since the molecular mass of the chimeric protein was estimated at around 43 kDa. Moreover, far-Western blotting using biotinylated cohesins from C. thermocellum as the probe confirmed the absence of the dockerin module in the supernatant produced by the FAEA-Doc (and Doc-FAEA) transformant, since no interaction signals were detected (data not shown). To exclude intracellular retention of the Doc-FAEA protein, we verified that the corresponding recombinant proteins had not accumulated in the mycelium cells of A. niger.
FIG. 2.
SDS-PAGE and Western blot analysis of extracellular proteins produced by the FAEA-Doc and Doc-FAEA transformants. The purified FAEA (lanes 1) and total proteins from the FAEA-Doc transformant (lanes 2), the Doc-FAEA transformant (lanes 3), and a nontransformed strain (lanes 4) were loaded onto an SDS-PAGE (11% polyacrylamide) gel. The gel was stained with Coomassie blue (A), or Western blotting using antibodies raised against A. niger FAEA protein was performed (B). Lane SD, molecular size standards.
Gene expression analysis.
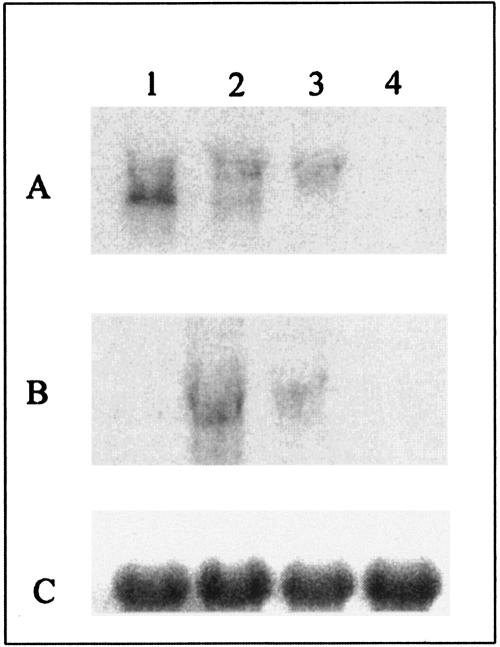
In order to analyze gene expression in both FAEA-Doc and Doc-FAEA transformants at the transcriptional level, Northern blot analysis was performed for 5-day-old cultures (Fig. 3). A transformant containing the faeA gene without dockerin was used for faeA gene transcription control (lane 1). An 18S gene probe was used as an internal control to monitor the amount of RNA loaded in each lane. By using FAEA and dockerin probes, similar levels of expression were detected for the FAEA-Doc and Doc-FAEA transformants (Fig. 3A and B, lanes 2 and 3). Moreover, for the two fusions, faeA-dockerin mRNAs of similar sizes were detected. Consequently, the absence of chimeric DF protein production was clearly not related to a transcriptional bottleneck.
FIG. 3.
Northern blot analysis of total RNAs isolated from biomass aliquots of A. niger transformants. Total RNAs (10 μg) from A. niger transformed with pF (lane 1), pFD (lane 2), or pDF (lane 3) and from nontransformed strain D15 (lane 4) were electroblotted and hybridized with faeA and dockerin cDNA probes from A. niger (A) and C. thermocellum (B), respectively. The PCR-amplified 18S DNA was used as a probe for the loading control (C).
Study of the chimeric Doc-FAEA protein production using a glucoamylase carrier strategy.
The results described above indicate that the localization of the dockerin module downstream of the FAEA leads to production of a truncated chimeric protein without dockerin. Moreover, when the bacterial dockerin-encoding sequence was expressed as a 5′ fusion to the fungal faeA gene, no protein was produced. Based on these results, we used a second strategy based on the fusion of the Doc-FAEA chimera to a carrier protein. With this strategy, the sequence encoding the first 514 amino acids of the well-secreted glucoamylase from A. niger (GLA1-514) is believed to improve the secretion efficiency of the Doc-FAEA protein by facilitating translocation and subsequent folding in the endoplasmic reticulum. In this genetic construction (pGDF [Table 1]), this sequence was fused upstream of the doc-faeA sequences, and a dibasic proteolytic processing site (kex-2) was introduced between the corresponding sequences encoding the GLA1-514 and Doc-FAEA proteins. Time course analysis of the feruloyl esterase activity produced by the best Doc-FAEA transformant showed a maximal activity of about 1.7 nkat/ml at day 8 (Fig. 4). Then esterase activity decreased, probably due to proteolytic degradation. This activity is approximately 20-fold-higher than the activity measured for the wild-type strain BRFM281 using maize bran as an inducer.
FIG. 4.
Time course activity of the feruloyl esterase produced by the Doc-FAEA transformant (pGDF construct). Activity was measured in the extracellular medium of the fungus by using MFA as the substrate.
Biochemical characterization of Doc-FAEA. (i) SDS-PAGE analysis of extracellular and intracellular proteins.
To monitor the production of the chimeric Doc-FAEA protein in the intracellular and extracellular fractions of the fungus, total proteins were analyzed by SDS-PAGE from days 3 to 10 (Fig. 5). From day 3, two predominant bands around 80 and 43 kDa were observed in the extracellular medium, in contrast to that of the wild-type strain D15, where no major band was present. The intensity of bands increased with incubation time, in accordance with the time course activity observations. These molecular masses were in complete agreement with those estimated for GLA1-514 and an intact chimeric Doc-FAEA protein, respectively, indicating correct processing of the GlaA carrier. With regard to total intracellular proteins, no clear protein accumulation was observed for the Doc-FAEA transformant.
FIG. 5.
Production of chimeric Doc-FAEA protein in the extracellular and intracellular fractions from A. niger transformants. Lanes: SD, molecular size standards; 1, purified FAEA; NT, nontransformed strain D15 at day 8; E and I, extracellular and intracellular fractions, respectively.
(ii) Detection of the chimeric Doc-FAEA protein by cohesin-dockerin interaction and Western blot analysis.
In order to confirm the production of an intact and functional chimeric Doc-FAEA protein, dockerin-cohesin interaction tests were carried out by using extracellular and intracellular fractions from day 3 to 10 (Fig. 6). Proteins from the untransformed strain D15 and engineered Cel9E (12) from C. cellulolyticum with a C. thermocellum dockerin appended served as negative and positive controls for dockerin-cohesin interactions, respectively. From day 6, two proteins with molecular masses around 43 and 7 kDa were detected in the extracellular medium. According to these molecular masses, we supposed that the labeled cohesins interacted with a whole Doc-FAEA protein (7 + 36 kDa) but also with a single dockerin module (7 kDa) cleaved from the Doc-FAEA protein. In the intracellular fraction, a very high molecular mass was detected from day 5, 1 day earlier than detection of secreted Doc-FAEA protein. This result suggests that Doc-FAEA is present in the secretion pathway, as an intermediary protein fused to the carrier glucoamylase, with a global molecular mass close to 120 kDa. This protein is processed in the late Golgi apparatus, where the fungal Kex-2-like protein is expected to be active (40). Consequently, this high-molecular-mass protein could be the immature Doc-FAEA form fused to glucoamylase.
FIG. 6.
Cohesin-dockerin interaction assay. Extracellular (E) and intracellular (I) media from days 3 to 10 were loaded onto an SDS-PAGE (11% acrylamide) gel, transferred, and probed with a cohesin-containing protein (C2CBD) labeled by biotinylation. Proteins from the nontransformed strain D15 and an engineered cellulase from C. cellulolyticum with a C. thermocellum dockerin appended (Cel9E) served as negative and positive controls for dockerin-cohesin interactions, respectively. Detection was performed using a streptavidin-peroxidase conjugate. SD, molecular size standards stained with Coomassie blue.
To complete and validate previous results, total extracellular and intracellular proteins were loaded onto an SDS-PAGE gel and immunodetected by using antibodies raised against FAEA protein (Fig. 7). In the extracellular medium (Fig. 7, lane 2), protein bands of approximately 43 and 36 kDa were detected. The first, large band corresponds to the whole Doc-FAEA protein, whereas the small band, with a molecular mass close to that of the purified FAEA, is a cleaved form of Doc-FAEA (Fig. 7, lane 1). In the intracellular fraction, both 36- and 43-kDa proteins could be observed. In addition, a protein with a molecular mass of around 120 kDa, corresponding to the noncleaved GLA1-514-Doc-FAEA form, was also detected.
FIG. 7.
Western blot analysis of extracellular and intracellular proteins from the Doc-FAEA transformant. Lane SD, molecular size standards; lane 1, purified FAEA; lanes 2 and 4, extracellular medium at day 9 from the Doc-FAEA transformant and the wild-type strain, respectively; lanes 3 and 5, intracellular medium at day 9 from the Doc-FAEA transformant and the wild-type strain, respectively.
DISCUSSION
The bacterial cellulosome is a very effective system for increasing the synergistic effect between enzymes. These positive effects were determined on crystalline cellulose by using hybrid cellulases containing dockerin domains of different clostridial species incorporated into chimeric scaffoldin protein (12, 14). In the present work, our aim was to produce a fungal enzyme different from bacterial cellulase, to be grafted into a cellulosome complex. This work will be included in future studies comparing the synergistic effects of free and grafted fungal and nonfungal enzymes for plant cell wall degradation. Therefore, we developed a strategy to secrete a chimeric fungal enzyme fused to a bacterial dockerin module in A. niger. This fungus is a well-known host for protein overproduction, producing complex enzymes that require posttranslational modifications, such as glycosylation. Thus, the production of chimeric proteins consisting of a complex eukaryotic enzyme fused to a bacterial dockerin domain provided an extension to the production of E. coli hybrid cellulases.
In the first approach, the dockerin module from C. thermocellum was fused to a well-secreted protein, A. niger FAEA. Both N-terminal and C-terminal fusions of the bacterial module were attempted to allow a correct folding of both partners. Feruloyl esterase activity in the extracellular medium from FAEA-Doc transformants was fivefold higher than that of the reference strain. This improvement factor is less than that obtained for homologous production of FAEA in A. niger (34). Apparently, as seen for other fusion proteins, the production yield was negatively affected by the presence of the bacterial domain (21, 31, 35, 36). In addition, we observed that FAEA-Doc protein was produced as a truncated form. The cleavage site is located at the beginning of the dockerin N-terminal end, since a very weak molecular mass difference between FAEA-Doc and native FAEA was observed. The C-terminal fusion of the bacterial domain generates an unstable structure, leading to a proteolytic or physical cleavage between the two partners, and as a consequence, the corresponding construct was not considered in the subsequent experiment.
The Doc-FAEA construct produced no chimeric protein, in agreement with the lack of feruloyl esterase activity in the extracellular medium. The detection of mRNA encoding the chimeric Doc-FAEA protein indicates that the bottleneck for Doc-FAEA production lies at a posttranscriptional level. Thus, the location of the bacterial domain next to the fungal protein is an essential parameter to consider for this overproduction. In earlier studies, methods were developed to alleviate limitations for protein production in fungal hosts. An efficient strategy is based on the use of translational fusions in which the targeted protein is fused to an endogenous secreted carrier protein at the N-terminal end of the heterologous protein (17, 35). The N-terminal carrier protein is believed to improve the secretion efficiency of the heterologous protein by facilitating translocation and folding in the endoplasmic reticulum. These results could explain differences between FAEA-Doc and Doc-FAEA protein production. Indeed, for the FAEA-Doc construction, the N-terminal FAEA played the role of carrier protein, in contrast to Doc-FAEA, where the FAEA was located C-terminal to the dockerin. In addition, Ward and coworkers (44) demonstrated that an autocatalytic release between the carrier and the heterologous protein was possible, which could explain the fact that only the truncated form of FAEA-Doc was detected.
We designed a new construct in which the Doc-FAEA sequence was located downstream of a partial sequence from A. niger glucoamylase (GLA1-514) and the kex-2 site (4, 6). Thus, the recombinant protein will be recognized and processed in vivo at this consensus dibasic proteolytic processing site (Kex2 site) in the trans-Golgi network by the kexin family of proteases (30, 40). By use of this strategy, the negative effect generated by the bacterial dockerin was reduced and an increased esterase activity was detected in the extracellular medium, demonstrating the efficiency of the fusion to the carrier protein. Moreover, the esterase activity was even 4- and 20-fold higher, respectively, than those obtained for the FAEA-Doc transformant and the wild-type strain under optimal induction conditions, confirming that glucoamylase is the carrier protein of choice for such a construct. Glucoamylase was used in previous work to successfully produce recombinant proteins such as porcine pancreatic prophospholipase A2, human interleukin-6, or hen egg white lysozyme (6, 17, 20, 21, 35). Based on the specific activity of purified Doc-FAEA, we estimated a production yield of 100 mg per liter. The cohesin-dockerin interaction assay revealed two extracellular bands of about 43 and 8 kDa interacting with the labeled scaffolding protein. The recombinant extracellular Doc-FAEA protein was partially cleaved, producing two forms: an intact Doc-FAEA protein containing a dockerin domain and a truncated form where the dockerin was separated from the enzymatic partner. This cleaved dockerin possessed two conserved duplicated sequences, each containing a calcium binding motif, allowing the dockerin domain to bind to cohesins (27). In addition to a unique extracellular band of 43 kDa interacting with cohesins, antibodies raised against FAEA recognized a second, minor band of about 36 kDa. This form corresponded to the FAEA without dockerin from partially cleaved Doc-FAEA, confirming previous results of cohesin-dockerin assays. Therefore, the cleavage occurred near the linker region between dockerin and FAEA, leading to two functional domains with the majority of the Doc-FAEA in the intact form, as shown by Western blot analysis. In order to improve the stability and the final recovery of intact Doc-FAEA, this linker region might be modified, i.e., by adding glycosylation sites [N-X-(S/T)]. In the cellular extract, a very high molecular weight protein was detected by the cohesin-dockerin interaction assay and Western blotting. This form was suggested to be a nonprocessed GlaA-Kex2-Doc-FAEA protein. Immunodetection also revealed two other proteins, corresponding to a major, intact Doc-FAEA and a minor, cleaved Doc-FAEA. The intracellular Doc-FAEA protein (around 43 kDa) could be observed in the terminal step of the secretion pathway, because the Kex2 site was already cleaved in the trans-Golgi apparatus. However, interaction of this Doc-FAEA protein form was not detected with labeled cohesins, indicating that this protein was not completely processed until the extracellular medium. No experiments fusing the FAEA-Doc construct to a carrier protein were carried out, because the aim of the study was achieved, but future work to investigate if the C-terminal dockerin end would be protected by the glucoamylase protein is envisaged.
The aim of this study was to produce in A. niger a chimeric protein fusing a fungal enzyme and a bacterial dockerin domain. In this work, a strategy was developed to produce the first fungal enzyme able to be incorporated in vitro into a bacterial cellulosome from C. thermocellum. Future work will be performed to modify the linker region between the dockerin and the fungal enzyme. Moreover, other complementary enzymes, such as metalloenzymes which cannot be produced in bacterial hosts, will be fused to dockerin from different species. All these chimeric enzymes might be assembled onto chimeric cellulosomes, and the resultant synergy of degradation could be estimated.
Acknowledgments
This research was supported by the GIS-EBL (Conseil Régional Provence-Alpes-Côte d'Azur, the Conseil Général 13, France), by ADEME (Agrice program), and by grants from the MENRT (Ministère de l'Education Nationale, de la Recherche et de la Technologie, France).
REFERENCES
- 1.Archer, D. B., and J. F. Peberdy. 1997. The molecular biology of secreted enzyme production by fungi. Crit. Rev. Biotechnol. 17:273-306. [DOI] [PubMed] [Google Scholar]
- 2.Bayer, E. A., E. Morag, and R. Lamed. 1994. The cellulosome—a treasure-trove for biotechnology. Trends Biotechnol. 12:379-386. [DOI] [PubMed] [Google Scholar]
- 3.Beguin, P. 1999. Hybrid enzymes. Curr. Opin. Biotechnol. 10:336-340. [DOI] [PubMed] [Google Scholar]
- 4.Calmels, T. P., F. Martin, H. Durand, and G. Tiraby. 1991. Proteolytic events in the processing of secreted proteins in fungi. J. Biotechnol. 17:51-66. [DOI] [PubMed] [Google Scholar]
- 5.Ciruela, A., H. J. Gilbert, B. R. Ali, and G. P. Hazlewood. 1998. Synergistic interaction of the cellulosome integrating protein (CipA) from Clostridium thermocellum with a cellulosomal endoglucanase. FEBS Lett. 422:221-224. [DOI] [PubMed] [Google Scholar]
- 6.Contreras, R., D. Carrez, J. R. Kinghorn, C. A. van den Hondel, and W. Fiers. 1991. Efficient KEX2-like processing of a glucoamylase-interleukin-6 fusion protein by Aspergillus nidulans and secretion of mature interleukin-6. Biotechnology (New York) 9:378-381. [DOI] [PubMed] [Google Scholar]
- 7.de Vries, R. P., B. Michelsen, C. H Poulsen., P. A. Kroon, R. H. H. van den Heuvel, C. B. Faulds, G. Williamson, J. P. T. W. van den Hombergh, and J. Visser. 1997. The faeA genes from Aspergillus niger and Aspergillus tubingensis encode ferulic acid esterases involved in degradation of complex cell wall polysaccharides. Appl. Environ. Microbiol. 63:4638-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vries, R. P., and J. Visser. 2001. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol. Mol. Biol. Rev. 65:497-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Vries, R. P., J. Visser, and L. H. de Graaff. 1999. CreA modulates the XlnR-induced expression on xylose of Aspergillus niger genes involved in xylan degradation. Res. Microbiol. 150:281-285. [DOI] [PubMed] [Google Scholar]
- 10.Doi, R. H., A. Kosugi, K. Murashima, Y. Tamaru, and S. O. Han. 2003. Cellulosomes from mesophilic bacteria. J. Bacteriol. 185:5907-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felix, C. R., and L. G. Ljungdahl. 1993. The cellulosome: the exocellular organelle of Clostridium. Annu. Rev. Microbiol. 47:791-819. [DOI] [PubMed] [Google Scholar]
- 12.Fierobe, H. P., E. A. Bayer, C. Tardif, M. Czjzek, A. Mechaly, A. Belaich, R. Lamed, Y. Shoham, and J. P. Belaich. 2002. Degradation of cellulose substrates by cellulosome chimeras. Substrate targeting versus proximity of enzyme components. J. Biol. Chem. 277:49621-49630. [DOI] [PubMed] [Google Scholar]
- 13.Fierobe, H. P., S. Pagès, A. Belaich, S. Champ, D. Lexa, and J. P. Belaich. 1999. Cellulosome from Clostridium cellulolyticum: molecular study of the dockerin/cohesin interaction. Biochemistry 38:12822-12832. [DOI] [PubMed] [Google Scholar]
- 14.Fierobe, H. P., A. Mechaly, C. Tardif, A. Belaich, R. Lamed, Y. Shoham, J. P. Belaich, and E. A. Bayer. 2001. Design and production of active cellulosome chimeras. Selective incorporation of dockerin-containing enzymes into defined functional complexes. J. Biol. Chem. 276:21257-21261. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Campayo, V., and P. Beguin. 1997. Synergism between the cellulosome-integrating protein CipA and endoglucanase CelD of Clostridium thermocellum. J. Biotechnol. 57:39-47. [DOI] [PubMed] [Google Scholar]
- 16.Gordon, C. L., D. B. Archer, D. J. Jeenes, J. H. Doonan, B. Wells, A. P. J. Trinci, and G. D. Robson. 2000. A glucoamylase::GFP gene fusion to study protein secretion by individual hyphae of Aspergillus niger. J. Microbiol. Methods 42:39-48. [DOI] [PubMed] [Google Scholar]
- 17.Gouka, R. J., P. J. Punt, and C. A. van den Hondel. 1997. Glucoamylase gene fusions alleviate limitations for protein production in Aspergillus awamori at the transcriptional and (post)translational levels. Appl. Environ. Microbiol. 63:488-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graf, E. 1992. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 13:435-448. [DOI] [PubMed] [Google Scholar]
- 19.Ho, S. N.; H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 20.Jeenes, D. J., B. Marczinke, D. A. MacKenzie, and D. B. Archer. 1993. A truncated glucoamylase gene fusion for heterologous protein secretion from Aspergillus niger. FEMS Microbiol. Lett. 107:267-271. [DOI] [PubMed] [Google Scholar]
- 21.Joosten, V., C. Lokman, C. A. van den Hondel, and P. J. Punt. 2003. The production of antibody fragments and antibody fusion proteins by yeasts and filamentous fungi. Microb. Cell Fact. 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kataeva, I., G. Guglielmi, and P. Beguin. 1997. Interaction between Clostridium thermocellum endoglucanase CelD and polypeptides derived from the cellulosome-integrating protein CipA: stoichiometry and cellulolytic activity of the complexes. Biochem. J. 326:617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Lam, T. B. T., K. Iiyama, and B. A. Stone. 1992. Cinnamic acid bridges between cell wall polymers in wheat and phalaris internodes. Phytochemistry 31:1179-1183. [Google Scholar]
- 25.Lesage-Meessen, L., A. Lomascolo, E. Bonnin, J. F. Thibault, A. Buleon, M. Roller, M. Asther, E. Record, B. C. Ceccaldi, and M. Asther. 2002. A biotechnological process involving filamentous fungi to produce natural crystalline vanillin from maize bran. Appl. Biochem. Biotechnol. 102-103:141-153. [DOI] [PubMed] [Google Scholar]
- 26.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 27.Lytle, B., and J. H. Wu. 1998. Involvement of both dockerin subdomains in assembly of the Clostridium thermocellum cellulosome. J. Bacteriol. 180:6581-6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oosterveld, A., J. H. Grabber, G. Beldman, J. Ralph, and A. G. J. Voragen. 1997. Formation of ferulic acid dehydrodimers through oxidative cross-linking of sugar beet pectin. Carbohydr. Res. 300:179-181. [Google Scholar]
- 29.Pagès, S., A. Belaich, C. Tardif, C. Reverbel-Leroy, C. Gaudin, and J. P. Belaich. 1996. Interaction between the endoglucanase CelA and the scaffolding protein CipC of the Clostridium cellulolyticum cellulosome. J. Bacteriol. 178:2279-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Punt, P. J., A. Drint-Kuijvenhoven, B. C. Lokman, J. A. Spencer, D. Jeenes, D. A. Archer, and C. A. M. J. van den Hondel. 2003. The role of the Aspergillus niger furin-type protease gene in processing of fungal proproteins and fusion proteins. Evidence for alternative processing of recombinant (fusion-) proteins. J. Biotechnol. 106:23-32. [DOI] [PubMed] [Google Scholar]
- 31.Punt, P. J., N. van Biezen, A. Conesa, A. Albers, J. Mangnus, and C. van den Hondel. 2002. Filamentous fungi as cell factories for heterologous protein production. Trends Biotechnol. 20:200-206. [DOI] [PubMed] [Google Scholar]
- 32.Punt, P. J., and C. A. van den Hondel. 1992. Transformation of filamentous fungi based on hygromycin B and phleomycin resistance markers. Methods Enzymol. 216:447-457. [DOI] [PubMed] [Google Scholar]
- 33.Ralet, M. C., C. B. Faulds, G. Williamson, and J. F. Thibault. 1994. Degradation of feruloylated oligosaccharides from sugar-beet pulp and wheat bran by ferulic acid esterases from Aspergillus niger. Carbohydr. Res. 263:257-269. [DOI] [PubMed] [Google Scholar]
- 34.Record, E., M. Asther, C. Sigoillot, S. Pagès, P. J. Punt, M. Delattre, M. Haon, C. A. van den Hondel, J. C. Sigoillot, L. Lesage-Meessen, and M. Asther. 2003. Overproduction of the Aspergillus niger feruloyl esterase for pulp bleaching application. Appl. Microbiol. Biotechnol. 62:349-355. [DOI] [PubMed] [Google Scholar]
- 35.Roberts, I. N., D. J. Jeenes, D. A. MacKenzie, A. P. Wilkinson, I. G. Sumner, and D. B. Archer. 1992. Heterologous gene expression in Aspergillus niger: a glucoamylase-porcine pancreatic prophospholipase A2 fusion protein is secreted and processed to yield mature enzyme. Gene 122:155-161. [DOI] [PubMed] [Google Scholar]
- 36.Roberts, I. N., R. P. Oliver, P. J. Punt, and C. A. van den Hondel. 1989. Expression of the Escherichia coli beta-glucuronidase gene in industrial and phytopathogenic filamentous fungi. Curr. Genet. 15:177-180. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., p. 7.43-7.45. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Schwarz, W. H. 2001. The cellulosome and cellulose degradation by anaerobic bacteria. Appl. Microbiol. Biotechnol. 56:634-649. [DOI] [PubMed] [Google Scholar]
- 39.Shoham, Y., R. Lamed, and E. A. Bayer. 1999. The cellulosome concept as an efficient microbial strategy for the degradation of insoluble polysaccharides. Trends Microbiol. 7:275-281. [DOI] [PubMed] [Google Scholar]
- 40.Siezen, R. J., and J. A. Leunissen. 1997. Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci. 6:501-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Hartingsveldt, W., I. E. Mattern, C. M. van Zeijl, P. H. Pouwells, and C. A. van den Hondel. 1987. Development of a homologous transformation system for Aspergillus niger based on the pyrG gene. Mol. Gen. Genet. 206:71-75. [DOI] [PubMed] [Google Scholar]
- 42.van Peij, N. N., M. M. Gielkens, R. P. de Vries, J. Visser, and L. H. de Graaff. 1998. The transcriptional activator XlnR regulates both xylanolytic and endoglucanase gene expression in Aspergillus niger. Appl. Environ. Microbiol. 64:3615-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Peij, N. N., J. Visser, and L. H. de Graaff. 1998. Isolation and analysis of xlnR, encoding a transcriptional activator co-ordinating xylanolytic expression in Aspergillus niger. Mol. Microbiol. 27:131-142. [DOI] [PubMed] [Google Scholar]
- 44.Ward, M., L. J. Wilson, K. H. Kodama, M. W. Rey, and R. M. Berka. 1990. Improved production of chymosin in Aspergillus by expression as a glucoamylase-chymosin fusion. Biotechnology (New York) 8:435-440. [DOI] [PubMed] [Google Scholar]
- 45.Wessels, J. G. H., G. H. Mulder, and J. Springer. 1987. Expression of dikaryon-specific and nonspecific mRNAs of Schizophylum commune: relation to environmental conditions and fruiting. J. Gen. Microbiol. 133:2557-2561. [Google Scholar]
- 46.Williamson, G., P. A. Kroon, and C. B. Faulds. 1998. Hairy plant polysaccharides: a close shave with microbial esterases, Microbiology 144:2011-2023. [DOI] [PubMed] [Google Scholar]
- 47.Yaron, S., E. Morag, E. A. Bayer, R. Lamed, and Y. Shoham. 1995. Expression, purification and subunit-binding properties of cohesins 2 and 3 of the Clostridium thermocellum cellulosome. FEBS Lett. 360:121-124. [DOI] [PubMed] [Google Scholar]