Abstract
Obesity increases inflammation, both peripherally and centrally, and exercise can ameliorate some of the negative health outcomes associated with obesity. Within the brain, the effect of obesity on inflammation has been well characterized in the hypothalamus and hippocampus, but has been relatively understudied in other brain regions. The current study was designed to address two primary questions; (1) whether western diet (high fat/high sucrose) consumption would increase markers of inflammation in the prefrontal cortex and (2) whether concurrent voluntary wheel running would ameliorate any inflammation. Adult male mice were exposed to a western diet or a control diet for 8 weeks. Concurrently, half the animals were given running wheels in their home cages, while half did not have access to wheels. At the conclusion of the study, prefrontal cortex was removed and expression of 18 proinflammatory genes was assayed. Expression of a number of proinflammatory molecules was upregulated by consumption of the western diet. For two chemokines, chemokine (C-C motif) ligand 2 (CCL2) and C-X-C motif chemokine 10 (CXCL10), voluntary exercise blocked the increase in the expression of these genes. Cluster analysis confirmed that the majority of the tested genes were upregulated by western diet, and identified another small cluster of genes that were downregulated by either diet or exercise. These data identify a proinflammatory phenotype within the prefrontal cortex of mice fed a western diet, and indicate that chemokine induction can be blocked by voluntary exercise.
Keywords: Western diet, Voluntary exercise, Chemokine, Cytokine, Prefrontal cortex, Mouse, Reward
1. Introduction
Chronic consumption of a high fat/high sugar diet (the so-called Western Diet, WD) contributes to weight gain, and subsequent negative health outcomes, including increased adiposity, hyperlipidemia, and hyperglycemia. Additionally, obesity is associated with an increase in inflammation, both in the periphery (Xu et al., 2003) as well as in brain (De Souza et al., 2005). This peripheral inflammation is thought to play a causative role in at least some of the obesity-related health problems, such as insulin resistance (Xu et al., 2003), while central inflammation is believed to play a contributing role in obesity and related complications (Dorfman and Thaler, 2015; Zhang et al., 2008), through direct negative effects on neurons within circuits that regulate energy balance (Thaler et al., 2012).
Exercise is one of the most effective strategies to combat the adverse effects of weight gain. By increasing energy expenditure while maintaining or decreasing energy intake, weight loss is initiated, with a subsequent improvement in blood glucose and lipid levels, and a reduction in adipose tissue. Peripherally, weight loss can decrease inflammation. In a study following weight loss patients for 33 weeks, a decrease in circulating levels of adipokines and inflammatory markers was observed after weight loss (de Mello et al., 2008). In a rodent study in which a 3-week exercise intervention followed a 12-week HFD exposure (and weight gain), exercise was found to reduce inflammation in skeletal muscle and liver, but interestingly, not adipose tissue (Jung et al., 2013). Inflammation in the brain may be similarly difficult to reverse. Following a 10 week exposure to HFD, hypothalamic inflammation was detectable, yet even after an 8 week return to chow feeding, adiposity levels normalized, but hypothalamic inflammation persisted (Wang et al., 2012).
With regard to HFD-induced inflammation in the brain, important regional differences exist. Inflammation associated with obesity and HFD has been consistently demonstrated in the hypothalamus (Thaler et al., 2012; Wu et al., 2014; Naznin et al., 2015), as well as in the hippocampus (Kang et al., 2016; Jeon et al., 2012). Fewer reports have examined the prefrontal cortex (PFC), and those that have generally failed to identify inflammation. In a two-week study with rats, in which animals were fed a cafeteria diet with or without the addition of sugar, there was no inflammation noted in cortex or hypothalamus (Beilharz et al., 2016). With a longer, 16 week HFD exposure in rats, increased expression of interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) was detected in the hippocampus, but not in the cortex (Dutheil et al., 2015), while another study using 16 week exposure to HFD, found that levels of IL-1, IL-6, and TNF-α were not elevated in hypothalamus, hippocampus, nor cortex (Boitard et al., 2014). However, in a study which involved HFD feeding for 5 months in rats, an increase in prostaglandin E2 (PGE2) and cyclooxygenase-2 (COX-2) was detected in the cortex (Zhang et al., 2005). Similarly, 4 months of high fat feeding in mice led to an increase in IL-1 and TNF-α mRNA in cortex (Jayaraman et al., 2014), though, the precise region of cortex was not identified. It is difficult to draw definitive conclusions from the current literature, as a range of protocols (differing diets, length of exposure, species) makes direct comparison between studies impossible. Further, the majority of the experiments examined only a few proinflammatory cytokines.
Recent work has examined the synergistic effects of exercise and HFD. In a recent report, 20 weeks of high fat diet induced an increase in the numbers of microglia and astrocytes, and TLR4-related proteins in hippocampus, and treadmill exercise normalized these proinflammatory changes (Kang et al., 2016). Similar responses, where exercise reversed or normalized the HFD-driven inflammatory responses have also been seen in liver (Jeong et al., 2015), lung (Warren et al., 2015) and spinal cord (Yoon et al., 2016). For the PFC, the literature is limited and mixed. Microglia and astrocyte numbers in the cortex were normalized by treadmill exercise in the aforementioned study (Kang et al., 2016), however no other pro-inflammatory molecules were measured in cortex. In a recent report, treadmill running for 26 weeks was found to attenuate microglia activation in the arcuate nucleus, but had no effect on microglia in the PFC or hippocampus (Yi et al., 2012). Therefore, the primary goal of the present study was to examine how concurrent exposure to a western diet (high fat/high sugar) and voluntary wheel running would affect proinflammatory gene expression in the PFC using a broader panel of immune-related genes. Further, behavioral assays of anxiety and reward were completed, as there is some evidence that HFD exposure can be anxiogenic (Dutheil et al., 2015), and exercise can be anxiolytic (Dubreucq et al., 2015).
2. Methods
2.1. Materials and methods
2.1.1. Animals
C57BL/6 male mice (Charles River Laboratories International, Inc., Wilmington, MA) at 9 weeks of age were singly housed in standard polyethylene cages in an environmentally controlled room (22–24 °C) with a 12 h light/dark cycle. Mice were randomized to ad lib access to either standard control diet (#5001, 13% fat (35% from saturated fat), 29.8% protein, and 56.7% carbohydrate (3.8% from sucrose)) or a western-style diet (# D12079B, 17% protein, 41% fat (62% of dietary fat is saturated fat), 43% carbohydrate (29% is from sucrose), Research Diets, New Brunswick, NJ). Randomly chosen mice (n = 10/group) from each dietary group received ad libitum access to a voluntary running wheel (MiniMitter, Bend Oregon) in their home cages, generating 4 experimental groups; (1) control (CTRL) diet + sedentary (SED), (2) WD diet + SED, (3) CTRL diet + exercise (EX), and (4) WD + EX. Body weight was measured at the beginning (T0), weekly, and conclusion of the study (at sacrifice). Sucrose preference was measured at the beginning of the study and after 6 weeks on the diet/exercise protocol, and the other behavioral assays were completed at week 7. At the end of week 8, animals were euthanized by carbon dioxide overdose, followed by cervical dislocation; a method recommended by the Panel on Euthanasia of the American Veterinary Medical Association. Animal housing and behavioral experiments took place at University of California at Los Angeles. Flash frozen brain samples were shipped to University of Pennsylvania for further experimentation. Experiments were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. The UCLA Chancellor’s Animal Research Committee approved all procedures used in this study.
2.1.2. Elevated plus maze
The elevated plus maze (EPM) test to assess anxiety-like behavior was carried out according to a previously established protocol (Walf and Frye, 2007). The EPM apparatus was made of laminated wood and consisted of 2 opposing open arms (10 × 50 cm) and 2 opposing closed arms (10 × 50 cm with 30 cm high walls). The maze was placed 60 cm above the floor. White curtains surrounded the maze and behavior was recorded by an overhead video camera. Each mouse was placed in the middle of the maze facing the open arm that faced away from the experimenter. The video camera recorded the time each mouse spent in each of the arms over a period of 5 min. A closed arm entry was counted when the mouse placed all four paws in a closed arm. An open arm entry was recorded when the mouse placed all four paws in an open arm or when the mouse’s hind-limbs were placed in the central area of the maze and both fore-limbs in an open arm with its head protruding into the open arm.
2.1.3. Open field test
The open field (OF) test to evaluate anxiety-like behavior was completed in a 1.2 m diameter circular tank with 60 cm walls. An inner circle, 80 cm in diameter, was marked on the tank floor to serve as a central arena. Testing began when each mouse was placed in the middle of the central arena and allowed to explore the field for 10 min. Mouse behavior was recorded by an overhead camera. Measurement included time spent and number of entries in central arena.
2.1.4. Sucrose preference
Consumption of sucrose engages the central reward circuitry, and sucrose preference is an indication of an animal’s response to a naturally rewarding stimulus. Mice were individually housed (n = 10/group) in standard cages for 3 days with one 200 ml bottle of 4% sucrose solution (w/v), another 200 ml bottle of tap water and house chow available ad libitum. Sucrose (ml), water (ml), and food consumption (g), were measured and the placement of the bottles was reversed daily. Preference was calculated using the averages from the last 2 days only as follows: preference % = [(sucrose consumption/sucrose + water consumption) × 100]. Sucrose preference test was performed at baseline and after 6 week diet and/or running wheel access.
2.1.5. Gene expression in prefrontal cortex
Animals were sacrificed and brains were placed in RNAlater and stored at −20C. The medial prefrontal cortex (PFC, consisting of anterior cingulate, prelimbic, and infralimbic) was dissected from a 2 mm coronal slice from bregma +2.3 to +0.3 and DNA and RNA was extracted using Qiagen AllPrep DNA/RNA Mini kit. 100 μg/μl cDNA was synthesized using Applied Biosystems High Capacity Reverse Transcriptase kit. Gene expression was assayed using Taqman primers on custom OpenArray real-time PCR plates assaying housekeeping genes (GAPDH, ACTB) and 18 genes of interest (see Table 1). The genes in the present panel include well studied pro-inflammatory genes (cytokines and chemokines) and molecules related to down-stream signaling (CREB, NOS, COX-2, NFkB) that are known to be expressed in the brain. Expression of housekeeping genes did not differ between groups. Expression of targets was normalized to the geometric mean of housekeeping genes and expressed as 2−△Ct (Schmittgen and Livak, 2008). The design of the OpenArray PCR plate allowed for analysis of n = 5 samples/group, so five samples were randomly chosen for gene expression analysis. 2 animals in the WD groups had very high Ct values for multiple housekeeping genes, suggesting these samples were of lower concentration or quality than expected, and were therefore eliminated. The resulting n’s for the gene expression experiment were as follows: n = 5 animals/group for control fed animals (Sed or Ex), and n = 4 for WD fed animals (Sed or EX). An additional single assay failed in the analysis of IL-6 and NOS, resulting in a reduced n for these genes.
Table 1.
Assay IDs for OpenArray gene expression experiment.
| Gene | Assay ID |
|---|---|
| ACTB | Mm00607939_s1 |
| CCR2 | Mm00438270_m1 |
| COX2 | Mm00478374_m1 |
| CREB | Mm00501607_m1 |
| CXCL10 | Mm00445235_m1 |
| GAPDH | Mm99999915_g1 |
| GFAP | Mm01253033_m1 |
| GR | Mm00433832_m1 |
| IkBalpha | Mm00477798_m1 |
| IL-18 | Mm00434225_m1 |
| IL-1β | Mm01336189_m1 |
| IL-1R | Mm00434237_m1 |
| IL-6 | Mm00446190_m1 |
| CCL2 | Mm00441242_m1 |
| NFkB (p65) | Mm00501346_m1 |
| NOS | Mm00440502_m1 |
| PGES | Mm00452105_m1 |
| SOCS3 | Mm00545913_s1 |
| TLR4 | Mm00445273_m1 |
| TNF-alpha | Mm00443258_m1 |
For the cluster analysis, normalized gene expression was converted to z-scores, which removes differences in endogenous expression levels between genes to highlight differences in expression levels between animals. We performed unsupervised hierarchical clustering of complete linkage (uncentered correlation) of genes and of treatment conditions based on gene expression levels using Cluster 3.0 and JavaTreeview (Eisen et al., 1998 as described in Grissom et al. (2015)). Complete linkage creates clusters of approximately equal size by associating elements (either genes or animals) that are most completely similar to each other across the expression of all genes, but does not require assumptions as to the number of clusters. The clustering produced by this algorithm was analyzed to interpret the number of clusters and their common elements. The relative expression level of genes was transformed into a yellow-blue heat map, where yellow indicated higher than average gene expression (positive z-scores), and blue indicated lower than average gene expression (negative z-scores).
2.2. Statistics
Data were analyzed with two-way ANOVA, followed by Newman-Keuls posthoc comparisons (two planned comparisons were conducted: SED-CONT vs SED-WD; to test the effect of WD in sedentary animals and SED-WD vs EX-WD; to test the effect of exercise in WD-fed animals). Repeated measures ANOVA was used for the analysis of the running wheel data.
3. Results
Body weight (Fig. 1A, B) was measured at the beginning of the experiment (T0) and weekly thereafter. There were no differences across the groups at the start of the experiment (T0, Fig. 1A). A significant diet × exercise × time (F(8,29) = 3.19, p < 0.01) interaction was noted, such that the rate of weight gain over time differed between the experimental groups (Fig. 1A). Animals on the control diet, regardless of exercise status, did not differ at any point. WD-SED animals weighed significantly more than all other groups from weeks 1–8, while the WED-EX group weighed more than the CONT-EX from week 5 onward. At the conclusion of the study, body weight differed significantly between the groups (significant interaction between diet and exercise (Fig. 1B, F(1,36) = 6.37, p < 0.02). Posthoc analyses revealed that animals on the WD weighed significantly more than control fed animals, however, animals on WD that were given running wheel access had a significantly attenuated body weight, as compared to WD-fed animals with no running wheel. Analysis of the distance run by the animals with wheels revealed a significant interaction between time and diet (Fig. 1B; F(7,126) = 4.75, p < 0.0001). Posthoc comparisons revealed that animals on the WD ran significantly more than control-fed mice during weeks 2–4, but beyond that, and importantly at the time of sacrifice, the distance run did not differ between the groups.
Fig. 1.
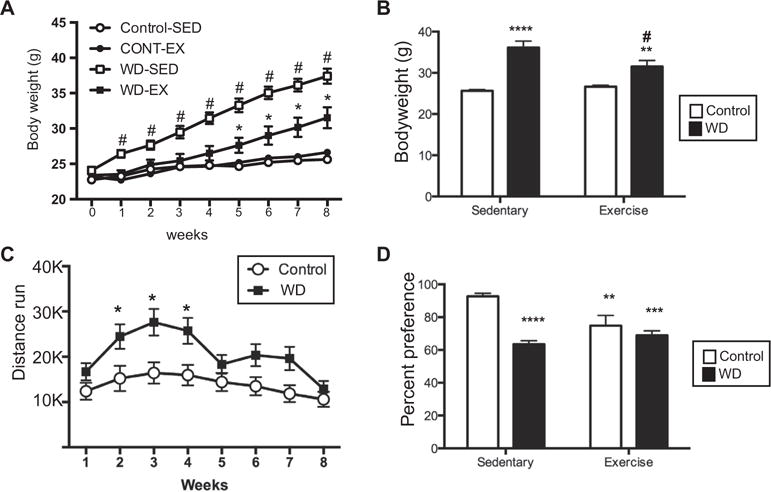
Body weight, running distance, and sucrose preference. (A) Body weight at T0 (start of the experiment) and weekly thereafter. #p < 0.01, WD-SED compared to each of the other three groups; *p < 0.05, WD-EX compared to CONT-EX. Circles: control-fed animals, squares: WD-fed animals, open symbol: SED, filled symbol: EX. (B) Body weight at the end of the experiment (8 weeks). Animals fed the western diet (WD; black bars) weighed significantly more than control fed animals (white bars). Access to a running wheel significantly attenuated the body weight gain. **p < 0.01, ****p < 0.0001; different from control-sedentary, #p < 0.01;different from WD-sedentary. (C) Animals fed the WD ran significantly more than control fed animals only during weeks 2–4. *p < 0.05. (D) All animals in experimental conditions had a significantly decreased sucrose preference as compared to Control-sedentary animals. **p < 0.01, ***p < 0.001, ****p < 0.0001.
Behavioral tasks were administered at week 6 (sucrose preference) and week 7 (elevated plus and open field). Sucrose preference was measured at T0, and there were no differences across the groups (data not shown). Analysis of the sucrose preference response revealed a significant interaction between diet and exercise (Fig. 1C; F(1,36) = 9.86, p = 0.0034), such that animals eating the WD and those given access to the running wheels, all had significantly reduced preference for sucrose. There were no significant differences observed in EPM or OF (data not shown).
At the end of week 8, animals were sacrificed and brains were collected for RNA analysis. Eighteen immune related genes were examined within the prefrontal cortex. Table 2 lists the genes and the results of the two-way ANOVA. Two notable patterns regarding the interaction of diet and exercise were observed. In the first pattern, WD increased expression of the gene, and exercise blocked this overexpression (Fig. 2A). This pattern was seen in CCL2 (F(1,14 = 31.75, p < 0.0001) and CXCL10 (F(1,14 = 14.73, p = 0.0018). Additionally, the proinflammatory cytokine, IL-6, demonstrated a similar pattern, however, the exercise effect was a non-significant trend (difference between WD-SED and WD-EX, p = 0.06). The second notable interaction showed that both diet and exercise increased the expression of the gene, and the effect was not additive (Fig. 2B). This pattern was seen in IL-1β (main effect for diet (F(1,14) = 11.86, p = 0.004, non-significant trend for exercise (F(1,14) = 4.35, p = 0.06), and significant interactions for IL-18 (F(1,14) = 5.88, p = 0.03) and CREB (F(1,14) = 5.70, p = 0.03).
Table 2.
Results from 2-way ANOVA analysis of gene expression data.
| Gene | Interaction | Main effect-diet | Main effect-exercise |
|---|---|---|---|
| CCL2 | F(1,14) = 31.75 p < 0.0001 |
F(1,14) = 33.16 p < 0.0001 |
F(1,14) = 8.93 p = 0.001 |
| CCR2 | F(1,14) = 0.22 N/S |
F(1,14) = 7.17 p = 0.018 |
F(1,14) = 0.008 N/S |
| CREB | F(1,14) = 5.699 p = 0.03 |
F(1,14) = 48.88 p < 0.0001 |
F(1,14) = 5.746 p = 0.03 |
| CXCL10 | F(1,14) = 14.73 p = 0.0018 |
F(1,14) = 24.46 p = 0.0002 |
F(1,14) = 1.833 N/S |
| GFAP | F(1,14) = 0.4043 N/S |
F(1,14) = 16.69 p = 0.0011 |
F(1,14) = 0.5465 N/S |
| IL-18 | F(1,14) = 5.878 p = 0.03 |
F(1,14) = 24.23 p = 0.0002 |
F(1,14) = 0.2159 N/S |
| IL-Iβ | F(1,14) = 1.447 N/S |
F(1,14) = 11.86 p = 0.004 |
F(1,14) = 4.345 p = 0.06 |
| IL-1R | F(1,14) = 0.3130 N/S |
F(1,14) = 3.233 N/S |
F(1,14) = 0.3318 N/S |
| IL-6 | F(1,13) = 7.002 p = 0.02 |
F(1,13) = 11.74 p = 0.005 |
F(1,13) = 0.2720 N/S |
| IKBα | F(1,14) = 0.3398 N/S |
F(1,14) = 0.001 N/S |
F(1,14) = 9.119 p = 0.009 |
| NOS2 | F(1,13) = 1.588 N/S |
F(1,13) = 0.4298 N/S |
F(1,13) = 0.5370 N/S |
| GR | F(1,14) = 0.4778 N/S |
F(1,14) = 5.436 p = 0.04 |
F(1,14) = 3.019 N/S |
| PGES | F(1,14) = 0.9570 N/S |
F(1,14) = 2.732 N/S |
F(1,14) = 0.3646 N/S |
| COX2 | F(1,14) = 2.365 N/S |
F(1,14) = 0.5350 N/S |
F(1,14) = 3.236 N/S |
| NFkB (p65) | F(1,14) = 1.960 N/S |
F(1,14) = 8.505 p = 0.01 |
F(1,14) = 1.847 N/S |
| SOCS3 | F(1,14) = 0.01392 N/S |
F(1,14) = 5.154 p = 0.04 |
F(1,14) = 3.556 p = 0.08 |
| TLR4 | F(1,13) = 0.5867 N/S |
F(1,13) = 8.643 p = 0.01 |
F(1,13) = 3.595 p = 0.08 |
| TNF-α | F(1,12) = 1.107 N/S |
F(1,12) = 0.5216 N/S |
F(1,12) = 3.647 p = 0.08 |
Fig. 2.
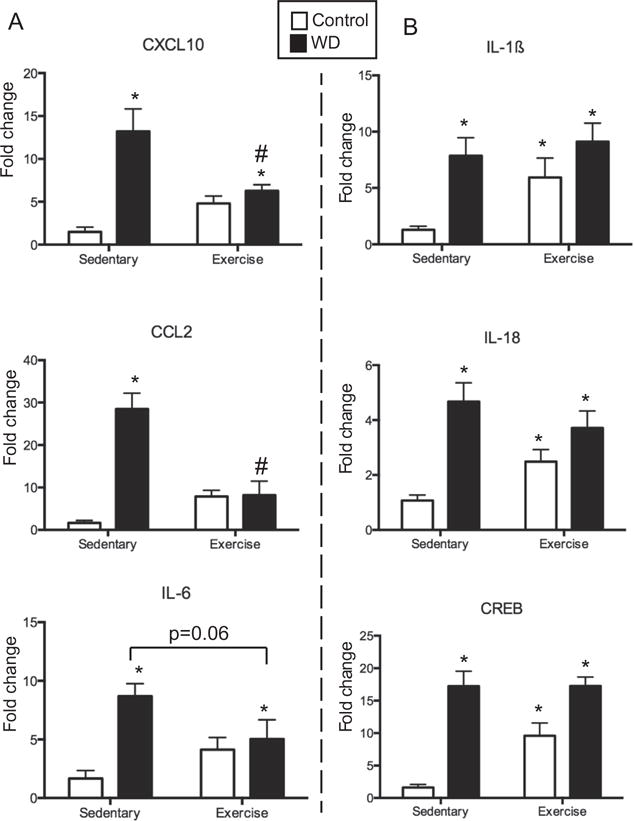
PFC genes showing a significant interaction between diet and exercise. (A) IL-1β, IL-18 and CREB all showed a similar pattern of expression such that WD and exercise increased expression to a similar, non-additive, extent. (B) CXCL10, CCL2, and IL-6 all showed a similar pattern of expression such that WD increased expression, while access to a running wheel decreased/normalized the expression. *p < 0.05, different from control-SED animals, #p < 0.005, different from WD-SED.
Interestingly, a comparison of the main effects of either diet or exercise revealed that in prefrontal cortex, diet affected significantly more inflammation-related genes when compared to exercise (13 versus 3, see summary in Fig. 3). More often, WD increased the expression of the inflammation-related genes (CCR2, TLR4, SOCS3, GFAP, Fig. 4), while WD decreased the expression of only 2 genes (GR and p65 subunit of NFkB, Fig. 5A). Exercise affected only one gene, IκBα, decreasing the expression (main effect, F(1,14) = 9.12, p = 0.009, Fig. 5B).
Fig. 3.
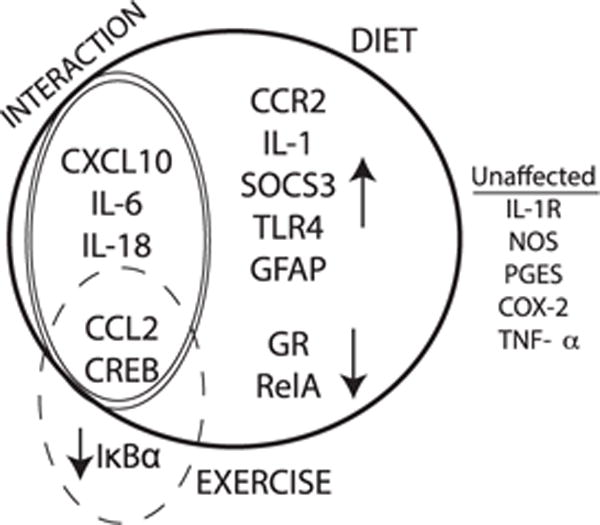
A schematic summarizing the expression patterns of genes in the prefrontal cortex in response to WD consumption and/or exercise. Genes within the bold black line represent all genes affected by diet and genes within the dashed line represent those affected by exercise, with the direction of the simple main effects shown via arrows. Genes within the double line are those for which there was a significant interaction, and unaffected genes are listed on the right.
Fig. 4.
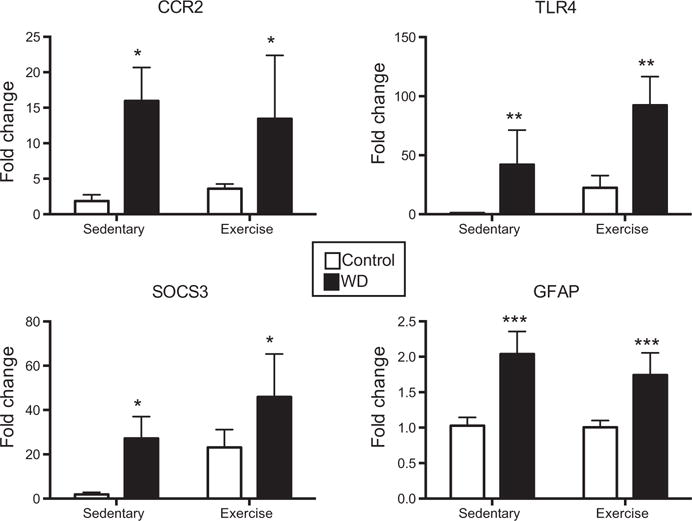
PFC genes upregulated by WD consumption. WD consumption (black bars) significantly increased the expression of CCR2, TLR4, SOCS3, and GFAP as compared to control fed animals (white bars). *p < 0.05, **p < 0.01, ***p < 0.001, main effect for diet.
Fig. 5.
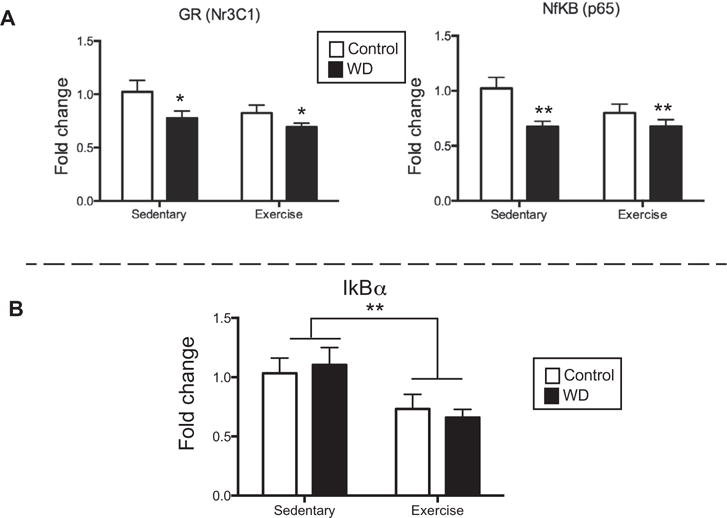
PFC genes downregulated by WD. (A) GR and NFkB were significantly downregulated in animals that consumed the WD (black bars), as compared to control fed animals (white bars). *p < 0.05, **p < 0.01, main effect for diet. (B) IkBa was significantly downregulated in animals with access to the running wheels (exercise) as compared to sedentary animals. **p < 0.01, main effect for exercise.
Complete linkage cluster analysis of genes and treatment conditions as a function of the expression of all genes in the PFC was completed and revealed two main clusters in genes (Fig. 6; unshaded and shaded) and three main clusters in treatment conditions (unshaded, light gray, dark gray). The largest cluster was defined by upregulation of 13 of the 18 genes (unshaded) assessed in a cluster comprising approximately half of the animals assessed (unshaded) including all but one of the animals on a high-fat diet (WD SED 6). These data support a general upregulation of pro-inflammatory gene expression in the prefrontal cortex of animals on a Western diet, regardless of exercise status. The second gene cluster was defined by upregulation of the remaining 5 of the 18 genes (shaded light gray) assessed in 4 animals on the control diet (shaded dark gray), 3 of which were sedentary. This cluster contains the glucocorticoid receptor, two components of NFkB signaling (p65 subunit and IKBα), and two enzymes which produce proinflammatory mediators, COX-2, which produces pros-taglandins, and NOS2, which synthesizes nitric oxide. These genes are functionally related, as GR can bind to NFkB, and NFkB activation induces both COX-2 and NOS2, and these data confirm this functional association in the PFC. Further, these data suggest that animals on the regular diet may have experienced a downregulation of these genes when exercising. Lastly, the only cluster that has a generally reduced pattern of expression across all genes (treatment conditions shaded in light gray) contains ¾ of the CON-EX animals, suggesting that for at least some animals on a regular diet, exercise can lead to a downregulation of proinflammatory genes in the PFC.
Fig. 6.
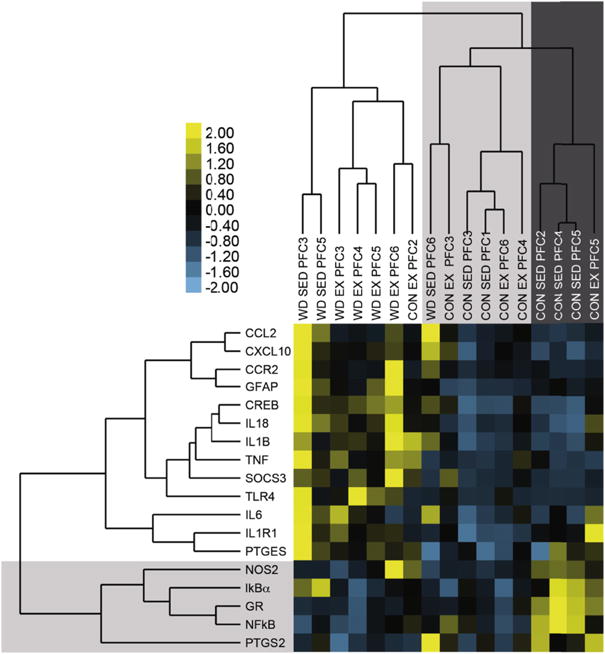
Complete linkage cluster analysis of genes (left) and treatment conditions (top) as a function of the expression of all genes in the prefrontal cortex The relative expression level of genes was transformed into a yellow-blue heat map, where yellow indicated higher than average gene expression (positive z-scores), and blue indicated lower than average gene expression (negative z-scores). Two primary clusters are identified, in the top, left corner and in the bottom, right corner. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In the present manuscript, we investigated the interaction of a calorie-dense, highly palatable, western diet with voluntary exercise on body weight, behavior and inflammation-related gene expression in the prefrontal cortex. Importantly, we identified a number of inflammatory genes that were increased in the pre-frontal cortex as a function of WD consumption, and identified two key chemokines whose overexpression was blocked by voluntary wheel running. Both WD consumption and voluntary exercise reduced sucrose preference, and genes in the IL-1β family showed an expression pattern that mirrored the pattern seen for sucrose preference. Using a broader panel of immune-related genes, the present work identifies a number of genes that are altered in the prefrontal cortex in response to WD consumption.
For two proinflammatory chemokines, CCL2 and CXCL10, WD increased the expression of these genes, and exercise blocked this increase. It is notable that these two cytokines responded similarly, as CCL2 and CXCL10 are both chemokines which recruit primarily lymphocytes and monocytes, with little activity for neutrophils. Previously, we have shown that in response to an acute immune challenge, the induced expression of CCL2 and CXCL10 is localized primarily to barrier-related cells of the brain (Reyes et al., 2003), ideally positioning these molecules at the interface of the peripheral circulating immune cells and the brain. A recent study using GFP+ bone marrow reconstitution of irradiated mice reported that 15 weeks HFD-feeding in mice increased the infiltration of monocytes from the periphery into the brain (Buckman et al., 2014), including the cortex. While chemokine levels were not assessed in the brain in this study, they did report an increase in the expression of CCL2 in adipose tissue. Interestingly, in a study which involved 12-week high fat diet exposure coupled with treadmill running (30–60 min day, 5 days/week), it was reported that exercise decreased the infiltration of macrophages into the liver that was typically seen in response to HFD consumption (Jeong et al., 2015), supporting the conclusion that exercise can moderate macrophage traffic into tissue. Altered expression of the chemokines CCL2 and CXCL10 are potential molecular links for this macrophage trafficking. While beyond the scope of the current experiments, future studies to examine protein levels and neuroanatomical and cellular localization will be important.
Sucrose preference was evaluated as a reward-related behavior, and both the WD and the presence of a running wheel decreased sucrose preference. Preference for sucrose has been shown to be reduced by a high fat diet (Vucetic et al., 2011) and by exercise (Moody et al., 2015). One interpretation of these findings is that sucrose preference is reduced due to the availability of other rewarding substances (e.g. WD or exercise). Consistent with this suggestion, voluntary running wheel access engages the central reward circuitry (Herrera et al., 2016), as does acute consumption of a calorie dense diet (Valdivia et al., 2014). Interestingly, the effects of WD and exercise were not additive, although this may have also been due to a floor effect.
A comparison of the gene expression response of the inflammatory genes in the PFC in the context of the sucrose preference data was informative. Three genes showed a pattern of responses that were similar to the pattern seen with sucrose preference. IL-1β, IL-18 and CREB were all induced by both WD and exercise, while sucrose preference was decreased. The grouping of these three molecules makes functional sense, as IL-1 and IL-18 are both members of the IL-1 family of cytokines. Further, IL-1 and IL-18 have been shown to be coordinately regulated in the brain (del Rey et al., 2013), and CREB is an important down-stream signaling molecule for both IL-1 and IL-18. These data suggest the possibility that increased IL-1 and/or IL-18 in PFC, acting through CREB may participate in modulating sucrose preference in response to palatable diet and exercise. This hypothesis is consistent with other reports in the literature. For example, an acute injection of LPS decreased sucrose preference, and concurrently, increased IL-1β expression in the PFC and altered pCREB (Ge et al., 2015). Further, CREB overexpression in the nucleus accumbens shell was shown to reduce sucrose preference (Barrot et al., 2002). Investigating a causative link between elevation of IL-1, IL-18 and CREB in PFC and decreased sucrose preference warrants further investigation.
Examination of the genes upregulated by WD consumption, reveals consistency across inflammatory molecules. For example, both CCL2 and CCR2 (the cognate receptor) were upregulated by WD. Similarly, both SOCS3 and IL-6 were upregulated, and SOCS3 is induced by IL-6. The two genes that were down-regulated by WD consumption, GR and NFkB, have been shown to share extensive cross-talk (Rao et al., 2011), with regard to their interaction with downstream targets. GFAP was also upregulated, indicating astrogliosis (Brahmachari et al., 2006). Astrogliosis has previously been observed in obese mice (both diet-induced and genetically-linked), an effect which was localized to the cerebral blood vessels (Buckman et al., 2013). Further this identifies astrocytes as a potential source for the increased expression of proinflammatory molecules, along with microglia, another likely source (although microglia were not evaluated in the current study).
Voluntary wheel running affected only one inflammation-related gene, IKBα (not considering CCL2 and CREB, as these genes demonstrated interactions). Few other studies in the literature have examined NFkB and exercise, however, in a similar experimental paradigm, neither cafeteria diet nor exercise were found to affect NFkB mRNA in hippocampus or PFC of mice (Auer et al., 2015), although the diet used in that study was significantly different from the Western diet used in the present study, which may partially explain the discrepant results. In humans, it has been shown that prolonged physical training also decreased mRNA expression of multiple NFKB-related genes in blood (Sousa e Silva et al., 2010). When considered on a single gene level, voluntary exercise (as opposed to forced) appeared to exert little effect on the proinflammatory profile in the prefrontal cortex. However, the cluster analysis suggested that when considered within a functional grouping, exercise appeared to generally reduce the expression of a subset of these genes, including both p65 subunit of NFkB and IKBα. Further, it is increasingly clear that exercise has the potential to mitigate inflammatory responses in the hippocampus (Kang et al., 2016), and as shown in the present data within the prefrontal cortex, particularly for the chemokines CXCL10 and CCL2. This beneficial effect of exercise on inflammation in the brain is also seen with other conditions, including chronic stress (Liu et al., 2013) and aging (Gomes da Silva et al., 2013).
Some methodological notes are required. The exercise paradigm employed in the current study was voluntary wheel running, as opposed to treadmill exercise, as the forced treadmill exercise approach can be considered a chronic stressor (Cook et al., 2013), and chronic stress can alter the inflammatory profile within the PFC (Couch et al., 2013). Further, the animals were single-housed for the duration of the experiment, which may also be considered a stressor. However, single housing as opposed to pair housing has been shown, particularly for male mice, to be associated with resiliency to stress (increased stress-coping behaviors) (Pan-Vazquez et al., 2015), as opposed to stress-inducing. The fact that exercise alone did little to affect gene expression in the PFC supports the conclusion that this exercise paradigm was not experienced as a chronic stressor for the animals, although verification of the absence of stress through additional measures such as HPA reactivity and/or thymus weight would be important in future studies. The duration of the experiment (8 weeks) was also relatively short, in comparison to the majority of work reported, which is important as it indicates that inflammatory responses in the PFC occur in this relatively brief time frame. The exercise was also provided concurrently with the western diet, and in future studies it would be interesting to investigate whether exercise before or after consumption of a calorie-dense diet would still be effective in moderating the CNS inflammatory response, as well as to study the time-course of the development of these responses. It is also important to note that body weight was reduced for those animals consuming the western diet who also had access to the running wheel, but at the conclusion of the study, they were still significantly heavier than the control/sedentary animals. This indicates that the reversal effects seen in the chemokine expression patterns were not likely due solely to changes in body weight. It is also important to note that early on (weeks 2–4) the animals on the WD ran significantly more than did the animals on the control diet, however, at the time of behavioral testing and sacrifice, the amount of running had normalized between the groups. Lastly, the current study involved male mice only, so no conclusions can be drawn regarding how females may respond in a similar experimental context. Given the notable sex differences in stress (Bale and Epperson, 2015) and immune (Lenz and McCarthy, 2015) physiology, future experiments should include direct sex comparisons.
Overall, the present data reveal that the PFC is susceptible to the effects of WD, with regard to the induction of proinflammatory molecules, a response that has not been detailed previously. Largely, exercise did not prevent the induction of most of the proinflammatory molecules. The notable exception was that voluntary wheel running was able to block the induction of two proinflammatory chemokines, CCL2 and CXCL10, indicating that exercise may help protect against the infiltration of monocytes that is associated with obesity. These data suggest that while concurrent voluntary exercise may be ineffective at stopping an initial proinflammatory response in the PFC, later exacerbation of that response, through the recruitment of additional proinflammatory cell types, may be mitigated through the reduction of chemokine expression.
Acknowledgments
This authors would like to thank Dr. Tim O’Brien and the Neurobehavior Testing Core at the University of Pennsylvania for running the EPM and OF tests.
Funding
This work was supported by the National Institutes of Health grants; NS50465 (F.G.P), TL1TR000138 (JLC), T32-GM008076 (JLC), and MH087978 (TMR).
Abbreviations
- CREB
cAMP response element binding protein
- CNS
central nervous system
- CCL2
chemokine (C-C Motif) ligand 2
- CCR2
chemokine (C-C Motif) receptor 2
- CTRL
control
- CXCL10
C-X-C motif chemokine 10
- COX-2
cyclooxygenase 2
- EPM
elevated plus maze
- EX RD
exercise-regular diet group
- EX-WD
exercise-western diet group
- GFAP
glial fibrillary acidic protein
- GR
glucocorticoid receptor
- HFD
high fat diet
- IL-1β
interleukin 1 beta
- IL-1
interleukin-1
- IL-18
interleukin-18
- IL-6
interleukin-6
- Iba-1
ionized calcium-binding adapter molecule 1
- LPS
lipopolysaccharides
- MR
mineralocorticoid receptor
- NOS2
nitric oxide synthase 2
- NFkB
nuclear factor kappa-light-chain-enhancer of activated B cells
- IkBa
nuclear factor of kappa B inhibitor alpha
- OF
open field
- pCREB
phosphorylated cAMP response element binding protein
- PFC
prefrontal cortex
- PGES
prostaglandin E synthase
- PGE2
prostaglandin E2
- SED-RD
sedentary-regular diet group
- SED-WD
sedentary-western diet group
- SOCS3
suppressor of cytokine signaling 3
- TLR4
toll-like receptor 4
- TNF-α
tumor necrosis factor alpha
- WD
Western diet
Footnotes
The authors have no financial interests or conflicts of interest to disclose.
References
- Auer MK, et al. Effects of a high-caloric diet and physical exercise on brain metabolite levels: a combined proton MRS and histologic study. J Cereb Blood Flow Metab. 2015;35(4):554–564. doi: 10.1038/jcbfm.2014.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18(10):1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99(17):11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz JE, Maniam J, Morris MJ. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav Brain Res. 2016;306:1–7. doi: 10.1016/j.bbr.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Boitard C, et al. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun. 2014;40:9–17. doi: 10.1016/j.bbi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Brahmachari S, Fung YK, Pahan K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci. 2006;26(18):4930–4939. doi: 10.1523/JNEUROSCI.5480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman LB, et al. Regional astrogliosis in the mouse hypothalamus in response to obesity. J Comp Neurol. 2013;521(6):1322–1333. doi: 10.1002/cne.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman LB, et al. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immun. 2014;35:33–42. doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MD, et al. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav Immun. 2013;33:46–56. doi: 10.1016/j.bbi.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch Y, et al. Microglial activation, increased TNF and SERT expression in the prefrontal cortex define stress-altered behaviour in mice susceptible to anhedonia. Brain Behav Immun. 2013;29:136–146. doi: 10.1016/j.bbi.2012.12.017. [DOI] [PubMed] [Google Scholar]
- de Mello VD, et al. Effect of weight loss on cytokine messenger RNA expression in peripheral blood mononuclear cells of obese subjects with the metabolic syndrome. Metabolism. 2008;57(2):192–199. doi: 10.1016/j.metabol.2007.08.024. [DOI] [PubMed] [Google Scholar]
- De Souza CT, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- del Rey A, et al. A cytokine network involving brain-borne IL-1beta, IL-1ra, IL-18, IL-6, and TNFalpha operates during long-term potentiation and learning. Brain Behav Immun. 2013;33:15–23. doi: 10.1016/j.bbi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Dorfman MD, Thaler JP. Hypothalamic inflammation and gliosis in obesity. Curr Opin Endocrinol Diabetes Obes. 2015;22(5):325–330. doi: 10.1097/MED.0000000000000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreucq S, Marsicano G, Chaouloff F. Duration- and environment-dependent effects of repeated voluntary exercise on anxiety and cued fear in mice. Behav Brain Res. 2015;282:1–5. doi: 10.1016/j.bbr.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Dutheil S, et al. High-fat diet induced anxiety and anhedonia: impact on brain homeostasis and inflammation. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, et al. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95(25):14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, et al. Resveratrol abrogates lipopolysaccharide-induced depressive-like behavior, neuroinflammatory response, and CREB/BDNF signaling in mice. Eur J Pharmacol. 2015;768:49–57. doi: 10.1016/j.ejphar.2015.10.026. [DOI] [PubMed] [Google Scholar]
- Gomes da Silva S, et al. Exercise-induced hippocampal anti-inflammatory response in aged rats. J Neuroinflamm. 2013;10:61. doi: 10.1186/1742-2094-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom NM, et al. Dissociable deficits of executive function caused by gestational adversity are linked to specific transcriptional changes in the prefrontal cortex. Neuropsychopharmacology. 2015;40(6):1353–1363. doi: 10.1038/npp.2014.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera JJ, et al. Neurochemical and behavioural indices of exercise reward are independent of exercise controllability. Eur J Neurosci. 2016;43(9):1190–1202. doi: 10.1111/ejn.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman A, Lent-Schochet D, Pike CJ. Diet-induced obesity and low testosterone increase neuroinflammation and impair neural function. J Neuroinflamm. 2014;11:162. doi: 10.1186/s12974-014-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon BT, et al. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 2012;61(6):1444–1454. doi: 10.2337/db11-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JH, et al. The effects of either resveratrol or exercise on macrophage infiltration and switching from M1 to M2 in high fat diet mice. J Exerc Nutr Biochem. 2015;19(2):65–72. doi: 10.5717/jenb.2015.15060203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung DY, et al. Short-term weight loss attenuates local tissue inflammation and improves insulin sensitivity without affecting adipose inflammation in obese mice. Am J Physiol Endocrinol Metab. 2013;304(9):E964–E976. doi: 10.1152/ajpendo.00462.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EB, et al. Neuroprotective effects of endurance exercise against high fat diet-induced hippocampal neuroinflammation. J Neuroendocrinol. 2016 doi: 10.1111/jne.12385. [DOI] [PubMed] [Google Scholar]
- Lenz KM, McCarthy MM. A starring role for microglia in brain sex differences. Neuroscientist. 2015;21(3):306–321. doi: 10.1177/1073858414536468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, et al. Swimming exercise ameliorates depression-like behavior in chronically stressed rats: relevant to proinflammatory cytokines and IDO activation. Behav Brain Res. 2013;242:110–116. doi: 10.1016/j.bbr.2012.12.041. [DOI] [PubMed] [Google Scholar]
- Moody L, et al. Wheel running decreases palatable diet preference in Sprague-Dawley rats. Physiol Behav. 2015;150:53–63. doi: 10.1016/j.physbeh.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naznin F, et al. Diet-induced obesity causes peripheral and central ghrelin resistance by promoting inflammation. J Endocrinol. 2015;226(1):81–92. doi: 10.1530/JOE-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan-Vazquez A, et al. Impact of voluntary exercise and housing conditions on hippocampal glucocorticoid receptor, miR-124 and anxiety. Mol Brain. 2015;8:40. doi: 10.1186/s13041-015-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NA, et al. Coactivation of GR and NFKB alters the repertoire of their binding sites and target genes. Genome Res. 2011;21(9):1404–1416. doi: 10.1101/gr.118042.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM, et al. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci. 2003;23(13):5607–5616. doi: 10.1523/JNEUROSCI.23-13-05607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sousa e Silva T, et al. Prolonged physical training decreases mRNA levels of glucocorticoid receptor and inflammatory genes. Horm Res Paediatr. 2010;74(1):6–14. doi: 10.1159/000313586. [DOI] [PubMed] [Google Scholar]
- Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia S, et al. Acute high fat diet consumption activates the mesolimbic circuit and requires orexin signaling in a mouse model. PLoS One. 2014;9(1):e87478. doi: 10.1371/journal.pone.0087478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Reyes TM. Chronic high fat diet drives postnatal epigenetic regulation of μ-opioid receptor in the brain. Neuropsychopharmacology. 2011;36(6):1199–1206. doi: 10.1038/npp.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Increased hypothalamic inflammation associated with the susceptibility to obesity in rats exposed to high-fat diet. Exp Diabetes Res. 2012;2012:847246. doi: 10.1155/2012/847246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KJ, et al. Exercise improves host response to influenza viral infection in obese and non-obese mice through different mechanisms. PLoS One. 2015;10(6):e0129713. doi: 10.1371/journal.pone.0129713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, et al. Central inflammation and leptin resistance are attenuated by ginsenoside Rb1 treatment in obese mice fed a high-fat diet. PLoS One. 2014;9(3):e92618. doi: 10.1371/journal.pone.0092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, et al. Exercise protects against high-fat diet-induced hypothalamic inflammation. Physiol Behav. 2012;106(4):485–490. doi: 10.1016/j.physbeh.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Yoon H, et al. Interplay between exercise and dietary fat modulates myelinogenesis in the central nervous system. Biochim Biophys Acta. 2016;1862(4):545–555. doi: 10.1016/j.bbadis.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, et al. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol. 2005;191(2):318–325. doi: 10.1016/j.expneurol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Zhang X, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135(1):61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]


