Abstract
CD4+ T-helper (Th) cells reactive against myelin antigens mediate the animal model experimental autoimmune encephalomyelitis (EAE) and have been implicated in the pathogenesis of multiple sclerosis (MS). It is currently debated whether encephalitogenic Th cells are heterogeneous or arise from a single lineage. In the current study, we challenge the dogma that stimulation with the monokine IL-23 is universally required for the acquisition of pathogenic properties by myelin-reactive T cells. We show that IL-12-modulated Th1 cells readily produce IFN-γ and GM-CSF in the central nervous system (CNS) and induce a severe form of EAE via an IL-23-independent pathway. Th1-mediated EAE is characterized by monocyte-rich CNS infiltrates, elicits a strong proinflammatory cytokine response in the CNS, and is partially CCR2-dependent. Conversely, IL-23-modulated, stable Th17 cells induce EAE with a relatively mild course via an IL-12-independent pathway. These data provide definitive evidence that autoimmune disease can be driven by distinct CD4+ T helper cell subsets and polarizing factors.
Keywords: Experimental autoimmune encephalomyelitis, IL-23, IL-12, Th1 cells, Th17 cells
Introduction
Multiple sclerosis (MS), an inflammatory demyelinating disease of the central nervous system (CNS), is believed to be autoimmune in etiology. In the animal model experimental autoimmune encephalomyelitis (EAE), active immunization with MHC Class II-restricted epitopes of myelin proteins or adoptive transfer of myelin-reactive CD4+ T cells results in an ascending paralysis with histopathological features that are reminiscent of MS. However, EAE studies have shown that not all myelin-reactive T cells are pathogenic [1–3]. A major goal in MS research is to elucidate the factors required for the differentiation and function of encephalitogenic T cells that might ultimately serve as biomarkers or novel therapeutic targets in the clinical setting.
It is widely believed that exposure of myelin-reactive T cells to the cytokine IL-23 is critical for their acquisition of pathogenic properties [1, 4, 5]. IL-23 promotes the expansion of IL-17-producing Th17 cells [4, 5]. Interestingly, “hallmark” Th17 cytokines (including IL-17A and IL-17F) are dispensable for the development of EAE [6]. Several recent studies suggest that the mechanism of action of IL-23 in EAE is to induce the production of GM-CSF, which drives the accumulation of pro-inflammatory myeloid cells in the CNS, culminating in axonopathy and demyelination [7, 8]. In contrast to the relationship between IL-23 and GM-CSF in mice, GM-CSF production is associated with the IL-12/Th1 axis in humans, including individuals with MS [9]. IL-12-polarized, IFN-γ-producing Th1 cells were once thought to be critical autoimmune effector cells in EAE as well as MS [10, 11]. However, their relevance was challenged by the observations that mice deficient in IL-12, the IL-12 receptor or IFN-γ are highly susceptible to EAE [12–14]. The notion that EAE is IL-23-dependent and IL-12-independent was primarily based on experiments in which mice on a C57BL/6 background are actively immunized with a peptide of myelin oligodendrocyte glycoprotein (MOG35–55) in CFA and injected with Bordetella pertussis toxin [5]. We questioned whether a non-redundant role of IL-23 is specific for that model.
In fact, we and others have shown that, in certain adoptive transfer paradigms, stimulation of ordinarily innocuous myelin-specific CD4+ T cells with recombinant IL-12 is sufficient to confer encephalitogenicity [2, 3, 15, 16]. Thakker et al. demonstrated that Th1 cells from IL-23-deficient mice are encephalitogenic following adoptive transfer into wild-type hosts; however, this study does not rule out the possibility that host-derived IL-23 rescues T cell encephalitogenicity following transfer [16]. More recently, fate mapping studies have shown that the majority of CD4+ T cells infiltrating the CNS of MOG-immunized C57BL/6 mice at peak EAE are Th17 cells that acquired Th1 characteristics (so-called exTh17 cells) [17]. This led some to speculate that in previous reports, disease mediated by Th1 cells was actually mediated by Th17 cells that had been exposed to endogenous IL-23 in vivo and became exTh17 cells during stimulation with IL-12 in vitro (so-called exTh17 cells), rather than by classical Th1 cells [18]. The goal of the current study was to determine whether classical Th1 cells, completely naive to IL-23 signaling,, are capable of mediating inflammatory demyelination.
Results and Discussion
IL-12- modulated Th1 cells and IL-23- modulated Th17 cells differentiate into GM-CSF- producing memory cells in the absence of the reciprocal polarizing cytokine
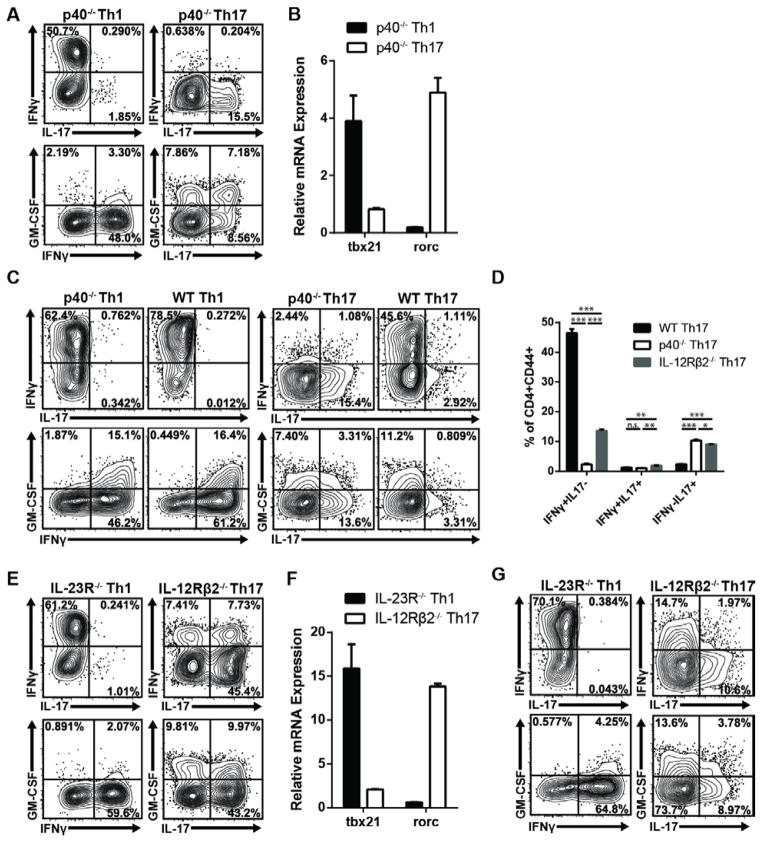
IL-12 and IL-23 are heterodimers composed of a common IL-12p40 chain and a unique IL-12p35 or IL-23p19 chain, respectively. We immunized C57BL/6 mice deficient in the IL-12p40 chain (and therefore unable to produce either bioactive IL-12 or IL-23) with MOG35–55 emulsified in CFA. To generate pure populations of Th1 and Th17 cells, draining lymph node cells (LNC) were obtained 10 days later and challenged ex vivo with antigen plus either recombinant IL-12 and IFN-γ or IL-23, IL-1α, and anti-IFN-γ to generate Th1 or Th17 cells, respectively. As expected, a significant percentage of IL-12-polarized IL-12p40−/− Th1 cells produced IFN-γ but not IL-17, while the converse was true of their IL-23-polarized Th17 counterparts (Fig. 1A). Th1 cells expressed high levels of tbet, while Th17 cells expressed high levels of rorc (Fig. 1B). Relatively few Th1 cells produced GM-CSF compared with Th17 cells derived from the same IL-12p40−/− donor pool (Fig. 1A). However, IL-12 can suppress GM-CSF production by murine Th1 cells [8]. We questioned whether committed Th1 cells would upregulate GM-CSF upon reactivation in the absence of IL-12. Indeed, a high percentage of IL-23-independent Th1 cells expressed GM-CSF during secondary antigenic challenge in vitro under neutral conditions (Fig. 1C). The reactivated Th1 cells expressed a high IFN-γ/low IL-17 profile, irrespective of donor phenotype. IL-23-polarized T cells derived from IL-12p40−/−, but not from WT, donors maintained a high level of IL-17 and low level of IFN-γ production during in vitro reactivation, indicating that they had not converted into exTh17 cells (Fig. 1C, D). Similar results were obtained in parallel experiments with Th1 and Th17 cells derived from donors deficient in the IL-23 receptor (IL-23R) or IL-12 receptor (IL-12Rβ2), respectively (Fig. 1D–G).
Figure 1. IL-12 and IL-23 promote the differentiation of stable Th1 and Th17 cells, respectively, in the absence of the reciprocal polarizing factor.
(A–D) LNC were harvested from IL-12p40−/− mice 10 days following immunization with MOG35–55 in CFA. Cells were cultured with antigen plus IL-12 and IFN-γ (Th1) or IL-23, IL-1α, and anti-IFN-γ (Th17), and after 4 days, cells were collected for cytokine and transcription factor analysis. (A) Cytokine expression in CD4+CD44+ T cells following stimulation with PMA/Ionomycin. (B) Transcription factor expression in purified CD4+ T cells, measured by quantitative RT-PCR and normalized to gapdh. (C,D) CD4+ T cells were purified from Th-polarized primary cultures, washed, rested for 2 days, and re-challenged with antigen and IL-12p40−/− T-depleted splenocytes under neutral conditions. CD4+CD44+ T cells were analyzed for cytokine expression at 24 hours. The bar graph represents biological replicates; n=3 per group. (E–G) LNC were harvested from MOG/CFA-immunized IL-23R−/− or IL-12Rβ2−/− mice, and cultured with antigen under Th1 or Th17 polarizing conditions for 4 days. (E) Cytokine expression in PMA/Ionomycin-stimulated CD4+CD44+ T cells and (F) transcription factor levels in purified CD4+ T cells, measured as described above. (G) MOG-specific cytokine production in CD4+CD44+ T cells following rest and re-challenge with WT T-depleted splenocytes. Data are representative of at least 10 (A,E), or 2–4 (B,C,D,F,G) independent experiments with n≥3 mice per group. (B,D,F) Data are shown as mean ± SEM. *p≤0.05, **p≤0.01, ***p≤0.001 using the two-tailed, unpaired t-test.
Th1 Cells Can Induce EAE Independently of IL-23
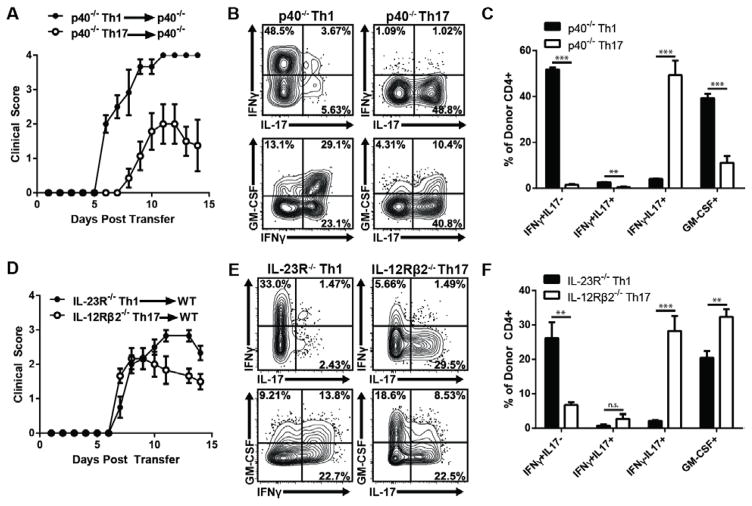
Next, we assessed the ability of highly polarized Th1 cells to induce EAE by an IL-23-independent pathway. MOG-primed T cells derived from IL-12p40−/− mice were polarized with either IL-12 or IL-23 and injected into IL-12p40−/− hosts. The IL-12-polarized cells reproducibly induced EAE in 100% of recipients, with an accelerated and more severe course than the disease induced by their IL-23-polarized counterparts (Fig. 2A). Non-polarized cells were incapable of inducing disease (Supp. Fig 1). Similarly, IL-12-polarized, MOG-specific IL-23R−/− T cells transferred clinical EAE to WT recipients at high incidence (Fig. 2D). In both experimental systems, donor Th1 cells continued to produce IFN-γ, but little IL-17, after infiltrating the CNS (Fig. 2B, C, E, F). They also produced GM-CSF, reminiscent of Th1 cells reactivated under neutral conditions in vitro (Fig. 1C). These data demonstrate that classical Th1 cells, bereft of IL-23 signaling during the priming, expansion, and effector stages, are potent encephalitogenic effectors. To our knowledge, this is the first report of EAE induction in the complete absence of IL-23. IL-23-polarized T cells derived from IL-12p40−/− or IL-12Rβ2−/− donors expressed a high ratio of IL-17 to IFN-γ in the CNS of IL-12p40−/− and WT recipients, respectively, demonstrating that the majority of transferred cells had retained a Th17 phenotype yet were still capable of mediating clinical EAE (Fig. 2B, C, E, F). Interestingly, most IL-12Rβ2−/− donor T cells did not transition to an exTh17 phenotype in WT hosts, despite their accessibility to IL-23 signaling. This finding is consistent with previous reports of EAE mediated by IL-23-polarized, T-bet-deficient CD4+ T cells, which also retain a polarized Th17 phenotype post-transfer, and suggests that IL-12 might play a distinctive role driving Th17 plasticity in vivo [19–21].
Figure 2. IL-12-polarized T cells induce EAE independent of IL-23 signaling.
(A–C) LNC from MOG/CFA-immunized CD45.2+ IL-12p40−/− mice were cultured for 4 days with antigen under Th1 or Th17 polarizing conditions. Purified CD4+ T cells were adoptively transferred into naïve CD45.1+ IL-12p40−/− hosts. (A) Mean clinical scores; n=6–7 mice per group, from one of four experiments with similar results. (B,C) At peak disease, leukocytes were recovered from the spinal cord, and stimulated with PMA/Ionomycin ex vivo. The figures show cytokine expression in CD45.2+ CD4+ donor T cells. (D–F) MOG35–55-reactive CD4+ IL-23R−/− Th1 or IL-12Rβ2−/− Th17 cells were purified and adoptively transferred into naive CD45.1+ WT hosts. (D) Mean clinical scores; n=6 mice per group, from one of three independent experiments with consistent results. (E,F) At peak disease, leukocytes were recovered from the spinal cord, and stimulated with PMA/Ionomycin ex vivo. The figures show cytokine expression in CD45.2+ CD4+donor T cells. Data are representative of two independent experiments with n≥3 mice per group. Bar graphs represent biological replicates. Data in A,C,D,F are shown as mean ± SEM. *p≤0.05, **p≤0.01, ***p≤0.001 using the two-tailed, unpaired t-test.
IL-23 independent Th1 cells induce inflammatory cytokine production and monocyte-rich infiltrates in the CNS
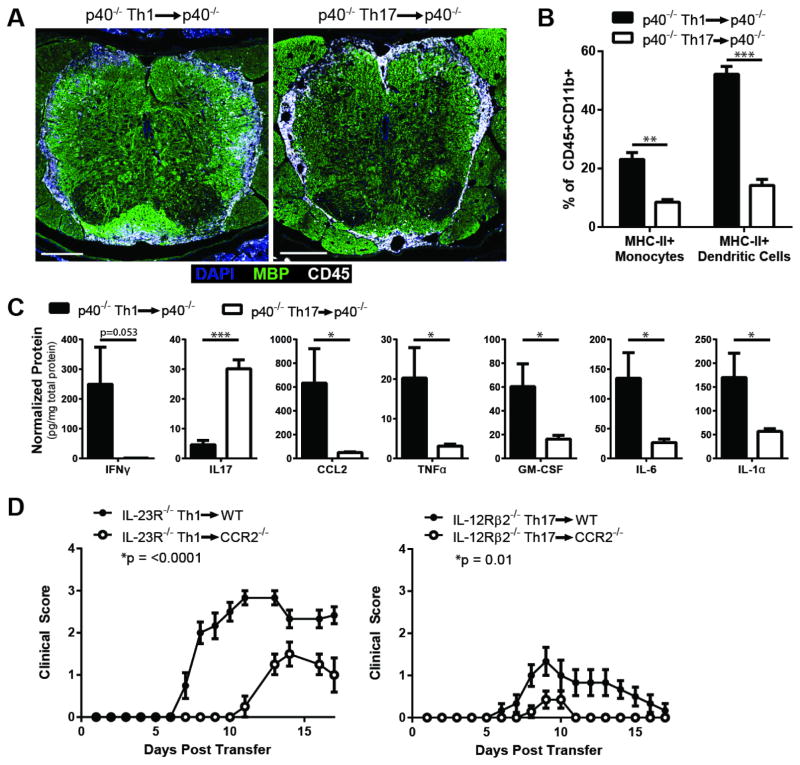
We next interrogated the characteristics of CNS infiltrates and the cytokine/chemokine profile induced by Th1 cells in our IL-23-independent EAE models. CNS tissues obtained from mice injected with IL-12-independent Th17 cells were analyzed as a foil. As in conventional models of EAE, inflammation was concentrated in the thoracolumbar spinal cord of both Th1 and Th17 recipients (Fig. 3A). The percent of MHC Class II+ monocytes and myeloid dendritic cells tended to be higher in infiltrates induced by IL-23-independent Th1 cells compared with stable Th17 cells (Fig. 3B). Th1 cells induced elevated levels of CCL2 in CNS homogenates, as well as other myeloid-related factors including TNFα, GM-CSF, IL-6, and IL-1α (Fig. 3C). Such an inflammatory milieu is likely to favor the recruitment of monocytes. CCR2−/− mice were relatively resistant to EAE following the transfer of either IL-23R−/− Th1 or IL-12Rβ2−/− Th17 donors cells, signifying a damaging role of inflammatory monocytes in both forms of EAE (Fig. 3D).
Figure 3. EAE mediated by IL-23-independent Th1 cells is characterized by monocyte-rich infiltrates and is partially CCR2-dependent.
(A–C) EAE was induced by adoptive transfer of MOG35–55-reactive IL-12p40−/− Th1 or Th17 cells. (A) Upon reaching a score of two, mice were perfused and spinal cords isolated for immunohistochemistry. Sections of the lumbar spinal cord were stained for DAPI (blue), myelin basic protein (MBP, green), and CD45 (white). Scale bar=200μm. (B) At disease onset, leukocytes were isolated from the spinal cord, and the frequencies of MHC-II+ monocytes (CD11b+CD11c−Ly6G−) and dendritic cells (CD11b+CD11c+) were determined. n=3 mice per group, from one of two independent experiments. (C) Spinal cords were isolated at disease onset, homogenized, and centrifuged to pellet tissue. Supernatants were subjected to a multiplex bead assay. Data were pooled from two independent experiments with 6–7 mice per group. (D) MOG35–55-reactive IL-23R−/− Th1 cells or IL-12Rβ2−/− Th17 cells were transferred into naïve syngeneic CCR2−/− or WT hosts. n=4–7 mice per group, from one of three independent experiments with consistent results. Data in B–D are shown as mean ± SEM. *p≤0.05, **p≤0.01, ***p≤0.001 using the two-tailed, unpaired t-test. Clinic courses were compared using 2-way ANOVA.
IFN-γ has been reported to have both protective and pathogenic effects in inflammatory demyelination [13, 22, 23]. The role of IFN-γ in IL-23-independent EAE has yet to be determined. We found that neutralization of IFN-γ did not suppress EAE induction by IL-23-independent Th1 cells (data not shown). These data contribute to a growing body of data demonstrating the dispensability of individual “hallmark” cytokines in autoimmune disease [6, 13]. Similarly, iNOS was highly upregulated in Th1 recipients, yet iNOS−/− mice remained highly susceptible to Th1-mediated, IL-23-independent EAE (data not shown).
Concluding Remarks
The current study challenges the dogma that IL-23 is universally critical for the development of EAE. And while IL-23-independent Th1 cells may possibly be exTh17 cells, these data confirm that their development and function does not require IL-23. Fate-mapping studies can further clarify the role of IL-12 in exTh17 cell development. (Supp Fig. 2). Collectively, our data substantiate and expand previous studies indicating that clinically similar forms of EAE may be mediated by distinct autoreactive T cell subsets via parallel inflammatory pathways [2, 24]. If these findings are translatable to human autoimmune disease, they suggest that the therapeutic targeting of proximal factors that promote the differentiation and/or stabilization of encephalitogenic T cells should be customized based on the predominant Th subset in an individual’s autoreactive repertoire. Conversely, we found that CNS GM-CSF production and monocyte recruitment are common features of EAE initiated by disparate effector T cells. Hence, targeting innate immune cells or factors that regulate them might have broader therapeutic efficacy.
Materials and Methods
Mice
C57BL/6 WT and CD45.1 congenic mice were obtained from Charles River Laboratories (Wilmington, MA). C57BL/6 IL-12Rβ2−/−, iNOS−/−, CCR2−/− and IFN-γR−/− mice were obtained from Jackson Labs (Bar Harbor, ME). IL-23R−/− were the generous gift of Dr. Nico Ghilardi (Genentech, South San Francisco, CA) [4]. IL-12p40−/− mice were originally obtained from J. Magram (Hoffman LaRoche, Nutley, NJ) [25]. All mice were bred and maintained under specific pathogen-free conditions in the University of Michigan animal housing facilities. All protocols were approved by the University of Michigan Committee on Use and Care of Animals.
Immunization and Cell Culture
Mice were subcutaneously immunized with an emulsion of complete Freund’s adjuvant (CFA; Difco, Detroit, MI) and 100 μg of myelin oligodendrocyte glycoprotein peptide 35–55 (Biosynthesis, Lewisville, Texas). 10–14 days later, draining lymph nodes were harvested and homogenized into a single cell suspension. Cells were cultured for 96 hours in the presence of MOG35–55 at 50 μg/ml and either Th1- or Th17-polarizing factors, as previously described [2, 20]. For rest and rechallenge experiments, in vitro polarized CD4+ T cells were purified by magnetic activated cell-sorting (MACS) (Miltenyi Biotech, San Diego, CA), and rested for 48 hours without exogenous cytokines. On the day of rechallenge, CD4+ T cells were harvested and co-cultured with T-depleted splenocytes (prepared using anti-CD4 MACS microbeads) in the presence or absence of MOG35–55 at 50 μg/ml.
Quantitative PCR
RNA was isolated from purified CD4+ T cells using TRIzol Reagent (Life Technologies, Grand Island, NY) and converted into cDNA using the High Capacity cDNA Reverse Transcription Kit (Life Technologies). qPCR was performed on an iQ Thermocycler (Bio-Rad, Hercules, CA) with predesigned Taqman assays (Life Technologies).
Induction of EAE
Naïve C57BL/6 WT or IL-12p40−/− mice were injected with 3–5x106 CD4+ T cells isolated from MOG-reactive Th cultures. Mice were scored daily by an examiner blinded to experimental groups according to the following scale: 1, limp tail; 2, hind-limb weakness; 3, partial hind-limb paralysis; 4, complete paralysis of hind-limbs; and 5, moribund state.
Multiplex Immunoassays and Isolation of CNS Mononuclear Cells
Mice were perfused with PBS via the intracardiac route. CNS tissues were homogenized in PBS and protease inhibitors using an 18G needle and syringe and centrifuged to pellet the tissue. Supernatants were analyzed with a customized Milliplex Mouse Cytokine/Chemokine Kit (MPXMCYTO-70K, Millipore, Billerica, MA) using the Bio-Plex 200 System (Bio-Rad). Cytokine and chemokine levels were normalized to total protein as quantified by NanoDrop (Thermo Scientific, Wilmington, DE). Solid tissue was digested in a solution of collagenase A (1 mg/mL, Roche, Indianapolis, IN) and DNase1 (1 mg/mL, Sigma-Aldrich, St. Louis, MO). Mononuclear cells were enriched on a 30%/70% Percoll gradient (GE Healthcare, Pittsburgh, PA).
Flow Cytometry
CNS mononuclear cells and cultured Th cells were stimulated with PMA (50 ng/mL) and ionomycin (2 μg/mL) in the presence of brefeldin A (10 μg/mL) for four hours. For rest and re-challenge experiments, brefeldin A (10ug/ml) was added for the final 4 hours of culture. For intracellular staining, cells were fixed with 4% paraformadehyde and permeabilized with 0.5% saponin. Fluorescent antibodies were purchased from eBioscience (San Diego, CA) and BD Biosciences (San Jose, CA). Data was acquired using a FACSCanto II flow cytometer (BD Biosciences) and analyzed using FlowJo software (Treestar, Ashland, OR). Full gating schemes are presented in Supplemental Figure 3.
Immunofluorescence
Mice were perfused with Tyrode’s solution followed by 4% paraformaldehyde in PBS. The spinal column was removed, post-fixed, decalcified, cryoprotected, embedded and frozen in OCT (Cellpath, Newton, UK). 12 μm sections were blocked with Avidin D and biotin (Vector Labs, Burlingame, CA), and stained with the following primary and secondary antibodies: goat anti-MBP (1:300, Millipore), Alexa Fluor 488-conjugated rabbit anti-goat IgG (1:300, Life Technologies), biotinylated rat anti-CD45 (1:100, eBioscience), and Alexa Fluor 647-conjugated streptavidin (1:300, Life Technologies). Nuclei were labeled with DAPI (Life Technologies). Images were acquired on a Nikon A1 confocal microscope (Nikon, Tokyo, Japan).
Statistics
Immune parameters were compared using the unpaired Student’s t test with Prism 6 software (GraphPad, La Jolla, CA). Clinical scores were compared by two-way ANOVA. p values: *<0.05, **<0.01, ***<0.001.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 NS057670 (to B.M.S.), Training Grants T32-AI007413 (H.M.G-W.) and 5T32GM7863-34 (D.A.G.), University of Michigan Rackham Merit Fellowship (H.M.G-W.) and Veterans Administration Merit Review Awards 1I01RX000416 and 1I01BX001387 (B.M.S). B.M.S. is a Scholar of the A. Alfred Taubman Medical Research Institute.
Abbreviations
- CNS
central nervous system
- MOG or MOG35–55
myelin oligodendrocyte glycoprotein fragment 35–55
- LNC
lymph node cells
Footnotes
Competing Interests
The authors have declared that no competing interests exist.
References
- 1.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467(7318):967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205(7):1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segal BM, Shevach EM. IL-12 unmasks latent autoimmune disease in resistant mice. J Exp Med. 1996;184(2):771–775. doi: 10.1084/jem.184.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10(3):314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 6.Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119(1):61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12(6):568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12(6):560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 9.Noster R, Riedel R, Mashreghi MF, Radbruch H, Harms L, Haftmann C, Chang HD, Radbruch A, Zielinski CE. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci Transl Med. 2014;6(241):241ra280. doi: 10.1126/scitranslmed.3008706. [DOI] [PubMed] [Google Scholar]
- 10.Voskuhl RR, Martin R, Bergman C, Dalal M, Ruddle NH, McFarland HF. T helper 1 (Th1) functional phenotype of human myelin basic protein-specific T lymphocytes. Autoimmunity. 1993;15(2):137–143. doi: 10.3109/08916939309043888. [DOI] [PubMed] [Google Scholar]
- 11.Segal BM. Experimental autoimmune encephalomyelitis: cytokines, effector T cells, and antigen-presenting cells in a prototypical Th1-mediated autoimmune disease. Curr Allergy Asthma Rep. 2003;3(1):86–93. doi: 10.1007/s11882-003-0017-6. [DOI] [PubMed] [Google Scholar]
- 12.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169(12):7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 13.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157(8):3223–3227. [PubMed] [Google Scholar]
- 14.Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170(4):2153–2160. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor RA, Prendergast CT, Sabatos CA, Lau CW, Leech MD, Wraith DC, Anderton SM. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181(6):3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thakker P, Leach MW, Kuang W, Benoit SE, Leonard JP, Marusic S. IL-23 is critical in the induction but not in the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;178(4):2589–2598. doi: 10.4049/jimmunol.178.4.2589. [DOI] [PubMed] [Google Scholar]
- 17.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12(3):255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duhen R, Glatigny S, Arbelaez CA, Blair TC, Oukka M, Bettelli E. Cutting edge: the pathogenicity of IFN-gamma-producing Th17 cells is independent of T-bet. J Immunol. 2013;190(9):4478–4482. doi: 10.4049/jimmunol.1203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grifka-Walk HM, Lalor SJ, Segal BM. Highly polarized Th17 cells induce EAE via a T-bet independent mechanism. Eur J Immunol. 2013;43(11):2824–2831. doi: 10.1002/eji.201343723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connor RA, Cambrook H, Huettner K, Anderton SM. T-bet is essential for Th1-mediated, but not Th17-mediated, CNS autoimmune disease. Eur J Immunol. 2013;43(11):2818–2823. doi: 10.1002/eji.201343689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vass K, Heininger K, Schafer B, Linington C, Lassmann H. Interferon-gamma potentiates antibody-mediated demyelination in vivo. Ann Neurol. 1992;32(2):198–206. doi: 10.1002/ana.410320212. [DOI] [PubMed] [Google Scholar]
- 23.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37(7):1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 24.Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16(4):406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segal BM, Dwyer BK, Shevach EM. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J Exp Med. 1998;187(4):537–546. doi: 10.1084/jem.187.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.