Abstract
We aimed to assess the prognostic significance of demographic factors, including age, sex, performance status, smoking status, obesity, and race in upper urinary tract urothelial carcinoma (UTUC) patients treated with radical nephroureterectomy through a systematic review and meta-analysis. We conducted PubMed search for all articles published until December 2014 according to Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines. Survival outcomes of interest were intravesical recurrence (IVR) free survival, progression free survival (PFS), cancer-specific survival (CSS), and overall survival (OS). Seventy-nine studies, including numbers of subjects ranging from 24 to 9899, met the inclusion criteria. Advanced age was significantly associated with worse PFS [hazard ratio (HR) 1.01] and OS (HR 1.05). The significant predictors of CSS were age (HR 1.02) and performance status (HR 1.35). Female gender (HR 0.81) and smoking (HR 1.38) were the significant predictors only for IVR free survival. No significant associations with survival outcomes were observed in obesity and race. Our study reveals that age is one of the most important demographic predictor of survival in UTUC. Also, male gender, poor performance status, and smoking are also significantly related to worse survival outcomes. However, large well-designed prospective studies are required to investigate the precise prognostic significance of demographics.
Keywords: urothelial carcinoma, urinary urinary tract, demographic, prognosis, survival
INTRODUCTION
Upper urinary tract urothelial carcinoma (UTUC) is a relatively rare malignancy, accounting for only 5–10% of urothelial carcinomas (UCs) [1]. Radical nephroureterectomy (RNU) with ipsilateral bladder cuff excision remains the standard treatment for patients with large, multifocal or high-grade tumors [1]. Despite definitive surgery, UTUC has high potential for local and distant recurrence, especially in patients with advanced disease [2, 3].
The identification of new prognostic factors could help guide decisions regarding administration of adjuvant chemotherapy and selective enrolment into clinical trials of novel therapies. Tumor stage and grade have been documented as the major prognostic factors in patients with UTUC [1, 2]. In addition, tumor size, architecture, location and multiplicity as well as lymphovascular invasion (LVI) have been suggested as potential prognostic factors [1]. However, to date, the definite risk factors for recurrence and survival in patients who received RNU are still unclear.
Previous reports showed that demographic factors, such as age [3], gender [4], performance status [5], smoking status [6] obesity [7] and race [8] may have prognostic value in UTUC.
However, because of the rarity of the disease and the contradictory conclusions among previous studies, the prognostic significance of demographics has not been clearly established in UTUC. Our purpose was to assess the value of demographic data as prognostic factors for UTUC after RNU through a systematic review of the literature and meta-analysis of the available data.
RESULTS
Study population
Table 1 shows individual data on the characteristics of the included studies and patient populations. We included 79 studies, comprising patients from Asia, North America, and Europe, with numbers of patients ranging from 24 to 9899. The studies were published between 1994 and 2014 and the patient recruitment periods ranged from 1986 to 2013. The median age ranged from 61 to 73 years and the median follow-up time was between 17.8 and 84 months. Only one was a prospective study [9]. Other pathologic characteristics of the eligible studies are reported in Supplementary Table 1.
Table 1. Study and patient characteristics of the eligible studies.
| Study | Year | Country | Recruitment period | No. of patients | Median age, range (years) | No. of sex (male/female) | Median follow-up, range (months) | No. of NACH | No. of ACH | No. of ART |
|---|---|---|---|---|---|---|---|---|---|---|
| Park [11] | 2004 | Korea | 1991–2001 | 86 | 59.5 (mean) | 81/5 | 43.8, 5-140 | 0 | 14 | 5 |
| Chen [12] | 2005 | Taiwan | 1993–2003 | 111 | 70.5 (mean), 38-91 | 69/42 | 49.3 (mean), 1-136 | NA | 9 | 4 |
| Ataus [13] | 2006 | Turkey | 1993–2003 | 24 | 61, 34-74 | 19/5 | 34.8 (mean), 5-97 | NA | NA | NA |
| Chung [14] | 2007 | Taiwan | 1996–2006 | 150 | NA | 66/84 | 47.5, 3-121 | NA | 0 | NA |
| Koda [15] | 2007 | Japan | 1995–2005 | 106 | 70.4 (mean) | NA | 17.5, 1-97 | NA | 25 | NA |
| Akao [16] | 2008 | Japan | 1992–2005 | 90 | 71 | 57/33 | 42, 2-179 | NA | 24 | 16 |
| Berger [17] | 2008 | USA | 1997–2005 | 100 | 73, 53-97 | 65/35 | 84, 24-120 | NA | NA | NA |
| Li [18] | 2008 | Taiwan | 1990–2005 | 260 | 65,23-87 | 125/135 | 56, 12-181 | 0 | 0 | NA |
| Soga [19] | 2008 | Japan | 1986–2005 | 46 | NA | 34/12 | NA | NA | 24 | NA |
| Capitanio [20] | 2009 | multination | 1987–2007 | 1249 | 27-97 | 846/403 | NA | 0 | 0 | NA |
| Chung [21] | 2009 | Taiwan | 1996–2006 | 76 | 66 (mean), 41-93 | 38/38 | 48, 15-88 | NA | 0 | NA |
| Kamihira [22] | 2009 | Japan | 1995–2005 | 1003 | 68.6 (mean), 27-92 | 718/285 | 20, 1-122 | NA | 181 | NA |
| Favaretto (I) [23] | 2010 | USA | 1995–2008 | 274 | NA | NA | NA | NA | NA | NA |
| Favaretto (II) [24] | 2010 | USA | 1995–2008 | 253 | 72, 64-77 (IQR) | 159/94 | 48 | 0 | NA | NA |
| Ishikawa [25] | 2010 | Japan | 1990–2005 | 208 | 70, 39-90 | 139/69 | 44, 1-212 | 0 | 0 | 0 |
| Kim [26] | 2010 | Korea | 1986–2006 | 238 | 64.1 (mean), 25-91 | 164/74 | 53.4, 3-240 | NA | NA | NA |
| Kobayashi [27] | 2010 | Japan | 2000–2004 | 221 | 72, 46-92 | 153/68 | 38.4, 0.8-92.2 | 0 | 35 | NA |
| Pieras [28] | 2010 | Spain | 1990–2006 | 79 | 67, 65-69 (95% CI) | 62/17 | 71, 59-84 (95% CI) | NA | NA | NA |
| Takaoka [29] | 2010 | Japan | 1989–2007 | 60 | 64.7 (mean), 40-83 | 40/20 | 51.3, 1.5-223.8 | 0 | 28 | NA |
| Cho [30] | 2011 | Korea | 1994–2009 | 87 | 62.2, 33-85 | 64/23 | 32, 1-131 | NA | 19 | 5 |
| Hou [31] | 2011 | Taiwan | 2003–2007 | 192 | 69.8 (mean), 43-89 | 79/113 | 43.8 (mean), 3.8-84.8 | NA | NA | NA |
| Ku [32] | 2011 | Korea | 1991–2006 | 181 | 63, 36-90 | 142/39 | 37.5, 1-174 | NA | 48 | NA |
| Walton [33] | 2011 | multination | 1987–2008 | 773 | 68, 61-75 (IQR) | 533/240 | 34, 15-65 (IQR) | 0 | 66 | NA |
| Ariane [34] | 2012 | France | 1995–2010 | 609 | 69.8, 61.9-76 | 415/194 | 27, 10-48 | 0 | NA | NA |
| Chen [35] | 2012 | China | 2007–2009 | 85 | NA | 58/24 | 28, 5-60 | NA | NA | NA |
| Chromecki [36] | 2012 | multination | 1987–2007 | 2492 | 69.2, 54.1-84.2 (IQR) | 1681/811 | 45, 0-101 (IQR) | NA | 247 | NA |
| Godfrey [37] | 2012 | USA | 1990–2010 | 211 | 70 (mean) | 124/87 | 27, 11-65.5 (IQR) | NA | NA | NA |
| Hirano [38] | 2012 | Japan | 1995–2010 | 151 | NA | 121/30 | 24, 3-162 | NA | 51 | NA |
| Kobayashi [39] | 2012 | Japan | 2005–2009 | 288 | 71.4, 32-89 | 197/91 | 20.2, 3-61.6 | 0 | 47 | 0 |
| Kuroda [40] | 2012 | Japan | 1999–2010 | 121 | 68, 38-86 | 92/29 | 44.4 (mean), 3.5-144.5 | 0 | 29 | NA |
| Liang [41] | 2012 | Taiwan | 1996–2004 | 340 | 68, 34-87 | 182/158 | 38, 1-176 | 0 | 20 | 4 |
| Cho [42] | 2013 | Korea | 1994–2009 | 78 | 61.1 (mean), 33-85 | 58/20 | 34, 12-132 | 0 | NA | NA |
| Ehdaie [43] | 2013 | USA | 1995–2008 | 288 | 71, 37-90 | 187/101 | 4.02 yr, 0.03-14.65 yr | NA | NA | NA |
| Elalouf [44] | 2013 | France | 1998–2011 | 237 | 69.3, 60-76 (IQR) | 161/76 | 44, 24-79 (IQR) | 0 | 23 | NA |
| Fairey [45] | 2013 | Canada | 1994–2009 | 849 | NA | 542/307 | 2.2 yr, 0.6-5 yr | NA | 94 | NA |
| Fujita [46] | 2013 | Japan | 1999–2011 | 139 | 72, 48-90 | 91/48 | 27, 1-139 | 11 | 23 | NA |
| Gunay [47] | 2013 | Turkey | 1987–2009 | 101 | 60.5 (mean) | 85/16 | 56.2 (mean) | NA | NA | NA |
| Hashimoto [48] | 2013 | Japan | 1997–2010 | 84 | 68.8 | 59/25 | NA | NA | NA | NA |
| Ito [49] | 2013 | Japan | 2005–2008 | 72 | NA | 43/29 | 24.9, 2.6-39.3 | 0 | 14 | NA |
| Kim [50] | 2013 | Korea | 2000–2013 | 65 | 60.4 (mean), 37-87 | 40/25 | 34, 12-114 | NA | 36 | NA |
| Kim [51] | 2013 | Korea | 1990–2010 | 422 | 64, 29-86 | 318/104 | 44, 3-257 | NA | 51 | NA |
| Kusuda [52] | 2013 | Japan | 2000–2009 | 502 | NA | 360/142 | 39 (mean), 6-134.8 | NA | NA | NA |
| Milojevic [53] | 2013 | Serbia | 1999–2010 | 183 | 66 (mean), 36-88 | 102/81 | 35, 1-154 | 0 | NA | NA |
| Morizane [54] | 2013 | Japan | 1995–2011 | 99 | 73, 44-86 | 71/28 | 37.9, 6.6-171.4 | NA | 33 | NA |
| Rink [55] | 2013 | multination | 1987–2007 | 864 | 70, 61-76 (IQR) | 553/311 | 50, 23-90 (IQR) | 0 | 63 | 0 |
| Sakano [56] | 2013 | Japan | 1995–2009 | 536 | 71, 32-93 | 370/166 | 40.9, 3-200 | 32 | NA | NA |
| Shimamoto [57] | 2013 | Japan | 1983–2008 | 105 | 66.9, 42-88 | 74/31 | 53 (mean) | 7 | 59 | NA |
| Takahara [58] | 2013 | Japan | 1996–2009 | 103 | 68.6 (mean), 62-75 (IQR) | 71/32 | 29, 14-63 (IQR) | NA | 12 | NA |
| Xylinas [59] | 2013 | France | 1995–2010 | 482 | 69.2, 60-76 (IQR) | 332/150 | 39.5, 25-60 (IQR) | 0 | 59 | NA |
| Zhang [60] | 2013 | China | 2000–2010 | 217 | 69, 62-81 | 130/87 | 52, 12-78 (IQR) | 0 | NA | NA |
| Aziz [61] | 2014 | Germany | 1990–2012 | 265 | NA | 169/96 | 23, 9-48 (IQR) | 0 | 47 | NA |
| Bachir [62] | 2014 | Canada | 1990–2010 | 644 | NA | NA | 24.5 | 28 | 76 | NA |
| Cho [63] | 2014 | Korea | 2004–2012 | 147 | 70, 44-84 | 41/106 | 33, 1-191 | 0 | 95 | NA |
| Choo [64] | 2014 | Korea | 2000–2011 | 319 | NA | 253/66 | NA | 6 | 85 | NA |
| Ehdaie [65] | 2014 | USA | 1995–2008 | 253 | 72, 63-77 (IQR) | 158/95 | NA | 0 | 0 | NA |
| Fang [66] | 2014 | China | 2000–2010 | 438 | 66, 20-88 | 187/251 | 45, 12-144 | 0 | NA | NA |
| Fradet [67] | 2014 | Canada | 1990–2010 | 743 | 69.7 (mean) | 438/304 | 24.8, 7.7-56.8 (IQR) | 0 | 73 | NA |
| Fujita [68] | 2014 | Japan | 1998–2012 | 226 | 70, 37-90 | 153/73 | 41, 1-164 | 8 | 44 | NA |
| Gandaglia [69] | 2014 | USA | 1988–2009 | 9899 | 73, 22-99 | 5823/4076 | 98, 0-263 | NA | NA | NA |
| Ichimura [70] | 2014 | Japan | 1996–2012 | 171 | NA | 119/52 | 56, 25-86 (IQR) | 0 | NA | NA |
| Ishioka (I) [71] | 2014 | Japan | 1995–2010 | 1014 | 70, 62-79 (IQR) | 718/296 | 38, 16-73 (IQR) | NA | 155 | NA |
| Ishioka (II) [72] | 2014 | Japan | 1995–2010 | 754 | 69, 62-75 (IQR) | 526/228 | 41, 18-75 (IQR) | NA | NA | NA |
| Ito [73] | 2014 | Japan | 1999–2012 | 70 | NA | 47/23 | 29.2, 1-157 | NA | 9 | NA |
| Kitamura [74] | 2014 | Japan | 1995–2010 | 110 | NA | NA | 60, 6-192 | NA | NA | NA |
| Kondo [75] | 2014 | Japan | 1988–2013 | 180 | NA | 128/52 | NA | NA | NA | NA |
| Lee [76] | 2014 | Taiwan | 2004–2010 | 250 | NA | 108/142 | 41 | NA | NA | NA |
| Liu (I) [77] | 2014 | China | 1998–2009 | 230 | NA | 179/51 | 67, 34-170 | 0 | 57 | NA |
| Liu (II) [78] | 2014 | China | 2002–2010 | 212 | 66.5, 31-84 | 123/89 | 39, 7-78 | 0 | 8 | NA |
| Ou [79] | 2014 | Taiwan | 2003–2011 | 61 | NA | 34/27 | 39.7, 12-96 | 0 | 0 | 0 |
| Park [80] | 2014 | Korea | 1991–2010 | 392 | 64, 29-86 | 299/93 | 47.6, 2-257 | 0 | 60 | NA |
| Sasaki [81] | 2014 | Japan | 1996–2012 | 171 | NA | 119/52 | NA | 0 | NA | NA |
| Shirotake [82] | 2014 | Japan | 1993–2011 | 873 | 70, 63-77 (IQR) | 608/231 | 32, 16-62 (IQR) | 0 | 129 | NA |
| Sung (I) [83] | 2014 | Korea | 1994–2011 | 410 | 64, 55-72 (IQR) | 312/98 | 40.2, 33-66.1 (IQR) | NA | 91 | NA |
| Sung (II) [84] | 2014 | Korea | 1994–2010 | 386 | NA | 293/93 | 39, 21.1-70.6 (IQR) | NA | NA | NA |
| Tanaka [85] | 2014 | Japan | 1994–2010 | 474 | 69, 61-76 (IQR) | 346/128 | 35, 17-68 (IQR) | 0 | 78 | NA |
| Xylinas (I) [86] | 2014 | multination | 1987–2007 | 519 | 70, 61-76 (IQR) | 330/189 | 37, 19-73 (IQR) | 0 | 53 | 0 |
| Xylinas (II) [87] | 2014 | multination | 1987–2007 | 2681 | 68.4, 54-84 | 1808/873 | 57.5, 1-271 | 0 | 264 | NA |
| Yafi [88] | 2014 | Canada | 1995–2010 | 1029 | 70, 62-77 (IQR) | 195/113 | 17.8, 5.5-46.8 | NA | 36 | NA |
| Zou [89] | 2014 | China | 1999–2013 | 122 | 64, 35-80 | 87/35 | 53, 3-159 | NA | NA | NA |
NACH: neoadjuvant chemotherapy, ACH: adjuvant chemotherapy, ART: adjuvant radiotherapy, NA: not available, IQR: interquartile range, CI: confidence interval.
Demographic predictors of outcomes
Age
In the 29 studies about intravesical recurrence (IVR)-free survival, there was an obvious inter-study heterogeneity (p = 0.02); thus, the random effect model was used to pool the results. The meta-analysis showed no association between age and IVR-free survival (hazard ratio (HR), 1.01; 95% confidence interval (CI), 0.99–1.02; p = 0.38) (Figure 1A). In the 10 studies about progression free survival (PFS) there was no obvious heterogeneity among studies (p = 0.17; I2 = 30%); thus, the fixed effects model was used to pool the results. The pooled HR for PFS was 1.01 (95% CI, 1.01–1.02; p = 0.0009) (Figure 1B), indicating a significant association between age and PFS. A total of 27 studies were included in the analysis of cancer-specific survival (CSS). Age was associated with worse CSS (HR, 1.02; 95% CI, 1.02–1.03; p < 0.00001). Since inter-study heterogeneity was not significant (p = 0.17; I2 = 30%), the fixed effects model was applied (Figure 1C). Seven studies were included in the analysis of overall survival (OS). Age was associated with worse OS (HR, 1.05; 95% CI, 1.03–1.07; p < 0.00001). There was significant heterogeneity (p = 0.02; I2 = 61%) (Figure 1D).
Figure 1. Forest plots of prognosis of age.
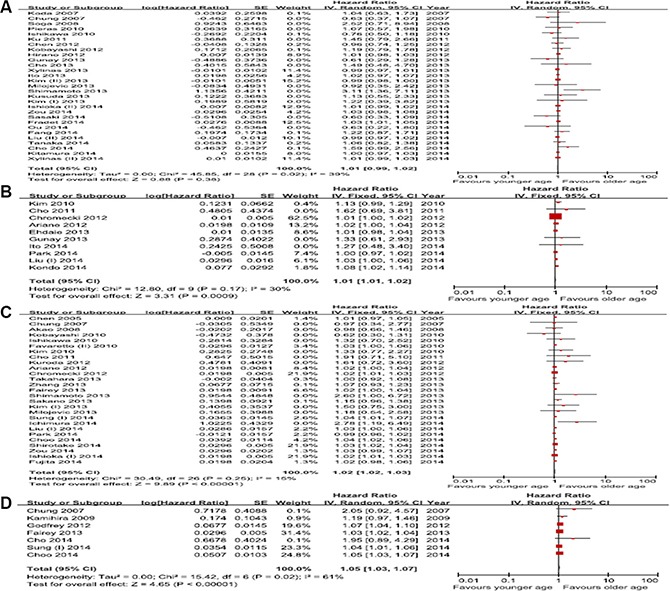
(A) Intravesical recurrence-free survival, (B) Progression-free survival, (C) Cancer-specific survival, (D) Overall survival.
Gender
Twenty-nine studies on the relationship between sex and IVR were included. The present meta-analysis indicated that female patients had a better IVR-free survival rate than male patients (HR, 0.81; 95% CI, 0.70–0.94; p = 0.004). However, the Cochrane Q test (p = 0.002) could not exclude a significant heterogeneity (Figure 2A). Nine studies provided information on PFS. There was no significant association between sex with PFS (HR, 1.06; 95% CI, 0.94–1.19; p = 0.33), and no heterogeneity was detected in the data (p = 0.96; I2 = 0%) (Figure 2B). A total of 27 studies evaluated the relationship between sex and CSS. The pooled HR (95% CI) of these studies for CSS was 1.04 (0.98–1.10; p = 0.22), and there was no heterogeneity (p = 0.45; I2 = 1%) (Figure 2C). The meta-analysis of 5 studies also demonstrated no significant association between sex and overall survival (OS) (HR, 0.95; 95% CI, 0.79–1.16; p = 0.63) in a fixed effects model (p = 0.54, I2 = 0%) (Figure 2D).
Figure 2. Forest plots of prognosis of sex.
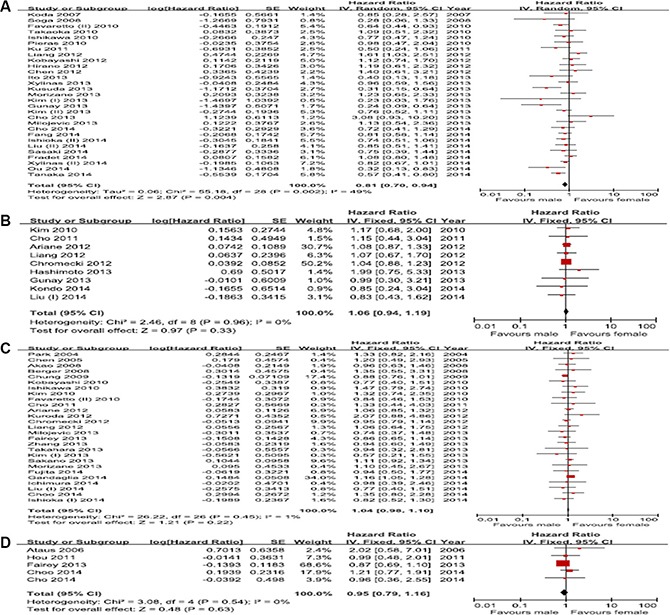
(A) Intravesical recurrence-free survival, (B) Progression-free survival, (C) Cancer-specific survival, (D) Overall survival.
Performance status
Four studies were included in the analysis of the correlation between performance status and IVR-free survival. The results indicated that performance status had no relationship with IVR-free survival (HR, 1.19; 95% CI, 0.85–1.66; p = 0.31), and there was between-study heterogeneity (p = 0.03; I2 = 64%) (Figure 3A). The pooled analysis of PFS was based on 6 studies. No significant association between performance status and PFS was observed (pooled HR, 1.74; 95% CI, 0.92–1.65; p = 0.16). The test for heterogeneity recorded an I2 value of 57% (p = 0.04), suggesting the presence of inter-study heterogeneity (Figure 3B). Nine eligible studies were analyzed to evaluate the impact of performance status on CSS, and the analysis revealed that performance status was significantly related to CSS (HR, 1.35; 95% CI, 1.15–1.57; p = 0.0002). No significant heterogeneity was observed (p = 0.05; I2 = 0%) (Figure 3C). The pooled HR estimate for OS was 2.03 (95% CI, 0.64–6.37; p = 0.23) in the analysis of 2 studies, indicating no clear correlation between performance status and OS. The result for the test for heterogeneity was significant (p = 0.01; I2 = 85%) (Figure 3D).
Figure 3. Forest plots of prognosis of performance status.
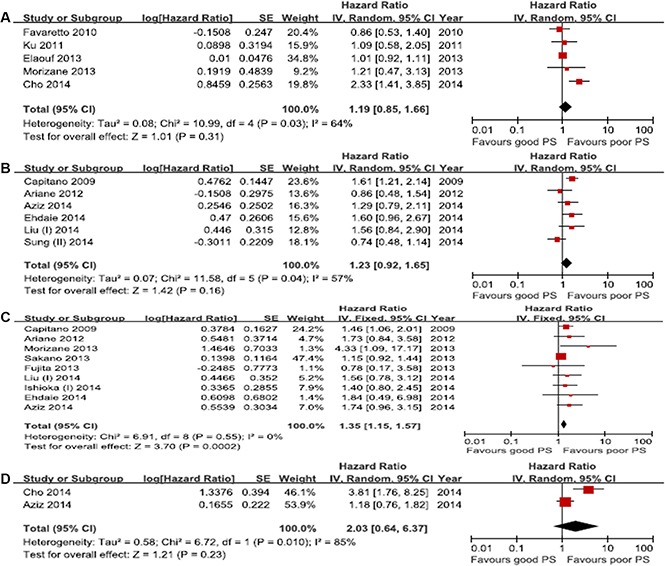
(A) Intravesical recurrence-free survival, (B) Progression-free survival, (C) Cancer-specific survival, (D) Overall survival.
Smoking history
Data on IVR-free survival was available from 5 studies. Smoking history was associated with a higher risk of IVR (HR, 1.38; 95% CI, 1.11–1.71; p = 0.003). Importantly, this analysis revealed no heterogeneity (p = 0.41; I2 = 0%) (Figure 4A). Six studies provided data on PFS. The results suggested that smoking status did not correlate with poor PFS (HR, 1.17; 95% CI, 0.79–1.73; p = 0.44), but there was significant heterogeneity (I2 = 59%) (Figure 4B). CSS rate was extracted from 7 studies. The meta-analysis indicated no significant association between smoking history and poor CSS (HR, 1.1; 95% CI, 0.89–1.37; p = 0.35). No significant heterogeneity was observed in the primary analysis (p = 0.12; I2 = 40%) (Figure 4C). The meta-analysis was not performed for OS because only 1 study was eligible for inclusion [10].
Figure 4. Forest plots of prognosis of smoking.
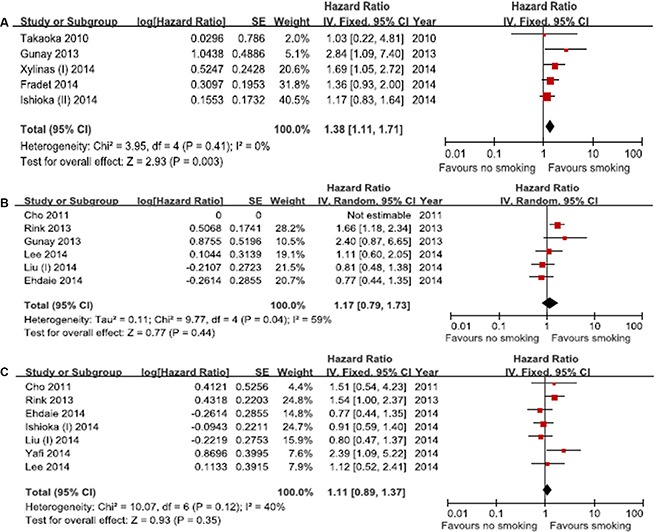
(A) Intravesical recurrence-free survival, (B) Progression-free survival, (C) Cancer-specific survival.
Obesity
In 3 studies about IVR-free survival, the pooled HR for IVR-free survival showed that obesity was not significantly associated with IVR-free survival (HR, 1.07; 95% CI, 0.65–1.75; p = 0.80). However, significant heterogeneity was observed (I2 = 64%) (Supplementary Figure 1A). Of 2 studies about PFS, the pooled HR for PFS was 0.98 (95% CI, 0.47–2.06; p = 0.96), which suggested no significant correlation between obesity and PFS. There was no significant heterogeneity among these studies (p = 0.46; I2 = 0%) (Supplementary Figure 1B). Four studies reported survival analysis for CSS. Obesity was not significantly related to worse CSS (HR, 0.99; 95% CI, 0.96–1.02; p = 0.38). However, there was an obvious inter-study heterogeneity (I2 = 53%) (Supplementary Figure 1C). Two studies were included in the analysis of OS. Obesity did not exhibit a correlation with worse OS (HR, 0.94; 95% CI, 0.80–1.19; p = 0.43). The inter-study heterogeneity was significant (I2 = 51%) (Supplementary Figure 1D).
Race
No studies were available for investigating the association between race and IVR-free survival. Furthermore, we could not perform meta-analysis for PFS and OS because only 1 study was eligible for PFS [11] and OS [12], respectively. Only 2 studies were evaluated for the impact of race on CSS. The pooled HR value was 1.02 (95% CI, 0.88–1.18; p = 0.79), which indicated no significant association between race and CSS. There was no heterogeneity between studies (p = 0.21; I2 = 37%) (Supplementary Figure 2).
Publication bias
Due to the small number of studies in most meta-analyses, the potential for publication bias could be reliably examined only in the meta-analyses of the correlation between age and IVR-free survival, PFS, and CSS, and between sex and IVR-free survival and CSS, which included ≥ 10 studies (Supplementary Figures 3 and 4). We found no strong evidence for publication bias by graphical inspection. However, Egger's test suggested there was a significant publication bias in the meta-analysis of the correlation between age and PFS (p = 0.03045). No publication bias was detected in the other meta-analyses (all p > 0.05, Egger's test).
DISCUSSION
UTUC is a heterogeneous disease showing a variety of clinical courses. In spite of the increased detection of earlier-stage tumors resulting from the recent improvement in imaging and endoscopic techniques, UTUC still remains an aggressive disease with high recurrence and progression rates [1]. According to the current European Association of Urology (EAU) guidelines, the prognostic predictors of UTUC can be largely divided into preoperative clinical (demographic) and postoperative pathological factors [1, 2]. Many studies have focused mainly on pathological factors, including tumor stage, tumor grade, concomitant CIS, lymph node invasion, tumor multifocality, tumor architecture, tumor necrosis, and LVI. In contrast, the studies mainly evaluating demographic factors, such as age, sex, obesity, performance status, smoking, and race, are relatively insufficient and have shown conflicting results; therefore, the prognostic significance of demographic data has not been definitely confirmed in UTUC. However, knowledge of the prognostic significance of preoperative demographic factors is important for the counselling and management of UTUC patients.
We performed a systematic review and meta-analysis to assess whether each demographic factor could predict the survival outcomes in patients treated with RNU for UTUC. This study aggregated the outcomes of 79 studies with numbers of patients ranging from 24 to 9899. On the basis of the available data, a total of 20 meta-analyses were conducted to investigate the association of each demographic factor with survival outcomes. To the best of our knowledge, this is the first study to systematically evaluate the association between each demographic factor and postoperative survival outcomes, including IVR-free survival, PFS, CSS, and OS. Our meta-analysis found an association between survival outcomes of UTUC and demographic factors. Advanced age was significantly associated with worse PFS, CSS, and OS, but not with IVR-free survival. Male sex and smoking history only correlated with poor IVR-free survival. Performance status was exclusively related to CSS. Obesity and race did not show any association with survival outcomes.
There are several limitations for this systematic review and meta-analysis. First, because we did not use sample-size restrictions, most meta-analyses included a small number of studies (less than 10). As a result, the evaluation for publication bias was possible only in 5 meta-analyses including more than 10 studies (effect of age on IVR-free survival, PFS, and CSS, and of sex on IVR-free survival and CSS). However, among the meta-analyses assessed for publication bias, no significant bias was observed (all p > 0.05 for Egger's test), except for the meta-analysis of the effect of age on PFS. Second, owing to scarcity or absence of data reported in the literature, the association between smoking and race and several survival outcomes (smoking and OS, race and IVR free survival, PFS, and PS) could not be evaluated in this meta-analysis. Third, there was a significant heterogeneity among studies included in half of the current meta-analyses. The meta-regression and subgroup analysis to identify the source of inter-study heterogeneity was not carried out in the present study. Although the random-effects model, which accounts for heterogeneity, was applied to analyze the studies with heterogeneity, the conclusions yielded in this systematic review and meta-analysis should be interpreted with caution [13, 14]. Fourth, the results of this systematic review and meta-analysis were partially drawn on the basis of unadjusted estimates from Kaplan-Meier or univariate Cox regression analyses because some studies did not provide detailed information. Furthermore, it should be kept in mind that a possible bias [15] may be due to the retrospective nature of almost all studies analyzed, except for one [9]. To the best of our knowledge, high quality prospectively, randomized controlled trials investigating the association between demographic factors and prognosis in UTUC have not been published to date. Finally, we cannot exclude the selection bias by including only English language written articles.
This first meta-analysis has yielded significant associations between demographic factors and IVR, progression and mortality in patients with UTUC who underwent RNU, although these findings need to be interpreted with caution. Based on a meta-analysis of the available data, age is one of the most important demographic marker, and is independently associated with mortality. We also identified male sex and smoking history as significant predictors of IVR. However, since the current evidence lacks prospective evaluation of this relationship, large, well-designed prospective studies are required to investigate the precise prognostic significance of demographic factors.
MATERIALS AND METHODS
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines [16].
Search strategy
PubMed was searched for all articles published till December 2014 on the topic of interest. Search terms used were (“nephroureterectomy”) and (“cancer” OR “carcinoma”). Article selection was performed by two independent evaluators (H.S.K., C.W.J.), and all the discrepancies between the two were resolved.
Inclusion and exclusion criteria
According to the PRISMA guidelines, we used the Population, Intervention, Comparator, Outcome, and Study design (PICOS) approach to define study eligibility [16].
Population: patients with UTUC.
Intervention: RNU.
Comparator: demographic data including age, sex, performance status, smoking status, obesity, and race.
Outcome: IVR-free survival, PFS, CSS, and OS.
Study design: univariate and/or multivariate Cox regression analysis.
The inclusion criteria were: (1) original article, (2) human research, (3) English language, (4) UTUC, (5) treatment exclusively with RNU, and (6) availability of Kaplan-Meier/Cox regression-derived results for the prognostic value of demographics on UTUC outcomes according to the REMARKS guidelines for assessment of prognostic markers [17]. No sample-size restrictions were used. Studies using analyses other than survival analysis were not included. In addition, studies which did not offer sufficient data to acquire the hazard ratio (HR) and its standard error (SE) were excluded. If duplicate study populations or analyses of repeated data were identified, only the most recent or most complete study was preferentially assessed.
In the present meta-analysis, IVR-free survival was defined as the interval between surgery and the occurrence of UC in the bladder. PFS was defined as the interval between surgery and the subsequent appearance of local failure, either at the operative site or in the regional lymph nodes, or distant metastasis, while recurrence in the bladder was not included in PFS analysis. We excluded from PFS analysis the studies in which PFS was defined as recurrence both in bladder and non-bladder lesions. CSS and OS were defined as the interval between surgery and death from UTUC or death from any cause, respectively.
Data extraction and analysis
Two authors (C.K. and H.H.K.) performed an independent review of 1,352 articles. A total of 1,037 articles were excluded upon examination of the titles and abstracts, while the full text of 315 articles was evaluated. In accordance with all previously mentioned inclusion criteria, a final selection of 79 articles was made [9–12, 18–92]; any discrepancies between the two evaluations were resolved. The PRISMA flow chart depicting the process for the systematic literature search and selection of the studies is shown in Figure 5.
Figure 5. Preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow chart.
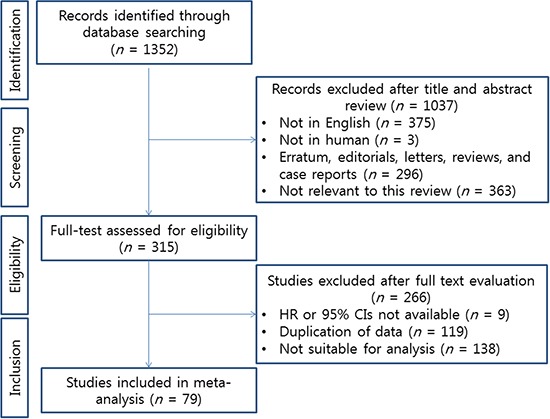
Data was independently extracted by two authors (H.S.K. and J.H.K.) from all the included studies and subsequently crosschecked to ensure accuracy. Any discrepancies between the two authors were resolved. Information was retrieved according to the REMARKS guidelines for reporting prognostic markers, including author, country, journal, publication year, prognostic factors examined, study design, study population (sample size, recruitment period, and follow-up), patient characteristics, treatment received, statistical method applied (with variables used for adjustment), and reported impact of examined factors on UTUC outcome using univariate or multivariate analyses.
Meta- analysis
We conducted a meta-analysis to summarize quantitatively the overall prognostic value of demographic factors. The cumulative effects of factors of interest were evaluated by the inverse variance method. If the 95% CI was not reported, due to the paucity of prognostic literature directly reporting these values, previously reported indirect methods were utilized to extract the log HR and variance [93]. Either the fixed-effects or the random-effects model was used, in case of absence or presence of between-study heterogeneity, respectively. Statistical heterogeneity was assessed using both the Cochran Q test and the I2 statistic, which describes the percentage of total variation across studies caused by heterogeneity rather than by chance. A value of p < 0.05 for the Cochran Q test or an I2 statistic > 50% indicated the presence of significant heterogeneity across selected studies [13, 14] which resulted in the use of the random-effects model based on the Der Simonian method for estimating the tau value [13]. To assess the risk of publication bias, we used a funnel plot and the Egger test for outcomes when at least 10 statistically significant studies were included in the meta-analysis [15]. The meta-analysis was performed using RevMan 5.0 statistical software (the Cochrane Collaboration, Copenhagen).
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
None.
Footnotes
CONFLICTS OF INTEREST
The authors have declared that no competing interests exist.
GRANT SUPPORT
None.
Authors’ contributions
Conceptualization, H.S.K. and J.H.K.; Methodology, C.W.J. and J.H.K.; Investigation, H.S.K., C.W.J. and J.H.K.; Writing Draft, H.S.K. and J.H.K.; Writing-Review & Editing, H.S.K. and J.H.K.; Supervision, C.K., H.H.K. and J.H.K.
REFERENCES
- 1.Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester R, Burger M, Cowan N, Bohle A, BW Van Rhijn, Kaasinen E, Palou J, European Shariat SF. Association of U. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63:1059–1071. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 3.Ploussard G, Xylinas E, Lotan Y, Novara G, Margulis V, Roupret M, Matsumoto K, Karakiewicz PI, Montorsi F, Remzi M, Seitz C, Scherr DS, Kapoor A, et al. Conditional survival after radical nephroureterectomy for upper tract carcinoma. Eur Urol. 2015;67:803–812. doi: 10.1016/j.eururo.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka N, Kikuchi E, Kanao K, Matsumoto K, Kobayashi H, Ide H, Miyazaki Y, Obata J, Hoshino K, Shirotake S, Akita H, Kosaka T, Miyajima A, et al. Metastatic Behavior of Upper Tract Urothelial Carcinoma After Radical Nephroureterectomy: Association with Primary Tumor Location. Ann Surg Oncol. 2014;21:1038–1045. doi: 10.1245/s10434-013-3349-z. [DOI] [PubMed] [Google Scholar]
- 5.Berod AA, Colin P, Yates DR, Ouzzane A, Audouin M, Adam E, Arroua F, Marchand C, Bigot P, Soulié M, Roumiguié M, Polguer T, Gardic S, et al. The role of American Society of Anesthesiologists scores in predicting urothelial carcinoma of the upper urinary tract outcome after radical nephroureterectomy: results from a national multi-institutional collaborative study. BJU Int. 2012;110:E1035–E1040. doi: 10.1111/j.1464-410X.2012.11195.x. [DOI] [PubMed] [Google Scholar]
- 6.Hagiwara M, Kikuchi E, Tanaka N, Matsumoto K, Ide H, Miyajima A, Masuda T, Nakamura S, Oya M. Impact of Smoking Status on Bladder Tumor Recurrence After Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma. J Urol. 2013;189:2062–2068. doi: 10.1016/j.juro.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Ehdaie B, Chromecki TF, Lee RK, Lotan Y, Margulis V, Karakiewicz PI, Novara G, Raman JD, Ng C, Lowrance WT, Scherr DS, Shariat SF. Obesity adversely impacts disease specific outcomes in patients with upper tract urothelial carcinoma. J Urol. 2011;186:66–72. doi: 10.1016/j.juro.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margulis V, Youssef RF, Karakiewicz PI, Lotan Y, Wood CG, Zigeuner R, Kikuchi E, Weizer A, Raman JD, Remzi M, Roscigno M, Montorsi F, Bolenz C, et al. Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract. J Urol. 2010;184:453–458. doi: 10.1016/j.juro.2010.03.142. [DOI] [PubMed] [Google Scholar]
- 9.Ito A, Shintaku I, Satoh M, Ioritani N, Aizawa M, Tochigi T, Kawamura S, Aoki H, Numata I, Takeda A, Namiki S, Namima T, Ikeda Y, et al. Prospective Randomized Phase II Trial of a Single Early Intravesical Instillation of Pirarubicin (THP) in the Prevention of Bladder Recurrence After Nephroureterectomy for Upper Urinary Tract Urothelial Carcinoma: The THP Monotherapy Study Group Trial. J Clin Oncol. 2013;31:1422–1427. doi: 10.1200/JCO.2012.45.2128. [DOI] [PubMed] [Google Scholar]
- 10.Yafi FA, Tanguay S, Rendon R, Jacobsen N, Fairey A, Izawa J, Kapoor A, Black P, Lacombe L, Chin J, So A, Lattouf J-B, Bell D, et al. Adjuvant chemotherapy for upper-tract urothelial carcinoma treated with nephroureterectomy: Assessment of adequate renal function and influence on outcome. Urol Oncol. 2014;32:31.e17–31.e24. doi: 10.1016/j.urolonc.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Walton TJ, Novara G, Matsumoto K, Kassouf W, Fritsche HM, Artibani W, Bastian PJ, Martinez-Salamanca JI, Seitz C, Thomas SA, Ficarra V, Burger M, Tritschler S, et al. Oncological outcomes after laparoscopic and open radical nephroureterectomy: results from an international cohort. BJU Int. 2011;108:406–412. doi: 10.1111/j.1464-410X.2010.09826.x. [DOI] [PubMed] [Google Scholar]
- 12.Godfrey MS, Badalato GM, Hruby GW, Razmjoo M, McKiernan JM. Prognostic indicators for upper tract urothelial carcinoma after radical nephroureterectomy: the impact of lymphovascular invasion. BJU Int. 2012;110:798–803. doi: 10.1111/j.1464-410X.2011.10893.x. [DOI] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Brit Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Brit Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA Statement for Reporting Systematic Reviews and. Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and ElaborationPRISMA: Explanation and Elaboration. Ann Intern Med. 2009;151:W–65. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 17.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Statistics Subcommittee of the NCIEWGoCD. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 18.Park S, Hong B, Kim CS, Ahn H. The impact of tumor location on prognosis of transitional cell carcinoma of the upper urinary tract. J Urol. 2004;171:621–625. doi: 10.1097/01.ju.0000107767.56680.f7. [DOI] [PubMed] [Google Scholar]
- 19.Chen WJ, Kuo JY, Chen KK, Lin AT, Chang YH, Chang LS. Primary urothelial carcinoma of the ureter: 11-year experience in Taipei Veterans General Hospital. J Chin Med Assoc. 2005;68:522–530. doi: 10.1016/S1726-4901(09)70087-5. [DOI] [PubMed] [Google Scholar]
- 20.Ataus S, Onal B, Tunc B, Erozenci A, Cekmen A, Kural AR, Oner A. Factors affecting the survival of patients treated by standard nephroureterectomy for transitional cell carcinoma of the upper urinary tract. Int Urol Nephrol. 2006;38:9–13. doi: 10.1007/s11255-005-3151-3. [DOI] [PubMed] [Google Scholar]
- 21.Chung SD, Huang KH, Lai MK, Huang CY, Chen CH, Pu YS, Yu HJ, Chueh SC. CKD as a risk factor for bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma. Am J Kidney Dis. 2007;50:743–753. doi: 10.1053/j.ajkd.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Koda S, Mita K, Shigeta M, Usui T. Risk factors for intravesical recurrence following urothelial carcinoma of the upper urinary tract: No relationship to the mode of surgery. Jpn J Clin Oncol. 2007;37:296–301. doi: 10.1093/jjco/hym016. [DOI] [PubMed] [Google Scholar]
- 23.Akao J, Matsuyama H, Yamamoto Y, Hara T, Kawai Y, Sakano S, Ohmi C, Gondo T, Naito K. Clinical significance of lymphovascular invasion in upper urinary tract urothelial cancer. BJU Int. 2008;102:572–575. doi: 10.1111/j.1464-410X.2008.07749.x. [DOI] [PubMed] [Google Scholar]
- 24.Berger A, Haber GP, Kamoi K, Aron M, Desai MM, Kaouk JH, Gill IS. Laparoscopic radical nephroureterectomy for upper tract transitional cell carcinoma: Oncological outcomes at 7 years. J Urol. 2008;180:849–854. doi: 10.1016/j.juro.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 25.Li CC, Chang TH, Wu WJ, Ke HL, Huang SP, Tsai PC, Chang SJ, Shen JT, Chou YH, Huang CH. Significant predictive factors for prognosis of primary upper urinary tract cancer after radical nephroureterectomy in Taiwanese patients. Eur Urol. 2008;54:1127–1134. doi: 10.1016/j.eururo.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 26.Soga N, Arima K, Sugimura Y. Adjuvant methotrexate, vinblastine, adriamycin, and cisplatin chemotherapy has potential to prevent recurrence of bladder tumors after surgical removal of upper urinary tract transitional cell carcinoma. Int J Urol. 2008;15:800–803. doi: 10.1111/j.1442-2042.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 27.Capitanio U, Shariat SF, Isbarn H, Weizer A, Remzi M, Roscigno M, Kikuchi E, Raman JD, Bolenz C, Bensalah K, Koppie TM, Kassouf W, Fernandez MI, et al. Comparison of oncologic outcomes for open and laparoscopic nephroureterectomy: a multi-institutional analysis of 1249 cases. Eur Urol. 2009;56:1–9. doi: 10.1016/j.eururo.2009.03.072. [DOI] [PubMed] [Google Scholar]
- 28.Chung SD, Wang SM, Lai MK, Huang CY, Liao CH, Huang KH, Pu YS, Chueh SC, Yu HJ. Lymphovascular invasion predicts poor outcome of urothelial carcinoma of renal pelvis after nephroureterectomy. BJU Int. 2009;103:1047–1051. doi: 10.1111/j.1464-410X.2008.08253.x. [DOI] [PubMed] [Google Scholar]
- 29.Kamihira O, Hattori R, Yamaguchi A, Kawa G, Ogawa O, Habuchi T, Kawauchi A, Uozumi J, Yokoi S, Tsujihata M, Hasui Y, Miyakoda K, Tada H, et al. Laparoscopic radical nephroureterectomy: a multicenter analysis in Japan. Eur Urol. 2009;55:1397–1407. doi: 10.1016/j.eururo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Favaretto RL, Shariat SF, Chade DC, Godoy G, Adamy A, Kaag M, Bochner BH, Coleman J, Dalbagni G. The Effect of Tumor Location on Prognosis in Patients Treated with Radical Nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur Urol. 2010;58:574–580. doi: 10.1016/j.eururo.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Favaretto RL, Shariat SF, Chade DC, Godoy G, Kaag M, Cronin AM, Bochner BH, Coleman J, Dalbagni G. Comparison Between Laparoscopic and Open Radical Nephroureterectomy in a Contemporary Group of Patients: Are Recurrence and Disease-Specific Survival Associated with Surgical Technique? Eur Urol. 2010;58:645–651. doi: 10.1016/j.eururo.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishikawa S, Abe T, Shinohara N, Harabayashi T, Sazawa A, Maruyama S, Kubota K, Matsuno Y, Osawa T, Shinno Y, Kumagai A, Togashi M, Matsuda H, et al. Impact of Diagnostic Ureteroscopy on Intravesical Recurrence and Survival in Patients With Urothelial Carcinoma of the Upper Urinary Tract. J Urol. 2010;184:883–887. doi: 10.1016/j.juro.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Kim DS, Lee YH, Cho KS, Cho NH, Chung BH, Hong SJ. Lymphovascular Invasion and pT Stage Are Prognostic Factors in Patients Treated with Radical Nephroureterectomy for Localized Upper Urinary Tract Transitional Cell Carcinoma. Urology. 2010;75:328–332. doi: 10.1016/j.urology.2009.07.1350. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi Y, Saika T, Manabe D, Nasu Y, Kumon H. Prognostic Factors Influencing Survival after Nephroureterectomy for Transitional Cell Carcinoma of the Upper Urinary Tract. Acta Med Okayama. 2010;64:27–31. doi: 10.18926/AMO/45274. [DOI] [PubMed] [Google Scholar]
- 35.Pieras E, Frontera G, Ruiz X, Vicens A, Ozonas M, Piza P. Concomitant carcinoma in situ and tumour size are prognostic factors for bladder recurrence after nephroureterectomy for upper tract transitional cell carcinoma. BJU Int. 2010;106:1319–1323. doi: 10.1111/j.1464-410X.2010.09341.x. [DOI] [PubMed] [Google Scholar]
- 36.Takaoka E, Hinotsu S, Joraku A, Oikawa T, Sekido N, Miyanaga N, Kawai K, Shimazui T, Akaza H. Pattern of intravesical recurrence after surgical treatment for urothelial cancer of the upper urinary tract: A single institutional retrospective long-term follow-up study. Int J Urol. 2010;17:623–628. doi: 10.1111/j.1442-2042.2010.02539.x. [DOI] [PubMed] [Google Scholar]
- 37.Cho DS, Hong SY, Kim YK, Kim SI, Kim SJ. Prognostic factors in transitional cell carcinoma of the upper urinary tract after radical nephroureterectomy. Korean J Urol. 2011;52:310–316. doi: 10.4111/kju.2011.52.5.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou C-P, Chang P-L, Chen C-L, Lin Y-H, Tsui K-H. Does adequate bladder cuff excision impact outcomes in patients undergoing nephroureterectomy for upper tract urothelial carcinoma. Chang Gung Med J. 2011;34:496–505. [PubMed] [Google Scholar]
- 39.Ku JH, Choi WS, Kwak C, Kim HH. Bladder cancer after nephroureterectomy in patients with urothelial carcinoma of the upper urinary tract. Urol Oncol. 2011;29:383–387. doi: 10.1016/j.urolonc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Ariane MM, Colin P, Ouzzane A, Pignot G, Audouin M, Cornu JN, Albouy B, Guillotreau J, Neuzillet Y, Crouzet S, Hurel S, Arroua F, Bigot P, et al. Assessment of Oncologic Control Obtained After Open Versus Laparoscopic Nephroureterectomy for Upper Urinary Tract Urothelial Carcinomas (UUT-UCs): Results from a Large French Multicenter Collaborative Study. Ann Surg Oncol. 2012;19:301–308. doi: 10.1245/s10434-011-1841-x. [DOI] [PubMed] [Google Scholar]
- 41.Chen MK, Ye YL, Zhou FJ, Liu JY, Lu KS, Han H, Liu ZW, Xu ZZ, Qin ZK. Clipping the extremity of ureter prior to nephroureterectomy is effective in preventing subsequent bladder recurrence after upper urinary tract urothelial carcinoma. Chinese Med J. 2012;125:3821–3826. [PubMed] [Google Scholar]
- 42.Chromecki TF, Cha EK, Fajkovic H, Margulis V, Novara G, Scherr DS, Lotan Y, Raman JD, Kassouf W, Bensalah K, Weizer A, Kikuchi E, Roscigno M, et al. The Impact of Tumor Multifocality on Outcomes in Patients Treated With Radical Nephroureterectomy. Eur Urol. 2012;61:245–253. doi: 10.1016/j.eururo.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 43.Hirano D, Okada Y, Nagane Y, Satoh K, Mochida J, Yamanaka Y, Hirakata H, Yamaguchi K, Kawata N, Takahashi S, Henmi A. Intravesical Recurrence after Surgical Management of Urothelial Carcinoma of the Upper Urinary Tract. Urol Int. 2012;89:71–77. doi: 10.1159/000338644. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi Y, Saika T, Miyaji Y, Saegusa M, Arata R, Akebi N, Takenaka T, Manabe D, Nasu Y, Kumon H. Preoperative positive urine cytology is a risk factor for subsequent development of bladder cancer after nephroureterectomy in patients with upper urinary tract urothelial carcinoma. World J Urol. 2012;30:271–275. doi: 10.1007/s00345-011-0731-y. [DOI] [PubMed] [Google Scholar]
- 45.Kuroda K, Asakuma J, Horiguchi A, Tasaki S, Yoshii H, Sato A, Ito K, Seguchi K, Sumitomo M, Asano T. Prognostic Factors for Upper Urinary Tract Urothelial Carcinoma after Nephroureterectomy. Urol Int. 2012;88:225–231. doi: 10.1159/000335274. [DOI] [PubMed] [Google Scholar]
- 46.Liang PI, Li WM, Wang YH, Wu TF, Wu WR, Liao AC, Shen KH, Wei YC, Hsing CH, Shiue YL, Huang HY, Hsu HP, Chen LT, et al. HuR cytoplasmic expression is associated with increased cyclin A expression and poor outcome with upper urinary tract urothelial carcinoma. BMC Cancer. 2012. p. 12. [DOI] [PMC free article] [PubMed]
- 47.Cho DS, Kim SI, Ahn HS, Kim SJ. Predictive Factors for Bladder Recurrence after Radical Nephroureterectomy for Upper Urinary Tract Urothelial Carcinoma. Urol Int. 2013;91:153–159. doi: 10.1159/000346086. [DOI] [PubMed] [Google Scholar]
- 48.Ehdaie B, Furberg H, Zabor EC, Ostroff JS, Shariat SF, Bochner BH, Coleman JA, Dalbagni G. Impact of smoking status at diagnosis on disease recurrence and death in upper tract urothelial carcinoma. BJU Int. 2013;111:589–595. doi: 10.1111/j.1464-410X.2012.11260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elalouf V, Xylinas E, Klap J, Pignot G, Delongchamps NB, Saighi D, Peyromaure M, Flam T, Zerbib M. Bladder recurrence after radical nephroureterectomy: Predictors and impact on oncological outcomes. Int J Urol. 2013;20:1078–1083. doi: 10.1111/iju.12121. [DOI] [PubMed] [Google Scholar]
- 50.Fairey AS, Kassouf W, Estey E, Tanguay S, Rendon R, Bell D, Izawa J, Chin J, Kapoor A, Matsumoto E, Black P, So A, Lattouf JB, et al. Comparison of oncological outcomes for open and laparoscopic radical nephroureterectomy: results from the Canadian Upper Tract Collaboration. BJU Int. 2013;112:791–797. doi: 10.1111/j.1464-410X.2012.11474.x. [DOI] [PubMed] [Google Scholar]
- 51.Fujita K, Tanigawa G, Imamura R, Nakagawa M, Hayashi T, Kishimoto N, Hosomi M, Yamaguchi S. Preoperative serum sodium is associated with cancer-specific survival in patients with upper urinary tract urothelial carcinoma treated by nephroureterectomy. Int J Urol. 2013;20:594–601. doi: 10.1111/j.1442-2042.2012.03228.x. [DOI] [PubMed] [Google Scholar]
- 52.Gunay LM, Akdogan B, Koni A, Inci K, Bilen CY, Ozen H. Upper urinary tract transitional cell carcinoma: is there a best? Clin Genitourin Cancer. 2013;11:39–44. doi: 10.1016/j.clgc.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Hashimoto T, Ohno Y, Nakashima J, Gondo T, Ohori M, Tachibana M. Clinical significance of preoperative peripheral blood neutrophil count in patients with non-metastatic upper urinary tract carcinoma. World J Urol. 2013;31:953–958. doi: 10.1007/s00345-012-0942-x. [DOI] [PubMed] [Google Scholar]
- 54.Kim KH, You D, Jeong IG, Hong JH, Ahn H, Kim CS. Muscle-invasive bladder cancer developing after nephroureterectomy for upper urinary tract urothelial carcinoma. Urol Oncol. 2013;31:1643–1649. doi: 10.1016/j.urolonc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 55.Kim TS, Oh JH, Rhew HY. The Efficacy of Adjuvant Chemotherapy for Locally Advanced Upper Tract Urothelial Cell Carcinoma. J Cancer. 2013;4:686–690. doi: 10.7150/jca.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kusuda Y, Miyake H, Terakawa T, Kondo Y, Miura T, Fujisawa M. Gender as a significant predictor of intravesical recurrence in patients with urothelial carcinoma of the upper urinary tract following nephroureterectomy. Urol Oncol. 2013;31:899–903. doi: 10.1016/j.urolonc.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 57.Milojevic B, Djokic M, Sipetic-Grujicic S, I Grozdic Milojevic, Vuksanovic A, Nikic P, Vukovic I, Djordjevic D, Bumbasirevic U, Tulic C. Prognostic significance of non-muscle-invasive bladder tumor history in patients with upper urinary tract urothelial carcinoma. Urol Oncol. 2013;31:1615–1620. doi: 10.1016/j.urolonc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Morizane S, Iwamoto H, Masago T, Yao A, Isoyama T, Sejima T, Takenaka A. Preoperative prognostic factors after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Int Urol Nephrol. 2013;45:99–106. doi: 10.1007/s11255-012-0347-1. [DOI] [PubMed] [Google Scholar]
- 59.Rink M, Xylinas E, Margulis V, Cha EK, Ehdaie B, Raman JD, Chun FK, Matsumoto K, Lotan Y, Furberg H, Babjuk M, Pycha A, Wood CG, et al. Impact of smoking on oncologic outcomes of upper tract urothelial carcinoma after radical nephroureterectomy. Eur Urol. 2013;63:1082–1090. doi: 10.1016/j.eururo.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakano S, Matsuyama H, Kamiryo Y, Hayashida S, Yamamoto N, Kaneda Y, Nasu T, Hashimoto O, Joko K, Baba Y, Shimabukuro T, Suga A, Yamamoto M, et al. Risk Group Stratification Based on Preoperative Factors to Predict Survival after Nephroureterectomy in Patients with Upper Urinary Tract Urothelial Carcinoma. Ann Surg Oncol. 2013;20:4389–4396. doi: 10.1245/s10434-013-3259-0. [DOI] [PubMed] [Google Scholar]
- 61.Shimamoto T, Inoue K, Kamata M, Kuno T, Karashima T, Shuin T. Pathological risk factors in upper urinary tract cancer. Asia Pac J Clin Oncol. 2013. [DOI] [PubMed]
- 62.Takahara K, Inamoto T, Komura K, Watsuji T, Azuma H. Post-operative urothelial recurrence in patients with upper urinary tract urothelial carcinoma managed by radical nephroureterectomy with an ipsilateral bladder cuff: Minimal prognostic impact in comparison with non-urothelial recurrence and other clinical indicators. Oncol Lett. 2013;6:1015–1020. doi: 10.3892/ol.2013.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xylinas E, Colin P, Audenet F, Phe V, Cormier L, Cussenot O, Houlgatte A, Karsenty G, Bruyere F, Polguer T, Ruffion A, Valeri A, Rozet F, et al. Intravesical recurrence after radical nephroureterectomy for upper tract urothelial carcinomas: predictors and impact on subsequent oncological outcomes from a national multicenter study. World J Urol. 2013;31:61–68. doi: 10.1007/s00345-012-0957-3. [DOI] [PubMed] [Google Scholar]
- 64.Zhang XH, Zhu ZW, Zhong S, Xu TY, Shen ZJ. Ureteral tumours showing a worse prognosis than renal pelvis tumours may be attributed to ureteral tumours more likely to have hydronephrosis and less likely to have haematuria. World J Urol. 2013;31:155–160. doi: 10.1007/s00345-012-0885-2. [DOI] [PubMed] [Google Scholar]
- 65.Aziz A, Rink M, Gakis G, Kluth LA, Dechet C, Miller F, Otto W, Gierth M, Denzinger S, Schwentner C, Stenzl A, Fisch M, Burger M, et al. Preoperative C-Reactive Protein in the Serum: A Prognostic Biomarker for Upper Urinary Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy. Urol Int. 2014;93:352–360. doi: 10.1159/000362248. [DOI] [PubMed] [Google Scholar]
- 66.Bachir BG, Aprikian AG, Izawa JI, Chin JL, Fradet Y, Fairey A, Estey E, Jacobsen N, Rendon R, Cagiannos I, Lacombe L, Lattouf JB, Kapoor A, et al. Effect of body mass index on the outcomes of patients with upper and lower urinary tract cancers treated by radical surgery: Results from a Canadian multicenter collaboration. Urol Oncol. 2014;32:441–448. doi: 10.1016/j.urolonc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 67.Cho YH, Seo YH, Chung SJ, Hwang I, Yu HS, Kim SO, Jung SI, Kang TW, Kwon DD, Park K, Hwang JE, Heo SH, Kim GS, et al. Predictors of intravesical recurrence after radical nephroureterectomy for upper urinary tract urothelial carcinoma: an inflammation-based prognostic score. Korean J Urol. 2014;55:453–459. doi: 10.4111/kju.2014.55.7.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choo MS, Jeong CW, Kwak C, Kim HH, Ku JH. Effect of sex on prognosis of urothelial carcinoma: propensity score matching analysis. Clin Genitourin Cancer. 2015;13:e113–121. doi: 10.1016/j.clgc.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Ehdaie B, Shariat SF, Savage C, Coleman J, Dalbagni G. Postoperative Nomogram for Disease Recurrence and Cancer-Specific Death for Upper Tract Urothelial Carcinoma: Comparison to American Joint Committee on Cancer Staging Classification. Urol J. 2014;11:1435–1441. [PubMed] [Google Scholar]
- 70.Fang D, Xiong GY, Li XS, Chen XP, Zhang L, Yao L, He ZS, Zhou LQ. Pattern and risk factors of intravesical recurrence after nephroureterectomy for upper tract urothelial carcinoma: a large Chinese center experience. J Formos Med Assoc. 2014;113:820–827. doi: 10.1016/j.jfma.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Fradet V, Mauermann J, Kassouf W, Rendon R, Jacobsen N, Fairey A, Izawa J, Kapoor A, Black P, Tanguay S, Chin J, So A, Lattouf JB, et al. Risk factors for bladder cancer recurrence after nephroureterectomy for upper tract urothelial tumors: Results from the Canadian Upper Tract Collaboration. Urol Oncol. 2014;32:839–845. doi: 10.1016/j.urolonc.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 72.Fujita K, Uemura M, Yamamoto Y, Tanigawa G, Nakata W, Sato M, Nagahara A, Kiuchi H, Nakai Y, Matsumiya K, Yamaguchi S, Nonomura N. Preoperative risk stratification for cancer-specific survival of patients with upper urinary tract urothelial carcinoma treated by nephroureterectomy. Int J Clin Oncol. 2015;20:156–163. doi: 10.1007/s10147-014-0695-1. [DOI] [PubMed] [Google Scholar]
- 73.Gandaglia G, Bianchi M, Trinh QD, Becker A, Larouche A, Abdollah F, Roghmann F, Tian Z, Shariat SF, Briganti A, Montorsi F, Karakiewicz PI, Sun M. Survival after nephroureterectomy for upper tract urothelial carcinoma: a population-based competing-risks analysis. Int J Urol. 2014;21:249–256. doi: 10.1111/iju.12267. [DOI] [PubMed] [Google Scholar]
- 74.Ichimura T, Morikawa T, Kawai T, Nakagawa T, Matsushita H, Kakimi K, Kume H, Ishikawa S, Homma Y, Fukayama M. Prognostic significance of CD204-positive macrophages in upper urinary tract cancer. Ann Surg Oncol. 2014;21:2105–2112. doi: 10.1245/s10434-014-3503-2. [DOI] [PubMed] [Google Scholar]
- 75.Ishioka J, Masuda H, Kijima T, Tatokoro M, Yoshida S, Yokoyama M, Matsuoka Y, Numao N, Koga F, Saito K, Fujii Y, Sakai Y, Arisawa C, et al. Bimodal pattern of the impact of body mass index on cancer-specific survival of upper urinary tract urothelial carcinoma patients. Anticancer Res. 2014;34:5683–5688. [PubMed] [Google Scholar]
- 76.Ishioka J, Saito K, Kijima T, Nakanishi Y, Yoshida S, Yokoyama M, Matsuoka Y, Numao N, Koga F, Masuda H, Fujii Y, Sakai Y, Arisawa C, et al. Risk stratification for bladder recurrence of upper urinary tract urothelial carcinoma after radical nephroureterectomy. BJU Int. 2015;115:705–712. doi: 10.1111/bju.12707. [DOI] [PubMed] [Google Scholar]
- 77.Ito K, Kuroda K, Asakuma J, Hamada S, Tachi K, Tasaki S, Sato A, Horiguchi A, Seguchi K, Asano T. Preoperative risk factors for extraurothelial recurrence in patients with ureteral cancer treated with radical nephroureterectomy. J Urol. 2014;191:1685–1692. doi: 10.1016/j.juro.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 78.Kitamura H, Maeda T, Tanaka T, Fukuta F, Kobayashi K, Nishiyama N, Takahashi S, Masumori N. Comparison of Laparoscopic, Hand-Assisted, and Open Surgical Nephroureterectomy. JSLS. 2014;18:288–293. doi: 10.4293/108680813X13794522666842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kondo T, Hara I, Takagi T, Kodama Y, Hashimoto Y, Kobayashi H, Iizuka J, Omae K, Ikezawa E, Yoshida K, Tanabe K. Possible role of template-based lymphadenectomy in reducing the risk of regional node recurrence after nephroureterectomy in patients with renal pelvic cancer. Jpn J Clin Oncol. 2014;44:1233–1238. doi: 10.1093/jjco/hyu151. [DOI] [PubMed] [Google Scholar]
- 80.Lee JN, Kwon SY, Choi GS, Kim HT, Kim TH, Kwon TG, Kim BW. Impact of surgical wait time on oncologic outcomes in upper urinary tract urothelial carcinoma. J Surg Oncol. 2014;110:468–475. doi: 10.1002/jso.23589. [DOI] [PubMed] [Google Scholar]
- 81.Liu JY, Li YH, Liu ZW, Zhang ZL, Ye YL, Yao K, Jiang LJ, Han H, Qin ZK, Zhou FJ. Influence of body mass index on oncological outcomes in patients with upper urinary tract urothelial carcinoma treated with radical nephroureterectomy. Int J Urol. 2014;21:136–142. doi: 10.1111/iju.12208. [DOI] [PubMed] [Google Scholar]
- 82.Liu YQ, Lu J, Hong K, Huang Y, Ma LL. Independent prognostic factors for initial intravesical recurrence after laparoscopic nephroureterectomy for upper urinary tract urothelial carcinoma. Urol Oncol. 2014;32:146–152. doi: 10.1016/j.urolonc.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 83.Ou CH, Yang WH. Impact of Earlier Ureteral Ligation on Intravesical Recurrence during Hand-Assisted Retroperitoneoscopic Nephroureterectomy. Urol Int. 2014;92:68–73. doi: 10.1159/000351744. [DOI] [PubMed] [Google Scholar]
- 84.Park J, Park S, Song C, Hong J, Kim CS, Ahn H. Peripelvic/periureteral fat invasion is independently associated with worse prognosis in pT3 upper tract urothelial carcinoma. World J Urol. 2014;32:157–163. doi: 10.1007/s00345-013-1073-8. [DOI] [PubMed] [Google Scholar]
- 85.Sasaki Y, Sasaki T, Kawai T, Morikawa T, Matsusaka K, Kunita A, Kume H, Aoki I, Homma Y, Fukayama M. HER2 protein overexpression and gene amplification in upper urinary tract urothelial carcinoma-an analysis of 171 patients. Int J Clin Exp Patho. 2014;7:699–708. [PMC free article] [PubMed] [Google Scholar]
- 86.Shirotake S, Kikuchi E, Tanaka N, Matsumoto K, Miyazaki Y, Kobayashi H, Ide H, Obata J, Hoshino K, Kaneko G, Hagiwara M, Kosaka T, Kanao K, et al. Impact of an Adjuvant Chemotherapeutic Regimen on the Clinical Outcome in High Risk Patients with Upper Tract Urothelial Carcinoma: A Japanese Multi-Institution Experience. J Urol. 2015;193:1122–1128. doi: 10.1016/j.juro.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 87.Sung HH, Jeon HG, Jeong BC, Seo SI, Jeon SS, Choi HY, Lee HM. Clinical significance of prognosis using the neutrophil-lymphocyte ratio and erythrocyte sedimentation rate in patients undergoing radical nephroureterectomy for upper urinary tract urothelial carcinoma. BJU Int. 2015;115:587–594. doi: 10.1111/bju.12846. [DOI] [PubMed] [Google Scholar]
- 88.Sung HH, Cho JH, Kwon GY, Jeon HG, Jeong BC, S Il Seo, Jeon SS, Choi HY, Lee HM. Clinical significance of micropapillary urothelial carcinoma of the upper urinary tract. J Clin Pathol. 2014;67:49–54. doi: 10.1136/jclinpath-2013-201799. [DOI] [PubMed] [Google Scholar]
- 89.Tanaka N, Kikuchi E, Kanao K, Matsumoto K, Shirotake S, Kobayashi H, Miyazaki Y, Ide H, Obata J, Hoshino K, Hayakawa N, Kosaka T, Oyama M, et al. The predictive value of positive urine cytology for outcomes following radical nephroureterectomy in patients with primary upper tract urothelial carcinoma: A multi-institutional study. Urol Oncol. 2014. p. 32. [DOI] [PubMed]
- 90.Xylinas E, Rink M, Cha EK, Clozel T, Lee RK, Fajkovic H, Comploj E, Novara G, Margulis V, Raman JD, Lotan Y, Kassouf W, Fritsche HM, et al. Impact of Distal Ureter Management on Oncologic Outcomes Following Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma. Eur Urol. 2014;65:210–217. doi: 10.1016/j.eururo.2012.04.052. [DOI] [PubMed] [Google Scholar]
- 91.Xylinas E, Kluth LA, Rieken M, Lee RK, Elghouayel M, Ficarra V, Margulis V, Lotan Y, Roupret M, Martinez-Salamanca JI, Matsumoto K, Seitz C, Karakiewicz PI, et al. Impact of smoking status and cumulative exposure on intravesical recurrence of upper tract urothelial carcinoma after radical nephroureterectomy. BJU Int. 2014;114:56–61. doi: 10.1111/bju.12400. [DOI] [PubMed] [Google Scholar]
- 92.Zou LJ, Zhang LM, Zhang H, Jiang HW, Ding Q. Comparison of post-operative intravesical recurrence and oncological outcomes after open versus laparoscopic nephroureterectomy for upper urinary tract urothelial carcinoma. World J Urol. 2014;32:565–570. doi: 10.1007/s00345-013-1160-x. [DOI] [PubMed] [Google Scholar]
- 93.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007. p. 8. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


