Abstract
Background
Obesity in pregnancy (MO) is a risk factor for maternal and/or fetal cardiovascular system disorders. This study evaluated maternal CVS expression of Micro-RNA-29 family and its target molecules in MO to test the hypotheses: CVS miR-29 concentrations are increased in pregnancy and decreased in MO.
Methods
Non-pregnant (n=4), pregnant obese (POb, n=4), and pregnant non-obese (PnOb, n=4) baboons (Papio spp.) were studied. Maternal left ventricle (LV), left atrium (LA), and aortic arch (AA) were collected at the end of gestation. Expression of MiR-29 and elastin (ELN) mRNA were quantified.
Results
LA miR-29 (a, c) expression was highest in PnOb. In the LV, miR-29b expression trended lower (p=0.059) for PnOb animals. ELN mRNA expression correlated positively with miR-29b expression in AA (r=0.76, p=0.03).
Conclusion
Maternal obesity diminishes miR-29 adaptation to pregnancy. Pharmacologic, tissue-specific targeting of miRNA-29 may represent a strategy for prevention and treatment of MO complications.
Keywords: Maternal obesity, non-human primate, vascular remodeling
INTRODUCTION
Recognized as a major health problem [8], maternal obesity is associated with an increased risk of cardiovascular system (CVS) complications in mothers and their offspring [5, 50, 56] through poorly understood mechanisms. Maternal CVS adaptations are critical for a successful pregnancy [6, 13, 44] and include local [10, 11] and systemic remodeling [2, 42]. Pathological remodeling is associated with CVS disorders [10] and fetal growth restriction (FGR) [63]. A central process of remodeling is extracellular matrix (ECM) reorganization [38], which includes changes in key molecules, determining CVS compliance-elastin and collagen [60]. Obesity, accompanied by arterial stiffness [24], cardiac fibrosis [36, 65] and consequent diminished diastolic function [31, 32], might interfere with normal pregnancy-related CVS remodeling,
MicroRNAs (miRNA) are small non-coding molecules (19–25 nucleotides long) that regulate biological processes by silencing target mRNAs [29] to repress translation of specific genes. The miR-29 microRNA family regulates at least 16 ECM genes and proteins involved in cardiovascular remodeling [4, 33, 57, 62], especially collagen (colA1, col 3A1), fibrillin, and elastin [39]. The regulatory elements for miR-29 are overrepresented in elastin and type 1 collagen genes [46]. miR-29 has been proposed as a marker for cardiac hypertrophy, fibrosis [15, 52] and atrial fibrillation [15]. MiR-29 is also involved in pregnancy regulation [30, 40]. Down-regulation of miR-29 in the ruminant model of FGR is associated with increased arterial stiffness [30] and up-regulation of this molecule is linked to the deceleration of postnatal body growth [26]. The central position of the miR-29 family in both CVS structural changes and in adaptations to pregnancy makes this microRNA family an ideal target and marker for adequate pregnancy-driven remodeling. The goal of this study was to evaluate maternal cardiovascular miR-29 family expression in obese and non-obese, pregnant and non-pregnant baboons. Based on data showing that pregnancy is associated with the left ventricular hypertrophy, that under physiologic conditions cardiac hypertrophy parallels an increase in miR-29 expression [57] and overfeeding-induced obesity decreases the concentrations of this molecule in the animal model [27], we hypothesized that cardiac miR-29 expression would be increased in pregnant animals and that maternal obesity would reduce these changes.
MATERIAL AND METHODS
Humane Care Guidelines
Colony characteristics and procedures have been described in detail elsewhere [55]. Briefly, pregnant baboons were housed in harem cages, typically housing one male and 10-15 females, in AALAC approved facilities at the Southwest National Primate Research Center at the Texas Biomedical Research Institute as described previously [19]. The harem cages were part of the complex with a covered area of 4988 sq. ft., of which 3200 sq. ft. is divided into eight 400-sq. ft. cages [20]. The Animals were fed LEO5 monkey diet with the composition identical to Purina 5038 (LEO 5, Purina, St. Louis, MO, USA) and given water ad libitum [43], environmental enrichment was provided in the form of toys, mirrors, and pools [53]. Subjects were divided into two groups, obese (POb, n=4) and non-obese (PnOb, n=4), based on the Rh index, which is an obesity index defined as body weight divided by the square of the crown-rump length [19]. Tissues from four non-pregnant, reproductive age animals were collected during routine pathological examinations at the department of pathology at the Southwest National Primate Center (Table 1). All procedures were approved by the Animal Care and Use Committee of the Texas Biomedical Research Institute.
Table 1. Demographic characteristics and cardiac morphometry of study baboons (mean ± SEM).
| Animal characteristics | Non-pregnant n=4 |
Pregnant | |
|---|---|---|---|
| non-obese n=4 |
Obese n=4 |
||
| Age (years) | 11.75 ± 1.1 | 10.5 ± 1.2 | 11 ± 1.6 |
| Parity | 3.5 ± 0.7 | 4 ± 0.7 | 3.5 ± 0.9 |
| Weight (kg)* | 14.4 ± 1.5 | 15.2 ± 0.7 | 16.7 ± 1.1 |
| Rh Index (kg/m2)* | NA | 39.1 ± 3.2A | 48.7 ± 1 |
| Heart weight (g)* | 52.48 ± 4.1 | 61.7 ± 2.09 | 57.1 ± 7.45 |
| Pericardial adipose tissue (g) | NA | 14.1 ± 2.4 | 29 ± 8.4 |
Tissue collection and processing
Cesarean sections were performed near term (92% of gestational length) with tissue collections as detailed by Farley et al. [19]. Animals were premedicated with ketamine hydrochloride (10 mg/kg) as described in (34). After intubation, isoflurane (2%, 2 l/min) was administered to maintain a surgical plane of anesthesia throughout surgery and fetal sampling [20, 54]. The euthanasia was performed according to the guidelines of the American Veterinary Medical Association (AVMA) Panel on Euthanasia [1] with exsanguination under general anesthesia. Samples of the maternal heart left ventricle (LV), left atrium (LA), and aortic arch (AA) were collected and divided into two parts in POb and PnOb animals. One part was flash-frozen in liquid nitrogen and stored at −80°C until evaluation. The second part was fixed in 10% neutral buffered formalin, embedded in paraffin, cut at 5 μm and processed for immunohistochemistry and morphometry as described below. NP baboon tissues were frozen and stored at −80°C until evaluation.
RT-PCR
Total RNA was extracted from 200 mg tissue from LA, LV, and AA using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), following the manufacturer’s instructions [9]. cDNA synthesis for elastin quantification was performed using a first-strand cDNA synthesis kit (Roche Diagnostics GmbH, Mannheim, Germany) from 1-5 μg of total RNA; amount of total RNA was the same within the pair transcript-gene of interest. MiRNA quantification was performed from 2.5 μg of total RNA using the NCode™ miRNA First-Strand cDNA Synthesis Kit (Life technologies, cat #45-6612) with GoTaq® qPCR Master Mix (Promega A6002, Madison, USA) [18, 45]. qPCR with 10 ng/reaction was performed on the CFX96™ Real-Time PCR Detection System using SYBR Green supermix (Kapa Bio systems Inc. Woburn, MA, USA). Quantitative PCR was performed in duplicates with sequence-specific oligonucleotides, that were custom-synthesized [59] (Thermo Fisher Scientific Inc, Waltham, MA, USA and Invitrogen, Carlsbad, CA, USA) and also commercially available (Roche, Applied sciences, Indianapolis, IN, USA) (Table 2) [16]. Data were collected on a LightCycler® 480 (Roche, Applied Sciences, Indianapolis, IN, USA) for ELN expression. Gene expressions were compared to suitable reference genes (i.e., β-actin, s-19, and UC6) and normalized using the 2−ΔΔCT method.
Table 2. Primers used in this study.
| Transcripts | Left (l) and Right (R) Primers | Reference |
|---|---|---|
| ELASTIN | L 5’-GGCCATTCCTGGTGGAGTTCC—3’ R5’-AACTGGCTTAAGAGGTTTGCCTCCA—3’ |
[16] |
| S-19 | L 5’GCTTGCTCCCTACGATGAGA-3’ R 5’- ACCCCGGAGGTACAGGTG-3’ |
Roche Applied Biosystems, Indianapolis, IN, USA |
| Β-ACTIN | L 5’-CCAACCGCGAGAAGATGA-3’ R 5’-CCAGAGGCGTACAGGGATAG-3’ |
[9] |
| MiR-29A | TAGCACCATCTGAAATCGGTTA | Invitrogen, Carlsbad, CA, USA |
| MiR-29B | TAGCACCATTTGAAATCAGTGTT | Invitrogen, Carlsbad, CA, USA |
| MiR-29C | TAGCACCATTTGAAATCGGTTA | Invitrogen, Carlsbad, CA, USA |
| U6 | L 5’-CTCGCTTCGGCAGCACA-3’ R 5’-ACGCTTCACGAATTTGCGT -3’ |
Invitrogen, Carlsbad, CA, USA |
Histomorphometry
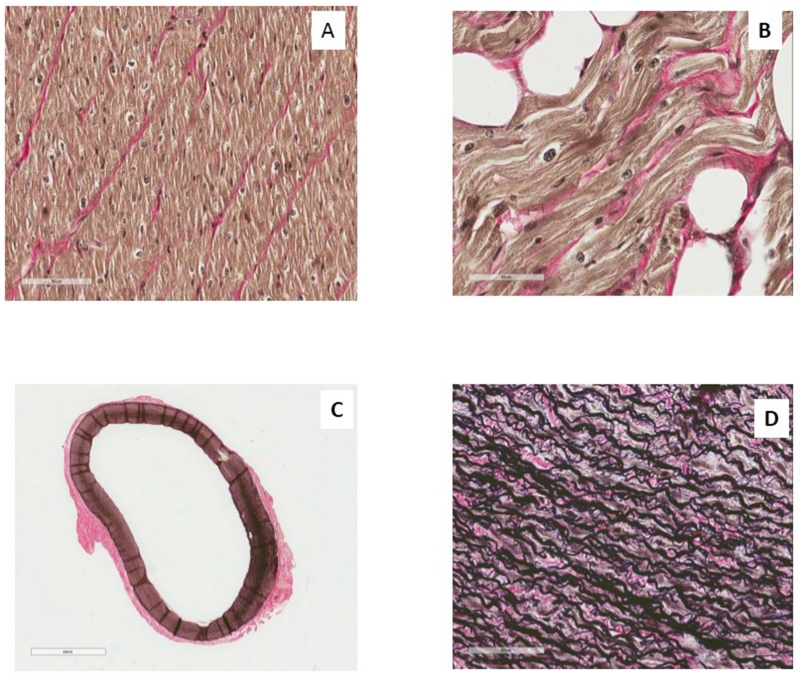
Elastin fibers were demonstrated using the Verhoeff-Van Gieson Elastic Stain Kit (Sigma Aldrich, LLC, USA) according to the manufacturer’s instructions. Slides where deparaffinized and hydrated with distilled water followed by the addition of Verhoeff working solution. After rinsing in distillated and tap water, each slide was differentiated individually in 2% ferric chloride with agitation, washed in tap water, then placed in Sodium Thiosulfate 5% Aqueous and counterstained with Van Gieson stain solution. Finally, the slides were dehydrated in 95% alcohol. This process resulted in elastic fibers and nuclei being blue/black to black and collagen being a red color (Fig.1).
Fig. 1.
Slides were scanned using an Aperio ScanScope® instrument at 40× magnificatoin. Image analysis with ImageScope™ v11.1.2.752 by Aperio® available positive pixel count algorithm quantified elastin content in LV and LA, using the histoscore calculation method as described in detail elsewhere (Fig. 1A) [9]. Minimal thickness of the aortic ring (Fig. 1C), number of elastic fibers (Fig. 1D) [58] and percentage of collagen fibers in LA and LV were evaluated as described previously [22].
Statistical methods
Kruskal-Wallis, non-parametric, one-way analysis compared three groups: obese, and non-obese pregnant groups, and the non-pregnant group. The Wilcoxon test was used for the analysis of obese and non-obese baboon groups and Wilcoxon Signed-rank test for differences between LA and LV for their collagen and elastin contents, aortic thickness and number of fibers. The association between two continuous variables was assessed by Spearman’s rank correlation. Data are presented as mean ± SE, unless indicated otherwise. Significance was set at 5%. Reported p values were not adjusted for multiplicity.
RESULTS
Maternal morphometry
The weight of maternal heart tended to be higher (p=0.08) in PnOB animals (61.68 ± 2.1g) compared to NP (52.48 ± 4.1g). The weight of maternal heart in POB animals (57.1± 7.5g ) was also higher than in NP, but less than PnOB animals. The weight of the pericardial adipose tissue was higher in POB than PnOB baboons, but did not reach significance (Table 1).
miR-29 expression
In the LA, combined expression of miR-29 (a, b, and c) was highest in PnOb baboons, while only miR-29a and miR-29c expression reached significance. (Table 3B). In the LV, combined expression of miR-29 (a, b, and c) was lowest in PnOb baboons compared to NP and POb non-pregnant baboons (p=0.056) (Table 3).
Table 3.
The miR29 expression in the heart of the pregnant obese (POb, n=3-4), pregnant non-obese (nOb, n=3-4) baboons at term and in the non–pregnant (NP, n=3-4) baboons (Papio spp.).
| Min | Median | Max | p-value | ||
|---|---|---|---|---|---|
| A. Aortic Arch | |||||
| miR-29a | NP | 0.71 | 1.25 | 2.67 | 0.11 |
| P-nOB | 2.01 | 4.01 | 6.54 | ||
| P-Ob | 1.70 | 2.23 | 10.15 | ||
| miR-29b | NP | 0.003 | 0.57 | 2.06 | 0.22 |
| P-nOB | 0.82 | 2.17 | 3.54 | ||
| P-Ob | 1.06 | 1.22 | 2.93 | ||
| miR-29c | NP | 0.05 | 1.14 | 2.34 | 0.33 |
| P-nOB | 1.15 | 3.09 | 4.83 | ||
| P-Ob | 1.10 | 1.43 | 6.40 | ||
| B. Left Atrium | |||||
| miR-29a | NP | 0.53 | 1.31 | 1.91 | 0.024 |
| P-nOB | 14.84 | 27.72 | 46.65 | ||
| P-Ob | 0.70 | 8.38 | 10.76 | ||
| miR-29b | NP | 0.37 | 1.54 | 3.85 | 0.080 |
| P-nOB | 6.29 | 6.84 | 16.94 | ||
| P-Ob | 1.39 | 1.57 | 11.53 | ||
| miR-29c | NP | 0.51 | 1.25 | 1.45 | 0.027 |
| P-nOB | 16.66 | 23.69 | 44.49 | ||
| P-Ob | 0.44 | 6.95 | 10.01 | ||
| C. Left Ventricle | |||||
| miR-29a | NP | 7.36 | 21.45 | 207.95 | 0.25 |
| P-nOB | 0.10 | 2.25 | 33.64 | ||
| P-Ob | 0.37 | 6.54 | 176.70 | ||
| miR-29b | NP | 8.29 | 10.09 | 62.06 | 0.059 |
| P-nOB | 0.51 | 1.92 | 6.32 | ||
| P-Ob | 0.86 | 3.81 | 27.87 | ||
| miR-29c | NP | 7.83 | 25.87 | 244.79 | 0.17 |
| P-nOB | 0.06 | 1.43 | 28.54 | ||
| P-Ob | 0.21 | 5.91 | 146.25 | ||
Elastin and collagen expression
ELN mRNA expression correlated positively with miR-29b expression in the AA (r=0.76, P =0 .03), but not in the LV and LA. There were no differences in elastin protein expression, collagen content, aortic thickness, or number of elastic fibers between the obese and non-obese animals (Table 4). In both groups of pregnant (PnOB and POB) animals elastin protein expression and collagen content differed between the left atrium and left ventricle (p=0.13), but did not reach significance (Table 4).
Table 4.
The histoscore of elastin stain and percentage of collagen in the left ventricle and left atrium; morphometry of aortic ring (data presented as mean ±SEM).
| Non-Obese | Obese | |
|---|---|---|
| Left Atrium | ||
| Elastin | 186.7 ± 4.7 | 181.9 ± 7.5 |
| Collagen (%) | 4.5 ± 0.01 | 8.5 ± 0.05 |
| Left Ventricle | ||
| Elastin | 214.5 ± 10.3 | 220.6 ± 12.7 |
| Collagen (%) | 2.1 ± 0.003 | 3.4 ± 0.01 |
| Aortic arch | ||
| Minimal thickness of aortic ring (μm) | 872.9 ± 108.1 | 809.4 ± 142.3 |
| Number of elastic fibers | 60.25 ± 14.0 | 54.0 ± 11.3 |
DISCUSSION
Structural and functional cardiac remodeling in obesity [3] and pregnancy [42] is well documented. Both conditions are associated with left ventricular hypertrophy and left atrial enlargement as a result of adaptation to endocrine and metabolic changes, and expansion of blood volume and inflammation that occur in pregnancy. In our study, only pregnancy, but not obesity in pregnancy, was paralleled by an increase in cardiac weight. This finding can possibly be explained by the diminished pregnancy driven heart remodeling in obesity. The mechanism could involve paracrine regulation of the myocardial growth by the cardiac fat [9, 12, 21, 28]. Epicardial, perivascular and intracardial fat are not separated from the underlying tissues by fascia, therefore the adipocytokines from adipose tissue have direct access to the cardiomyocytes [35, 47] (Fig 1B).
Atrial and ventricular changes
Obesity, in general, is a profibrotic state [31] with such common complications as atrial fibrillation (AF) [64]. AF is associated with pathological atrial remodeling including perivascular fibrosis [14]. Interestingly miR-29 regulates cardiac fibrosis [4, 62] and expression of this micro-RNA is decreased in atrial fibrillation [15]. Conceivably, obesity in pregnancy may stimulate cardiac fibrosis through an miR-29 related pathway. In our study, atrial miR-29a and miR-29c (but not miR-29b) expressions were decreased in obese pregnant animals, however miR-29 expression did not correlate with atrial collagen content. This discrepancy could be explained by additional mechanisms that regulate collagen expression, e.g., mechanical changes in the blood flow parameters [7] and pregnancy-specific changes [41]. Interestingly, miR-29 regulates the gene cluster associated with body growth, down regulating growth-promoting genes, including Igf1 [26]. Thus the observed decrease in miR-29 might represent obesity-specific cardiac growth promoting adaptations.
MiR 29b regulated LV specific collagen content in mouse models infected with a miR-29b sponge carried by a cardiotropic adeno-associated virus [15]. Additionally recently, antagomir 29b had been shown to inhibit endometrial fibrosis [37]. MiR-29c is decreased in cardiac hypertrophy [61]. In agreement with this observation, our model displayed decreased miR-29b expression paralleled by the increased cardiac weight in pregnant, compared to the non-pregnant animals.
The topographic differences in miR-29 expression (in the LA, but not in the LV) between non-obese and obese animals are in agreement with the fact that miR-29 pathway [51, 66] is under the control of the major mediators of differential molecular signaling between LA and LV [17, 23, 37, 48, 49].
Changes in the aortic arch
The Framingham offspring cohort study demonstrated that arterial stiffness precedes increases in systolic blood pressure in obesity [25]. Pregnancy decreases total vascular resistance by at least 25%. The obese animals in this study did not manifest structural changes in the AA, but their ELN expression correlated positively with miR-29 expression. This relationship supports the reported role of miR-29 in profibrotic changes of other tissues, namely urinary, hepatic and pulmonary fibroses [48, 51].
CONCLUSION
This study’s data describes the maternal cardiac changes in miR-29 and its down-stream molecules in pregnancy and obesity.
Several strengths of this study include the use of the experimental animal model closest to humans thus allowing translation to the events in pregnant women; the uniform, controlled environment of animal housing (i.e., temperature, humidity, physical activity, dietary composition, etc.) would be impossible to achieve in human population studies. A unique strength of this study was the ability to obtain myocardial tissues from precise topographic locations at precise times for pregnant and non-pregnant animals of reproductive age. Weaknesses of this study include its retrospective design, small sample sizes, and heterogeneity among the non-pregnant cohort. Pharmacologic, tissue-specific targeting of miR-29 may represent a novel strategy to prevent and treat the early consequences of maternal obesity, as it has been shown to suppress lipogenic programs in liver [34].
ACKNOWLEDGEMENTS
This investigation used resources which were supported by the Southwest National Primate Research Center grant P51 RR013986 from the National Center for Research Resources, National Institutes of Health and which are currently supported by the Office of Research Infrastructure Programs through P51 OD011133. This investigation was conducted in facilities constructed with support from the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through Grant Number C06 RR015456 and C06 RR014578. NIH grant HD21350 to Dr. Peter Nathanielsz (UTHSC San Antonio), UTHSCSA ERC New Investigator Award to N.S.-L., NCRR grant P51 RR013986 to the Southwest National Primate Research Center, NIH HL grant P50 084922 to R.A.P, and 1R01AR056666-01A2 and R21 AR066505-01A1 to AS also helped facilitate this study. The authors are grateful to Dr. Anand Kulkarni and Ms. Ashley Ezekiel for their expertise with an Aperio ScanScope. Dr. M. Fan’s expertise in miR-29 biology has been highly appreciated. The authors also acknowledge the assistance of L. Williams and S. Zhang with molecular biology (RNA, cDNA synthesis, and q-RT-PCR) and thank Ms. Meifin Lu for her expertise in immunohistochemistry. The authors are grateful to Melissa Waggoner for editing this paper.
Footnotes
The authors report no conflict of interest.
This work was performed at the Southwest National Primate Research Center and University of Tennessee Health Sciences Center
REFERENCES
- 1.2000 Report of the AVMA Panel on Euthanasia. Journal of the American Veterinary Medical Association. 2001;218:669–696. doi: 10.2460/javma.2001.218.669. [DOI] [PubMed] [Google Scholar]
- 2.Abbas AE, Lester SJ, Connolly H. Pregnancy and the cardiovascular system. Int J Cardiol. 2005;98:179–189. doi: 10.1016/j.ijcard.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiological reviews. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abonnenc M, Nabeebaccus AA, Mayr U, Barallobre-Barreiro J, Dong X, Cuello F, Sur S, Drozdov I, Langley SR, Lu R, Stathopoulou K, Didangelos A, Yin X, Zimmermann WH, Shah AM, Zampetaki A, Mayr M. Extracellular matrix secretion by cardiac fibroblasts: role of microRNA-29b and microRNA-30c. Circ Res. 2013;113:1138–1147. doi: 10.1161/CIRCRESAHA.113.302400. [DOI] [PubMed] [Google Scholar]
- 5.Aviram A, Hod M, Yogev Y. Maternal obesity: implications for pregnancy outcome and long-term risks-a link to maternal nutrition. Int J Gynaecol Obstet. 2011;115(Suppl 1):S6–10. doi: 10.1016/S0020-7292(11)60004-0. [DOI] [PubMed] [Google Scholar]
- 6.Avni B, Frenkel G, Shahar L, Golik A, Sherman D, Dishy V. Aortic stiffness in normal and hypertensive pregnancy. Blood Press. 2010;19:11–15. doi: 10.3109/08037050903464535. [DOI] [PubMed] [Google Scholar]
- 7.Basu P, Sen U, Tyagi N, Tyagi SC. Blood flow interplays with elastin: collagen and MMP: TIMP ratios to maintain healthy vascular structure and function. Vascular health and risk management. 2010;6:215–228. doi: 10.2147/vhrm.s9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briley AL, Barr S, Badger S, Bell R, Croker H, Godfrey KM, Holmes B, Kinnunen TI, Nelson SM, Oteng-Ntim E, Patel N, Robson SC, Sandall J, Sanders T, Sattar N, Seed PT, Wardle J, Poston L. A complex intervention to improve pregnancy outcome in obese women; the UPBEAT randomised controlled trial. BMC Pregnancy Childbirth. 2014;14:74. doi: 10.1186/1471-2393-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brocato B, Zoerner AA, Janjetovic Z, Skobowiat C, Gupta S, Moore BM, 2nd, Slominski A, Zhang J, Schenone M, Phinehas R, Ferry RJ, Jr., Dick E, Jr., Hubbard GB, Mari G. Schlabritz-Loutsevitch N: Endocannabinoid crosstalk between placenta and maternal fat in a baboon model (Papio spp.) of obesity. Placenta. 2013;34:983–989. doi: 10.1016/j.placenta.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol. 1999;181:718–724. doi: 10.1016/s0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- 12.Cappellano G, Uberti F, Caimmi PP, Pietronave S, Mary DA, Dianzani C, Micalizzi E, Melensi M, Boldorini R, Nicosia G, Crosio E, Chiocchetti A, Aina F, Prat M, Dianzani U, Vacca G, Ariatti C, Grossini E. Different expression and function of the endocannabinoid system in human epicardial adipose tissue in relation to heart disease. The Canadian journal of cardiology. 2013;29:499–509. doi: 10.1016/j.cjca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Conrad KP. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am J Physiol Regul Integr Comp Physiol. 2011;301:R267–275. doi: 10.1152/ajpregu.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corradi D. Atrial fibrillation from the pathologist’s perspective. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2014;23:71–84. doi: 10.1016/j.carpath.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Dawson K, Wakili R, Ordog B, Clauss S, Chen Y, Iwasaki Y, Voigt N, Qi XY, Sinner MF, Dobrev D, Kaab S, Nattel S. MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation. 2013;127:1466–1475. 1475e1461–1428. doi: 10.1161/CIRCULATIONAHA.112.001207. [DOI] [PubMed] [Google Scholar]
- 16.Deslee G, Woods JC, Moore CM, Liu L, Conradi SH, Milne M, Gierada DS, Pierce J, Patterson A, Lewit RA, Battaile JT, Holtzman MJ, Hogg JC, Pierce RA. Elastin expression in very severe human COPD. The European respiratory journal. 2009;34:324–331. doi: 10.1183/09031936.00123008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabritz L, Kirchhof P. Selective atrial profibrotic signalling in mice and man. Cardiovascular research. 2013;99:592–594. doi: 10.1093/cvr/cvt188. [DOI] [PubMed] [Google Scholar]
- 18.Fan M, Krutilina R, Sun J, Sethuraman A, Yang CH, Wu ZH, Yue J, Pfeffer LM. Comprehensive analysis of microRNA (miRNA) targets in breast cancer cells. The Journal of biological chemistry. 2013;288:27480–27493. doi: 10.1074/jbc.M113.491803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farley D, Tejero ME, Comuzzie AG, Higgins PB, Cox L, Werner SL, Jenkins SL, Li C, Choi J, Dick EJ, Jr., Hubbard GB, Frost P, Dudley DJ, Ballesteros B, Wu G, Nathanielsz PW, Schlabritz-Loutsevitch NE. Feto-placental adaptations to maternal obesity in the baboon. Placenta. 2009;30:752–760. doi: 10.1016/j.placenta.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frost PA, Hubbard GB, Dammann MJ, Snider CL, Moore CM, Hodara VL, Giavedoni LD, Rohwer R, Mahaney MC, Butler TM, Cummins LB, McDonald TJ, Nathanielsz PW, Schlabritz-Loutsevitch NE. White monkey syndrome in infant baboons (Papio species) Journal of medical primatology. 2004;33:197–213. doi: 10.1111/j.1600-0684.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 21.Gualillo O, Gonzalez-Juanatey JR, Lago F. The emerging role of adipokines as mediators of cardiovascular function: physiologic and clinical perspectives. Trends in cardiovascular medicine. 2007;17:275–283. doi: 10.1016/j.tcm.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Hadi AM, Mouchaers KT, Schalij I, Grunberg K, Meijer GA, Vonk-Noordegraaf A, van der Laarse WJ, Belien JA. Rapid quantification of myocardial fibrosis: a new macro-based automated analysis. Cell Oncol (Dordr) 2011;34:343–354. doi: 10.1007/s13402-011-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanna N, Cardin S, Leung TK, Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovascular research. 2004;63:236–244. doi: 10.1016/j.cardiores.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Iancu ME, Copaescu C, Serban M, Ginghina C. Favorable changes in arterial elasticity, left ventricular mass, and diastolic function after significant weight loss following laparoscopic sleeve gastrectomy in obese individuals. Obes Surg. 2014;24:364–370. doi: 10.1007/s11695-013-1097-6. [DOI] [PubMed] [Google Scholar]
- 25.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA : the journal of the American Medical Association. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamran F, Andrade AC, Nella AA, Clokie SJ, Rezvani G, Nilsson O, Baron J, Lui JC. Evidence That Up-Regulation of MicroRNA-29 Contributes to Postnatal Body Growth Deceleration. Molecular endocrinology (Baltimore, Md) 2015;29:921–932. doi: 10.1210/me.2015-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karere GM, Glenn JP, VandeBerg JL, Cox LA. Differential microRNA response to a high-cholesterol, high-fat diet in livers of low and high LDL-C baboons. BMC genomics. 2012;13:320. doi: 10.1186/1471-2164-13-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karmazyn M, Rajapurohitam V. Leptin as a cardiac pro-hypertrophic factor and its potential role in the development of heart failure. Current pharmaceutical design. 2014;20:646–651. doi: 10.2174/13816128113199990023. [DOI] [PubMed] [Google Scholar]
- 29.Karunakaran D, Rayner KJ. MicroRNAs in cardiovascular health: from order to disorder. Endocrinology. 2013;154:4000–4009. doi: 10.1210/en.2013-1299. [DOI] [PubMed] [Google Scholar]
- 30.Khorram O, Han G, Bagherpour R, Magee TR, Desai M, Ross MG, Chaudhri AA, Toloubeydokhti T, Pearce WJ. Effect of maternal undernutrition on vascular expression of micro and messenger RNA in newborn and aging offspring. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298:R1366–1374. doi: 10.1152/ajpregu.00704.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosmala W, Jedrzejuk D, Derzhko R, Przewlocka-Kosmala M, Mysiak A, Bednarek-Tupikowska G. Left ventricular function impairment in patients with normal-weight obesity: contribution of abdominal fat deposition, profibrotic state, reduced insulin sensitivity, and proinflammatory activation. Circ Cardiovasc Imaging. 2012;5:349–356. doi: 10.1161/CIRCIMAGING.111.969956. [DOI] [PubMed] [Google Scholar]
- 32.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, Marwick TH. Fibrosis and cardiac function in obesity: a randomised controlled trial of aldosterone blockade. Heart. 2013;99:320–326. doi: 10.1136/heartjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- 33.Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics. 2012;44:237–244. doi: 10.1152/physiolgenomics.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurtz CL, Fannin EE, Toth CL, Pearson DS, Vickers KC, Sethupathy P. Inhibition of miR-29 has a significant lipid-lowering benefit through suppression of lipogenic programs in liver. Scientific reports. 2015;5:12911. doi: 10.1038/srep12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai YH, Liu CC, Kuo JY, Hung TC, Wu YJ, Yeh HI, Bulwer BE, Hung CL. Independent Effects of Body Fat and Inflammatory Markers on Ventricular Geometry, Midwall Function, and Atrial Remodeling. Clinical cardiology. 2014 doi: 10.1002/clc.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leopoldo AS, Sugizaki MM, Lima-Leopoldo AP, do Nascimento AF, Luvizotto Rde A, de Campos DH, Okoshi K, Dal Pai-Silva M, Padovani CR, Cicogna AC. Cardiac remodeling in a rat model of diet-induced obesity. Can J Cardiol. 2010;26:423–429. doi: 10.1016/s0828-282x(10)70440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Du S, Sheng X, Liu J, Cen B, Huang F, He Y. MicroRNA-29b Inhibits Endometrial Fibrosis by Regulating the Sp1-TGF-beta1/Smad-CTGF Axis in a Rat Model. Reproductive sciences (Thousand Oaks, Calif) 2015 doi: 10.1177/1933719115602768. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Umar S, Amjedi M, Iorga A, Sharma S, Nadadur RD, Regitz-Zagrosek V, Eghbali M. New frontiers in heart hypertrophy during pregnancy. Am J Cardiovasc Dis. 2012;2:192–207. [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Zhang Y, Kuruba R, Gao X, Gandhi CR, Xie W, Li S. Roles of microRNA-29a in the antifibrotic effect of farnesoid X receptor in hepatic stellate cells. Molecular pharmacology. 2011;80:191–200. doi: 10.1124/mol.110.068247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li P, Guo W, Du L, Zhao J, Wang Y, Liu L, Hu Y, Hou Y. microRNA-29b contributes to pre-eclampsia through its effects on apoptosis, invasion and angiogenesis of trophoblast cells. Clin Sci (Lond) 2013;124:27–40. doi: 10.1042/CS20120121. [DOI] [PubMed] [Google Scholar]
- 41.Mattioli AV, Pennella S, Demaria F, Farinetti A. Atrial Remodeling in Pregnant Hypertensive Women: Comparison between Chronic and Gestational Hypertension. The open cardiovascular medicine journal. 2012;6:9–14. doi: 10.2174/1874192401206010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melchiorre K, Sharma R, Thilaganathan B. Cardiac structure and function in normal pregnancy. Curr Opin Obstet Gynecol. 2012;24:413–421. doi: 10.1097/GCO.0b013e328359826f. [DOI] [PubMed] [Google Scholar]
- 43.NATALIA SCHLABRITZ-LOUTSEVITCH AGC, MAHANEY MICHAEL, HUBBARD GENEB, DICK EDWARDJ, KOCAK M, GUPTA SONALI, CARRILLO MAIRA, SCHENONE MAURO, POSTLETHWAITE ARNOLD, SLOMINSKI ANDRZEJ. SERUM VITAMIN D CONCENTRATIONS IN BABOONS (PAPIO SPP.) Comparative Medicine. 2015 in press. [PMC free article] [PubMed] [Google Scholar]
- 44.Nichols WWORM. McDonald’s blood flow in arteries theoretical, experimental and clinical principles. Oxford University Press; New York: 1998. [Google Scholar]
- 45.Niu J, Shi Y, Tan G, Yang CH, Fan M, Pfeffer LM, Wu ZH. DNA damage induces NF-kappaB-dependent microRNA-21 up-regulation and promotes breast cancer cell invasion. The Journal of biological chemistry. 2012;287:21783–21795. doi: 10.1074/jbc.M112.355495. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Ott CE, Grunhagen J, Jager M, Horbelt D, Schwill S, Kallenbach K, Guo G, Manke T, Knaus P, Mundlos S, Robinson PN. MicroRNAs differentially expressed in postnatal aortic development downregulate elastin via 3′ UTR and coding-sequence binding sites. PloS one. 2011;6:e16250. doi: 10.1371/journal.pone.0016250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. Journal of cellular and molecular medicine. 2010;14:2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, Sung JJ, Lan HY. TGF-beta/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22:1462–1474. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahmutula D, Marcus GM, Wilson EE, Ding CH, Xiao Y, Paquet AC, Barbeau R, Barczak AJ, Erle DJ, Olgin JE. Molecular basis of selective atrial fibrosis due to overexpression of transforming growth factor-beta1. Cardiovascular research. 2013;99:769–779. doi: 10.1093/cvr/cvt074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, Sarwar N, Lee AJ, Norman JE. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539. doi: 10.1136/bmj.f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M, Tacke F, Trautwein C, Luedde T. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 52.Roncarati R, Viviani Anselmi C, Losi MA, Papa L, Cavarretta E, Da Costa Martins P, Contaldi C, Saccani Jotti G, Franzone A, Galastri L, Latronico MV, Imbriaco M, Esposito G, De Windt L, Betocchi S, Condorelli G. Circulating miR-29a, Among Other Up-Regulated MicroRNAs, Is the Only Biomarker for Both Hypertrophy and Fibrosis in Patients With Hypertrophic Cardiomyopathy. J Am Coll Cardiol. 2014;63:920–927. doi: 10.1016/j.jacc.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 53.Schlabritz-Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL, Bill Cummins L, Frost PA, McDonald TJ, Nathanielsz PW. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. Journal of medical primatology. 2004;33:117–126. doi: 10.1111/j.1600-0684.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 54.Schlabritz-Loutsevitch NE, Hubbard GB, Dammann MJ, Jenkins SL, Frost PA, McDonald TJ, Nathanielsz PW. Normal concentrations of essential and toxic elements in pregnant baboons and fetuses (Papio species) Journal of medical primatology. 2004;33:152–162. doi: 10.1111/j.1600-0684.2004.00066.x. [DOI] [PubMed] [Google Scholar]
- 55.Schlabritz-Loutsevitch NE, Moore CM, Lopez-Alvarenga JC, Dunn BG, Dudley D, Hubbard GB. The baboon model (Papio hamadryas) of fetal loss: maternal weight, age, reproductive history and pregnancy outcome. Journal of medical primatology. 2008;37:337–345. doi: 10.1111/j.1600-0684.2008.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmiegelow MD, Andersson C, Kober L, Andersen SS, Olesen JB, Jensen TB, Azimi A, Nielsen MB, Gislason G, Torp-Pedersen C. Prepregnancy obesity and associations with stroke and myocardial infarction in women in the years after childbirth: a nationwide cohort study. Circulation. 2014;129:330–337. doi: 10.1161/CIRCULATIONAHA.113.003142. [DOI] [PubMed] [Google Scholar]
- 57.Soci UP, Fernandes T, Hashimoto NY, Mota GF, Amadeu MA, Rosa KT, Irigoyen MC, Phillips MI, Oliveira EM. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol Genomics. 2011;43:665–673. doi: 10.1152/physiolgenomics.00145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sokolis DP, Boudoulas H, Kavantzas NG, Kostomitsopoulos N, Agapitos EV, Karayannacos PE. A morphometric study of the structural characteristics of the aorta in pigs using an image analysis method. Anatomia, histologia, embryologia. 2002;31:21–30. doi: 10.1046/j.1439-0264.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- 59.Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic acids research. 2010;38:D792–799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stephanis CG, Mourmouras DE, Tsagadopoulos DG. On the elastic properties of arteries. J Biomech. 2003;36:1727–1731. doi: 10.1016/s0021-9290(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 61.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vasapollo B, Valensise H, Novelli GP, Altomare F, Galante A, Arduini D. Abnormal maternal cardiac function precedes the clinical manifestation of fetal growth restriction. Ultrasound Obstet Gynecol. 2004;24:23–29. doi: 10.1002/uog.1095. [DOI] [PubMed] [Google Scholar]
- 64.Wang TJ, Parise H, Levy D, D’Agostino RB, Sr., Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA : the journal of the American Medical Association. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 65.Weber KT, Pick R, Jalil JE, Janicki JS, Carroll EP. Patterns of myocardial fibrosis. J Mol Cell Cardiol. 1989;21(Suppl 5):121–131. doi: 10.1016/0022-2828(89)90778-5. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Ru Huang X, Wei LH, Chung AC, Yu CM, Lan HY. miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-beta/Smad3 signaling. Molecular therapy : the journal of the American Society of Gene Therapy. 2014 doi: 10.1038/mt.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]