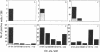Abstract
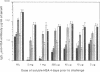
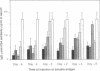
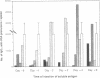
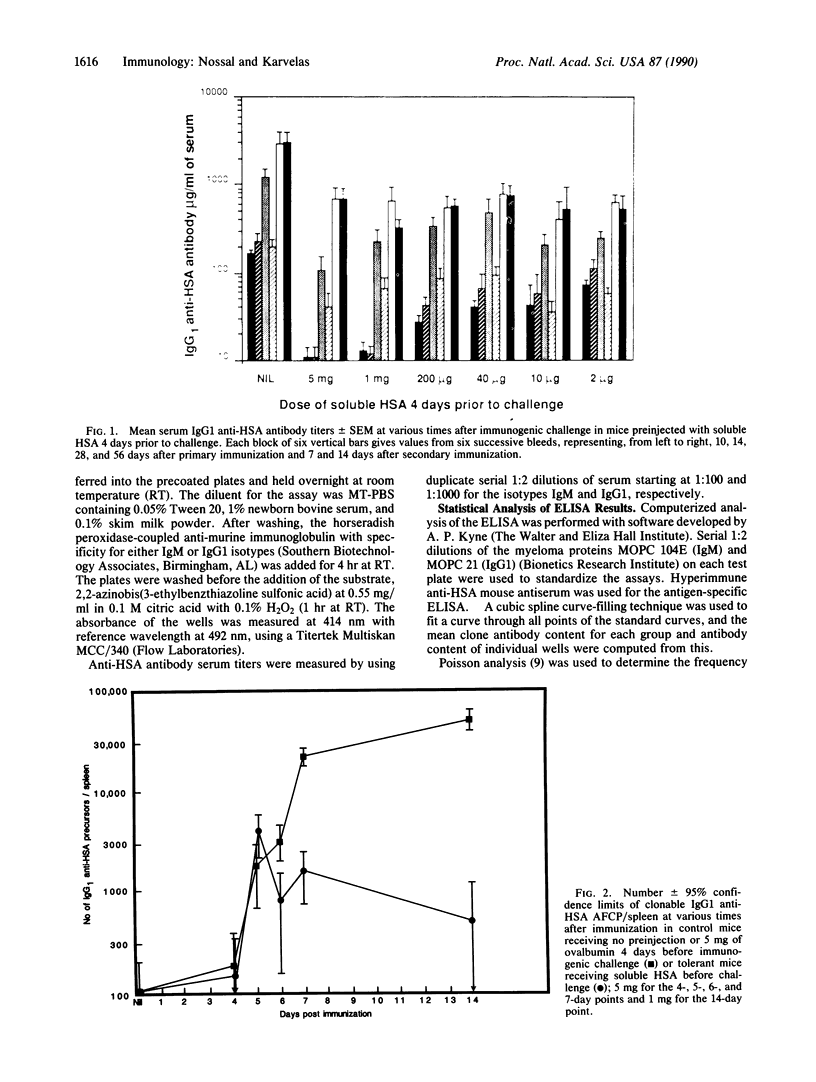
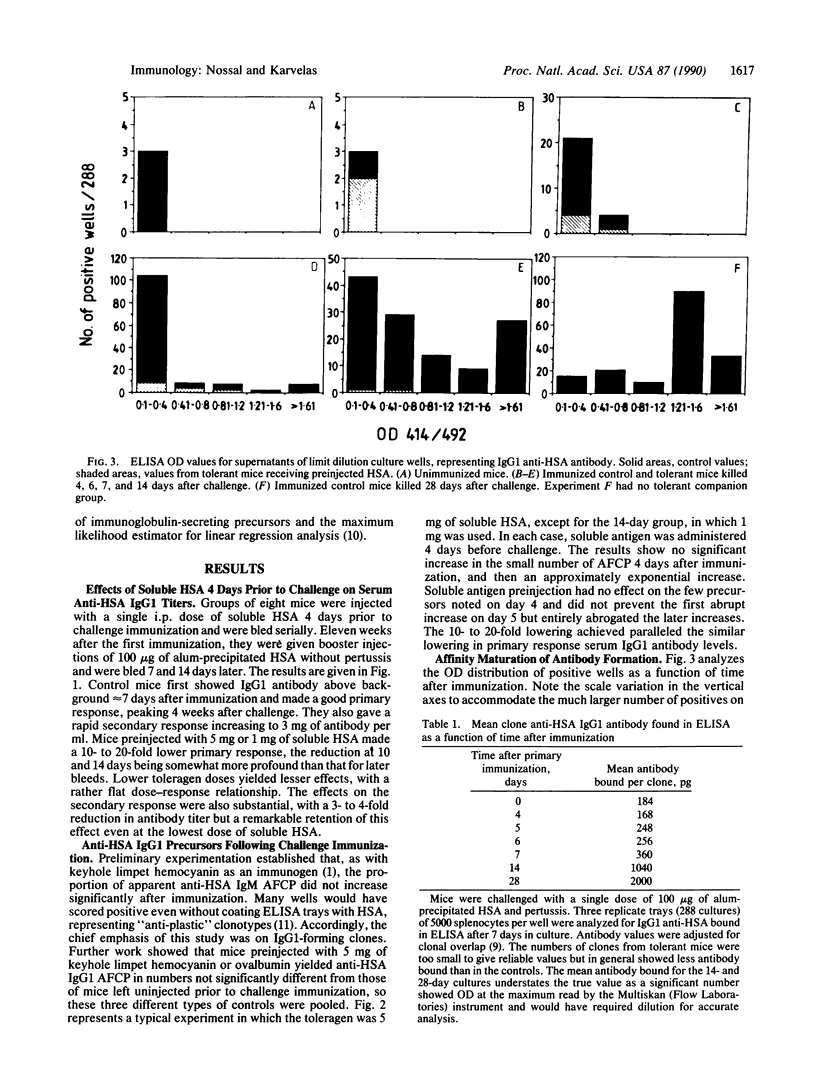
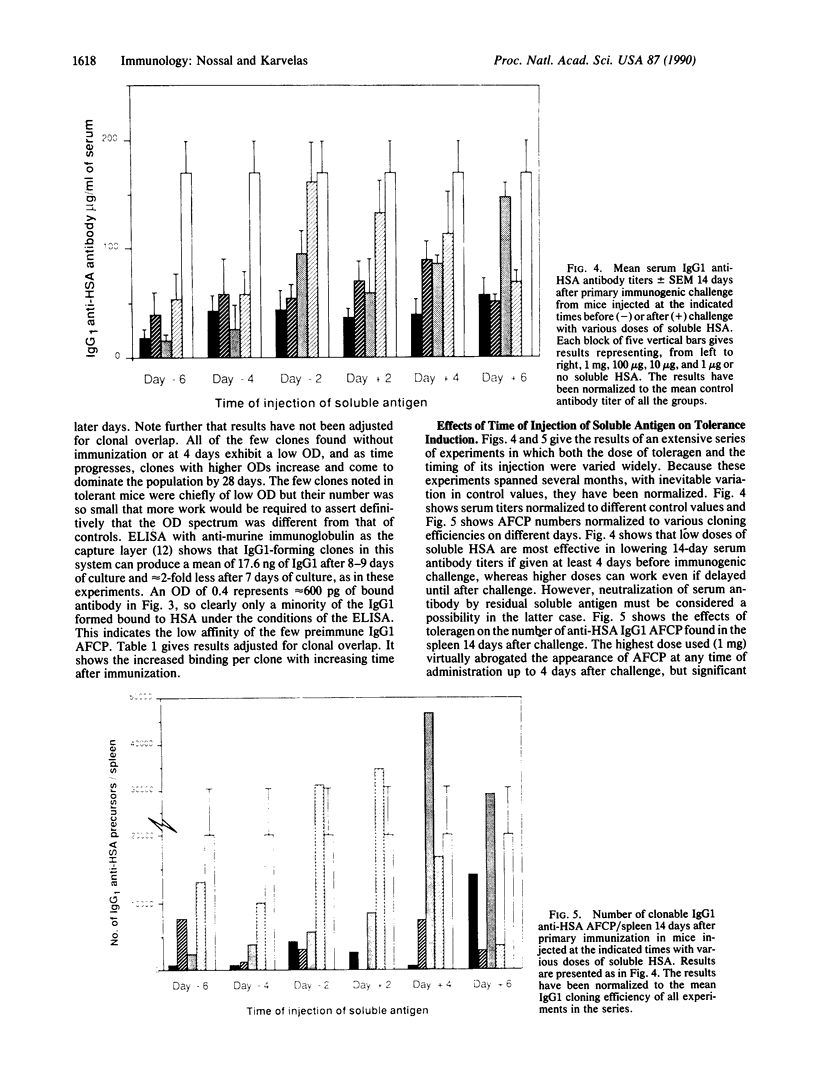
The anti-human serum albumin (HSA) B-cell repertoire of C57BL/6 mice was examined by culturing splenocytes at limiting dilution following polyclonal stimulation with Escherichia coli lipopolysaccharide and a lymphokine mixture. The frequency of anti-HSA precursors was determined before and after immunization with alum-precipitated HSA and 10(9) killed Bordetella pertussis organisms, by submitting clonal supernatants to an ELISA. Anti-HSA IgG1-forming precursors were rare in unimmunized spleens, representing approximately equal to 1 in 500,000 splenocytes or only approximately equal to 100 cells per spleen. Between day 5 and day 7 after immunization, this figure increased to approximately equal to 20,000 cells per spleen. Over the following 3 weeks, there was a progressive increase in the mean optical density generated in the clonal ELISA, presumably due to affinity maturation of the B-cell population. When freshly deaggregated HSA was injected before or even up to 4 days after challenge immunization, the appearance of anti-HSA IgG1-forming cell precursors was largely prevented. The effect was most marked with 5 mg or 1 mg of soluble HSA, but impressive partial effects could be seen with as little as 10 micrograms of HSA if administered before challenge immunization. Most of the few clones seen after the higher doses of the toleragen appeared to make antibody of low affinity. The capacity to influence the B-cell pool by soluble antigen administered just 1-2 days before the sudden appearance of IgG1 precursors argues against the totality of the effect being due to T-cell-mediated suppression and in favor of a direct effect on B cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basten A., Miller J. F., Loblay R., Johnson P., Gamble J., Chia E., Pritchard-Briscoe H., Callard R., McKenzie I. F. T cell-dependent suppression of antibody production. I. Characteristics of suppressor T cells following tolerance induction. Eur J Immunol. 1978 May;8(5):360–370. doi: 10.1002/eji.1830080513. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Klaus G. G. Crosslinking of surface immunoglobulin and Fc receptors on B lymphocytes inhibits stimulation of inositol phospholipid breakdown via the antigen receptors. J Exp Med. 1985 Dec 1;162(6):1825–1836. doi: 10.1084/jem.162.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger J. D., Pike B. L., Nossal G. J. Analysis of the B lymphocyte repertoire by polyclonal activation. Hindrance by clones yielding antibodies which bind promiscuously to plastic. J Immunol Methods. 1988 Feb 10;106(2):181–189. doi: 10.1016/0022-1759(88)90195-0. [DOI] [PubMed] [Google Scholar]
- Gershon R. K. A disquisition on suppressor T cells. Transplant Rev. 1975;26:170–185. doi: 10.1111/j.1600-065x.1975.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Good M. F., Boyd A. W., Nossal G. J. Analysis of true anti-hapten cytotoxic clones in limit dilution microcultures after correction for "anti-self" activity: precursor frequencies, Ly-2 and Thy-1 phenotype, specificity, and statistical methods. J Immunol. 1983 May;130(5):2046–2055. [PubMed] [Google Scholar]
- Kaartinen M., Griffiths G. M., Hamlyn P. H., Markham A. F., Karjalainen K., Pelkonen J. L., Mäkelä O., Milstein C. Anti-oxazolone hybridomas and the structure of the oxazolone idiotype. J Immunol. 1983 Feb;130(2):937–945. [PubMed] [Google Scholar]
- MacLennan I. C., Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986 Jun;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G. Combinations of interleukins 2, 4 and 5 regulate the secretion of murine immunoglobulin isotypes. Eur J Immunol. 1989 Nov;19(11):2025–2030. doi: 10.1002/eji.1830191109. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G., Nossal G. J. Clonal analysis of autoantibody-producing cell precursors in the preimmune B cell repertoire. J Immunol. 1988 Dec 15;141(12):4118–4123. [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Single cell studies on the antibody-forming potential of fractionated, hapten-specific B lymphocytes. Immunology. 1976 Feb;30(2):189–202. [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Riedel C. Sudden appearance of anti-protein IgG1-forming cell precursors early during primary immunization. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4679–4683. doi: 10.1073/pnas.86.12.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N. E., Parker D. C. Cross-linking of B lymphocyte Fc gamma receptors and membrane immunoglobulin inhibits anti-immunoglobulin-induced blastogenesis. J Immunol. 1984 Feb;132(2):627–632. [PubMed] [Google Scholar]
- Pike B. L., Vaux D. L., Clark-Lewis I., Schrader J. W., Nossal G. J. Proliferation and differentiation of single hapten-specific B lymphocytes is promoted by T-cell factor(s) distinct from T-cell growth factor. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6350–6354. doi: 10.1073/pnas.79.20.6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K., Förster I., Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987 Nov 20;238(4830):1088–1094. doi: 10.1126/science.3317826. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Ohara J., Myers C. D., Layton J. E., Krammer P. H., Paul W. E. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985 Nov 1;162(5):1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Weigle W. O., Chiller J. M., Habicht G. S. Effect of immunological unresponsiveness on different cell populations. Transplant Rev. 1972;8:3–25. doi: 10.1111/j.1600-065x.1972.tb01562.x. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Shortman K. The separation of different cell classes from lymphoid organs. IX. A simple and rapid method for removal of damaged cells from lymphoid cell suspensions. J Immunol Methods. 1973 Apr;2(3):293–301. doi: 10.1016/0022-1759(73)90055-0. [DOI] [PubMed] [Google Scholar]