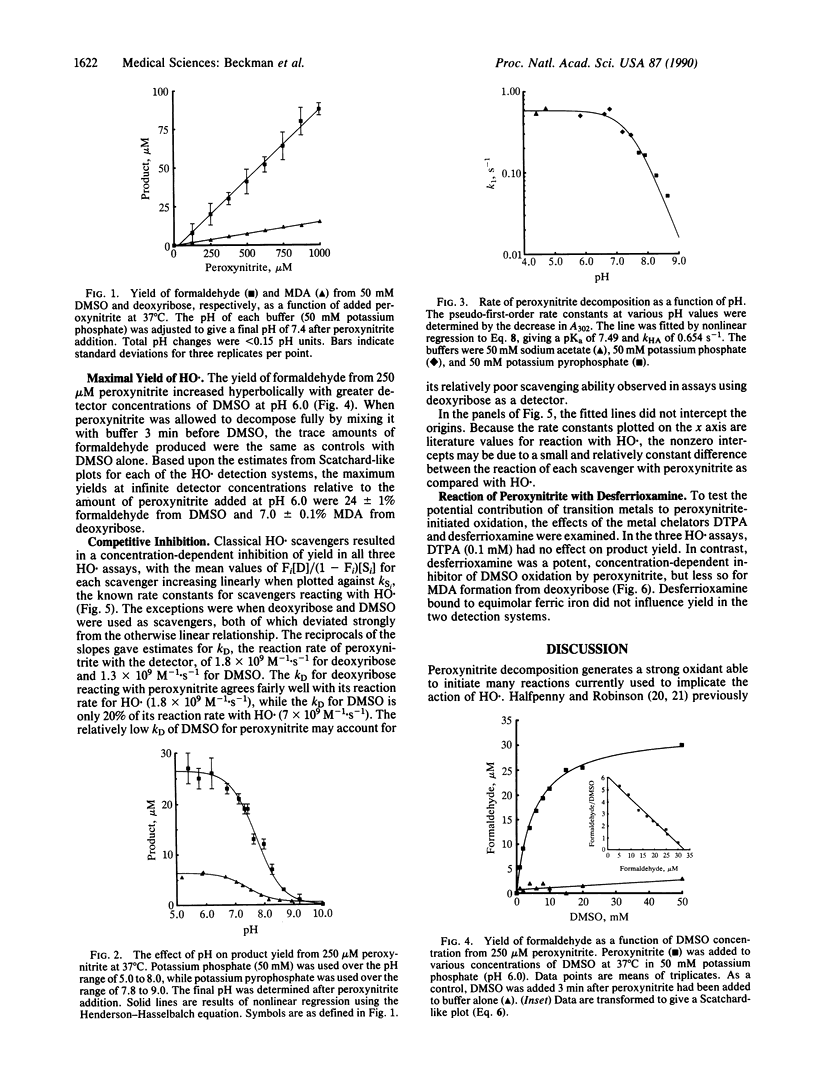
Abstract
Superoxide dismutase reduces injury in many disease processes, implicating superoxide anion radical (O2-.) as a toxic species in vivo. A critical target of superoxide may be nitric oxide (NO.) produced by endothelium, macrophages, neutrophils, and brain synaptosomes. Superoxide and NO. are known to rapidly react to form the stable peroxynitrite anion (ONOO-). We have shown that peroxynitrite has a pKa of 7.49 +/- 0.06 at 37 degrees C and rapidly decomposes once protonated with a half-life of 1.9 sec at pH 7.4. Peroxynitrite decomposition generates a strong oxidant with reactivity similar to hydroxyl radical, as assessed by the oxidation of deoxyribose or dimethyl sulfoxide. Product yields indicative of hydroxyl radical were 5.1 +/- 0.1% and 24.3 +/- 1.0%, respectively, of added peroxynitrite. Product formation was not affected by the metal chelator diethyltriaminepentaacetic acid, suggesting that iron was not required to catalyze oxidation. In contrast, desferrioxamine was a potent, competitive inhibitor of peroxynitrite-initiated oxidation because of a direct reaction between desferrioxamine and peroxynitrite rather than by iron chelation. We propose that superoxide dismutase may protect vascular tissue stimulated to produce superoxide and NO. under pathological conditions by preventing the formation of peroxynitrite.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbs C. F., Griffin D. W. Scatchard analysis of methane sulfinic acid production from dimethyl sulfoxide: a method to quantify hydroxyl radical formation in physiologic systems. Free Radic Biol Med. 1989;6(5):493–503. doi: 10.1016/0891-5849(89)90042-7. [DOI] [PubMed] [Google Scholar]
- Busse R., Lückhoff A., Bassenge E. Endothelium-derived relaxant factor inhibits platelet activation. Naunyn Schmiedebergs Arch Pharmacol. 1987 Nov;336(5):566–571. doi: 10.1007/BF00169315. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Anoxia and endothelium-dependent reactivity of the canine femoral artery. J Physiol. 1983 Feb;335:65–74. doi: 10.1113/jphysiol.1983.sp014519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986 May 15;247(1):1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Gorren A. C., de Boer E., Wever R. The reaction of nitric oxide with copper proteins and the photodissociation of copper-NO complexes. Biochim Biophys Acta. 1987 Nov 5;916(1):38–47. doi: 10.1016/0167-4838(87)90208-1. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Johansson L. H., Borg L. A. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal Biochem. 1988 Oct;174(1):331–336. doi: 10.1016/0003-2697(88)90554-4. [DOI] [PubMed] [Google Scholar]
- Klein S. M., Cohen G., Cederbaum A. I. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical generating systems. Biochemistry. 1981 Oct 13;20(21):6006–6012. doi: 10.1021/bi00524a013. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978 Jul 10;253(13):4697–4699. [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Superoxide anion release by human endothelial cells: synergism between a phorbol ester and a calcium ionophore. J Cell Physiol. 1986 May;127(2):207–210. doi: 10.1002/jcp.1041270203. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Boughton-Smith N. K., Palmer R. M., Whittle B. J., Moncada S. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J. 1989 Jul 1;261(1):293–296. doi: 10.1042/bj2610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Patel J. M., Block E. R. Nitrogen dioxide-induced changes in cell membrane fluidity and function. Am Rev Respir Dis. 1986 Dec;134(6):1196–1202. doi: 10.1164/arrd.1986.134.5.1196. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Lightsey J. W. Mechanisms of nitrogen dioxide reactions: initiation of lipid peroxidation and the production of nitrous Acid. Science. 1981 Oct 23;214(4519):435–437. doi: 10.1126/science.214.4519.435. [DOI] [PubMed] [Google Scholar]
- Prütz W. A., Mönig H., Butler J., Land E. J. Reactions of nitrogen dioxide in aqueous model systems: oxidation of tyrosine units in peptides and proteins. Arch Biochem Biophys. 1985 Nov 15;243(1):125–134. doi: 10.1016/0003-9861(85)90780-5. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol. 1986 May;250(5 Pt 2):H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- Salaris S. C., Babbs C. F. A rapid, widely applicable screen for drugs that suppress free radical formation in ischemia/reperfusion. J Pharmacol Methods. 1988 Dec;20(4):335–345. doi: 10.1016/0160-5402(88)90057-5. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Nau H., Wittfoht W., Gerlach J., Prescher K. E., Klein M. M., Niroomand F., Böhme E. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988 Sep 13;154(2):213–216. doi: 10.1016/0014-2999(88)90101-x. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Comparison of superoxide with other reducing agents in the biological production of hydroxyl radicals. Biochem J. 1979 Aug 15;182(2):625–628. doi: 10.1042/bj1820625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. The ability of scavengers to distinguish OH. production in the iron-catalyzed Haber-Weiss reaction: comparison of four assays for OH. Free Radic Biol Med. 1987;3(1):33–39. doi: 10.1016/0891-5849(87)90037-2. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]


