Abstract
Introduction
Skeletal muscle impairment is an important feature of chronic obstructive pulmonary disease (COPD). Renin–angiotensin system activity influences muscle phenotype, so we wished to investigate whether it affects the response to pulmonary rehabilitation.
Methods
Two studies are described; in the first, the response of 168 COPD patients (mean forced expiratory volume in one second 51.9% predicted) to pulmonary rehabilitation was compared between different ACE insertion/deletion polymorphism genotypes. In a second, independent COPD cohort (n=373), baseline characteristics and response to pulmonary rehabilitation were compared between COPD patients who were or were not taking ACE inhibitors or angiotensin receptor antagonists (ARB).
Results
In study 1, the incremental shuttle walk distance improved to a similar extent in all three genotypes; DD/ID/II (n=48/91/29) 69(67)m, 61 (76)m and 78 (78)m, respectively, (p>0.05). In study 2, fat free mass index was higher in those on ACE-I/ARB (n=130) than those who were not (n=243), 17.8 (16.0, 19.8) kg m−2 vs 16.5 (14.9, 18.4) kg/m2 (p<0.001). However change in fat free mass, walking distance or quality of life in response to pulmonary rehabilitation did not differ between groups.
Conclusions
While these data support a positive association of ACE-I/ARB treatment and body composition in COPD, neither treatment to reduce ACE activity nor ACE (I/D) genotype influence response to pulmonary rehabilitation.
Keywords: Pulmonary Rehabilitation, COPD Pathology
Key Messages.
Treatment with an ACE-I/angiotensin receptor blocker is associated with higher fat free mass in people with COPD.
Neither treatment with ACE-I/angiotensin receptor blocker nor ACE (I/D) polymorphism appear to influence response to pulmonary rehabilitation.
Background
Skeletal muscle impairment is a common and important feature of chronic obstructive pulmonary disease (COPD), occurring in about one-third of patients irrespective of the severity of their airflow obstruction.1–3 It is associated with reduced exercise capacity,4 as well as impaired quality of life,5 and quadriceps weakness has been shown to predict mortality in COPD independent of lung function.6 Physical inactivity is clearly a key driver of muscle weakness in COPD,3 7 8 although other factors, for example inflammation, hypoxia and hormonal factors, as well as genetic predisposition, may be important.5 9–11 Moreover, there is strong evidence that pulmonary rehabilitation (PR), a programme of supervised exercise and education, can produce significant improvements in quality of life, muscle strength and endurance, as well as exercise capacity, in patients with COPD.12 13
The circulating (endocrine) renin–angiotensin system (RAS) plays an important role in circulatory homeostasis, degrading vasodilator bradykinin and synthesising vasoconstrictor (and renal sodium-retaining) angiotensin II. However, local RAS also exists in diverse tissues14 including skeletal muscle.15 The presence (insertion, I) rather than the absence (deletion, D) of a 287 base pair sequence in intron 16 of the human ACE inhibitors gene is associated with lower tissue16 17 and circulating ACE activity.18 19 In turn, an extensive literature supports an association between ACE genotype and physical performance, the I allele being associated with endurance performance, and the D with power/sprint performance.19 20 The ACE (I/D) polymorphism, as well as polymorphisms of genes for bradykinin type 2 (BK(2)R) and vitamin D receptors, have been shown to influence strength and body composition in COPD.9–11 Furthermore, ACE-I use in patients with hypertension has been associated with preservation of quadriceps strength and walking speed compared to those on other medications or controls.21
Given these data, we wished to establish whether factors known to affect RAS activity might influence response to PR in patients with COPD. We investigated this by conducting two studies. The first explored the impact of the ACE (I/D) polymorphism on responses to PR in patients with COPD. The second, in a separate cohort, investigated the effects of concomitant ACE-I or angiotensin II receptor blocker (ARB) use in patients with COPD, testing the hypothesis that this would be associated with preserved fat free mass (FFM) and an enhanced response to PR.
Methods
Study 1, investigating the effect of ACE genotype, was approved by the Ethics Committee of King's College Hospital (05/Q0703/134) and funded by The British Lung Foundation and started in 2004. Participants provided written informed consent. The study was retrospective and involved contacting patients with a clinical diagnosis of COPD who had completed a PR programme at King's College or Royal Brompton and Harefield Hospitals. The PR programmes consisted of an 8 week course of aerobic and strength activities with two supervised and one or more home sessions per week. The initial exercise prescription was based on the outcome of their baseline incremental shuttle walk test distance (ISWD),22 and workloads were increased through the programme as tolerated. Programmes were multidisciplinary with an educational component covering issues including exercise, medication use, diet and coping strategies. Patients with COPD who attended at least 75% of their scheduled rehabilitation sessions and had ISWD measured pre-rehabilitation and immediately post-rehabilitation were invited to take part. Either a blood sample or a mouth swab was obtained from each patient to collect cells from which the PCR was used to determine ACE genotype.11 None of the patients or clinical research staff involved in the study knew the genotype of participants until after the phenotypic outcomes database had been finalised.
The association of ACE genotype with baseline characteristics and response to PR was assessed across all three genotypes by ANOVA and also between those with or without the D or I allele by unpaired t-test. The primary end point was change in ISWD immediately after PR. A p value of <0.05 was taken as significant and StatView 4.0 used for analysis.
In study 2, routinely collected data from a different cohort of patients with COPD who had been referred for a course of PR at Harefield Hospital between 2009 and 2011 were used. The Ethics Committee of Royal Brompton Hospital has determined that ethical approval is not required for the retrospective analysis of routinely collected clinical data. The primary outcome was differences in the response to PR of ISWD between patients who were or were not on an ACE-I or ARB (determined by patient self-report). Additional outcomes were fat free mass index (FFMI) determined by bioelectrical impedance analysis using a disease-specific regression equation,23 and the chronic respiratory disease questionnaire (CRQ)24 and the COPD assessment test score (CAT).13 25 Data from 72 of the patients in study 2 were included in a previous publication.13
Results
Study 1: effect of ACE genotype on response to PR
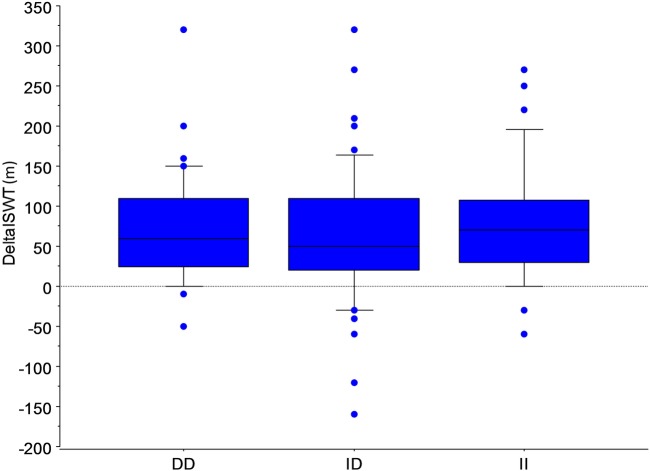
Data were available for 168 individuals who had participated in PR; 92 (53.8%) women, forced expiratory volume in one second (FEV1) 51.9 (22.7)% predicted. ACE genotypes were DD 48 (28%); ID 91 (53%); II 29 (19%) (table 1). There was no significant difference in patients' characteristics by ACE genotype either across all three possible genotypes (figure 1) or comparing those with or without an I or a D allele. Exercise capacity improved following PR in the whole study population with a mean increase in ISWD of 67.5(74.7)m and for each genotype (all p<0.0001), but the response did not differ significantly between genotypes (ANOVA, p=0.5).
Table 1.
Study 1: patient characteristics for whole group and separated by ACE genotype
| All n=168 |
DD n=48 (28%) |
ID n=91 (53%) |
II n=29 (19%) |
|
|---|---|---|---|---|
| Age | 68.7 (9.0) | 69.2 (10.4) | 67.8 (8.8) | 71.0 (6.9) |
| Gender (n(%) female) | 92 (54.8) | 23 (47.9) | 52 (57.1) | 15 (51.7) |
| FEV1 (% predicted) | 51.9 (22.7) | 50.4 (22.3) | 52.8 (24.4) | 50.1 (18.7) |
| FEV1/FVC (%) | 48.1 (17.6) | 48.0 (17.7) | 48.7 (19.3) | 46.2 (13.2) |
| ISWD baseline (m) | 251.4 (149.8) | 261.7 (184.0) | 253.0 (140.2) | 223.4 (103.9) |
| ISWD end (m) | 318.9 (170.6) | 331.0 (200.0) | 313.6 (165.3) | 301.7 (109.4) |
| ΔISWD (m) | 67.5 (74.7) | 69.4 (66.6) | 60.6 (76.2) | 78.3 (78.2) |
| ΔISWD (%) | 43.3 (66.7) | 45.6 (76.9) | 36.0 (53.2) | 59.2 (84.5) |
Values are mean (SD). All p>0.05 ANOVA across genotypes.
FEV1, forced expiratory volume in one second; FVC, forced vital capacity; ISWD, incremental shuttle walk test distance.
Figure 1.
Plot of change in incremental shuttle walk test following pulmonary rehabilitation according to ACE (insertion/deletion) polymorphism. A total of 168 COPD patients took part, DD 48 (28%); ID 91 (53%); II 29 (19%). The horizontal line represents median value. Boxes represent 25th/75th centiles; whiskers 10th/90th centiles (ANOVA, p=0.5).
Study 2: effect of ACE-I or ARB on response to PR
Baseline data from 373 consecutive COPD patients (213M:160F; mean age 68.3; median FEV1 41% predicted) referred to an outpatient PR programme were analysed (table 2). Of these, 130 reported taking either an ACE-I (n=82), ARB (n=45) or both (n=3). The groups had similar gender distribution and long-term oral corticosteroid use. Patients on ACE-I or ARB were older, had less severe airflow obstruction but similar values for ISWD, CRQ, Medical Research Council dyspnoea score (MRC) and CAT. However, the patients receiving ACE-I or ARB had significantly higher FFM and FFMI 17.8 kg/m2 (16.0, 19.8) versus 16.5 kg/m2 (14.9, 18.4) (p<0.0001 when adjusted for difference in FEV1 and age).
Table 2.
Study 2: baseline patient characteristics separated by whether patients were or were not taking an ACE-I or ARB.
| ARB or ACE-I n=130 |
No ARB or ACE-I n=243 |
p Value | |
|---|---|---|---|
| Age (years) | 71 (64, 78) | 67.6 (9.8) | 0.004 |
| FEV1 (% predicted) | 44.5 (32.3, 60.8) | 39.0 (26.0, 58.5) | 0.007 |
| FFM (kg) | 51.1 (11.2) | 45.5 (40.1, 52.0) | <0.001 |
| FFMI (kg/m2) | 17.8 (16.0, 19.8) | 16.5 (14.9, 18.4) | <0.001 |
| ISWD (m) | 140 (60, 250) | 160 (80, 280) | 0.10 |
| CRQ | 71.5 (55.8, 91.0) | 68.0 (56.0, 87.0) | 0.45 |
| MRC dyspnoea score | 4 (3, 5) | 4 (3, 5) | 0.79 |
| CAT score | 23.0 (8.0) | 22.0 (7.0) | 0.76 |
p Values are for unpaired t-tests. Data are presented as median (25th, 75th centiles) or (SD).
ACE-I, ACE inhibitor; ARB, AT II receptor antagonist; CAT, COPD assessment test score; CRQ, chronic respiratory disease questionnaire; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FFM, fat free mass; FFMI, fat free mass index; ISWD, incremental shuttle walk test distance.
A total of 255 patients completed the PR programme, 76 (30%) of whom were taking an ACE-I/ARB. Responses did not differ between those who were or were not on an ACE-I or ARB (table 3), with improvements in ISWD and CRQ exceeding the minimum clinically important difference.
Table 3.
Study 2: response to pulmonary rehabilitation in COPD patients who were or were not taking an ACE-I or ARB.
| ARB or ACE-I n=76 |
No ARB or ACE-I n=179 |
p value | |
|---|---|---|---|
| ΔFFM (kg) | −1.7 (2.7) | 1.8 (1.4) | 0.21 |
| ΔISWD (m) | 104 (21) | 63 (15) | 0.13 |
| ΔCRQ | 16.2 (3.8) | 17.7 (2.5) | 0.75 |
ACE-I, ACE inhibitors; ARB, AT II receptor antagonist; FFM, fat free mass; ISWD, incremental shuttle walk test distance; CRQ, chronic respiratory disease questionnaire.
All p>0.05. Data are presented as mean (SD).
Discussion
The present data suggest that the beneficial response to PR in COPD patients was not strongly influenced by their ACE (I/D) genotype, or by pharmacological RAS antagonism. However, long-term use of an ACE-I/ARB was associated with relatively preserved FFM in patients referred for PR.
Rationale for studying the RAS in COPD
In patients with COPD, the quadriceps muscle displays muscle fibre atrophy and a shift away from an endurance phenotype, with a reduced proportion of type I slow twitch, fatigue-resistant fibres together with reduced capillarity and oxidative enzymes.26–30 The RAS and thus ACE inhibitors and functional ACE gene polymorphisms have the potential to influence these processes through a number of mechanisms. These include effects on muscle atrophy/hypertrophy signalling, fibre shift, systemic inflammation and remodelling.31 Angiotensin II opposes the action of the insulin-like growth factor (IGF-1) system activating the ubiquitin-proteasome proteolytic pathway via IGF-1 and via NF-kB,32 33 and IGF-1 levels are reduced in the quadriceps of COPD patients in the stable state compared to healthy controls.34 Increases in exercise capacity and fibre size in COPD patients undergoing PR are associated with upregulation of IGF-1 and its splice variant mechano-growth factor (MGF).35
The I allele of the ACE gene polymorphism is associated with a higher proportion of type I fibres,36 and there is evidence that ACE inhibitors and AT II receptor antagonists interact with peroxisome proliferator-activated receptors (PPARs)37 38 which are major regulators of cell metabolism mediating type II (anaerobic) to type I (aerobic) fibre shift and regulate mitochondrial activity as well as muscle oxidative status.39–41 This is potentially of particular relevance in COPD given that PPAR-delta protein content is decreased in the skeletal muscle of these patients.42 The DD genotype has also been associated with systemic inflammation in COPD.43 In stable COPD patients, the deletion allele (D) of the ACE gene polymorphism has been associated with increased quadriceps strength, in contrast to age-matched healthy controls where this relationship was not observed.11
Effect of ACE inhibition in COPD
In the present cross-sectional study, patients taking an ACE-I/ARB did have relatively preserved FFM, though this was not associated with differences in exercise capacity. Patients had not been randomly allocated to treatment so these data need to be treated with caution, but they are consistent with a beneficial effect of reduced ACE activity on body composition.44
In keeping with this, in healthy older people the use of ACE-I as a treatment for hypertension is associated with relative preservation of lower limb muscle mass,45 and with a reduced rate of loss of knee extensor strength21 compared to patients using other antihypertensives or to those not treated for hypertension. The ACE-I perindopril has been shown to increase 6 min walk distance in older people.46 A small number of studies have investigated the effects of RAS inhibition in COPD patients.47–50 In one study captopril improved pulmonary haemodynamics during exercise in patients with the ID or II genotype47 though other studies have not found similar effects.51 52 A double-blind, placebo-controlled study by Di Marco et al evaluated the effects of 4 weeks treatment with enalapril on exercise performance in 21 COPD patients finding that it increased peak work rate in the treatment group compared to placebo, an effect not significantly modified by ACE genotype.53 A randomised controlled trial of fosinopril in 80 patients with COPD selected for quadriceps weakness found no benefit,49 and enalapril did not enhance the effect of PR on improvements in exercise performance in COPD.50 Of note, these two studies excluded people with a clinical indication for an ACE-I who, by definition, are the subject of the present paper.
Epidemiological data suggest a survival benefit in patients with COPD who are on an ACE-I.54 55 However, in the present study, treatment with an ACE-I was not associated with greater strength or exercise capacity. Interestingly, the patients on ACE-I/ARB had less severe airflow obstruction but similar health status and dyspnoea. It is therefore possible that comorbidities such as cardiac impairment were contributing to their overall symptom burden and exercise limitation which might have had an effect on response to PR.
We found no association of the ACE(I/D) genotype with response to PR. This contrasts with Gosker et al who found, in a study of 95 COPD patients undergoing PR, that the improvement in peak VO2 during cycle ergometry was significantly less in patients with the DD genotype.56 However in that study, those with an I allele had a lower exercise capacity initially so may have been more detrained. The difference could also be due to the test modalities employed in the two studies (walking vs cycling) or a regression to the mean effect.
Critique of methods
Functional exercise capacity is an integrative end point subject to respiratory, cardiac, skeletal muscle and motivational limitation, so the absence of an apparent effect of ACE genotype or ACE-I on response does not preclude the possibility of some physiological impact which might have been more apparent with a more controlled exercise end point such as metabolic parameters at a particular workload. Since the RAS is active at a number of levels, it may be that impacts on muscle strength, muscle endurance and the systemic and pulmonary vascular system may have opposing effects which a walking test cannot separate.
This paper addresses the question of whether either the genotype or treatment with drugs that influence the ACE system has an effect, in clinical practice, on outcome measures accepted as clinically relevant in international guidelines for PR—health status and exercise capacity assessed using a walking test.13 There is of course ongoing debate about the different information conveyed by laboratory and field tests of exercise performance as well as walking versus cycling, but there is certainly no reason to ascribe greater clinical relevance to VO2 max, etc than to performance on a field walking test when considering daily physical activity or patient-relevant outcomes.
Patient recruitment for the genotyping study was retrospective, so it is conceivable that some survival or other bias was in operation. Genotype data were not available for the cohort in study 2, so it is not possible to comment on possible interactions between genotype and treatment with ACE-I/ARB. It is possible that disease processes for which RAS antagonists were prescribed were themselves associated with differences in body composition. All participants were taking part in clinical PR programmes and data were entered prospectively, but because they were clinical programmes the full range of possible phenotypes were not recorded as might have been the case in a prospective study, such as exacerbation frequency and multimorbidities, as well as more detailed lung function parameters or gas transfer.57
Patient treatments were based on self-report, so it is possible that an effect of ACE-I or ARB was underestimated because of poor compliance with medication.
Conclusions
Although treatment with an ACE-I/ARB was associated with a higher FFM in patients with COPD, neither the ACE (I/D) polymorphism nor treatment with an ACE/ARB appear to influence response to PR despite previous data showing an association with quadriceps strength. Although trial data do not support a beneficial effect from the addition of an ACE-I in COPD patients who do not have a conventional clinical indication,49 50 the present data do not suggest that there is any advantage to avoiding or stopping ACE-I in COPD patients in whom they are indicated.
Acknowledgments
The study was funded by a Trevor Clay grant from The British Lung Foundation (TC 04/4) and supported by the NIHR Respiratory Biomedical Research Unit at Royal Brompton and Harefield Hospital and Imperial College who part fund MIP's salary. WD-CM is an NIHR Clinician Scientist and supported by a MRC New Investigator Research Grant. DS was funded by the MRC G0701628. ZP is a NIHR Doctorate Research Fellow. SSCK and CJJ were funded by the MRC.
Footnotes
Contributors: NSH, WD-CM, HM, MIP and JM conceived the study. SSCK, CJJ and DS collected data. JRAS and ZP performed genotyping. NSH and SSCK produced the first draft which all authors subsequently contributed to and approved in this final version. NSH is guarantor for the study.
Funding: Medical Research Council (grant number G0701628) and British Lung Foundation (grant number TC 04/4).
Competing interests: HM has held a consultancy with ARK therapeutics relating to ACE-I and muscle efficiency. The other authors have no conflicts of interest to declare.
Ethics approval: Ethics Committee of King's College Hospital (05/Q0703/134).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Requests for data sharing can be made to the corresponding author.
References
- 1.Seymour JM, Spruit MA, Hopkinson NS et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J 2010;36:81–8. doi:10.1183/09031936.00104909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shrikrishna D, Patel M, Tanner RJ et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J 2012;40:1115–22. doi:10.1183/09031936.00170111 [DOI] [PubMed] [Google Scholar]
- 3.Kelly JL, Elkin SL, Fluxman J et al. Breathlessness and skeletal muscle weakness in patients undergoing lung health screening in primary care. COPD 2013;10:40–54. doi:10.3109/15412555.2012.727923 [DOI] [PubMed] [Google Scholar]
- 4.Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996;153:976–80. doi:10.1164/ajrccm.153.3.8630582 [DOI] [PubMed] [Google Scholar]
- 5.Shrikrishna D, Hopkinson NS. Chronic obstructive pulmonary disease: consequences beyond the lung. Clin Med 2012;12:71–4. doi:10.7861/clinmedicine.12-1-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swallow EB, Reyes D, Hopkinson NS et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007;62:115–20. doi:10.1136/thx.2006.062026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hopkinson NS, Polkey MI. Does physical inactivity cause chronic obstructive pulmonary disease?. Clin Sci (Lond) 2010;118:565–72. doi:10.1042/CS20090458 [DOI] [PubMed] [Google Scholar]
- 8.Gimeno-Santos E, Frei A, Steurer-Stey C et al. Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax 2014;69:731–9. doi:10.1136/thoraxjnl-2013-204763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopkinson NS, Eleftheriou KI, Payne J et al. +9/+9 Homozygosity of the bradykinin receptor gene polymorphism is associated with reduced fat-free mass in chronic obstructive pulmonary disease. Am J Clin Nutr 2006;83:912–17. [DOI] [PubMed] [Google Scholar]
- 10.Hopkinson NS, Li KW, Kehoe A et al. Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr 2008;87:385–90. [DOI] [PubMed] [Google Scholar]
- 11.Hopkinson NS, Nickol AH, Payne J et al. Angiotensin converting enzyme genotype and strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:395–9. doi:10.1164/rccm.200304-578OC [DOI] [PubMed] [Google Scholar]
- 12.McCarthy B, Casey D, Devane D et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015(2):CD003793 doi:10.1002/14651858.CD003793.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodd JW, Hogg L, Nolan J et al. The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax 2011;66:425–9. doi:10.1136/thx.2010.156372 [DOI] [PubMed] [Google Scholar]
- 14.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 2006;86:747–803. doi:10.1152/physrev.00036.2005 [DOI] [PubMed] [Google Scholar]
- 15.Cabello-Verrugio C, Morales MG, Rivera JC et al. Renin–angiotensin system: an old player with novel functions in skeletal muscle. Med Res Rev 2015;35:437–63. doi:10.1002/med.21343 [DOI] [PubMed] [Google Scholar]
- 16.Costerousse O, Allegrini J, Lopez M et al. Angiotensin I-converting enzyme in human circulating mononuclear cells: genetic polymorphism of expression in T-lymphocytes. Biochem J 1993;290:33–40. doi:10.1042/bj2900033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danser AHJ, Schalekamp MADH, Bax WA et al. Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation 1995;92:1387–8. doi:10.1161/01.cir.92.6.1387 [DOI] [PubMed] [Google Scholar]
- 18.Tiret L, Rigat B, Visvikis S et al. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet 1992;51:197–205. [PMC free article] [PubMed] [Google Scholar]
- 19.Jones A, Woods DR. Skeletal muscle RAS and exercise performance. Int J Biochem Cell Biol 2003;35:855–66. doi:10.1016/S1357-2725(02)00342-4 [DOI] [PubMed] [Google Scholar]
- 20.Ma F, Yang Y, Li X et al. The Association of Sport Performance with ACE and ACTN3 Genetic Polymorphisms: a systematic review and meta-analysis. PLoS One 2013;8:e54685 doi:10.1371/journal.pone.0054685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onder G, Penninx BWJH, Balkrishnan R et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet 2002;359:926–30. doi:10.1016/s0140-6736(02)08024-8 [DOI] [PubMed] [Google Scholar]
- 22.Singh SJ, Morgan MD, Scott S et al. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992;47:1019–24. doi:10.1136/thx.47.12.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiner MC, Barton RL, Singh SJ et al. Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J 2002;19:626–31. doi:10.1183/09031936.02.00279602 [DOI] [PubMed] [Google Scholar]
- 24.Guyatt GH, Berman LB, Townsend M et al. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;42:773–8. doi:10.1136/thx.42.10.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones PW, Harding G, Berry P et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648–54. doi:10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 26.Shrikrishna D, Hopkinson NS. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Respir Med 2009;5:7–13. doi:10.1016/j.rmedu.2009.01.002 [Google Scholar]
- 27.Maddocks M, Shrikrishna D, Vitoriano S et al. Skeletal muscle adiposity is associated with physical activity, exercise capacity and fibre shift in COPD. Eur Respir J 2014;44:1188–98. doi:10.1183/09031936.00066414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natanek SA, Gosker HR, Slot IGM et al. Heterogeneity of quadriceps muscle phenotype in chronic obstructive pulmonary disease (COPD); implications for stratified medicine?. Muscle Nerve 2013;48:488–97. doi:10.1002/mus.23784 [DOI] [PubMed] [Google Scholar]
- 29.Swallow EB, Gosker HR, Ward KA et al. A novel technique for nonvolitional assessment of quadriceps muscle endurance in humans. J Appl Physiol 2007;103:739–46. doi:10.1152/japplphysiol.00025.2007 [DOI] [PubMed] [Google Scholar]
- 30.Evans RA, Kaplovitch E, Beauchamp MK et al. Is quadriceps endurance reduced in COPD?: a systematic review. Chest 2015;147:673–84. doi:http://dx.doi.org/10.1378/chest.14-1079 [DOI] [PubMed] [Google Scholar]
- 31.Shrikrishna D, Astin R, Kemp PR et al. Renin–angiotensin system blockade: a novel therapeutic approach in chronic obstructive pulmonary disease. Clin Sci 2012;123:487–98. doi:10.1042/cs20120081 [DOI] [PubMed] [Google Scholar]
- 32.Song YH, Li Y, Du J et al. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest 2005;115:451–8. doi:10.1172/JCI22324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell ST, Wyke SM, Tisdale MJ. Mechanism of induction of muscle protein degradation by angiotensin II. Cell Signal 2006;18:1087–96. doi:10.1016/j.cellsig.2005.09.009 [DOI] [PubMed] [Google Scholar]
- 34.Crul T, Spruit MA, Gayan-Ramirez G et al. Markers of inflammation and disuse in vastus lateralis of chronic obstructive pulmonary disease patients. Eur J Clin Invest 2007;37:897–904. doi:doi:10.1111/j.1365-2362.2007.01867.x [DOI] [PubMed] [Google Scholar]
- 35.Vogiatzis I, Stratakos G, Simoes DCM et al. Effects of rehabilitative exercise on peripheral muscle TNF{alpha}, IL-6, IGF-I and MyoD expression in patients with COPD. Thorax 2007;62:950–6. doi:10.1136/thx.2006.069310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang B, Tanaka H, Shono N et al. The I allele of the angiotensin-converting enzyme gene is associated with an increased percentage of slow-twitch type I fibers in human skeletal muscle. Clin Genet 2003;63:139–44. doi:10.1034/j.1399-0004.2003.00029.x [DOI] [PubMed] [Google Scholar]
- 37.Feng X, Luo Z, Ma L et al. Angiotensin II receptor blocker telmisartan enhances running endurance of skeletal muscle through activation of the PPAR-delta/AMPK pathway. J Cell Mol Med 2011;15:1572–81. doi:10.1111/j.1582-4934.2010.01085.x JCMM1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoll J, Monassier L, Garnier A et al. ACE inhibition prevents myocardial infarction-induced skeletal muscle mitochondrial dysfunction. J Appl Physiol 2006;101:385–91. doi:10.1152/japplphysiol.01486.2005 [DOI] [PubMed] [Google Scholar]
- 39.Luquet S, Lopez-Soriano J, Holst D et al. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J 2003;17:2299–301. doi:10.1096/fj.03-0269fje [DOI] [PubMed] [Google Scholar]
- 40.Sathyapala SA, Kemp P, Polkey MI. Decreased muscle PPAR concentrations: a mechanism underlying skeletal muscle abnormalities in COPD?. Eur Respir J 2007;30:191–3. doi:30/2/191 [pii] 10.1183/09031936.00068907 [DOI] [PubMed] [Google Scholar]
- 41.Arany Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev 2008;18:426–34. doi:10.1016/j.gde.2008.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Remels AH, Schrauwen P, Broekhuizen R et al. Peroxisome proliferator-activated receptor expression is reduced in skeletal muscle in COPD. Eur Respir J 2007;30:245–52. doi:10.1183/09031936.00144106 [DOI] [PubMed] [Google Scholar]
- 43.Tkacova R, Joppa P. Angiotensin-converting enzyme genotype and C-reactive protein in patients with COPD. European Respir J 2007;29:816–17. doi:10.1183/09031936.00147506 [DOI] [PubMed] [Google Scholar]
- 44.Montgomery H, Clarkson P, Barnard M et al. Angiotensin-converting-enzyme gene insertion/deletion polymorphism and response to physical training. Lancet 1999;353:541–5. doi:10.1016/S0140-6736(98)07131-1 [DOI] [PubMed] [Google Scholar]
- 45.Di Bari M, Van De Poll-Franse LV, Onder G et al. Antihypertensive medications and differences in muscle mass in older persons: The Health, Aging and Body Composition Study. J Am Geriatr Society 2004;52:961–6. doi:10.1111/j.1532-5415.2004.52265.x [DOI] [PubMed] [Google Scholar]
- 46.Sumukadas D, Witham MD, Struthers AD et al. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ 2007;177:867–74. doi:177/8/867 [pii] 10.1503/cmaj.061339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanazawa H, Hirata K, Yoshikawa J. Effects of captopril administration on pulmonary haemodynamics and tissue oxygenation during exercise in ACE gene subtypes in patients with COPD: a preliminary study. Thorax 2003;58:629–31. doi:10.1136/thorax.58.7.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bertoli L, Lo Cicero S, Busnardo I et al. Effects of captopril on hemodynamics and blood gasses in chronic obstructive lung disease with pulmonary hypertension. Respiration 1986;49:251–6. doi:10.1159/000194887 [DOI] [PubMed] [Google Scholar]
- 49.Shrikrishna D, Tanner RJ, Lee JY et al. A randomized controlled trial of angiotensin-converting enzyme inhibition for skeletal muscle dysfunction in COPD. Chest 2014;146:932–40. doi:10.1378/chest.13-2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curtis KJ, Meyrick VM, Mehta B et al. Angiotensin-converting enzyme inhibition as an adjunct to pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2016;194:1349–57. doi:10.1164/rccm.201601-0094OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrell NW, Higham MA, Phillips PG et al. Pilot study of losartan for pulmonary hypertension in chronic obstructive pulmonary disease. Respir Res 2005;6:88 doi:10.1186/1465-9921-6-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zielinski J, Hawrylkiewicz I, Gorecka D et al. Captopril effects on pulmonary and systemic hemodynamics in chronic cor pulmonale. Chest 1986;90:562–5. doi:10.1378/chest.90.4.562 [DOI] [PubMed] [Google Scholar]
- 53.Di Marco F, Guazzi M, Vicenzi M et al. Effect of enalapril on exercise cardiopulmonary performance in chronic obstructive pulmonary disease: a pilot study. Pulm Pharmacol Ther 2010;23:159–64. doi:10.1016/j.pupt.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 54.Mortensen EM, Copeland LA, Pugh MJ et al. Impact of statins and ACE inhibitors on mortality after COPD exacerbations. Respir Res 2009;10:45 doi:10.1186/1465-9921-10-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mancini GB, Etminan M, Zhang B et al. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol 2006;47:2554–60. doi:10.1016/j.jacc.2006.04.039 [DOI] [PubMed] [Google Scholar]
- 56.Gosker HR, Pennings HJ, Schols AM. ACE gene polymorphism in COPD. Am J Respir Crit Care Med 2004;170:572.; author reply 72–3 doi:10.1164/ajrccm.170.5.950 [DOI] [PubMed] [Google Scholar]
- 57.Boutou AK, Shrikrishna D, Tanner RJ et al. Lung function indices for predicting mortality in chronic obstructive pulmonary disease. Eur Respir J 2013;42:616–25. doi:10.1183/09031936.00146012 [DOI] [PMC free article] [PubMed] [Google Scholar]