Abstract
Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) act on gonadal cells to promote steroidogenesis and gametogenesis. Clarifying the in vivo roles of LH and FSH permits a feasible approach to contraception involving selective blockade of gonadotropin action. One way to address these physiologically important problems is to generate mice with an isolated LH deficiency and compare them with existing FSH loss-of-function mice. To model human reproductive disorders involving loss of LH function and to define LH-responsive genes, we produced knockout mice lacking the hormone-specific LHβ-subunit. LHβ-null mice are viable but demonstrate postnatal defects in gonadal growth and function resulting in infertility. Mutant males have decreased testes size, prominent Leydig cell hypoplasia, defects in expression of genes encoding steroid biosynthesis pathway enzymes, and reduced testosterone levels. Furthermore, spermatogenesis is blocked at the round spermatid stage, causing a total absence of the elongated spermatids. Mutant female mice are hypogonadal and demonstrate decreased levels of serum estradiol and progesterone. Ovarian histology demonstrates normal thecal layer, defects in folliculogenesis including many degenerating antral follicles, and absence of corpora lutea. The defects in both sexes are not secondary to aberrant FSH regulation, because FSH levels were unaffected in null mice. Finally, both male and female null mice can be pharmacologically rescued by exogenous human chorionic gonadotropin, indicating that LH-responsiveness of the target cells is not irreversibly lost. Thus, LHβ null mice represent a model to study the consequences of an isolated deficiency of LH ligand in reproduction, while retaining normal LH-responsiveness in target cells.
Keywords: gonads, pituitary, testosterone, spermatogenesis, folliculogenesis
Luteinizing hormone (LH) is a member of the glycoprotein hormone family that includes follicle-stimulating hormone (FSH) and thyroid-stimulating hormone (1, 2). These hormones are heterodimers consisting of a common α-subunit noncovalently linked to a hormone-specific β-subunit. LH binds to G-protein coupled receptors on Leydig cells in the testis and granulosa and theca cells in the ovary (1, 2). During pubertal and postpubertal gonad development, LH promotes steroidogenesis required for normal reproductive function (1, 2). Less clear are its functions during embryonic gonad development, although evidence suggests that early gonad development is independent of gonadotropin stimulation (3, 4).
Intercellular communication within the testis is essential for normal spermatogenesis (5, 6). In response to FSH, the Sertoli cells secrete various factors that affect Leydig cell function (7, 8). Similarly, testosterone produced from Leydig cells is required for spermatogenesis (7, 8). In females, ovarian folliculogenesis and ovulation are critically dependent on synchronized activities of both FSH and LH (9, 10). Previously, we and others have used genetic models, including FSHβ, FSH-receptor, and LH-receptor knockout mice, to study the physiological roles of gonadotropins in gonad development and function (11–15). Whereas FSHβ and FSH-receptor knockout mice mostly phenocopy human ovarian dysgenesis disease (16, 17), LH-receptor mutant mice demonstrate most of the phenotypes associated with inactivating LH-receptor mutations in human males, including Leydig cell hypoplasia (18, 19). Only a single male patient with an inactivating mutation in the LHβ gene has been described (20). The consequences of absence of LH ligand in female reproduction are unknown. To date, there is no loss-of-function mouse model available for an in vivo analysis of the roles of LH ligand. Further, there are no known naturally occurring mutations at the LHβ locus in mice. Hence, it is unknown whether mice with a loss of LH ligand function would be distinct or phenocopy the LH-receptor knockout mice.
In hypogonadal (hpg) and α-glycoprotein subunit knockout mice, both LH and FSH are either suppressed or absent (21, 22). Although these models were useful for understanding the modifier roles of gonadotropins in tumor-prone inhibin α knockout mice (23), they are not useful for studying an isolated deficiency of only LH or FSH in normal reproductive physiology. A mouse model lacking only LH ligand eventually permits defining LH-responsive genes that play critical roles in gonad development and function. To study the effects of only LH independent of FSH in reproductive physiology, to generate mouse models for human reproductive disorders involving selective loss of LH function, and to define the role of LH in gonadal tumor development in inhibin α-null mice, we characterized LHβ knockout mice. These mice have normal FSH levels, demonstrate hypogonadism, have defects in steroid biosynthesis, and are infertile. Furthermore, mutant mice were pharmacologically rescued by exogenous human chorionic gonadotropin (hCG), indicating that the responses to LH stimulation were not irreversibly lost in these mutants.
Materials and Methods
ES Cell Manipulation and Generation of LHβ Knockout Mice. Targeting vector to disrupt the coding sequence of the mouse LHβ gene was electroporated into the hypoxanthine phosphoribosyltransferase (hprt) -negative AB2.2 ES cell line, and cell clones were selected by the double-selection enrichment method described in refs. 11 and 24. DNA from the expanded clones was analyzed by Southern blot analysis, and targeted ES cell clones were injected into blastocysts to obtain chimeras as described in refs. 11 and 24. Multiple male chimeras were bred to C57BL/6J females to generate initially heterozygous and subsequently homozygous LHβ mutant mice. Southern blot analysis of tail DNA was performed as described in ref. 11.
Fertility Analysis. Mutant mice were mated to controls beginning at 42 days of age. The number of litters and pups per litter born over a 6-month period were recorded. Histological analysis was performed as described in refs. 11 and 25.
RNA Analysis. Total RNA was isolated by using TRIzol (Invitrogen). RNA blots were hybridized with an Hsd3b6 probe, washed, and exposed to autoradiographic film as described in refs. 11 and 23. Blots were stripped and rehybridized with a cyclophilin A cDNA probe to check for equal loading of RNA.
RT-PCR. Total RNA (1 μg) was reverse-transcribed, and the first-strand cDNA templates were amplified with each of the primer pairs (see Table 1, which is published as supporting information on the PNAS web site) in independent sets of PCR reactions. The concentration of Mg2+ and the linear range of amplification of cDNAs with each primer pair first were optimized, and cDNAs subsequently were tested. The expression of cyclophilin A was used as a control.
Western Blot Analysis. Proteins (20 μg) were separated on 10% SDS-polyacrylamide gels, and transferred to polyvinylidene fluoride membranes (Bio-Rad). Immunodetection was performed with an enhanced chemiluminescence detection system (Amersham Pharmacia) and appropriate rabbit primary antibodies. The blots were stripped and reprobed with a rabbit β-tubulin antiserum to confirm equal loading of proteins. All antibodies used were purchased from Santa Cruz Biotechnology, except for rabbit anti-mouse histone H1-like linker protein (gift from W. Yan, University of Nevada School of Medicine, Reno, NV), anti-FSHβ (purchased from Genzyme), and anti-hCGβ serum that crossreacts with mouse LHβ (gift from I. Boime, Washington University School of Medicine, St. Louis, MO).
Pituitary Hormone Immunofluorescence. Cryosections of pituitaries were used for double immunofluorescence with LHβ and FSHβ antisera and visualized with dye-conjugated second antibodies as described in ref. 26.
Hormone Assays. Serum samples of LH and FSH were assayed by the standard protocols of the Ligand Assay and Analysis Core Laboratory (University of Virginia, Charlottesville, VA). Androstenedione and testosterone levels were estimated by using RIA kits (Diagnostic Systems Laboratories, Webster, TX) according to the manufacturer's instructions. Anti-Müllerian hormone (AMH) was measured by ELISA using human AMH reagents according to the manufacturer's instructions (Diagnostic Systems Laboratories).
Pharmacological Rescue. Immature mice (21–23 days old) were injected with 5 units of hCG, and 24 h later, testes were isolated and total RNA was isolated as described above. Superovulation of immature female mice was performed as described in ref. 11. Testosterone pellets (Innovative Research of America) were implanted s.c. into male mice, and 1 week later, testes were collected for RNA isolation and RT-PCR assays as described above.
Statistical Analyses. Student's t test or one-way ANOVA was carried out with excel (Version 6.0, Microsoft) software. Differences were considered significant at P < 0.05.
Results
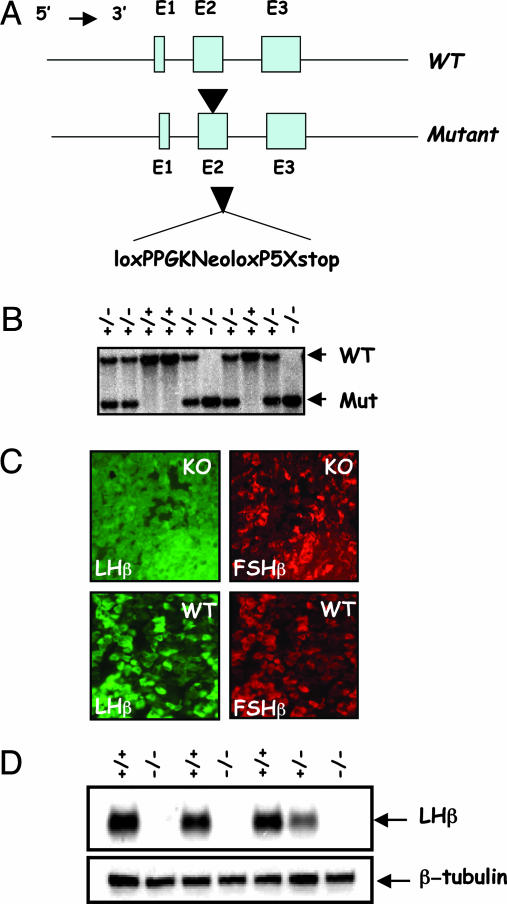
Gene Targeting at the LHβ Locus. Heterodimerization of the LHβ-subunit with the α-glycoprotein subunit is essential for the biological activity of LH (1, 2). LHβ mature protein is encoded by exons 2 and 3. To generate mice lacking LH, hormone-specific LHβ gene was disrupted by inserting a loxP–neomycin–loxP cassette into exon 2 by homologous recombination in ES cells (Fig. 1A). This strategy led to the generation of a mutant LHβ allele resulting in translational disruption of the LHβ polypeptide and, hence, LH deficiency. Southern blot analysis identified all genotypes of mutant mice at the weaning age in a Mendelian ratio of 1:2:1 for WT/heterozygous/null, suggesting that LH deficiency did not affect embryonic development and viability (Fig. 1B). To confirm whether the mutation resulted in absence of LH, we assayed immunoreactive LH by three methods. First, immunofluorescent analysis of the pituitaries from WT mice indicated the presence of gonadotropes to which both LHβ and FSHβ antibodies colocalized. In contrast, pituitary sections from homozygous mutants showed the absence of LHβ-specific staining but the presence of FSHβ-specific staining and grossly normal numbers of gonadotropes (Fig. 1C). Second, Western blot analysis indicated the absence of an immunoreactive band corresponding to LHβ in the pituitary extracts of null (–/–) mice but not in those of heterozygous (+/–) or WT (+/) control mice (Fig. 1D). Finally, serum levels of LH were undetectable in null mice (data not shown). These results indicate that we engineered a null mutation at the LHβ locus that led to LH deficiency.
Fig. 1.
Gene targeting at the LHβ locus. (A) A phosphoglycerate kinase-neomycin expression cassette was engineered to contain a 5′ loxP sequence and on the 3′ side another loxP sequence followed by five stop codons. This cassette was inserted into exon 2 of the mouse LHβ gene in ES cells by homologous recombination (arrowhead). The floxed phosphoglycerate kinase-neomycin cassette was flanked by 6.3 kb of 5′ and 6 kb of 3′ LHβ gene sequences originally cloned from a 129S6/SvEv mouse genomic library. (B) Southern blot shows WT and mutant alleles identified by their size (7.3 and. 3.4 kb, respectively) to distinguish the genotypes of mice. (C) Dual immunofluorescence of pituitary sections shows that LHβ-specific staining (labeled green) was absent in a pituitary section from the null mouse (KO) compared with that in control (WT). FSHβ-specific staining (in red) is seen in both control and null mice. (D) Immunoblot of pituitary proteins shows the presence of LHβ-reactive band only in control pituitary extracts (+/+ and +/–) but not in that of the null (–/–) mice, thus confirming that we engineered a null mutation at the LHβ locus. Because heterodimer assembly confers biological activity to glycoprotein hormones, absence of LHβ-subunit leads to LH deficiency in these null mice.
LHβ Knockout Mice Are Infertile. Heterozygous matings, similar to WT mice, produced normal number of pups per litter (8.8 ± 0.3 pups per litter in 22 litters for heterozygous mice vs. 8.4 ± 0.2 pups per litter in 107 litters for WT mice; P > 0.05). In contrast, matings between either male (5 of 5) or female (10 of 10) null mice and controls did not result in any pups over a 6-month period, indicating that LHβ null mice were infertile.
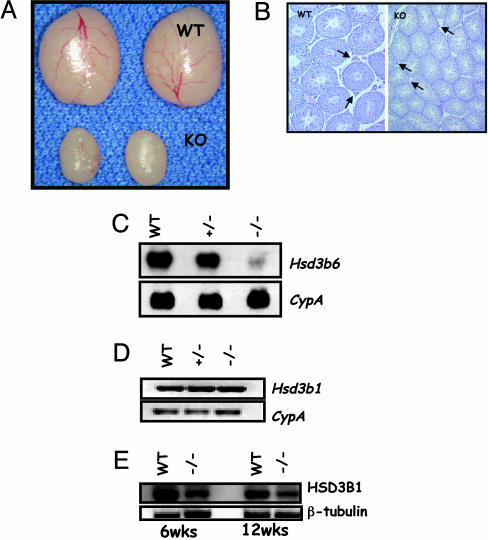
Hypogonadism, Leydig Cell Defects, and Hypoplastic Accessory Sex Glands in LHβ Knockout Male Mice. Morphological examination of the mutants indicated hypoplastic external genitalia, suggestive of possible defects in steroid biosynthesis. To further define the causes of infertility of null males, we examined the testes morphologically and histologically. The testis size was decreased significantly in homozygous mutants compared with that in controls at 42 days and in all ages tested (Fig. 2A and Fig. 5A, which is published as supporting information on the PNAS web site; data not shown). Whereas abundant Leydig cells were readily apparent in the testis interstitium of adult control mice, the null testes demonstrated insignificant interstitium containing very few Leydig cells of smaller size (Fig. 2B). Consistent with these findings, serum and testicular testosterone levels were significantly suppressed in mutants (Fig. 5B). Furthermore, serum levels of androstenedione, a testosterone precursor, were elevated significantly in mutants (Fig. 5B). LH promotes steroidogenesis in Leydig cells. To determine which of the Leydig cell genes encoding testosterone biosynthesis pathway enzymes are altered in the absence of LH, we analyzed the expression of various markers. We found that Leydig cell markers, including Hsd3b6, Cyp17a1, and Hsd 17b3, were suppressed in the absence of LH (Figs. 2C and 5C). Although mRNA levels of Hsd3b1, another Leydig cell marker, were not affected (Fig. 2D), the corresponding protein (HSD3B1) levels decreased with age in the mutant testes (Fig. 2E).
Fig. 2.
Male reproductive phenotypes of LHβ knockout mice. (A) Morphology of testes from adult (6 weeks) control (WT) and null (KO) male mice. Note the reduction in size due to the absence of LHβ.(B Left) Low-power histology shows many normal Leydig cells in the WT testis (arrows), whereas the mutant testis (Right) shows sparse interstitium and small Leydig cells (arrows). Note the reduction in tubule size in KO testis section. (Photographed at ×5 magnification.) (C Upper) Northern blot analysis indicates that expression of adult Leydig cell specific 3β-hydroxysteroid dehydrogenase type VI (Hsd3b6) is suppressed in the null (–/–) testes. (Lower) Hybridization with a cyclophilin (CypA) probe confirms that an equal amount of RNA was loaded in each lane. (D and E) Expression of 3β-hydroxysteroid dehydrogenase type I in the mutant testes assessed by RT-PCR assay (D) and Western blot analysis (E) shows that although the steady-state levels of RNA were not changed, the protein is reduced in the null (–/–) testes. CypA amplification was used as a control for RT-PCR assay, and β-tubulin expression was used as an internal control for Western blot analysis.
Previous studies suggested that fetal Leydig cells originate independent of LH signaling. To verify whether fetal Leydig cells are present in the mutant testes, we analyzed the expression of thrombospondin 2 (Thbs2), a fetal Leydig cell marker, which is normally very low in the adult testis (27, 28). We observed that expression of Thbs2 is persistent in the testes of adult null mice (Fig. 5D). These data confirm that fetal Leydig cells continue to persist in the mutant testes despite the complete absence of LH.
In the adult testis, LH stimulates proliferation and differentiation of immature Leydig cells. These terminally differentiated cells produce testosterone and undergo minimal proliferation. To test two key markers of cell proliferation, we determined the levels of p27, a cell-cycle inhibitor, and proliferating cell nuclear antigen. Levels of p27 were decreased, whereas expression of proliferating cell nuclear antigen was increased with age in the mutant testes as determined by Western blot analysis (Fig. 5E), confirming aberrant cell-cycle regulation in the testes of LHβ knockout mice.
As a secondary consequence to the above Leydig cell defects and testosterone production, the accessory glands, epididymides and seminal vesicles, were reduced in size compared with those in controls (Fig. 5A). Collectively, the above data indicate that absence of LH in the male results in hypogonadism, a block in Leydig cell differentiation, severely reduced testosterone levels, and hypoplastic accessory sex glands.
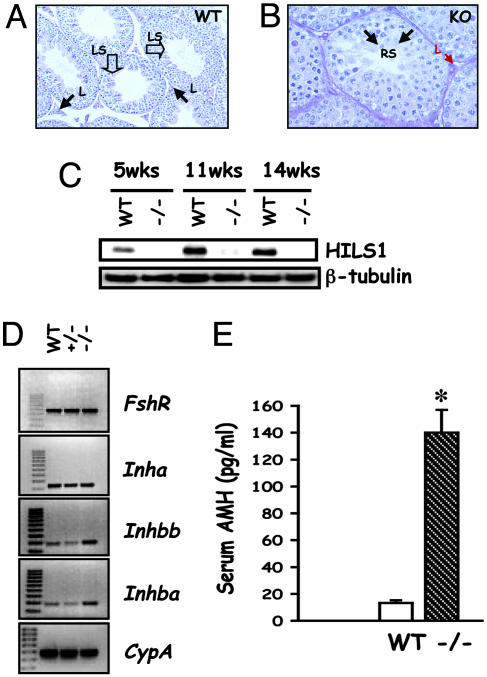
Defects in Spermatogenesis and Aberrant Sertoli Cell Gene Expression. LH signaling is critical for testosterone production from the Leydig cells, and testosterone is important for spermatogenesis. To analyze the consequences of significantly reduced testosterone on spermatogenesis in mutants, the testes were analyzed histologically, and the expression of spermatogenesis-specific markers was assessed. The mutant testes, like those of the control, contained spermatogonia, meiotic cells (spermatocytes), and round spermatids (Fig. 3 A and B). However, elongated and late-stage spermatids were completely absent with no sperm present in the lumen of the tubules in the mutant testes (Fig. 3B). Consistent with these histological findings, expression of histone H1-like linker protein (HILS1), a late-stage spermatid marker, was completely suppressed in the mutant testes (Fig. 3C). These results confirm that spermatogenesis was arrested at the round spermatid stage in the absence of LH.
Fig. 3.
Spermatogenesis arrest and Sertoli cell marker expression. (A and B) High-power histology of WT (A) and KO (B) testis sections shows Leydig cells (arrows, L). In contrast to many late-stage spermatids (open arrows, LS) and abundant sperm in the lumen of WT testis (A), spermatogenesis proceeds up to the round-spermatid stage (arrows, RS) in the KO testis (B). Note that other stages of spermatogenesis grossly appear normal in the KO testis section (B). (Magnification: A, ×10; B, ×20.) (C) Western blot analysis shows that HILS1, a late spermatid stage-specific marker, is not expressed in the mutant testis and confirms the block in spermatogenesis at the transition between round to elongated spermatid step. (D) RT-PCR analysis of testis cDNAs identifies that expression of Sertoli cell markers, including activin subunits, Inhba and Inhbb was increased, whereas that of FSH receptor and inhibin-α in the mutant testes remained unchanged. (E) Assay of serum levels of AMH indicates that this Sertoli cell marker was elevated in the mutant (–/–). *, P < 0.05 (n = 4).
Sertoli cells are major targets of androgen action within the testis. Leydig cell-derived testosterone is important for various aspects of Sertoli cell development and function. Members of the transforming growth factor β family, including inhibins, activins, and AMH, normally are secreted from Sertoli cells. To test whether Sertoli cell gene expression was affected due to the absence of LH, we performed RT-PCR assays and identified that, although expression of FSH receptor and inhibin α-subunit was not affected, expression of both inhibin βA- and βB-subunits was up-regulated in the absence of LH signaling (Fig. 3D). Similarly, serum levels of AMH were elevated in null mice compared with controls (Fig. 3E). These data indicate that Sertoli cell markers are regulated aberrantly because of LH deficiency.
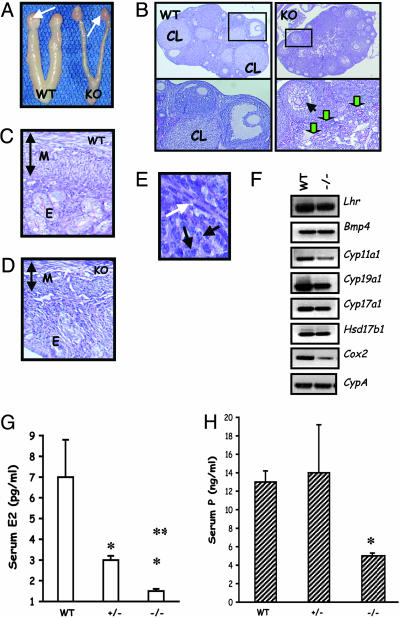
Ovarian Folliculogenesis Is Blocked at the Antral Stage in LHβ Knockout Female Mice. Consistent with the external genitalia findings, female LHβ null mice demonstrated small ovaries and thin uteri (Fig. 4A). Histological analysis of the ovaries from adult mutants revealed the absence of healthy antral, preovulatory follicles and corpora lutea, confirming impaired estrous cycles (Fig. 4B Right). Although primary and secondary follicles appeared normal, many antral follicles looked abnormal, collapsing with degenerating oocytes (Fig. 4B Right Lower). Occasionally, non-hemorrhagic cysts in some of the ovary sections were observed (data not shown). In many ovarian sections, periodic acid-Schiff/hematoxylin reagent-stained zona pellucida remnants were obvious, indicating prominent apoptosis of oocyte/follicles (shown with green arrows in Fig. 4B Right Lower). Although granulosa cells looked abnormal in many degenerating antral follicles, a prominent thecal cell layer was present in multiple follicles at different stages of progression (Fig. 4E and data not shown). Within the ovary, LH acts on thecal cells that express LH receptors. Expression analysis indicated that thecal differentiation markers, including LH receptor, Bmp4, and Cyp17a1, all were expressed in the null ovaries. This finding confirms that differentiation of thecal layer was not impaired in the absence of LH (Fig. 4F). Although an intact theca was present in the mutant ovary, expression of many genes encoding steroid biosynthesis enzymes, including Cyp11a1, Cyp19a1, and Cyp17a1, were reduced. Additionally, expression of cyclooxygenase 2 (Cox2), a marker for ovulation, also was reduced in the null ovary (Fig. 4F). Consistent with these defects in steroidogenic markers, serum estradiol and progesterone levels both were decreased in the adult null females compared with those in WT controls (Fig. 4 G and H). This reduction in steroid hormone levels was reflected further in the histology of the null uteri that displayed severe hypoplasia with complete reduction of all three layers of the uterus (Fig. 4 C and D). Collectively, these data indicate that absence of LH does not affect theca formation but leads to decreased steroid production, defects in ovarian folliculogenesis, and hypoplastic uteri.
Fig. 4.
Female reproductive phenotypes. (A) Morphological analysis of the female reproductive tract indicates hypoplastic uteri and ovaries in the mutant (KO, Right) compared with those in a WT control (Left) at 9 weeks. White arrows denote ovaries. (B) Low-power histology of the ovary shows many corpora lutea (CL) in the WT (Left) but not in the mutant (KO, Right). Multiple follicles at different stages are present in the mutant section, but preovualtory follicles are not present (Right Upper). (Left Lower) A magnified region (rectangle) of the WT section that contains CL, an antral follicle with a healthy oocyte. (Right Lower) A magnified region (rectangle) of the KO ovary section with a follicle containing a collapsed oocyte (black arrow) and many degenerating follicles with remnants of zona pellucida (green arrows). (Magnification: ×5.) (C and D) Histology of a 9-week-old null (KO, D) mouse uterus shows a thin myometrium (M) and hypoplastic endometrium (E), compared with that from a control (WT) mouse (C). The vertical doublehead arrows indicate the relative thickness of the myometrium in both cases. (E) High-power image of a follicle from KO ovary shows theca (white arrow) is formed in the absence of LH. Black arrows indicate granulosa cells for reference to the interior of the follicle. (Magnification: ×20.) (F) RT-PCR assay indicates that thecal cell markers LH receptor and BMP4 are expressed in the null (–/–) ovary. Many steroidogenic pathway enzymes and cycloxygenase 2 are suppressed in the null ovary in the absence of LH. Lhr, LH receptor; Bmp4, bone morphogenetic protein 4; Cyp11a1, P450 side chain cleavage enzyme11a1; Cyp19a1, cytochrome P450 aromatase; Cyp17a1, cytochrome P450 17-α hydroxylase; Hsd17b1, 17-β dehydrogenase type I; Cox2, cycloxygenase 2. (G and H) Consistent with the ovarian and uterine phenotype, serum estradiol (E2; G) and progesterone (P; H) are suppressed in the null mutants (–/–), but only E2 levels are suppressed in the heterozygous mutants (+/–). *, P < 0.05 vs. WT; **, P < 0.05 vs. ±.
Responsiveness to LH Is Retained in the Gonads of LHβ Knockout Mice. Expression of LH receptor is maintained in the gonads of LHβ knockout mice despite multiple defects in the target cells. To test whether mutant mice respond to exogenous LH, immature males were injected with hCG, which binds and activates LH receptors. In this rescue experiment, expression of Hsd3b6 and Cyp17a RNAs (suppressed in the mutants compared with WT controls) was restored in mutants injected with hCG to comparable levels in control WT mice (see Fig. 6A, which is published as supporting information on the PNAS web site). Similarly, female mice responded to exogenous hCG as indicated by activation of ovary-specific marker genes (Fig. 6B). Furthermore, superovulation of immature LHβ null females resulted in the release of a comparable number of ova obtained with similarly treated immature control mice (Fig. 6C). These results support the hypothesis that LH responsiveness is not irreversibly lost in the absence of LH in LHβ knockout mice.
The absence of circulating LH and a severe reduction in serum and testicular testosterone in LHβ knockout mice permit an in vivo analysis of identifying LH-responsive vs. testosterone-responsive genes in the Leydig cells. To further confirm whether short-term steroid replacement pharmacologically rescues expression of the Leydig cell markers, we performed RT-PCR assays and analyzed the expression of three important Leydig cell genes after 1-week treatment of WT and LHβ knockout male mice with testosterone pellets. Whereas expression of both Hsd3b1 and Hsd3b6 was rescued (see rows 1 and 2 of Fig. 7, which is published as supporting information on the PNAS web site), Cyp17a1 remained suppressed (Fig. 7, row 3) in the mutant after 1 week. These data indicate that Cyp17a1 is regulated in mouse Leydig cells directly by LH action that does not require testosterone.
FSH Levels Are Unchanged in LHβ Knockout Mice. LH and FSH are both synthesized in the gonadotropes and share a common α-subunit. Absence of LHβ in gonadotropes may affect the heterodimer assembly of α-FSHβ-subunits and lead to aberrant FSH synthesis and/or secretion. To determine whether absence of LHβ leads to changes in FSHβ production, we performed Western blot analysis of pituitary proteins from male and female mice by using a FSHβ-specific monoclonal antibody. We found that expression of the FSHβ-subunit was not altered significantly in the pituitaries of null mutants compared with that in controls (see Fig. 8 A and C, which is published as supporting information on the PNAS web site). In accordance with this finding, serum levels of FSH in both male and female LHβ knockout mice were comparable with those in control mice (Fig. 8 B and D). These data indicate that FSH homeostasis is unaffected in the absence of LH, and the resultant phenotypes of LHβ knockout mice did not manifest because of FSH imbalance.
Discussion
With the availability of previously reported mouse models (11–15, 23, 29–31), and the LHβ knockout mice described here, gonadotropin action now can be manipulated in vivo by at least four methods. These include gain-of-function and loss-of-function models for FSH and LH function. LHβ knockout mice mostly phenocopy LH-receptor knockout mice, although LH receptors are expressed much earlier than LHβ-subunit in the pituitary during mouse embryogenesis (15, 32, 33). Similar to LH-receptor knockout mice, absence of LH ligand does not affect embryonic development and survival. These remarkable similarities in LH ligand and its cognate receptor knockout mice suggest that crosstalk in vivo with other structurally similar ligand-receptor pairs is unlikely. Although LHβ is synthesized in the pituitary in greater abundance than FSHβ-subunit, the null mutation at the LHβ locus did not affect the synthesis of FSHβ or circulating FSH heterodimer in the mutants. Accordingly, this model, unlike the LH-receptor knockout mice, which have elevated serum LH and FSH levels, permits an analysis of isolated LH ligand deficiency without affecting FSH production.
In contrast to male mice lacking FSHβ or FSH receptor, LHβ knockout male mice are infertile. These null males demonstrate defects in Leydig cells, including an arrested development at the immature stage and suppression of testosterone levels. Consistent with these findings, expression of many steroidogenic enzymes is suppressed in the mutant testes. Leydig cell development occurs in two distinct phases: one that specifies fetal Leydig cells and the other that specifies adult Leydig cell fate (8, 34–36). In the adult mouse, few fetal-type Leydig cells exist normally, whereas adult-type Leydig cells predominate (8, 34–36). The persistent expression of Thbs2, a fetal Leydig cell marker, in the testes of adult null mice confirms that LH signaling is not essential for fetal Leydig cell lineage. This finding is consistent with similar observations made by others using various genetic models (3, 4).
Spermatogenesis is blocked at the round spermatid stage, and lack of HILS1 protein clearly indicated that elongated and late-stage spermatids are absent in the mutant testis. This failure in spermatogenesis is similar to that in recently described mutant mice that selectively lack androgen receptor function in Sertoli cells but is distinct from that of androgen-insensitive tfm mice (37–39). The incomplete spermatogenesis in LHβ knockout mice suggests that FSH and low levels of testosterone cannot drive spermatogenesis past the round spermatid stage. Whether the block can be reversed by testosterone supplementation or an intact LH signaling is required for complete spermatogenesis remains to be established. Leydig cell-derived testosterone regulates Sertoli cell function, and we observed that some of the Sertoli cell-specific genes were up-regulated in the absence of LH. However, we do not know whether these changes reflect increased Sertoli cell number or lack of testosterone itself. Future stereological studies will clarify whether the Sertoli cell number is affected in LHβ knockout mice.
Similar to the phenotypes in males, LHβ knockout female mice are infertile because of folliculogenesis defects. Whereas early stage follicle recruitment is unaffected in the absence of LH, late-stage follicles at and beyond the antral stage are blocked in differentiation. Clearly, preovulatory follicles and corpora lutea are absent in the mutant ovary, and this block in folliculogenesis is distinct from that observed in FSHβ knockout mice that have a block at the preantral stage of follicle development (11). Although folliculogenesis progresses up to the antral stage in the absence of LH, the majority of the antral follicles are abnormal and appear to undergo apoptosis as evident from many zona pellucida remnants in the mutant ovary. It is not known how LH action maintains physiologically low levels of apoptotic machinery in the follicle; LHβ knockout mice may provide a useful model to address this issue that has clinical implications for reproduction in aging women.
The initial ovarian targets of LH action are thecal cells, where androgens are produced and converted to estrogens in granulosa cells (10). It is unknown whether LH signaling is essential for thecal cell recruitment and differentiation. Despite the absence of LH signaling, the thecal cell recruitment appears normal in the ovaries of null mice. As molecular evidence for this, expression of thecal cell markers including Bmp4 and LH receptor is observed in the mutant ovary. Furthermore, expression of the majority of the steroidogenic pathway enzymes is affected to varying levels in the absence of LH. This change is reflected in suppressed levels of serum estradiol and progesterone and hypoplastic uteri in null female mice. These data support the “two-cell, two-gonadotropin” hypothesis, although there is evidence to suggest gonadotropin-independent steroid production during early postnatal development in the mouse ovary (40–42).
One advantage of a mouse model lacking LH ligand is that it is possible to perform rescue experiments by providing exogenous ligands with LH activity. We pharmacologically rescued immature female null mice that superovulated in response to pregnant mare serum gonadotropin and hCG stimulation. Similarly, in the immature mutant testes, expression of Hsd3b6 and Cyp17a was restored to that in control mice. The results with rescue experiments provide molecular markers that are sensitive to the absence of LH signaling and indicate that LH responsiveness is not irreversibly lost in target cells in LHβ knockout mice. Furthermore, short-term testosterone replacement rescued the expression of only Hsd3b1 and Hsd3b6 but not Cyp17a1. This pharmacological rescue strategy provides a convenient model system for future gene-expression profiling and identifying LH- and steroid-responsive genes during distinct phases of gonad development.
In conclusion, we have developed an LHβ knockout mouse model in which LH ligand is selectively absent and pharmacologically rescued LH deficiency. LHβ knockout mice have normal FSH levels; they demonstrate infertility and defects in steroid biosynthesis and have distinct developmental blocks in spermatogenesis and folliculogenesis. These mice are useful models for further analyzing Leydig cell biology, defects in spermiogenesis, and ovarian failure. These mice are also useful for developing novel strains of mutant mice that lack both FSH and LH with an intact gonadotropin-releasing hormone-signaling pathway.
Supplementary Material
Acknowledgments
We thank Ms. Bisong Haupt for help with the mouse blastocyst injections, Drs. Irving Boime and Anita Payne (Stanford University Medical Center, Stanford, CA) for reagents, and Dr. Y. Radhakrishnan for technical assistance. This work was supported in part by Baylor College of Medicine, Department of Pathology start-up funds, a grant from The Moran Foundation (to T.R.K.), and National Institutes of Health Grant CA60651 (to M.M.M.). Serum FSH was assayed by the Hormone Assay Core at the University of Virginia, supported by National Institutes of Health Grant U54-HD28-934.
Author contributions: X.M., Y.D., and T.R.K. performed research; T.R.K. designed research; T.R.K. contributed new reagents/analytic tools; T.R.K. analyzed data; T.R.K. wrote the paper; and M.M.M. helped with ES cell work and in generating chimeric mice.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone; hCG, human chorionic gonadotropin; AMH, anti-Müllerian hormone; Thbs2, thrombospondin 2; Hsd3b, 3β-hydroxysteroid dehydrogenase.
References
- 1.Pierce, J. G. & Parsons, T. F. (1981) Annu. Rev. Biochem. 50, 465–495. [DOI] [PubMed] [Google Scholar]
- 2.Bousfield, G. R., Perry, W. M. & Ward, D. N. (1994) in The Physiology of Reproduction, eds. Knobil, E. & Neill, J. D. (Raven, New York), pp. 1749–1792.
- 3.O'Shaughnessy, P. J., Baker, P., Sohnius, U., Haavisto, A. M., Charlton, H. M. & Huhtaniemi, I. (1998) Endocrinology 139, 1141–1146. [DOI] [PubMed] [Google Scholar]
- 4.Pakarinen, P., Kimura, S., El-Gehani, F., Pelliniemi, L. J. & Huhtaniemi, I. (2002) Endocrinology 143, 4477–4482. [DOI] [PubMed] [Google Scholar]
- 5.Saez, J. M., Perrard-Sapori, M. H., Chatelain, P. G., Tabone, E. & Rivarola, M. A. (1987) J. Steroid. Biochem. 27, 317–329. [DOI] [PubMed] [Google Scholar]
- 6.Saez, J. M., Avallet, O., Lejeune, H. & Chatelain, P. G. (1991) Horm. Res. 36, 104–115. [DOI] [PubMed] [Google Scholar]
- 7.Saez, J. M., Avallet, O., Naville, D., Perrard-Sapori, M. H. & Chatelain, P. G. (1989) Ann. N.Y. Acad. Sci. 564, 210–231. [DOI] [PubMed] [Google Scholar]
- 8.Saez, J. M. (1994) Endocr. Rev. 15, 574–626. [DOI] [PubMed] [Google Scholar]
- 9.Richards, J. S. (2001) Endocrinology 142, 2184–2193. [DOI] [PubMed] [Google Scholar]
- 10.Richards, J. S., Russell, D. L., Ochsner, S., Hsieh, M., Doyle, K. H., Falender, A. E., Lo, Y. K. & Sharma, S. C. (2002) Recent Prog. Horm. Res. 57, 195–220. [DOI] [PubMed] [Google Scholar]
- 11.Kumar, T. R., Wang, Y., Lu, N. & Matzuk, M. M. (1997) Nat. Genet. 15, 201–204. [DOI] [PubMed] [Google Scholar]
- 12.Dierich, A., Sairam, M. R., Monaco, L., Fimia, G. M., Gansmuller, A., LeMeur, M. & Sassone-Corsi, P. (1998) Proc. Natl. Acad. Sci. USA 95, 13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abel, M. H., Wootton, A. N., Wilkins, V., Huhtaniemi, I., Knight, P. G. & Charlton, H. M. (2000) Endocrinology 141, 1795–1803. [DOI] [PubMed] [Google Scholar]
- 14.Zhang, F. P., Poutanen, M., Wilbertz, J. & Huhtaniemi, I. (2001) Mol. Endocrinol. 15, 172–183. [DOI] [PubMed] [Google Scholar]
- 15.Lei, Z. M., Mishra, S., Zou, W., Xu, B., Foltz, M., Li, X. & Rao, C. V. (2001) Mol. Endocrinol. 15, 184–200. [DOI] [PubMed] [Google Scholar]
- 16.Aittomaki, K., Lucena, J. L., Pakarinen, P., Sistonen, P., Tapanainen, J., Gromoll, J., Kaskikari, R., Sankila, E. M., Lehvaslaiho, H., Engel, A. R., et al. (1995) Cell 82, 959–968. [DOI] [PubMed] [Google Scholar]
- 17.Aittomaki, K., Herva, R., Stenman, U. H., Juntunen, K., Ylostalo, P., Hovatta, O. & de la Chapelle, A. (1996) J. Clin. Endocrinol. Metab. 81, 3722–3726. [DOI] [PubMed] [Google Scholar]
- 18.Themmen, A. P. N. & Huhtaniemi, I. T. (2000) Endocr. Rev. 21, 551–583. [DOI] [PubMed] [Google Scholar]
- 19.Themmen, A. P. & Verhoef-Post, M. (2002) Semin. Reprod. Med. 20, 199–204. [DOI] [PubMed] [Google Scholar]
- 20.Weiss, J., Axelrod, L., Whitcomb, R. W., Harris, P. E., Crowley, W. F. & Jameson, J. L. (1992) N. Engl. J. Med. 326, 179–183. [DOI] [PubMed] [Google Scholar]
- 21.Mason, A. J., Pitts, S. L., Nikolics, K., Szonyi, E., Wilcox, J. N., Seeburg, P. H. & Stewart, T. A. (1986) Science 234, 1372–1378. [DOI] [PubMed] [Google Scholar]
- 22.Kendall, S. K., Samuelson, L. C., Saunders, T. L., Wood, R. I. & Camper, S. A. (1995) Genes Dev. 9, 2007–2019. [DOI] [PubMed] [Google Scholar]
- 23.Kumar, T. R., Palapattu, G., Wang, P., Woodruff, T. K., Boime, I., Byrne, M. C. & Matzuk, M. M. (1999) Mol. Endocrinol. 13, 851–865. [DOI] [PubMed] [Google Scholar]
- 24.Matzuk, M. M., Finegold, M. J., Su, J. G., Hsueh, A. J. & Bradley, A. (1992) Nature 360, 313–319. [DOI] [PubMed] [Google Scholar]
- 25.Matzuk, M. M., DeMayo, F. J., Hadsell, L. A. & Kumar, T. R. (2003) Biol. Reprod. 69, 338–346. [DOI] [PubMed] [Google Scholar]
- 26.Kumar, T. R., Fairchild-Huntress, V. & Low, M. J. (1992) Mol. Endocrinol. 6, 81–90. [DOI] [PubMed] [Google Scholar]
- 27.O'Shaughnessy, P. J., Willerton, L. & Baker, P. J. (2002) Biol. Reprod. 66, 966–975. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, F. P., Pakarainen, T., Poutanen, M., Toppari, J. & Huhtaniemi, I. (2003) Proc. Natl. Acad. Sci. USA 100, 13692–13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Risma, K. A., Hirshfield, A. N. & Nilson, J. H. (1997) Endocrinology 138, 3540–3547. [DOI] [PubMed] [Google Scholar]
- 30.Rulli, S. B., Kuorelahti, A., Karaer, O., Pelliniemi, L. J., Poutanen, M. & Huhtaniemi, I. (2002) Endocrinology 143, 4084–4095. [DOI] [PubMed] [Google Scholar]
- 31.Rulli, S. B., Ahtiainen, P., Makela, S., Toppari, J., Poutanen, M. & Huhtaniemi, I. (2003) Endocrinology 144, 4980–4990. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, F. P., Pakarainen, T., Zhu, F., Poutanen, M. & Huhtaniemi, I. (2004) Endocrinology 145, 1453–1463. [DOI] [PubMed] [Google Scholar]
- 33.Huhtaniemi, I. (1995) Reprod. Fertil. Dev. 7, 1025–1035. [DOI] [PubMed] [Google Scholar]
- 34.Benton, L., Shan, L. X. & Hardy, M. P. (1995) J. Steroid. Biochem. Mol. Biol. 53, 61–68. [DOI] [PubMed] [Google Scholar]
- 35.Habert, R., Lejeune, H. & Saez, J. M. (2001) Mol. Cell. Endocrinol. 179, 47–74. [DOI] [PubMed] [Google Scholar]
- 36.Lejeune, H., Habert, R. & Saez, J. M. (1998) J. Mol. Endocrinol. 20, 1–25. [DOI] [PubMed] [Google Scholar]
- 37.Holdcraft, R. W. & Braun, R. E. (2004) Development (Cambridge, U.K.) 131, 459–467. [DOI] [PubMed] [Google Scholar]
- 38.Chang, C., Chen, Y. T., Yeh, S. D., Xu, Q., Wang, R. S., Guillou, F., Lardy, H. & Yeh, S. (2004) Proc. Natl. Acad. Sci. USA 101, 6876–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He, W. W., Kumar, M. V. & Tindall, D. J. (1991) Nucleic Acids Res. 19, 2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csernus, V. (1986) Exp. Clin. Endocrinol. 88, 1–5. [PubMed] [Google Scholar]
- 41.Mannan, M. A. & O'Shaughnessy, P. J. (1991) J. Endocrinol. 130, 101–106. [DOI] [PubMed] [Google Scholar]
- 42.Sokka, T. A., Hamalainen, T. M., Kaipia, A., Warren, D. W. & Huhtaniemi, I. T. (1996) Biol. Reprod. 55, 663–670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.