ABSTRACT
As the rates of systemic fungal infections continue to rise and antifungal drug resistance becomes more prevalent, there is an urgent need for new therapeutic options. This issue is exacerbated by the limited number of systemic antifungal drug classes. However, the discovery, development, and approval of novel antifungals is an extensive process that often takes decades. For this reason, there is growing interest and research into the possibility of combining existing therapies with various adjuvants that either enhance activity or overcome existing mechanisms of resistance. Reports of antifungal adjuvants range from plant extracts to repurposed compounds, to synthetic peptides. This approach would potentially prolong the utility of currently approved antifungals and mitigate the ongoing development of resistance.
KEYWORDS: antifungals, chemosensitizer, combination therapy, resistance, synergy
Introduction
The incidence of life-threatening invasive fungal infections has increased significantly in recent decades. This is the result of several factors including a rise in immune suppressive disorders and aggressive medical intervention that has led to a growing population of immune compromised individuals. These individuals are particularly susceptible to infection by fungal pathogens which are typically opportunistic in nature, although it is worth noting that immune competent individuals are also at risk in some cases. The prevalence of these infections is particularly concerning, especially when considering their high associated morbidity and mortality even with therapeutic intervention. Depending on the medical resources available and the site of infection, mortality rates range from 20–40% for Candida albicans, 20–70% for Cryptococcus neoformans, and 50–90% for Aspergillus fumigatus.1,2
However, despite the global health burden these infections present, there are very few treatment options available. One universal limitation confronted by those attempting to develop compounds with antifungal activity is that fungi are eukaryotes and therefore share many of the same biochemical processes as their host. This results in difficulties in identifying targets that provide sufficient fungal selectivity. The limited number of distinct targets being exploited by current antifungals is a serious concern as it increases the likelihood for the development of cross-resistance. At present, there are only 4 classes of systemic antifungals, each with their own array of limitations in activity, spectrum, and patient toxicity.
Additionally, antifungal development efforts have not kept pace with the rapid increase in incidence of these infections. The pressing need for new systemic antifungals has resulted in an increased research efforts, both in academia and industry. While this is an encouraging sign, these efforts will require time to progress from bench top to bedside. In fact, it is estimated that the average drug takes 12 y from discovery to approval, although in the field of antifungal development, 30 y may be more realistic.3,4
As a means of addressing the limitations of current treatment options and to help mediate the development of resistance to current and future antifungals, there is growing interest in combining antifungal therapies with compounds that either enhance the antifungal's activity directly or that alter the pathogen's susceptibility through circumventing resistance mechanisms. This approach has been used successfully in other fields, most notably in the field of oncology where the rapid development of drug resistance and the need for selectivity closely parallel the situation observed with fungal pathogens.
Limitations of antifungal therapy for serious fungal infections
There are currently only 4 classes of systemic antifungals available: polyenes, a pyrimidine analog, azoles, and echinocandins. Only one representative of the first 2 classes are used to treat invasive disease, amphotericin B and 5-flucytosine, respectively. While amphotericin B has been used for over 50 y and remains the gold standard for the treatment of some fungal diseases, its use is limited by lack of oral bioavailability and significant toxicity.5 While new lipid-based formulations have been developed that improve toxicity, they are often cost-prohibitive.5 5-Flucytosine has potent activity and excellent fungal selectivity but is associated with significant toxicity.6 However, its mechanism of action predisposes it to the rapid development of resistance so it is relegated to use solely in combination therapy.7 The azoles are the most prolific and widely used class, due to their broad spectrum of activity and limited toxicity profiles.8 However, they are fungistatic and therefore depend on the host immune response to resolve the infection. The inability of the azoles to kill the invading pathogen affords the fungus an opportunity for adaptation and development of resistance. Moreover, this antifungal class is associated with a number of drug-drug interactions.9 The echinocandins are the newest class of antifungals and are beginning to supplant the azoles as the agents of choice for candidiasis since they are fungicidal and associated with limited drug-drug interactions, however, they are only available as an intravenously administered formulation and resistance is developing.10 Echinocandins are also limited by a narrower spectrum of activity than the azoles and lack activity against Cryptococcus, a pathogen that kills nearly 650,000 people annually, and several other medically important fungi.2
Potential for improving effectiveness of existing antifungal agents
In contrast to the limited repertoire of antifungals, there are abundant reports of compounds, ranging from traditional herbal remedies to FDA-approved medications, that enhance antifungal activity or mitigate resistance. This primarily occurs through inhibition of fungal stress responses that are required for survival in the presence of antifungals or increasing intracellular drug concentrations by either increasing cell permeability or decreasing drug efflux.11-13 Less frequently, combinations that inhibit or disrupt biofilm formation have also been reported. While reports exist for a wide range of fungi and a plethora of compounds, in this review, we have chosen to focus on the most prevalent human fungal pathogens and examples where the interaction has been mechanistically examined.
Enhancing activity
One approach to improving antifungal therapy is by enhancing the activity of existing antifungals. The most prevalent example of this is through converting a fungistatic monotherapy into a fungicidal combination. This may aid in resolving infections rapidly and limit the development of drug resistance.14 In some cases, these combinations also improve antifungal activity against resistant strains and isolates, without directly impacting the mechanism(s) of resistance.
Iron homeostasis inhibitors
Iron is essential for a variety of biological processes including oxygen transport, electron transfer, and DNA synthesis and is required for all heme containing proteins. However, excess free iron can be toxic so the concentration and availability of iron is tightly regulated by the host, such that iron is often growth limiting for pathogens during infection.15 Host mechanisms of iron sequestration result in free iron concentrations of approximately 10-18 M in serum.16 Since iron acquisition is crucial for survival, microbes have developed an array of mechanisms to meet this need, including within the host. While these mechanisms are sufficient to support pathogen growth, iron is often still a limiting nutrient and the addition of small molecule iron chelators can disturb the balance of iron acquisition and utilization.
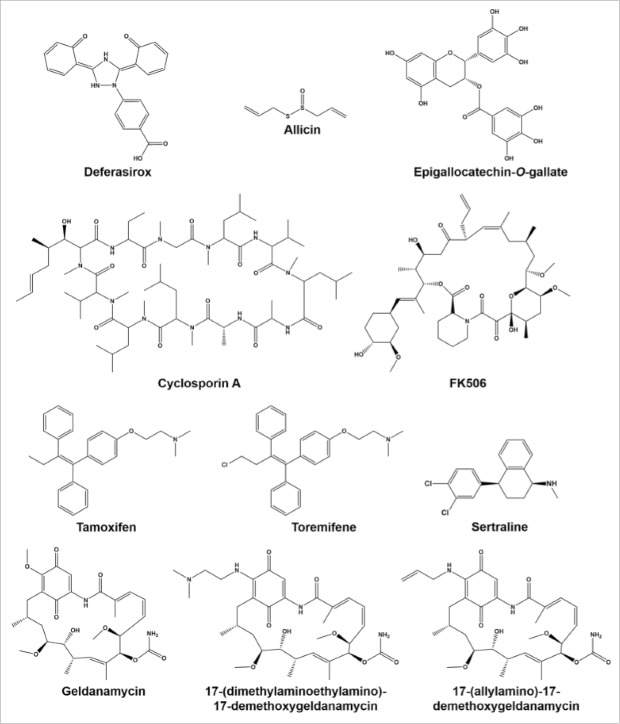
Several structurally-distinct iron chelators have been shown to have antifungal activity including doxycycline and other tetracycline antibiotics, deferasirox, and lactoferrin.17-19 However, while standalone activity generally occurs at concentrations above those therapeutically achievable, doxycycline has been shown to enhance the activity of various azoles at much lower concentrations.17 Azoles inhibit lanosterol demethylase, a key component of the ergosterol biosynthesis pathway. When confronted with this challenge, fungi upregulate several of the ergosterol biosynthesis genes to compensate for the inhibition of lanosterol demethylase.20 Many of these enzymes require heme cofactors to function and their upregulation increases the cell's demand for iron. Thus constricting the availability of iron inhibits an azole stress response resulting in improved antifungal activity. Additionally, iron chelation has been shown to improve outcome in combination with amphotericin B in a murine model of aspergillosis indicating that the need for iron to tolerate drug stress is not an azole specific pheno-menon.18
Calcium homeostasis inhibitors
Calcium acts as a second messenger and its release can trigger a range of biological responses in eukaryotes, depending on concentration and location. Calcium signaling is central to multiple processes including cell division, polarized growth, and stress responses in fungi, including those that occur in response to drug exposure.21 As such intracellular calcium levels are very tightly controlled and adjusted through the expression and activity of a network of transmembrane pumps at both the plasma membrane and the vacuole, the primary site of intracellular calcium storage.22 Disruption of the delicate balance between calcium import and storage can result in impaired signaling and an inability to respond appropriately to antifungals.21
Depletion of calcium levels, either by chelating extracellular calcium with ethylene diamine tetra-acetic acid (EDTA) or inhibition of calcium importers with benidipine and nifedipine, have been reported to enhance azole activity against C. albicans.23,24 Alternatively, treatment of various fungi with low doses of amiodarone, an antiarrhythmic, results in a rapid increase in the cytosolic calcium concentration through inducing calcium influx and the release of internal calcium stores.25 While amiodarone alone has modest antifungal activity, its use has been shown to reduce the MIC of fluconazole even against highly resistant strains of C. albicans in vitro.
Calcineurin/calmodulin inhibitors
The calmodulin/calcineurin signaling pathway is highly conserved in eukaryotes. In response to stress, calcium is released and binds to calmodulin, which then binds to and activates the phosphatase, calcineurin. Calcineurin in turn dephosphorylates the transcription factor Crz1, which then translocates from the cytoplasm to the nucleus and transcribes a variety of stress related genes. While the components of this pathway are highly conserved, there has been significant rewiring such that the signals this cascade responds to and the processes it is required for vary among fungi. In Candida albicans calcineurin is required for growth in serum, for Cryptococcus neoformans this pathway is essential for growth at elevated temperatures, and in Aspergillus fumigatus it is essential for conidia formation. However in all 3 pathogens mentioned above, calcineurin signaling plays an essential role in antifungal tolerance. Thus, it is not surprising that inhibition of the pathway by genetic or chemical manipulation result in decreased virulence and hyper-susceptibility to various antifungals in Candida, Cryptococcus, and Aspergillus.26
Calcineurin inhibitors
Cyclosporin A and FK506 (tacrolimus) are both immunosuppressives that bind to cyclophilin A and FKBP12, respectively, and form an inhibitory complex with calcineurin.27,28 These drugs are frequently used in organ transplant recipients and there is some clinical evidence that patients receiving calcineurin inhibitor based immunosuppressive therapy develop fewer invasive fungal infections than those on other regimens.26
Disruption of calcineurin function has minimal effect on Candida albicans growth under normal laboratory conditions. However, these strains are sensitive to growth under a variety of stress conditions, including growth in serum, and attenuated in virulence.29 As such, inhibiting calcineurin has significant in vivo potential. Additionally, when combined with azoles there is a potent synergy and a fungicidal effect.30 This enhanced activity has been observed with several azoles and against other Candida species that are intrinsically more resistant to the azoles, C. glabrata and C. krusei.31
In Cryptococcus, it has been shown that calcineurin signaling is required for growth at elevated temperatures.32 It is therefore not surprising that calcineurin inhibitors have anticryptococcal activity when tested at 37°C, but not at 24°C.33 While FK506 is active alone, it also enhances the activity of caspofungin and the fluconazole and result in fungicidal combinations which is a particularly important outcome for the treatment of cryptococcosis.34,35 In the case of fluconazole, this enhanced activity is not due to calcineurin inhibition but its ability to inhibit multidrug efflux pumps which would result in higher intracellular fluconazole concentrations. FK506 has also been shown to enhance the activity of caspofungin against Cryptococcus, in a fully calcineurin dependent manner, although not to a clinically relevant extent since there is only a modest increase in susceptibility and Cryptococcus is inherently resistant to echinocandins.
Loss of calcineurin activity in Aspergillus results in defects in hyphal extension and invasive growth. In vivo this defect manifests as reduced host tissue damage and significantly reduced mortality.36 Alone, calcineurin inhibitors have minimal in vitro activity against Aspergillus species. However, in combination with either azoles or echinocandins there was enhanced, fungicidal activity.37,38
Finally, several groups have independently examined clinical isolates of C. albicans, C. neoformans, and A. fumigatus, from patients receiving long-term calcineurin-inhibitor based immune suppression.39-41 No differences in susceptibility to various calcineurin inhibitors were detected, indicating that resistance is not being selected for in this patient population. Despite these positive interactions in vitro, the potent immune suppressive effects of cyclosporine A and FK506 limit their potential for use as adjuvants with antifungal therapy.26 For this reason, non-immunosuppressive analogs of both compounds have been developed and investigated.33 These fungal specific analogs retain their synergistic activity in vitro, however there is little in the literature to indicate that they are efficacious in vivo and it appears that they are no longer being pursued.
Calmodulin inhibitors
More recently, it has been shown that inhibiting the calcineurin pathway further upstream also results in enhanced azole activity both in Candida and Cryptococcus.42 Structurally diverse calmodulin inhibitors have been shown to enhance fluconazole activity, and some combinations resulted in fungicidal activity as demonstrated by time-kill assays.43 Some of the most promising of these are the triphenylethylene estrogen receptor antagonists, tamoxifen and toremifene. Tamoxifen and toremifene both have antifungal activity on their own at clinically relevant concentrations, but when combined with fluconazole at subinhibitory concentrations produce a fungicidal effect. The combination of subinhibitory concentrations of fluconazole and tamoxifen has been shown to reduce brain fungal burden in a murine model of cryptococcosis.44
Hsp90 inhibitors
Heat-shock protein 90 is a molecular chaperone and regulator of a variety of stress responses, including calcineurin signaling, in many fungi. It is also required for several morphogenic changes associated with host environmental adaptation.11 Recently, it has been shown that depletion of Hsp90 interferes with the development of resistance to azoles and echinocandins.45,46 As such, inhibition of Hsp90 by the natural product geldanamycin and synthetic analogs, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG) and 17-(allylamino)-17- demethoxygeldanamycin (17-AAG) which are being developed as anti-tumor agents, has gained favor as a potential therapeutic strategy.
The addition of any of the above named Hsp90 inhibitors to the growth media reduced the concentration of fluconazole required to inhibit C. albicans growth, even though they have no detectable growth inhibitory activity alone. The combination of geldanamycin with fluconazole has also been shown to be fungicidal against C. albicans within 24hrs by time-kill analysis. In a Galleria mellonella larval model of systemic candidiasis, the combination of either 17-AAG or 17-DMAG with fluconazole resulted in complete rescue and survival of the G. mellonella. Additionally, there are reports of Hsp90 inhibitors preventing C. albicans biofilm formation, although there are currently no studies to the best of our knowledge that explore the impact of Hsp90 inhibitors on established biofilms. In Aspergillus, inhibition of Hsp90 function enhances the activity of both caspofungin and voriconazole in vitro. However, in a similarly designed G. mellonella experiment, the combination of geldanamycin and caspofungin prolonged larvae survival but had minimal impact on total mortality.47
Selective serotonin reuptake inhibitors
Sertraline is a commonly prescribed selective serotonin reuptake inhibitor (SSRI), a class of compounds used to treat a variety of psychological disorders including depression. Its antifungal activity was first described in 2001 when 3 women receiving sertraline therapy to treat premenstrual dysmorphic disorder experienced remission of their recurrent vulvovaginal candidiasis.48 In vitro testing confirmed that sertraline exhibited fungicidal activity against several Candida strains. It was later confirmed that sertraline, as well as several other SSRIs, have activity against Aspergillus as well.49 Unfortunately, the MICs against these organisms were relatively high compared to established serum levels and interest in translating sertraline to antifungal use stalled.
A decade later, sertraline was rediscovered as a potentiater of the antifungal effect of fluconazole against several yeast species, including Cryptococcus.50 In humans, sertraline inhibits the 5-hydroxytryptamine transporter and in tumor cells has been shown to inhibit translation by disrupting signaling through the mTOR pathway. However, there are no clear fungal orthologs for these targets so genome wide screens were conducted to identify the mechanism(s) by which sertraline exerts its antifungal effect. The cumulative results indicate that sertraline has a variety of effects on fungal cells including altering membrane organization, vesicle transport, and inhibiting protein translation.51,52 Follow-up studies confirmed that it possesses potent anti-cryptococcal activity alone at concentrations that are similar to the levels that can be achieved in the cerebrospinal fluid and the brain in humans, an extremely relevant niche given the neurotropism of Cryptococcus. This activity, as well as the additive effect in combination with fluconazole, has been demonstrated in a murine model of cryptococcosis.52 Efforts to progress sertraline as an adjunctive therapy for cryptococcosis are on-going and thus far have resulted in a dose finding clinical study.53
Statins
Statins are a class of drugs that are commonly prescribed for the treatment of high cholesterol. It has however been observed that they have a variety of other properties including antioxidant, anti-inflammatory, and antimicrobial activity. These compounds have been shown to inhibit the activity of fungal HMG-CoA reductase.54 HMG-CoA reductase functions in the isoprenoid pathway which is upstream of the ergosterol biosynthesis pathway. Alone, the concentrations of statins required to inhibit fungal growth are not therapeutically achievable but have been shown to reduce MICs when used in combination with various azoles.
Although there are some discrepancies as to the underlying mechanism(s) of the antifungal effect of statins, it is likely a multifactorial effect. Inhibition of the isoprenoid pathway reduces the availability of the substrates required for sterol synthesis which is then further inhibited by the azoles. It has also been shown that treatment of C. albicans with lovastatin results in downregulation of multiple genes whose products are required for sterol biosysnthesis.55 Finally, there is some evidence to suggest that treatment with statins alters membrane fluidity and may therefore increase cell permeability resulting in higher intracellular drug accumulation.54
The interaction between a panel of commercially available statins and several azoles against 2 Candida and Aspergillus species has been explored. Although the exact nature of the interaction depended on the specific statin/azole/strain combination, antagonism was never observed and the majority of the interactions resulted in growth inhibition at reduced azole concentrations.56 Notably, this enhancement of azole activity occurred at statin concentrations that are achievable in human serum.
Nonsteriodal anti-inflammatory drugs
Prostaglandins are small lipid molecules that are produced and secreted by a variety of organisms, including humans and some pathogenic fungi, that serve as signaling molecules. In humans, prostaglandins are produced by the activity of 2 cyclooxygenase isozymes, COX-1 and COX-2, which are inhibited by a variety of anti-inflammatory drugs including many over the counter pain relievers. C. albicans and C. neoformans both produce and secrete prostaglandins and although their function is not fully understood, they may regulate gene expression and serve a role in host immune modulation. Furthermore, addition of prostaglandins to C. albicans cultures has been shown to induce filamentation. Treatment with various COX inhibitors decreases the ability of both C. albicans and C. neoformans to produce and secrete prostaglandins.57 Additionally, they reduce fungal viability, although it is not clear if the decrease in viability is due to the loss of prostaglandins or an additional role of cyclooxygenases in fungal metabolism. While their standalone antifungal activity occurs at concentrations much higher than serum concentrations of people on anti-inflammatory regimens, several of these compounds have been shown to be synergistic with fluconazole against C. albicans.58 High-dose ibuprofen causes direct damage to the cytoplasmic membrane which likely underlies its ability to synergize with fluconazole.59
Plant extracts and essential oils
There are extensive reports of the antifungal activity of various plant extracts, particularly from aromatic herbs. The majority of this work has been conducted using crude extracts and the active component or components are not clearly identified or well characterized. However, in some cases, purified constituents responsible for activity have been identified and studied.
Thymol, a major component of extracts from thyme, has been shown to negatively affect membrane integrity and reduce ergosterol content.60 Additionally, thymol is synergistic with amphotericin B, fluconazole, and itraconazole against C. albicans, and synergistic, neutral, and additive, respectively, against C. neoformans.61 It has been shown to be additive in combination with amphotericin B, fluconazole and ketoconazole against A. fumigatus.62 The ability of thymol to undermine membrane integrity and diminish ergosterol content is likely at the root of its ability to sensitize these fungi to cell membrane perturbing antifungals. Carvacrol, also found in thyme extracts, shares this mechanism of chemosensitization.63 Eugenol and methyleugenol, which are present in high levels in the extracts of basil and clove, have also been reported to disrupt ergosterol biosynthesis and synergize with fluconazole against both fluconazole-susceptible and resistant isolates of C. albicans.64 In addition to their membrane active mechanisms, thymol and eugenol have been shown to inhibit the plasma membrane H+ATPase resulting in cellular acidification.65
Several other phenolic compounds found in plant extracts, including berberine, curcumin, and dihydrobenzaldehydes have been reported to enhance fluconazole activity against C. albicans and C. neoformans through inhibition of the oxidative stress response pathway.61,66,67 Interesting, cinnamaldehyde, which is structurally similar, acts through a distinct mechanism. In vitro assays have demonstrated that it inhibits β-(1,3)-glucan synthase and chitin synthase from Saccharomyces cerevisiae.68 The combination of cell wall and membrane disruption results in improved activity of fluconazole against C. albicans and C. neoformans.61 Allicin, a component of garlic extract, synergizes with amphotericin B and fluconazole against C. albicans.69,70 The primary mechanism appears to be disruption of vacuolar function, but only when ergosterol is present in the membrane. The ability to enhance azole activity has been confirmed in a murine model of systemic candidiasis.70 Epigallocatechin-O-gallate, found in black tea, is a dihydrofolate reductase inhibitor which in turn inhibits ergosterol biosynthesis. In combination with amphotericin B it enhanced antifungal activity and has been shown to improve the outcome in a murine model of systemic candidiasis.71
Overcoming resistance
Another, complimentary approach to improving antifungal treatment is overcoming existing resistance mechanisms. This approach has been successfully used in the treatment of bacterial infections, where many therapies include inhibitors of detoxifying enzymes, and in oncology, where efflux pump inhibitors are routinely used to increase the intracellular concentration of the active agent. The most commonly encountered mechanisms of antifungal resistance are alterations in drug target sequence, increased expression of the drug target, and increased drug efflux.
Histone deacetylase inhibitors
Gene expression is a highly dynamic process with several layers of regulation including alterations in histone acetylation. In C. albicans, treatment with fluconazole leads to the upregulation of ERG11 which encodes sterol demethylase, the target of the azoles, and several efflux pumps.20,72, 73 This ability to alter gene expression in response to drug treatment increases MIC and is thought to contribute to the phenomenon of trailing growth, which is continued growth at drug concentrations well above the MIC.74 The ability of Candida to adapt to growth at very high azole concentrations is a therapeutic challenge. To address this issue, the use of histone deacetylase inhibitors in combination with azoles has been explored. Alone, several HDAC inhibitors, including trichostatin A, apicidin, and sodium butyrate, had no or minimal effect on C. albicans growth in rich media at 35°C. Additionally, while the inclusion of a trichostatin did not reduce the fluconazole MIC at 24 hours, it had a profound impact on the extent of trailing growth. This reduction in trailing growth was also observed with itraconazole and 2 closely related Candida species, C. tropicalis and C. parapsilosis, although it was not observed with the more distant relatives, C. glabrata and C. krusei. Finally, it has been demonstrated that the addition of HDAC inhibitors prevents the azole induced upregulation of several ergosterol biosynthesis genes and efflux pumps.75
Efflux pump inhibitors
Upregulation of efflux pumps is an extremely common mechanism of antifungal drug resistance. This results in increased antifungal efflux and reduces the intracellular concentration of the active agents. As such, efflux pump inhibitors are an attractive strategy for overcoming resistance. ATP-binding cassette (ABC) efflux pumps are the predominate type that contribute to drug resistance, although major facilitator superfamily (MFS) efflux pumps also have a role. In Candida albicans the ABC transporters that are most commonly overexpressed in drug resistant isolates are Cdr1 and Cdr2 while overexpression of the MFS transporter Mdr1 appear to be less prevalent.76 These pumps have varying substrate specificities and limited structural similarities, so it is not surprising that inhibitors are rarely active against both classes.77
ABC transporter inhibitors
As mentioned above, it has been shown that FK506 has some ABC efflux inhibitory activity, as does a structurally unrelated propafenone, GP382. To demonstrate this activity, C. albicans Cdr1 and Cdr2 were heterologously expressed in a Saccharomyces cerevisiae efflux null mutant and shown to be functional. It was then shown that the addition of FK506 could reverse ketoconazole resistance in this strain. Finally, a rhodamine efflux assay was used to confirm that treatment with either FK506 or GP382 resulted in decreased efflux of the rhodamine dye, Rh-6G, which is a known substrate of several ABC transporters.78
In an effort to identify novel efflux pump inhibitors one group conducted a screen of natural products from fermentation extracts that potentiated the activity of fluconazole in strains of C. albicans and C. glabrata that overexpressed Cdr1. Several extracts were identified as enhancing fluconazole activity against the Cdr1-overexpressing strains. Active components of these extracts were isolated and several, including enniatins, beauvericins, and milbemycins, were confirmed as ABC efflux pump inhibitors in a whole cell efflux assay. Of these compounds, it was decided that the milbemycins warranted further exploration as they are also being explored as insecticides and antiparasitics. Nearly two dozen milbemycins were isolated and tested for efflux inhibition against a panel of Candida strains and isolates. The addition of milbemycin α9 significantly decreased both the MIC50 and MIC90 of posaconazole against all Candida species tested. This broad spectrum of activity is extremely promising since the infecting species is not always known at the onset of treatment for candidiasis.79
Multiple large scale screens of D-octopeptide combinatorial libraries have identified ABC-efflux pump inhibitors of varying specificity. The original screen was conducted using a strain of Saccharomyces cerevisiae that over-expressed Pdr5p in the presence of a subinhibitory concentration of fluconazole. In this manner, KN20 was identified. This peptide derivative inhibits efflux by Pdr1p, Cdr1p, Cdr2p, and Mdr1p. Not surprisingly, addition of KN20 sensitized a collection of Candida species, including C. albicans, C. dubliniensis, C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis to fluconazole.80 It is worth noting, however, that it had minimal effect on fluconazole's activity against C. neoformans. It is not clear if this is due to an inability to inhibit the efflux pumps or if it is unable to penetrate the polysaccharide capsule that surrounds C. neoformans. More recently, a highly selective D-octopeptide inhibitor of C. albicans Cdr1p, RC21, was identified by a similar screening strategy. However, this strategy employed several counter screens to eliminate hits with promiscuous activity. Addition of RC21 did not reduce fluconazole resistance in strains overexpressing a variety of other ABC efflux pumps, Mdr1p, or Erg11p but did sensitize several azole-resistant isolates of C. albicans.81
Figure 1.
Compounds that have been tested in vivo in combination with existing antifungals and enhanced efficacy.
MFS transport inhibitors
While searching specifically for inhibitors of ABC transporters, another compound, cytochalasin H, was identified which was isolated from hit extracts and shown to have modest inhibitory activity against Mdr1, a member of the major facilitator superfamily.79
Recently, a screen for compounds that potentiate the activity of fluconazole against an Mdr1 overexpressing strain was conducted using a synthetic small molecule library. Two, structurally similar compounds, MCC1189 and MCC1375, were identified as having minimal antifungal activity alone but enhanced fluconazole activity. Efflux assays confirmed inhibition of Mdr1-mediated efflux but not Cdr-mediated efflux. Both compounds were shown to be synergistic with fluconazole against C. albicans and the combination of fluconazole and MCC1189 resulted in fungicidal activity.82
General inhibitors
While many screens for efflux pump inhibitors are based on sensitizing cells to the presence of a subinhibitory concentration of an antifungal, usually fluconazole, one group took an alternative approach. In a repurposing screen, cells were loaded with one of 2 fluorescent efflux pump substrates and incubated in the presence of compounds in the Prestwick library. Actively effluxing cells decreased in fluorescence while those with inhibited efflux retained the fluorescent substrate and high fluorescence. This approach identified clorgyline, a monoamine oxidase A inhibitor, as a multi-efflux class inhibitor. This compound inhibited both ABC and MFS efflux pumps. Furthermore, addition of clorgyline sensitized azole resistant isolates both C. albicans and C. glabrata to fluconazole.83
Both thymol and carvacrol, which are discussed above, have also been shown to reduce drug efflux through disruption of the proton gradient used to drive MFS pumps.84 This is why the degree of sensitization is greater in fluconazole resistant isolates. Baicalein, a component of many Chinese herbs, has alsobeen shown to reduce efflux in C. albicans and improve fluconazole activity, especially in highly resistant strains.85
Biofilm active combinations
As mentioned above, Candida biofilms are a significant therapeutic challenge. These multicellular communities form on both inert and biological surfaces and include cells in a variety of morphological and metabolic states surrounded by a dense extracellular matrix. Biofilms are inherently resistant to antifungal treatment due to both reduced drug penetration through the matrix and the presence of cells that are metabolically quiescent and not actively dividing. In addition to being difficult to treat, biofilms can act as a reservoir of fungal cells that can break off and seed infections throughout the body making their eradication crucial for the successful treatment of systemic candidiasis. To acheive this, compounds that inhibit biofilm formation, as well as those that are active against mature biofilms, are needed.
Doxycycline
As discussed above, the addition of various tetracyclines to fluconazole treatment against planktonic cells enhances antifungal activity. It has also been shown that doxycycline is synergistic with fluconazole against early stage biofilm (4–12 hours). However, mature biofilms exhibited resistance to this combination.86 While it is tempting to assume that this enhanced activity is due to iron chelation, as is the case for planktonic cultures, that has not yet been confirmed to our knowledge.
Calcineurin inhibitors
In addition to resulting in fungicidal activity against planktonic cells, the combination of either FK506 or cyclosporine A and fluconazole has dramatic activity against mature C. albicans biofilms. Interestingly, while calcineurin function was not required for the formation of apparently normal biofilms, mutants in this pathway demonstrated increased fluconazole susceptibility. In vitro biofilms treated with the drug combination not only had reduced metabolic activity measured by XTT assay compared to either drug alone, but they were also substantially thinner after 24 hours of treatment. These finding where then confirmed in an in vivo rat catheter model. Finally, the ability of FK506 to enhance fluconazole activity against C. albicans biofilms was confirmed to be calcineurin dependent.87
Nonsteriodal anti-inflammatory drugs
A variety of nonsteroidal anti-inflammatory drugs have been shown to inhibit C. albicans biofilm formation and some even have activity against mature biofilms.88 This is likely due to their ability to interfere with prostaglandin production and decrease C. albicans filamentation which has a detrimental impact on adherence and the development and maintenance of biofilms. However, this occurs at relatively high concentrations, limiting their use as single agent therapies. However, it has since been shown that the addition of aspirin to amphotericin B has enhances its activity against C. albicans and C. parapsilosis biofilms.89
Plant extracts
The ability of several plant-derived compounds to inhibit biofilm formation and improve the activity of antifungals against mature biofilms has been investigated. One group investigated the activity of cinnamaldyhyde, citral, geraniol, and eugenol. While each compound has some antifungal activity, their inclusion in the growth media at sub-growth inhibitory concentrations reduced biofilm formation alone, with eugenol and cinnamaldehyde showing the greatest activity. Importantly, eugenol and cinnamaldehyde were also synergistic with fluconazole against mature biofilms. Scanning electron microscopy of biofilms treated with eugenol or cinnamaldehyde showed decreased secreted polysaccharide matrix and rough, shriveled cell membranes.90 This loss of biofilm structure and organization results in a loss of fluconazole resistance.
Repurposed compounds
Recently, a screen of the Pharmakon 1600 library was conducted to identify compounds that enhanced the activity of amphotericin B against C. albicans biofilms. Test compounds were included at the initiation of biofilm formation and amphotericin B was added 24 hours later. To differentiate between compounds that prevented or inhibited biofilm alone and those that enhanced amphotericin B activity, biofilms treated with the test compounds were subjected to concentration gradient of amphotericin B. Three compounds were found to enhance amphotericin B activity including drospirenone, perhexiline, and toremifine. These compounds were then shown to enhance the antifungal activity of amphotericin B and caspofungin against both C. albicans and C. glabrata biofilms. It is worth noting that perhexiline and toremifene have antifungal activity linked to the inhibition of calmodulin function, however this has mechanism has not been investigated in relation to their ability to enhance activity of known antifungals against biofilms.91
Conclusions
When examining the literature for this review it was surprising how many reports there were, but also how diffuse this body of work appears. The terminology used varies widely between investigators and includes enhancers, chemosensitizers, potentiators, and synergizers with often overlapping definitions.92,93 The methodologies and strains used also vary significantly which has resulted in conflicting reports with specific combinations being reported as synergistic, additive, neutral, and antagonistic. It is our belief that as focus shifts toward identifying these combinations and the subfield of antifungal adjuvant research becomes more cohesive, standard methodologies will be established and issues with ambiguous terminology will be resolved. In many cases, a compound's ability to improve the activity of established antifungals seems to be investigated almost as an afterthought and is rarely mechanistically characterized and examined in in vivo studies. This is understandable and by no means meant to be disparaging to these investigators, but instead is intended to highlight the broad base of knowledge in this area with extensive possibilities for further research and development.
As screening technologies become more advanced there is growing interest in a more systematic approach to identifying multi-component therapies and these approaches may yield novel compounds that lack inherent antifungal activity.50,51, 94 For example, this approach is at the root of the discovery of many of the compounds discussed in the overcoming resistance section of this review and is how the majority of efflux pump inhibitors where identified as well as some of the compounds in the anti-biofilm section. Another exciting strategy is the idea of targeting virulence attributes in combination with existing antifungal therapy for improving efficacy. Work toward exploiting this approach in both C. albicans and C. neoformans has been reviewed recently.95,96
While it is generally believed that only fungal specific structures and processes, such as the cell wall and ergosterol biosynthesis, are acceptable antifungal targets, it is worth noting the vast majority of targets exploited above have human homologs. Specificity is a concept that is rarely black and white, and it is important to realize that specificity can be built in parts.
It is encouraging to see that several groups appear to be actively pursuing this approach for improving antifungal activity and are conducting in depth mechanistic characterization efforts and exploring the effects in vivo. It is also promising that individuals outside of academia are beginning to take notice and embrace the idea of antifungal adjuvants as a viable path forward in the treatment of invasive fungal infections. It has now been 14 y since the newest class of systemic antifungals received FDA approval.3 Even with state-of-the-art therapies, mortality rates for invasive fungal infections often exceed 50%. It is crucial that we not only embrace the idea of antifungal adjuvants, but push forward to move this approach from conceptually interesting to clinically applicable.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Dr. Rogers's and Dr. Palmer's work on antifungal drug discovery and resistance is supported by NIH grants R01 AI058145 and R01 AI099080, respectively.
References
- [1].Lai CC, Tan CK, Huang YT, Shao PL, Hsueh PR. Current challenges in the management of invasive fungal infections. J Infect Chemother 2008; 14:77-85; PMID:18622668; http://dx.doi.org/ 10.1007/s10156-007-0595-7 [DOI] [PubMed] [Google Scholar]
- [2].Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009; 23:525-30 10.1097/QAD.0b013e328322ffac; PMID:19182676; http://dx.doi.org/ 10.1097/QAD.0b013e328322ffac [DOI] [PubMed] [Google Scholar]
- [3].Butts A, Krysan DJ. Antifungal drug discovery: something old and something new. PLoS Pathog 2012; 8:e1002870; PMID:22969422; http://dx.doi.org/ 10.1371/journal.ppat.1002870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 2007; 6:29-40; PMID:17159923; http://dx.doi.org/ 10.1038/nrd2201 [DOI] [PubMed] [Google Scholar]
- [5].Donovick R, Gold W, Pagano JF, Stout HA. Amphotericins A and B, antifungal antibiotics produced by a streptomycete. I. In vitro studies. Antibiot Annu 1955; 3:579-86; PMID:13355330 [PubMed] [Google Scholar]
- [6].Vandevelde AG, Mauceri AA, Johnson JE 3rd. 5-fluorocytosine in the treatment of mycotic infections. Ann Intern Med 1972; 77:43-51; PMID:4559539; http://dx.doi.org/ 10.7326/0003-4819-77-1-43 [DOI] [PubMed] [Google Scholar]
- [7].Normark S, Schonebeck J. In vitro studies of 5-fluorocytosine resistance in Candida albicans and Torulopsis glabrata. Antimicrob Agents Chemother 1972; 2:114-21; PMID:4597703; http://dx.doi.org/ 10.1128/AAC.2.3.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fromtling RA. Overview of medically important antifungal azole derivatives. Clin Microbiol Rev 1988; 1:187-217; PMID:3069196; http://dx.doi.org/ 10.1128/CMR.1.2.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brüggemann RJM, Alffenaar J-WC, Blijlevens NMA, Billaud EM, Kosterink JGW, Verweij PE, Burger DM, Saravolatz LD. Clinical Relevance of the Pharmacokinetic Interactions of Azole Antifungal Drugs with Other Coadministered Agents. Clinical Infectious Diseases 2009; 48:1441-58; PMID:19361301; http://dx.doi.org/ 10.1086/598327 [DOI] [PubMed] [Google Scholar]
- [10].Denning DW. Echinocandins: a new class of antifungal. J Antimicrob Chemoth 2002; 49:889-91; http://dx.doi.org/ 10.1093/jac/dkf045 [DOI] [PubMed] [Google Scholar]
- [11].Cowen LE. Hsp90 orchestrates stress response signaling governing fungal drug resistance. PLoS Pathog 2009; 5:e1000471; PMID:19714223; http://dx.doi.org/ 10.1371/journal.ppat.1000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cannon RD, Lamping E, Holmes AR, Niimi K, Tanabe K, Niimi M, Monk BC. Candida albicans drug resistance – another way to cope with stress. Microbiology+ 2007; 153:3211-7; PMID:17906120 [DOI] [PubMed] [Google Scholar]
- [13].Cowen LE, Steinbach WJ. Stress, Drugs, and Evolution: the Role of Cellular Signaling in Fungal Drug Resistance. Eukaryotic Cell 2008; 7:747-64; PMID:18375617; http://dx.doi.org/ 10.1128/EC.00041-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lewis JS 2nd, Graybill JR. Fungicidal versus Fungistatic: what's in a word? Expert Opin Pharmacother 2008; 9:927-35; PMID:18377336; http://dx.doi.org/ 10.1517/14656566.9.6.927 [DOI] [PubMed] [Google Scholar]
- [15].Sutak R, Lesuisse E, Tachezy J, Richardson DR. Crusade for iron: iron uptake in unicellular eukaryotes and its significance for virulence. Trends Microbiol 2008; 16:261-8; PMID:18467097; http://dx.doi.org/ 10.1016/j.tim.2008.03.005 [DOI] [PubMed] [Google Scholar]
- [16].Almeida RS, Wilson D, Hube B. Candida albicans iron acquisition within the host. FEMS Yeast Res 2009; 9:1000-12; PMID:19788558; http://dx.doi.org/ 10.1111/j.1567-1364.2009.00570.x [DOI] [PubMed] [Google Scholar]
- [17].Fiori A, Van Dijck P. Potent synergistic effect of doxycycline with fluconazole against Candida albicans is mediated by interference with iron homeostasis. Antimicrob Agents Chemother 2012; 56:3785-96; PMID:22564841; http://dx.doi.org/ 10.1128/AAC.06017-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ibrahim AS, Gebremariam T, French SW, Edwards JE Jr., Spellberg B. The iron chelator deferasirox enhances liposomal amphotericin B efficacy in treating murine invasive pulmonary aspergillosis. J Antimicrob Chemother 2010; 65:289-92; PMID:19942619; http://dx.doi.org/ 10.1093/jac/dkp426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kobayashi T, Kakeya H, Miyazaki T, Izumikawa K, Yanagihara K, Ohno H, Yamamoto Y, Tashiro T, Kohno S. Synergistic antifungal effect of lactoferrin with azole antifungals against Candida albicans and a proposal for a new treatment method for invasive candidiasis. Jpn J Infect Dis 2011; 64:292-6; PMID:21788703 [PubMed] [Google Scholar]
- [20].Henry KW, Nickels JT, Edlind TD. Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob Agents Chemother 2000; 44:2693-700; PMID:10991846; http://dx.doi.org/ 10.1128/AAC.44.10.2693-2700.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Edlind T, Smith L, Henry K, Katiyar S, Nickels J. Antifungal activity in Saccharomyces cerevisiae is modulated by calcium signalling. Mol Microbiol 2002; 46:257-68; PMID:12366848; http://dx.doi.org/ 10.1046/j.1365-2958.2002.03165.x [DOI] [PubMed] [Google Scholar]
- [22].Cui J, Kaandorp JA, Sloot PM, Lloyd CM, Filatov MV. Calcium homeostasis and signaling in yeast cells and cardiac myocytes. FEMS Yeast Res 2009; 9:1137-47; PMID:19678847; http://dx.doi.org/ 10.1111/j.1567-1364.2009.00552.x [DOI] [PubMed] [Google Scholar]
- [23].Shi W, Chen Z, Chen X, Cao L, Liu P, Sun S. The combination of minocycline and fluconazole causes synergistic growth inhibition against Candida albicans: an in vitro interaction of antifungal and antibacterial agents. FEMS Yeast Res 2010; 10:885-93; PMID:20707818; http://dx.doi.org/ 10.1111/j.1567-1364.2010.00664.x [DOI] [PubMed] [Google Scholar]
- [24].Liu S, Yue L, Gu W, Li X, Zhang L, Sun S. Synergistic Effect of Fluconazole and Calcium Channel Blockers against Resistant Candida albicans. PLoS One 2016; 11:e0150859; PMID:26986478; http://dx.doi.org/ 10.1371/journal.pone.0150859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gamarra S, Rocha EM, Zhang YQ, Park S, Rao R, Perlin DS. Mechanism of the synergistic effect of amiodarone and fluconazole in Candida albicans. Antimicrob Agents Chemother 2010; 54:1753-61; PMID:20194694; http://dx.doi.org/ 10.1128/AAC.01728-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Steinbach WJ, Reedy JL, Cramer RA Jr., Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol 2007; 5:418-30; PMID:17505522; http://dx.doi.org/ 10.1038/nrmicro1680 [DOI] [PubMed] [Google Scholar]
- [27].Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 1992; 357:695-7; PMID:1377362; http://dx.doi.org/ 10.1038/357695a0 [DOI] [PubMed] [Google Scholar]
- [28].Hemenway CS, Heitman J. Calcineurin. Structure, function, and inhibition. Cell Biochem Biophys 1999; 30:115-51; PMID:10099825; http://dx.doi.org/ 10.1007/BF02737887 [DOI] [PubMed] [Google Scholar]
- [29].Blankenship JR. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot Cell 2003; 2:422-30; PMID:12796287; http://dx.doi.org/ 10.1128/EC.2.3.422-430.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cruz MC. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J 2002; 21:546-59; PMID:11847103; http://dx.doi.org/ 10.1093/emboj/21.4.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Onyewu C, Blankenship JR, Del Poeta M, Heitman J. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob Agents Chemother 2003; 47:956-64; PMID:12604527; http://dx.doi.org/ 10.1128/AAC.47.3.956-964.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Odom A. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J 1997; 16:2576-89; PMID:9184205; http://dx.doi.org/ 10.1093/emboj/16.10.2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Odom A, Del Poeta M, Perfect J, Heitman J. The immunosuppressant FK506 and its nonimmunosuppressive analog L-685,818 are toxic to Cryptococcus neoformans by inhibition of a common target protein. Antimicrob Agents Chemother 1997; 41:156-61; PMID:8980772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Del Poeta M, Cruz MC, Cardenas ME, Perfect JR, Heitman J. Synergistic Antifungal Activities of Bafilomycin A1, Fluconazole, and the Pneumocandin MK-0991/Caspofungin Acetate (L-743,873) with Calcineurin Inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob Agents Chemother 2000; 44:739-46; PMID:10681348; http://dx.doi.org/ 10.1128/AAC.44.3.739-746.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bicanic T, Muzoora C, Brouwer AE, Meintjes G, Longley N, Taseera K, Rebe K, Loyse A, Jarvis J, Bekker LG, et al.. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis 2009; 49:702-9; PMID:19613840; http://dx.doi.org/ 10.1086/604716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Steinbach WJ. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot Cell 2006; 5:1091-103; PMID:16835453; http://dx.doi.org/ 10.1128/EC.00139-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kontoyiannis DP, Lewis RE, Osherov N, Albert ND, May GS. Combination of caspofungin with inhibitors of the calciuneurin pathway attenuates growth in vitro in Aspergillus species. J Antimicrob Chemother 2003; 51:313-6; PMID:12562696; http://dx.doi.org/ 10.1093/jac/dkg090 [DOI] [PubMed] [Google Scholar]
- [38].Steinbach WJ. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob Agents Chemother 2004; 48:1664-9; PMID:15105118; http://dx.doi.org/ 10.1128/AAC.48.5.1664-1669.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Reedy JL. Immunotherapy with tacrolimus (FK506) does not select for resistance to calcineurin inhibitors in Candida albicans isolates from liver transplant patients. Antimicrob Agents Chemother 2006; 50:1573-7; PMID:16569889; http://dx.doi.org/ 10.1128/AAC.50.4.1573-1577.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Blankenship JR, Singh N, Alexander BD, Heitman J. Cryptococcus neoformans isolates from transplant recipients are not selected for resistance to calcineurin inhibitors by current immunosuppressive regimens. J Clin Microbiol 2005; 43:464-7; PMID:15635017; http://dx.doi.org/ 10.1128/JCM.43.1.464-467.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Steinbach WJ. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus isolates from transplant and nontransplant patients. Antimicrob Agents Chemother 2004; 48:4922-5; PMID:15561883; http://dx.doi.org/ 10.1128/AAC.48.12.4922-4925.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dolan K, Montgomery S, Buchheit B, Didone L, Wellington M, Krysan DJ. Antifungal activity of tamoxifen: in vitro and in vivo activities and mechanistic characterization. Antimicrob Agents Chemother 2009; 53:3337-46; PMID:19487443; http://dx.doi.org/ 10.1128/AAC.01564-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Butts A, DiDone L, Koselny K, Baxter BK, Chabrier-Rosello Y, Wellington M, Krysan DJ. A repurposing approach identifies off-patent drugs with fungicidal cryptococcal activity, a common structural chemotype, and pharmacological properties relevant to the treatment of cryptococcosis. Eukaryot Cell 2013; 12:278-87; PMID:23243064; http://dx.doi.org/ 10.1128/EC.00314-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Butts A, Koselny K, Chabrier-Roselló Y, Semighini CP, Brown JCS, Wang X, Annadurai S, DiDone L, Tabroff J, Childers WE, et al.. Estrogen Receptor Antagonists Are Anti-Cryptococcal Agents That Directly Bind EF Hand Proteins and Synergize with Fluconazole In Vivo. MBio 2014; 5:e00765-13; PMID:24520056; http://dx.doi.org/ 10.1128/mBio.00765-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cowen LE, Carpenter AE, Matangkasombut O, Fink GR, Lindquist S. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot Cell 2006; 5:2184-8; PMID:17056742; http://dx.doi.org/ 10.1128/EC.00274-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 2005; 309:2185-9; PMID:16195452; http://dx.doi.org/ 10.1126/science.1118370 [DOI] [PubMed] [Google Scholar]
- [47].Cowen LE, Singh SD, Kohler JR, Collins C, Zaas AK, Schell WA, Aziz H, Mylonakis E, Perfect JR, Whitesell L, et al.. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci U S A 2009; 106:2818-23; PMID:19196973; http://dx.doi.org/ 10.1073/pnas.08133-94106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lass-Florl C, Dierich MP, Fuchs D, Semenitz E, Ledochowski M. Antifungal activity against Candida species of the selective serotonin-reuptake inhibitor, sertraline. Clin Infect Dis 2001; 33:E135-6; PMID:11700578; http://dx.doi.org/ 10.1086/324589 [DOI] [PubMed] [Google Scholar]
- [49].Lass-Florl C, Dierich MP, Fuchs D, Semenitz E, Jenewein I, Ledochowski M. Antifungal properties of selective serotonin reuptake inhibitors against Aspergillus species in vitro. J Antimicrob Chemother 2001; 48:775-9; PMID:11733460; http://dx.doi.org/ 10.1093/jac/48.6.775 [DOI] [PubMed] [Google Scholar]
- [50].Spitzer M, Griffiths E, Blakely KM, Wildenhain J, Ejim L, Rossi L, De Pascale G, Curak J, Brown E, Tyers M, et al.. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol Syst Biol 2011; 7:499; PMID:21694716; http://dx.doi.org/ 10.1038/msb.2011.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Robbins N, Spitzer M, Yu T, Cerone Robert P, Averette Anna K, Bahn Y-S, Heitman J, Sheppard Donald C, Tyers M, Wright Gerard D. An Antifungal Combination Matrix Identifies a Rich Pool of Adjuvant Molecules that Enhance Drug Activity against Diverse Fungal Pathogens. Cell Reports 13:1481-92; PMID:26549450; http://dx.doi.org/ 10.1016/j.celrep.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhai B, Wu C, Wang L, Sachs MS, Lin X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother 2012; 56:3758-66; PMID:22508310; http://dx.doi.org/ 10.1128/AAC.00212-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rhein J, Morawski BM, Hullsiek KH, Nabeta HW, Kiggundu R, Tugume L, Musubire A, Akampurira A, Smith KD, Alhadab A, et al.. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 2016; 16:809-18; PMID:26971081; http://dx.doi.org/ 10.1016/S1473-3099(16)00074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lorenz RT, Parks LW. Effects of lovastatin (mevinolin) on sterol levels and on activity of azoles in Saccharomyces cerevisiae. Antimicrob Agents Chemother 1990; 34:1660-5; PMID:2285278; http://dx.doi.org/ 10.1128/AAC.34.9.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Song JL, Lyons CN, Holleman S, Oliver BG, White TC. Antifungal activity of fluconazole in combination with lovastatin and their effects on gene expression in the ergosterol and prenylation pathways in Candida albicans. Med Mycol 2003; 41:417-25; PMID:14653518; http://dx.doi.org/ 10.1080/1369378031000137233 [DOI] [PubMed] [Google Scholar]
- [56].Nyilasi I, Kocsube S, Krizsan K, Galgoczy L, Pesti M, Papp T, Vagvolgyi C. In vitro synergistic interactions of the effects of various statins and azoles against some clinically important fungi. FEMS Microbiol Lett 2010; 307:175-84; PMID:20636975; http://dx.doi.org/ 10.1111/j.1574-6968.2010.01972.x [DOI] [PubMed] [Google Scholar]
- [57].Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun 2001; 69:2957-63; PMID: 11292712; http://dx.doi.org/ 10.1128/IAI.69.5.2957-2963.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Scott EM, Tariq VN, McCrory RM. Demonstration of synergy with fluconazole and either ibuprofen, sodium salicylate, or propylparaben against Candida albicans in vitro. Antimicrob Agents Chemother 1995; 39:2610-4; PMID:8592988; http://dx.doi.org/ 10.1128/AAC.39.12.2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Pina-Vaz C, Sansonetty F, Rodrigues AG, Martinez-De-Oliveira J, Fonseca AF, Mardh PA. Antifungal activity of ibuprofen alone and in combination with fluconazole against Candida species. J Med Microbiol 2000; 49:831-40; PMID:10966233; http://dx.doi.org/ 10.1099/0022-1317-49-9-831 [DOI] [PubMed] [Google Scholar]
- [60].Guo N, Liu J, Wu X, Bi X, Meng R, Wang X, Xiang H, Deng X, Yu L. Antifungal activity of thymol against clinical isolates of fluconazole-sensitive and -resistant Candida albicans. J Med Microbiol 2009; 58:1074-9; PMID:19528168; http://dx.doi.org/ 10.1099/jmm.0.008052-0 [DOI] [PubMed] [Google Scholar]
- [61].Faria NC, Kim JH, Goncalves LA, Martins Mde L, Chan KL, Campbell BC. Enhanced activity of antifungal drugs using natural phenolics against yeast strains of Candida and Cryptococcus. Lett Appl Microbiol 2011; 52:506-13; PMID:21332761; http://dx.doi.org/ 10.1111/j.1472-765X.2011.03032.x [DOI] [PubMed] [Google Scholar]
- [62].Kim J, Campbell B, Mahoney N, Chan K, Molyneux R, May G. Chemosensitization prevents tolerance of Aspergillus fumigatus to antimycotic drugs. Biochem Biophys Res Commun 2008; 372:266-71; PMID:18486603; http://dx.doi.org/ 10.1016/j.bbrc.2008.05.030 [DOI] [PubMed] [Google Scholar]
- [63].Pinto E, Pina-Vaz C, Salgueiro L, Goncalves MJ, Costa-de-Oliveira S, Cavaleiro C, Palmeira A, Rodrigues A, Martinez-de-Oliveira J. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J Med Microbiol 2006; 55:1367-73; PMID:17005785; http://dx.doi.org/ 10.1099/jmm.0.46443-0 [DOI] [PubMed] [Google Scholar]
- [64].Ahmad A, Khan A, Khan LA, Manzoor N. In vitro synergy of eugenol and methyleugenol with fluconazole against clinical Candida isolates. J Med Microbiol 2010; 59:1178-84; PMID:20634332; http://dx.doi.org/ 10.1099/jmm.0.020693-0 [DOI] [PubMed] [Google Scholar]
- [65].Ahmad A, Khan A, Yousuf S, Khan LA, Manzoor N. Proton translocating ATPase mediated fungicidal activity of eugenol and thymol. Fitoterapia 2010; 81:1157-62; PMID:20659536; http://dx.doi.org/ 10.1016/j.fitote.20-10.07.020 [DOI] [PubMed] [Google Scholar]
- [66].Iwazaki RS, Endo EH, Ueda-Nakamura T, Nakamura CV, Garcia LB, Filho BP. In vitro antifungal activity of the berberine and its synergism with fluconazole. Antonie Van Leeuwenhoek 2010; 97:201-5; PMID:19882381; http://dx.doi.org/ 10.1007/s10482-009-9394-8 [DOI] [PubMed] [Google Scholar]
- [67].Sharma M, Manoharlal R, Negi AS, Prasad R. Synergistic anticandidal activity of pure polyphenol curcumin I in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res 2010; 10:570-8; PMID:20528949 [DOI] [PubMed] [Google Scholar]
- [68].Bang KH, Lee DW, Park HM, Rhee YH. Inhibition of fungal cell wall synthesizing enzymes by trans-cinnamaldehyde. Biosci Biotechnol Biochem 2000; 64:1061-3; PMID:10879482; http://dx.doi.org/ 10.1271/bbb.64.1061 [DOI] [PubMed] [Google Scholar]
- [69].Borjihan H, Ogita A, Fujita K, Hirasawa E, Tanaka T. The vacuole-targeting fungicidal activity of amphotericin B against the pathogenic fungus Candida albicans and its enhancement by allicin. J Antibiot (Tokyo) 2009; 62:691-7; PMID:19876074; http://dx.doi.org/ 10.1038/ja.2009.103 [DOI] [PubMed] [Google Scholar]
- [70].Guo N, Wu X, Yu L, Liu J, Meng R, Jin J, Lu H, Wang X, Yan S, Deng X. In vitro and in vivo interactions between fluconazole and allicin against clinical isolates of fluconazole-resistant Candida albicans determined by alternative methods. FEMS Immunol Med Microbiol 2010; 58:193-201; PMID:19878317; http://dx.doi.org/ 10.1111/j.1574-695X.2009.00620.x [DOI] [PubMed] [Google Scholar]
- [71].Han Y. Synergic anticandidal effect of epigallocatechin-O-gallate combined with amphotericin B in a murine model of disseminated candidiasis and its anticandidal mechanism. Biol Pharm Bull 2007; 30:1693-6; PMID:17827722; http://dx.doi.org/ 10.1248/bpb.30.1693 [DOI] [PubMed] [Google Scholar]
- [72].Schubert S, Barker KS, Znaidi S, Schneider S, Dierolf F, Dunkel N, Aid M, Boucher G, Rogers PD, Raymond M, et al.. Regulation of efflux pump expression and drug resistance by the transcription factors Mrr1, Upc2, and Cap1 in Candida albicans. Antimicrob Agents Chemother 2011; 55:2212-23; PMID:21402859; http://dx.doi.org/ 10.1128/AAC.01343-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].White TC. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother 1997; 41:1482-7; PMID:9210670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Revankar SG, Kirkpatrick WR, McAtee RK, Fothergill AW, Redding SW, Rinaldi MG, Patterson TF. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J Clin Microbiol 1998; 36:153-6; PMID:9431939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Smith WL, Edlind TD. Histone deacetylase inhibitors enhance Candida albicans sensitivity to azoles and related antifungals: correlation with reduction in CDR and ERG upregulation. Antimicrob Agents Chemother 2002; 46:3532-9; PMID:12384361; http://dx.doi.org/ 10.1128/AAC.46.11.3532-3539.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Flowers SA, Barker KS, Berkow EL, Toner G, Chadwick SG, Gygax SE, Morschhauser J, Rogers PD. Gain-of-function mutations in UPC2 are a frequent cause of ERG11 upregulation in azole-resistant clinical isolates of Candida albicans. Eukaryot Cell 2012; 11:1289-99; PMID:22923048; http://dx.doi.org/ 10.1128/EC.00215-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Del Sorbo G, Schoonbeek H, De Waard MA. Fungal transporters involved in efflux of natural toxic compounds and fungicides. Fungal Genet Biol 2000; 30:1-15; PMID:10955904; http://dx.doi.org/ 10.1006/fgbi.2000.1206 [DOI] [PubMed] [Google Scholar]
- [78].Schuetzer-Muehlbauer M, Willinger B, Egner R, Ecker G, Kuchler K. Reversal of antifungal resistance mediated by ABC efflux pumps from Candida albicans functionally expressed in yeast. Int J Antimicrob Agents 2003; 22:291-300; PMID:13678837; http://dx.doi.org/ 10.1016/S0924-8579(03)00213-9 [DOI] [PubMed] [Google Scholar]
- [79].Lee MD, Galazzo JL, Staley AL, Lee JC, Warren MS, Fuernkranz H, Chamberland S, Lomovskaya O, Miller GH. Microbial fermentation-derived inhibitors of efflux-pump-mediated drug resistance. Farmaco 2001; 56:81-5; PMID:11347972; http://dx.doi.org/ 10.1016/S0014-827X(01)01002-3 [DOI] [PubMed] [Google Scholar]
- [80].Niimi K, Harding DR, Parshot R, King A, Lun DJ, Decottignies A, Niimi M, Lin S, Cannon RD, Goffeau A, et al.. Chemosensitization of fluconazole resistance in Saccharomyces cerevisiae and pathogenic fungi by a D-octapeptide derivative. Antimicrob Agents Chemother 2004; 48:1256-71; PMID:15047528; http://dx.doi.org/ 10.1128/AAC.48.4.1256-1271.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Niimi K, Harding DR, Holmes AR, Lamping E, Niimi M, Tyndall JD, Cannon RD, Monk BC. Specific interactions between the Candida albicans ABC transporter Cdr1p ectodomain and a D-octapeptide derivative inhibitor. Mol Microbiol 2012; 85:747-67; PMID:22788839; http://dx.doi.org/ 10.1111/j.1365-2958.2012.08140.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Keniya MV, Fleischer E, Klinger A, Cannon RD, Monk BC. Inhibitors of the Candida albicans Major Facilitator Superfamily Transporter Mdr1p Responsible for Fluconazole Resistance. PLoS One 2015; 10:e0126350; PMID:25951180; http://dx.doi.org/ 10.1371/journal.pone.0126350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Holmes AR, Keniya MV, Ivnitski-Steele I, Monk BC, Lamping E, Sklar LA, Cannon RD. The monoamine oxidase A inhibitor clorgyline is a broad-spectrum inhibitor of fungal ABC and MFS transporter efflux pump activities which reverses the azole resistance of Candida albicans and Candida glabrata clinical isolates. Antimicrob Agents Chemother 2012; 56:1508-15; PMID:22203607; http://dx.doi.org/ 10.1128/AAC.05706-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ahmad A, Khan A, Manzoor N. Reversal of efflux mediated antifungal resistance underlies synergistic activity of two monoterpenes with fluconazole. Eur J Pharm Sci 2013; 48:80-6; PMID:23111348; http://dx.doi.org/ 10.1016/j.ejps.2012.09.016 [DOI] [PubMed] [Google Scholar]
- [85].Huang S, Cao YY, Dai BD, Sun XR, Zhu ZY, Cao YB, Wang Y, Gao PH, Jiang YY. In vitro synergism of fluconazole and baicalein against clinical isolates of Candida albicans resistant to fluconazole. Biol Pharm Bull 2008; 31:2234-6; PMID:19043205; http://dx.doi.org/ 10.1248/bpb.31.2234 [DOI] [PubMed] [Google Scholar]
- [86].Gao Y, Zhang C, Lu C, Liu P, Li Y, Li H, Sun S. Synergistic effect of doxycycline and fluconazole against Candida albicans biofilms and the impact of calcium channel blockers. FEMS Yeast Res 2013; 13:453-62; PMID:23577622; http://dx.doi.org/ 10.1111/1567-1364.12048 [DOI] [PubMed] [Google Scholar]
- [87].Uppuluri P, Nett J, Heitman J, Andes D. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob Agents Chemother 2008; 52:1127-32; PMID:18180354; http://dx.doi.org/ 10.1128/AAC.01397-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Alem MA, Douglas LJ. Effects of aspirin and other nonsteroidal anti-inflammatory drugs on biofilms and planktonic cells of Candida albicans. Antimicrob Agents Chemother 2004; 48:41-7; PMID:14693516; http://dx.doi.org/ 10.1128/AAC.48.1.41-47.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhou Y, Wang G, Li Y, Liu Y, Song Y, Zheng W, Zhang N, Hu X, Yan S, Jia J. In vitro interactions between aspirin and amphotericin B against planktonic cells and biofilm cells of Candida albicans and C. parapsilosis. Antimicrob Agents Chemother 2012; 56:3250-60; PMID:22391539; http://dx.doi.org/ 10.1128/AAC.06082-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Khan MS, Ahmad I. Antibiofilm activity of certain phytocompounds and their synergy with fluconazole against Candida albicans biofilms. J Antimicrob Chemother 2012; 67:618-21; PMID:22167241; http://dx.doi.org/ 10.1093/jac/dkr512 [DOI] [PubMed] [Google Scholar]
- [91].Delattin N, De Brucker K, Vandamme K, Meert E, Marchand A, Chaltin P, Cammue BP, Thevissen K. Repurposing as a means to increase the activity of amphotericin B and caspofungin against Candida albicans biofilms. J Antimicrob Chemother 2014; 69:1035-44; PMID:24284780; http://dx.doi.org/ 10.1093/jac/dkt449 [DOI] [PubMed] [Google Scholar]
- [92].Campbell BC, Chan KL, Kim JH. Chemosensitization as a means to augment commercial antifungal agents. Front Microbiol 2012; 3:79; PMID:22393330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Liu S, Hou Y, Chen X, Gao Y, Li H, Sun S. Combination of fluconazole with non-antifungal agents: a promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery. Int J Antimicrob Agents 2014; 43:395-402; PMID:24503221; http://dx.doi.org/ 10.1016/j.ijantimicag.2013.12.009 [DOI] [PubMed] [Google Scholar]
- [94].Borisy AA, Elliott PJ, Hurst NW, Lee MS, Lehar J, Price ER, Serbedzija G, Zimmermann GR, Foley MA, Stockwell BR, et al.. Systematic discovery of multicomponent therapeutics. Proc Natl Acad Sci U S A 2003; 100:7977-82; PMID:12799470; http://dx.doi.org/ 10.1073/pnas.1337-088100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cui J, Ren B, Tong Y, Dai H, Zhang L. Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence 2015; 6:362-71; PMID:26048362; http://dx.doi.org/ 10.1080/21505594.2015.1039885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Coelho C, Casadevall A. Cryptococcal therapies and drug targets:the old, the new and the promising. Cell Microbiol 2016; 18(6):792-9; PMID:26990050 [DOI] [PMC free article] [PubMed] [Google Scholar]