Abstract
The intensity and duration of TGF-β signaling determine the cellular biological response. How this is negatively regulated is not well understood. Here, we identified a novel negative regulator of TGF-β signaling, transmembrane p24-trafficking protein 10 (TMED10). TMED10 disrupts the complex formation between TGF-β type I (also termed ALK5) and type II receptors (TβRII). Misexpression studies revealed that TMED10 attenuated TGF-β-mediated signaling. A 20-amino acid-long region from Thr91 to Glu110 within the extracellular region of TMED10 was found to be crucial for TMED10 interaction with both ALK5 and TβRII. Synthetic peptides corresponding to this region inhibit both TGF-β-induced Smad2 phosphorylation and Smad-dependent transcriptional reporter activity. In a xenograft cancer model, where previously TGF-β was shown to elicit tumor-promoting effects, gain-of-function and loss-of-function studies for TMED10 revealed a decrease and increase in the tumor size, respectively. Thus, we determined herein that TMED10 expression levels are the key determinant for efficiency of TGF-β receptor complex formation and signaling.
Keywords: epithelial-mesenchymal transition (EMT), serine/threonine protein kinase, SMAD transcription factor, transforming growth factor β (TGF-β), tumor
Introduction
Transforming growth factor (TGF)-β is a secreted cytokine that modulates proliferation, differentiation, apoptosis, immune responses, and other cellular functions in many different cell types in a highly context-dependent manner (1–5). Other secreted proteins, including bone morphogenetic proteins (BMP)3 and activins, are similar to TGF-β in their protein structure and function; TGF-β-related proteins are collectively termed the TGF-β family (6). The TGF-β/Smad pathway is initiated by ligand binding to TGF-β type II receptor (TβRII), in which the intracellular domain has constitutive serine/threonine kinase activity (7). Subsequently, TβRII forms a heteromeric complex with TGF-β type I receptor (TβRI or activin receptor-like kinase 5 (ALK5)) and phosphorylates serine and threonine residues within the glycine-serine repeat (GS) domain and is present at the juxtamembrane region in ALK5 (8). Then, the intracellular serine/threonine kinase activity in ALK5 becomes active. The active ALK5 kinase catalyzes the phosphorylation of the two extreme C-terminal serine residues of certain receptor-regulated (R-Smad) proteins, i.e. Smad2 and Smad3 (7). These phosphorylated R-Smad form a binary or ternary complex with common-mediated Smad (Co-Smad, i.e. Smad4), which is followed by their translocation to the nucleus, where they transcriptionally regulate TGF-β target genes together with other transcriptional factors, coactivators, and corepressors (9–11). Besides the canonical Smad pathway, the TGF-β receptor complex can also initiate intracellular non-Smad pathways. Mitogen-activated protein kinases (MAPK) including Erk, JNK, p38, PI3K/Akt, RhoA/Rock1, mTORC, Par6, and Shc become activated by TGF-β contextually in certain cell types (12–16). In some cases, non-Smad pathways cooperate with the Smad pathway to adequately elicit TGF-β-dependent responsiveness in cells (17).
TGF-β signaling is of key importance in embryogenesis and tissue homeostasis; hence, dysregulation of the TGF-β signaling pathway invokes congenital abnormalities as well as a number of diseases including cancer, fibrosis, and vascular defects (18–22). Thus, TGF-β signaling needs to be intricately regulated. This intricate regulation occurs at all steps in the TGF-β signal transduction cascade, for example, in extracellular environments in which the bioavailability of the ligand is controlled by extracellular matrix ligand-binding proteins (23), ligand traps (2, 24), auxiliary coreceptors (2, 25), and decoy receptors (26). Frequently, these negative extracellular and intracellular regulators are induced by TGF-β, thereby exerting negative feedback functions. Consequently, cells are prohibited from being exposed by an excessive intensity and duration of TGF-β signaling (27, 28).
Transmembrane p24-trafficking protein 10 (TMED10), alternatively termed p23, TMP21 (transmembrane protein with type I topology 21), Tmp-21-I, and p24δ, is a member of the EMP24 (endomembrane protein precursor of 24 kDa)/GP25L (glycoprotein 25L)/p24 family, which is involved in COP (coat protein) vesicle cargo receptors (29). TMED10, a type I transmembrane protein, is located to the plasma membrane, microsomal membranes, and zymogen granule membranes (30). Indeed, it has been reported that TMED10 contributes to the recruitment of the small GTPase ADP-ribosylation factor 1 (ARF1) to the Golgi apparatus owing to actin assembly (31–33). TMED10 has roles that extend beyond trafficking; for example, TMED10 interacts with presenilin complexes to modulate the activity of γ-secretase without any effect of ϵ-secretase in decreasing the secretion of amyloid-β (34, 35). Thus, TMED10 may be involved in Alzheimer disease (36). In addition, TMED10 can limit the activity of PKCδ via its association with PKCδ in the prostate cancer cell line LNCaP to inhibit apoptosis (37, 38) and activate the AMPK/mTOR (mammalian target of rapamycin) pathway to modulate cell growth (39). Furthermore, TMED10 preferentially binds to MHC I heavy chains that dissociate with β2-microglobulin (40). Therefore, TMED10, dependent on the context, may act as a multipotent protein in cells.
In this study, we found that TMED10 attenuates TGF-β signaling via dissociation of the TGF-β type I/type II heteromeric receptor complex. In particular, the extracellular domain of TMED10 is required for TMED10 to bind to both TβRII and ALK5. Interestingly, the short peptide derived from the extracellular domain of TMED10 can antagonize TGF-β signaling. Thus, a low molecular weight mimetic based on the structure of this peptide might be therapeutically suitable for patients with diseases with underlying excessive TGF-β receptor signaling.
Results
Identification of ALK5-interacting Proteins
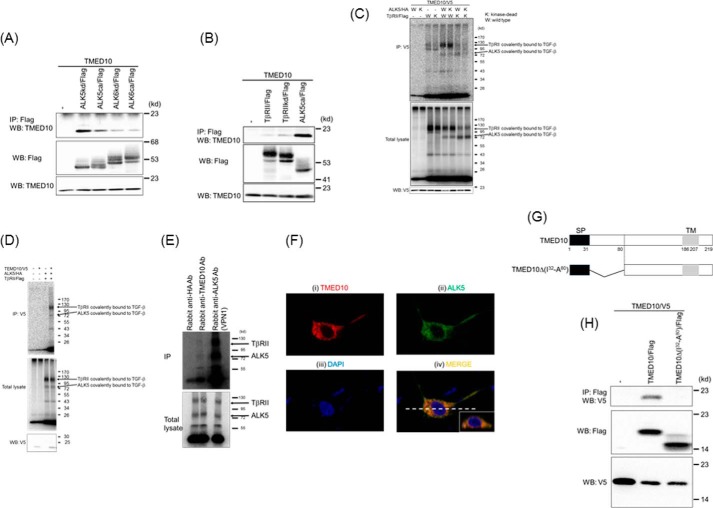
To elucidate the regulatory mechanisms that underlie TGF-β signaling via modulation of TGF-β receptor activity, we identified putative ALK5-interacting proteins by high throughput analysis of proteins coimmunoprecipitated with epitope-tagged human constitutively active ALK5 (ALK5ca) in HEK293 cells by use of liquid chromatography-mass spectrometry and liquid chromatography (LC-MS/MS) analysis. One of the proteins we identified was TMED10. It was selected from among 13 candidates because TMED10 was always isolated using the above method in four independent experiments. To validate this finding, we analyzed whether TMED10 interacts with human ALK5ca when coexpressed in COS7 cells (Fig. 1A). We also tested whether the kinase activity of ALK5 influences its interaction with TMED10. We found that the interaction of TMED10 with ALK5ca was weaker than that with kinase-dead ALK5 (ALK5kd) (Fig. 1A). To investigate the specificity of the interaction of TMED10 with ALK5, we also investigated whether ALK6, a BMP type IB receptor, forms a complex with TMED10 upon their coexpression in COS7 cells. The interaction of TMED10 with either kinase-dead ALK6 (ALK6kd) or constitutively active (ALK6ca) was only marginally observed. In addition, TMED10 possessed the subtle ability to bind to TβRII and kinase-dead TβRII (TβRIIkd) (Fig. 1, A and B). These results indicate that TMED10 has selectivity to interact with ALK5 when compared with other tested type I and type II receptors. Next, we performed affinity-labeling experiments using 125I-TGF-β in COS1 cells transfected with TMED10, ALK5 (or its kinase-inactive mutant), TβRII (or its kinase-inactive mutant), or combinations thereof. The kinase activity of ALK5 was not required for ALK5 to interact with TMED10, whereas the loss of TβRII kinase activity made the interaction between TβRII and TMED10 weaker. The reason that the intensity of the band corresponding to TβRII covalently bound to 125I-TGF-β becomes strong is that TβRII alone, but not ALK5 alone, can bind to TGF-β (Fig. 1C) (41, 42). On the other hand, we could not detect any interaction between TMED10 and 125I-TGF-β when TMED10 alone was transfected in COS1 cells (Fig. 1D). Furthermore, we tried to show endogenous interaction between TGF-β receptors and TMED10 using 125I-TGF-β in HaCaT cells. The immunoprecipitation of the receptor complex with a rabbit anti-TMED10 antibody could be detected, although rabbit anti-HA antibody as a control antibody could not isolate any receptor complex (Fig. 1E). Thus, TMED10 endogenously interacts with the TGF-β receptor complex on the cell membrane.
FIGURE 1.
Interaction of TMED10 with TGF-β receptors. A, interaction of TMED10 with ALK5. COS7 cells were transfected with the indicated plasmids and harvested for coimmunoprecipitation (co-IP) experiments. The interaction between TMED10 and either ALK5 or ALK6 is shown in the top panel. The total expressions of FLAG-ALK and TMED10 are indicated in the middle and bottom panels, respectively. B, interaction of TMED10 with TβRII. COS7 cells were transfected with the indicated plasmids and harvested for the co-IP experiments. The interaction of TMED10 with TβRII is shown in the top panel. The total expressions of FLAG-TβRII and TMED10 are indicated in the middle and bottom panels, respectively. ALK5ca/FLAG was used as the positive control. C, association of TMED10 with the TGF-β receptor complex. COS1 cells were transfected with the indicated plasmids. Forty hours after transfection, the TGF-β receptors were covalently affinity-labeled with iodinated TGF-β. The cell lysates were subjected to immunoprecipitation with an anti-V5 antibody and analyzed by SDS-PAGE and a PhosphorImager (top panel). The expression of the receptors was determined by analyzing an aliquot of cell lysates by SDS-PAGE without immunoprecipitation (middle panel). The expression of TMED10 was detected by Western blotting analysis (WB) with an anti-V5 antibody using cell lysates without the addition of iodinated TGF-β (bottom panel). D, no direct interaction between TMED10 and TGF-β. COS1 cells were transfected with the indicated plasmids. Forty hours after transfection, iodinated TGF-β was added to the medium followed by covalent binding between cell surface molecules and iodinated TGF-β with disuccinimidyl suberate. The cell lysates were subjected to immunoprecipitation with an anti-V5 antibody and analyzed by SDS-PAGE and a PhosphorImager (top panel). The expression of receptors was determined by analyzing an aliquot of cell lysates by SDS-PAGE without immunoprecipitation (middle panel). The expression of TMED10 was detected by Western blotting analysis with an anti-V5 antibody using cell lysates without addition of iodinated TGF-β (bottom panel). E, endogenous interaction between TMED10 and TGF-β receptors. After iodinated TGF-β was added to the media in which HaCaT cells had been cultured, the cell lysates were prepared. The cell lysates were immunoprecipitated with anti-TMED10 antibody followed by SDS-PAGE. Expression of the receptors was determined by analyzing an aliquot of cell lysates by SDS-PAGE without immunoprecipitation. As the negative and positive controls, anti-HA (Y-11) and anti-ALK5 (VPN1) antibodies were used for immunoprecipitation. F, colocalization of TMED10 with ALK5. TMED10/V5 was transfected with ALK5/HA in 911 cells. Twenty-four hours after transfection, the cells were stimulated with 5 ng/ml TGF-β for 2 h. Subsequently, the cells were fixed and stained with mouse anti-V5 monoclonal and rabbit ALK5 (V-22) polyclonal antibodies. Then, Alexa 555-conjugated goat anti-mouse (i) and CF488A goat anti-rabbit IgG antibodies (ii) were used for visualization. Colocalization can be seen in the merged panel as the yellow portin (iv). Nuclear staining (blue) was carried out using DAPI (iii). The insert in panel iv shows the vertical image. G, schematic presentation of TMED10Δ(I32-A80). SP, signal peptide; TM, transmembrane. H, homodimer formation of TMED10. COS7 cells were transfected with the indicated plasmids and harvested for the co-IP experiments. The homodimer formation of TMED10 is shown in the top panel. The total expressions of TMED10/FLAG or its mutant and TMED10/V5 are indicated in the middle and bottom panels, respectively.
The physiological interaction between TMED10 and ALK5 prompted us to examine their subcellular colocalization. When human embryonic retinoblast 911 cells were transfected with both TMED10 and ALK5, both of them could be seen mainly in the cytosol (Fig. 1F, upper panels), because both proteins are synthesized in the rough endoplasmic reticulum (ER) and transported to the cell membrane via the Golgi apparatus as transmembrane proteins. However, the vertical image shows that their colocalization can be observed along the cell membrane in part (Fig. 1F, insert in lower right panel). These data suggest that TMED10 associates with ALK5 at the cell membrane.
ALK5 and TβRII are present as homodimers at the cell surface (43). To examine whether this is also the case for TMED10, we transfected two differentially epitope-tagged TMED10 (TMED10/FLAG and TMED10/V5) (Fig. 1H) in COS7 cells was followed by coimmunoprecipitation with an anti-FLAG antibody and Western blotting analysis with an anti-V5 antibody. The result showed evidence for homo-oligomerization of TMED10. On the other hand, a mutant TMED10 containing an internal deletion of TMED10 between Ile32 and Ala80 (Fig. 1G, TMED10Δ(I32-A80)) was unable to interact with wild-type TMED10 (Fig. 1H). Taken together, these results suggest that the N-terminal region of TMED10 plays a key role in the homo-oligomerization of TMED10.
Negative Regulation of TGF-β Signaling by TMED10
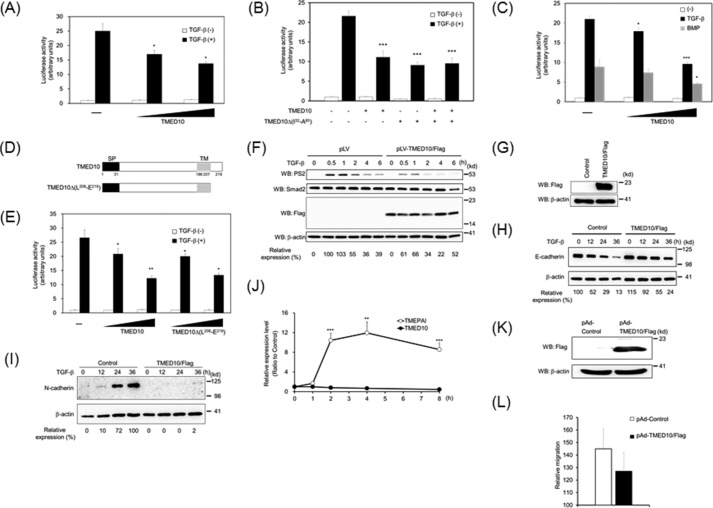
To investigate whether TMED10 affects TGF-β signaling, we tested the effect of TMED10 on the TGF-β-induced Smad-driven transcriptional (SBE)4-luc reporter (44), we and observed dose-dependent inhibition of TMED10 (Fig. 2A). Interestingly, TMED10Δ(I32-A80) could also inhibit TGF-β signaling like TMED10. Thus, the oligomerization of TMED10 is not required for the inhibition of TGF-β signaling (Fig. 2B). TMED10 also has the ability to interfere with BMP signaling, albeit with relatively weak inhibition (Fig. 2C). Thus, we focused on the inhibitory effect of TMED10 on TGF-β signaling in the subsequent experiments. To examine the possibility that the short C-terminal intracellular tail of TMED10 plays a role in the perturbation of TGF-β signaling, TMED10Δ(L208-E219) was constructed (Fig. 2D) and then transfected and subsequently stimulated with TGF-β in HepG2 cells. As seen in Fig. 2E, TMED10Δ(L208-E219) had an inhibitory action comparable to that of wild-type TMED10. We further examined whether TMED10 had an ability to hamper TGF-β-mediated Smad2 phosphorylation. Ectopic expression of TMED10 in human keratinocyte HaCaT (Fig. 2F), 293T (data not shown), and mouse mammary gland epithelial NMuMG cells (data not shown) inhibited phosphorylation of endogenous Smad2 upon TGF-β stimulation. It is well known that TGF-β potentiates the epithelial-mesenchymal transition (EMT) in NMuMG cells (45). During this process, a switch from E-cadherin to N-cadherin expression takes place (46). Adenoviral infection of TMED10 in NMuMG cells blocked TGF-β-mediated suppression of E-cadherin expression (Fig. 2, G and H) and TGF-β-mediated induction of N-cadherin expression (Fig. 2I).
FIGURE 2.
Inhibitory action of TMED10 on TGF-β signaling. A, dose-dependent inhibition of TGF-β-driven reporter activity by TMED10. HepG2 cells were transfected with (SBE)4-luc, which can be activated upon not only by TGF-β but also BMP signals (44), pCH110, and the indicated plasmids at two different doses. Twenty-four hours later, the cells were stimulated with 5 ng/ml TGF-β for 18 h. Significant differences from the control in the presence of TGF-β are indicated with asterisks. B, effect of TMED10Δ(I32-A80) on the activity of TGF-β-driven transcriptional reporter. The experiments were performed as described in A. The combined total amount of TMED10 with TMED10Δ(I32-A80) was the same in all columns. Significant differences from the control in the presence of TGF-β are indicated with asterisks. C, inhibitory ability of TMED10 on BMP signaling. The experiments were performed as described in A except for the addition of 25 ng/ml BMP-6. Significant differences from the control in the presence of TGF-β or BMP are indicated with asterisks. D, illustration of C-terminal deletion of TMED10. SP, signal peptide; TM, transmembrane E, dispensation of the C-terminal end of TMED10 for its inhibitory action. The experiments were performed as described in A. Significant differences from the control in the presence of TGF-β are indicated with asterisks. F, inhibition of Smad2 phosphorylation by TMED10 in HaCaT cells. HaCaT cells carrying TMED10/FLAG by the method of lentiviral gene transfer were stimulated with 0.5 ng/ml TGF-β for the indicated times. After preparation of the cell lysates, anti-phosphorylated Smad2 (PS2) (top panel), anti-Smad2 (second panel), anti-FLAG (third panel), and anti-β-actin antibodies (bottom panel) were used for Western blotting analyses (WB). The expression of phosphorylated Smad2 upon TGF-β stimulation was normalized using the intensity of the band corresponding to Smad2. Relative expression was calculated relative to the value for pLV-CMV-IRES-Puro-infected cells in the absence of TGF-β. G, overexpression of TMED10/FLAG by adenoviral transfer. NMuMG cells were infected with TMED10/FLAG-expressing adenovirus. After preparation of the cell lysates, anti-FLAG (top panel) or anti-β-actin antibody (bottom panel) was used. H, extension of E-cadherin expression in NMuMG cells expressing TMED10/FLAG upon TGF-β stimulation. TMED10 were introduced into NMuMG cells by an adenoviral gene transfer system as described in G. Forty hours after infection, the cells were stimulated with 0.5 ng/ml TGF-β for the indicated times. After preparation of the cell lysates, anti-E-cadherin (top panel) and anti-β-actin antibodies (bottom panel) were used for Western blotting analyses. The expression of E-cadherin upon TGF-β stimulation was normalized using the intensity of the band corresponding to β-actin. Relative expression was calculated relative to the value for control cells in the absence of TGF-β. I, inhibition of N-cadherin expression by TMED10 in NMuMG cells. After gene transfer of TMED10/FLAG by adenovirus, the cells were cultured for 40 h. Subsequently, the cells were stimulated with 0.5 ng/ml TGF-β for the indicated times. After preparation of the cell lysates, anti-N-cadherin (top panel) and anti-β-actin antibodies (bottom panel) were used for Western blotting analyses. The expression of N-cadherin upon TGF-β stimulation was normalized using the intensity of the band corresponding to β-actin. Relative expression was calculated relative to the value for control cells in the absence of TGF-β. J, expression of TMED10 mRNA upon TGF-β stimulation. HepG2 cells were stimulated with 5 ng/ml TGF-β at the different time points. After preparation of total RNA from the cells, PCR was carried out using specific primer sets. As the positive control, the TMEPAI gene, which is well known as a TGF-β target gene, was used. Before qPCR, the amplified PCR product using each primer set could be seen in the agarose gel as a single band. Significant differences from the cells without the treatment of TGF-β are indicated with asterisks. K, overexpression of TMED10/FLAG in A549 cells by adenoviral gene transfer. The experiment was performed as described in G. L, inhibition of TGF-β-induced cell migration by TMED10. After adenoviral infection as described in K, A549 cells were seeded on the upper membrane of the Boyden chamber. Subsequently, 5 ng/ml TGF-β was added to the lower chamber for 18 h. The cells were then stained with hematoxylin/eosin solution, and the number of transmigrated cells was counted. Probability values below 0.05, 0.01, and 0.001 were considered significant: *, p < 0.05, **, p < 0.01,***, p < 0.001.
Negative regulators of TGF-β signaling such as Smad7, SnoN, TGIF, Smurf1, and TMEPAI are known to be direct target genes of TGF-β signaling (28). However, TGF-β did not influence the expression of TMED10 mRNA, whereas it did influence the expression of TMEPAI mRNA (Fig. 2J).
Because cell motility is potentiated in some epithelial cells by TGF-β, we tried to confirm that TMED10 inhibits cell migration upon TGF-β stimulation. In the Transwell assay, we observed a trend for the inhibition by TMED10 on TGF-β-induced cell migration when TMED10 was overexpressed by adenoviral gene transfer (Fig. 2, K and L).
Enhancement of TGF-β Signaling due to Knockdown of TMED10
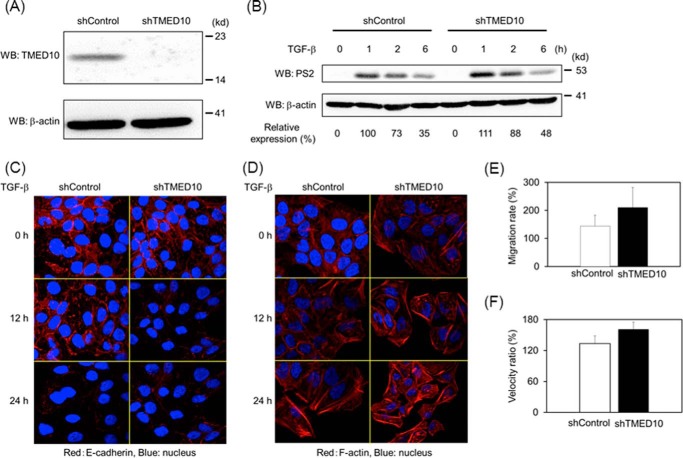
Because of the inhibitory effect of TMED10 on TGF-β signaling, we investigated whether loss-of-function of TMED10 enhances TGF-β signaling. When we reduced the expression of TMED10 in HaCaT cells by lentiviral transfer of TMED10-specific shRNA (Fig. 3A), an extension of TGF-β-induced Smad2 phosphorylation could be observed (Fig. 3B). Consistently, E-cadherin expression in HaCaT cells carrying shTMED10 decreased more quickly than in HaCaT cells carrying control shRNA when the cells were stimulated with TGF-β (Fig. 3C). We also confirmed the enhancement of stress fiber formation in HaCaT cells carrying TMED10 shRNA (Fig. 3D). Next we explored the effect of TMED10 depletion on cell motility. As seen in Fig. 3, E and F, not only the cell migration (using a Boyden chamber) but also the migration speed, measured by a wound healing assay, was raised, albeit weakly, in TMED10 knocked-down cells when cells received TGF-β signaling. Taken together, these results indicate that TMED10 interferes with TGF-β/Smad signaling.
FIGURE 3.
Loss-of-function analyses for TMED10. A, the expression of TMED10 in HaCaT cells carrying shTMED10 by lentiviral gene transfer. After establishment of shTMED10-expressing HaCaT cells by puromycin selection, the total cell lysates were prepared. Western blotting analyses (WB) were carried out using anti-TMED10 (top panel) and anti-β-actin (bottom panel) antibodies. B, extension of TGF-β-induced Smad2 phosphorylation in shTMED10-expressing HaCaT cells. The cells were stimulated with TGF-β for the indicated times. After cell lysis, Western blotting analysis was performed as described in the legend for Fig. 2F. shControl, HaCaT cells carrying the empty pLKO.1 vector. The expression of phosphorylated Smad2 upon TGF-β stimulation was normalized using the intensity of the band corresponding to β-actin. Relative expression was calculated relative to the value for HaCaT cells carrying the empty pLKO.1 vector in the absence of TGF-β. C, E-cadherin expression by immunofluorescence staining in shTMED10-expressing HaCaT cells. The cells were stimulated with 0.5 ng/ml TGF-β for the indicated times. Subsequently, the cells were fixed and stained with anti-E-cadherin antibody Then Alexa 555-conjugated goat anti-mouse antibody was used for visualization. Nuclear staining (blue) was carried out using DAPI. D, stress fiber formation upon TGF-β stimulation in HaCaT cells carrying shTMED10. The experiments were done as described in D above, except for the use of Alexa 555-conjugated phalloidin instead of anti-E-cadherin and Alexa 555-conjugated goat anti-mouse antibodies. E, TGF-β-mediated chemotaxis in shTMED10-expressing HaCaT cells. Experiments were performed as described in the legend for Fig. 2L. The value derived by dividing the cell number in the presence of TGF-β by that in the absence of TGF-β is shown. F, TGF-β-induced cell migration in shTMED10-expressing HaCaT cells. Cells were seeded confluently in 12-well plates. After cell scratching, 0.5 ng/ml TGF-β was added to the media. The cell movement was then measured by microscopy. The velocity of the cell movement was calculated. Then the value derived by dividing the velocity in the presence of TGF-β by that in the absence of TGF-β was shown.
Disruption of the TGF-β Receptor Complex by TMED10
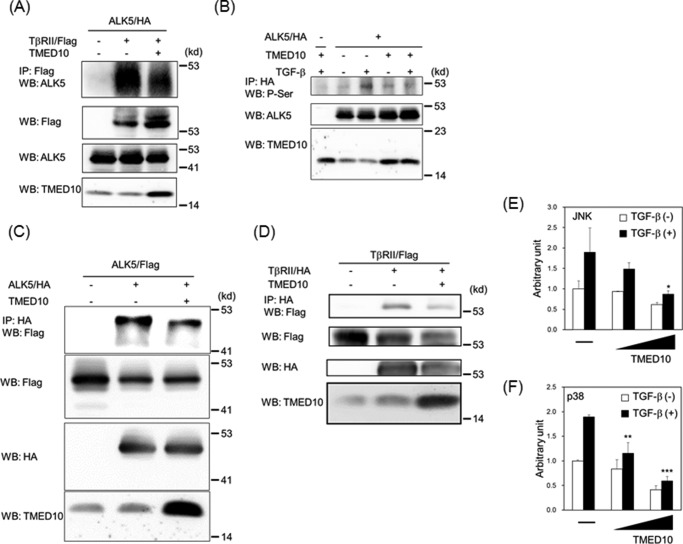
Having shown that TMED10 can interact not only with ALK5 but also with TβRII, we hypothesized that TMED10 could dissociate the complex between ALK5 and TβRII. Fig. 4A revealed that TMED10 disrupts the TGF-β receptor complex between ALK5 and TβRII when both receptors are overexpressed together with TMED10 in COS7 cells. After TGF-β binds to TβRII, TβRII recruits ALK5 to phosphorylate serine and threonine residues in its GS domain close to the juxtamembrane region (47). When ALK5/HA was transfected into 911 cells, we could observe the phosphorylation of serine residues in ALK5 1h after TGF-β stimulation. Co-transfection with TMED10 decreased the level of serine phosphorylation in ALK5 (Fig. 4B). We also checked whether TMED10 could disrupt ALK5 or TβRII homodimerization. Besides the assembly of heteromeric complexes between ALK5 and TβRII, both ALK5 and TβRII homodimer formations were attenuated upon introduction of TMED10 into cells (Fig. 4, C and D). Thus, TMED10 inhibits not only ligand-independent homomeric but also TGF-β-induced heteromeric complex formation between ALK5 and TβRII. Because TMED10 blocks TGF-β receptor complex formation, there is a possibility that TMED10 also interferes with non-Smad pathways upon TGF-β receptor activation (12–16). As expected, the TGF-β-mediated JNK and p38 pathways could be inhibited by TMED10 (Fig. 4, E and F).
FIGURE 4.
Disruption of the TGF-β receptor complex by TMED10. A, disruption of the receptor complex between ALK5 and TβRII by TMED10. COS7 cells were transfected with the indicated plasmids and harvested for co-IP experiments using anti-FLAG antibody. The interaction between ALK5 and TβRII is shown in the top panel. The total expressions of TβRII/FLAG, ALK5/HA, and TMED10 are indicated in the second, third, and bottom panels, respectively. The bands corresponding to TMED10 in the lane without overexpression of TMED10 seem to be endogenous TMED10. B, inhibition of TGF-β-induced ALK5 phosphorylation by TMED10. The 911 cells were transfected with the indicted plasmids. After the cells had been stimulated with 5 ng/ml TGF-β for 1 h, they were harvested for immunoprecipitation using anti-HA3F10 antibody followed by Western blotting analysis (WB) using anti-pSer antibody (top panel). Total expressions of ALK5 (middle panel) and TMED10 (bottom panel) were detected with anti-ALK5 (V-22) and anti-TMED10 antibodies, respectively. C, hampering of homo-oligomer formation of ALK5 by TMED10. The COS7 cells were transfected with the indicated plasmids and harvested for the co-IP experiments using anti-HA3F10 antibody. The homodimer formation of ALK5 is shown in the top panel. Total expressions of ALK5/FLAG, ALK5/HA, and TMED10 are indicated in the second, third, and bottom panel, respectively. D, interference of TβRII homo-oligomer formation by TMED10. The COS7 cells were transfected with the indicated plasmids and harvested for the co-IP experiments using anti-HA3F10 antibody. The homodimer formation of TβRII is shown in the top panel. Total expressions of TβRII/FLAG, TβRII/HA, and TMED10 are indicated in the second, third, and bottom panel, respectively. E and F, dose-dependent inhibition of TGF-β-mediated JNK and p38 activities by TMED10. E, HepG2 cells were transfected with pFR-luc, pFA2-cJun, and pCH110 (17) together with two different doses of TMED10 expression plasmids. Twenty-four hours later, the cells were stimulated with 5 ng/ml TGF-β for 18 h. F, HepG2 cells were transfected with pFR-luc, pFA-CHOP (17), and pCH110 together with two different doses of TMED10 expression plasmids. Twenty-four hours later, the cells were stimulated with 5 ng/ml TGF-β for 18 h. Significant differences from the control in the presence of TGF-β are indicated with asterisks. Probability values below 0.05, 0.01, and 0.001 were considered significant: *, p < 0.05, **, p < 0.01,***, p < 0.001.
Determination of the Interaction Domain of TMED10 with TGF-β Receptors
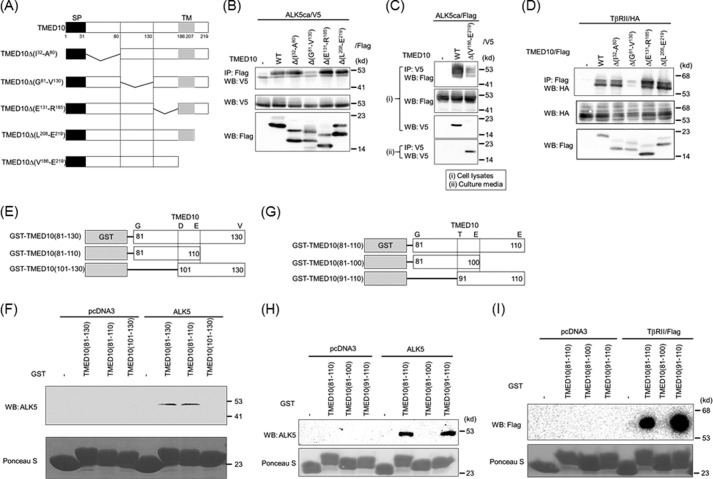
To determine the binding region of TMED10 with the TGF-β receptor, we made several TMED10 mutants, as seen in Fig. 5A. Upon deletion of TMED10 from Gly81 to Val130 (TMED10Δ(G81-V130)), the interaction between ALK5ca and TMED10 was lost (Fig. 5B). Although TMED10Δ(V186-E219), which lacks a C-terminal region, became a secretory protein (Fig. 5C, bottom panel), ALK5ca could associate with TMED10Δ(V186-E219). Thus, the transmembrane and intracellular domains of TMED10 were not required for TMED10 to interact with ALK5 (Fig. 5C). Similarly, TβRII also required the same 50-amino acid-long region from Gly81 to Val130 in TMED10 to interact with TβRII (Fig. 5D). To further narrow the binding region of TMED10 with the TGF-β receptor, we performed a pulldown assay using three GST fusion proteins (Fig. 5E). As observed in Fig. 5F, the 30-amino acid-long peptide composed of the region between Gly81and Glu110 in TMED10 was sufficient for TMED10 to interact with ALK5. We next divided this region into two fragments, GST-TMED10(81–100) and GST-TMED10(91–110) (Fig. 5G). GST-TMED10(91–110), but not GST-TMED10(81–100) associated with ALK5 (Fig. 5H) as well as with TβRII (Fig. 5I).
FIGURE 5.
Determination of the TGF-β receptor-interacting domain in TMED10. A, illustration of TMED10 and its mutants. SP, signal peptide; TM, transmembrane. B, interaction of TMED10 or its mutants with ALK5. COS7 cells were transfected with the indicated plasmids and harvested for co-IP experiments. The interaction between ALK5 and either TMED10 or its mutants is shown in the top panel. Total expressions of ALK5ca/V5 and TMED10/FLAG (or its mutants) are shown in the middle and bottom panel, respectively. C, non-necessity of both the transmembrane and the intracellular domains of TMED10 to interact with ALK5. The COS7 cells were transfected with the indicated plasmids. Section I, after cell lysis, anti-V5 antibodies were used for immunoprecipitation. The immunoprecipitates were separated by SDS-PAGE followed by Western blotting analysis (WB) using anti-FLAG antibody to detect their interaction (top panel). Total expressions of ALK5ca/FLAG and TMED10/V5 (or its mutants) in cells are shown in the second and third panel, respectively. Section II, because TMED10Δ(V186-E219) seemed to be a secretory protein, immunoprecipitation of TMED10Δ(V186-E219) into the media in which the cells were cultured was performed to detect secretory TMED10Δ(V186-E219) (bottom panel). D, interaction of TMED10 or its mutants with TβRII. Co-IP was performed as described in B above. The interaction between TβRII and TMED10 or it mutants is shown in the top panel. Total expressions of TβRII and TMED10 or its mutants were detected using anti-HA12CA5 (middle panel) and anti-FLAG antibodies (bottom panel), respectively. E, schematic presentation of GST-TMED10 fusion proteins. The region from Gly81 to Val130 in TMED10 was split into two pieces to make GST fusion proteins. F, requirement of the region consisting of 30 amino acids from Gly81 to Glu110 within TMED10 to interact with ALK5. A GST pulldown assay was performed using the GST fusion proteins described in E above. ALK5 ectopically expressed in COS7 cells was mixed with GST fusion protein. After loading the protein(s) bound to GSH-Sepharose 4B in SDS-PAGE, Western blotting analysis was performed using anti-ALK5 (V-22) antibody (top panel). The cell lysates from COS7 cells transfected with pcDNA3 were used as the negative control. As loading controls, GST and GST fusion proteins were visible with Ponceau S staining (bottom panel). G, illustration of GST-TMED10 fusion proteins. The region from Gly81 to Glu110 in TMED10 was divided into two pieces to make GST fusion proteins. H, determination of the ALK5-binding region in TMED10. A GST pulldown assay was carried out as described in F above. The top and bottom panels show the interaction of ALK5 with GST fusion proteins and the loading controls with Ponceau S staining. I, requirement of a 20-amino acid-long region in TMED10 to associate with TβRII. A GST pulldown assay was carried out as described in F above. The upper and lower panels show the interaction of TβRII with GST fusion proteins and the loading controls with Ponceau S staining.
Blockage of TGF-β Signaling by Short Peptides Derived from TMED10
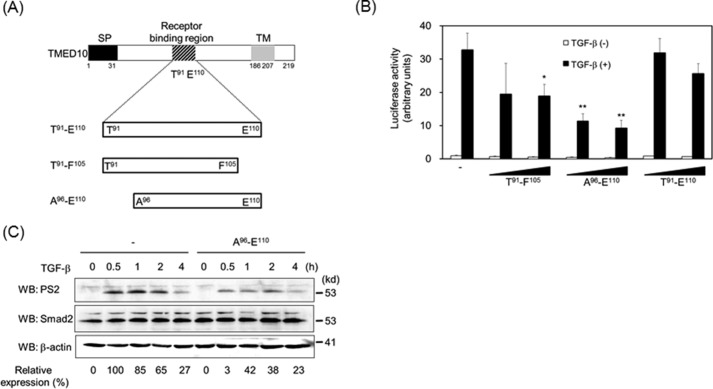
Because the 20-amino acid-long peptide derived from TMED10 is critical for TMED10 to interact with TGF-β receptors, it is possible that the peptides within these 20 amino acids disrupt TGF-β signaling. To test this possibility, three peptides were synthesized to investigate whether they could perturb TGF-β signaling (Fig. 6A). All three peptides inhibited TGF-β-mediated luciferase reporter activity to a certain extent. However, the addition of peptide in the media without Xfect protein transfection reagent did not significantly inhibit this response. Although we do not know the exact reasons, one possibility is that peptides incorporated into the Golgi apparatus and/or ER bind to immature biosynthetic intermediate TGF-β receptors (ALK5 and/or TβRII), thereby leading to decreased homodimer and/or heterodimer formations of TGF-β receptors at the cell surface. Among the three peptides, the peptide composed of 15 amino acids (Ala96–Glu110) was the most effective inhibitor (Fig. 6B). We do not know why Thr91–Glu110 has the weakest inhibitory activity among the three peptides. One possibility is that the active site (Ala96–Phe105) might be hidden because of the steric structure of Thr91–Glu110. Further experiments will be needed to confirm this possibility. When Ala96–Glu110 was added to the cells and then stimulated with TGF-β, Smad2 phosphorylation was inhibited (Fig. 6C).
FIGURE 6.
Fifteen-amino acid-long peptide as a unique inhibitor of TGF-β signaling. A, illustration of the synthesized peptides. SP, signal peptide; TM, transmembrane. B, inhibition of TGF-β-driven reporter activity by the synthesized peptides. The experiments were performed as described in Fig. 2A except for the addition of 200 nm or 1 μm peptide 2 h prior to TGF-β (5 ng/ml) stimulation. Significant differences from the control in the presence of TGF-β are indicated with asterisks. C, inhibition of TGF-β-induced Smad2 phosphorylation by peptides from Ala96 to Glu110. Two hours after the addition of 1 μm peptide, HepG2 cells were stimulated with 0.5 ng/ml TGF-β for the indicated times. Western blotting analyses (WB) were performed as described in Fig. 2F. The top, middle, and bottom panels show the expressions of phosphorylated Smad2, Smad2, and β-actin, respectively. The expression of phosphorylated Smad2 upon TGF-β stimulation was normalized using the intensity of the band corresponding to Smad2. Relative expression was calculated relative to the value without peptide in the absence of TGF-β. Probability values below 0.01 and 0.001 were considered significant: **, p < 0.01,***, p < 0.001.
Decreased and Enhanced Tumor Formation of JygMC(A) Cells with Gain and Loss of TMED10 Expression in Nude Mice
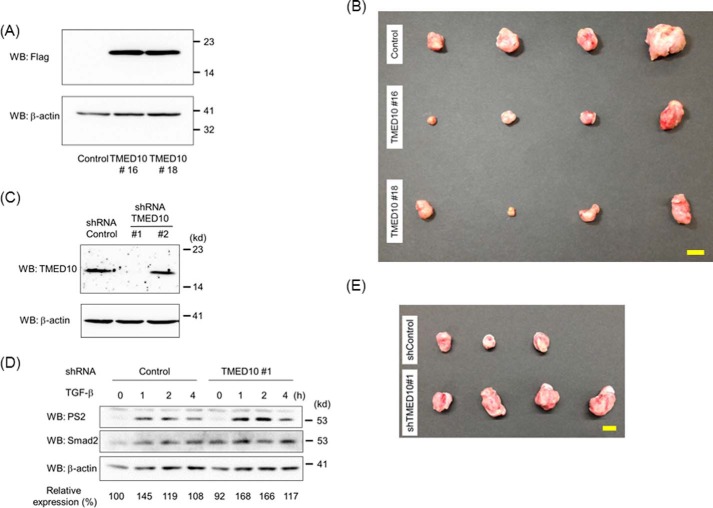
Mouse mammary carcinoma JygMC(A) cells are known to proliferate in the presence of TGF-β under low serum conditions (48). Additionally, the inhibition of TGF-β signaling in JygMC(A) cells by gene transfer of Smad7 was reported to suppress metastasis in vivo as well as in epithelial-mesenchymal transition and cell motility in vitro (49). These lines of evidence encouraged us to investigate whether increased and reduced expression of TMED10 in JygMC(A) cells would result in decreased and increased tumorigenicity in vivo, respectively. When we implanted two TMED10-expressing JygMC(A) cells (TMED10#16 and TMED10#18) (Fig. 7A) into the mammary fat pad of each nude mouse, the primary tumors from TMED10#16 and TMED10#18 were smaller than those from the control (Fig. 7B and Table 1). Next, we established two cell lines with descending levels of TMED10 expression (TMED10#1 and TMED10#2). Then, low expression of TMED10 could be observed in the TMED10#1 cells when compared with the control cells (Fig. 7C). Consistently, the TGF-β-induced duration of Smad2 phosphorylation was extended in the TMED10#1 cells (Fig. 7D). Subsequently, we injected JygMC(A) cells carrying shTMED10#1 or shControl cells according to the method described in the legend for Fig. 7B. The primary tumors in the mammary glands from mice injected with JygMC(A) cells carrying shTMED10#1 were bigger than those carrying control shRNA (Fig. 7E and Table 2). These lines of evidence demonstrated that TMED10 is implicated as a negative regulator of TGF-β-induced pro-oncogenic signaling in these breast cancer cells.
FIGURE 7.
Xenograft implantation of mammary tumors carrying TMED10/FLAG or TMED10 shRNA. A, expression of TMED10/FLAG in JygMC(A) cells with TMED10. After JygMC(A) cells carrying control, TMED10#16, or TMED#18 were established, the cell lysates were prepared. Western blotting analyses (WB) were then carried out using anti-FLAG (top panel) and anti-β-actin (bottom panel) antibodies. B, effect of TMED10 expression in JygMC(A) cells on primary tumors. Photos of tumors derived from JygMC(A) cells carrying control, TMED10#16, or TMED#18 are shown. Scale bar: 10 mm. C, expression of TMED10 in JygMC(A) cells with the introduced TMED10 shRNA. After JygMC(A) cells carrying control, shTMED10#1, or shTMED10#2 were established, the cell lysates were prepared. Western blotting analyses were then carried out using anti-TMED10 (top panel) and anti-β-actin (bottom panel) antibodies. D, extension of TGF-β-induced Smad2 phosphorylation in JygMC(A) cells in which the TMED10 expression was reduced. Cells were stimulated with 0.25 ng/ml TGF-β for the indicated times. Western blotting analyses were performed as described in Fig. 2F. The top, middle, and bottom panels show the expressions of phosphorylated Smad2, Smad2, and β-actin, respectively. The expression of phosphorylated Smad2 upon TGF-β stimulation was normalized using the intensity of the band corresponding to Smad2. Relative expression was calculated relative to the value for control cells in the absence of TGF-β. E, effect of shTMED10 expression in JygMC(A) cells on primary tumors. Photos of tumors derived from JygMC(A) cells carrying control shRNA or shTMED10#1 are shown. Scale bar: 10 mm.
TABLE 1.
Quantification of volumes and weights for primary tumors after inoculation of JygMC(A) cells carrying TMED10
The data represent means ± S.D. (n = 4). Primary tumors in mammary glands were enucleated 5 weeks after orthotopic transplantation. Probability values below 0.05 were considered significant: *, p < 0.05.
| Cell line | Average tumor volume | Average tumor weight |
|---|---|---|
| mm3 | g | |
| Control | 1631.1 ± 1191.4 | 2.0 ± 1.5 |
| pAd-TMED10#16 | 403.3 ± 286.9* | 0.7 ± 0.7 |
| pAd-TMED10#18 | 450.8 ± 290.9 | 0.5 ± 0.4 |
TABLE 2.
Quantification of volumes and weights for primary tumors after inoculation of JygMC(A) cells carrying TMED10 shRNA
The data represent means ± S.D. (n = 3 or 4). Primary tumors in mammary glands were enucleated 5 weeks after xenograft transplantation.
| shRNA | Average tumor volume | Average tumor weight |
|---|---|---|
| mm3 | g | |
| shControl | 533.0 ± 94 | 0.88 ± 0.25 |
| shTMED10#1 | 2967.5 ± 1198 | 3.02 ± 0.70 |
Discussion
During the more than two decades of study of Smads since they were first discovered, Smad signaling has been characterized as intricately regulated by a great number of molecules (4, 11, 50, 51). Because not only hyper-TGF-β signaling but also hypo-TGF-β signaling is known to be involved in various malformations and severe diseases in humans, it is important to understand overall TGF-β signaling from the extracellular microenvironment to the nucleus.
Until now, it has been acknowledged that negative regulators of TGF-β family signaling act mainly at the Smad level, including phosphatases, ubiquitin ligases, inhibitory Smads (I-Smads), TMEPAI, c-Ski, and SnoN (27, 28). Only a few molecules, such as BAMBI (BMP and activin membrane-bound inhibitor) (26) and ETV6-NTRK3 (52), have been implicated in the disruption of TGF-β receptor complexes. Therefore, we tried to identify novel interactors for ALK5 through comprehensive screening using mass spectrometry analysis. Among the positive candidates in the primary screening, TMED10 was further characterized in our current study as a potent negative regulator of TGF-β signaling by acting on TGF-β receptors. It is possible that stoichiometric conversion of ALK5 by TβRII kinase is involved in the interaction between ALK5 and TMED10. Although it was curious how one molecule of TMED10 could simultaneously associate with two receptors using the same 20-amino acid-long region of TMED10, we obtained evidence that TMED10 could oligomerize through its N-terminal region. Besides ALK5, TMED10 also interacted with ALK6, despite its relatively weaker interaction with ALK6 than with ALK5. BAMBI, which is closely related to BMP receptor type I without its C-terminal kinase domain, is known to bind not only to ALK5 but also to ALK6 (26). Like BAMBI, TMED10 tends to interact with other type I and type II receptors of the TGF-β family via its N-terminal extracellular region (data not shown), although its inhibitory action on TGF-β signaling seems to be stronger than that on BMP signaling. Thus, TMED10 interrupts TGF-β receptor complex formation without sequestering the TGF-β ligand. Because the protein structure of TMED10 resembles that of the EMP24/GP25L/p24 family, TMED10 may be localized in the Golgi apparatus or the ER (53, 54). However, Blum and Lepier (55) reported that two different domains in TMED10 determine the destination of TMED10 in cells: either the ER/Golgi apparatus or the plasma membrane. Our experiments using confocal microscopy also supported their conclusion that parts of TMED10 were localized in the cell membrane and the remaining parts were present in the cytosol, although we did not identify the organelles associated with TMED10.
Blum and Lepier (55) show that the extracellular domain and extreme C terminus including KKLIE in TMED10 are involved in the retention of TMED10 in the plasma membrane and the ER, respectively. Mutation analyses of TMED10 demonstrated that the N-terminal domain following the signal peptide and the middle domain from Thr91 to Glu110 was needed for TMED10 to make a homo-oligomer and a hetero-oligomer with TGF-β receptors, respectively. In addition, a secreted form of TMED10 lacking a transmembrane and an intracellular domain could interfere with TGF-β signaling because of the presence of the region between Thr91 to Glu110. Therefore, TMED10 can be classified into three parts at least: a homo-oligomeric domain from Ile32 to Ala80, a hetero-oligomeric domain from Thr91 to Glu110, and a C-terminal short region including KKLIE. After the receptor-binding domain was narrowed down, the 15-amino acid-long peptide was evaluated as an antagonist for TGF-β signaling. Therefore, TMED10 probably reveals its inhibitory action through its N-terminal extracellular domain, which has at least three functional domains: homodimer formation, heterodimer formation of TGF-β receptors, and retention of the plasma membrane. As TMED10 is not induced by TGF-β, we supposed that TMED10 could serve as a way to set up a threshold for activating TGF-β signaling in cells.
To examine the antagonistic effect of TMED10 on tumor growth, JygMC(A) cells, which are known to proliferate in the presence of TGF-β under low serum conditions (48), were implanted into murine mammary glands after TMED10 expression had been increased and reduced by gain-of-function or loss-of-function methods, respectively. Expectedly, the increase and the decrease in TMED10 expression suppressed and promoted tumor growth in vivo, respectively. Thus, high expression of TMED10 in JygMC(A) cells, in which TGF-β acts as a tumor-promoting factor, suppressed tumorigenicity. In contrast, the Human Protein Atlas Web database reveals that most tumors show high expression of TMED10. Consistently, TMED10 was expressed at a relatively high level in MCF10CA1a, characterized as a model of high grade breast carcinoma, whereas the expression of TMED10 was low in MCF10A1, characterized as a model of normal breast epithelium, and MCF10AT1, categorized as a premalignant epithelium (supplemental Fig. 1). However, TGF-β signaling is known to act context-dependently in the human body (11). These lines of evidence may support the notion that most tumors probably overcome TGF-β-mediated growth arrest through high expression of TMED10. It is possible that cancer cells themselves alter the expression of TMED10 depending on the way in which TGF-β signaling in cancer cells acts on their survival or death.
In conclusion, TMED10 is a novel antagonist of TGF-β signaling that preferentially disrupts ALK5 and TβRII. Thus, TMED10 can limit the duration of Smad phosphorylation followed by transcriptional regulation of direct target genes for TGF-β signaling. Because the short peptide derived from the N-terminal extracellular domain of TMED10 could interfere with TGF-β signaling, this peptide or its derivatives might become a potential anti-tumor therapeutic agent.
Experimental Procedures
Antibodies
Antibodies were obtained from the following sources: anti-FLAG M2 mouse monoclonal antibody (mAb) (F3165-1MG, Sigma); anti-DYKDDDDK tag mAb (catalog No. 040-30953, Wako), corresponding to anti-FLAG antibody; anti-V5 tag mouse mAb (catalog No. 011-23594, Wako); anti-HA12CA5 mouse mAb (catalog No. 11583816001, Roche Applied Science); anti-HA3F10 rat mAb (catalog No. 11867423001, Roche Applied Science); anti-β-actin mouse mAb (AC-15) (catalog No. sc-69879, Santa Cruz Biotechnology); anti-ALK5 (V-22) rabbit polyclonal antibody (pAb) (catalog No. sc-398, Santa Cruz Biotechnology), corresponding to anti-ALK5 antibody; anti-E-cadherin mouse mAb (catalog No. 610920, BD Biosciences); anti-HA rabbit pAb Y-11 (catalog No. sc-805, Santa Cruz); anti-N-cadherin mouse mAb (catalog No. 610921, BD Biosciences); anti-phosphoserine mouse mAb (clone 4A4) (catalog No. 05-1000, Merck); anti-mouse IgG HRP-linked sheep pAb (catalog No. NA931-1ML, GE Healthcare); and anti-rabbit IgG HRP-linked donkey F(ab)2 fragment (catalog No. NA9340–1ML, GE Healthcare).
To generate anti-TMED10 rabbit pAb, synthetic peptides (Asn97–Met114) were used to immunize rabbits (Sigma). After the sera were obtained, they were purified using a protein A-IgG purification kit (catalog No. 44557, Thermo Fisher Scientific). Anti-phosphorylated Smad2 (PS2), anti-Smad2, and anti-ALK5 (VPN) rabbit pAb were homemade (42, 56, 57).
Cell Culture
NMuMG, HaCaT, 911, HEK293T, 293A, A549, COS1, and COS7 cells were cultured in Dulbecco's modified Eagle's medium (Nacalai Tesque or Sigma) containing 10% fetal calf serum (FCS) (Invitrogen). JygMC(A) cells provided by RIKEN BioResource Center were also maintained in DMEM/10% FCS. HepG2 cells were maintained in minimum essential medium (Wako) containing 10% FCS, nonessential amino acids (Nacalai Tesque), and sodium pyruvate (Nacalai Tesque). The expression of TMED10 in the cell lines used is shown in supplemental Fig. 1.
Expression Plasmids
Human TMED10 cDNA was cloned by RT-PCR. All of the TMED10 mutants were made using PrimeStar HS DNA polymerase (Takara). TMED10 and its mutants were inserted into pcDNA3.1-V5-His-A (Invitrogen), pcDNA3-HA, or pcDNA3-FLAG (58). Subsequently, cDNA fused with each tag sequence were excised to ligate them to a pcDEF3 vector (59). All TMED10 constructs possessed the FLAG-, HA-, or V5 epitope tag at their C terminus. Additionally, TMED10 or its mutants without the tags were constructed using pcDEF3. Adenoviruses expressing TMED10/FLAG were generated using the pAd/CMV/V5-DEST vector (Thermo Fisher Scientific). The resulting plasmids were transfected into 293T cells, and the adenoviruses were amplified. A lentiviral expression vector for TMED10/FLAG was made with the pLV-CMV-IRES-Puro vector (60) and then co-transfected with VSV, GAG, and REV expression vectors in 293T cells to obtain viral particles. The other constructs have been described previously (17, 44, 61–63).
Lentiviral shRNAs
Short double-stranded DNA were inserted into the pLKO.1-TRC vector (64) purchased from Addgene. Lentiviral vectors expressing shTMED10 were transfected into 293A cells together with psPAX2 and pMD2.G. After 48 h of transfection, the media were collected as a source of lentiviruses. The lentiviruses were simultaneously incubated in DMEM containing 8 μg/ml Polybrene (Sigma) for 2 h and then added to the cell culture dishes. Twelve hours after infection, the cells were washed and cultured in medium. Infected HaCaT and JygMC(A) cells, which became puromycin-resistant, were used for the experiments. The short double-stranded DNA used here were as follows: 5′-CCGGCGCTTCTTCAAGGCCAAGAAACTCGAGTTTCTTGGCCTTGAAGAAGCGTTTTTG-3′/3′-GCGAAGAAGTTCCGGTTCTTTGAGCTCAAAGAACCGGAACTTCTTCGCAAAAACTTAA-5′ for human shTMED10; 5′-CCGGAACTCTCGCAAGTGTCTCCGACTCGAGTCGGAGACACTTGCGAGAGTTTTTTTG-3′/TTGAGAGCGTTCACAGAGGCTGAGCTCAGCCTCTGTGAACGCTCTCAAAAAAACTTAA-5′ for mouse shTMED10#1; and 5′-CCGGAAGATCACAGATTCTGCTGGCCTCGAGGCCAGCAGAATCTGTGATCTTTTTTTG-3′/3′-TTCTAGTGTCTAAGACGACCGGAGCTCCGGTCGTCTTAGACACTAGAAAAAAACTTAA-5′ for mouse shTMED10#2.
Protein Identification by LC/MS Analysis
FLAG-tagged human ALK5ca was expressed in HEK 293T cells, and the associated proteins were recovered from the cell extracts by immunoprecipitation with anti-FLAG M2 antibody. The ALK5ca-associated protein complexes were digested with lysyl endopeptidase (Lys-C, Wako), and the resulting peptides were analyzed using a direct nanoflow LC-MS/MS system as described previously (65).
Transcriptional Reporter Assays
One day before transfection, HepG2 cells were seeded at 1.2 × 105 cells/well in 12-well plates. The cells were transfected with a reporter gene, pCH110 (GE Healthcare), and the indicated plasmids by use of polyethylenimine (Polysciences). Where indicated, 5 ng/ml TGF-β or 25 ng/ml BMP-6 was added to the wells 24 h after transfection. Subsequently, the cells were cultured in the absence of FCS for 18 h. In all experiments, β-galactosidase activity was measured to normalize for transfection efficiency. Each transfection was carried out in triplicate and repeated at least twice.
Immunoprecipitation and Western Blotting Analysis
To detect interactions among the proteins, plasmids were transfected into COS7 cells (5 × 105 cells/6-cm dish) by use of polyethylenimine. Forty hours after the transfection, the cells were lysed in 500 μl of TNE buffer (10 mm Tris (pH 7.4), 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40, 1 mm PMSF, 5 μg/ml leupeptin, 100 units/ml aprotinin, 2 mm sodium vanadate, 40 mm NaF, and 20 mm β-glycerophosphate). The cell lysates were precleared with protein G-Sepharose beads (GE Healthcare) for 30 min at 4 °C and then incubated with the indicated antibodies for 2 h at 4 °C. The protein complexes were immunoprecipitated by incubation with protein G-Sepharose beads for 30 min at 4 °C and then washed three times with TNE buffer. The immunoprecipitated proteins and aliquots of the total cell lysates were boiled for 5 min in sample buffer, separated by SDS-PAGE, and transferred to either Hybond-C Extra membrane (GE Healthcare) or UltraCruz nitrocellulose pure transfer membrane (Santa Cruz Biotechnology). The membranes were probed with the primary antibodies, which were detected with horseradish peroxidase-conjugated secondary antibodies and chemiluminescent substrate (Western BLoT Quant HRP substrate, Takara). The protein expression in the total cell lysates was evaluated by Western blotting analysis.
Iodination of Ligands and Affinity Cross-linking
TGF-β was iodinated using the chloramine T method according to Frolik et al. (66). For the ectopic expression system, COS1 cells were transfected with the indicated plasmids 40 h prior to incubation with 125I-TGF-β. Cross-linking was performed as described previously (67). Complexes of TMED10 and affinity-labeled receptors were immunoprecipitated with an anti-V5 or anti-TMED10 antibody. To investigate the expression levels of the receptors, aliquots of the cell lysates were analyzed directly by SDS-polyacrylamide gel electrophoresis.
GST Pulldown Assay
TMED10 mutants were subcloned in pGEX4T-1 (GE Healthcare). GST fusion proteins from Escherichia coli were purified according to the manufacturer's instructions (GE Healthcare). Cell lysates were prepared from COS7 cells transfected with ALK5, TβRII/FLAG, or an empty vector precleared with GST immobilized to GSH-Sepharose 4B (GE Healthcare) for 30 min at 4 °C. Subsequently, the above cell lysates were incubated with GST-TMED10 mutants immobilized to GSH-Sepharose 4B for 2 h at 4 °C and washed three times with 50 mm Tris (pH 7.4) containing 100 mm NaCl, 2 mm MgCl2, 10% glycerol, 1% Nonidet P-40, 1 mm PMSF, 5 μg/ml leupeptin, 20 units/ml aprotinin, and 5 mm benzamidine. After the samples had been loaded on SDS-PAGE, the proteins were blotted on the nitrocellulose membrane and detected with either an anti-ALK5 (V-22) or an anti-FLAG antibody using a chemiluminescent substrate (Takara). To show the quantity of GST fusion proteins in each sample, Ponceau S staining was performed after blotting.
Immunofluorescence Staining
Immunofluorescence staining for detection of colocalization was performed as described previously (68). Briefly, 911 cells grown on cover glasses were transfected with the indicated plasmids. After 24 h of transfection, the cells were stimulated with TGF-β for 2 h. Then, the cover glasses were washed once with PBS, fixed for 10 min with 4% paraformaldehyde, washed three times with PBS, permeabilized with 0.5% Triton X-100 in PBS for 10 min, and again washed three times with PBS. The cover glasses were blocked with 5% bovine serum albumin (BSA) in PBS at 37 °C for 1 h and incubated with 5% BSA in PBS containing the indicated antibodies at 4 °C overnight. The cover glasses were then washed three times with PBS, incubated with 5% BSA in PBS containing both Alexa Fluor 555-conjugated goat anti-mouse IgG antibody (diluted 1:250; A-21422, Thermo Fisher Scientific) and CF488A-conjugated goat anti-rabbit IgG antibody (diluted 1:250; catalog No. 20012, Biotium, Inc.) at room temperature for 1 h, and washed three times with PBS. The nuclei were stained with 4′,6-diamidino-2-phenylidole (DAPI). To visualize the fluorescence, a confocal microscope (A1Rsi, Nikon) was used. For E-cadherin expression, we performed the same experiments except using the anti-E-cadherin antibody as the primary antibody and the Alexa Fluor 555-conjugated goat anti-mouse IgG antibody (A21422, Thermo Fisher Scientific) as the secondary antibody instead of the above combination of primary and second antibodies. To detect stress fiber formation, we carried out the same experiment except for using Alexa Fluor 555-phalloidin (A34055, Thermo Fisher Scientific).
RNA Preparation and Quantitative Real-time PCR (qPCR) Analysis
Total RNA from HepG2 cells was extracted using a ReliaPrep RNA cell miniprep system (Promega). Reverse transcription was performed with a high-capacity RNA-to-cDNA kit (Thermo Fisher Scientific). qPCR was performed with FastStart SYBR Green Master Mix (Roche Applied Science). All reactions were carried out on a StepOnePlus system (Applied Biosystems Inc.). Each sample was analyzed in triplicate at least twice for each PCR measurement. Melting curves were checked to ensure specificity. The relative quantification of mRNA expression was calculated using the standard curve method with the β-actin level. Before qPCR, the DNA fragment amplified using each primer set was detected to be a single band with the correct size by agarose gel electrophoresis. The following primer sets were used to amplify TMED10, TMEPAI, and β-actin cDNA: 5′-GAGATGCGTGATACCAACGA-3′ and 5′-TTCTTGGCCTTGAAGAAGCG-3′ for human TMED10; 5′-TGTCCTCAGAAGGATGCCTG-3′ and 5′-CACTGTCGAAGATGGTTCTG-3′ for human TMEPAI; and 5′-caagagatggccacggctgct-3′ and 5′-tccttctgcatcctgtcggca-3′ for human β-actin.
Migration Assay
Cell migration assays were performed using a Boyden chamber. Costar nucleopore filters (8-μm pore diameter) were coated with 10 μg/ml fibronectin (Sigma) overnight at 4 °C. The chambers were washed three times with PBS. Cells cultured for 12 h without FCS were added to the top of each migration chamber at a density of 1.5 × 105 cells/chamber in 150 μl of DMEM with 0.5% FCS. The cells were allowed to migrate to the underside of the chamber in the presence or absence of 1 ng/ml TGF-β in the lower chamber. After 24 h, the cells were fixed in 4% paraformaldehyde and stained with hematoxylin (Leica) and eosin (Muto Chemicals). The upper surface was wiped with cotton swabs to remove nonmigrating cells. The cells present on the lower surface were counted. Each experiment was carried out in triplicate and repeated more than twice.
Scratch Assay
Cells were seeded in 12-well plates, grown until confluent, and wounded with a 200-μl tip. After wounding, 0.5 ng/ml TGF-β was added. Photographs were then taken under a microscope (Nikon) for 24 h. The wound distance from each well was measured in duplicate at three randomly defined wound gap locations per frame recorded per experiment, and at least three independent scratch assays were used for the calculation. Each experiment was carried out in triplicate and repeated several times.
Introduction of Peptides
Three peptides (Thr91–Glu110, TKGKFAFTTEDYDMFEVCFE; Thr91–Phe105, TKGKFAFTTEDYDMF; and Ala96–Glu110, AFTTEDYDMFEVCFE) were synthesized (Bio-Synthesis or CS Bio Co.). We mixed 200 nm or 1 μm peptides and Xfect protein transfection reagent (Clontech) for 30 min. After the cells had been incubated with the peptides for 1 h, the cell media were refreshed. The cells were then cultured for 2 h followed by stimulation of the cells with TGF-β for the indicated times.
Xenograft Model
Six-week-old female BALB/c nu/nu mice were purchased from Japan SLC Inc. JygMC(A) cells (1 × 107 cells) carrying TMED10/FLAG or TMED10 shRNA were implanted into the mammary fat pad of each mouse. Five weeks later, the primary tumors and lungs were removed after the mice had been sacrificed. Tumor volumes were calculated using the following formula: 0.4 × (longest axis) × (shortest perpendicular axis)2 (48). The mice were housed in the animal facilities of Showa Pharmaceutical University under specific pathogen-free conditions at a constant temperature and humidity and fed a standard diet. They were treated in accordance with the institutional guidelines of the Animal Care and Use Program of Showa Pharmaceutical University.
Statistical Analysis
Data were expressed as means ± SD unless otherwise mentioned. Significance was assessed using the t test. Probability values below 0.05, 0.01, and 0.001 were considered significant: *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
Author Contributions
N. N., Y. T., K. K., K. U., S. Ikeno, E. O., M. S., A. N., N. S., F. I., and M. v. D. performed the in vitro experiments as well as the cell-based experiments. K. S., E. O., and Y. N. carried out the in vivo xenograft tumor model. S. Iemura identified ALK5-interacting proteins by TOF-MASS. T. N., P. t. D., and S. Itoh designed most aspects of the research, interpreted the data, and drafted the manuscript.
Supplementary Material
Acknowledgment
We thank F. Miyamasu for excellent English proofreading.
This research was supported by Grant-in-aid for Young Scientists (B) 15K18866 (to N. N.); the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to S. Itoh); the Takeda Science Foundation (to S. Itoh); the Smoking Research Foundation (to S. Itoh); the Daiichi-Sankyo Foundation of Life Science (to S. Itoh); the Naito Foundation (to S. Itoh); the Vehicle Racing Commemorative Foundation (to S. Itoh); Grant 2013-2017 from the MEXT-supported Program for the Strategic Research Foundation at Private Universities (to N. N. and S. Itoh); and a Grant-in-aid for Young Scientists from Showa Pharmaceutical University (to N. N.). This research also was supported by the Joint Usage/Research Program of the Medical Research Institute, Tokyo Medical and Dental University, and the Japan Society for the Promotion of Science Core-to-Core Program, “Cooperative International Framework in TGF-β Family Signaling”. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Fig. 1.
- BMP
- bone morphogenetic protein
- TβRI
- TGF-β type I receptor
- TβRII
- TGF-β type II receptor
- ALK
- activin receptor-like kinase
- ALK5ca
- constitutively active ALK5
- R-Smad
- receptor-regulated Smad protein(s)
- kd
- kinase-dead
- ER
- endoplasmic reticulum
- SBE
- Smad-binding element
- BAMBI
- BMP and activin membrane-bound inhibitor
- pAb
- polyclonal antibody
- qPCR
- quantitative real-time PCR
- co-IP
- coimmunoprecipitation.
References
- 1. Derynck R., and Zhang Y. E. (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 2. Shi Y., and Massagué J. (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113, 685–700 [DOI] [PubMed] [Google Scholar]
- 3. Feng X. H., and Derynck R. (2005) Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 21, 659–693 [DOI] [PubMed] [Google Scholar]
- 4. Weiss A., and Attisano L. (2013) The TGFβ superfamily signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2, 47–63 [DOI] [PubMed] [Google Scholar]
- 5. Morikawa M., Derynck R., and Miyazono K. (2016) TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology. Cold Spring Harb. Perspect. Biol. 10.1101/cshperspect.a021873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Derynck R., and Miyazono K. (2008) TGF-β and the TGF-β family, in The TGF-β Family (Derynck R., and Miyazono K., eds) pp. 29–43, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 7. Macias M. J., Martin-Malpartida P., and Massagué J. (2015) Structural determinants of Smad function in TGF-β signaling. Trends Biochem. Sci. 40, 296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Massagué J. (1998) TGF-β signal transduction. Annu. Rev. Biochem. 67, 753–791 [DOI] [PubMed] [Google Scholar]
- 9. Derynck R., and Akhurst R. J. (2007) Differentiation plasticity regulated by TGF-β family proteins in development and disease. Nat. Cell Biol. 9, 1000–1004 [DOI] [PubMed] [Google Scholar]
- 10. Ikushima H., and Miyazono K. (2010) Cellular context-dependent “colors” of transforming growth factor-β signaling. Cancer Sci. 101, 306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massagué J. (2012) TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 13, 616–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moustakas A., and Heldin C. H. (2005) Non-Smad TGF-β signals. J. Cell Sci. 118, 3573–3584 [DOI] [PubMed] [Google Scholar]
- 13. Hoover L. L., and Kubalak S. W. (2008) Holding their own: the noncanonical roles of Smad proteins. Sci. Signal. 1, pe48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kang J. S., Liu C., and Derynck R. (2009) New regulatory mechanisms of TGF-β receptor function. Trends Cell Biol. 19, 385–394 [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y. E. (2009) Non-Smad pathways in TGF-β signaling. Cell Res. 19, 128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mu Y., Gudey S. K., and Landström M. (2012) Non-Smad signaling pathways. Cell Tissue Res. 347, 11–20 [DOI] [PubMed] [Google Scholar]
- 17. Itoh S., Thorikay M., Kowanetz M., Moustakas A., Itoh F., Heldin C. H., and ten Dijke P. (2003) Elucidation of Smad requirement in transforming growth factor-β type I receptor-induced responses. J. Biol. Chem. 278, 3751–3761 [DOI] [PubMed] [Google Scholar]
- 18. ten Dijke P., and Arthur H. M. (2007) Extracellular control of TGFβ signalling in vascular development and disease. Nat. Rev. Mol. Cell Biol. 8, 857–869 [DOI] [PubMed] [Google Scholar]
- 19. Gordon K. J., and Blobe G. C. (2008) Role of transforming growth factor-β superfamily signaling pathways in human disease. Biochim. Biophys. Acta 1782, 197–228 [DOI] [PubMed] [Google Scholar]
- 20. Orlova V. V., Liu Z., Goumans M. J., and ten Dijke P. (2011) Controlling angiogenesis by two unique TGF-β type I receptor signaling pathways. Histol. Histopathol. 26, 1219–1230 [DOI] [PubMed] [Google Scholar]
- 21. Katsuno Y., Lamouille S., and Derynck R. (2013) TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr. Opin. Oncol. 25, 76–84 [DOI] [PubMed] [Google Scholar]
- 22. Meng X. M., Nikolic-Paterson D. J., and Lan H. Y. (2016) TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338 [DOI] [PubMed] [Google Scholar]
- 23. Robertson I. B., and Rifkin D. B. (2013) Unchaining the beast; insights from structural and evolutionary studies on TGFβ secretion, sequestration, and activation. Cytokine Growth Factor Rev. 24, 355–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piek E., Heldin C. H., and Ten Dijke P. (1999) Specificity, diversity, and regulation in TGF-β superfamily signaling. FASEB J. 13, 2105–2124 [PubMed] [Google Scholar]
- 25. Massagué J. (2000) How cells read TGF-β signals. Nat. Rev. Mol. Cell Biol. 1, 169–178 [DOI] [PubMed] [Google Scholar]
- 26. Onichtchouk D., Chen Y. G., Dosch R., Gawantka V., Delius H., Massagué J., and Niehrs C. (1999) Silencing of TGF-β signalling by the pseudoreceptor BAMBI. Nature 401, 480–485 [DOI] [PubMed] [Google Scholar]
- 27. Itoh S., and ten Dijke P. (2007) Negative regulation of TGF-β receptor/Smad signal transduction. Curr. Opin. Cell Biol. 19, 176–184 [DOI] [PubMed] [Google Scholar]
- 28. Itoh S., and Itoh F. (2011) Inhibitory machinery for the TGF-β family signaling pathway. Growth Factors 29, 163–173 [DOI] [PubMed] [Google Scholar]
- 29. Strating J. R., and Martens G. J. (2009) The p24 family and selective transport processes at the ER-Golgi interface. Biol. Cell 101, 495–509 [DOI] [PubMed] [Google Scholar]
- 30. Blum R., Feick P., Puype M., Vandekerckhove J., Klengel R., Nastainczyk W., and Schulz I. (1996) Tmp21 and p24A, two type I proteins enriched in pancreatic microsomal membranes, are members of a protein family involved in vesicular trafficking. J. Biol. Chem. 271, 17183–171839 [DOI] [PubMed] [Google Scholar]
- 31. Zhao L., Helms J. B., Brunner J., and Wieland F. T. (1999) GTP-dependent binding of ADP-ribosylation factor to coatomer in close proximity to the binding site for dilysine retrieval motifs and p23. J. Biol. Chem. 274, 14198–14203 [DOI] [PubMed] [Google Scholar]
- 32. Majoul I., Straub M., Hell S. W., Duden R., and Söling H. D. (2001) KDEL-cargo regulates interactions between proteins involved in COPI vesicle traffic: measurements in living cells using FRET. Dev. Cell 1, 139–153 [DOI] [PubMed] [Google Scholar]
- 33. Fucini R. V., Chen J. L., Sharma C., Kessels M. M., and Stamnes M. (2002) Golgi vesicle proteins are linked to the assembly of an actin complex defined by mAbp1. Mol. Biol. Cell 13, 621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen F., Hasegawa H., Schmitt-Ulms G., Kawarai T., Bohm C., Katayama T., Gu Y., Sanjo N., Glista M., Rogaeva E., Wakutani Y., Pardossi-Piquard R., Ruan X., Tandon A., Checler F., et al. (2006) TMP21 is a presenilin complex component that modulates γ-secretase but not ϵ-secretase activity. Nature 440, 1208–1212 [DOI] [PubMed] [Google Scholar]
- 35. Pardossi-Piquard R., Böhm C., Chen F., Kanemoto S., Checler F., Schmitt-Ulms G., St George-Hyslop P., and Fraser P. E. (2009) TMP21 transmembrane domain regulates γ-secretase cleavage. J. Biol. Chem. 284, 28634–28641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bromley-Brits K., and Song W. (2012) The role of TMP21 in trafficking and amyloid-β precursor protein (APP) processing in Alzheimer's disease. Curr. Alzheimer Res. 9, 411–424 [DOI] [PubMed] [Google Scholar]
- 37. Wang H., and Kazanietz M. G. (2010) p23/Tmp21 differentially targets the Rac-GAP β2-chimaerin and protein kinase C via their C1 domains. Mol. Biol. Cell 21, 1398–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang H., Xiao L., and Kazanietz M. G. (2011) p23/Tmp21 associates with protein kinase Cδ (PKCδ) and modulates its apoptotic function. J. Biol. Chem. 286, 15821–15831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu X., Gao H., Qin J., He L., and Liu W. (2015) TMP21 modulates cell growth in papillary thyroid cancer cells by inducing autophagy through activation of the AMPK/mTOR pathway. Int. J. Clin. Exp. Pathol. 8, 10824–10831 [PMC free article] [PubMed] [Google Scholar]
- 40. Jun Y., and Ahn K. (2011) Tmp21, a novel MHC-I interacting protein, preferentially binds to β2-microglobulin-free MHC-I heavy chains. BMB Rep. 44, 369–374 [DOI] [PubMed] [Google Scholar]
- 41. Attisano L., Cárcamo J., Ventura F., Weis F. M., Massagué J., and Wrana J. L. (1993) Identification of human activin and TGF β type I receptors that form heteromeric kinase complexes with type II receptors. Cell 75, 671–680 [DOI] [PubMed] [Google Scholar]
- 42. Franzén P., ten Dijke P., Ichijo H., Yamashita H., Schulz P., Heldin C. H., and Miyazono K. (1993) Cloning of a TGF β type I receptor that forms a heteromeric complex with the TGF β type II receptor. Cell 75, 681–692 [DOI] [PubMed] [Google Scholar]
- 43. Yamashita H., ten Dijke P., Franzén P., Miyazono K., and Heldin C. H. (1994) Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-β. J. Biol. Chem. 269, 20172–20178 [PubMed] [Google Scholar]
- 44. Jonk L. J., Itoh S., Heldin C. H., ten Dijke P., and Kruijer W. (1998) Identification and functional characterization of a Smad binding element (SBE) in the JunB promoter that acts as a transforming growth factor-β, activin, and bone morphogenetic protein-inducible enhancer. J. Biol. Chem. 273, 21145–21152 [DOI] [PubMed] [Google Scholar]
- 45. Miettinen P. J., Ebner R., Lopez A. R., and Derynck R. (1994) TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J. Cell Biol. 127, 2021–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bhowmick N. A., Ghiassi M., Bakin A., Aakre M., Lundquist C. A., Engel M. E., Arteaga C. L., and Moses H. L. (2001) Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell 12, 27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wrana J. L., Attisano L., Wieser R., Ventura F., and Massagué J. (1994) Mechanism of activation of the TGF-β receptor. Nature 370, 341–347 [DOI] [PubMed] [Google Scholar]
- 48. Ehata S., Hanyu A., Hayashi M., Aburatani H., Kato Y., Fujime M., Saitoh M., Miyazawa K., Imamura T., and Miyazono K. (2007) Transforming growth factor-β promotes survival of mammary carcinoma cells through induction of antiapoptotic transcription factor DEC1. Cancer Res. 67, 9694–9703 [DOI] [PubMed] [Google Scholar]
- 49. Azuma H., Ehata S., Miyazaki H., Watabe T., Maruyama O., Imamura T., Sakamoto T., Kiyama S., Kiyama Y., Ubai T., Inamoto T., Takahara S., Itoh Y., Otsuki Y., Katsuoka Y., et al. (2005) Effect of Smad7 expression on metastasis of mouse mammary carcinoma JygMC(A) cells. J. Natl. Cancer Inst. 97, 1734–1746 [DOI] [PubMed] [Google Scholar]
- 50. Gaarenstroom T., and Hill C. S. (2014) TGF-β signaling to chromatin: how Smads regulate transcription during self-renewal and differentiation. Semin. Cell Dev. Biol. 32, 107–118 [DOI] [PubMed] [Google Scholar]
- 51. Zhao B., and Chen Y. G. (2014) Regulation of TGF-β signal transduction. Scientifica (Cairo) 2014, 874065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jin W., Kim B. C., Tognon C., Lee H. J., Patel S., Lannon C. L., Maris J. M., Triche T. J., Sorensen P. H., and Kim S. J. (2005) The ETV6-NTRK3 chimeric tyrosine kinase suppresses TGF-β signaling by inactivating the TGF-β type II receptor. Proc. Natl. Acad. Sci. U.S.A. 102, 16239–16244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Blum R., Pfeiffer F., Feick P., Nastainczyk W., Kohler B., Schäfer K. H., and Schulz I. (1999) Intracellular localization and in vivo trafficking of p24A and p23. J. Cell Sci. 112, 537–548 [DOI] [PubMed] [Google Scholar]
- 54. Nickel W., Sohn K., Bünning C., and Wieland F. T. (1997) p23, a major COPI-vesicle membrane protein, constitutively cycles through the early secretory pathway. Proc. Natl. Acad. Sci. U.S.A. 94, 11393–11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Blum R., and Lepier A. (2008) The luminal domain of p23 (Tmp21) plays a critical role in p23 cell surface trafficking. Traffic 9, 1530–1550 [DOI] [PubMed] [Google Scholar]
- 56. Nakao A., Imamura T., Souchelnytskyi S., Kawabata M., Ishisaki A., Oeda E., Tamaki K., Hanai J., Heldin C. H., Miyazono K., and ten Dijke P. (1997) TGF-β receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 16, 5353–5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Persson U., Izumi H., Souchelnytskyi S., Itoh S., Grimsby S., Engström U., Heldin C. H., Funa K., and ten Dijke P. (1998) The L45 loop in type I receptors for TGF-β family members is a critical determinant in specifying Smad isoform activation. FEBS Lett. 434, 83–87 [DOI] [PubMed] [Google Scholar]
- 58. Kawabata M., Inoue H., Hanyu A., Imamura T., and Miyazono K. (1998) Smad proteins exist as monomers in vivo and undergo homo- and hetero-oligomerization upon activation by serine/threonine kinase receptors. EMBO J. 17, 4056–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Goldman L. A., Cutrone E. C., Kotenko S. V., Krause C. D., and Langer J. A. (1996) Modifications of vectors pEF-BOS, pcDNA1 and pcDNA3 result in improved convenience and expression. BioTechniques 21, 1013–1015 [DOI] [PubMed] [Google Scholar]
- 60. Zhou F., Zhang L., van Laar T., van Dam H., and Ten Dijke P. (2011) GSK3β inactivation induces apoptosis of leukemia cells by repressing the function of c-Myb. Mol. Biol. Cell 22, 3533–3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dennler S., Itoh S., Vivien D., ten Dijke P., Huet S., and Gauthier J. M. (1998) Direct binding of Smad3 and Smad4 to critical TGF β-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 17, 3091–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Itoh F., Asao H., Sugamura K., Heldin C. H., ten Dijke P., and Itoh S. (2001) Promoting bone morphogenetic protein signaling through negative regulation of inhibitory Smads. EMBO J. 20, 4132–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Watanabe Y., Itoh S., Goto T., Ohnishi E., Inamitsu M., Itoh F., Satoh K., Wiercinska E., Yang W., Shi L., Tanaka A., Nakano N., Mommaas A. M., Shibuya H., Ten Dijke P., and Kato M. (2010) TMEPAI, a transmembrane TGF-β-inducible protein, sequesters Smad proteins from active participation in TGF-β signaling. Mol. Cell 37, 123–134 [DOI] [PubMed] [Google Scholar]
- 64. Moffat J., Grueneberg D. A., Yang X., Kim S. Y., Kloepfer A. M., Hinkle G., Piqani B., Eisenhaure T. M., Luo B., Grenier J. K., Carpenter A. E., Foo S. Y., Stewart S. A., Stockwell B. R., Hacohen N., et al. (2006) A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 [DOI] [PubMed] [Google Scholar]
- 65. Natsume T., Yamauchi Y., Nakayama H., Shinkawa T., Yanagida M., Takahashi N., and Isobe T. (2002) A direct nanoflow liquid chromatography-tandem mass spectrometry system for interaction proteomics. Anal. Chem. 74, 4725–4733 [DOI] [PubMed] [Google Scholar]
- 66. Frolik C. A., Wakefield L. M., Smith D. M., and Sporn M. B. (1984) Characterization of a membrane receptor for transforming growth factor-β in normal rat kidney fibroblasts. J. Biol. Chem. 259, 10995–11000 [PubMed] [Google Scholar]
- 67. Souchelnytskyi S., Nakayama T., Nakao A., Morén A., Heldin C. H., Christian J. L., and ten Dijke P. (1998) Physical and functional interaction of murine and Xenopus Smad7 with bone morphogenetic protein receptors and transforming growth factor-β receptors. J. Biol. Chem. 273, 25364–25370 [DOI] [PubMed] [Google Scholar]
- 68. Nakano N., Maeyama K., Sakata N., Itoh F., Akatsu R., Nakata M., Katsu Y., Ikeno S., Togawa Y., Vo Nguyen T. T., Watanabe Y., Kato M., and Itoh S. (2014) C18 ORF1, a novel negative regulator of transforming growth factor-β signaling. J. Biol. Chem. 289, 12680–12692 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.