Highlights
-
•
We propose a harmonized set of age bins for assessing risks from chemical exposure.
-
•
The set of early life age groups will facilitate consistency with recent guidance.
-
•
The age bins allow results from longitudinal birth cohort studies to be combined.
-
•
Region-specific exposure factors and monitoring data are needed to apply the bins.
Keywords: Exposure assessment, Risk assessment, Harmonized early life age groups, Childhood life stages, Windows of exposure, Windows of susceptibility, Cultural and geographic modifying factors, Developmental changes in children, Exposure to environmental contaminants, Exposure factors
Abstract
In this paper, we summarize exposure-related issues to consider in determining the most appropriate age ranges and life stages for risk assessment. We then propose a harmonized set of age bins for monitoring and assessing risks from exposures to chemicals for global use. The focus is on preconception through adolescence, though the approach should be applicable to additional life stages. A two-tiered set of early life age groups is recommended. The first tier involves the adoption of guidance similar to the childhood age groups recommended by the U.S. Environmental Protection Agency, whereas the second tier consolidates some of those age groups to reduce the burden of developing age-specific exposure factors for different regions. While there is no single “correct” means of choosing a common set of age groups to use internationally in assessing early life exposure and risk, use of a set of defined age groups is recommended to facilitate comparisons of potential exposures and risks around the globe, the collection of data and analyses of aggregate exposure and cumulative risk. Application of these age groups for robust assessment of exposure and risk for specific populations will require region-specific exposure factors as well as local environmental monitoring data.
1. Introduction
A significant challenge associated with monitoring and assessing individual- and population-level exposure to and risk from exposure to environmental chemicals is associated with the need to rigorously consider changes in behavior and physiology that are related to age and life stage. Age- and life stage-related differences will determine windows of highest exposure as well as the appropriate distribution of exposure factors required to address specific exposure scenarios. Age and life stage differences in how people interact with the environment may be a major determinant for identifying the individual or population most vulnerable to risks from particular exposures to environmental contaminants. Identifying the most vulnerable age range or life stage for a particular population and exposure scenario requires a better scientific basis. Currently available approaches are limited in scope and potentially in applicability to the full range of geographic, social, cultural and economic diversity in populations worldwide. In addition, there is a need to better link or coordinate hazard and exposure assessment (the need to identify the most vulnerable based on windows of greatest susceptibility as well as windows of highest exposure, and then to incorporate that knowledge in a population-based risk assessment). Therefore, the World Health Organization (WHO) convened a group of experts to review these issues and provide guidance on how to better identify critical life stages for use in exposure and risk assessment.
The objective of this exercise was to propose a fit-for-purpose set of life stages independent of exposure context and exposure scenario. In this context, the group considered the following steps towards development and application of common life stages for exposure assessment:
-
•
Define age bins by carefully identifying the particular characteristics that distinguish them.
-
•
Decide how finely the overall life stage of childhood should be divided into age bins.
-
•
Describe how additional factors, such as sex, culture and geography, might modify the significance of standard age bins.
-
•
Recognize that there may be cases in which a specific factor (e.g. mouthing behavior) is a more significant indicator of exposure than age.
-
•
Identify the most pressing gaps in the base of scientific knowledge that would justify standard age bins and in the exposure factor data required to use the age bins for risk assessment.
In this paper, we summarize important exposure-related issues to consider in determining the most appropriate age ranges and life stages for risk assessment. We then propose a harmonized set of age bins for monitoring and assessing risks from exposures to chemicals for use globally. The focus is on preconception through adolescence, though the approach should be applicable to addressing additional life stages. Information collated here was developed as follows. A review of previous efforts to establish standardized age bins was conducted, and previously proposed bins were used as a starting point for harmonization. Important developmental changes underpinning extant binning approaches were identified. A literature review was conducted to identify potential modifying factors and impacts on development, exposure and vulnerability to risk. The influence of social structure and geography on exposure factors was considered, and proposed age bins were evaluated based on important contextual elements.
2. Background
According to the United Nations Convention on the Rights of the Child, which has been ratified by 192 countries and is a legally binding international instrument, a “child means every human being below the age of eighteen years unless under the law applicable to the child, majority is attained earlier”.
Life stage is defined as “a distinguishable timeframe in an individual’s life characterized by unique and relatively stable behavioral and/or physiological characteristics that are associated with development and growth” (Firestone et al., 2007). The evolution of the use of a life stage-specific approach to assessing risks associated with the exposure of children to environmental contaminants is noted in a number of publications that relate mainly to the development of specific age categories to determine what the most critical “windows” of exposure are for particular health outcomes, such as cardiovascular disease, chronic diseases and cancers (Adams et al., 2000, Armstrong et al., 2000, Barr et al., 2000, Brown et al., 2008, Daston et al., 2004, Faustman et al., 2000, Makris et al., 2008, Olshan et al., 2000, Pohl and Abadin, 2008, Selevan et al., 2000, Stevens, 2006, Weiss and Bellinger, 2006, West, 2002). This approach views childhood as a sequence of life stages, from conception through fetal development, infancy and adolescence, rather than characterizing children as a population subgroup.
Life stages can be defined by referring to specific characteristics related to changes in anatomy, physiology, metabolism and behavior that can lead to differences in potential for exposure and/or risk—i.e. children may experience higher exposures to chemicals and greater risks from those exposures compared with adults. Table 1 illustrates different aspects of toxic substance exposure as described by Sexton et al. (1995). Again, the focus on children or childhood is highlighted in this paper because of their potential vulnerabilities (Bruckner, 2000, Graeter and Mortensen, 1996, Makri et al., 2004, Schwenk et al., 2003, Walker, 2005).
Table 1.
Aspects of contact between people and toxic substances.
| Aspects of contact | Examples |
|---|---|
| Agent(s) | Biological, chemical, physical, single agent, multiple agents, mixtures |
| Source(s) | Anthropogenic (of human origin) or non-anthropogenic, area or point, stationary or mobile, indoor or outdoor |
| Transport medium | Air, water, soil, dust, food, product or item |
| Exposure pathway(s) | Eating contaminated food, breathing contaminated workplace air, touching residential surfaces |
| Exposure concentration | mg/kg (food), mg/L (water) |
| Exposure route(s) | Inhalation, dermal contact, ingestion, multiple routes |
| Exposure duration | Seconds, minutes, hours, days, weeks, months, years, lifetime |
| Exposure frequency | Continuous, intermittent, cyclic, random, rare |
| Exposure setting(s) | Occupational or non-occupational, residential or non-residential, indoors or outdoors |
| Exposed population | General population, population groups |
Source: Sexton et al. (1995).
“Although there is no single ‘correct’ set of age groups, adopting a common convention for defining age groups will enable scientists to better understand differences in exposure and risk across life stages and the factors that may account for such differences, such as nutritional status, prevalence of certain diseases, ethnic/cultural norms regarding activity or behavior patterns, population genetic characteristics, meteorological conditions, geography, and social stress” (Firestone, 2010). This improved understanding will facilitate health-protective decisions and policy.
Harmonizing exposure and risk assessment approaches and tools requires consideration of a range of life stage-specific issues. Relevant issues include:
-
•
identification of the relevant changes in behavior and physiology;
-
•
guidance on use of available data to identify the age range at which important behavioral and physiological changes occur;
-
•
approaches for incorporating factors influencing age- or life stage-related differences in behavior, physiology and exposures (e.g. nutritional status and endemic disease) for a given population and in different geographic regions, and the influence of social structure on these parameters;
-
•
approaches for determining age ranges to conduct exposure assessment when data are limited or unavailable;
-
•
approaches for determining age ranges to conduct hazard assessment when data are limited or unavailable;
-
•
selection of important age ranges to consider in designing and conducting exposure and health studies;
-
•
approaches for coordinating windows of highest exposure with windows of greatest susceptibility to hazardous effects.
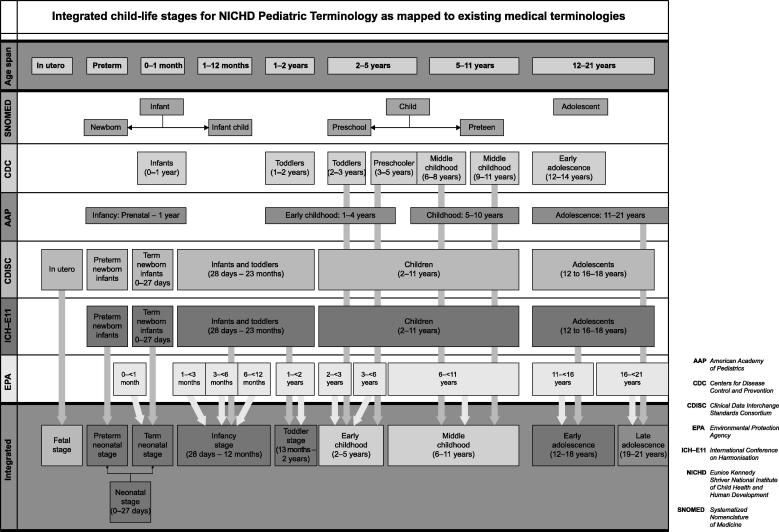
The WHO group began by reviewing existing standardized age groups used by other organizations, including those developed recently by the U.S. Environmental Protection Agency (US EPA). The issues delineated above were among those considered when the US EPA undertook a significant effort to develop a consistent set of age groups for assessing childhood exposure to and potential dose of environmental contaminants (Firestone et al., 2007). This effort consisted of integrating scientific knowledge in disparate fields through a series of workshops and extensive input from a variety of experts in pediatric development, exposure assessment and risk assessment. It was undertaken in part to aid the US EPA in implementing regulatory initiatives requiring federal agencies to ensure that standards take into account special risks to children. The US EPA pediatric life stage categories as well as those of other national and international agencies are summarized in Table 2. These and some other childhood integrated life stages are mapped and presented in Fig. 1 (NCS, 2011).
Table 2.
Pediatric life stage definition by different agencies.
| Agency | Age bracket | Descriptor | Reference |
|---|---|---|---|
| US Environmental Protection Agency | Birth to <1 month | – | US EPA (2005b) |
| 1 to <3 months | – | ||
| 3 to <6 months | – | ||
| 6 to <12 months | – | ||
| 1 to <2 years | – | ||
| 2 to <3 years | – | ||
| 3 to <6 years | – | ||
| 6 to <11 years | – | ||
| 11 to <16 years | – | ||
| 16 to <21 years | – | ||
| U.S. Food and Drug Administration | – | Preterm newborn infants | US FDA (2000) |
| 0–27 days | Term newborn infants | ||
| 28 days to 23 months | Infants and toddlers | ||
| 2–11 years | Children | ||
| 12 to 16–18 yearsb | Adolescents | ||
| World Health Organizationa | Birth to 28 days | Neonate | WHO (2006) |
| 28 days to 1 year | Infant | ||
| 1–4 years | Young child | ||
| 2–3 years | Toddler | ||
| 5–12 years | Older child | ||
| 12–18 yearsc | Adolescent |
Developmental stages.
Dependent on region.
Usual age range; beginning with the appearance of secondary sexual characteristics to achievement of full maturity.
Fig. 1.
Mapping of integrated childhood life stages (NCS, 2011).
3. Developmental changes in children
Children’s physiology changes over time in ways that can impact both their exposures to environmental contaminants and their susceptibility to certain health effects. Children’s behavior also changes over time in ways that can have an important impact on exposure to environmental contaminants. These developmental changes occur as a continuum that contributes to an exposure function over all ages. However, typically existing information is not adequate to construct an exposure function that reflects continuous behavioral and anatomical development. In these cases, a consistent, default approach using age group “bins” is required to provide a reasonable surrogate for the continuous function.
Two aspects of physiological changes are relevant for risk assessment. The first is anatomical changes resulting from physical growth. The second is changes in toxicokinetics and toxicodynamics that affect the absorption, distribution, excretion and effects of environmental contaminants. The following discussion focuses on how to consider age-related changes in behavior and anatomy as these impact potential for exposure. (Toxicodynamics and toxicokinetics as these impact dose–response relationships have been addressed previously. For more on toxicokinetic and toxicodynamic changes across development, see WHO, 2006.)
Although the issues are organized in two categories – issues associated with behavioral changes in children and those associated with anatomical changes and physical growth – to facilitate the development of harmonized age bins, it is understood that these two categories are considerably intertwined.
3.1. Behavioral changes during child development and their impact on exposure to environmental contaminants
Changes in childhood behavior over time are linked to physical and mental growth and can influence where children spend their time, what physical activities they engage in and what foods they eat. To define standard age bins, aspects of behavior most important for characterizing exposure and risk must be identified, as well as critical changes in these behaviors over the course of development.
In developing the proposed age bins for harmonized risk assessment, the following behavior-specific issues were considered:
-
•
important developmental milestones in children’s behavior;
-
•
for each milestone, the range of ages during which the behaviors are typically observed;
-
•
variability among children with respect to the age of onset and the age of abandonment (if applicable) for these behaviors;
-
•
observed changes in behavior associated with these milestones that are likely to affect children’s exposure to environmental contaminants, such as mouthing hands and objects and crawling;
-
•
for those behaviors that are likely to have an important impact on exposure, existing information that is representative of the impact of this behavior on exposure;
-
•
how these behaviors and milestones impact exposure by different routes (e.g. dermal, inhalation and ingestion).
These issues were addressed during an expert’s peer involvement workshop sponsored by the US EPA in 2000, focusing on children’s behavior and anatomy/physiology (US EPA, 2001). Table 3 summarizes the behavioral factors that are likely to affect children’s exposures and associated developmental windows.
Table 3.
Examples of factors considered in deriving age groups reflecting behavioral development.
| Age group | Characteristics relevant to oral and dermal exposure | Characteristics relevant to inhalation exposure |
|---|---|---|
| Birth to <3 months | Breastfeeding and bottle feeding. Hand-to-mouth activities | Time spent sleeping/sedentary |
| 3 to <6 months | Solid food may be introduced. Contact with surfaces increases. Object/hand-to-mouth activities increase | Breathing zone close to the floor |
| 6 to <12 months | Food consumption expands. Floor mobility increases (surface contact). Children are increasingly likely to mouth non-food items | Development of personal dust clouds |
| 12 to <24 months | Children consume full range of foods. They participate in increased play activities, are extremely curious and exercise poor judgment. Breastfeeding and bottle feeding cease | Children walk upright, run and climb. They occupy a wider variety of breathing zones and engage in more vigorous activities |
| 2 to <6 years | Children begin wearing adult-style clothing. Hand-to-mouth activities begin to moderate | Occupancy of outdoor spaces increases |
| 6 to <11 years | There is decreased oral contact with hands and objects as well as decreased dermal contact with surfaces | Children spend time in school environments and begin playing sports |
| 11 to <16 years | Smoking may begin. There is an increased rate of food consumption | Increased independence (more time out of home). Workplace exposure can begin |
| 16 to <21 years | High rate of food consumption begins | Independent driving begins. Expanded work opportunities |
3.2. Anatomical changes and physical growth during child development and their impact on exposure to environmental contaminants
Children’s physiological changes over time include anatomical changes resulting from physical growth. To define and apply standard age bins for risk assessment, anatomical changes relating directly to commonly used exposure factors (e.g. body weight, skin surface area, skin permeability, gut absorption and inhalation rate) are especially important.
In developing the proposed age bins for harmonized risk assessment, the following anatomy-specific issues were considered:
-
•
important developmental milestones for anatomical changes related to physical growth in children;
-
•
for each milestone, the range of ages during which the anatomical characteristics are typically observed;
-
•
variability among children with respect to the age of onset for the anatomical characteristics;
-
•
observed characteristics associated with these milestones that are likely to affect children’s exposure to environmental contaminants;
-
•
for those anatomical characteristics that are likely to have an important impact on exposure, existing information that is representative of the impact of these characteristics on exposure;
-
•
how these anatomical characteristics and milestones impact exposure by different routes (e.g. dermal, inhalation and ingestion).
These issues were also addressed during the expert’s peer involvement workshop sponsored by the US EPA in 2000 (US EPA, 2001). Table 4 summarizes the anatomical and physiological factors that are likely to affect children’s exposures and associated developmental windows.
Table 4.
Examples of factors considered in deriving age groups reflecting anatomical and physiological development.a
| Age group | Anatomy/physiology characteristics |
|---|---|
| Birth to <1 month | Rapid growth and weight gain. Proportion of body fat increases. Increased skin permeability. Deficiencies in hepatic enzyme activity. Immature immune system functions. High oxygen requirements (leading to higher inhalation rates). Stomach more alkaline. Increases in extracellular fluid. Renal function less than predicted by surface area |
| 1 to <3 months | Rapid growth and weight gain. Proportion of body fat increases. Deficiencies in hepatic enzyme activity. Immature immune system functions. High oxygen requirements (leading to higher inhalation rates). Stomach more alkaline. Increases in extracellular fluid. Renal function less than predicted by surface area |
| 3 to <6 months | Rapid growth and weight gain. Proportion of body fat increases. Deficiencies in hepatic enzyme activity. Immature immune system functions. Increases in extracellular fluid. Renal function less than predicted by surface area |
| 6 to <12 months | Rapid growth and weight gain. Body fat increase begins to level off. Deficiencies in hepatic enzyme activity. Immature immune system functions. Rapid decrease in extracellular fluid. Can begin predicting renal function by surface area |
| 1 to <3 years | Some hepatic enzyme activities peak, then fall back to adult range. Most immune system functions have matured. Extracellular fluid becomes more consistently related to body size |
| 3 to <8/9 years | Period of relatively stable weight gain and skeletal growth (as opposed to a period marked by growth spurts) |
| 8/9 to <16/18 years | Rapid skeletal growth. Epiphyseal closure (may take until age 20). Rapid reproductive and endocrine system changes, inclusive of puberty |
Source: US EPA (2005b).
Many of the characteristics listed in this table are repeated across age groups (especially for ages up to <12 months, e.g. rapid growth and weight gain). In determining the range of ages to include in a particular age group, the rate of change in these characteristics was often a key factor discussed at the workshop held in 2000 that led to the development of the guidance document on selecting age groups for monitoring and assessing childhood exposures to environmental contaminants (US EPA, 2005b).
4. Modifying factors and impacts on development, exposure and vulnerability to risk
Exposure assessment and risk assessment require population- and community-specific information or exposure factors that may vary significantly based on geography and cultural practices. These factors are reviewed here, and a framework is described to facilitate systematic consideration of these contextual factors for exposure and risk assessment.
4.1. Epigenetics/mother and immediate caregivers
Exposure in utero and during early childhood is connected in a number of ways to health outcomes later in life, especially outcomes related to the development of chronic and terminal diseases. For example, chemical exposures in utero result in gene expression changes in the fetus (i.e. epigenetic changes) that may confer susceptibility to disease (Fry et al., 2007, Jirtle and Skinner, 2007). The influence of the mother and/or immediate caregivers in terms of their own exposure to chemicals via substance use or abuse, nutrition and use of body care products and household chemicals is therefore of particular importance in determining the exposure of the fetus in utero and of the child in early childhood (Chapin et al., 2004, Delemarre-van de Waal, 1993, Lee et al., 2009, McMillen et al., 2008, Neri et al., 2006, Perera and Herbstman, 2010, Sood et al., 2001, Wells, 2007, Worthman and Kuzara, 2005).
4.2. Exposure-modifying factors associated with geography and culture
Geographic and cultural factors may modify the different aspects of exposure described in Table 1. Geography and culture may influence exposure pathways, routes, duration and frequency. Geography and culture may also impact the exposure settings that determine the agents, sources and transport media that children contact within different contexts.
In order to develop frameworks that can be used in describing different modifying pathways in different contexts, we need to simplify the different combinations that may have an effect in particular contexts. The point has to be made that different pathways become relevant in different contexts, implying that users of this information need to make certain qualitative judgments on the relative “weight” or importance of different modifiers in their particular contexts.
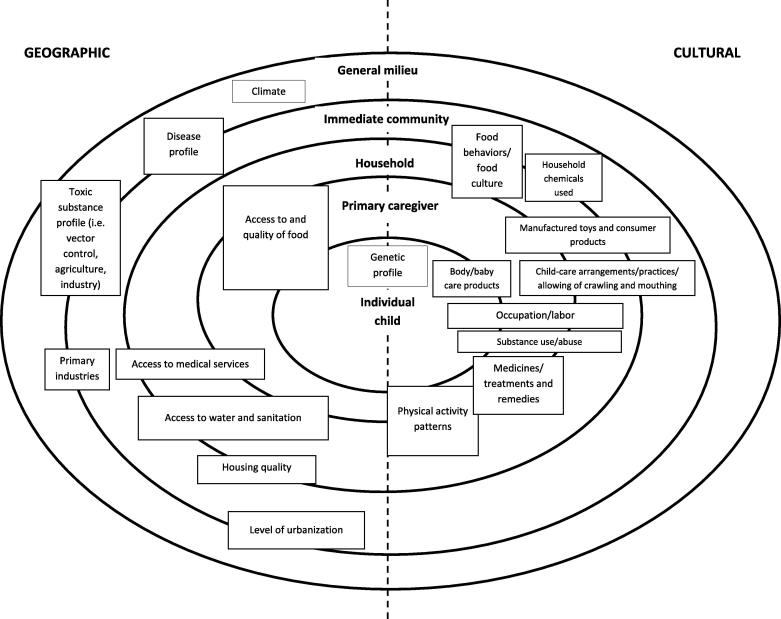
From the literature, five main levels or layers of impact can be identified. These layers are nested within each other, describing levels wherein modifying factors are located. The location of modifying factors (geographic and cultural) within the layers of impact can be moved depending on a particular context. The five levels or layers of impact are:
-
1.
Individual (child or fetus)
-
2.
Primary caregiver/mother/immediate caregivers
-
3.
Household
-
4.
Immediate community
-
5.
Extended community or general milieu
These five levels or layers of impact are visually represented in Fig. 2, together with the different geographical and cultural modifying factors that operate on these levels or layers. It is important to note that these interactions often intersect the levels or layers of impact and may combine in different ways in different contexts.
Fig. 2.
Framework of modifying factors for exposure associated with geography and culture.
A literature review was conducted to identify potential modifying factors and to explore evidence for these factors. For many of the potential modifying factors discussed in the literature, studies have not been conducted or published that actually associate the factors with a particular impact. However, the objective of the review conducted here and of the resulting framework was to consider life stage-specific aspects of these modifying factors and to understand how these are addressed by the proposed life stage-specific age bins or groups. In essence, as is the case for the full range of exposure-related factors, use of the age bins or groups to conduct robust assessment of exposure of and risk to specific populations will require information on national or regional modifying factors.
4.2.1. Geographic factors
In many instances, exposure relates to both the climate and the toxic substance profile of an area or region. The toxic substance profile refers mainly to the history of chemical use in that area, often related to the area’s primary industry (Bjørling-Poulsen et al., 2008, Counter et al., 1998, Goldman and Koduru, 2000, Guillette et al., 1998, Handal et al., 2007, Jain and Hu, 2006, Mendola et al., 2002, Muruka and Muruka, 2007, Röllin et al., 2005). Another key geographic factor relates to the quality of housing and the materials used for building, as well as for heating and cooking indoors (Barnes et al., 2006, Montgomery and Mathee, 2004).
The geographic modifying factors that may potentially operate or combine with the five levels of impact in the framework to produce particular impacts (via exposure) are:
-
1.
Climate (impacts on level of general milieu)
-
•
Includes reference to disease profile and specific environmental adaptations (e.g. malaria)
-
•
Often associated with play/crawl areas for children (inside or outside)
-
•
To a large extent determines the infectious disease profile of people (e.g. malaria, respiratory conditions)
-
•
Living at different altitudes may result in adaptations during pregnancy and early childhood
-
2.
Disease profile (impacts on levels of general milieu and immediate community)
-
•
See above association with climate
-
•
Reclaimed land for residences (e.g. landfills, rubbish dumps), low-lying areas, groundwater
-
•
Disease/vector control measures (e.g. the pesticide DDT is still used in some areas to control malaria)
-
•
Socioeconomic drivers of disease occurrence (social epidemiology, e.g. combination of factors that results in high levels of human immunodeficiency virus infection in particular places)
-
3.
Toxic substance profile (impacts on levels of general milieu and immediate community)
-
•
Disease/vector control measures (e.g. DDT is still used in some areas to control malaria)
-
•
Level of urbanization (often associated with exposure to traffic-related pollutants, e.g. lead and carbon monoxide, and carcinogenic compounds, e.g. benzene and polycyclic aromatic hydrocarbons; also associated with play areas for children, access to medical care, specific toxic substances that have a history in that area)
-
•
Primary industry (e.g. agricultural areas and pesticide exposure)
-
4.
Primary industries (impacts on levels of general milieu and immediate community)
-
•
Air, ground and water pollution from industry
-
5.
Level of urbanization (impacts on levels of general milieu and immediate community)
-
•
Proximity to industry
-
•
Proximity to major roads
-
•
Associated with play areas for children (inside or outside)
-
•
Associated with access to medical care
-
6.
Housing quality (impacts on levels of immediate community and household)
-
•
Building materials, ventilation, paints used, asbestos
-
•
Sources of fuel for heating and cooking (e.g. paraffin, coal and wood)
-
•
Reclaimed land for residences (e.g. landfills, rubbish dumps), low-lying areas, groundwater
-
7.
Access to services (impacts on levels of immediate community, household and primary caregiver)
-
•
Access to clean water
-
•
Access to sanitation
-
•
Access to medical care
-
8.
Access to and quality of food (impacts on household, primary caregiver and individual child)
-
•
Access and quality restricted by drought, flooding or other weather-related events
-
•
Quality of food (e.g. pesticide residues, steroid hormonal residues, additives for food preservation and enhancement, antimicrobials in animal feed)
4.2.2. Cultural factors
Cultural factors may modify a range of exposure-related practices, including activity patterns, chemical and substance use, labor practices and diet.
Physical activity patterns and contact with different surfaces are well described (Beamer et al., 2008, Beamer et al., 2009, Black et al., 2005, Rowlands and Eston, 2007, Tulve et al., 2002, Xue et al., 2007). The uses of particular medicines and treatments (especially in traditional contexts) are also well described in the literature (Brand et al., 2009, Green et al., 1994, Woywodt and Kiss, 2002). Exposures related to work and labor practices are described, especially in terms of farm workers and their exposure to pesticides and chemicals (Quandt et al., 2006), as well as practices such as recycling/reclaiming of electronics, scavenging on dumpsites and artisanal mining. Practices around food and feeding (especially related to women’s practice of breastfeeding and how it impacts their other activities) are described as an important factor in determining the exposure of children to environmental contaminants and are in fact well described in the literature for their effects on childhood growth and development (Allen, 1995, Asefa et al., 1998, Fawzi et al., 1997, Koletzko et al., 1998, LaKind et al., 2005, Mamabolo et al., 2004, Solem et al., 1992).
The behavioral modifying factors that potentially operate or combine with the levels of impact to produce particular impacts (via exposure) are:
-
1.
Substance use/abuse (impacts on levels of household, primary caregiver and individual)
-
•
Smoking, alcohol and medicine/substance use/abuse during pregnancy
-
•
Smoking, alcohol and medicine/substance use/abuse by people in immediate surroundings during early childhood
-
•
Smoking, alcohol, solvents and other substance use/abuse by young children and adolescents in the early teenage years (e.g. glue sniffing by street children in South Africa)
-
2.
Household chemicals used (impacts on levels of immediate community and household)
-
•
Affects inhalation and dermal exposure
-
•
Associated with play areas for children, indoor/outdoor crawling and mouthing
-
3.
Manufactured toys and consumer products (impacts on levels of immediate community, household and primary caregiver)
-
•
Chemicals used in manufacturing products for or associated with children
-
o
Paint/coating materials (e.g. toys, playground and play area equipment painted with lead-based paint)
-
o
Plastics (e.g. bisphenol A released by baby bottles when warmed in the microwave)
-
o
Synthetic fibers and textiles (e.g. flammable material)
-
4.
Body/baby care products (impacts on levels of primary caregiver and individual)
-
•
Chemicals used to manufacture care products
-
•
Baby powders and lotions
-
•
Detergents
-
5.
Child-care arrangements/practices/allowing of crawling and mouthing (impacts on levels of immediate community, household and primary caregiver)
-
•
Activity patterns associated with the physical state of childhood (being an infant, toddler, child, pubescent, etc.), such as crawling and mouthing, are considered
-
•
Playing and/or crawling inside or outside (associated with climate conditions)
-
•
Child-care arrangements (how much is an infant picked up or played with)
-
•
Mouthing (inside or outside play areas)
-
•
Household chemicals on surfaces (inhalation and dermal exposure)
-
6.
Physical activity patterns (impacts on levels of household, primary caregiver and individual)
-
•
Possibility to play outdoors
-
•
Type of toys determines activity patterns (e.g. electronic devices, computers vs. football)
-
•
Ways of measuring activity patterns also important
-
•
Standard definitions of developmental milestone measures in different contexts often adjusted (individual indicators related to the domains of language and socialization, almost never related to physical growth and the attainment of motor skills)
-
7.
Food behaviors/food culture (impacts on levels of immediate community, household and primary caregiver)
-
•
Food availability
-
•
Urban and rural food availability
-
•
Poverty – income as well as own food production
-
•
Differential understanding of nutritional value and what makes “good” food
-
•
Secular trend hypothesis: over past century, better “western” nutrition (plus social factors around child care) has resulted in higher stature and earlier onset of puberty in certain populations
-
•
History of pesticide/chemical use in an area
-
•
Practices and beliefs around breastfeeding and breastfeeding interval
-
•
Livelihood strategies and the role of women (associated with breastfeeding interval and the introduction of other foods)
-
•
The beliefs of parents around “normal” growth/development
-
•
Levels of heavy metals and other toxic substances in human milk
-
•
Food additives (preservatives and colorants)
-
8.
Occupation/labor (impacts on levels of household, primary caregiver and individual)
-
•
Marginalized groups with few choices in work/income are often exposed, with a lack of legislation or control over working conditions and occupational safety
-
•
In this instance, those working in agriculture are particularly highlighted, for exposure to pesticides in their work and home environments
-
•
History of pesticide and/or chemical use in a particular environment (long-term presence of certain chemicals)
-
•
Outsourced tasks that are done in households (beedi rolling, reclaiming and recycling materials, e.g. heavy metals such as lead and mercury from car batteries and electronics)
-
•
Artisanal mining (Africa and Latin America)
-
•
Child labor (artisanal mining and outsourced tasks) highly illegal
-
•
Livelihood activities (associated with the economic circumstances and activities of the household, e.g. farming)
-
9.
Medicines/treatments and remedies (impacts on levels of household, primary caregiver and individual)
-
•
Various traditional ways of understanding disease and consequent treatment
-
•
Effects (often unintended) on common illnesses among infants and young children
-
•
Example of “impila” (Callilepis laureola) for protection in utero and in early childhood
-
•
Antenatal “modes” of care: e.g. “Isihlambezo” or traditional herbal antenatal care (also Ayurvedic medicine, Chinese herbal remedies)
-
•
“Muti” medicine/generic names for certain “concoctions”
-
•
Geophagic practice among different populations
-
•
Remedies for pregnant women
-
•
Remedies for infants and small children
-
•
Effect of medicines on activity patterns; interaction of medicines with environmental contaminants in the body
5. Methodological considerations in the collection of data and the design of cohort studies
5.1. Methodological designs
The literature survey highlighted the issues involved in designing studies that can produce the data needed to evaluate exposure to environmental chemicals and risk associated with such exposure at different developmental stages of a child’s life. The most suitable approach for the determination of exposure and risk at different life stages is the longitudinal birth cohort study. Several such studies are being planned or are under way in various parts of the world. Because of the enormous expense of undertaking a longitudinal cohort study, most of these studies have been undertaken in high-income countries. WHO held several consultations to promote longitudinal cohort studies (2003–2007), which resulted in the publication of “A Guide to Undertaking a Birth Cohort Study: Purposes, Pitfalls and Practicalities” as a supplement to the journal Paediatric and Perinatal Epidemiology (Golding et al., 2009a).
Important in this discussion are some of the issues in play when attempting to measure developmental stages and growth in babies and young children, across many different genetic, social and economic contexts. This raises the issue of cross-cultural (or, rather, cross-population) application of standard measures (Abubakar et al., 2008, Aina and Morakinyo, 2005, Bornstein, 2004, Carter-Pokras et al., 2007, Cheung et al., 2001, de Onis, 2006, de Onis et al., 2006, Dibley et al., 1987, Gladstone et al., 2010, Holding et al., 2004, Kelly et al., 2006, Lung et al., 2010, Miyahara and Meyers, 2008, Onyango et al., 2007).
In the past 20 years, birth cohort studies to assess the risks to developing children from exposure to chemicals in air, water and food have been undertaken in many countries. These birth cohort studies usually started during pregnancy and followed children through adolescence or beyond. Even the largest of these birth cohort studies, however, were not big enough to study rare outcomes, such as sudden infant death syndrome or childhood cancer. To increase the sample size, investigators working on these older cohort studies are now making an effort to pool their data. Their efforts are hampered by the fact that the older studies did not use agreed-upon disease outcome definitions, time periods of measurement or methods for measuring biomarkers and chemical contaminants in air, water and food. This makes pooling data extremely difficult, if not impossible.
To avoid such problems in the new birth cohort studies, WHO is currently working with investigators from various countries undertaking large-scale birth cohort studies to invest time up front to agree on when during pregnancy, infancy and childhood to assess disease outcomes, measure biomarkers and measure environmental exposures. A harmonized set of age bins for assessing exposures will greatly enhance the ability to conduct cohort studies that can then be combined in the future, yielding studies with more power to identify positive results.
5.2. Analysis options
Various analysis options are described in the literature, relating to the types of variables that work well in certain types of statistical approaches (continuous, dichotomous and categorical variable types) and statistical testing that allows for the use of large datasets over time (Eldred and Darrah, 2010, Longnecker et al., 2003, Wigle et al., 2008).
5.3. Methodological considerations/problems
Some literature has been produced on the problems encountered in existing cohort studies, from the type of variable collected, the range of issues considered and the design of collection tools and questionnaires to analysis and interpretation (Barr et al., 2005, Bradman and Whyatt, 2005, Cohen Hubal et al., 2000, Dietrich et al., 2005, Landrigan et al., 2004, Luo et al., 2010, Needham and Sexton, 2000, Samet, 2004, Savitz and Harlow, 1991, Williams et al., 2006, Wilson et al., 2004).
5.4. Data points/variables of interest
At this stage in the development of cohort studies, particular data points and variables have emerged as particularly important when considering different levels of analysis of this type of longitudinal data. There are certain pieces of information that are best collected via biological samples, such as blood samples, whereas other information (especially that related to geography and behavior) needs to be collected by carefully designed survey data collection tools (Emmett, 2009, Golding, 2009, Golding and Jones, 2009, Golding et al., 2009b, Jones and Golding, 2009).
6. Key issues for applying age bins to assess exposure and risk
6.1. Problem formulation
The need for, and use of, the recommended age bins will depend on the purpose of any given assessment. Where a specific outcome with a known window of susceptibility is of primary interest, the age associated with this window should be assessed, and the bins are not required. However, where a unique window of susceptibility cannot be identified and an assessment is required to evaluate potential for highest exposures compared against potential for impacts at multiple developmental time points, use of these age bins is encouraged to develop estimates of life stage-specific exposures.
6.2. Applying age bins to support life stage-based exposure assessment
When assessing long-term exposures to environmental toxicants, it is desirable to integrate age-specific values for both exposure and toxicity/potency, where such data are available and appropriate (US EPA, 2005a). Historically, chronic risks, including cancer risks, have been assessed assuming that risk is proportional to the lifetime average daily dose for a “typical” adult. A life stage integrative approach is a departure from this approach, because it assesses risk by summing time-weighted exposures or risks across all relevant age groups, including those of childhood, adulthood and old age, as well as maternal–fetal exposures during pregnancy, and then averages across the total exposure period.
For example, when assessing risks from exposure to carcinogens with a mutagenic mode of action, the US EPA applies different toxic potency adjustments for exposure of children less than 2 years of age and between 2 and 16 years of age (US EPA, 2005b). In Table 5, the exposure duration and potency adjustments for the US EPA-recommended set of childhood age groups are presented.
Table 5.
Integrating the US EPA’s supplemental guidance for assessing susceptibility from early life exposure to carcinogens (US EPA, 2005a) with the guidance on selecting age groups for monitoring and assessing childhood exposures to environmental contaminants (US EPA, 2005b).a
| Exposure age groupings | Exposure duration (years) | Age-dependent adjustment factor (ADAF) |
|---|---|---|
| Birth to <1 month | 0.083 | 10× |
| 1 to <3 months | 0.167 | 10× |
| 3 to <6 months | 0.25 | 10× |
| 6 to <12 months | 0.5 | 10× |
| 1 to <2 years | 1 | 10× |
| 2 to <3 years | 1 | 3× |
| 3 to <6 years | 3 | 3× |
| 6 to <11 years | 5 | 3× |
| 11 to <16 years | 5 | 3× |
| 16 to <21 years | 5 | 1× |
| >21 years (21 to <70 years) | 49 | 1× |
Source: US EPA (2005b).
Cancer potency adjustments, or age-dependent adjustment factors, apply only to carcinogens that act via a mutagenic mode of action.
6.3. Variability
Variability is a key challenge for children’s exposure assessment. Children of the same age can exhibit tremendous variability in development and behavior. This presents a challenge for identifying fixed age ranges to use for assessing children’s exposure and risk. However, it remains useful to address this variability in development of the exposure factor data while maintaining a standard set of age bins. In this case, with sufficient data, the standardized bins facilitate understanding of the contextual and confounding factors that are driving differences in exposure and risk, even within each bin.
6.4. Representativeness
Another challenge when assessing children’s exposure is the extent to which the available exposure data represent the population of interest (Thompson, 1999). Exposure data are collected for a specific group of people, in a specific place and at a specific time. They can be used in a risk assessment only to the extent that they are sufficiently relevant to the population being assessed in the current time and place. The rapid pace of social and behavioral change may diminish the relevance of study data. In addition, social and behavioral differences may be significant from one community to another and from one population to another. Here again, the need for a common exposure metric facilitated by a standard set of life stages will improve understanding of similarities and differences among and across study populations.
6.5. Coordinating exposure and hazard assessment
As noted previously, there is a need to better link or coordinate hazard and exposure assessment (the need to identify the most vulnerable based on windows of greatest susceptibility as well as windows of highest exposure and then to incorporate that knowledge in a population-based risk assessment). Approaches for coordinating and linking exposure and hazard assessment will necessarily be fit to purpose. If risk for a specific health outcome with known life stage-specific etiology is being assessed, then the window of exposure associated with the known window of biological susceptibility should be assessed, and the full set of recommended age bins need not be considered. If, however, there is some uncertainty about the key drivers of a particular outcome or these are multifactorial and complex, information on critical windows of susceptibility should be mapped to exposure-related age bins for further assessment and/or data collection.
7. WHO recommendation on harmonized early life age groups
To harmonize exposure assessment for comparison across time, place and culture, we need to define a standard framework within which to analyze population-specific information. Defining standard age ranges for children will also facilitate collection of data and analyses of aggregate exposure and cumulative risk.
Given the range of scientific and policy-related needs for a harmonized set of age groups, the following tiered set of early life age groups (Table 6) is recommended for international use to facilitate some level of consistency with recently developed age grouping guidance currently in use in some regions:
Tier 1: Adopt guidance similar to the US EPA’s (2005b) recommended childhood age groups.
Tier 2: Consolidate some of the age groups defined above in order to reduce the burden of developing age-specific exposure factor data for different countries or regions.
Table 6.
WHO-recommended tiered set of early life age groups.
| Life stage descriptor | Tier 1 age groups | Tier 2 age groups |
|---|---|---|
| Preconception | Preconception | – |
| Fetal | Prenatal | Conception to birth |
| Newborn (neonatal) | Birth to <1 month | Birth to <1 month |
| Infant | 1 to <3 months | 1 to <12 months |
| 3 to <6 months | ||
| 6 to <12 months | ||
| Toddler | 1 to <2 years | 1 to <2 years |
| Early childhood | 2 to <3 years | 2 to <6 years |
| 3 to <6 years | ||
| Middle childhood | 6 to <11 years | 6 to <11 years |
| Early adolescence | 11 to <16 years | 11 to <16 years |
| Late adolescence | 16 to <21 years | 16+ years |
Tier 1 is preferred in those cases where significant differences in exposure early in life can greatly impact health risks from acute or subchronic exposure to toxins. For example, fluid consumption on a body weight basis is on average almost 3 times greater shortly after birth (birth to <1 month) than for infants 6 to <12 months of age and almost twice the time-weighted average for the entire first year of life (Table 7).
Table 7.
Recommended mean drinking water ingestion rates, consumers only,a by age group.
| Age group | Intake (mL/kg body weight per day) | Ratio to adults ⩾21 years |
|---|---|---|
| Birth to <1 month | 137 | 9 |
| 1 to <3 months | 119 | 7 |
| 3 to <6 months | 80 | 5 |
| 6 to <12 months | 53 | 3 |
| 1 to <2 years | 27 | 2 |
| Time-weighted average for birth to <12 months | 78 | 5 |
| ⩾21 years (adults) | 16 | 1 |
Source: Data from Recommended Values for Drinking Water Ingestion Rates, Table 3, Table 1 (http://www.epa.gov/ncea/efh/pdfs/efh-chapter03.pdf) in US EPA (2011).
Consumer-only intake represents the quantity of water consumed only by individuals that reported consuming water during the survey period.
The above recommendation builds on several recent activities and fills gaps identified in recent publications that focus on assessing risks from exposures of children to environmental contaminants. The US EPA document titled Guidance on Selecting Age Groups for Monitoring and Assessing Childhood Exposures to Environmental Contaminants (US EPA, 2005b) presents recommended age bins for children based on physiology and behavior. The scope of this document narrowly focuses on birth through 18 years of age and is designed specifically to promote a more uniform approach for exposure assessments conducted across US EPA program offices and regions. Prenatal and preconception periods were identified as important periods for consideration in assessing health risks from early life exposures, and these life stages were added to the US EPA-recommended age bins in the US EPA (2006) document titled A Framework for Assessing Health Risks of Environmental Exposures to Children. The WHO document titled Principles for Evaluating Health Risks in Children Associated with Exposure to Chemicals (WHO, 2006) cites the US EPA guidance document (US EPA, 2006) in the exposure section. However, the lack of harmonization in determining age ranges for life stages became apparent during development of the WHO (2006) document. In a few instances, life stages defined at the beginning of the document consistent with WHO terminology were slightly different from the US EPA-recommended exposure bins that were used in the exposure chapter of the WHO (2006) document. Even with the focus on children in these three documents, there is not a uniform approach for identifying the important life stage (age range), exposure factors specific to the exposure/risk assessment question of interest or the characteristics of a particular population that might modify these. In addition, very few institutions outside the United States have addressed this issue, and there are likely some different factors that might be important for non-US populations that should be considered for a harmonized approach.
While there is no single “correct” means of choosing a common set of age groups to use internationally in assessing early life exposure and risk, use of a set of defined age groups is recommended to facilitate comparisons of potential exposures and risks around the globe.
Application of these age groups for robust assessment of exposure and risk for specific populations will then require country- or region-specific exposure factor information as well as local environmental monitoring data (and/or characterization of local sources).
Conflict of interest
None.
Acknowledgments
A draft version of this article was released on the WHO International Programme on Chemical Safety (IPCS) web site for comment in December 2011, and 15 responses were received from individual experts and institutions. A draft version was presented and discussed at a WHO Workshop on Risk Assessment Methodologies, held in Bonn, Germany, on 28 March 2012. The authors gratefully acknowledge the comments and suggestions made by reviewers.
Footnotes
The authors alone are responsible for the views expressed in this publication, which do not necessarily represent the decisions or policies of the World Health Organization or the U.S. Environmental Protection Agency.
This is an Open Access article published without any waiver of WHO’s privileges and immunities under international law, convention, or agreement. This article should not be reproduced for use in association with the promotion of commercial products, services or any legal entity. There should be no suggestion that WHO endorses any specific organization or products. The use of the WHO logo is not permitted. This notice should be preserved along with the article’s original URL.
Contributor Information
Elaine A. Cohen Hubal, Email: Hubal.Elaine@epamail.epa.gov.
Thea de Wet, Email: tdewet@uj.ac.za.
Lilo Du Toit, Email: Lilo.Dutoit@wits.ac.za.
Michael P. Firestone, Email: Firestone.michael@epa.gov.
Mathuros Ruchirawat, Email: mathuros@cri.or.th.
Jacqueline van Engelen, Email: jacqueline.van.engelen@rivm.nl.
Carolyn Vickers, Email: vickersc@who.int.
References
- Abubakar A., Holding P., Van Baar A., Newton C.R.J.C., Van de Vijver F.J.R. Monitoring psychomotor development in a resource limited setting: an evaluation of the Kilifi Developmental Inventory. Ann. Trop. Pediatr. 2008;28:217–226. doi: 10.1179/146532808X335679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J., Barone S., Jr., LaMantia A., Philen R., Rice D.C., Spear L., Susser E. Workshop to identify critical windows of exposure for children’s health: neurobehavioral work group summary. Environ. Health Perspect. 2000;108(Suppl. 3):535–544. doi: 10.1289/ehp.00108s3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aina O.F., Morakinyo O. Normative data on mental and motor development in Nigerian children. West Afr. J. Med. 2005;24(2):151–156. doi: 10.4314/wajm.v24i2.28187. [DOI] [PubMed] [Google Scholar]
- Allen L.H. Malnutrition and human function: a comparison of conclusions from the INCAP and nutrition CRSP studies. Am. Inst. Nutr. 1995;125(4):1119–1126. doi: 10.1093/jn/125.suppl_4.1119S. [DOI] [PubMed] [Google Scholar]
- Armstrong T.W., Hushka L.J., Tell J.G., Zaleski R.T. A tiered approach for assessing children’s exposure. Environ. Health Perspect. 2000;108(6):469–474. doi: 10.1289/ehp.00108469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asefa M., Hewison J., Drewett R. Traditional nutritional and surgical practices and their effects on the growth of infants in south-west Ethiopia. Paediatr. Perinat. Epidemiol. 1998;12(2):182–198. doi: 10.1046/j.1365-3016.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- Barnes B., Mathee A., Bruce I.N., Thomas L. Protecting children from indoor air pollution exposure through outdoor cooking in rural South Africa. Boiling Point. 2006;52:11–13. [Google Scholar]
- Barr M., Jr., DeSesso J.M., Lau C.S., Osmond C., Ozanne S.E., Sadler T.W., Simmons R.A., Sonawane B.R. Workshop to identify critical windows of exposure for children’s health: cardiovascular and endocrine work group summary. Environ. Health Perspect. 2000;108(Suppl. 3):569–571. doi: 10.1289/ehp.00108s3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr D.B., Wang R.Y., Needham L.L. Biologic monitoring of exposure to environmental chemicals throughout the life stages: requirements and issues for consideration for the National Children’s Study. Environ. Health Perspect. 2005;113(8):1083–1091. doi: 10.1289/ehp.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer P., Key M.E., Ferguson A.C., Canales R.A., Auyeung W., Leckie J.O. Quantified activity pattern data from 6 to 27-month-old farmworker children for use in exposure assessment. Environ. Res. 2008;108:239–246. doi: 10.1016/j.envres.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer P.I., Canales R.A., Bradman A.S.A., Leckie J.O. Farmworker children’s residential non-dietary exposure estimates from micro-level activity time series. Environ. Int. 2009;35(8):1202–1209. doi: 10.1016/j.envint.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørling-Poulsen M., Andersen H.R., Grandjean P. Potential developmental neurotoxicity of pesticides used in Europe. Environ. Health. 2008;7:50. doi: 10.1186/1476-069X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black K., Shalat S.L., Freeman N.C., Jimenez M., Donnelly K.C., Calvin J.A. Children’s mouthing and food-handling behavior in an agricultural community on the US/Mexico border. J. Expo. Anal. Environ. Epidemiol. 2005;15(3):244–251. doi: 10.1038/sj.jea.7500398. [DOI] [PubMed] [Google Scholar]
- Bornstein M.H. Cross-cultural developmental comparisons: the case of Japanese-American infant and mother activities and interactions. What we know, what we need to know, and why we need to know. Dev. Rev. 2004;9(2):171–204. [Google Scholar]
- Bradman A., Whyatt R.M. Characterizing exposures to nonpersistent pesticides during pregnancy and early childhood in the National Children’s Study: a review of monitoring and measurement methodologies. Environ. Health Perspect. 2005;113(8):1092–1099. doi: 10.1289/ehp.7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand C.E., de Jager L., Ekosse G.E. Possible health effects associated with human geophagic practise: an overview. Med. Technol. South Afr. 2009;23(13):11–13. [Google Scholar]
- Brown R.C., Barone S., Jr., Kimmel C.A. Children’s health risk assessment: incorporating a lifestage approach into the risk assessment process. Birth Defects Res. B Dev. Reprod. Toxicol. 2008;83(6):511–521. doi: 10.1002/bdrb.20172. [DOI] [PubMed] [Google Scholar]
- Bruckner J.V. Differences in sensitivity of children and adults to chemical toxicity: the NAS panel report. Regul. Toxicol. Pharmacol. 2000;31(3):280–285. doi: 10.1006/rtph.2000.1393. [DOI] [PubMed] [Google Scholar]
- Carter-Pokras O., Zambrana R.E., Popell C.F., Logie L.A., Guerrero-Preston R. The environmental health of Latino children. J. Pediatr. Health Care. 2007;21(5):307–314. doi: 10.1016/j.pedhc.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin R.E., Robbins W.A., Schieve L.A., Sweeney A.M., Tabacova S.A., Tomashek K.M. Off to a good start: the influence of pre- and periconceptional exposures, parental fertility, and nutrition on children’s health. Environ. Health Perspect. 2004;112(1):69–78. doi: 10.1289/ehp.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Y.B., Yip P.S.F., Karlberg J.P.E. Fetal growth, early postnatal growth and motor development in Pakistani infants. Int. J. Epidemiol. 2001;30:66–72. doi: 10.1093/ije/30.1.66. [DOI] [PubMed] [Google Scholar]
- Cohen Hubal E.A., Sheldon L.S., Burke J.M., McCurdy T.R., Berry M.R., Rigas M.L., Zartarian V.G., Freeman N.C. Children’s exposure assessment: a review of factors influencing children’s exposure, and the data available to characterize and assess that exposure. Environ. Health Perspect. 2000;108(6):475–486. doi: 10.1289/ehp.108-1638158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter S.A., Buchanan L.H., Rosas H.D., Ortega F. Neurocognitive effects of chronic lead intoxication in Andean children. J. Neurol. Soc. 1998;160(1):47–53. doi: 10.1016/s0022-510x(98)00180-4. [DOI] [PubMed] [Google Scholar]
- Daston G., Faustman E., Ginsberg G., Fenner-Crisp P., Olin S., Sonawane B., Bruckner J., Breslin W., McLaughlin T.J. A framework for assessing risks to children from exposure to environmental agents. Environ. Health Perspect. 2004;112(2):238–256. doi: 10.1289/ehp.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Onis M. Assessment of sex differences and heterogeneity in motor milestone attainment among populations in the WHO Multicentre Growth Reference Study. Acta Paediatr. Suppl. 2006;450(95):66–75. doi: 10.1111/j.1651-2227.2006.tb02377.x. [DOI] [PubMed] [Google Scholar]
- de Onis M., Onyango A.W., Borghi E., Garza C., Yang H. Comparison of the World Health Organization (WHO) child growth standards and the National Center for Health Statistics/WHO international growth reference: implications for child health programmes. Public Health Nutr. 2006;9:942–947. doi: 10.1017/phn20062005. [DOI] [PubMed] [Google Scholar]
- Delemarre-van de Waal H.A. Environmental factors influencing growth and pubertal development. Environ. Health Perspect. 1993;101(Suppl. 2):39–44. doi: 10.1289/ehp.93101s239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibley M.J., Goldsby J.B., Staehling N.W., Towbridge F.L. Development of normalized curves for the international growth reference: historical and technical considerations. Am. J. Clin. Nutr. 1987;46(5):736–748. doi: 10.1093/ajcn/46.5.736. [DOI] [PubMed] [Google Scholar]
- Dietrich K.N., Eskenazi B., Schantz S., Yolton K., Rauh V.A., Johnson C.B., Alkon A., Canfield R.L., Pessah I.N., Berman R.F. Principles and practices of neurodevelopmental assessment in children: lessons learned from the Centers for Children’s Environmental Health and Disease Prevention Research. Environ. Health Perspect. 2005;113(10):1437–1446. doi: 10.1289/ehp.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldred K., Darrah J. Using cluster analysis to interpret the variability of gross motor scores of children with typical development. Phys. Ther. 2010;90(10):1510–1518. doi: 10.2522/ptj.20090308. [DOI] [PubMed] [Google Scholar]
- Emmett P. Assessing diet in longitudinal birth cohort studies. Paediatr. Perinat. Epidemiol. 2009;23(Suppl. 1):154–173. doi: 10.1111/j.1365-3016.2009.01015.x. [DOI] [PubMed] [Google Scholar]
- Faustman E.M., Silbernagel S.M., Fenske R.A., Burbacher T.M., Ponce R.A. Mechanisms underlying children’s susceptibility to environmental toxicants. Environ. Health Perspect. 2000;108(Suppl. 1):13–21. doi: 10.1289/ehp.00108s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawzi W.W., Forman M.R., Levy A., Graubard B.I., Naggan L., Berendes H.W. Maternal anthropometry and infant feeding practices in Israel in relation to growth in infancy: the North African Infant Feeding Study. Am. J. Clin. Nutr. 1997;65(6):1731–1737. doi: 10.1093/ajcn/65.6.1731. [DOI] [PubMed] [Google Scholar]
- Firestone M.J. Protecting children from environmental risks throughout each stage of their childhood. J. Expo. Sci. Environ. Epidemiol. 2010;20(3):227–228. doi: 10.1038/jes.2010.10. [DOI] [PubMed] [Google Scholar]
- Firestone M., Moya J., Cohen Hubal E., Zartarian V. Identifying childhood age groups for exposure assessments and monitoring. Risk Anal. 2007;27(3):701–714. doi: 10.1111/j.1539-6924.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- Fry R.C., Navasumrit P., Valiathan C., Svensson J.P., Hogan B.J., Luo M., Bhattacharya S., Kandjanapa K., Soontararuks S., Nookabkaew S., Mahidol C., Ruchirawat M., Samson L.D. Activation of inflammation/NF-κB signaling in infants born to arsenic-exposed mothers. PLoS Genet. 2007;3(11):e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone M., Lancaster G.A., Umar E., Nyirenda M., Kayira E., van den Broek N.R., Smyth R.L. The Malawi Developmental Assessment Tool (MDAT): the creation, validation, and reliability of a tool to assess child development in rural African settings. PLoS Med. 2010;7(5):1–14. doi: 10.1371/journal.pmed.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J. Measuring outcomes in a longitudinal birth cohort. Paediatr. Perinat. Epidemiol. 2009;23(Suppl. 1):185–200. doi: 10.1111/j.1365-3016.2009.01016.x. [DOI] [PubMed] [Google Scholar]
- Golding J., Jones R. Sources of data for a longitudinal birth cohort. Paediatr. Perinat. Epidemiol. 2009;23(Suppl. 1):51–62. doi: 10.1111/j.1365-3016.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- Golding J., Birmingham K., Jones R. Special issue: a guide to undertaking a birth cohort study: purposes, pitfalls and practicalities. Paediatr. Perinat. Epidemiol. 2009;23(Suppl. 1):1–236. [Google Scholar]
- Golding J., Jones R., Preece A., Brune M.N., Pronczuk J. Choice of environmental components for a longitudinal birth cohort study. Paediatr. Perinat. Epidemiol. 2009;23(Suppl. 1):134–153. doi: 10.1111/j.1365-3016.2009.01014.x. [DOI] [PubMed] [Google Scholar]
- Goldman L.R., Koduru S. Chemicals in the environment and developmental toxicity to children: a public health and policy perspective. Environ. Health Perspect. 2000;108(Suppl. 3):443–448. doi: 10.1289/ehp.00108s3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeter L.J., Mortensen M.E. Kids are different: developmental variability in toxicology. Toxicology. 1996;111:15–20. doi: 10.1016/0300-483x(96)03389-6. [DOI] [PubMed] [Google Scholar]
- Green E.C., Jurg A., Djedje A. The snake in the stomach: child diarrhea in Central Mozambique. Med. Anthropol. Q. 1994;8(1):4–24. [Google Scholar]
- Guillette E.A., Meza M.M., Aquilar M.G., Soto A.D., Garcia I.E. An anthropological approach to the evaluation of preschool children exposed to pesticides in Mexico. Environ. Health Perspect. 1998;106(6):347–353. doi: 10.1289/ehp.98106347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handal A.J., Lozoff B., Breihl J., Harlow S.D. Effect of community of residence on neurobehavioral development in infants and young children in a flower-growing region of Ecuador. Environ. Health Perspect. 2007;115(1):128–133. doi: 10.1289/ehp.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holding P.A., Taylor H.G., Kazungu S.D., Mkala T., Gona J., Mwamuye B., Mbonani L., Stevenson J. Assessing cognitive outcomes in a rural African population: development of a neuropsychological battery in Kilifi District, Kenya. J. Int. Neuropsychol. Soc. 2004;10(2):246–260. doi: 10.1017/S1355617704102166. [DOI] [PubMed] [Google Scholar]
- Jain N.B., Hu H. Childhood correlates of blood lead levels in Mumbai and Delhi. Environ. Health Perspect. 2006;114(3):466–470. doi: 10.1289/ehp.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirtle R.L., Skinner M.K. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007;8(4):253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R., Golding J. Choosing the types of biological sample to collect in longitudinal birth cohort studies. Paediatr. Perinat. Epidemiol. 2009;23(Suppl. 1):103–113. doi: 10.1111/j.1365-3016.2008.01000.x. [DOI] [PubMed] [Google Scholar]
- Kelly Y., Sacker A., Schoon I., Nazroo J. Ethnic differences in achievement of developmental milestones by 9 months of age: the Millennium Cohort Study. Dev. Med. Child Neurol. 2006;48(10):825–830. doi: 10.1017/S0012162206001770. [DOI] [PubMed] [Google Scholar]
- Koletzko B., Aggett P.J., Bindels J.G., Bung P., Ferré P., Gil A., Lentze M.J., Roberfroid M., Strobel S. Growth, development and differentiation: a functional food science approach. Br. J. Nutr. 1998;80(Suppl. 1):S5–S45. doi: 10.1079/bjn19980104. [DOI] [PubMed] [Google Scholar]
- LaKind J.S., Brent R.L., Dourson M.L., Kacew S., Koren G., Sonawane B., Tarzian A.J., Uhl K. Human milk biomonitoring data: interpretation and risk assessment issues. J. Toxicol. Environ. Health. 2005;68(20):1713–1769. doi: 10.1080/15287390500225724. [DOI] [PubMed] [Google Scholar]
- Landrigan P.J., Kimmel C.A., Correa A., Eskenazi B. Children’s health and the environment: public health issues and challenges for risk assessment. Environ. Health Perspect. 2004;112(2):257–265. doi: 10.1289/ehp.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.H., Jabocs D.R., Porta M. Hypothesis: a unifying mechanism for nutrition and chemicals as lifelong modulators of DNA hypomethylation. Environ. Health Perspect. 2009;117(12):1799–1802. doi: 10.1289/ehp.0900741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker M.P., Bellinger D.C., Crews D., Eskenazi B., Silbergeld E.K., Woodruff T.J., Susser E.S. An approach to assessment of endocrine disruption in the National Children’s Study. Environ. Health Perspect. 2003;111(13):1691–1697. doi: 10.1289/ehp.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung F.-W., Chiang T.-L., Lin S.-J., Fen J.-Y., Chen P.-F., Shu B.-C. Gender differences of children’s developmental trajectory from 6 to 60 months in the Taiwan Birth Cohort Pilot Study. Res. Dev. Disabil. 2010;32(1):100–106. doi: 10.1016/j.ridd.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Luo Z.C., Liu J.M., Fraser W.D. Large prospective birth cohort studies on environmental contaminants and child health – goals, challenges, limitations and needs. Med. Hypotheses. 2010;74(2):318–324. doi: 10.1016/j.mehy.2009.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makri A., Goveia M., Balbus J., Parkin R. Children’s susceptibility to chemicals: a review by developmental stage. J. Toxicol. Environ. Health B Crit. Rev. 2004;7(6):417–434. doi: 10.1080/10937400490512465. [DOI] [PubMed] [Google Scholar]
- Makris S.L., Thompson C.M., Euling S.Y., Selevan S.G., Sonawane B. A life stage-specific approach to hazard and dose–response characterization for children’s health risk assessment. Birth Defects Res. B Dev. Reprod. Toxicol. 2008;86(6):530–546. doi: 10.1002/bdrb.20176. [DOI] [PubMed] [Google Scholar]
- Mamabolo R.L., Alberts M., Mbenyane G.X., Steyn N.P., Nthangeni N.G., Delemarre-van de Waal H.A., Levitt N.S. Feeding practices and growth of infants from birth to 12 months in the central region of the Limpopo Province of South Africa. Nutr. Afr. 2004;20(3):327–333. doi: 10.1016/j.nut.2003.11.011. [DOI] [PubMed] [Google Scholar]
- McMillen I.C., MacLaughlin S.M., Muhlhausler B.S., Gentili S., Duffield J.L., Morrison J.L. Developmental origins of adult health and disease: the role of periconceptional and foetal nutrition. Basic Clin. Pharmacol. Toxicol. 2008;102(2):82–89. doi: 10.1111/j.1742-7843.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- Mendola P., Selevan S.G., Gutter S., Rice D. Environmental factors associated with a spectrum of neurodevelopmental deficits. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8(3):188–197. doi: 10.1002/mrdd.10033. [DOI] [PubMed] [Google Scholar]
- Miyahara J., Meyers C. Early learning and development standards in East Asia and the Pacific: experiences from eight countries. Int. J. Early Child. 2008;40(2):17–31. [Google Scholar]
- Montgomery M., Mathee A. A preliminary study of residential paint lead concentrations in Johannesburg. Environ. Res. 2004;98:279–283. doi: 10.1016/j.envres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Muruka C., Muruka A. Guidelines for environmental health management in children’s homes in sub-Sahara Africa. Int. J. Environ. Res. Public Health. 2007;4(4):319–331. doi: 10.3390/ijerph200704040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCS, 2011. Briefing material for the National Children’s Study Environmental Summit, May 2011, Bethesda, Maryland. Available from: <http://www.nationalchildrensstudy.gov/research/workshops/Pages/child-development-Stages.pdf> [accessed November 30, 2011].
- Needham L.L., Sexton K. Introduction and overview assessing children’s exposure to hazardous environmental chemicals: an overview of selected research challenges and complexities. J. Expo. Anal. Environ. Epidemiol. 2000;10:611–629. doi: 10.1038/sj.jea.7500142. [DOI] [PubMed] [Google Scholar]
- Neri M., Bonassi S., Knudsen L.E., Sram R.J., Holland N., Ugolini D., Merlo D.F. Children’s exposure to environmental pollutants and biomarkers of genetic damage. Overview and critical issues. Mutat. Res. 2006;612(1):1–13. doi: 10.1016/j.mrrev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Olshan A.F., Anderson L., Roman E., Fear N., Wolff M., Whyatt R., Vu V., Diwan B.A., Potischman N. Workshop to identify critical windows of exposure for children’s health: cancer work group summary. Environ. Health Perspect. 2000;108(Suppl. 3):595–597. doi: 10.1289/ehp.00108s3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango A.W., de Onis M., Caroli M., Shah U., Sguassero Y., Redondo N., Carroli B. Field-testing the WHO child growth standards in four countries. J. Nutr. 2007;137(1):149–152. doi: 10.1093/jn/137.1.149. [DOI] [PubMed] [Google Scholar]
- Perera F., Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 2010;31(3):363–373. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl H.R., Abadin H.G. Chemical mixtures: evaluation of risk for child-specific exposures in a multi-stressor environment. Toxicol. Appl. Pharmacol. 2008;233:116–125. doi: 10.1016/j.taap.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Quandt S.A., Hernández-Valero M.A., Grzywacz J.G., Hovey J.D., Gonzales M., Arcury T.A. Workplace, household, and personal predictors of pesticide exposure for farmworkers. Environ. Health Perspect. 2006;114(6):943–952. doi: 10.1289/ehp.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röllin H., Mathee A., Levin J., Theodoru P., Wewers F. Blood manganese concentrations among first-grade schoolchildren in two South African cities. Environ. Res. 2005;97:93–99. doi: 10.1016/j.envres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rowlands A.V., Eston R.G. The measurement and interpretation of children’s physical activity. J. Sports Sci. Med. 2007;6:270–276. [PMC free article] [PubMed] [Google Scholar]
- Samet J.M. Risk assessment and child health. Pediatrics. 2004;113(Suppl. 3):952–956. [PubMed] [Google Scholar]
- Savitz D.A., Harlow S.D. Selection of reproductive health end points for environmental risk assessment. Environ. Health Perspect. 1991;90:159–164. doi: 10.1289/ehp.90-1519470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk M., Gundert-Remy U., Heinemeyer G., Olejniczak K., Stahlmann R., Kaufmann W., Bolt H.M., Greim H., von Keutz E., Gelbke H.P., DGPT Children as a sensitive subgroup and their role in regulatory toxicology: DGPT workshop report. Arch. Toxicol. 2003;77(1):2–6. doi: 10.1007/s00204-002-0416-9. [DOI] [PubMed] [Google Scholar]
- Selevan S.G., Kimmel C.A., Mendola P. Identifying critical windows of exposure for children’s health. Environ. Health Perspect. 2000;108(Suppl. 3):451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton K., Callahan M.A., Ryan E.F., Saint C.G., Wood W.P. Informed decisions about protecting and promoting public health: rationale for a national human exposure assessment survey. J. Expo. Anal. Environ. Epidemiol. 1995;5(3):233–256. [PubMed] [Google Scholar]
- Solem B.J., Norr K.F., Gallo A.M. Infant feeding practices of low-income mothers. J. Pediatr. Health Care. 1992;6(2):54–59. doi: 10.1016/0891-5245(92)90121-j. [DOI] [PubMed] [Google Scholar]
- Sood B., Delaney-Black V., Covington C., Nordstrom-Klee B., Ager J., Templin T., Janisse J., Martier S., Sokol R.J. Prenatal alcohol exposure and childhood behavior at age 6–7 years: I. Dose–response effect. Pediatrics. 2001;108(2):e34. doi: 10.1542/peds.108.2.e34. [DOI] [PubMed] [Google Scholar]
- Stevens G.D. Gradients in the health status and developmental risks of young children: the combined influences of multiple social risk factors. Matern. Child Health J. 2006;10(2):187–199. doi: 10.1007/s10995-005-0062-y. [DOI] [PubMed] [Google Scholar]
- Thompson K.M. Developing univariate distributions from data for risk analysis. Hum. Ecol. Risk Assess. 1999;5(4):755–783. [Google Scholar]
- Tulve N.S., Suggs J.C., McCurdy T., Cohen Hubal E.A., Moya J. Frequency of mouthing behavior in young children. J. Expo. Anal. Environ. Epidemiol. 2002;12(4):259–264. doi: 10.1038/sj.jea.7500225. [DOI] [PubMed] [Google Scholar]
- US EPA, 2001. Summary Report of the Technical Workshop on Issues Associated with Considering Developmental Changes in Behavior and Anatomy When Assessing Exposure to Children. U.S. Environmental Protection Agency, Risk Assessment Forum, Washington, DC (630/R-00/005).
- US EPA, 2005a. Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC (EPA/630/R-03/003F).
- US EPA, 2005b. Guidance on Selecting Age Groups for Monitoring and Assessing Childhood Exposures to Environmental Contaminants. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC (EPA/630/P-03/003F).
- US EPA, 2006. A Framework for Assessing Health Risks of Environmental Exposures to Children. National Center for Environmental Assessment. Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC (EPA/600/R-05/093F).
- US EPA . US Environmental Protection Agency; Washington, DC: 2011. Exposure Factors Handbook. [Google Scholar]
- US FDA, 2000. Guidance for Industry: E11 Clinical Investigation of Medicinal Products in the Pediatric Population. Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research, Food and Drug Administration, U.S. Department of Health and Human Services, Rockville, MD.
- Walker B. Pediatric environmental health. J. Natl Med. Assoc. 2005;97(2):262–269. [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Bellinger D.C. Social ecology of children’s vulnerability to environmental pollutants. Environ. Health Perspect. 2006;114(10):1479–1485. doi: 10.1289/ehp.9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J.C.K. The thrifty phenotype as an adaptive maternal effect. Biol. Rev. 2007;82(1):143–172. doi: 10.1111/j.1469-185X.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- West L.J. Defining critical windows in the development of the human immune system. Hum. Exp. Toxicol. 2002;21(9–10):499–505. doi: 10.1191/0960327102ht288oa. [DOI] [PubMed] [Google Scholar]
- WHO, 2006. Principles for Evaluating Health Risks in Children Associated with Exposure to Chemicals. International Programme on Chemical Safety, World Health Organization, Geneva (Environmental Health Criteria 237).
- Wigle D.T., Arbuckle T.E., Turner M.C., Bérubé A., Yang Q., Liu S., Krewski D. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J. Toxicol. Environ. Health. 2008;11(5–6):373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- Williams P.R., Patterson J., Briggs D.W. VCCEP pilot: progress on evaluating children’s risks and data needs. Risk Anal. 2006;26(3):781–801. doi: 10.1111/j.1539-6924.2006.00766.x. [DOI] [PubMed] [Google Scholar]
- Wilson N.K., Chuang J.C., Iachan R., Lyu C., Gordon S.M., Morgan M.K., Ozkaynak H., Sheldon L.S. Design and sampling methodology for a large study of preschool children’s aggregate exposures to persistent organic pollutants in their everyday environments. J. Expo. Anal. Environ. Epidemiol. 2004;14(3):260–274. doi: 10.1038/sj.jea.7500326. [DOI] [PubMed] [Google Scholar]
- Worthman C.M., Kuzara J. Life history and the early origins of health differentials. Am. J. Hum. Biol. 2005;17(1):95–112. doi: 10.1002/ajhb.20096. [DOI] [PubMed] [Google Scholar]
- Woywodt A., Kiss A. Geophagia: a history of earth-eating. J. R. Soc. Med. 2002;95(3):143–146. doi: 10.1258/jrsm.95.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J., Zartarian V., Moya J., Freeman N., Beamer P., Black K., Tulve N., Shalat S. A meta-analysis of children’s hand-to-mouth frequency data for estimating nondietary ingestion exposure. Risk Anal. 2007;27(2):411–420. doi: 10.1111/j.1539-6924.2007.00893.x. [DOI] [PubMed] [Google Scholar]