Abstract
Streptococcus pyogenes an adapted human pathogen asymptomatically colonizes the nasopharynx, among other polymicrobial communities. However, information on the events leading to the colonization and expression of virulence markers subject to interspecies and host-bacteria interactions are limited. The interference of acyl homoserine lactones (AHLs) with the hemolytic activity and viability of S. pyogenes M6 S165 was examined. AHLs, with fatty acid side chains ≥12 carbon atoms, inhibited hemolytic activity by downregulating the expression of the sag operon involved in the production of streptolysin S. Inhibitory AHLs upregulated the expression of transcriptional regulator LuxR. Electrophoretic mobility shift assays revealed the interaction of LuxR with the region upstream of sagA. AHL-mediated bactericidal activity observed at higher concentrations (mM range) was an energy-dependent process, constrained by the requirement of glucose and iron. Ferrichrome transporter FtsABCD facilitated transport of AHLs across the streptococcal membrane. The study demonstrates a previously unreported role for AHLs in S. pyogenes virulence.
S. pyogenes is a common colonizer of the nasopharynx, a site inhabited by a plethora of other opportunistic Gram-positive and Gram-negative bacteria1,2. The bacterium is an adapted human pathogen, and colonization is usually asymptomatic. The bacterium can also readily colonize the skin; the other infrequent sites of colonization include the gastrointestinal tract and the lower female genital tract3. However, in certain cases, this bacterium is the causative agent of severe pharyngitis, streptococcal toxic shock syndrome, and necrotizing fasciitis4. Infections caused by S. pyogenes are epidemic and worldwide; the past decade has seen a steep increase in streptococcal infections5,6. The bacterium possesses the machinery to modulate virulence factors enabling adherence, invasion, and spread within the human host7.
In opportunistic pathogens, the expression of virulence factors is tightly regulated, a feat achieved by the phenomenon of quorum sensing8. The S. pyogenes quorum sensing systems that control various virulence attributes are categorized into the LuxS/AI-2, Sil, lantibiotics, and Rgg systems9,10. Canonically, the bacteria detect the quorum sensing molecule that they synthesize and thus generate a coordinated response11. However, in a polymicrobial community, several species of bacteria have been shown to detect the signaling molecules that they did not synthesize, a process known as eavesdropping12,13,14. Through the process of interspecies signaling, bacteria can sense the surrounding populations in a polymicrobial environment. Quorum sensing is an essential part of the interspecies competition, and the process of interspecies signaling/eavesdropping keeps competitors in check in a polymicrobial setting15. Therefore, interspecies strategies that interfere with quorum sensing signals can be explored to develop new-generation antimicrobials. In addition, data on interspecies quorum sensing signaling interference will provide insights into bridging the gap in knowledge regarding asymptomatic colonization.
The secretion of AHLs and use of quorum sensing molecules in the regulation of various virulence phenotypes have been reported for Gram-negative bacteria16. However, a documented role for AHLs in the pathogenesis of Gram-positive bacteria is lacking. Therefore, the present study aimed to investigate the role of AHLs in the regulation of S. pyogenes M6 S165 virulence and growth.
Results
AHLs inhibit the hemolytic activity of S. pyogenes M6 S165
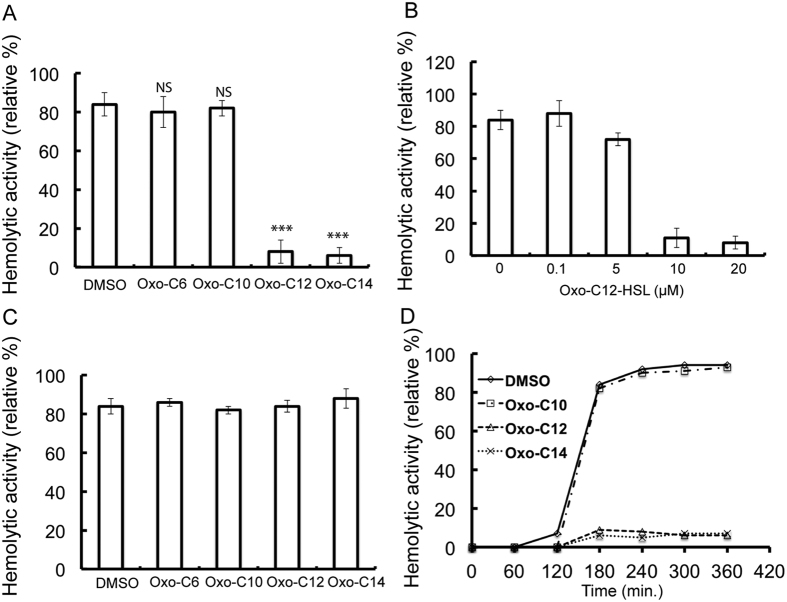
The present study investigated the effect of AHLs on the hemolytic activity of S. pyogenes M6 S165. S. pyogenes M6 S165 was cultured in the presence of different AHLs, which varied in the number of carbon atoms in their fatty acid side chain, and then assayed for hemolytic activity. The AHLs with fatty acid side chains containing ≥12 carbon atoms significantly (P < 0.001) inhibited the hemolytic activity of S. pyogenes M6 S165 (Fig. 1A). The inhibitory effect of the AHLs fell within the micromolar concentration range (Fig. 1B). The SLS-mediated hemolytic activity was confirmed using trypan blue as SLS gets inhibited in the presence of trypan blue17. The hemolytic activity noted in the growth supernatant was completely abolished by the addition of trypan blue (Supplementary Figure S1), indicating that streptolysin S (SLS) was the primary erythrolysin present in the supernatant. To determine if the inhibitory AHLs inactivated the SLS secreted in the growth media, the supernatant from the growth of S. pyogenes M6 S165 was mixed with different AHLs (20 μM) and assayed for hemolytic activity. A noticeable decrease in hemolytic activity was not observed, suggesting that AHLs did not inactivate the secreted SLS (Fig. 1C). Furthermore, the reduction in the S. pyogenes M6 S165 hemolytic activity due to AHLs could not be attributed to a defect in the growth rate (Supplementary Figure S1). The decrease in hemolytic activity by the inhibitory AHLs was not transient, but rather it was observed throughout the growth phase (Fig. 1D). These results indicate that observed inhibitory effects of oxo-C12-HSL and oxo-C14-HSL on hemolytic activity was not through inactivation of secreted SLS.
Figure 1. Effect of oxo-AHL on the hemolytic activity of S. pyogenes.
(A) Hemolytic activity of S. pyogenes M6 S165 grown in the presence of different oxo-AHLs (20 μM). (B) Hemolytic activity of S. pyogenes M6 S165 co-incubated with different concentrations of oxo-C12-HSL. (C) S. pyogenes M6 S165 was grown in the presence of varying concentrations of oxo-C12-HSL, and the supernatant from the growth was analyzed for hemolytic activity. (D) Hemolytic activity of S. pyogenes M6 S165 in the presence of inhibitory oxo-AHLs during different growth stages. The hemolytic activity is measured in relative to caused by water.
AHLs interfere with the expression of the sag operon
For group A Streptococcus, the contiguous nine-gene sag operon (sagABCDEFGHI) encodes the functional SLS18,19. The effect of AHLs on the expression of the sag operon was monitored by qPCR. A significant (P < 0.05) decrease in the expression of sagA was observed when S. pyogenes M6 S165 was cultured in the presence of oxo-C12-HSL (10 μM) and oxo-C14-HSL (10 μM) (Fig. 2A). However, the sagA expression was not altered when S. pyogenes M6 S165 was cultured in the presence of oxo-C10-HSL. Thus, a decrease in the sagA transcripts was detected only in S. pyogenes M6 S165 cultured with the inhibitory AHLs. The transcript levels of the slo gene encoding streptolysin O remained unaffected (Fig. 2B), thereby indicating that the inhibition of the hemolytic activity due to the oxo-AHLs is the result of a decrease in SLS. To further determine if oxo-C12-HSL and oxo-C14-HSL inhibited the expression of SLS at the transcriptional level, a promoter assay utilizing a fusion of the promoter region of the sag operon to a promoterless luciferase reporter gene was conducted20. A significant (P < 0.001) decrease in luciferase activity was observed for the luciferase gene-containing S. pyogenes M6 S165 cultured in the presence of oxo-C12-HSL (10 μM) and oxo-C12-HSL (10 μM) (Fig. 2C). These results suggested that oxo-C12-HSL and oxo-C14-HSL inhibit the hemolytic activity of S. pyogenes M6 S165 by regulating the expression of the sag operon.
Figure 2. Oxo-AHLs inhibit transcription of saga.
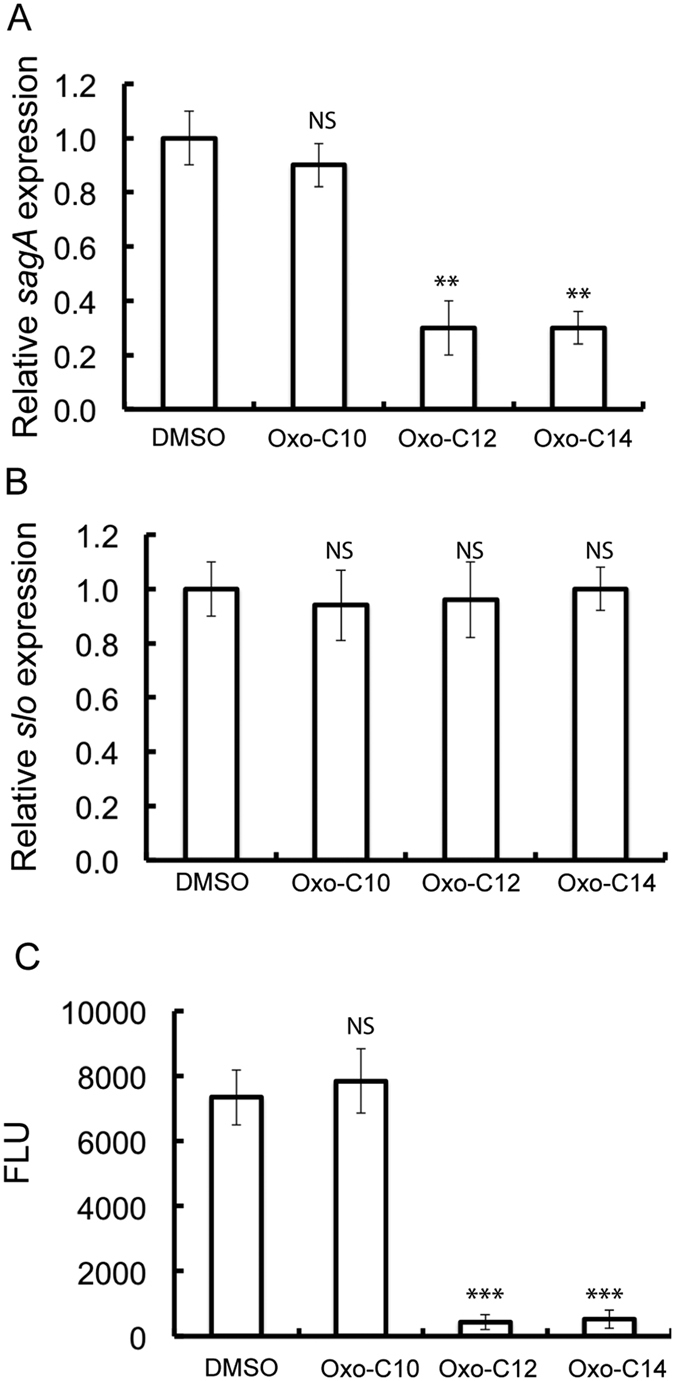
(A) Expression of sagA during co-incubation with inhibitory oxo-AHLs. (B) Expression of slo during co-incubation with inhibitory oxo-AHLs. (C) Luciferase assay to determine the effect of inhibitory oxo-AHLs on the promoter activity of sagA.
P. aeruginosa growth supernatant inhibits the hemolytic activity of S. pyogenes M6 S165
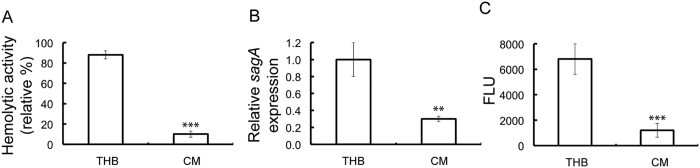
P. aeruginosa has been shown to secrete AHLs with varying fatty acid side chains. Conditioned media (CM) was prepared from the growth supernatant of P. aeruginosa21,22,23. The growth of S. pyogenes M6 S165 was not affected in the CM (Supplementary Figure S3). A significant (P < 0.001) decrease in the hemolytic activity of S. pyogenes M6 S165 grown in CM compared to S. pyogenes M6 S165 grown in THB was observed (Fig. 3A). The expression of sagA was significantly (P < 0.05) reduced in S. pyogenes M6 S165 cultured in CM compared to S. pyogenes M6 S165 cultured in THB (Fig. 3B). In addition, a significant (P < 0.001) reduction in the luciferase activity of S. pyogenes M6 S165 harboring the sagA promoter fused to the luciferase reporter gene was observed when S. pyogenes M6 S165 was cultured in CM (Fig. 3C). Therefore, the CM obtained from the growth of P. aeruginosa inhibits the SLS-mediated hemolytic activity of S. pyogenes M6 S165.
Figure 3. P. aeruginosa growth supernatant inhibits the hemolytic activity of S. pyogenes.
(A) Hemolytic activity of S. pyogenes M6 S165 grown in THB and CM obtained from growth of P. aeruginosa. (B) Expression of sagA in S. pyogenes M6 S165 grown in THB and CM obtained from growth of P. aeruginosa. (C) Luciferase assay to determine the effect of CM on the promoter activity of sagA.
Inhibition of the hemolytic activity due to AHLs is strain-specific
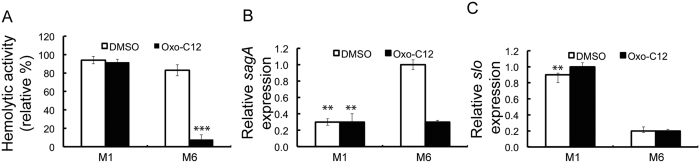
The effect of AHLs on the hemolytic activity of S. pyogenes M1 strain was examined and no decrease was recorded in the presence of oxo-C12-HSL (10 μM) (Fig. 4A). In addition, the sagA transcript levels of S. pyogenes M1 grown in the presence of oxo-C12-HSL (10 μM) remained unaffected (Fig. 4B). The change in the expression of sagA due to oxo-C12-HSL (10 μM) was observed only for S. pyogenes M6 S165. However, the transcript levels of sagA were significantly (P < 0.05) lower in S. pyogenes M1 compared to those of S. pyogenes M6 S165 (Fig. 4B). In addition, a significantly (P < 0.05) higher amount of slo transcripts were detected in S. pyogenes M1 compared to the amount detected in S. pyogenes M6 S165 (Fig. 4C). The transcript level of slo remained unaffected for both strains grown in the presence of oxo-C12-HSL (10 μM). Altogether, these results demonstrated that the slo-mediated hemolytic activity predominates in S. pyogenes M1 and is unaffected by AHL. Therefore, the AHL-mediated inhibition of the hemolytic activity can be attributed to the reduced expression of SLS.
Figure 4. Strain specificity in the inhibition of hemolytic activity due to oxo-AHLs.
(A) Hemolytic activity of two different strains of S. pyogenes in the presence of oxo-AHLS. (B) Expression of sagA in S. pyogenes M1 and M6 during co-incubation with oxo-C12-HSL. (C) Expression of slo in S. pyogenes M1 and M6 during co-incubation with oxo-C12-HSL.
AHLs influence the expression of luxR and the intracellular iron concentration
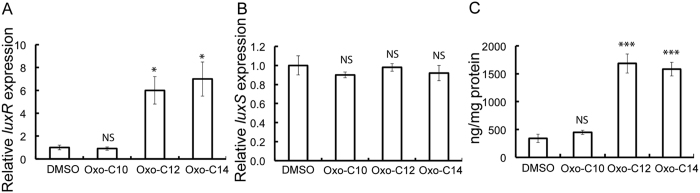
The role of AHLs in the activation of the transcriptional regulator LuxR and influx of iron was examined. It has been demonstrated that expression and activity of LuxR family of transcriptional regulators are affected by AHLs24. Therefore, we investigated if the AHLs have an influence on the expression of luxR. The genome sequence of strain S. pyogenes MGAS 10394 from the NCBI database was used for reference. The gene M6_Spy1777 has been annotated as LuxR in MGAS 10394 and some other S. pyogenes strains, the protein has DNA binding domain. It has been shown that AHLs generate the dissociation product, tetramic acid, with an ability to bind iron25. Therefore we speculate that AHLs could result in increased levels of intracellular iron. The expression of luxR and luxS in S. pyogenes M6 S165 was assessed by qPCR. A significant (P < 0.05) increase in the expression of luxR was observed in S. pyogenes M6 S165 grown in the presence of oxo-C12-HSL (10 μM) and oxo-C14-HSL (10 μM) (Fig. 5A), whereas the presence of oxo-C10-HSL (10 μM) had no effect on luxR expression. The expression levels of luxS remained unaltered in S. pyogenes M6 S165 grown in the presence of any of the AHLs (Fig. 5B). A marked (P < 0.001) increase in the intracellular iron concentration in S. pyogenes M6 S165 grown in the presence of oxo-C12-HSL (10 μM) and oxo-C14-HSL (10 μM) was observed (Fig. 5C). These results demonstrate an increase in the transcriptional activity of luxR in addition to a heavy influx of intracellular iron.
Figure 5. Effect of oxo-AHLs on the transcriptional activity of luxR and luxS and the total intracellular iron content.
(A) Expression of luxR during co-incubation with different oxo-AHLs. (B) Expression of luxS during co-incubation with different oxo-AHLs. (C) Total intracellular iron content during co-incubation with different oxo-AHLs.
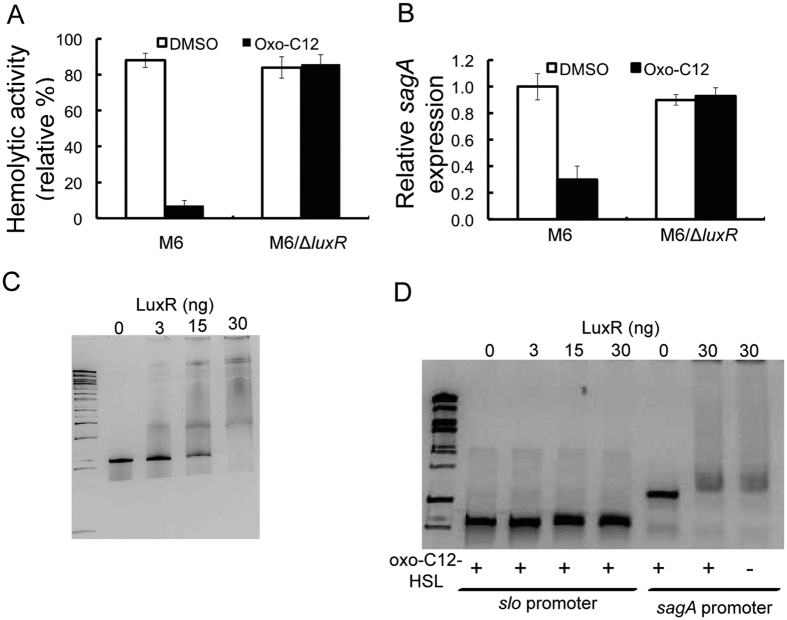
Role of LuxR in the AHL-mediated inhibition of SLS activity
To verify the role of LuxR in the regulation of the sag operon, an insertional mutant construct for the luxR gene was established in S. pyogenes M6 S165. The hemolytic activity of the luxR mutant strain grown in the presence of oxo-C12-HSL remained unaffected (Fig. 6A). Moreover, the transcript levels of sagA in the luxR mutant strain grown in the presence of oxo-C12-HSL were unaltered (Fig. 6B). The role of luxR in the regulation of the sag operon was also examined by EMSA. The 334 bp probe contained the sequence of the upstream segment of the initiation sequence of sagA. Purified LuxR was found to interact with the promoter region of sagA (Fig. 6C). No interaction of LuxR with the DNA from slo promoter region was detected from the gel shift assay (Fig. 6D). It was also found that oxo-C12-HSL was not required for binding of LuxR to DNA from the promoter region of sagA (Fig. 6D). Furthermore, when luxR was expressed under a constitutive promoter of gyrA inhibition in the hemolytic activity was observed (Supplementary Figure 4). However, there was a slight reduction in growth of the S. pyogenes M6 165 strain harboring the luxR construct under gyrA promoter (Supplementary Figure S4). Thus, LuxR negatively regulates the expression of the sag operon.
Figure 6. Role of luxR in the oxo-AHL-mediated inhibition of SLS activity.
(A) Hemolytic activity of S. pyogenes M6 S165 and its luxR mutant grown in the presence of oxo-C12-HSL. (B) Expression of sagA in S. pyogenes M6 S165 and its luxR mutant grown in the presence of oxo-C12-HSL. (C) EMSA to demonstrate the binding of luxR to the promoter region of sagA. (D) EMSA to demonstrate the bindiding of luxR is specific to DNA from the promoter region of sagA and not affected by AHL. No gel shift for DNA from slo promoter is observed when incubated with oxo-C12-HSL (10 μM). Also, sagA promoter a gel shift was observed even in the absence of oxo-C12-HSL.
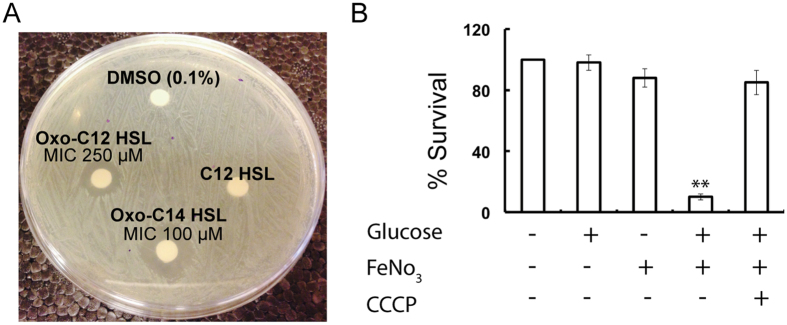
Higher concentrations of AHLs inhibit the growth of S. pyogenes M6 S165
The minimum inhibitory concentrations (MICs) of AHLs against S. pyogenes M6 S165 were determined by the serial dilution assay. The MICs of oxo-C12-HSL and oxo-C14-HSL were 250 and 100 μM, respectively. The disk diffusion assay was used to analyze the effect of AHLs on S. pyogenes M6 S165 growth. Oxo-C12-HSL (250 μM) and oxo-C14-HSL (100 μM) inhibited the growth of S. pyogenes M6 S165 (Fig. 7A), while C12-HSL (250 μM) did not exhibit growth inhibition. This suggests that the antimicrobial effect was not due to the fatty acid side chain. Therefore, to determine if Oxo-C12-HSL exert bactericidal effect on S. pyogenes M6 S165 a survival assay was performed. A survival assay, which used PBS, was then conducted to assess the conditions required for the bactericidal activity of oxo-C12-HSL (Fig. 7B). Oxo-C12-HSL (1 mM) in PBS alone was not found to be lethal to S. pyogenes M6 S165. Moreover, in the presence of either glucose or FeNO3, the survival of S. pyogenes was unaffected by oxo-C12-HSL (1 mM). However, in the case of co-incubation with both glucose and FeNO3, the survival of S. pyogenes M6 S165 decreased by 95%. The addition of carobonyl cyanide m-chlorophenyl hydrazine (CCCP), a chemical inhibitor of oxidative phosphorylation, abrogated the bactericidal activity of oxo-C12-HSL even when PBS was supplemented with both glucose and FeNO3, thereby indicating that the bactericidal activity of oxo-C12-HSL was dependent on the membrane potential.
Figure 7. Bactericidal effect of oxo-AHLs.
(A) Disk diffusion assay to demonstrate the inhibition of S. pyogenes M6 S165 growth by the oxo-AHLs. (B) Effect of glucose and iron on the S. pyogenes M6 S165 growth inhibition by oxo-C12-HSL.
Similar results were also obtained by the M1 strain of S. pyogenes (Supplementary figure S2). Thus, high concentrations of AHLs inhibit the growth of both S. pyogenes M1 and M6 strains reliant on the availability of glucose and iron.
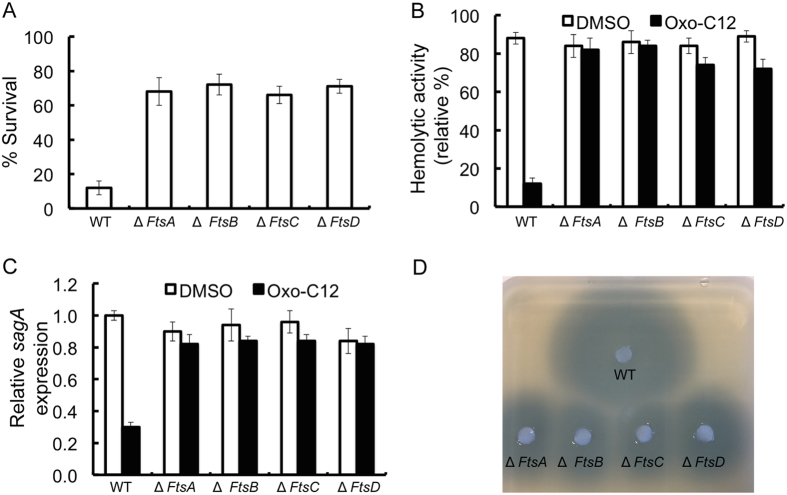
Ferrichrome transporter FtsABCD is involved in the uptake of AHLs by S. pyogenes M6 S165
To investigate the transporter involved in the uptake of AHLs, we constructed an insertional mutants for the ferrichrome transporter FtsABCD in S. pyogenes M6 S165. The bactericidal effect of oxo-C12-HSL (1 mM) was nullified in all the S. pyogenes M6 S165 FtsABCD mutants (Fig. 8A). The inhibitory effect of oxo-C12-HSL on hemolytic activity was also lost in all of the S. pyogenes M6 S165 FtsABCD mutants (Fig. 8B). Additionally, transcript levels of sagA in the S. pyogenes M6 S165 FtsABCD mutants grown in the presence of oxo-C12-HSL were unchanged (Fig. 8C). An Agrobacterium tumefaciens-based bioassay was conducted to estimate the intracellular concentration of oxo-C12-HSL in the wild type S. pyogenes and FtsABCD mutants cultured in the presence 20 μM oxo-C12-HSL (Fig. 8D). The bioassay revealed the presence of lower intracellular amounts of oxo-C12-HSL in the FtsABCD mutants compared to concentrations present in the wild type. This data suggests a potential role for FtsABCD in the uptake of AHLs.
Figure 8. Role of the ferrichrome transporter in the oxo-AHL-mediated killing of S. pyogenes.
(A) Survival assay of S. pyogenes M6 S165 and its ferrichrome transporter mutants in the presence of oxo-C12-HSL. (B) Hemolytic activity of S. pyogenes M6 S165 and its ferrichrome transporter mutants grown in the presence of oxo-C12-HSL. (C) Expression of sagA in S. pyogenes M6 S165 and its ferrichrome transporter mutants grown in the presence of oxo-C12-HSL. (D) Bioassay to determine the intracellular accumulation of oxo-C12-HSL in S. pyogenes M6 S165 and its ferrichrome transporter mutants grown in the presence of oxo-C12-HSL.
Discussion
The current study aimed to investigate the role of AHLs, a group of quorum sensing molecules released by several members of the host microbiota, in the virulence of S. pyogenes. We demonstrated that the AHLs with fatty acid side chains of ≥12 carbon atoms inhibit S. pyogenes M6 S165 SLS-mediated hemolytic activity by negatively regulating the expression of the sag operon. Furthermore, the study revealed that the transport of AHLs across the streptococcal cell membrane is an energy driven process facilitated by the ferrichrome transporter FtsABCD.
S. pyogenes encodes SLS, which is a non-immunogenic post-translationally modified secreted virulence peptide with the ability to lyse mammalian erythrocytes19. In addition, SLS impairs the membranes of lymphocytes, neutrophils, platelets, lysosomes, and mitochondria26,27,28,29, clearly playing a vital role in streptococcal virulence. We, therefore, investigated the role of AHLs in the expression of SLS. In the present study, we found that SLS was the primary hemolysin secreted into the growth media, and its expression was detected towards the latter part of the exponential phase, indicating a cell-density-dependent phenomenon. A similar cell density-dependent expression of SLS was previously reported and speculated to act as a quorum sensing molecule in S. pyogenes30,31. The SLS-mediated hemolytic activity was completely abolished only when S. pyogenes M6 S165 was cultured in media containing oxo-C12-HSL or oxo-C14-HSL. The presence of oxo-C6-HSL or oxo-C10-HSL in the growth media had no inhibitory effect on hemolytic activity. The data from the qPCR analysis and transcriptional fusion assays revealed that the inhibitory AHLs decreased the hemolytic activity by negatively regulating the promoter activity of the sag operon, thereby downregulating the expression of SLS. The inhibitory effect of the AHLs was found to be specific towards SLS, as revealed by the data obtained from the S. pyogenes M1 strain, which predominantly produced SLO in the THB growth media used in the hemolytic assay experiments. The sag operon is highly conserved among group A Streptococcus19. Therefore, ambiguity in the strain-specific secretion of SLS into the THB growth media remains unresolved and requires further investigation. Together, this study provides experimental evidence that the AHLs with fatty acid side chains of ≥12 carbon atoms specifically inhibit (within the micromolar range) the SLS-mediated hemolytic activity of S. pyogenes M6 S165 without altering the growth rate. A similar effect of AHLs with fatty acid side chains of ≥12 carbon atoms has been reported in Staphylococcus aureus wherein AHLs interact with the cytoplasmic membrane and downregulate the exotoxin production and agr mediated quorum sensing32.
The observations suggest a scenario wherein S. pyogenes, when colonizing in the vicinity of microbes capable of secreting AHLs with fatty acid side chains of ≥12 carbon atoms, will be attenuated in its ability to produce the virulence marker SLS. P. aeruginosa is reported to secrete a variety of AHLs including oxo-C12-HSL and oxo-C14-HSL21,22. In addition, P. aeruginosa is a frequent colonizer of the respiratory tract, identified as the causative agent of chronic lung infections and the most prevalent pathogen associated with cystic fibrosis2,33. Therefore, it was speculated that the CM obtained from the growth of P. aeruginosa should negatively affect the hemolytic activity of S. pyogenes. To confirm this hypothesis, the supernatant from the culture of S. pyogenes M6 S165 in CM was analyzed for hemolytic activity. The CM from P. aeruginosa was able to inhibit S. pyogenes M6 S165 SLS-mediated hemolytic activity by downregulating the expression of sagA.
To gain mechanistic insights into the inhibition of SLS-mediated hemolytic activity by AHLs, the roles of luxR, luxS, and the intracellular iron concentration were examined. One of the mechanisms in bacterial quorum sensing involves the synthesis of the autoinducers through the evolutionarily conserved LuxI family of autoinducer synthases and its homologs, while the responses towards particular autoinducers are generated through the evolutionary conserved LuxR family of transcriptional regulators24,34. In S. pyogenes, luxS is involved in the synthesis of the quorum sensing molecule autoinducer 235. In the present study, an increase in the transcript levels of luxR was observed for S. pyogenes M6 S165 grown in the presence of oxo-C12-HSL and oxo-C14-HSL. However, no effect on the expression of luxS was observed. The SLS-mediated hemolytic activity and expression of sagA remained unaffected in the S. pyogenes M6 S165 luxR mutant. EMSAs revealed that LuxR could potentially bind to the region upstream of sagA, thereby affecting its promoter activity. Hence, we hypothesize that the inhibitory AHLs induce the expression of the transcriptional regulator LuxR which in turn negatively regulates the sag operon. The production of SLS in S. pyogenes is reportedly influenced by the concentration of iron in the growth medium36; however, the underlying mechanism has yet to be elucidated. Nevertheless, a role for SLS in the acquisition of iron has been proposed37,38. We observed a 3-fold increase in the S. pyogenes M6 S165 intracellular iron concentration grown in the presence of inhibitory AHLs. Hence, the AHL-mediated inhibition of hemolytic activity might be the result of an excess accumulation of intracellular iron.
The growth of S. pyogenes M6 S165 was not affected by either oxo-C12-HSL or oxo-C14-HSL when used in micromolar quantities. However, at higher concentrations, bactericidal effects were observed. A similar bactericidal activity of AHLs affecting Gram-positive bacteria has been reported by Kaufmann et al.25. Davis et al. have demonstrated that oxo-C12-HSL and oxo-C14-HSL are capable to interact with the cellular membrane in the micromolar range and cause changes in the membrane dipole potential39. The survival assay demonstrated that the antibacterial effect of oxo-C12-HSL on S. pyogenes M6 S165 was an energy-driven process with an absolute requirement for glucose and iron. The AHLs generate the dissociation product, tetramic acid, with an ability to bind iron25. In S. pyogenes, the ABC transporter FtsABCD has been implicated as a transporter involved in the uptake of Fe3+ ferrichrome40. Therefore, we sought to determine if FtsABCD participates in the uptake of oxo-C12-HSL. Although, the bioassay was qualitative it provides clue that the ferrichrome transporter FtsABCD was involved in the transport of the oxo-C12-HSL across the membrane. Moreover, FtsABCD mutants were insensitive towards AHL-mediated inhibition of hemolytic activity and killing. The results indicate a potential interaction of oxo-C12-HSL with iron to form a complex, with subsequent uptake facilitated by the ferrichrome transporter FtsABCD.
In summary, this study provides evidence for the role of AHLs in the virulence of the Gram-positive bacteria S. pyogenes M6 S165. We demonstrated that the inhibitory effect of the AHLs on hemolytic activity was due to LuxR-mediated downregulation of the sag operon. In addition, the ferrichrome transporter FtsABCD facilitated the transport of AHLs across the S. pyogenes membrane. However the study is limited with its results based on single strain, it will be intriguing to further investigate differences in SLS/SLO expression profiles in different S. pyogenes strains and the resultant hemolytic activity as affected by different quorum sensing molecules such as AHLs. These intriguing results highlight the importance of additional studies needed to elucidate the role of inter-bacterial communications in the expression of virulence markers.
Methods
Bacterial strains and growth conditions
The clinical S. pyogenes isolates S165 (emm6) and S291 (emm1) are blood isolates from patients with severe invasive streptococcal disease41. The strain Agrobacterium tumefaciens NTL4 (pZLR4) used in AHL bioassays was a kind gift from Prof. Stephen Farrand at the University of Illinois, USA. S. pyogenes was grown on GC agar (Acumedia; Lansing, MI, USA). Liquid cultures of S. pyogenes were grown in Todd Hewitt Broth (THB, Acumedia) at 37 °C and 5% CO2. A. tumefaciens NTL4 (pZLR4) was grown on Luria Agar (Acumedia) with 30 μg/mL of gentamicin at 28 °C. The AHLs (Sigma-Aldrich) oxo-C6-HSL, oxo-C10-HSL, oxo-C12-HSL, and oxo-C14-HSL were added at varying concentration to the THB. Pseudomonas aeruginosa PA01 was a kind gift from Dr. Klaus Udekwu at the Stockholm University, Sweden. The genome sequence of strain S. pyogenes MGAS 10394 from the NCBI database was used for reference in construction of the primers employed in this study.
Hemolytic activity
The assay for hemolytic activity was performed as described previously20. Horse blood (Håtunalab AB, defibrinated) was used to obtain red blood cells (RBCs). Briefly, S. pyogenes was cultured in THB with or without 5 μM AHLs from A600 ≈ 0.1 to A600 ≈ 1.0. The cells were harvested by centrifugation; the supernatant obtained was passed through a 0.2 μm filter and used for the hemolytic assay. The filtrate was diluted 1:10 in THB, mixed with RBCs at a 1:1 ratio, and incubated at 37 °C in 5% CO2 for 1 h. The hemolysis was estimated at A404, and the activity is reported as the percentage relative to water alone. For the hemolytic assays describing the non-transient inhibitory activity of AHLs, the supernatant was obtained every hour until the growth reached the stationary phase.
Quantitative PCR assays
S. pyogenes was cultured in THB with or without AHLs from A600 ≈ 0.1 to A600 ≈ 1.0. The cells were harvested by centrifugation, treated with mutanolysin for 1 h at 37 °C, and subjected to total RNA isolation with the RNeasy Mini Kit (Qiagen). The cDNA was synthesized from 200 ng RNA using SuperScript VILOTM master mix (ThermoFisher Scientific). The primers used for the quantification of sagA, luxS, luxR, and gyrA transcripts are listed in Supplementary Table 1. The PCR reaction conditions were as follows: an initial denaturation step at 95 °C for 10 min and 45 cycles at 95 °C for 15 s and 60 °C for 1 min. The fold change was calculated relative to the housekeeping gene gyrA.
Luciferase assay
The construction of the transcriptional fusion of the sagA promoter to the promoterless firefly luciferase gene used in the present study was previously reported20. The S. pyogenes M6 strain harboring this construct was grown in THB from A600 ≈ 0.1 to A600 ≈ 1.0 with or without AHLs, and the luciferase activity was measured using the Luciferase Assay Kit (Promega) according to the manufacturer’s instructions.
Conditioned media (CM)
The CM from the growth of P. aeruginosa was prepared as described previously42. P. aeruginosa was grown in THB at an initial density of A600 ≈ 0.1 until the culture reached A600 ≈ 1.0. The cells were harvested by centrifugation and the supernatant was filtered through a 0.2 μm filter. The filtrate was adjusted to pH 7.8 and used as CM.
S. pyogenes M6 S165 gene manipulation
The S. pyogenes M6 S165 mutants for slo, luxR, FtsA, FtsB, FtsC, and FtsD were constructed by directed insertional inactivation using the pSPC18 plasmid as described by Lyon et al.43. Briefly, the internal region of interest in the gene was amplified and inserted into the BamHI site of pSPC18. The construct was electroporated into S. pyogenes M6 S165 to obtain the desired gene disruption via homologous recombination, which was confirmed by PCR. The strains were then maintained with 30 μg/mL spectinomycin. For construction of luxR under gyrA promoter, a fusion PCR was performed with the luxR coding sequence and 350 bp fragment upstream of gyrA. The resultant product was inserted into BamHI and NdeI site of the Streptococcus shuttle vector PJRS525 and electroporated to S. pyogenes M6 S16544. The primers used for gene amplification are listed in Supplementary Table 2.
Measurement of intracellular iron
S. pyogenes M6 was cultured in THB with or without AHLs. The cells were harvested at A600 ≈ 1.0 and subjected to intracellular iron measurements using the Iron Assay Kit (Sigma-Aldrich) according to manufacturer’s instructions. The values represent the total iron content in ng/mg protein. The total protein content was estimated by Bradford assay.
Electrophoretic mobility shift assay (EMSA)
LuxR was His-tagged at the N-terminal using the pTrcHis TOPO® TA Expression Kit (Invitrogen, USA). For cloning, a 3075 bp region consisting of the luxR ORF was amplified using the primers luxR-his F 5′-ATGACGAAGGGTATTCGATTTC-3′ and luxR-his R 5′-TTAGTTGTTAGAGGAGAATTGC-3′. The tagged LuxR was purified using standard Ni-NTA agarose (Qiagen) purification protocols. The purity of the protein was assessed by standard SDS-PAGE, and the purified protein was stored in 15% glycerol at −80 °C.
For the EMSAs, a 334 bp region upstream of the sagA start codon was amplified with primers sagA-Shift-F 5′-GGATGAAGTAAAGATATTAGCTAGGG-3′ and sagA-Shift-R 5′-GGTTTACCTCCTTATCTAATAAGTAAC-3′ and a 273 bp region upstream of slo was amplified with primers slo-Shift 5′ ACCCAATTGAAAGCTAACATCG-3′ and slo-Shift-R 5′-TGTTCTTTCGACCATATCAAGCA-3′. The product was purified using DNA Clean & Concentrator™-25 (Zymo Research, USA). The DNA binding assay was conducted in binding buffer (20 mM Tris-HCl, pH 7.4; 50 mM KCl; 1 mM EDTA; 1 mM DTT; 5% glycerol; and 100 μg/mL BSA) as described previously45. The binding buffer was supplemented with 10 μM oxo-C12-HSL. The reactions consisted of 50 ng purified DNA and increasing amounts of N-terminal His-tagged LuxR (3–30 ng).
Survival assay
S. pyogenes was grown in THB and harvested at the mid-exponential phase. The cells were washed three times with PBS and resuspended in PBS at a cell density of 107 cfu/mL; 100 μL of the cell suspension was incubated with 1 mM oxo-C12-HSL in PBS at 37 °C for 3 h. Glucose was added at a final concentration of 0.4%. FeNO3 was used at a final concentration of 25 μM. Following incubation, the suspension was serially diluted and plated on GC agar to determine viability. The minimum inhibitory concentrations for the AHLs were determined by standard disk diffusion assays.
AHL bioassay
The bioassay to measure the uptake of AHLs by S. pyogenes was performed using the A. tumefaciens NTL4 (pZLR4) strain. Although the A. tumefaciens NTL4 (pZLR4) strain does not produce AHLs, it responds to a variety of AHLs46. S. pyogenes was grown in THB with or without 10 μM oxo-C12-HSL. The cells were harvested at a density of A600 ≈ 1.0, treated with mutanolysin at 37 °C for 1 h, and lysed by sonication. Next, 10 μL of the lysate after centrifugation was applied to Whatman filter disks and allowed to dry. 500 μl of the overnight growth of A. tumefaciens NTL4 (pZLR4) was added to LBA cooled to 50 °C supplemented with 25 μg/mL gentamicin and poured on standard petri dishes. The plate was overlaid with 0.7% bacteriological agar containing Bluo-Gal (Sigma-Aldrich). The disk with the lysate was placed on the overlaid agar. The plate was incubated at 37 °C for 16 h, and the precipitation zone due to β-galactosidase activity was measured.
Statistical analysis
All experiments were performed in triplicate and repeated three times. Analysis of variance (ANOVA) and the Student’s t-test were employed to analyze the difference between the groups for statistical significance. P < 0.05 was considered statistically significant. The data is represented as the mean ± standard deviation. The stars in the bar graph denote statistical significance.
Additional Information
How to cite this article: Saroj, S. D. et al. Inhibitory role of acyl homoserine lactones in hemolytic activity and viability of Streptococcus pyogenes M6 S165. Sci. Rep. 7, 44902; doi: 10.1038/srep44902 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank Prof. Stephen K. Farrand, University of Illinois, USA, for providing the Agrobacterium tumefaciens strains used in the bioassay for AHLs. We also extend our sincere gratitude to Prof. Kevin S. McIver, University of Maryland, USA, for providing pKSM720. We thank Prof. Zehava Eichenbaum, Gerogia State University, USA for providing pJRS525. We would like to thank Prof. Michael G. Caparon, Washington University School of Medicine, USA, for providing the pSPC18 plasmid.
Footnotes
The authors declare no competing financial interests.
Author Contributions Conceived and designed the experiments: S.S., A.-B.J. Performed the experiments: S.S. Screening of A.H.L. inhibitory activity: S.S., L.H. Screening of ferrichrome transporter mutants: S.S., J.B. Analyzed the data and wrote the manuscript: S.S., A.-B.J.
References
- Siegel S. J. & Weiser J. N. Mechanisms of Bacterial Colonization of the Respiratory Tract. Annu Rev Microbiol 69, 425–444, doi: 10.1146/annurev-micro-091014-104209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. Colonization and infection of the respiratory tract: What do we know? Paediatr Child Health 9, 21–24 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobbs A. H., Lamont R. J. & Jenkinson H. F. Streptococcus adherence and colonization. Microbiol Mol Biol Rev 73, 407–450, Table of Contents, doi: 10.1128/MMBR.00014-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca-Harari B. et al. Clinical and Microbiological Characteristics of Severe Streptococcus pyogenes Disease in Europe. J Clin Microbiol 47, 1155–1165, doi: 10.1128/Jcm.02155-08 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. S. Y. & Yuen K. Y. Streptococcus pyogenes and re-emergence of scarlet fever as a public health problem. Emerg Microbes Infec 1, doi: 10.1038/emi.2012.9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. L. The flesh-eating bacterium: what’s next? J Infect Dis 179 Suppl 2, S366–374, doi: 10.1086/513851 (1999). [DOI] [PubMed] [Google Scholar]
- Cunningham M. W. Pathogenesis of group A streptococcal infections and their sequelae. Adv Exp Med Biol 609, 29–42 (2008). [DOI] [PubMed] [Google Scholar]
- Rutherford S. T. & Bassler B. L. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2, doi: 10.1101/cshperspect.a012427 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez J. C. & Federle M. J. Quorum sensing in group A Streptococcus. Front Cell Infect Mi 4, doi: 10.3389/fcimb.2014.00127 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes L. C., Ferreira R. B., Buckner M. M. & Finlay B. B. Quorum sensing in bacterial virulence. Microbiology 156, 2271–2282, doi: 10.1099/mic.0.038794-0 (2010). [DOI] [PubMed] [Google Scholar]
- Miller M. B. & Bassler B. L. Quorum sensing in bacteria. Annu Rev Microbiol 55, 165–199, doi: 10.1146/annurev.micro.55.1.165 (2001). [DOI] [PubMed] [Google Scholar]
- Case R. J., Labbate M. & Kjelleberg S. AHL-driven quorum-sensing circuits: their frequency and function among the Proteobacteria. Isme Journal 2, 345–349, doi: 10.1038/ismej.2008.13 (2008). [DOI] [PubMed] [Google Scholar]
- Ryan R. P. & Dow J. M. Diffusible signals and interspecies communication in bacteria. Microbiol-Sgm 154, 1845–1858, doi: 10.1099/mic.0.2008/017871-0 (2008). [DOI] [PubMed] [Google Scholar]
- Smith J. L., Fratamico P. M. & Yan X. Eavesdropping by bacteria: the role of SdiA in Escherichia coli and Salmonella enterica serovar Typhimurium quorum sensing. Foodborne Pathog Dis 8, 169–178, doi: 10.1089/fpd.2010.0651 (2011). [DOI] [PubMed] [Google Scholar]
- Chandler J. R., Heilmann S., Mittler J. E. & Greenberg E. P. Acyl-homoserine lactone-dependent eavesdropping promotes competition in a laboratory co-culture model. ISME J 6, 2219–2228, doi: 10.1038/ismej.2012.69 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga M. E. & Bassler B. L. Chemical communication among bacteria. Proc Natl Acad Sci USA 100, 14549–14554, doi: 10.1073/pnas.1934514100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loridan C. & Alouf J. E. Purification of RNA-core induced streptolysin S, and isolation and haemolytic characteristics of the carrier-free toxin. J Gen Microbiol 132, 307–315, doi: 10.1099/00221287-132-2-307 (1986). [DOI] [PubMed] [Google Scholar]
- Borgia S. M., Betschel S., Low D. E. & de Azavedo J. C. Cloning of a chromosomal region responsible for streptolysin S production in Streptococcus pyogenes. Adv Exp Med Biol 418, 733–736 (1997). [DOI] [PubMed] [Google Scholar]
- Molloy E. M., Cotter P. D., Hill C., Mitchell D. A. & Ross R. P. Streptolysin S-like virulence factors: the continuing sagA. Nat Rev Microbiol 9, 670–681, doi: 10.1038/nrmicro2624 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saroj S. D., Maudsdotter L., Tavares R. & Jonsson A. B. Lactobacilli interfere with Streptococcus pyogenes hemolytic activity and adherence to host epithelial cells. Front Microbiol 7, 1176, doi: 10.3389/fmicb.2016.01176 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. P. et al. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA 91, 197–201, doi: 10.1073/pnas.91.1.197 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. P., Passador L., Iglewski B. H. & Greenberg E. P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA 92, 1490–1494 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson M. K. et al. Multiple N-Acyl-L-Homoserine Lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 92, 9427–9431, doi: 10.1073/pnas.92.20.9427 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K. M. & Garey J. R. The evolution of bacterial LuxI and LuxR quorum sensing regulators. Microbiology 147, 2379–2387, doi: 10.1099/00221287-147-8-2379 (2001). [DOI] [PubMed] [Google Scholar]
- Kaufmann G. F. et al. Revisiting quorum sensing: Discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. P Natl Acad Sci USA 102, 309–314, doi: 10.1073/pnas.0408639102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser H., Weissmann G. & Bernheimer A. W. Studies on lysosomes. Iv. Solubilization of enzymes during mitochondrial swelling and disruption of lysosomes by streptolysin S and other hemolytic agents. J Cell Biol 22, 101–113 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W. & Schwartz L. L. Lysosomal disruption by bacterial toxins. J Bacteriol 87, 1100–1104 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniewicz W. & Pryjma J. Effect of streptolysin S on human and mouse T and B lymphocytes. Infect Immun 16, 730–733 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W. & Schwartz L. L. Effect of staphylococcal and other bacterial toxins on platelets in vitro. J Pathol Bacteriol 89, 209–223 (1965). [DOI] [PubMed] [Google Scholar]
- Betschel S. D., Borgia S. M., Barg N. L., Low D. E. & De Azavedo J. C. Reduced virulence of group A streptococcal Tn916 mutants that do not produce streptolysin S. Infect Immun 66, 1671–1679 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim K. Y., de Azavedo J. C., Bast D. J. & Cvitkovitch D. G. Role for sagA and siaA in quorum sensing and iron regulation in Streptococcus pyogenes. Infect Immun 75, 5011–5017, doi: 10.1128/IAI.01824-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi S. et al. N-acylhomoserine lactones antagonize virulence gene expression and quorum sensing in Staphylococcus aureus. Infect Immun 74, 910–919, doi: 10.1128/IAI.74.2.910-919.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keravec M. et al. Insights into the respiratory tract microbiota of patients with cystic fibrosis during early Pseudomonas aeruginosa colonization. Springerplus 4, doi: 10.1186/s40064-015-1207-0 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua W. C., Winans S. C. & Greenberg E. P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176, 269–275 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marouni M. J. & Sela S. The luxS gene of Streptococcus pyogenes regulates expression of genes that affect internalization by epithelial cells. Infect Immun 71, 5633–5639, doi: 10.1128/Iai.71.10.5633-5639.2003 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths B. B. & Mcclain O. The Role of iron in the growth and hemolysin (Streptolysin S) production in Streptococcus pyogenes. J Basic Microb 28, 427–436, doi: 10.1002/jobm.3620280703 (1988). [DOI] [PubMed] [Google Scholar]
- Bates C. S., Montanez G. E., Woods C. R., Vincent R. M. & Eichenbaum Z. Identification and characterization of a Streptococcus pyogenes operon involved in binding of hemoproteins and acquisition of iron. Infect Immun 71, 1042–1055, doi: 10.1128/Iai.71.3.1042-1055.2003 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum Z., Muller E., Morse S. A. & Scott J. R. Acquisition of iron from host proteins by the group A Streptococcus. Infect Immun 64, 5428–5429 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. M., Jensen R., Williams P. & O’Shea P. The interaction of N-acylhomoserine lactone quorum sensing signaling molecules with biological membranes: implications for inter-kingdom signaling. PLoS One 5, e13522, doi: 10.1371/journal.pone.0013522 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks T. S., Liu M., McClure M. J. & Lei B. ABC transporter FtsABCD of Streptococcus pyogenes mediates uptake of ferric ferrichrome. BMC Microbiol 5, 62, doi: 10.1186/1471-2180-5-62 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjolinder H. et al. The ScpC protease of Streptococcus pyogenes affects the outcome of sepsis in a murine model. Infect Immun 76, 3959–3966, doi: 10.1128/Iai.00128-08 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saroj S. D. & Rather P. N. Streptomycin inhibits quorum sensing in Acinetobacter baumannii. Antimicrob Agents Chemother 57, 1926–1929, doi: 10.1128/AAC.02161-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon W. R., Madden J. C., Levin J. C., Stein J. L. & Caparon M. G. Mutation of luxS affects growth and virulence factor expression in Streptococcus pyogenes. Mol Microbiol 42, 145–157 (2001). [DOI] [PubMed] [Google Scholar]
- McIver K. S. & Scott J. R. Role of mga in growth phase regulation of virulence genes of the group A streptococcus. J Bacteriol 179, 5178–5187 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski A. L., Lostroh C. P. & Greenberg E. P. Reversible acyl-homoserine lactone binding to purified Vibrio fischeri LuxR protein. J Bacteriol 186, 631–637, doi: 10.1128/Jb.186.3.631-637.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z. Q. & Farrand S. K. Cloning and characterization of a tetracycline resistance determinant present in Agrobacterium tumefaciens C58. J Bacteriol 181, 618–626 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.