Abstract
Endometriosis is a major cause of infertility and pelvic pain, affecting more than 10% of reproductive-aged women. Progesterone resistance has been observed in the endometrium of women with this disease, as evidenced by alterations in progesterone-responsive gene and protein expression. cAMP-Response Element-Binding 3-like protein 1 (Creb3l1) has previously been identified as a progesterone receptor (PR) target gene in mouse uterus via high density DNA microarray analysis. However, CREB3L1 function has not been studied in the context of endometriosis and uterine biology. In this study, we validated progesterone (P4) regulation of Creb3l1 in the uteri of wild-type and progesterone receptor knockout (PRKO) mice. Furthermore, we observed that CREB3L1 expression was significantly higher in secretory phase human endometrium compared to proliferative phase and that CREB3L1 expression was significantly decreased in the endometrium of women with endometriosis. Lastly, by transfecting CREB3L1 siRNA into cultured human endometrial stromal cells (hESCs) prior to hormonal induction of in vitro decidualization, we showed that CREB3L1 is required for the decidualization process. Interestingly, phosphorylation of ERK1/2, critical factor for decidualization, was also significantly reduced in CREB3L1-silenced hESCs. It is known that hESCs from patients with endometriosis show impaired decidualization and that dysregulation of the P4-PR signaling axis is linked to a variety of endometrial diseases including infertility and endometriosis. Therefore, these results suggest that CREB3L1 is required for decidualization in mice and humans and may be linked to the pathogenesis of endometriosis in a P4-dependent manner.
Keywords: CREB3L1, Decidualization, Endometrium, Endometriosis, Progesterone, Uterus
Introduction
The uterine endometrium is comprised of epithelial and stromal cell compartments that undergo dynamic molecular and morphological changes to allow for embryo implantation and development. These components are tightly regulated by the ovarian steroid hormones, estrogen (E2) and progesterone (P4) [1]. E2 is known to stimulate uterine epithelial cell proliferation whereas P4 inhibits this E2 effect, presumably through coordination of stromal-epithelial cell cross-talk. The progesterone receptor (PR) is required for successful implantation in both humans and rodents. PR signaling has been shown to regulate implantation-associated events including the development of uterine receptivity and endometrial stromal cell decidualization [2]. Studies utilizing a transgenic mouse model with a null mutation in the PR gene (PRKO) demonstrate the essential role for PR in P4-mediated uterine responses [3, 4], and have led to the identification of several P4-PR- signaling pathways within the uterus [5].
During early pregnancy, endometrial stromal cells are transformed into decidual cells in a P4-dependent manner. Decidualization is unique to species with hemichorial placentation, such as human, non-human primates and rodents and serves to protect the maternal uterus during trophoblast invasion and provide nourishment to the early embryo [6]. Endometrial stromal cells undergoing decidualization become plumper, acquire a secretory epithelioid-like morphology and secrete a variety of factors, including prolactin (PRL) and insulin-like growth factor binding protein 1 (IGFBP1) [7]. Moreover, this transformation results in extensive changes in cellular gene expression, including alterations in steroid hormone receptor, extracellular matrix (ECM) and cytoskeletal gene profiles [8, 9]. Multiple transgenic mouse models have shown that the decidualization process is essential for the maintenance of pregnancy [3, 10, 11].
Imbalances in steroid signaling have been linked with multiple pathologies, including infertility, endometriosis, endometrial carcinoma, polycystic ovarian syndrome, and leiomyomas [12–15]. Each of these diseases displays a characteristic dysregulation of ER and PR expression profiles as well as their downstream target genes. All of these disorders are associated with increased E2 sensitivity, as characterized by decreased E2 metabolism and increased ER expression [12–15]. However, mounting evidence now links P4 dysregulation to these diseases as well. Loss of functional PR signaling has been shown to contribute to P4 resistance, at either the level of PR itself or its downstream target genes, which ultimately can disrupt critical epithelial-stromal cross talk within the uterus [16–18].
Endometriosis, a benign gynecological disease defined as the presence of endometrial cells outside the uterus, seriously impacts the quality of life and reproductive ability of 6–10% of reproductive-aged women and is strongly associated with chronic pelvic pain and infertility [19]. Disrupted steroidal control of uterine cell proliferation as well as abnormal decidualization have been shown in women with this disease [20]. Furthermore, P4 exposure is a negative risk factor for endometriosis [21], as pregnancy or progestin-based therapies are associated with disease regression in some women [22, 23]. However, a subset of women with this disease is non-responsive to frontline progestin treatments. Moreover, P4-induced molecular changes in the eutopic (intrauterine) endometrial tissue of this cohort are either blunted or undetectable. These in vivo observations suggest a resistance to P4 action in endometriosis [24]. Therefore, understanding the role of the progesterone receptor and its target genes will be key to the development of new therapeutic strategies [25, 26].
cAMP-Response Element-Binding 3-like protein 1 (CREB3L1), also known as Old Astrocyte Specifically Induced Substrate (OASIS), is an endoplasmic reticulum unfolded protein response (UPR) transducer [27]. In mammalian cells, the UPR family is comprised of three members: Inositol Requiring Element 1 (IRE1), PKR-like ER kinase (PERK), and Activating Transcription Factor (ATF-6) [27]. CREB3L1 is most structurally similar to ATF-6 [28, 29], a basic leucine zipper (bZIP) transcription factor, and located at the ER membrane under normal conditions. Upon UPR signaling, the N-terminal cytoplasmic domain of CREB3L1 is cleaved, translocates into the nucleus and alters target gene expression [29–31]. CREB3L1 is expressed in a variety of tissues including pancreas, prostate, and intestine [32, 33]. Furthermore, previous studies suggest that CREB3L1 has a role in the differentiation and development of astrocytes, osteoblasts, odontoblasts, pancreatic beta-cells and large intestine goblet cells [28–30, 33–35].
Previous studies have identified Creb3l1 as a PR target gene using high density DNA microarray analysis [36]. However, the function of CREB3L1 in the female reproductive tract is unclear. In this study, we examined the endometrial expression profile of CREB3L1 during the human menstrual cycle as well as its expression in women with and without endometriosis. To investigate the function of CREB3L1, we used the well-characterized in vitro primary human endometrial stromal cell decidualization model. Our results show that attenuation of CREB3L1 expression via small interfering RNA (siRNA) significantly reduced decidualization. We also observed reduced expression of CREB3L1 in the eutopic endometrium of women with endometriosis, as compared to healthy controls, suggesting that loss of CREB3L1 expression is an important factor in the pathogenesis of endometriosis.
Materials and Methods
Animal and tissue collection
Animals were maintained in a designated animal care facility according to the Michigan State University’s Institutional Guidelines for the care and use of laboratory animals. All animal procedures were approved by the Institutional Animal Care and Use Committee of Michigan State University. To examine the P4 regulation of Creb3l1 expression, wild type C57BL/6 mice and PRKO mice at six weeks age were ovariectomized. After two weeks, the mice were given a subcutaneous injection of either vehicle (sesame oil) or P4 (1 mg/mice) (n=3 per genotype). Six hours later, uterine tissues were dissected, flash-frozen and stored at −80°C for RNA /protein extraction and/or fixed in 4% (v/v) paraformaldehyde for immunohistochemistry.
Human endometrium samples
The human endometrial samples used to examine CREB3L1 expression patterns were obtained from Michigan State University’s Center for Women’s Health Research Female Reproductive Tract Biorepository, the Greenville Hospital System, and the University of North Carolina in accordance with the guidelines set by the Institutional Review Boards of Michigan State University (Grand Rapids, MI), Greenville Health System (Greenville, SC) and University of North Carolina (Chapel Hill, NC), respectively. Written informed consent was obtained from all participants. For experiments examining CREB3L1 expression throughout the menstrual cycle, endometrial samples were analyzed from 25 (n=6 proliferative and n=19 secretory) cycling premenopausal women undergoing hysterectomy for benign conditions who were surgically negative for endometriosis and had not been on any hormonal therapies for at least three months prior to surgery. Endometrial menstrual staging was confirmed by an experienced pathologist familiar with female reproduction. To compare CREB3L1 expression patterns in the eutopic endometrium of women with and without endometriosis, 6 control samples were compared to 17 endometriotic samples obtained during the early secretory phase. Samples used for immunohistochemistry were fixed in 10% buffered formalin prior to embedding in paraffin wax.
Immunohistochemistry
Uterine cross sections from paraffin-embedded tissue were cut into 6 μm sections, mounted on silane-coated slides, deparaffinized and rehydrated in a graded alcohol series. Sections were pre-incubated with 10% normal goat serum in phosphate-buffered saline (PBS; pH 7.5) and then incubated with anti-CREB3L1 (Santa Cruz, Santa Cruz, CA) antibody in PBS supplemented with 10% normal goat serum overnight at 4°C. The next day, sections were washed with PBS and incubated with secondary antibody conjugated to horseradish peroxidase (Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. Immunoreactivity was detected using diaminobenzidine (DAB-Vector Laboratories, Burlingame, CA) then counterstained with hematoxylin and coverslipped with permount. Imuunostaining was analyzed using microscopy software from NIS Elements, Inc. (Nikon, Melville, NY).
Human endometrial stromal cell culture and in vitro decidualization
Human endometrial stromal cells (hESCs), obtained from The Michigan State University’s Center for Women’s Health Research Female Reproductive Tract Biorepository with MSU Biological Institutional Review Board approval, were isolated by digesting endometrial tissue samples with DNase I (Sigma-Aldrich, St. Louis, MO) and collagenase (Sigma-Aldrich, St. Louis, MO), followed by filtration. Isolated hESCs were then maintained in phenol red–free RPMI-1640 medium (Gibco, Grand Island, NY) containing 0.1 mM sodium pyruvate (Gibco, Grand Island, NY), 10% fetal bovine serum (FBS; Gibco, Grand Island, NY) depleted of steroids by pre-treatment with dextran-coated charcoal (Sigma Aldrich, St. Louis, MO) (Charcoal-stripped FBS; CS-FBS), and 1% penicillin streptomycin (P/S; Gibco, Grand Island, NY). Cells were cultured in monolayer at 37°C in 5% CO2. To induce in vitro decidualization, cells were washed with PBS and transferred to OPTI-MEM medium (Gibco, Grand Island, NY) containing 2% CS-FBS, 10 nM estradiol (E2; Sigma-Aldrich, St. Louis, MO), 1 mM medroxyprogesterone acetate (MPA; Sigma-Aldrich, St. Louis, MO), 50 μM cAMP (Sigma-Aldrich, St. Louis, MO), and 1% P/S. Differentiation medium was changed every 48 hours for a total of 6 days. For CREB3L1 knockdown, small interfering RNA (siRNA) was obtained from Dharmacon (Lafayette, CO), RNAi Techologies. Human CREB3L1 siRNA was transfected using Lipofectamine 2000 reagent (Invitrogen Crop., Carlsbad, CA) prior to in vitro decidualization.
Immunofluorescence
hESCs were grown on glass coverslips to 90% confluency and subjected to decidualization treatment as described above. Upon completion of treatment, coverslips were washed with PBS, fixed with 4% paraformaldehyde and permeabilized with 0.1% of Triton X-100 (Sigma-Aldrich, St. Louis, MO). After further washing, hESCs were exposed to anti-CREB3L1 (Santa Cruz, Santa Cruz, CA), anti-phospoho-ERK1/2 (Cell Signaling, Danvers, MA), or ERK1/2 (Cell Signaling, Danvers, MA) antibodies overnight at 4°C and secondary antibody for 2 hour at room temperature. Washed coverslips were then mounted onto microscope slides with a DAPI-impregnated mounting media (Vector Laboratories, Burlingame, CA) to enable nuclear visualization and images captured via fluorescent microscopy (Nikon Instruments Inc., Melville, NY) using software from NIS Elements, Inc. (Nikon, Melville, NY).
Western blotting
Cellular proteins were extracted using lysis buffer (10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2.5 mM EDTA, and 0.125% Nonidet P-40 (vol/vol)) supplemented with both a protease inhibitor cocktail (Roche, Indianapolis, IN) and a phosphatase inhibitor cocktail (Sigma Aldrich, St. Louis, MO). Twenty μg of protein lysates were electrophoresed via SDS-PAGE and transferred onto polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA). Membrane was blocked with Casein (0.5% v/v) prior to exposure to anti-phospho ERK1/2 (Cell Signaling, Danvers, MA), anti-ERK1/2 (Cell Signaling, Danvers, MA), or anti-Actin (Santa Cruz, Santa Cruz, CA) antibodies. Immunoreactivity was visualized by incubation with a horseradish peroxidase-linked secondary antibody followed by exposure to Electrochemiluminescence reagents (ECL) according to manufacturer’s instructions (GE Healthcare Biosciences, Piscataway, NJ).
RNA isolation and quantitative real-time PCR
Total RNA was isolated using the RNeasy total RNA isolation kit (Qiagen, Valencia, CA) according to manufacturer’s instruction. As a template for quantitative real-time PCR, cDNAs were synthesized using quantitative PCR random hexamers and MMLV Reverse Transcriptase (Invitrogen Crop., Carlsbad, CA). The expression of CREB3L1, IGFBP1, and PRL were quantified by real-time PCR using a CFX96 Real-time Detection System (Bio-Rad Laboratories, Hercules, CA) and iQ™ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). RPL7 expression was included in each treatment group for normalization. Gene specific primers used are listed in Table 1.
Table 1.
List of gene specific primers used in real-time PCR analysis.
| Species | Gene name | Primer sequence | |
|---|---|---|---|
| Mouse | CREB3L1 | F | 5′-GCCTTCCTGCATTCTCTTCC-3′ |
| R | 5′-CCTCTGGCCAGGTCTCTCTC-3′ | ||
|
| |||
| RPL7 | F | 5′-TCAATGGAGTAAGCCCAAAG-3′ | |
| R | 5′-CAAGAGACCGAGCAATCAAG-3′ | ||
|
| |||
| Human | CREB3L1 | F | 5′-ACAATGCGCACTTTCCTGAG-3′ |
| R | 5′-GAGGGCTCTTCTCATCCAGC-3′ | ||
|
| |||
| IGFBP1 | F | 5′-CTATGATGGCTCGAAGGCTC-3′ | |
| R | 5′-TTCTTGTTGCAGTTTGGCAG -3′ | ||
|
| |||
| PRL | F | 5′-CATCAACAGCTGCCACACTT-3′ | |
| R | 5′-CGTTTGGTTTGCTCCTCAAT-3′ | ||
|
| |||
| RPL7 | F | 5′-AAGAAGCGAATTGCTTTGAC-3′ | |
| R | 5′-CAAATCCTCCATGCAGATGA-3′ | ||
Statistical Analysis
This semiquantitative approach was used to generate a H-Score for each immunohistochemistry sample: the percentage of positive tumor cells per slide (0%–100%) was multiplied by intensity strength of staining (0, negative or trace; 1, weak; 2, moderate; 3, intense). The overall score ranged from 0 to 300. Statistical analysis were performed using one-way ANOVA analysis Tukey’s post hoc multiple range test or Student’s t-tests using the Instat package from GraphPad (San Diego, CA). p<0.05 was considered statistically significant.
Results
Creb3l1 is identified as P4 and PR target gene in the murine uterus
P4-PR signaling is critical to implantation, decidualization, and glandular development [3, 37]. Creb3l1 was previously identified as a PR target gene via high density DNA microarray analysis [36]. To validate whether Creb3l1 is a P4-PR target gene, ovariectomized wild-type or PRKO mice were treated with vehicle (sesame oil) or P4 for 6 hours, then the levels of Creb3l1 mRNA were examined using real-time PCR. As shown Figure 1A, the expression of Creb3l1 mRNA was significantly increased in the uteri of wild-type mice treated with P4 compared to vehicle. However, this induction was significantly decreased in PRKO mice compared to wild-type mice. These results indicate that the expression of Creb3l1 mRNA is mediated by PR. To analyze the uterine localization of CREB3L1 proteins following P4 treatment, we performed immunohistochemistry using tissues from vehicle or P4-treated wild-type and PRKO mice. In the control ovariectomized mouse uterus, CREB3L1 proteins were weakly detected in the stroma, and luminal and glandular epithelium. P4 treatment remarkably increased the localization of CREB3L1 proteins in the mouse uterus. However, CREB3L1 proteins were weakly detectable in PRKO mice treated with either vehicle and P4 (Figure 1B). These results suggest that Creb3l1 is a novel target gene of the P4-PR signaling axis in the uterus.
Figure 1.
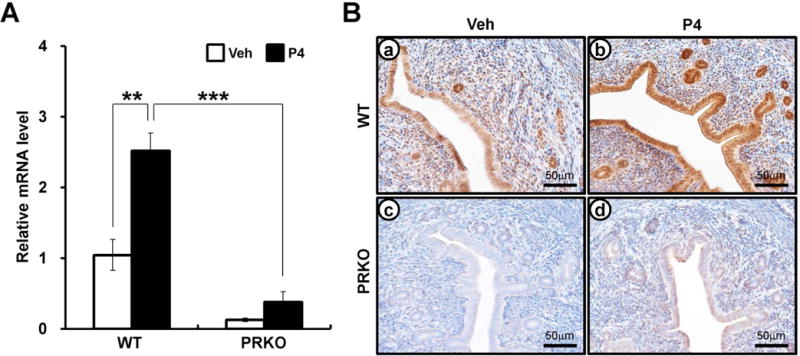
The expression of CREB3L1 in wild-type (WT) or progesterone receptor knock-out (PRKO) mouse. (A) The expression level of Creb3l1 from Progesterone (P4) injected WT or PRKO uteri by real-time RT-PCR assay. The samples were prepared from wild-type or PRKO uterus that was injected vehicle (Sesami oil) or P4 after 6 hours. The results represent the mean ± SEM. **p<0.01, ***p<0.001. (B) The localization pattern of CREB3L1 by immunohistochemistry in the vehicle or P4-treated uterus. Uterine sections were collected from P4 or vehicle treated WT and PRKO mice for 6 hours. Nuclei were counterstained with hematoxylin.
To investigate the expression of CREB3L1 during early pregnancy, the level of CREB3L1 was examined in the uteri of female mice during early pregnancy (Figure 2). Immunohistochemical analysis of uterine sections revealed that the expression of CREB3L1 was weakly detected at 0.5 dpc (days post coitum). Interestingly, strong expression of CREB3L1 was present in luminal and glandular epithelium as well as stroma after 2.5 dpc. The expression of CREB3L1 was extended into the primary decidual zone at 5.5 dpc, and was next observed in the secondary decidual zone at 7.5 dpc. These results show that CREB3L1 may be involved in decidualization during early pregnancy.
Figure 2.
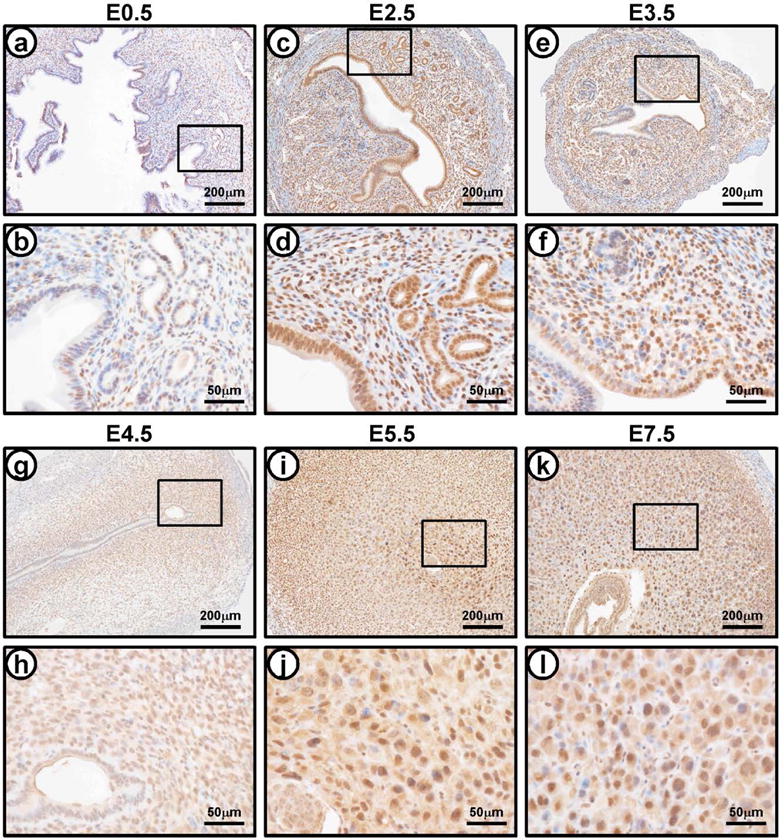
The expression of CREB3L1 during early pregnancy. The localization pattern of CREB3L1 during natural pregnancy by immunohistochemistry were determined at 0.5 dpc (a and b), 2.5 dpc (c and d), 3.5 dpc (e and f), 4.5 dpc (g and h), 5.5 dpc (i and j), and 7.5 dpc (k and l).
CREB3L1 is decreased in human endometrium from women with endometriosis
We next used immunohistochemical analysis to examine the cell-specific expression of CREB3L1 in endometrium from proliferative phase (n=6), early (n=6), mid (n=8), and late (n=5) secretory phase in women (Figure 3). CREB3L1 expression was only weakly detectable in epithelial cells of the proliferative phase. CREB3L1 protein was significantly higher in endometrial stromal and epithelial cells of the secretory phase as compared to the proliferative phase (Figure 3B). Interestingly, we observed that decidual cells have strong CREB3L1 staining in the late secretory phase (Figure 3Ah). Because the secretory phase is a time of elevated circulating P4 levels, this result suggests P4 regulation of CREB3L1 in human endometrium.
Figure 3.
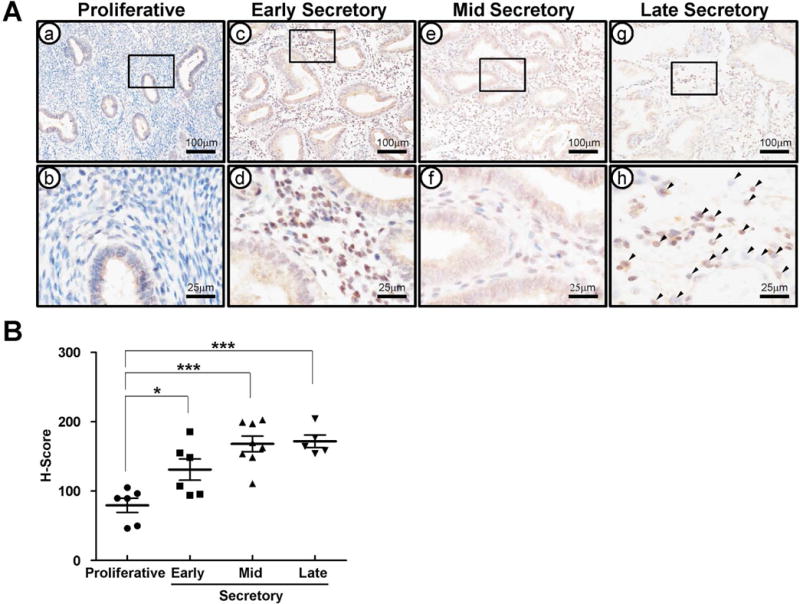
The expression profile of CREB3L1 in human endometrium during estrus cycle. (A) The expression level and localization of CREB3L1 in proliferative (a and b) and early (c and d), mid (e and f), and late (g and h) secretory phase of human endometrium. CREB3L1 expression was high expression in secretary phase endometrial epithelium. Black arrow head indicates a decidualized cell. (B) was H-Score by measuring expression intensity of endometrial cells. CREB3L1 expression intensity was significantly high level in secretary phase endometrium. The results represent the mean ± SEM. *p<0.05, ***p<0.001.
We hypothesize that the molecular basis for progesterone resistance in endometriosis is related to an overall reduction in progesterone receptor levels (PRs), leading to dysregulation of downstream PR target genes [12, 17, 24]. Therefore, to confirm the association of CREB3L1 with PR, we examined CREB3L1 expression in secretory endometrium from women with (n=17) or without (n=19) endometriosis by immunohistochemistry. Interestingly, CREB3L1 protein is significantly lower in endometrium from women with endometriosis as compared to controls during the secretory phase (Figure 4).
Figure 4.
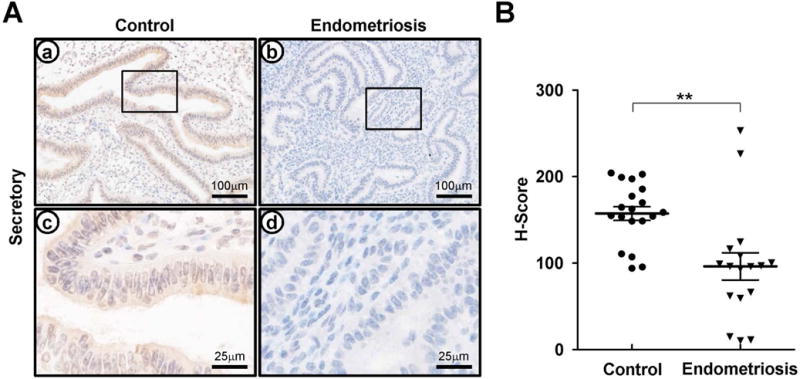
The expression profile of CREB3L1 in secretory endometrium from women with or without endometriosis (A) The expression level and localization of CREB3L1 in human endometrium with or without endometriosis. CREB3L1 expression was low expression in human endometrium with endometriosis. Nuclei were counterstained with hematoxylin. (B) H-Score of CREB3L1 expression in human endometrium with or without endometriosis. CREB3L1 expression intensity was significantly low level in human endometrium with endometriosis. The results represent the mean ± SEM. **p<0.01.
CREB3L1 is activated in human endometrial stromal cells (hESCs) during in vitro decidualization
P4 signaling is crucial for the decidualization of the endometrial stromal cells during early pregnancy [2]. Previous studies have shown that eutopic human primary endometrial stromal cells (hESCs) from patients with endometriosis showed impaired decidualization [38, 39]. To examine the role of CREB3L1 in hESC decidualization, we induced decidualization in cultured hESCs by treating the cells with estrogen, MPA, and cAMP [54] and examined expression of the known decidualization markers, prolactin (PRL) and insulin-like growth factor-binding protein 1 (IGFBP1), as well as cellular morphology. CREB3L1 mRNA expression levels were unaltered during in vitro decidualization in hESC (data not shown). Since CREB3L1 protein is activated by cleavage of its N-terminal domain which translocates from the ER membrane to the nucleus [28–31], we also examined CREB3L1 protein localization during decidualization by immunofluorescent staining. Our result shows that CREB3L1 was consistently expressed in cytoplasm of nondecidualized hESCs. However, upon induction of in vitro decidualization, CREB3L1 protein translocated to the nucleus on day 3 and day 6 (Figure 5) of treatment. This result suggests that CREB3L1 protein is activated during in vitro decidualization of hESCs.
Figure 5.
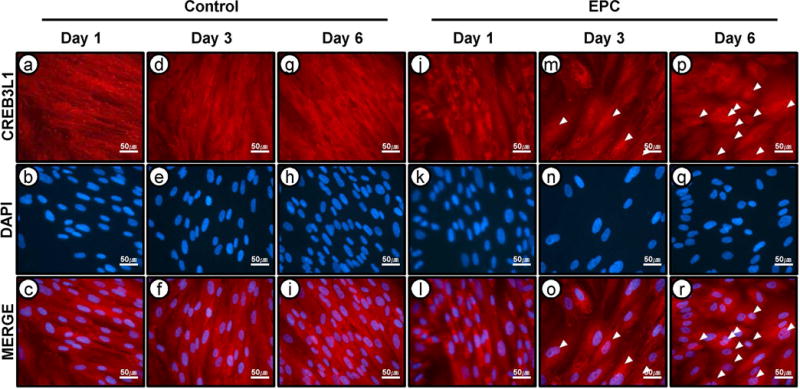
The nuclear translocalization of CREB3L1 during in vitro decidualization of human endometrial stromal cells (hESCs). Nuclear localization of CREB3L1 was detected at day 3 and day 6 in vitro decidualization hESCs with EPC. Neclei were conterstained with DAPI staining.
CREB3L1 is required for decidualization in hESCs
To further analyze the role of CREB3L1 in hESC decidualization, we performed siRNA-mediated knockdown of CREB3L1 expression (Figure 6A). To confirm CREB3L1 attenuation, we performed real-time PCR and observed decreased CREB3L1 expression in the CREB3L1 siRNA group as compared to non-targeting pool siRNA (Figure 6A). Surprisingly, hESCs morphology changed markedly upon decidualization treatment, showing a reduction in size and shape on day 6 (Figure 6B). The expression of decidualization marker genes, IGFBP1 and PRL, were also significantly reduced on day 6 of decidualization treatment (Figures 6C and D). These results suggest that CREB3L1 is required for both marker gene expression and morphology changes accompanying decidualization in hESCs.
Figure 6.
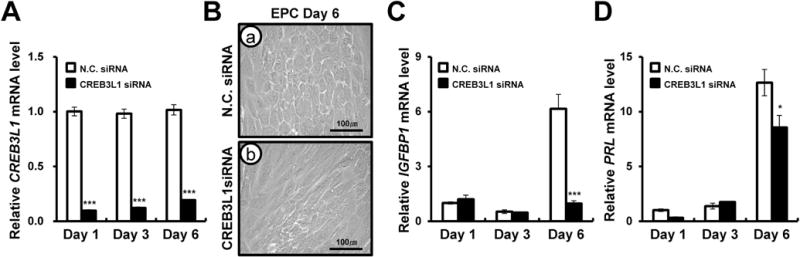
The effect of inhibition of CREB3L1 in human endometrial stromal cells (hESCs) during in vitro decidualization. CREB3L1 expression was inhibited by treatment with CREB3L1 siRNA during in vitro decidualization. (A) The expression of CREB3L1 gene was examined during in vitro decidualization. CREB3L1 were significantly decreased in hESCs treated with CREB3L1 siRNA during in vitro decidualization. The results represent the mean ± SEM. ***p<0.001. (B) Morphological change of hESCs was observed during in vitro decidualization on day 3 and day 6. The expression of decidualization marker gene IGFBP1(C) and PRL (D) were examined during in vitro decidualization. PRL and IGFBP1 were significantly decreased in transfected CREB3L1 siRNA hESC during in vitro decidualization. The results represent the mean ± SEM. *p<0.05, ***p<0.001.
CREB3L1 regulates phosphorylation of ERK1/2 in decidualization process
ERK1/2 is a member of the MAPK pathway previously shown to be required for endometrial stromal cell decidualization [40]. To determine whether phosphorylation of ERK1/2 is regulated by CREB3L1 during in vitro decidualization, we performed western blot analysis on protein from hESCs decidualized in the presence or absence of CREB3L1 siRNA prior to in vitro decidualization. We observed decreased phosphorylation of ERK1/2 (phospho-ERK1/2) on day 3 and day 6 in hESC treated with CREB3L1 siRNA as compared to the non-targeting pool siRNA (Figure 7A). Quantification of phospho-ERK1/2 revealed a statistically significantly decrease in phospho-ERK1/2 expression on day 6, as well as a marked decrease in phospho-ERK1/2 on day 3, but the latter did not reach statistical significance (Figure 7B). It is known that phospho-ERK1/2 translocates from the cytoplasm to the nucleus, where it binds to and transcriptionally activates downstream target genes [41, 42]. Thus, we investigated the localization of both total and phospho-ERK1/2 throughout in vitro decidualization of hESCs in the presence or absence of CREB3L1 siRNA transduction. This immunofluorescence result showed that levels of nuclear phospho-ERK1/2 were markedly reduced in decidualized hESCs transfected with CREB3L1 siRNA as compared to those transfected with non-targeting pool siRNA (Figure 7C). However, total ERK1/2 levels in hESCs were unchanged by CREB3L1 siRNA transfection (Supplemental Figure 1). These results suggest that CREB3L1 regulates the activation of phospho-ERK1/2 during the decidualization process.
Figure 7.
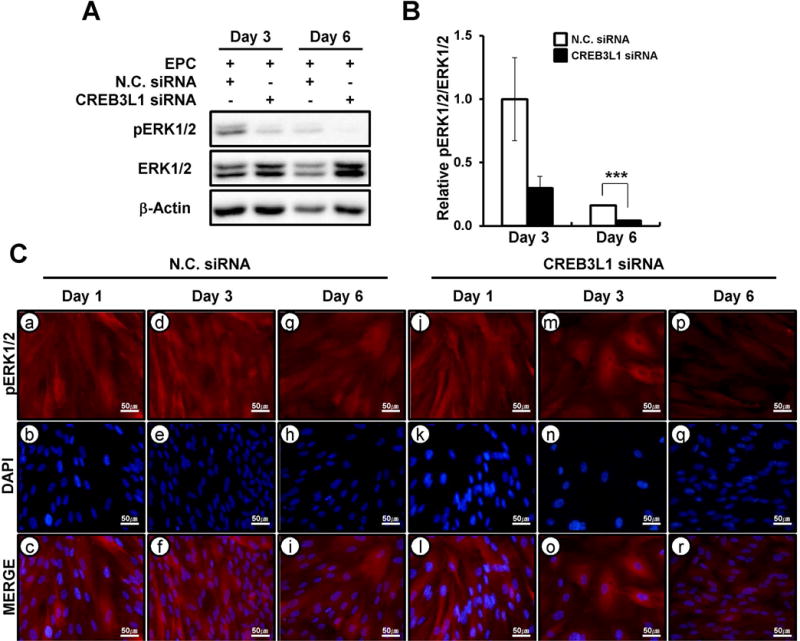
The regulation of phospho-ERK1/2 by CREB3L1 during hESCs decidualization. (A) The expression level of ERK1/2 phosphorylation during CREB3L1 inhibited hESCs decidualization. ERK1/2 phosphorylation was decreased at day 3 and day 6 in CREB3L1 siRNA treatment. (B) Quantification of phospho-ERK1/2 protein level during in vitro decidualization with or without CREB3L1 target siRNA treatment. The results represent the mean ± SEM. ***p<0.001. (C) Nuclear phospho-ERK1/2 was reduced at day 3 and day 6 in vitro decidualization hESCs with CREB3L1 siRNA compared to transfected with non targeting pool siRNA. Neclei were conterstained with DAPI staining.
Discussion
In this study, we have characterized Creb3l1 as a P4-PR regulated gene in mouse uterus. P4 is a known critical regulator of the reproductive events associated with embryo implantation, decidualization and the maintenance of pregnancy [43–45]. Previous studies have shown that P4 regulation is dependent upon progesterone receptor (PR) [3, 4]. The binding of P4 to PR results in PR nuclear translocation and subsequent alteration of P4 target gene transcription. Recently, Rubel et al. identified uterine PR-regulated mechanisms and downstream targets via chromatin immunoprecipitation followed by deep sequencing (ChIP-Seq) [36]. This study provided an important dataset for identification of target genes in the uterus regulated by the P4-PR axis. These ChIP-Seq results also show that PR directly binds to the proximal promoter region of Creb3l1 gene in uteri of ovariectomized mice treated with P4. Our study has shown that CREB3L1 proteins were strongly detected in luminal and glandular epithelium as well as stroma of mouse uteri after 2.5 dpc. CREB3L1 proteins were significantly increased in both epithelial and stromal compartments of the human endometrium at the secretory phase as compared to the proliferative phase. The secretory phase of humans and 2.5 dpc of mice are characterized by elevated P4 levels [46]. Furthermore, decidual cells also have strong CREB3L1 staining in mice and humans. Therefore, our results support that CREB3L1 may play an important role for P4-PR signaling in both mice and humans.
Progesterone resistance has been hypothesized as a crucial element in the development of endometriosis [12, 24]. The interruption of P4 signaling, occurring from either loss of the PR itself or its interacting partners/downstream effectors, leads to a physiological state of P4 resistance [36]. Aberrant expression of critical P4 target genes such as aromatase, leukaemia-inhibitory factor, matrix metalloproteinases and the progesterone receptor has previously been shown in the endometrium of women with endometriosis [47–50]. This study demonstrates that CREB3L1 expression is also altered in the endometrium from women with endometriosis. Similar identification of P4 target genes and signaling pathways will be crucial for understanding the uterine dysfunction caused by this disease and contribute to the development of new therapeutic interventions.
Our study shows that inhibition of CREB3L1 by siRNA treatment impaired decidualization capacity in hESCs, as evidenced by suppression of PRL and IGFBP1 expression, known decidualization marker genes. P4 is a steroid hormone closely associated with endometrial stromal cell decidualization [2]. hESC from patients with endometriosis [39] and PRKO mice demonstrate a decidualization defect, supporting a critical role for P4-PR signaling in decidualization in both humans and mice [3]. Previous studies have shown that P4 activates Ihh signaling to induce expression of chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) during decidualization in hESCs [51, 52]. COUP-TFII, has been shown to promote decidualization of hESCs via induction of bone morphogenetic protein 2 (BMP2) and inhibition of ERα activation [51]. It has also been shown that STAT3 and PR crosstalk is required for successful embryo implantation and stromal cell decidualization in mice [53, 54]. However, the expression profiles of IHH, COUP-TFII, BMP2, and STAT3 were not altered in our decidualized hESCs following treatment with CREB3L1 siRNA (data not shown), suggesting that CREB3L1 is acting down-stream of these key molecules or has an independent function with respect to decidualization.
CREB3L1 functions within the endoplasmic reticulum as an unfolding protein response (UPR) sensor [55]. Activation of CREB3L1 is stimulated by the UPR, leading to cleavage of its N-terminal domain by the basic leucine zipper (b-ZIP), causing translocation from the cytoplasm into the nucleus [29–31]. The cleaved CREB3L1 N-terminal domain then transcriptionally activates downstream target genes, such as collagen type I genes, protein-folding chaperones, and the antiproliferative gene [29–31]. This study reveals that during endometrial stromal cell decidualization, CREB3L1 also translocates from the cytoplasm to the nucleus, suggesting a role for activated CREB3L1 in decidualization.
ERK1/2 is activated by phosphorylation, resulting in its translocation from the cytoplasm into the nucleus where it phosphorylates target transcription factors [56, 57]. Previous studies have shown that ERK1/2 activity is increased in hESCs from women with endometriosis [58] and have linked the enhanced proliferation and survival of hESCs derived from women with endometriosis with alterations in ERK1/2 signaling [59, 60]. Abnormal decidualization of endometrial stromal cells has been correlated with unexplained infertility, miscarriage and endometrial pathologies such as endometriosis [20, 61–63]. It has been reported that endometriosis patients have a reduced decidualization capacity. Our previous studies showed that activation of ERK1/2 signaling coincided with the onset of decidualization in mice and humans [40]. Inhibition of ERK1/2 phosphorylation significantly decreased this decidualization process. TGF-β superfamily members are key regulators of female reproduction including decidualization. TGF-β stimulates activation of CREB3L1 [64] and ERK1/2 [65]. Additionally, many studies have demonstrated a potential link between ERK1/2 signaling and endometriosis [58, 59, 66]. These results suggest that tight regulation of CREB3L1 and ERK1/2 activity is required during the process of decidualization.
In this study, total ERK1/2 levels and its localization within hESCs was unchanged by CREB3L1 silencing during decidualization. However, phosphorylation of ERK1/2 was significantly reduced by CREB3L1 silencing and CREB3L1 expression was required for nuclear translocation of phospho-ERK1/2 during hESC decidualization. These results suggest that CREB3L1 regulates the accumulation and translocation of phospho-ERK1/2 during the decidualization process. Previous studies have also shown that in vitro decidualization of hESCs from women with endometriosis is characterized by aberrantly regulated ERK1/2 phosphorylation [58] and that pretreatment with the ERK1/2 inhibitor, U0126, can prevent decidualization [40]. In light of these previous results, we hypothesize that phospho-ERK1/2 expression is tightly regulated by CREB3L1 during decidualization.
In summary, Creb3l1 is identified as a target gene of P4-PR in the uterus. Our study observed dysregulation of CREB3L1 in endometrium from women with endometriosis as compared with healthy controls. Inhibition of CREB3L1 activity by siRNA-mediated knockdown suppresses decidualization of hESCs. We also show that phosphorylation of ERK1/2, a critical factor for decidualization, was regulated by CREB3L1. These results suggest that CREB3L1 is required for successful decidualization in mice and humans and have implications for our understanding of endometriosis and other endometrial pathologies.
Supplementary Material
Supplemental Figure 1. The expression of ERK1/2 by CREB3L1 during hESCs decidualization. Expression levels of total ERK1/2 were not changed during in vitro decidualization of hESCs with CREB3L1 siRNA compared to transfected with non targeting pool siRNA. Neclei were conterstained with DAPI staining.
Acknowledgments
We would like to thank the Human Female Reproductive Tract Biorepository and the Spectrum Health Medical Group, Department of Obstetrics, Gynecology and Reproductive Biology (especially Elizabeth Leary, M.D., Calvin Leazenby, M.D., Diana Bitner, M.D., and Christine Heisler, M.D.) and Meighan L. McAuliffe for their help in obtaining human samples for research use. We would also like to thank Amanda Sterling for manuscript preparation. This work was supported by Bio-industry Technology Development Program (IPET312060-5), Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea to J.M.L., NIH R01 HD067721 to S.L.Y and B.A.L and NIH R01 HD057873 and American Cancer Society Research Grant RSG-12-084-01-TBG to J.W.J.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
References
- 1.Critchley HO, Saunders PT. Hormone receptor dynamics in a receptive human endometrium. Reprod Sci. 2009 Feb;16(2):191–9. doi: 10.1177/1933719108331121. Epub 2009/02/12. eng. [DOI] [PubMed] [Google Scholar]
- 2.Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003 Nov;68(10–13):771–8. doi: 10.1016/s0039-128x(03)00126-0. Epub 2003/12/12. eng. [DOI] [PubMed] [Google Scholar]
- 3.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes & development. 1995 Sep 15;9(18):2266–78. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 4.Lydon JP, DeMayo FJ, Conneely OM, O’Malley BW. Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol. 1996 Jan;56(1–6 Spec No):67–77. doi: 10.1016/0960-0760(95)00254-5. Epub 1996/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 5.Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, et al. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology. 2005 Aug;146(8):3490–505. doi: 10.1210/en.2005-0016. [DOI] [PubMed] [Google Scholar]
- 6.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002 Nov;187(5):1416–23. doi: 10.1067/mob.2002.127305. Epub 2002/11/20. eng. [DOI] [PubMed] [Google Scholar]
- 7.Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007 Nov;25(6):445–53. doi: 10.1055/s-2007-991042. Epub 2007/10/26. eng. [DOI] [PubMed] [Google Scholar]
- 8.Popovici RM, Kao LC, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology. 2000 Sep;141(9):3510–3. doi: 10.1210/endo.141.9.7789. [DOI] [PubMed] [Google Scholar]
- 9.Brar AK, Handwerger S, Kessler CA, Aronow BJ. Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiological genomics. 2001 Dec 21;7(2):135–48. doi: 10.1152/physiolgenomics.00061.2001. [DOI] [PubMed] [Google Scholar]
- 10.Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, et al. Bmp2 is critical for the murine uterine decidual response. Molecular and cellular biology. 2007 Aug;27(15):5468–78. doi: 10.1128/MCB.00342-07. Pubmed Central PMCID: 1952078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart CL, Cullinan EB. Preimplantation development of the mammalian embryo and its regulation by growth factors. Dev Genet. 1997;21(1):91–101. doi: 10.1002/(SICI)1520-6408(1997)21:1<91::AID-DVG11>3.0.CO;2-D. Epub 1997/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 12.Bulun SE. Endometriosis. The New England journal of medicine. 2009 Jan 15;360(3):268–79. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 13.Ito K, Utsunomiya H, Yaegashi N, Sasano H. Biological roles of estrogen and progesterone in human endometrial carcinoma–new developments in potential endocrine therapy for endometrial cancer. Endocr J. 2007 Dec;54(5):667–79. doi: 10.1507/endocrj.kr-114. Epub 2007/09/06. eng. [DOI] [PubMed] [Google Scholar]
- 14.Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003 Jun;111(8):1037–54. doi: 10.1289/ehp.5787. Epub 2003/06/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giudice LC. Endometrium in PCOS: Implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab. 2006 Jun;20(2):235–44. doi: 10.1016/j.beem.2006.03.005. Epub 2006/06/15. eng. [DOI] [PubMed] [Google Scholar]
- 16.Balleine RL, Earls PJ, Webster LR, Mote PA, deFazio A, Harnett PR, et al. Expression of progesterone receptor A and B isoforms in low-grade endometrial stromal sarcoma. Int J Gynecol Pathol. 2004 Apr;23(2):138–44. doi: 10.1097/00004347-200404000-00008. Epub 2004/04/16. eng. [DOI] [PubMed] [Google Scholar]
- 17.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007 Aug;148(8):3814–26. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 18.Arnett-Mansfield RL, deFazio A, Wain GV, Jaworski RC, Byth K, Mote PA, et al. Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Res. 2001 Jun 1;61(11):4576–82. Epub 2001/06/05. eng. [PubMed] [Google Scholar]
- 19.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012 Sep;98(3):511–9. doi: 10.1016/j.fertnstert.2012.06.029. Epub 2012/07/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006 Mar;85(3):564–72. doi: 10.1016/j.fertnstert.2005.08.046. Epub 2006/02/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosskinsky CM, Halme J. Endometriosis: the host response. Baillieres Clin Obstet Gynaecol. 1993 Dec;7(4):701–13. doi: 10.1016/s0950-3552(05)80459-6. [DOI] [PubMed] [Google Scholar]
- 22.Kaunitz AM. Injectable depot medroxyprogesterone acetate contraception: an update for U.S. clinicians. Int J Fertil Womens Med. 1998 Mar-Apr;43(2):73–83. Epub 1998/06/03. eng. [PubMed] [Google Scholar]
- 23.Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best practice & research Clinical obstetrics & gynaecology. 2004 Apr;18(2):319–28. doi: 10.1016/j.bpobgyn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Molecular and cellular endocrinology. 2006 Mar 27;248(1–2):94–103. doi: 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 25.Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Molecular and cellular endocrinology. 2012 Jun 24;357(1–2):108–18. doi: 10.1016/j.mce.2011.10.028. Pubmed Central PMCID: 3443857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kistner RW. The use of newer progestins in the treatment of endometriosis. Am J Obstet Gynecol. 1958 Feb;75(2):264–78. doi: 10.1016/0002-9378(58)90384-3. [DOI] [PubMed] [Google Scholar]
- 27.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007 Jul;8(7):519–29. doi: 10.1038/nrm2199. Epub 2007/06/15. eng. [DOI] [PubMed] [Google Scholar]
- 28.Kondo S, Murakami T, Tatsumi K, Ogata M, Kanemoto S, Otori K, et al. OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat Cell Biol. 2005 Feb;7(2):186–94. doi: 10.1038/ncb1213. Epub 2005/01/25. eng. [DOI] [PubMed] [Google Scholar]
- 29.Murakami T, Saito A, Hino S, Kondo S, Kanemoto S, Chihara K, et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol. 2009 Oct;11(10):1205–11. doi: 10.1038/ncb1963. Epub 2009/09/22. eng. [DOI] [PubMed] [Google Scholar]
- 30.Murakami T, Kondo S, Ogata M, Kanemoto S, Saito A, Wanaka A, et al. Cleavage of the membrane-bound transcription factor OASIS in response to endoplasmic reticulum stress. J Neurochem. 2006 Feb;96(4):1090–100. doi: 10.1111/j.1471-4159.2005.03596.x. Epub 2006/01/19. eng. [DOI] [PubMed] [Google Scholar]
- 31.Denard B, Seemann J, Chen Q, Gay A, Huang H, Chen Y, et al. The membrane-bound transcription factor CREB3L1 is activated in response to virus infection to inhibit proliferation of virus-infected cells. Cell Host Microbe. 2011 Jul 21;10(1):65–74. doi: 10.1016/j.chom.2011.06.006. Epub 2011/07/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omori Y, Imai J, Suzuki Y, Watanabe S, Tanigami A, Sugano S. OASIS is a transcriptional activator of CREB/ATF family with a transmembrane domain. Biochem Biophys Res Commun. 2002 Apr 26;293(1):470–7. doi: 10.1016/S0006-291X(02)00253-X. Epub 2002/06/11. eng. [DOI] [PubMed] [Google Scholar]
- 33.Asada R, Saito A, Kawasaki N, Kanemoto S, Iwamoto H, Oki M, et al. The endoplasmic reticulum stress transducer OASIS is involved in the terminal differentiation of goblet cells in the large intestine. J Biol Chem. 2012 Mar 9;287(11):8144–53. doi: 10.1074/jbc.M111.332593. Epub 2012/01/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vellanki RN, Zhang L, Guney MA, Rocheleau JV, Gannon M, Volchuk A. OASIS/CREB3L1 induces expression of genes involved in extracellular matrix production but not classical endoplasmic reticulum stress response genes in pancreatic beta-cells. Endocrinology. 2010 Sep;151(9):4146–57. doi: 10.1210/en.2010-0137. Epub 2010/07/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bae CH, Kim TH, Chu JY, Cho ES. New population of odontoblasts responsible for tooth root formation. Gene Expr Patterns. 2013 Jun-Jul;13(5–6):197–202. doi: 10.1016/j.gep.2013.04.001. Epub 2013/04/23. eng. [DOI] [PubMed] [Google Scholar]
- 36.Rubel CA, Lanz RB, Kommagani R, Franco HL, Lydon JP, DeMayo FJ. Research resource: Genome-wide profiling of progesterone receptor binding in the mouse uterus. Molecular endocrinology. 2012 Aug;26(8):1428–42. doi: 10.1210/me.2011-1355. Pubmed Central PMCID: 3404303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke CL, Sutherland RL. Progestin regulation of cellular proliferation. Endocrine reviews. 1990 May;11(2):266–301. doi: 10.1210/edrv-11-2-266. [DOI] [PubMed] [Google Scholar]
- 38.Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, et al. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Molecular human reproduction. 2007 May;13(5):323–32. doi: 10.1093/molehr/gam005. [DOI] [PubMed] [Google Scholar]
- 39.Minici F, Tiberi F, Tropea A, Orlando M, Gangale MF, Romani F, et al. Endometriosis and human infertility: a new investigation into the role of eutopic endometrium. Hum Reprod. 2008 Mar;23(3):530–7. doi: 10.1093/humrep/dem399. [DOI] [PubMed] [Google Scholar]
- 40.Lee CH, Kim TH, Lee JH, Oh SJ, Yoo JY, Kwon HS, et al. Extracellular signal-regulated kinase 1/2 signaling pathway is required for endometrial decidualization in mice and human. PloS one. 2013;8(9):e75282. doi: 10.1371/journal.pone.0075282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenormand P, Sardet C, Pages G, L’Allemain G, Brunet A, Pouyssegur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993 Sep;122(5):1079–88. doi: 10.1083/jcb.122.5.1079. Epub 1993/09/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Molecular and cellular biology. 1992 Mar;12(3):915–27. doi: 10.1128/mcb.12.3.915. Epub 1992/03/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubel CA, Jeong JW, Tsai SY, Lydon JP, Demayo FJ. Epithelial-stromal interaction and progesterone receptors in the mouse uterus. Semin Reprod Med. 2010 Jan;28(1):27–35. doi: 10.1055/s-0029-1242990. [DOI] [PubMed] [Google Scholar]
- 44.Kim TH, Lee DK, Franco HL, Lydon JP, Jeong JW. ERBB receptor feedback inhibitor 1 regulation of estrogen receptor activity is critical for uterine implantation in mice. Biology of reproduction. 2010 Apr;82(4):706–13. doi: 10.1095/biolreprod.109.081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conneely OM, Jericevic BM. Progesterone regulation of reproductive function through functionally distinct progesterone receptor isoforms. Reviews in endocrine & metabolic disorders. 2002 Sep;3(3):201–9. doi: 10.1023/a:1020020308980. [DOI] [PubMed] [Google Scholar]
- 46.Lee KY, DeMayo FJ. Animal models of implantation. Reproduction. 2004 Dec;128(6):679–95. doi: 10.1530/rep.1.00340. [DOI] [PubMed] [Google Scholar]
- 47.Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000 Aug;85(8):2897–902. doi: 10.1210/jcem.85.8.6739. Epub 2000/08/18. eng. [DOI] [PubMed] [Google Scholar]
- 48.Afshar Y, Hastings J, Roqueiro D, Jeong JW, Giudice LC, Fazleabas AT. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biology of reproduction. 2013 Feb;88(2):44. doi: 10.1095/biolreprod.112.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang S, Fang Z, Suzuki T, Sasano H, Zhou J, Gurates B, et al. Regulation of aromatase P450 expression in endometriotic and endometrial stromal cells by CCAAT/enhancer binding proteins (C/EBPs): decreased C/EBPbeta in endometriosis is associated with overexpression of aromatase. J Clin Endocrinol Metab. 2002 May;87(5):2336–45. doi: 10.1210/jcem.87.5.8486. Epub 2002/05/08. eng. [DOI] [PubMed] [Google Scholar]
- 50.Tabibzadeh S, Mason JM, Shea W, Cai Y, Murray MJ, Lessey B. Dysregulated expression of ebaf, a novel molecular defect in the endometria of patients with infertility. J Clin Endocrinol Metab. 2000 Jul;85(7):2526–36. doi: 10.1210/jcem.85.7.6674. Epub 2000/07/21. eng. [DOI] [PubMed] [Google Scholar]
- 51.Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS genetics. 2007 Jun;3(6):e102. doi: 10.1371/journal.pgen.0030102. Pubmed Central PMCID: 1892047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nature genetics. 2006 Oct;38(10):1204–9. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- 53.Liu T, Ogle TF. Signal transducer and activator of transcription 3 is expressed in the decidualized mesometrium of pregnancy and associates with the progesterone receptor through protein-protein interactions. Biology of reproduction. 2002 Jul;67(1):114–8. doi: 10.1095/biolreprod67.1.114. Epub 2002/06/25. eng. [DOI] [PubMed] [Google Scholar]
- 54.Lee JH, Kim TH, Oh SJ, Yoo JY, Akira S, Ku BJ, et al. Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013 Jul;27(7):2553–63. doi: 10.1096/fj.12-225664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asada R, Kanemoto S, Kondo S, Saito A, Imaizumi K. The signalling from endoplasmic reticulum-resident bZIP transcription factors involved in diverse cellular physiology. J Biochem. 2011 May;149(5):507–18. doi: 10.1093/jb/mvr041. Epub 2011/04/02. eng. [DOI] [PubMed] [Google Scholar]
- 56.Eldredge ER, Korf GM, Christensen TA, Connolly DC, Getz MJ, Maihle NJ. Activation of c-fos gene expression by a kinase-deficient epidermal growth factor receptor. Molecular and cellular biology. 1994 Nov;14(11):7527–34. doi: 10.1128/mcb.14.11.7527. Epub 1994/11/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukuda M, Gotoh I, Adachi M, Gotoh Y, Nishida E. A novel regulatory mechanism in the mitogen-activated protein (MAP) kinase cascade. Role of nuclear export signal of MAP kinase kinase. J Biol Chem. 1997 Dec 19;272(51):32642–8. doi: 10.1074/jbc.272.51.32642. Epub 1998/01/24. eng. [DOI] [PubMed] [Google Scholar]
- 58.Velarde MC, Aghajanova L, Nezhat CR, Giudice LC. Increased mitogen-activated protein kinase kinase/extracellularly regulated kinase activity in human endometrial stromal fibroblasts of women with endometriosis reduces 3′,5′-cyclic adenosine 5′-monophosphate inhibition of cyclin D1. Endocrinology. 2009 Oct;150(10):4701–12. doi: 10.1210/en.2009-0389. Epub 2009/07/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yotova IY, Quan P, Leditznig N, Beer U, Wenzl R, Tschugguel W. Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis. Hum Reprod. 2011 Apr;26(4):885–97. doi: 10.1093/humrep/der010. Epub 2011/02/10. eng. [DOI] [PubMed] [Google Scholar]
- 60.Mormile R, Vittori G. MAPK signaling pathway and endometriosis: what is the link? Arch Gynecol Obstet. 2013 Apr;287(4):837–8. doi: 10.1007/s00404-012-2587-9. Epub 2012/10/12. eng. [DOI] [PubMed] [Google Scholar]
- 61.Laird SM, Tuckerman EM, Li TC. Cytokine expression in the endometrium of women with implantation failure and recurrent miscarriage. Reprod Biomed Online. 2006 Jul;13(1):13–23. doi: 10.1016/s1472-6483(10)62011-1. Epub 2006/07/06. eng. [DOI] [PubMed] [Google Scholar]
- 62.Karpovich N, Klemmt P, Hwang JH, McVeigh JE, Heath JK, Barlow DH, et al. The production of interleukin-11 and decidualization are compromised in endometrial stromal cells derived from patients with infertility. J Clin Endocrinol Metab. 2005 Mar;90(3):1607–12. doi: 10.1210/jc.2004-0868. Epub 2004/12/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryan IP, Taylor RN. Endometriosis and infertility: new concepts. Obstet Gynecol Surv. 1997 Jun;52(6):365–71. doi: 10.1097/00006254-199706000-00021. Epub 1997/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 64.Chen Q, Lee CE, Denard B, Ye J. Sustained induction of collagen synthesis by TGF-beta requires regulated intramembrane proteolysis of CREB3L1. PloS one. 2014;9(10):e108528. doi: 10.1371/journal.pone.0108528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003 Oct 9;425(6958):577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 66.Mormile R, Vittori G. MAPK signaling pathway and endometriosis: what is the link? Arch Gynecol Obstet. 2012 Oct 11; doi: 10.1007/s00404-012-2587-9. Epub 2012/10/12. Eng. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. The expression of ERK1/2 by CREB3L1 during hESCs decidualization. Expression levels of total ERK1/2 were not changed during in vitro decidualization of hESCs with CREB3L1 siRNA compared to transfected with non targeting pool siRNA. Neclei were conterstained with DAPI staining.


