Abstract
Intracranial aneurysm (IA) is pathological dilatations of the cerebral artery and rupture of IAs can cause subarachnoid hemorrhage, which has a high ratio of fatality and morbidity. However, the pathogenesis of IAs remains unknown. We performed long noncoding RNA (lncRNA) and messenger RNA (mRNA) expression profiles in IA tissues and superficial temporal arteries (STAs). A total of 4129 differentially expressed lncRNAs and 2926 differentially expressed mRNAs were obtained from the microarrays (P < 0.05). Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses showed that up-regulated mRNAs were enriched in immune response, inflammatory response, regulation of immune response and lysosome, et al; while the down-regulated mRNAs were enriched in muscle contraction, smooth muscle contraction, cGMP-PKG signaling pathway and vascular smooth muscle contraction, et al. The lncRNA-mRNA co-expression networks were represented in immune response, inflammatory response, muscle contraction and vascular smooth muscle contraction. These findings may gain insight in the pathogenesis of IAs and provide clues to find key roles for IA patients.
Keywords: intracranial aneurysm, long non-coding RNA, microarray, gene ontology, pathway analysis
INTRODUCTION
Intracranial aneurysm (IA) is pathological dilatations of the cerebral artery and its overall prevalence is about 3.2% with a mean age of 50 years [1, 2]. Rupture of IAs can cause subarachnoid hemorrhage, which has a high ratio of fatality and morbidity [3]. Although several genome-wide association studies have been performed worldwide [4–7], the pathogenesis of IAs remains unknown.
Long noncoding RNA (LncRNA) is defined as longer than 200 nucleotides and lack of protein-coding ability [8]. Many studies have revealed a wide range of functional activities of lncRNAs and it suggests that some lncRNAs are involved in common cardiovascular diseases, including atherosclerosis, myocardial infarction, and aneurysms, et al [9–11]. ANRIL is identified as a genetic susceptibility locus associated with IA and abdominal aortic aneurysm [12–14]. HIF1a-AS1 is overexpressed in the thoracoabdominal aortic aneurysm and plays a key role in the proliferation and apoptosis of vascular smooth muscle cells in vitro [15]. Therefore, lncRNAs may paly important role in the formation of IA.
In our study, we performed microarrays to investigate lncRNA and messenger RNA (mRNA) expression profiles in aneurismal tissues from IA patients and controls-superficial temporal arteries (STAs). The microarray analysis of the differential lncRNAs and mRNAs is necessary to gain insight in the pathogenesis of the formation and development of IAs.
RESULTS
The clinical characteristics of included IA patients
We included 27 IA patients (12 ruptured IAs and 15 unruptured IAs) in our study. The clinical characteristics, including gender, age, IA size, hypertension, smokers and drinkers, were shown in Table 1. There were more patients with hypertension and smokers in ruptured IAs group than those in unruptured IAs group (11 vs. 6; 3 vs. 1). LncRNA and mRNA expression profiles were obtained from 27 samples.
Table 1. Clinical characteristics of included patients.
| Items | Ruptured IAs (n=12) |
Unruptured IAs (n=15) |
STAs (n=27) |
|---|---|---|---|
| Gender (Female %) | 7 (58.33%) | 13 (86.67%) | 20 (74.07%) |
| Age (Years, mean±SD) | 52.83±6.44 | 49.87±13.28 | 51.19±10.89 |
| IA Size (mm, mean±SD) | 10.27±7.32 | 16.97±7.11 | ND |
| Hypertension % | 11 (91.67%) | 6 (40%) | 17 (62.96%) |
| Smokers % | 3 (25%) | 1 (6.67%) | 4 (14.81%) |
| Drinkers % | 1 (8.33%) | 1 (6.67%) | 2 (7.41%) |
IA, intracranial aneurysm; STA, superficial temporal artery. ND, no data.
Identification of differentially expressed lncRNAs and mRNAs
The workflow of the entire experiment was summarized in Figure 1. We identified 4129 differentially expressed lncRNAs (876 up-regulated; 3253 down-regulated) from the Agilent lncRNA microarrays of 12 IA patients and controls arteries (P < 0.05). Volcano plots were performed to identify differences of lncRNAs (Figure S1A, S1B). A total of 2926 differentially expressed mRNAs were identified from two mRNA microarrays (Agilent, Affymetrix; P < 0.05; Figure S1C-S1F). Of those, 1511 up-regulated mRNAs and 1415 down-regulated mRNAs were screened out in 27 IAs compared with STAs, respectively (Table S1).
Figure 1. Schematic overview of the workflow.
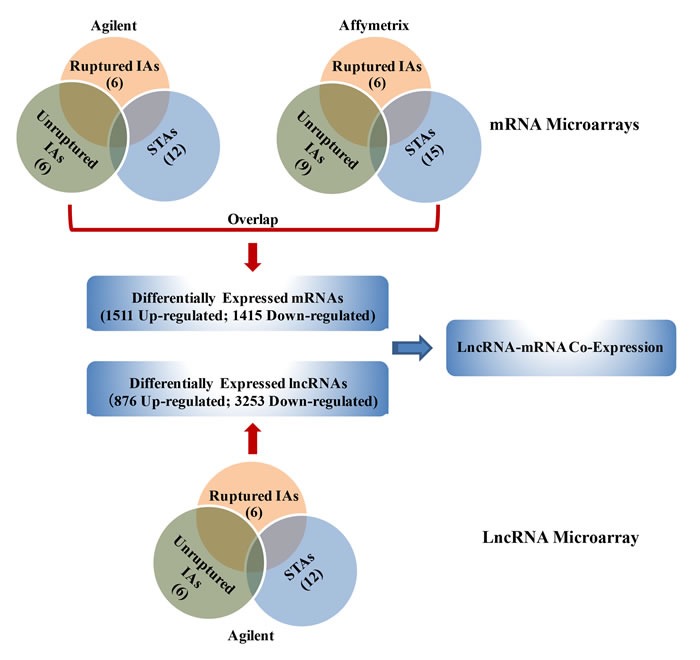
IA, intracranial aneurysm; STA, superficial temporal artery.
The heat map of the differentially expressed lncRNAs and mRNAs separated by IAs and STAs was showed in Figure 2.
Figure 2. Heat map of differentially expressed lncRNAs and mRNAs of IAs and STAs.
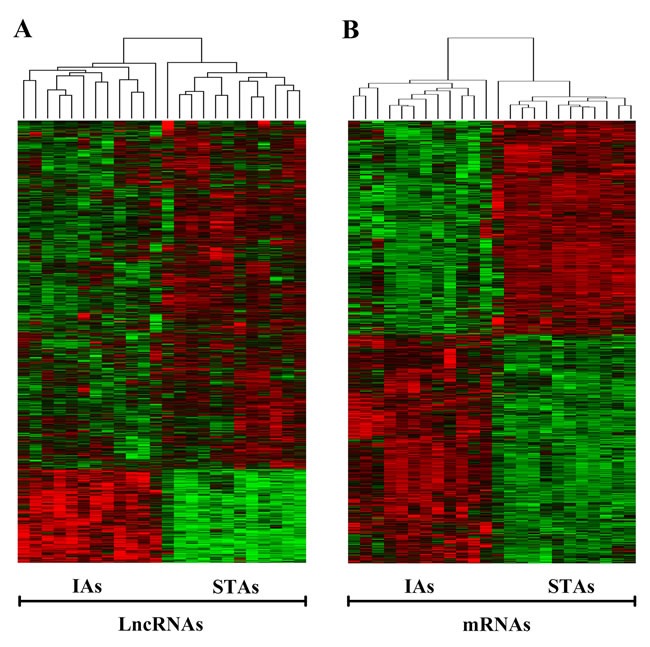
IA, intracranial aneurysm; STA, superficial temporal artery.
Function exploration of the differentially expressed mRNAs
The 2926 differentially expressed mRNAs were conducted GO and KEGG pathway analyses using DAVID (The Database for Annotation, Visualization and Integrated Discovery,https://david-d.ncifcrf.gov/home.jsp). GO analysis showed that up-regulated genes were enriched in immune response, inflammatory response and regulation of immune response, et al (Figure 3A); while the down-regulated genes were enriched in muscle contraction, muscle organ development, positive regulation of glucose import and smooth muscle contraction, et al (Figure 3B). Moreover, KEGG pathway analysis showed that the up-regulated genes enriched in lysosome, phagosome and staphylococcus aureus infection, et al (Figure 4A); while the down-regulated genes were enriched in cGMP-PKG signaling pathway, vascular smooth muscle contraction and proteoglycans in cancer, et al (Figure 4B).
Figure 3. Gene ontology of differentially expressed mRNAs.
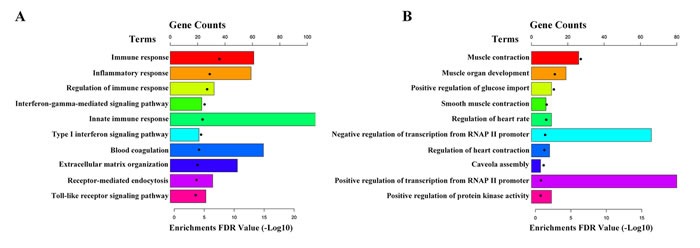
A. The top 10 GO terms up-regulated in IAs compared with STAs. B. The top 10 GO terms down-regulated in IAs compared with STAs.
Figure 4. KEGG pathway analysis of differentially expressed mRNAs.
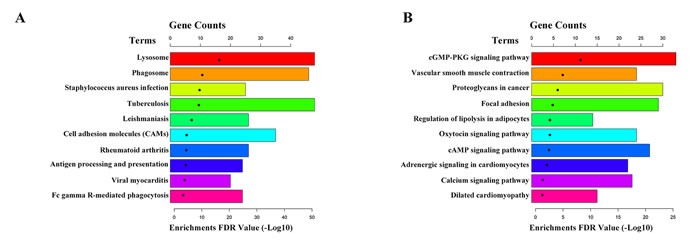
A. The top 10 pathways up-regulated in IAs compared with STAs. B. The top 10 pathways down-regulated in IAs compared with STAs.
LncRNA-mRNA co-expression networks
We further performed lncRNA-mRNA co-expression network analysis and the co-expression networks were represented in immune response, inflammatory response, muscle contraction pathway and vascular smooth muscle contraction pathway (Table S2). There were 24 lncRNAs that interacted with 8 mRNAs in the vascular smooth muscle contraction pathway (Figure 5A), 10 lncRNAs interacted with 10 mRNAs in the GO term of immune response (Figure 5B), 7 lncRNAs interacted with 7 mRNAs in the GO term of inflammatory response (Figure 5C) and 31 lncRNAs interacted with 9 mRNAs in the muscle contraction pathway (Figure 5D).
Figure 5. LncRNA-mRNA co-expression networks in four meaningful pathways.
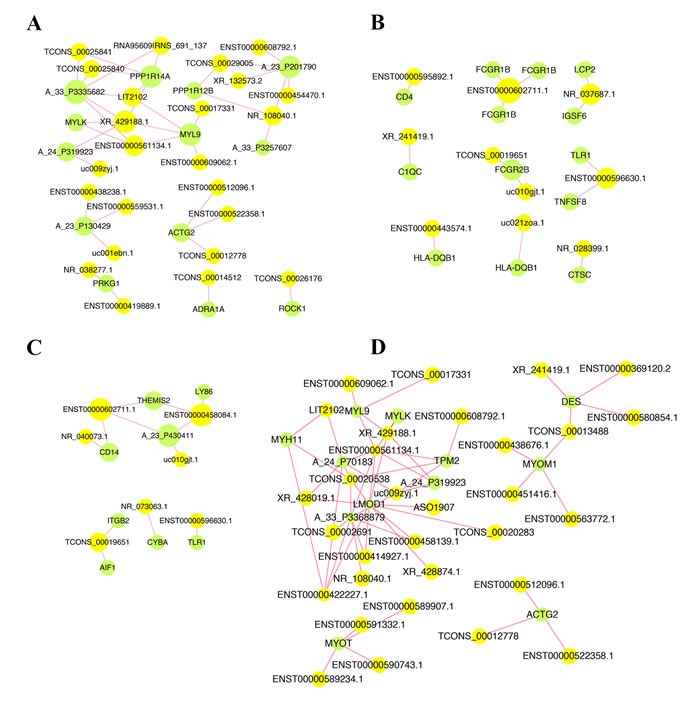
A. 24 lncRNAs interacted with 8 mRNAs in the vascular smooth muscle contraction pathway. B. 10 lncRNAs interacted with 10 mRNAs in the GO term of immune response. C. 7 lncRNAs interacted with 7 mRNAs in the GO term of inflammatory response. D. 31 lncRNAs interacted with 9 mRNAs in the muscle contraction pathway. Yellow, lncRNA; Green, mRNA.
DISCUSSION
The prevalence of IAs was about 7% in Chinese adults aged 35 to 75 years [16] and the standard treatments are endovascular treatment and open surgery. Many studies have provided evidence of the associations between lncRNA and mRNA [17–19]. However, there are fewer studies focused on the lncRNA-mRNA co-expression study of IAs and the molecular mechanisms behind IA formation remain poorly understood. Therefore, lncRNA-mRNA co-expression analysis could provide clues to detect differentially expressed lncRNAs and mRNAs in meaningful pathways. A total of 575 lncRNAs and 110 mRNAs were included in networks of four meaningful pathways.
The neighboring mRNAs tend to have high correlation with lncRNAs in IAs. We studied the genomic position of differentially expressed lncRNA and mRNA to identify the nearest coding genes in our microarray dataset. Moreover, we’ve redone the lncRNAs-mRNAs (4129 lncRNAs, 2926 mRNAs) co-expression analysis for IAs. Surprisingly, 47.33% of IA mRNAs were adjacent to lncRNAs within 1KB up- and downstream of lncRNAs (Correlation coefficient (Cor) ≥ 0.9 or ≤ -0.9; P < 0.05). Even focusing on the Cor ≥ 0.8 or ≤ -0.8, there were still about 29.85% of the nearest mRNAs surrounded by lncRNAs. The genomic position and orientation of lncRNAs and mRNAs do have correlation with their Cor value. LncRNAs may regulate the expression of neighboring mRNAs at the level of chromatin modification, transcription and post-transcriptional processing [9, 20]. Some positive/negative correlations of lncRNAs and neighboring mRNAs were observed in the microarray. LncRNAs can regulate transcription by acting as enhancers or co-factors and they can influence gene promoters through interacting with initiation complex [21–23]. Moreover, some lncRNAs, like antisense lncRNAs, have the ability to regulate mRNAs expression through splicing, editing and translation in the post-transcriptional processing [24, 25].
It reported that AGTR1 could regulate blood pressure and promote angiogenesis [26]. MYLK can encode smooth muscle and nonmuscle isoforms. Causal mutation of MYLK was identified in thoracic aortic aneurysm patients [27]. Recently, Yan et al. performed a genetic study of IAs and it indicated that ADAMTS15 might have antiangiogenic activity [4]. Therefore, these genes played important roles in regulating vascular smooth muscle contraction pathway (Figure 5A). Besides, the differentially expressed microRNAs (miRNAs) were crucial to the pathophysiology of IAs [6, 28]; the miRNA/mRNA profiling and regulatory network of IAs also showed similar results in vitro or in vivo [7, 29]. Serum miRNAs might be novel biological markers that were useful in assessing the likelihood of IAs occurrence and development [30]. These miRNAs also have warning effect for the rupture [31].
The vascular remodeling and inflammation response played important roles in the pathogenesis of IAs [32, 33]. The rupture of IAs often lead to extremely high morbidity and mortality [34]. It reported immune response and inflammatory response were more enhanced in ruptured aneurysms [35–37]. Macrophage infiltration and M1/M2 imbalance were strongly associated with aneurysm rupture [38, 39]. Smooth muscle cells (SMCs) migrated into the intima and produced myointimal hyperplasia due to endothelial injury and proliferation [40]. Concurrently, SMCs underwent phenotypic modulation to a proinflammatory phenotype which led to IA formation [41]. Therefore, we overlapped the differentially expressed mRNAs between ruptured IAs or unruptured IAs and STAs to avoid the genetic basis of their development.
There are limitations in our study. Agilent mRNAs profile was performed on 12 IA patients and Affymetrix mRNA profile was performed on 15 patients, so many differentially expressed mRNAs may be filtered due to the different platforms. However, the mRNAs were isolated from both Agilent and Affymetrix platforms to enlarge the number of samples and increase the statistical power. The identified mRNAs of two microarrays overlapped were more likely to be differentially expressed genes. STAs were selected because of the difficulties in obtaining intracranial arteries from humans and they have been widely used as controls in previous studies [42–44]. The morphological and phenotypic differences between STAs and intracranial arteries may affect the final results, which caused inevitable bias. Moreover, this study was mainly based on microarray analysis and bioinformatics analysis. LncRNAs involved in the identified pathways may further narrow the scope of future explorations. Therefore, further studies were needed to confirm these lncRNAs functions with knockdown and over-expression experiments. The manuscript was our preliminary work and future work remains to be done.
In conclusion, we identified 4129 differentially expressed lncRNAs and 2926 differentially expressed mRNAs from the microarrays of IA patients and STAs. The co-expression networks also indicated the correlations between lncRNAs and mRNAs in IA patients. These findings may partly explain the pathogenesis of the formation and development of IAs and provide clues to find key roles for IA patients.
MATERIALS AND METHODS
Patients and samples
We enrolled 27 IA patients who were diagnosed with saccular cerebral aneurysms and underwent microsurgical clipping in Beijing Tiantan Hospital. Further, we collected 27 STAs that were injured during the pterional craniotomies and lateral frontal craniotomies in our study. The informed consent was obtained at enrollment and our study was approved by the Ethics Committee in Beijing Tiantan hospital. The study was carried out in accordance with the Declaration of Helsinki and all methods were performed in accordance with the relevant guidelines and regulations.
The clinical characteristics of all IA patients are summarized in Table 1. We obtained aneurismal and STAs tissues in operations and stored at -80°C freezer until RNA extraction. RNA was extracted by using TRIzol reagent (Invitrogen, Grand Island, NY, USA) according to the manufacturer's instructions. The quality evaluation was determined by using Spectrophotometer (NanoDrop ND-1000) and Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
LncRNA and mRNA microarrays
The Agilent Human 4 × 180K lncRNA and mRNA Microarrays (Agilent, Santa Clara, CA, USA) were performed on 12 IA tissues and 12 STAs by Gene Expression Hybridization Kit (Agilent, Santa Clara, CA, US) according to the manufacturer's instructions. The Affymetrix Human Genome U133 GeneChip Microarrays (Affymetrix, Santa Clara, California) were performed on 15 IA tissues and 15 STAs according to the manufacturer's instructions. We selected several genes randomly and examined their expression levels with quantitative real-time polymerase chain reaction (qRT-PCR). The qRT-PCR results matched well with the microarray data. The microarray data can be obtained at the Gene Expression Omnibus (GEO) database (GSE75436;http://www.ncbi.nlm.nih.gov/geo). Analyses of the arrays were performed using R software (version 3.2.3).
The differentially expressed lncRNAs and mRNAs were filtered by at least P < 0.05 and false discovery rate (FDR) < 0.05. To avoid the factors of IA rupture, we compared the differentially expressed lncRNAs and mRNAs between ruptured IA and unruptured IA with STAs, respectively (Figure 1).
Statistical analysis
All statistical data was analyzed by using SPSS (version 22; SPSS Inc., Chicago, IL, USA). A two-sided P value of < 0.05 was regarded as statistically significant. The raw data was normalized using Quantile Algorithm in Gene Spring Software 13.0 (Agilent technologies, CA, USA).
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed by DAVID (The Database for Annotation, Visualization and Integrated Discovery,https://david-d.ncifcrf.gov/home.jsp).
SUPPLEMENTARY MATERIALS AND TABLES
ACKNOWLEDGMENTS AND FUNDING
This work was supported by grants from National High Technology Research and Development Program (No. 2012AA02A508), the “13th Five-Year Plan” National Science and Technology supporting plan (No. 2015BAI09B04).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
REFERENCES
- 1.Krings T, Mandell DM, Kiehl TR, Geibprasert S, Tymianski M, Alvarez H, terBrugge KG, Hans FJ. Intracranial aneurysms: from vessel wall pathology to therapeutic approach. Nat Rev Neurol. 2011;7:547–559. doi: 10.1038/nrneurol.2011.136. [DOI] [PubMed] [Google Scholar]
- 2.Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10:626–636. doi: 10.1016/S1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 3.Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8:635–642. doi: 10.1016/S1474-4422(09)70126-7. [DOI] [PubMed] [Google Scholar]
- 4.Yan J, Hitomi T, Takenaka K, Kato M, Kobayashi H, Okuda H, Harada KH, Koizumi A. Genetic study of intracranial aneurysms. Stroke. 2015;46:620–626. doi: 10.1161/STROKEAHA.114.007286. [DOI] [PubMed] [Google Scholar]
- 5.Yu L, Fan J, Wang S, Zhang D, Wang R, Zhao Y, Zhao J. Gene expression profiles in intracranial aneurysms. Neurosci Bull. 2014;30:99–106. doi: 10.1007/s12264-013-1398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu D, Han L, Wu X, Yang X, Zhang Q, Jiang F. Genome-wide microRNA changes in human intracranial aneurysms. BMC neurology. 2014;14:188. doi: 10.1186/s12883-014-0188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Y, Zhang M, He H, Chen J, Zeng H, Li J, Duan R. MicroRNA/mRNA profiling and regulatory network of intracranial aneurysm. BMC medical genomics. 2013;6:36. doi: 10.1186/1755-8794-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowalczyk MS, Higgs DR, Gingeras TR. Molecular biology: RNA discrimination. Nature. 2012;482:310–311. doi: 10.1038/482310a. [DOI] [PubMed] [Google Scholar]
- 9.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 10.Fang Y, Roles Fullwood MJ. Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genomics Proteomics Bioinformatics. 2016;14:42–54. doi: 10.1016/j.gpb.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang X, Ning Q. The emerging roles of long noncoding RNAs in common cardiovascular diseases. Hypertens Res. 2015;38:375–379. doi: 10.1038/hr.2015.26. [DOI] [PubMed] [Google Scholar]
- 12.Pasmant E, Sabbagh A, Vidaud M, Bieche I. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB J. 2011;25:444–448. doi: 10.1096/fj.10-172452. [DOI] [PubMed] [Google Scholar]
- 13.Helgadottir A, Thorleifsson G, Magnusson KP, Gretarsdottir S, Steinthorsdottir V, Manolescu A, Jones GT, Rinkel GJ, Blankensteijn JD, Ronkainen A, Jaaskelainen JE, Kyo Y, Lenk GM, et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- 14.Foroud T, Koller DL, Lai D, Sauerbeck L, Anderson C, Ko N, Deka R, Mosley TH, Fornage M, Woo D, Moomaw CJ, Hornung R, et al. Genome-wide association study of intracranial aneurysms confirms role of Anril and SOX17 in disease risk. Stroke. 2012;43:2846–2852. doi: 10.1161/STROKEAHA.112.656397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Q, Tan J, Yu B, Shi W, Liang K. Long noncoding RNA HIF1A-AS1A reduces apoptosis of vascular smooth muscle cells: implications for the pathogenesis of thoracoabdominal aorta aneurysm. Pharmazie. 2015;70:310–315. [PubMed] [Google Scholar]
- 16.Li MH, Chen SW, Li YD, Chen YC, Cheng YS, Hu DJ, Tan HQ, Wu Q, Wang W, Sun ZK, Wei XE, Zhang JY, Qiao RH, et al. Prevalence of unruptured cerebral aneurysms in Chinese adults aged 35 to 75 years: a cross-sectional study. Ann Intern Med. 2013;159:514–521. doi: 10.7326/0003-4819-159-8-201310150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Zhang F, Gao C, Ma X, Peng X, Kong D, Hao J. Microarray Analysis of lncRNA and mRNA Expression Profiles in Patients with Neuromyelitis Optica. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sui J, Li YH, Zhang YQ, Li CY, Shen X, Yao WZ, Peng H, Hong WW, Yin LH, Pu YP, Liang GY. Integrated analysis of long non-coding RNAassociated ceRNA network reveals potential lncRNA biomarkers in human lung adenocarcinoma. Int J Oncol. 2016;49:2023–2036. doi: 10.3892/ijo.2016.3716. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Wang W, Zhang L, Lan Q, Wang J, Cao Y, Zhao J. Identification of a Long Noncoding RNA-Associated Competing Endogenous RNA Network in Intracranial Aneurysm. World Neurosurg. 2016 doi: 10.1016/j.wneu.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Mattick JS, Gagen MJ. The evolution of controlled multitasked gene networks: the role of introns and other noncoding RNAs in the development of complex organisms. Mol Biol Evol. 2001;18:1611–1630. doi: 10.1093/oxfordjournals.molbev.a003951. [DOI] [PubMed] [Google Scholar]
- 21.Ashe HL, Monks J, Wijgerde M, Fraser P, Proudfoot NJ. Intergenic transcription and transinduction of the human beta-globin locus. Genes Dev. 1997;11:2494–2509. doi: 10.1101/gad.11.19.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng J, Bi C, Clark BS, Mady R, Shah P, Kohtz JD. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20:1470–1484. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martianov I, Ramadass A, A Serra Barros, Chow N, Akoulitchev A. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature. 2007;445:666–670. doi: 10.1038/nature05519. [DOI] [PubMed] [Google Scholar]
- 24.He Y, Vogelstein B, Velculescu VE, Papadopoulos N, Kinzler KW. The antisense transcriptomes of human cells. Science. 2008;322:1855–1857. doi: 10.1126/science.1163853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beltran M, Puig I, Pena C, Garcia JM, Alvarez AB, Pena R, Bonilla F, de Herreros AG. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev. 2008;22:756–769. doi: 10.1101/gad.455708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh E, Kim JY, Cho Y, An H, Lee N, Jo H, Ban C, Seo JH. Overexpression of angiotensin II type 1 receptor in breast cancer cells induces epithelial-mesenchymal transition and promotes tumor growth and angiogenesis. Biochimica et biophysica acta. 2016;1863:1071–1081. doi: 10.1016/j.bbamcr.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Proost D, Vandeweyer G, Meester JA, Salemink S, Kempers M, Ingram C, Peeters N, Saenen J, Vrints C, Lacro RV, Roden D, Wuyts W, Dietz HC, Mortier G, Loeys BL, Van Laer L. Performant Mutation Identification Using Targeted Next-Generation Sequencing of 14 Thoracic Aortic Aneurysm Genes. Hum Mutat. 2015;36:808–814. doi: 10.1002/humu.22802. [DOI] [PubMed] [Google Scholar]
- 28.Bekelis K, Kerley-Hamilton JS, Teegarden A, Tomlinson CR, Kuintzle R, Simmons N, Singer RJ, Roberts DW, Kellis M, Hendrix DA. MicroRNA and gene expression changes in unruptured human cerebral aneurysms. J Neurosurg. 2016:1–10. doi: 10.3171/2015.11.JNS151841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holcomb M, Ding YH, Dai D, McDonald RJ, McDonald JS, Kallmes DF, Kadirvel R. RNA-Sequencing Analysis of Messenger RNA/MicroRNA in a Rabbit Aneurysm Model Identifies Pathways and Genes of Interest. AJNR Am J Neuroradiol. 2015;36:1710–1715. doi: 10.3174/ajnr.A4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li P, Zhang Q, Wu X, Yang X, Zhang Y, Li Y, Jiang F. Circulating microRNAs serve as novel biological markers for intracranial aneurysms. Journal of the American Heart Association. 2014;3:e000972. doi: 10.1161/JAHA.114.000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin H, Li C, Ge H, Jiang Y, Li Y. Circulating microRNA: a novel potential biomarker for early diagnosis of intracranial aneurysm rupture a case control study. Journal of translational medicine. 2013;11:296. doi: 10.1186/1479-5876-11-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penn DL, Witte SR, Komotar RJ, E Sander Connolly., Jr The role of vascular remodeling and inflammation in the pathogenesis of intracranial aneurysms. Journal of clinical neuroscience. 2014;21:28–32. doi: 10.1016/j.jocn.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013;44:3613–3622. doi: 10.1161/STROKEAHA.113.002390. [DOI] [PubMed] [Google Scholar]
- 34.Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R. and International Subarachnoid Aneurysm Trial Collaborative G. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 35.Kleinloog R, Verweij BH, van der Vlies P, Deelen P, Swertz MA, de Muynck L, P Van Damme, Giuliani F, Regli L, van der Zwan A, JW Berkelbach van der Sprenkel, Han KS, et al. RNA Sequencing Analysis of Intracranial Aneurysm Walls Reveals Involvement of Lysosomes and Immunoglobulins in Rupture. Stroke. 2016;47:1286–1293. doi: 10.1161/STROKEAHA.116.012541. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Wan JQ, Zhou JP, Fan YL, Jiang JY. Gene expression analysis of ruptured and un-ruptured saccular intracranial aneurysm. European review for medical and pharmacological sciences. 2013;17:1374–1381. [PubMed] [Google Scholar]
- 37.Marchese E, Vignati A, Albanese A, Nucci CG, Sabatino G, Tirpakova B, Lofrese G, Zelano G, Maira G. Comparative evaluation of genome-wide gene expression profiles in ruptured and unruptured human intracranial aneurysms. Journal of biological regulators and homeostatic agents. 2010;24:185–195. [PubMed] [Google Scholar]
- 38.Frosen J, Piippo A, Paetau A, Kangasniemi M, Niemela M, Hernesniemi J, Jaaskelainen J. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35:2287–2293. doi: 10.1161/01.STR.0000140636.30204.da. [DOI] [PubMed] [Google Scholar]
- 39.Hasan D, Chalouhi N, Jabbour P, Hashimoto T. Macrophage imbalance (M1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: preliminary results. Journal of neuroinflammation. 2012;9:222. doi: 10.1186/1742-2094-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosierkiewicz TA, Factor SM, Dickson DW. Immunocytochemical studies of atherosclerotic lesions of cerebral berry aneurysms. Journal of neuropathology and experimental neurology. 1994;53:399–406. doi: 10.1097/00005072-199407000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Nakajima N, Nagahiro S, Sano T, Satomi J, Satoh K. Phenotypic modulation of smooth muscle cells in human cerebral aneurysmal walls. Acta neuropathologica. 2000;100:475–480. doi: 10.1007/s004010000220. [DOI] [PubMed] [Google Scholar]
- 42.Peters DG, Kassam AB, Feingold E, Heidrich-O’Hare E, Yonas H, Ferrell RE, Brufsky A. Molecular anatomy of an intracranial aneurysm: coordinated expression of genes involved in wound healing and tissue remodeling. Stroke. 2001;32:1036–1042. doi: 10.1161/01.str.32.4.1036. [DOI] [PubMed] [Google Scholar]
- 43.Shi C, Awad IA, Jafari N, Lin S, Du P, Hage ZA, Shenkar R, Getch CC, Bredel M, Batjer HH, Bendok BR. Genomics of human intracranial aneurysm wall. Stroke. 2009;40:1252–1261. doi: 10.1161/STROKEAHA.108.532036. [DOI] [PubMed] [Google Scholar]
- 44.Nakaoka H, Tajima A, Yoneyama T, Hosomichi K, Kasuya H, Mizutani T, Inoue I. Gene expression profiling reveals distinct molecular signatures associated with the rupture of intracranial aneurysm. Stroke. 2014;45:2239–2245. doi: 10.1161/STROKEAHA.114.005851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


