Abstract
Prostate cancer can be targeted by ligands to the prostate-specific membrane antigen (PSMA). We aimed to evaluate dosimetry, safety and efficacy of 177Lu-PSMA-617 radioligand therapy (RLT) in patients with metastatic castration-resistant prostate cancer (mCRPC).
Fifteen patients each received two cycles of 3.7 GBq (n = 5) or 6.0 GBq (n = 10) 177Lu-PSMA-617 at an eight to ten weeks interval. For safety monitoring, each treatment was followed by dosimetry with serial quantitative SPECT as well as inpatient and outpatient recording of adverse events. Response to RLT was primarily determined by baseline to follow-up change in 68Ga-PSMA PET/CT (RECIST1.1), as well as change in prostate-specific antigen (PSA), quality of life (QoL, FACT-P scale), and pain (Brief Pain Inventory) as secondary endpoints.
Radiation dose delivered to the tumor (6.1 Gy/GBq) was six to twelve-fold higher than to critical organs (kidney left/right 0.5/0.6 Gy/GBq each, salivary glands 1.0 Gy/GBq). Total radiation dose per kidney did not exceed 23 Gy in any patient. Three patients had sub-acute and latent grade 3 events, i.e. anemia, leukocytopenia, and nausea. No acute events, grade ≥4 events or high grade events for salivary gland or kidney function were observed. After two RLT cycles, 4 (27%) patients had partial response, 6 (40%) had stable disease, and 5 (33%) had progressive disease according to RECIST. Any PSA decline was observed in 12/15 (80%) patients during RLT. Significant pain relief was documented in 7/10 (70%) symptomatic patients and QoL improved in 9/15 (60%) patients.
177Lu-PSMA-617 therapy proved safe and indicated promising response rates for both objective and patient-reported outcomes in our small group of mCRPC patients.
Keywords: mCRPC, PET, prostate cancer, PSMA, lutetium
INTRODUCTION
After non-melanoma skin tumors, prostate cancer (PCa) is the second most common malignancy in men, and ultimately causes more than 250,000 deaths worldwide each year [1]. Radionuclide therapy has become an effective treatment option for metastatic disease with the approval of bone seeking radiopharmaceuticals; systemic application of the calcium ion mimetic 223Ra improves survival in patients with castration-resistant PCa and symptomatic bone metastases [2]. However, about one third of patients with metastatic castration-resistant prostate cancer (mCRPC) present with lymph node or visceral metastases, which are un-responsive to bone-seeking agents [3]. Radiolabeled ligands to the prostate-specific membrane antigen (PSMA) have recently been developed to image and target systemic disease. PSMA is over-expressed in PCa, and this over expression increases further in cases of de-differentiated, metastatic or hormone-refractory disease [4]. The 177Lu labeled small molecule ligand DKFZ-PSMA-617 (177Lu-PSMA-617) binds with high affinity to PSMA in vitro and in vivo [5-6]. The potential of 177Lu-PSMA-617 for radioligand therapy (RLT) has been explored in several preclinical and clinical studies, among which our previous report on human dosimetry demonstrated favorable body distribution and high tumor-to-organ uptake ratios [6]. Based on our dosimetry calculations, we identified kidney as activity-limiting organ and recommended 6.0 GBq as an appropriate activity for the initial 177Lu-PSMA-617 RLT cycle [6], giving high tumor dosing without excessive irradiation of vulnerable healthy organs. Following upon this result, we now aim to provide data on clinical safety and efficacy of 177Lu-PSMA-617 RLT in an expanded patient cohort after a total of 30 177Lu-PSMA-617 RLT cycles. Efficacy was based both on objective endpoints and patient-reported metrics, including pain intensity and quality of life (QoL) scores. To complete dose safety evaluation, we further aimed to perform accurate dosimetry of salivary glands, the organ with highest absorbed radiation dose.
RESULTS
Characteristics of the study cohort
Baseline clinical and demographic characteristics are given in Table 1. All patients completed two cycles of RLT. Five patients were unfit for chemotherapy prior to RLT. 14 of 15 (93%) patients underwent Abiraterone or Enzalutamide therapy before 177Lu-PSMA-617 RLT. One patient did not tolerate second or third line hormonal therapy. Patients had undergone a median of 3 (range, 1 to 5) courses of hormonal therapy prior to admission. Patients with ongoing hormonal therapy at the start of RLT continued their therapy until at least the final follow-up in the present study. None of the patients discontinued gonadotropin-releasing hormone analogs within three months before RLT.
Table 1. Baseline characteristics of the patients.
| Characteristic (n = 15) | Median (range) or total number (%) | |
|---|---|---|
| Age | 73 | (54 - 81) |
| ECOG | ||
| 0 | 5 | (33%) |
| 1 | 5 | (33%) |
| 2 | 5 | (33%) |
| Gleason sum | ||
| 7 | 1 | (7%) |
| 8 | 1 | (7%) |
| 9-10 | 13 | (87%) |
| Sites of metastases | ||
| Bone | 14 | (93%) |
| Lymph node | 12 | (80%) |
| Liver | 3 | (20%) |
| Other | 2 | (13%) |
| Lung | 1 | (7%) |
| Mean pain intensity score at baseline (0-10) | ||
| 0 | 5 | (33%) |
| 1-5 | 8 | (53%) |
| 6-10 | 2 | (13%) |
| Biochemical values | ||
| Lactate dehydrogenase (U/L) | 287 | (167 - 1220) |
| Hemoglobin (g/dL) | 12.3 | (8.3 - 15.5) |
| Total alkaline phosphatase (U/L) | 147 | (49 - 420) |
| PSA (μg/L) | 388 | (3.2 - 10661) |
| No. of prior hormonal therapies | ||
| 1 | 1 | (7%) |
| 2 | 4 | (27%) |
| 3 | 7 | (47%) |
| ≥4 | 3 | (20%) |
| No. of prior chemotherapy regimens | ||
| 0 | 5 | (33%) |
| 1 | 5 | (33%) |
| ≥2 | 5 | (33%) |
| Prior 223Ra | 5 | (33%) |
| Prior EBRT | 9 | (60%) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group Performance Status; EBRT, External Beam Radiation Therapy.
Dosimetry
Mean±SD radiation dose by organ calculated from serial quantitative SPECT and blood activity concentrations is given in Table 2. The highest organ radiation dose was observed for salivary glands (1.0±0.6 Gy/GBq) and kidneys (right/left 0.6±0.2/0.5±0.3 Gy/GBq). The mean radiation dose to tumor lesions was 6.1±4.9 Gy/GBq resulting in a mean tumor-to-kidney dose ratio of 11.1 and mean tumor-to-salivary-gland ratio of 6.1. Liver, spleen and bone marrow all received doses ≤ 0.1 Gy/GBq. Maximum cumulative kidney dose (10.3 Gy) and salivary gland dose (16.5 Gy) were below the dose range reported as critical (23 Gy for kidney [7]; 26-50 Gy [8-9] for salivary glands).
Table 2. Radiation dose after 30 cycles of .
| Organ (n = 30) | Mean (Gy/GBq) | SD |
|---|---|---|
| Dose limiting organs | ||
| Kidney left | 0.5 | 0.3 |
| Kidney right | 0.6 | 0.2 |
| Salivary glands* | 1.0 | 0.6 |
| Non-dose limiting organs | ||
| Liver | 0.1 | 0.1 |
| Spleen | 0.1 | 0.1 |
| Bone marrow | 0.002 | 0.005 |
| Tumor‡ | 6.1 | 4.9 |
determined in ten patients with 2×6.0 GBq 177Lu-PSMA-617 RLT, n = 20; ‡up to three lesions with highest tracer uptake per patient and cycle (n = 5 visceral metastases, n = 12 LN metastases, n = 22 bone metastases).
Safety
No acute or grade ≥4 adverse events were observed. Sub-acute and latent adverse events occurring in at least one patient are listed in Table 3. Grade 1 and 2 adverse events occurred almost equally often in patients with 3.7 versus 6.0 GBq RLT (3.4 events/patient versus 4.2 events/patient). During inpatient stay, one instance of grade 3 anemia, and during follow-up one case of grade 3 leukocytopenia were noted in patients with 6.0 GBq RLT; both conditions had improved at final follow-up without requiring transfusion or bone marrow stimulation. No event for neutropenia was recorded. At intermediate follow-up, grade 3 nausea was noted in one patient after the second cycle of 3.7 GBq RLT, which responded well to antiemetic medication. Mild or transient xerostomia was reported both for patients with salivary gland dose above (n = 4) versus below (n = 3) the median. One patient presented with new onset of epigastric pain and markedly elevated gamma-glutamyl transferase at final follow-up. PET/CT demonstrated progression of liver metastases with infiltration of hepatic veins and intrahepatic cholestasis. The patient died six weeks later from acute liver failure associated with progressive tumor infiltration. In total, four patients died from PCa-related events during the observation period, at 24, 28, 36 and 42 weeks after start of RLT.
Table 3. Adverse events after 30 cycles of .
| Adverse event | Latent | Sub-acute | ||
|---|---|---|---|---|
| All grades | Grade 3 | All grades | Grade 3 | |
| Renal | ||||
| Glomerular filtration rate (GFR) | 8 (53%) | 0 | 1 (7%) | 0 |
| Tubular extraction rate (TER) | 2 (13%) | 0 | - | - |
| Hyperkalemia | 4 (27%) | 0 | 0 | 0 |
| Hematologic | ||||
| Anemia | 3 (20%) | 0 | 9 (60%) | 1 (7%) |
| Thrombocytopenia | 2 (13%) | 0 | 1 (7%) | 0 |
| Leukocytes | 7 (47%) | 1 (7%) | 1 (7%) | 0 |
| Liver | ||||
| Bilirubin | 0 | 0 | 1 (7%) | 0 |
| AST/ALT | 2 (13%) | 0 | 1 (7%) | 0 |
| Other | ||||
| Fatigue | 5 (33%) | 0 | 0 | 0 |
| Dry mouth | 7 (47%) | 0 | 0 | 0 |
| Nausea | 5 (33%) | 1 (7%) | 0 | 0 |
| Dysgeusia | 3 (20%) | 0 | 0 | 0 |
Absolute number of adverse events (%) are given.
Efficacy
Response after one cycle and two cycles of RLT is given separately in Table 4. Ten (67%) patients had measurable target lesions by RECIST1.1. According to RECIST1.1, 4 (27%) patients had PR, 6 (40%) patients had SD, and 5 (33%) patients had PD. Images from two patients with partial response by CT criteria are shown in Figures 1 and 2. Each 7 (47%) patients had biochemical partial response by PSA level after the first and second cycle, respectively. Bone pain was completely resolved in 3 (20%) and responded well in 4 (27%) symptomatic patients after two RLT cycles. One and 3 (20%) patients had increase in pain intensity after the first and second RLT cycle, respectively. QoL was improved in 8 (53%) patients and 3 (20%) patients had a 30% or more increase in QoL score after the second cycle.
Table 4. Response after two cycles .
| n=15 | Objective response | Patient reported outcomes | ||||
|---|---|---|---|---|---|---|
| RECIST* | PSA | Pain | QoL | |||
| After one cycle | ||||||
| CR | - | 0 | 1 (7%) | - | ||
| PR | - | 7 (47%)** | 6 (40%) | - | ||
| SD | - | 5 (33%) | 2 (13%) | - | ||
| PD | - | 3 (20%) | 1 (7%) | - | ||
| After two cycles | ||||||
| CR | 0 | 0 | 3 (20%) | 0 | ||
| PR | 4 (27%) | 7 (47%)** | 4 (27%) | 3 (20%) | ||
| SD | 6 (40%) | 5 (33%) | 0 | 11 (73%) | ||
| PD | 5 (33%) | 3 (20%) | 3 (20%) | 1 (7%) | ||
primary outcome,
≥30% decline in PSA; Abbreviations: RECIST, Response Evaluation Criteria in Solid Tumors; PSA, Prostate-Specific Antigen; QoL, quality of life; SD, stable disease; PR, partial response; PD, progressive disease; CR, complete response.
Figure 1. Response after two cycles of 6.0 GBq 177Lu-PSMA-617 RLT.
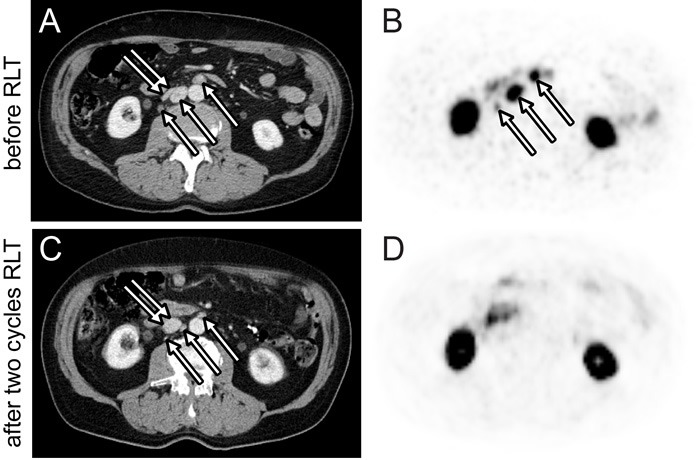
Axial 68Ga-PSMA PET B. and D. and CT A. and C. images of the abdomen before (A and B) and after (C and D) two RLT cycles. Lymph node metastases demonstrate a >30% baseline to follow-up decrease in short axis diameter (A and C, arrows) and SUVmax (B, arrows). Compression of the inferior vena cava by lymph node metastases at baseline (A, double arrow) was resolved at follow-up (C, double arrow).
Figure 2. Response after two cycles of 6.0 GBq 177Lu-PSMA-617 RLT.
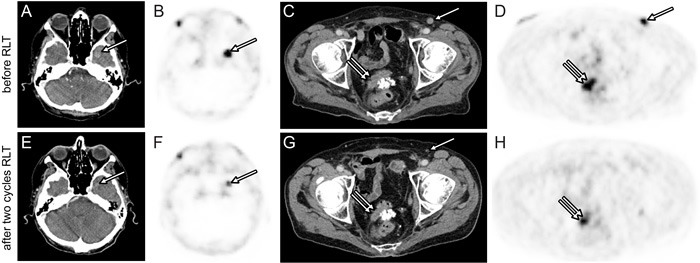
Axial 68Ga-PSMA PET B., D., F., H. and CT A., C., E., G. images of the base of the skull and the pelvis before (A, B, C, D) and after (E, F, G, H) two RLT cycles. Brain metastasis (A, B, E, F, arrow) and local recurrence (C, D, G, H, double arrow) demonstrate a >30% baseline to follow-up decrease in largest diameter and SUVmax. Inguinal lymph node metastasis (C, D, G, arrow) shows complete response on follow-up PET/CT.
Best response observed for PSA, pain score and final response by QoL score are shown for each patient in Figure 3. Nine (60%) patients had a PSA decline of 50% or more during RLT. Response by baseline to follow-up change in AP level or 68Ga-PSMA uptake are given in Supplemental Table 1.
Figure 3. Best response after 30 cycles of 177Lu-PSMA-617 RLT in 15 patients.
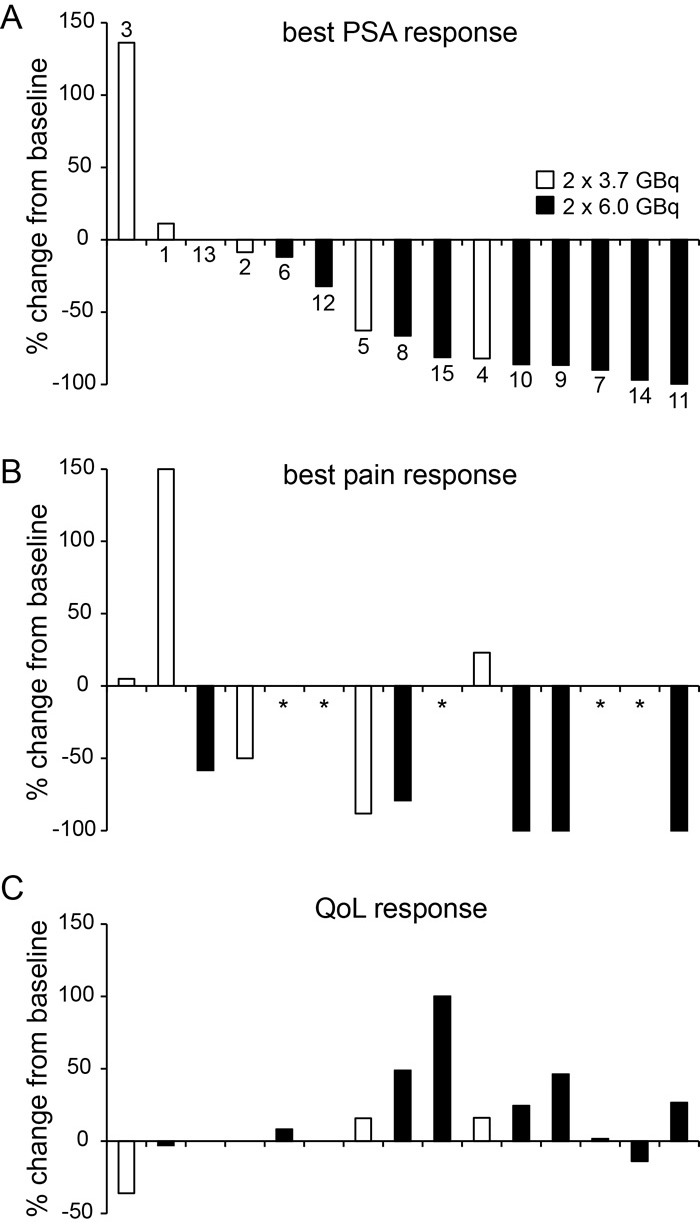
Individual response by lowest PSA A., lowest pain B. and quality of life (QoL) level C. from start until follow-up after the second RLT cycle is shown. Individual results were arranged by best PSA response and separated by regimen (white bars: 2×3.7 GBq; black bars: 2×6.0 GBq). Patient number is given in (A). Bars indicate baseline to follow-up change in percent. *no baseline pain noted.
DISCUSSION
Dosimetry, safety and efficacy of 177Lu-PSMA-617 RLT were analyzed in 15 patients after a total of 30 applications. Treatment response was determined by RECIST1.1 and a range of secondary endpoints, including change in PSA and patient reported outcomes (pain, quality of life). In general, we find that 177Lu-PSMA-617 RLT was tolerated well for both activity regimens, i.e. 3.7 or 6.0 GBq per cycle. Dose limits for critical organs (kidney, 23 Gy [7]; salivary glands, 26-50 Gy [8-9]) were not exceeded in any patient. Eight to ten weeks after the second application, tumor extent on CT had stabilized or showed significant reduction in 10 patients corresponding to a disease control rate of 67%. Approximately half of patients experienced pain relief and improvement in QoL after RLT.
Endoradiotherapy using chelator-bound ligands present several advantages over conventional therapy for metastatic prostate cancer. Whereas the 68Ga-labelled compound provides pre-therapeutic staging and target expression levels to PET, the corresponding 177Lu-labelled compound enables tumor irradiation through focal beta emission, while also enabling imaging and dosimetry through partial gamma emission. High efficacy and safety of 177Lu-based RLT was recently proven in a phase III study of patients with advanced-stage mid-gut neuroendocrine tumors (NET). In that study, somatostatin analogue combination therapy with octreotide plus 177Lu-DOTATATE versus octreotide alone demonstrated approximately 80% risk reduction for NET progression, with few serious adverse events [10]. Chelating ligands with high affinity to PSMA have been developed for imaging (68Ga-HBED-PSMA) and RLT (177Lu-PSMA-617) in order to translate this concept into prostate cancer patients [5, 11]. We proved favourable dose distribution, and determined safe initial activity for 177Lu-PSMA-617 RLT (i.e. 6.0 GBq) in an earlier dosimetry trial [6]. This cohort was now expanded by ten patients completing 20 cycles of 6.0 GBq RLT so as to analyze better the clinical safety and efficacy of this regimen. In this expanded cohort, mean tumor-to-kidney dose ratio was higher than the ratio reported for 177Lu-DOTATATE, which is the clinical standard for RLT in NET patients [12]. More accurate dosimetry using quantitative SPECT reveals an approximately 20 to 30% lower radiation dose to salivary glands than previously estimated by planar scintigraphy [6, 13-14]. Accordingly, the critical dose limits bringing loss of salivary function were not exceeded in any patient. Indeed, serious adverse events for kidney or salivary gland function were not recorded, indicating an excellent safety profile of 177Lu-PSMA-617 RLT. Consistent with dosimetry and previous clinical findings [14-17] the rate of hematologic adverse events was low and few hematologic conditions were transient and not life threatening.
Several trials have earlier shown effective reduction in tumor burden and serum PSA levels after administration of 177Lu-PSMA-617 [14-20]. About one third to half of our fifteen patients showed significant improvement after two cycles based on CT size and PSA level. Most remarkably, one patient with left temporal brain metastasis refractory to external beam radiotherapy demonstrated partial response after RLT (Figure 2). PSA decline of 50% and more during RLT was recorded in nine patients, corresponding to a best PSA response rate of 60%. This rate is consistent with prior reports from Ahmadzadehfar et al [15], Kratochwil et al [14] and with findings for other radio-labelled PSMA ligands [21-22]. Rahbar et al and Heck et al demonstrate similar rates for PSA decline after one and two RLT cycles respectively [16, 23]. When compared to findings from a recent study on mCRPC patients, the best PSA response was roughly similar to that for Abiraterone treatment and higher than for Enzalutamide treatment [24]. Notably, all patients with RECIST partial response and 85% of patients with ≥30% final PSA decline underwent the 6.0 GBq RLT regimen. However, single biomarker tests for treatment response are unreliable in end-stage prostate cancer patients [25]. Accuracy of CT response is limited by tumor dissemination and the frequent presence of non-target bone lesions. Change in PSA or AP levels show only modest correlation with clinical benefit [26]. We thus chose to systematically record patient-reported outcomes using established instruments (Brief Pain Inventory, FACT-P) in order to understand better the clinical response to RLT. Patient reported outcome was favourable in those of our patients with decreasing PSA level during 177Lu-PSMA-617 RLT: Seven (47%) patients experienced significant pain relief and 8 (53%) reported increased QoL score after the second cycle.
By including broad range of objective and patient-reported biomarker endpoints, our study provides a detailed analysis of the efficacy of 177Lu-PSMA-617 RLT. Safety was thoroughly documented by dosimetry and frequent follow-up visits for all patients. The present study however has limitations. Although patient inclusion criteria and treatment protocols were based upon pre-defined institutional standards, our retrospective data analysis may have increased the risk for underreporting of adverse events. Furthermore, our inclusion criteria resulted in a small patient cohort, which was nonetheless representative of mCRPC patients seen at our clinic. Furthermore 10 to 20% error for radiation dose calculation might be caused by omitting late SPECT acquisition as previously estimated for planar scintigraphy [27]. Overall results are promising however must be confirmed in a randomized trial with longer follow-up period in order to monitor for radiation induced, delayed events.
In summary, two cycles of 177Lu-PSMA-617 RLT were safe and resulted in promising response rates for objective and patient-reported outcomes in fifteen patients with metastatic castration-resistant prostate cancer. Based on dosimetry more than three cycles of 6.0 GBq RLT can be performed with acceptable safety margin. Our preliminary data encourage future investigations of 177Lu-PSMA-617 RLT in a multicentre study of prospective design.
MATERIALS AND METHODS
Patients
Between September 2014 and May 2016 thirty cycles of 177Lu-PSMA-617 RLT were applied in 15 consecutive patients. The initial five patients received 2 × 3.7 GBq (n = 10 cycles). Those patients had also been included in our previous report on 177Lu-PSMA-617 dosimetry [6]. The following five patients received a higher dose (2 x 6.0 GBq, n = 20 cycles) based on our previous recommendation [6]. RLT was offered in accordance with the current Declaration of Helsinki, paragraph 37 “Unproven Interventions in Clinical Practice” and in accordance with German compassionate use regulations. Indication was confirmed by both a nuclear medicine physician and a urologist or oncologist. Patients meeting the following criteria, consistent with the German Society of Nuclear Medicine consensus panel recommendation [28], were considered for RLT: (a) prostate cancer proven by histopathology, (b) unresectable metastases, (c) castration resistant disease, (d) completed on-label treatment options for castration-resistant disease or unfit for such treatments, (e) objective disease progression by PSA level and imaging, (f) PSMA-avid lesions on pre-therapeutic 68Ga-HBED-PSMA positron emission tomography/computed tomography (PET/CT), (g) white blood cell count (WBC) >3000/μl and platelet count >75000/μl, (h) creatinine <2-fold the upper limit of normal (ULN), (i) AST and ALT <5-fold ULN, and (j) no myelosuppressive therapy within six weeks prior to RLT. All patients gave written informed consent to undergo RLT with subsequent follow-up. Analyses presented in this study were performed retrospectively on anonymized patient data. The study protocol was approved by the local ethics committee and all subjects had provided prior written informed consent.
177Lu-PSMA-617 RLT
DOTA-PSMA-617 precursor was obtained from ABX GmbH (Radeberg, Germany). In brief, DOTA-PSMA-617 was dissolved in 1.5 ml of acetate buffer (pH 4.8) containing 10 mg/ml dihydroxybenzoic acid. The solution was added to no-carrier-added 177LuCl3 in 0.04M HCl obtained from ITG GmbH (Garching, Germany), and heated for 30 min at 90 to 100°C. The amount of precursor was 20 μg/GBq 177Lu. Radiochemical yield of 177Lu-PSMA-617 was greater than 95% and radiochemical purities greater than 99% were achieved in each final PBS-buffered preparation. Each treatment was performed during a four days stay at the Nuclear Medicine ward. 177Lu-PSMA-617 was given by slow i.v. application over twenty minutes. Patients received 50 mg prednisolone and 1L of 0.9% NaCl i.v. or equivalent amounts of p.o. fluids daily until discharge. In order to reduce blood flow and radioligand uptake in parotid and submandibular glands, ice packs were applied locally from ten min prior to 177Lu-PSMA-617 administration, and continued for six hours thereafter.
Safety and dosimetry
A flow diagram for treatment and follow-up is given in Supplemental Figure S1. All patients were monitored daily as in-patients for sub-acute toxicity, with physical examination and routine bloodwork for electrolytes, hematology, liver and kidney function. All patients had two follow-up examinations at four to five and eight to ten weeks after each RLT cycle, including routine bloodwork and one 99mTc-MAG3 renal scintigraphy to monitor latent toxicity. The observation period ended on January 31st 2016. Adverse events for total WBC were graded by CTCAE 3.0 [29]. Adverse events for tubular extraction rate (TER) were defined as follows: grade 1, 67-100% lower limit of normal (LLN); grade 2, 33-66% LLN; grade 3, 0-32% LLN. The National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 was used for all other toxicity [30].
Whole body and organ dosimetry was obtained at each cycle (n = 30) as described previously [6]. Additional quantitative SPECT/CT of the head was performed approximately 24, 48 and 72h after each application of 6.0 GBq 177Lu-PSMA-617 in 10 patients (n = 20) to accurately calculate radiation dose to salivary glands, the organ with highest absorbed radiation dose in our initial study [6].
Response to RLT and statistical analysis
68Ga-HBED-PSMA PET/CT had been performed one to two weeks before the first RTL cycle, and again at eight to ten weeks following the second RLT cycle, as reported previously [31]. Primary endpoint for efficacy was response as determined by RECIST 1.1. Secondary endpoints were change in PSA level, pain intensity score, and QoL score. PSA and, in case of bone metastases, AP blood levels were measured at baseline and each subsequent visit. Progressive disease (PD) was defined as 30% or more increase, partial response (PR) as 30% or more decrease in final PSA/AP levels relative to baseline. Symptomatic patients reported all four pain severity items of the Brief Pain Inventory (BPI) [32] at baseline and again at eight to ten weeks after each cycle. In addition, any change in pain medication was documented. Here, PD was defined as a 30% or greater increase in pain score or increase in pain medication, whereas PR was defined as corresponding 30% or greater decrease or decrease in pain medication. CR was defined as pain score 0 on final follow-up. Patients reported QoL based on Functional Assessment of Cancer Therapy - Prostate questionnaire (FACT-P) [33] at baseline and at eight to ten weeks after the second cycle, with PD and PR defined as changes of at least 30% relative to baseline QoL score. Stable disease (SD) was each defined as non-PD/PR. Results are presented as total number (percent), mean ± standard deviation (SD), or median (range). Change in baseline to follow-up SUVmax was determined as exploratory data for up to three lesions with highest SUVmax on baseline 68Ga-HBED-PSMA PET. Details for AP and PET response are given in the Supplemental Material.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
The authors acknowledge manuscript revision by Inglewood Biomedical Editing. We thank Vera Wenter and our colleagues from the Nuclear Medicine ward and outpatient clinic for their participation in data collection. We thank Anika Brunegraf, Astrid Gosewisch, and nuclear medicine technicians who participated in imaging and dosimetry studies.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
GRANT SUPPORT
None.
REFERENCES
- 1.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker C, Nilsson S, Heinrich D, Helle SI, O'sullivan JM, Fossa SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 3.Pond GR, Sonpavde G, de Wit R, Eisenberger MA, Tannock IF, Armstrong AJ. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol. 2014;65:3–6. doi: 10.1016/j.eururo.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–539. doi: 10.1002/jcb.10661. [DOI] [PubMed] [Google Scholar]
- 5.Benesova M, Schafer M, Bauder-Wust U, Afshar-Oromieh A, Kratochwil C, Mier W, Haberkorn U, Kopka K, Eder M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J Nucl Med. 2015;56:914–920. doi: 10.2967/jnumed.114.147413. [DOI] [PubMed] [Google Scholar]
- 6.Delker A, Fendler WP, Kratochwil C, Brunegraf A, Gosewisch A, Gildehaus FJ, Tritschler S, Stief CG, Kopka K, Haberkorn U, Bartenstein P, Boning G. Dosimetry for (177)Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:42–51. doi: 10.1007/s00259-015-3174-7. [DOI] [PubMed] [Google Scholar]
- 7.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 8.Gensheimer MF, Liao JJ, Garden AS, Laramore GE, Parvathaneni U. Submandibular gland-sparing radiation therapy for locally advanced oropharyngeal squamous cell carcinoma: patterns of failure and xerostomia outcomes. Radiat Oncol. 2014;9:255. doi: 10.1186/s13014-014-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hey J, Setz J, Gerlach R, Janich M, Hildebrandt G, Vordermark D, Gernhardt CR, Kuhnt T. Parotid gland-recovery after radiotherapy in the head and neck region—36 months follow-up of a prospective clinical study. Radiat Oncol. 2011;6:125. doi: 10.1186/1748-717X-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FROM ECC 2015-neuroendocrine cancer: SSA therapies-(177)Lu-DOTATATE is a better one in NETTER-1. Nat Rev Clin Oncol. 2015;12:684. [Google Scholar]
- 11.Eder M, Schafer M, Bauder-Wust U, Hull WE, Wangler C, Mier W, Haberkorn U, Eisenhut M. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–697. doi: 10.1021/bc200279b. [DOI] [PubMed] [Google Scholar]
- 12.Gupta SK, Singla S, Thakral P, Bal CS. Dosimetric analyses of kidneys, liver, spleen, pituitary gland, and neuroendocrine tumors of patients treated with 177Lu-DOTATATE. Clin Nucl Med. 2013;38:188–194. doi: 10.1097/RLU.0b013e3182814ac1. [DOI] [PubMed] [Google Scholar]
- 13.Kabasakal L, AbuQbeitah M, Aygun A, Yeyin N, Ocak M, Demirci E, Toklu T. Pre-therapeutic dosimetry of normal organs and tissues of (177)Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:1976–1983. doi: 10.1007/s00259-015-3125-3. [DOI] [PubMed] [Google Scholar]
- 14.Kratochwil C, Giesel FL, Stefanova M, Benesova M, Bronzel M, Afshar-Oromieh A, Mier W, Eder M, Kopka K, Haberkorn U. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with Lu-177 labeled PSMA-617. J Nucl Med. 2016;57:1170–6. doi: 10.2967/jnumed.115.171397. [DOI] [PubMed] [Google Scholar]
- 15.Ahmadzadehfar H, Eppard E, Kurpig S, Fimmers R, Yordanova A, Schlenkhoff CD, Gartner F, Rogenhofer S, Essler M. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016;7:12477–88. doi: 10.18632/oncotarget.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, Claesener M, Ahmadzadehfar H. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. J Nucl Med. 2016 doi: 10.2967/jnumed.116.173757. [DOI] [PubMed] [Google Scholar]
- 17.Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A, Bal C. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2016 doi: 10.1007/s00259-016-3481-7. [DOI] [PubMed] [Google Scholar]
- 18.Ahmadzadehfar H, Rahbar K, Kurpig S, Bogemann M, Claesener M, Eppard E, Gartner F, Rogenhofer S, Schafers M, Essler M. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;5:114. doi: 10.1186/s13550-015-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kratochwil C, Giesel FL, Eder M, Afshar-Oromieh A, Benesova M, Mier W, Kopka K, Haberkorn U. [(177)Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:987–988. doi: 10.1007/s00259-014-2978-1. [DOI] [PubMed] [Google Scholar]
- 20.Rahbar K, Bode A, Weckesser M, Avramovic N, Claesener M, Stegger L, Bogemann M. Radioligand Therapy With 177Lu-PSMA-617 as A Novel Therapeutic Option in Patients With Metastatic Castration Resistant Prostate Cancer. Clin Nucl Med. 2016;41:522–528. doi: 10.1097/RLU.0000000000001240. [DOI] [PubMed] [Google Scholar]
- 21.Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, Schottelius M, Mueller D, Klette I, Wester HJ. Lutetium-177 PSMA Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J Nucl Med. 2016;57:1006–13. doi: 10.2967/jnumed.115.168443. [DOI] [PubMed] [Google Scholar]
- 22.Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B, Joyal J, Kopka K, Debus J, Babich JW, Haberkorn U. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41:1280–1292. doi: 10.1007/s00259-014-2713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heck MM, Retz M, D’Alessandria C, Rauscher I, Scheidhauer K, Maurer T, Storz E, Janssen F, Schottelius M, Wester HJ, Gschwend JE, Schwaiger M, Tauber R, et al. Systemic Radioligand Therapy with 177Lu Labeled Prostate Specific Membrane Antigen Ligand for Imaging and Therapy in Patients with Metastatic Castration Resistant Prostate Cancer. J Urol. 2016;196:382–91. doi: 10.1016/j.juro.2016.02.2969. [DOI] [PubMed] [Google Scholar]
- 24.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris MJ, Autio KA, Basch EM, Danila DC, Larson S, Scher HI. Monitoring the clinical outcomes in advanced prostate cancer: what imaging modalities and other markers are reliable? Semin Oncol. 2013;40:375–392. doi: 10.1053/j.seminoncol.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Verbel DA, Heller G, Kelly WK, Scher HI. Quantifying the amount of variation in survival explained by prostate-specific antigen. Clin Cancer Res. 2002;8:2576–2579. [PubMed] [Google Scholar]
- 27.Hohberg M, Eschner W, Schmidt M, Dietlein M, Kobe C, Fischer T, Drzezga A, Wild M. Lacrimal Glands May Represent Organs at Risk for Radionuclide Therapy of Prostate Cancer with [(177)Lu]DKFZ-PSMA-617. Mol Imaging Biol. 2016;18:437–445. doi: 10.1007/s11307-016-0942-0. [DOI] [PubMed] [Google Scholar]
- 28.Fendler WP, Kratochwil C, Ahmadzadehfar H, Rahbar K, Baum RP, Schmidt M, Pfestroff A, Lutzen U, Prasad V, Heinzel A, Heuschkel M, Ruf J, Bartenstein P, et al. [177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer] Nuklearmedizin. 2016;55:123–128. [PubMed] [Google Scholar]
- 29.Institute NC. Common Terminology Criteria for Adverse Events v3.0.: NCI, NIH, DHHS 2006 [Google Scholar]
- 30.Institute NC. Common Terminology Criteria for Adverse Events v4.0.: NCI, NIH, DHHS) 2009 [Google Scholar]
- 31.Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C, Gildehaus FJ, Stief CG, Gratzke C, Fendler WP. Ga-PSMA Positron Emission Tomography/Computed Tomography Provides Accurate Staging of Lymph Node Regions Prior to Lymph Node Dissection in Patients with Prostate Cancer. Eur Urol. 2016 doi: 10.1016/j.eururo.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 32.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 33.Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


