Abstract
This article summarizes (1) the recent achievements to further improve symptomatic therapy of motor Parkinson’s disease (PD) symptoms, (2) the still-few attempts to systematically search for symptomatic therapy of non-motor symptoms in PD, and (3) the advances in the development and clinical testing of compounds which promise to offer disease modification in already-manifest PD. However, prevention (that is, slowing or stopping PD in a prodromal stage) is still a dream and one reason for this is that we have no consensus on primary endpoints for clinical trials which reflect the progression in prodromal stages of PD, such as in rapid eye movement sleep behavior disorder (RBD) —a methodological challenge to be met in the future.
Keywords: Parkinson's disease, prodromal stage, motor symptoms, non-motor symptoms, disease modifying treatment
Introduction
The decades of focus on the nigrostriatal system and dopamine replacement therapy
Parkinson’s disease (PD) is a devastating disorder of the human nervous system and the second most common progressive chronic neurodegenerative disease. The three cardinal motor symptoms, akinesia in combination with either tremor at rest or rigidity ( 1 UK brain bank criteria), are—200 years after their description—still the basis of the clinical diagnosis. Up to 2016, we still have no treatment to slow down or even stop the progression of the disease. Available therapy is symptomatic. This article presents evidence that for the first time in history substances with a potentially disease-modifying effect for PD are under development and thus offer hope for the patient, the spouse, and the treating physician.
For more than 100 years, we know the neuropathological hallmarks of the disorder: the so-called Lewy bodies 2 (proteinaceous intracytoplasmic inclusion bodies) containing aggregations of the protein alpha-synuclein 3 and the loss of pigmented melanin containing neurons in the midbrain 4. The latter reflects the neurodegeneration of dopaminergic neurons in the substantia nigra (SN) leading to a marked dopamine deficit in the striatum. Since 1961 5, L-Dihydroxyphenylalanine (L-Dopa), a symptomatic dopamine replacement therapy, has been available for PD for more than 50 years. As L-Dopa, the precursor of dopamine—and subsequently ergot- and non-ergot dopamine agonists— are highly effective in reducing motor symptoms, PD was—for a long time—predominantly considered as a movement disorder. This focus was even enforced by the unraveling of the motor circuitry of the basal ganglia, of its imbalances in PD and the dramatic therapeutic effect of deep brain stimulation (DBS) of the subthalamic nucleus or the globus pallidus. These symptomatic therapeutic achievements may explain why the development of therapies for the wide range of disabling non-motor symptoms (NMSs) the patient with PD has throughout the course of the disease has been neglected. In addition, research efforts on the development of disease-modifying drugs were largely performed in acute toxin-induced rodent models (6-hydroxy-dopamine, or MPTP) and their neuroscientific results failed to translate into clinically successful drugs (reviewed in 6). Thus, apart from few cases of toxin-induced Parkinson syndromes, firm knowledge at the molecular level on the etiopathogenesis of PD was lacking until the year 1996.
Genetic and neuropathological research revolutionize the understanding of Parkinson’s disease
Two discoveries between 1996 and 2006 changed the field:
-
1)
The description of a mutation in the gene for the protein alpha-synuclein, causing a rare form of autosomal-dominant PD 7; the discovery that gene duplication of this gene for normal wild-type alpha-synuclein (that is, the presence of three alleles instead of two alleles leading to a production of 150% of normal alpha-synuclein) causes PD; and the presence of pathological alpha-synuclein-aggregations in the Lewy bodies in the SN 3.
-
2)
The publication of the Braak staging of PD 8 combined with the “dual hit theory” 9 (see below) proposing that the manifestation of motor PD symptoms is a late-stage phenotype preceded for years, if not decades, by three prodromal stages ( Figure 2).
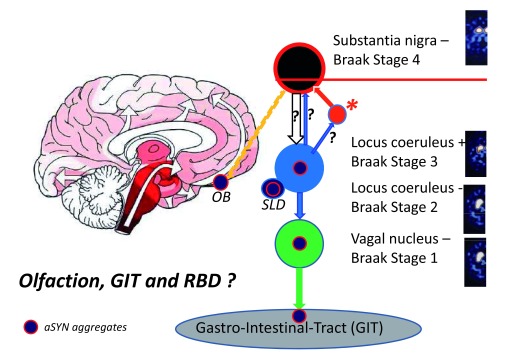
Figure 2. Braak’s hypothesis: staging of Parkinson’s disease.
This simplified hypothetical scheme shows how Parkinson’s disease may develop based on the hypothesis of the Braak staging 8 and the “dual hit theory” 9. OB, olfactory bulb, SLD, sublaterodorsal nucleus of the subcoeruleus/coeruleus complex; lesion of the SLD leads to rapid-eye-movement sleep behavior disorder (RBD). The orange line illustrates the afferent connection from the substantia nigra to the olfactory bulb. The α-synuclein (aSYN) aggregates (red-circled icon) may retrogradely spread from the GIT via the dorsal motor nucleus of the vagal nerve to the locus coeruleus and subsequently ascend to the substantia nigra (Hit 1). It is discussed that the later propagation may either take place by means of direct anterograde transport (thin light blue arrow with question mark – from the LC to the SN) or retrograde transport from the LC to the SN (empty arrow with question mark), or via an intermediate structure (orange circle) such as the amygdala complex (asterix). Likewise the aSYN aggregates (red-circled icon) may retrogradely spread from the OB to the substantia nigra (Hit 2). Dopamine transporter single-photon emission computed tomography (DAT-SPECT): On the right side, images of the striatal presynaptic terminals of the nigrostriatal pathway are visualized by DAT-SPECT. In Braak stage 1, the DAT-SPECT is normal. In Braak stage 2, the DAT-SPECT is still normal (Locus coeruleus −). In Braak stage 3, the nigrostriatal pathway is partially degenerated but less than necessary to cause parkinsonian motor symptoms and signs. The image shows a partial loss of the nigrostriatal dopaminergic terminals in the dorsolateral aspect of the striatum (Locus coeruleus +). The red line indicates the threshold for the extent of the nigrostriatal lesion associated with parkinsonian motor symptoms. In Braak stage 4, the loss of the nigrostriatal terminals in the striatum is so marked that the patient will experience parkinsonian motor symptoms and signs.
The shift from research on symptomatic therapy to the search for Parkinson’s disease-modifying therapy
Based on these findings, the majority of cases with PD (the so-called idiopathic form of PD) were assumed to present an alpha-synucleinopathy. Drug development shifted its focus from transmitters, transmitter-related receptor agonists and antagonists, and transmitter-synthesizing and -degrading enzymes to the protein chemistry, synthesis, transport, aggregation, and degradation of alpha-synuclein and other proteins related to neurodegenerative disorders, such as MAP-Tau or beta-amyloid. A now-20-year-long effort in neuroscience and drug development appears to provide the first results.
This article summarizes (1) the recent achievements to further improve symptomatic therapy of motor PD symptoms, (2) the still-limited attempts to provide symptomatic therapy for NMSs in PD, and (3) the advances in the development and clinical testing of compounds, which promise to offer disease modification in already-manifest PD. However, prevention (that is, slowing or stopping PD in a prodromal stage) is still a dream and one reason for this is that we have no consensus on primary endpoints for clinical trials, which reflect the progression in prodromal stages of PD, such as in rapid eye movement (REM) sleep behavior disorder (RBD), the most specific prodromal stage of PD - a methodological challenge to be met in the future.
Present therapy 2017
Present therapy (2017) available for Parkinson’s disease is symptomatic
In 1960, the lack of dopamine in brains of patients with PD was discovered, and the first rationally derived therapy was introduced to neurology when the “miraculous” improvement of the motor symptoms under therapy with intravenous L-Dopa, the blood-brain barrier-passing precursor of dopamine 5, was reported. Since then, generations of medical students have learned that the symptoms of PD are caused by a dopamine deficit, leading to an imbalance of the motor, cognitive, and emotional loops in the basal ganglia circuitry. Although hard to believe, 56 years after its discovery, L-Dopa is still the gold standard for any of the available multiple symptomatic therapies for PD. Owing to L-Dopa’s short plasma half-life (1–2 hours), repeated intake results in a pulsatile plasma profile. With progressing neurodegeneration of the nigrostriatal pathway, the storage capacity of the central nervous system (CNS) for L-Dopa and dopamine declines, thus in the intermediate to advanced stages of PD, the duration of the central L-Dopa effect will mimic the pulsatile plasma profile of the medication. The longer the disease lasts, the more patients with PD experience “motor complications”, consisting of motor fluctuations (a change between phases of akinesia (OFF: no or low, therapeutically ineffective L-Dopa level) and normal movement (ON: therapeutically effective L-Dopa level) and also of an excess of movements (choreatic “dyskinesia”) at the peak of the L-Dopa curve in the blood and thus in the CNS. To delay or ameliorate these L-Dopa therapy-associated motor complications, several other classes of drugs are available to be prescribed before the use of or in combination with L-Dopa. The L-Dopa effect can be enhanced and prolonged by (1) the combination with peripheral inhibitors—(a) of the degrading enzyme L-DOPA-decarboxylase (that is, benserazide, carbidopa – standard combination) and (b) of the degrading enzyme catechol- O-methyl transferase (COMT) (that is, by adding the short-acting COMT-inhibitor entacapone or intermediate-acting tolcapone)—or with (2) centrally active inhibitors of the degrading enzyme monoamino-oxidase B (MAO-B), such as selegiline or rasagiline. As an advanced therapeutic option, L-Dopa emulsion can be applied by an external pump via a percutaneous tubing into the jejunal cavity in order to provide a nearly constant continuous supply of L-Dopa to the blood and thus to the CNS. In addition, physicians have at hand five non-ergot dopamine agonists, mainly of the dopamine-2-receptor type: pramipexole, ropinirole and piribedil (only registered in Europe) for oral intake, rotigotine by 24-hour transdermal application, and apomorphine, which needs a parenteral administration (that is, subcutaneous: bolus or pump assisted infusion). As pramipexole and ropinirole are available—besides the standard release formulation—as slow release preparations, four non-ergot agonists can offer continuous dopamine-2-receptor stimulation for a 24-hour period. Ergot dopamine agonists are indicated only as a second-line choice, as they run the risk of inducing fibrosis of the heart valves or the retroperitoneum. Furthermore, the N-methyl-D-aspartate (NMDA) (glutamate subtype) receptor antagonist amantadine is considered to improve PD motor symptoms and at the same time to reduce motor complications, especially dyskinesia.
Finally, numerous pharmacotherapies are available for individual NMSs 10 though mostly by employing a given compound approved to treat the symptom or disease per se and not a particular symptom as part of the spectrum of NMSs in PD (for example, using an antidepressant for depression in PD; see below and Seppi et al. 11)
The introduction of DBS further increased the therapeutic options for patients with PD. The pacemaker assisted stimulation of stereotactically, to the millimeter precisely placed electrodes—either in the subthalamic nucleus or the internal part of the globus pallidus—allows reduction of the imbalance in the above-mentioned motor circuitry of the basal ganglia 12. This procedure has been shown to be effective not only in very advanced PD patients but also in PD patients, who just have started to develop motor complications 13. DBS in turn allows one to decrease the amount of pharmacotherapy and this measure leads to less motor (see above) or neuropsychiatric (see below) adverse effects or both, which can occur with the above-mentioned combination of different pharmaceuticals in all stages of PD.
In addition, in the last decade, an increasing number of partly well-designed and conducted studies have investigated the effect of non-medical/non-surgical supportive “activating” therapies; these include physical exercise, physiotherapy (more than 30 trials) 14, 15, 82, 83, dance interventions (4 trials), and logopedic training of dysphagia (2 trials) on the neurological symptoms of PD patients and their quality of life (comprehensively reviewed in 15, 16). Despite all of these achievements until today, we still treat PD at an entirely symptomatic level 17.
The challenge of treating motor AND non-motor symptoms
In summary, neurologists, and especially movement disorder experts, can choose from a large number of compounds to treat the motor symptoms in PD effectively for several years, if not decades. In addition, owing to advances in the field of internal medicine, the surgical disciplines, and anesthesia, patients with PD live longer, but with a price or rather trade off to find the optimal therapeutic balance between motor- and non-motor symptoms with a minimum of adverse effects:
1) With increasing age and duration of PD, gait problems—non-responsive to dopamimetic therapy, combined with the increased risk to fall and to incur fractures—and other NMSs appear. These NMSs include autonomic dysfunctions (for example, urinary incontinence and severe obstipation), sleep impairment, pain syndromes, and most important, neuropsychiatric symptoms, including depression, impulse control disorders, punding, hallucinations, overt psychosis (in part induced by dopamimetic therapy), and cognitive impairment progressing to dementia 10. The NMSs substantially impair the quality of life not only of the PD-patient but also of their family. Thus, in the very advanced stages, the family and the physician face—in many cases—a disoriented, often demented PD patient, who either is mobile, if not hypermobile (dyskinetic), and may hallucinate and even endanger him- or herself or others or who is akinetic-rigid.
2) With increasing age, the extent of comorbidity increases (for example, orthopedic syndromes, diabetes mellitus and metabolic syndrome 16, heart failure, and stroke). This comorbidity in patients with PD is a major challenge in the ambulatory care. Hardly any studies have been performed to assess the efficacy, benefit-risk ratio, and tolerability of available symptomatic PD therapy in these real-life “wild-type” PD patients.
Thus, the weight of therapeutic need has shifted from “just” making or keeping the PD patient mobile to the challenge to fine-tune a therapeutic combination of drugs for (1) the treatment of motor and non-motor symptoms, (2) motor and non-motor complications (including hallucinations, or psychosis induced by dopamimetic therapy, particularly in cognitively impaired patients with PD), and (3) treatment that, in accordance with other medical treatments and care, is acceptable to the patient and the caring partner.
New developments in Parkinson’s disease therapy
With this situation in mind, efforts over the last 20 years to develop new therapies for PD can be divided in two categories: (1) improving symptomatic therapy of (1a) motor and (1b) non-motor symptoms and (2) addressing potential causes of PD, with a focus on the protein alpha-synuclein, its chemistry, synthesis, aggregation, degradation, and interaction with other proteins in order to develop a disease modifying treatment.
New developments in symptomatic therapy of motor symptoms
To improve the available symptomatic therapy for motor symptoms, several drugs have recently been approved or are still under testing. These developments include the improvement of the pump device for infusing L-Dopa in the jejunal cavity (likely available in 2017 or 2018 18) and the approval of a long-acting (5- to 6-hour duration of action) L-Dopa (currently available in the USA under the tradename Rytary 19– 25; Table 1). The latter compound is absorbed not only in the duodenum but also in the ileum; thus, its absorption may depend less on food intake/diet 26. Surprisingly little information is available about its use in daily practice, and no active comparator trial (for example, against the intrajejunal L-Dopa-infusion or DBS) is under way or in planning in PD patients with motor complications.
Table 1. New developments in symptomatic therapy of Parkinson’s disease.
Part A. For motor symptoms and motor complications by means of dopaminergic mode of action: new compound or new formulation, mode of action (fully or in part) provided by other approved compound.
| Compound/
Trade name (Company [C], Sponsor [S]) |
Indication | Mode of action | Phase of
development |
Commentary and
approved dose |
Reference |
|---|---|---|---|---|---|
|
Melevodopa/
Carbidopa Sirio (Chiesi [C]) |
Motor | Modified form of
L-Dopa soluble tablet |
Approved | Marketed in Italy | Zangaglia
et al. 2010
85
Fasano et al. 2014 86 |
|
Opicapone
Ongentys (BIAL [C]) |
Motor
wearing-off |
COMT-inhibitor,
long-acting, add-on to L-Dopa |
Approved | Reimbursed in EU
50 mg/day |
Ferreira
et al. 2015
33
Ferreira et al. 2015 87 Rocca et al. 2016 35 Fabbri et al. 2016 34 |
|
Safinamide
Xadago (Zambon [C]) |
Motor
wearing-off |
MAO-B-inhibitor,
glutamate modulator, add-on to L-Dopa |
Approved | Reimbursed in EU,
active comparator study to other MAO-B- inhibitors not available 50 or 100 mg/day |
Stocchi + Torti 2016
32
Cattaneo et al. 2016 31 Borgohain et al. 2014 29 Borgohain et al. 2014 30 Schapira et al. 2013 28 Stocchi et al. 2012 27 |
|
“XP066”
Rytary (Impax [C]) |
Motor
wearing-off |
L-Dopa/
Carbidopa (4/1) long-acting, extended release |
Approved | Reimbursed in USA
95, 145, 195, or 245 mg L-Dopa capsules |
Yao
et al. 2016
25
Mao + Modi 2016 24 Waters et al. 2015 22 Hsu et al. 2015 23 Stocchi et al. 2014 21 Pahwa et al. 2014 20 Hauser et al. 2013 19 |
Part B. For motor symptoms, motor complications or non-motor symptoms by means of a non-dopaminergic mode of action: new compounds or new formulation.
| Compound | Indication | Mode of action | Phase of
development |
Commentary | Reference |
|---|---|---|---|---|---|
|
Amantadine
Extended release (Adamas [C]) |
Motor
dyskinesia off-time |
NMDA-receptor
antagonist, long-acting |
Phase III
completed |
Likely to be registered
2017 or 2018 as 340 mg/day |
Pahwa
et al. 2016
37
Pahwa et al. 2015 36 |
|
Droxidopa
L-Threo-3,4- Dihydroxy-Phenylserine Northera (Lundbeck [C]) |
Motor and
Non-Motor freezing Neurogenic orthostatic hypotension |
Noradrenaline
precursor |
Approved in Japan, approved in USA 3×100 mg/capsule max. 3×6 capsules (max. daily dose 1,800 mg) |
Hauser
et al. 2014
56
Espay et al. 2014 57 Mathias et al. 2001 55 |
|
|
Istradefylline
Nouriast (Kyowa-Hakko- Kirin [C]) |
Motor
wearing-off |
Adenosine
2A receptor antagonist |
Phase III
positive Phase III ongoing in EU |
Approved in Japan
20 mg/once daily (40 mg/daily possible) |
Vorovenci + Antonini
2015 43 Kondo et al. 2015 40 Pinna 2014 44 Mizuno et al. 2013 39 Pourcher et al. 2012 42 Factor et al. 2010 41 Mizuno et al. 2010 38 |
|
Tozadenant
(Biotie [C]) |
Motor
dyskinesia wearing-off |
Adenosine
2A receptor antagonist |
Phase III
ongoing |
Michel
et al. 2015
88
Hauser et al. 2014 89 Perez-Lloret + Morello 2014 90 |
|
|
Pimavanserin
Nuplazid (Acadia [C]) |
Non-motor
psychosis |
5HT2A inverse
agonist |
Phase III
positive |
Approved in USA
2x17 mg/ once daily |
Cummings
et al. 2014
53
Hacksell et al. 2014 91 |
Modified from Oertel and Schulz 17 (2016).
Part C. For motor complications or non-motor symptoms by means of a non-dopaminergic mode of action: drug approved in another indication, now tested in Parkinson’s disease.
| Compound | Indication | Mode of action | Phase of
development |
Commentary | Reference |
|---|---|---|---|---|---|
|
Donepezil
Eisai (C) |
Non-motor
falls, gait disorder, dementia in Parkinson’s disease (PD) |
Acetylcholin-
esterase- inhibitor |
Phase IIIb
ongoing |
Approved
for therapy of Alzheimer dementia |
Chung
et al. 2010
92
Ravina et al. 2005 93 |
|
Duloxetine
Cimbalta, Xeristar (University of Toulouse [S]) |
Non-motor
pain |
SSNRI | Phase III
ongoing |
Approved for
therapy of pain and of depression |
|
|
Oxycodone/
Naloxone Targin (MundiPharma [C]) |
Severe pain
syndrome in PD |
Opioid | Phase III
positive |
Approved for
therapy of pain |
Trenkwalder et al. 2015 54 |
Modified from Oertel and Schulz 17 (2016).
To prolong and enhance the action of L-Dopa, the new reversible MAO B-inhibitor safinamide 27– 32—with the claim that this compound also influences glutamatergic transmission—and the long-lasting COMT inhibitor opicapone 33– 35 have been approved (both at present in Europe). An extended-release (24-hour long-acting) formulation of amantadine has been recently reported to markedly reduce the severity and extent of L-Dopa-induced dyskinesia and also to reduce off-time 36, 37.
Based on the restricted localization of adenosine-2A-receptors in the striatopallidal indirect pathway of the basal ganglia circuitry, adenosine-2A-receptor antagonists have been developed for the therapy of motor fluctuations and are now approved in Japan (istradefylline, tradename Nouriast 38– 40, whereas registration trials 41– 43 in Europe with this compound and clinical testing with a second compound of this class (tozadenant) are ongoing ( Table 1).
The development of a third compound of this class, preladenant, was stopped because of negative results in phase III trials 44.
Likewise employing the primary endpoint of “reduction of L-Dopa induced dyskinesia”, the results of clinical phase III trials with the metabotropic-glutamate-receptor-5-antagonist (mavoglurant) 45– 48 and the nicotine-alpha-7-partial agonist AQW051 49 were negative and thus their development stopped, whereas the clinical testing of glutamate-receptor-4-antagonists and allosteric modulators of the metabotropic-glutamate-receptor-5—employing the same endpoint (reduction of dyskinesia)—are ongoing.
For the field of DBS, improved hardware and software (rechargeable battery, multipolar electrodes) now offer a higher flexibility and precision to individually target the different motor symptoms (akinesia, rest tremor) and to avoid stimulation-related side effects (reviewed in 50– 52).
New developments in symptomatic therapy of non-motor symptoms
In 2011, the International Movement Disorder Society published a comprehensive evidence-based medicine review on the therapy of NMSs of PD 10, 11. On one hand, it provides profound guidance for how to treat the individual NMSs in PD. On the other hand, this review demonstrates how few clinical randomized placebo or active comparator multicenter prospective trials of “evidence-based medicine class I and class II” existed thus far for the therapy of NMSs in PD—in contrast to the numerous prospective multicenter trials on motor symptoms and motor complications. Since then, increased efforts in this field have led to the approval of new therapies. For the treatment of dopamimetic-induced psychosis in PD, the 5HT2A inverse agonist pimavanserin has been approved in the USA 53; thus, for the first time, an alternative to the currently employed antipsychotic compounds quetiapine or clozapine is available—although an active comparator trial between pimavanserin and, for example, clozapine is missing. In regard to severe pain syndromes, the slow-release preparation of oxycodone/naloxone has been successfully tested in PD 54. Furthermore, the precursor of noradrenaline droxidopa, also known as L-threo-DOPS, has been approved for the treatment of neurogenic orthostatic hypotension, one of the troubling autonomic symptoms in advanced PD and even more so in multiple system atrophy (MSA), another alpha-synucleinopathy 55– 57. In regard to DBS, a large recent study has shown beneficial effects of this neurosurgical procedure on NMSs 58. Given the major impact of NMS and therapy-related non-motor complications on the quality of life for PD patients and their partners, this field clearly needs priority in future clinical trials.
In summary, these compounds and techniques allow fine tuning of the available symptomatic therapy of motor and in part of NMSs in PD. However, they do not represent a major innovation. A true, highly needed innovation would be a compound with disease-modifying properties in order to slow down, if not stop, the progressive pathophysiology of PD (that is, most likely the spreading of the alpha-synucleinopathy in the central, peripheral, autonomic, and gastrointestinal nervous system of patients with PD).
The revolution in genetic research in Parkinson’s disease
In 1997, the world of PD research changed. For the first time, though very rare, an autosomal dominant mutation (termed PARK1) responsible for the protein alpha-synuclein was described 7. By 2016, at least eight monogenic causes for PD are known. The autosomal dominant forms relate either to a mutation of alpha-synuclein or to LRRK2, whereas autosomal recessive forms ( PARK2, PINK1, DJ1) cause mitochondrial dysfunction. The third major discovery was the fact that 3–7% of patients with idiopathic PD carry a heterozygous mutation for the gene glucocerebrosidase A. Genome-wide association studies have confirmed—besides the role of alpha-synuclein—the importance of the microtubule-associated protein tau (MAPT) in the etiopathogenesis of PD. Furthermore, at least 28 genetic risk (susceptibility) factors have been identified, and it is likely that this number will further increase (reviewed in 59). These discoveries have already had a major impact on the development of new therapies, especially in regard to potentially disease-modifying compounds—as will be discussed in relation to the alpha-synuclein “spreading hypothesis” below.
The Braak staging of Parkinson’s disease, the spreading hypothesis, the search for prodromal stages of Parkinson’s disease: advantages and limitations
Shortly after the discovery that a mutation of alpha-synuclein causes PD, alpha-synuclein aggregates were identified in the Lewy bodies in the post-mortem SN samples of patients with idiopathic PD 3. Therefore, the majority of patients with idiopathic PD are now considered to suffer from an alpha-synucleinopathy. Based on the distribution of these Lewy bodies in the nervous system, Braak et al. 8 postulated that most likely PD—as it is defined with its motor symptoms by the neurologist—is a late-stage phenotype of a disease which has been going on for decades.
The Braak staging of Parkinson’s disease: the spreading hypothesis
The Braak 8 staging hypothesis, combined with the “dual hit theory” 9, proposes that PD starts either in the olfactory bulb and related areas or in the gastrointestinal system. Thus, a pathological agent either would retrogradely reach the SN via an only recently discovered connection between the olfactory bulb and the SN 60 or may move retrogradely from the gastrointestinal system up to the dorsal motor nucleus of the vagal nerve, would then—in a caudorostral ascending direction—propagate upwards in the brainstem reaching the locus coeruleus complex, and over the next 5 to 10 years finally affect the SN (see Figure 2 and its legend).
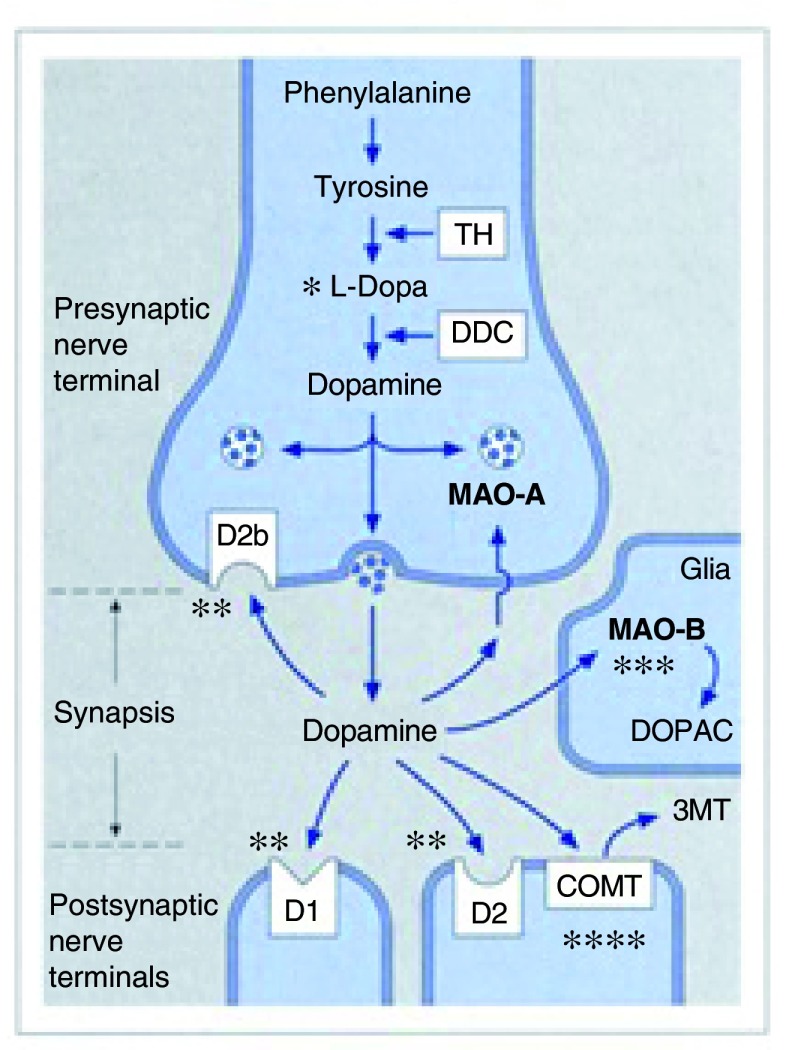
Figure 1. Scheme of a dopaminergic synapse with the different sites of actions for symptomatic Parkinson’s disease therapy.
COMT, catechol-O-methyl-transferase (inhibitor: entacapone, opicapone
a, tolcapone); D1, D1 receptor: agonist (new compound in phase III); D2, D2/D3 receptor (non-ergot agonist: apomorphine, piribedil, pramipexole, ropinirole, rotigotine); D2b, presynaptic D2-autoreceptor; DDC, L-DOPA-decarboxylase; DOPAC, dihydroxyphenylacetic acid; L-DOPA
a, L-dihydroxyphenylalanine (extended release); MAO-A, monoaminooxidase A; MAO-B, monoaminooxidase B (inhibitor: rasagiline, safinamide
a, selegiline); TH, tyrosine hydroxylase; 3MT, 3 methoxy-tyramine; *, effect of L-DOPA (converted into dopamine); **, effect of dopamine agonists (mimics dopamine at D1 or D2/3 receptor and at D2b autoreceptor); ***, effect of MAO-B-inhibitor (blocks centrally degradation of dopamine, enhances and prolongs dopamine action); ****, effect of COMT-inhibitor (blocks peripherally degradation of L-DOPA, enhances and prolongs presence of L-DOPA in blood and thus enhances and prolongs action of central dopamine action);
 , vesicle; the round structure represents the symbol for the vesicle
, vesicle; the round structure represents the symbol for the vesicle
 , the arrow represents the uptake mechanism - dopamine reuptake mechanism.
aRecently approved for therapy of Parkinson’s disease (
Table 1).
, the arrow represents the uptake mechanism - dopamine reuptake mechanism.
aRecently approved for therapy of Parkinson’s disease (
Table 1).
There is considerable clinical and experimental evidence in favor of this “dual hit theory” 9, although it is not proven beyond doubt and likely not to be applicable for a minority of patients with PD. That alpha-synuclein can spread in the human nervous system was reported in 2008 61: Lewy bodies arise in healthy fetal mesencephalic cells several years after their transplantation into the striatum of patients with PD. This fact is most likely explained by trans-synaptic spreading of pathological alpha-synuclein from the host neurons into the donor neurons. Intense research in experimental animal models of PD is ongoing to understand the pathogenic role of the different forms (such as monomers versus oligomers versus fibrils) of aggregated alpha-synuclein and to analyze the mechanism behind the postulated “prion-like” spreading of alpha-synuclein. For example, research in a transgenic alpha-synuclein-overexpressing and germ-free mouse model of PD recently demonstrated a marked effect of the gut microbiota on the development of the alpha-synucleinopathy and the manifestation of motor impairment, thus strengthening the hypothesis that the gastrointestinal system can play a role in the etiopathogenesis or progression (or both) of PD 62.
Clinical research on prodromal Parkinson’s disease
In the clinical situation, manifest PD—according to Braak et al. 8—is preceded by years, if not decades, by prodromal phases. To screen for prodromal (premotor) phases, the NMS hyposmia, constipation, depression, and the sleep-dream phase disorder RBD (REM sleep behavior disorder) are now considered prodromal indicators. Whereas the first three are sensitive but not specific, RBD is now accepted as the most specific phenotype of the PD prodromal phases with a risk of more than 80% to convert into PD, or dementia with Lewy bodies (DLB) or less frequently into multiple system atrophy(MSA)—in 10 to 15 years 63, 64. Similar research on prodromal stages takes place with “at risk relatives” of Parkinson patients, who are either heterozygous for the LRRK2 gene or are homozygous for one of the autosomal-recessive genes for a mitochondrial dysfunction in PD ( PAKR2, PINK1, DJ1; for a comprehensive review on Mendelian and non-Mendelian inheritance of PD, see 59) or possess a mutation of the gene for glucocerebrosidase 1 ( GBA1) and thus are classified as PD-GBA1 65.
The Braak hypothesis: advantages and limitations
The advantage of the Braak hypothesis is that it can clinically be tested. By carefully screening and following up patients at risk for PD, such as patients with hyposmia or RBD or with both 66, clinicians can identify a subgroup of patients with prodromal PD who present with and develop the sequence of symptoms related to the postulated prodromal PD stages. This type of study may allow us to discover endpoints for future neuroprotective trials in prodromal PD.
Limitations of the Braak hypothesis are related to post-mortem analysis of PD brains 67: they show, for example, that the density of Lewy bodies in the medullary areas is lower than in the cortex. In addition, a similar distribution of Lewy neuropathology is observed in patients with incidental Lewy body disease (that is, individuals with the hallmark Lewy pathology in brain, who did not present when alive with motor Parkinson features).
This observation does not appear to be consistent with a caudorostral spreading of alpha-synuclein aggregates. But if Lewy bodies are considered a mechanism to reduce the amount of soluble toxic alpha-synuclein oligomers in the cell, then the density of Lewy bodies in a given brain area may reflect its “defense” capability. In addition, if the caudorostral ascending process is tightly linked to the connectome of the involved structure, the locus coeruleus—with its lack of connections to the basal ganglia and, on the other hand, its strong projections to cortical areas—might drive the alpha-synuclein load of cortical areas many years longer than the SN might influence the alpha-synuclein load of the basal ganglia. This speculation is again testable in animal models and in post-mortem studies.
Search for Parkinson’s disease-modifying therapy delivers first results
The ultimate therapeutic challenge remains. Every person who knows a patient with PD asks why there is not a treatment that prevents the (at least) motor manifestation of PD. Already-diagnosed PD patients and their families dream of a treatment that can slow down or even partially reverse the progression of this devastating disorder with all its motor and non-motor symptoms and complications in the advanced stage. These dreams may be fulfilled in the not-too-distant future.
Two therapeutic strategies are currently followed. The first is based on epidemiological findings and large clinical prospective trials reporting a correlation between a reduced occurrence or prevalence (or both) of PD and the consumption of compounds such as caffeine or nicotine (e.g., 68). Table 2 lists examples of these generic substances with a postulated disease-modifying potential for PD.
Table 2. Therapy with compounds of disease-modifying potential: generic substances.
| Compound | Indication | Mode of action | Phase of
development |
Reference |
|---|---|---|---|---|
| Caffeine
(University of Montreal, Canada [C]) |
Motor early
Parkinson’s disease (PD) |
Adenosine-receptor
antagonist |
Phase IIIb
ongoing |
Wills
et al. 2013
94
Postuma et al. 2012 95 |
| Inosine
(Michael J. Fox Foundation [MJFF] [C]) |
Motor early PD | Precursor of urate,
antioxidant |
Phase IIb
ongoing |
Bhattacharyya
et al. 2016
96
Ascherio et al. 2009 97] |
| Isradipine – STEADY-PDIII
(NIH-NINDS, Novartis, University of Chicago [C]) |
Motor early PD | Dihydropyridine calcium
channel blocker |
Phase IIIb
ongoing |
Simuni
et al. 2016
98
Simuni et al. 2013 99 |
| Nicotine - NIC-PD
(German Parkinson Study Group, Parkinson Study Group USA; MJFF, IPF, NP, DPG, Novartis Germany [C]) |
Motor de novo PD | Cholinergic, modulation of
α-synuclein aggregation? |
Phase IIIb
completed |
Oertel
et al. 2016
100
Quik et al. 2008 101 Hong et al. 2009 102 |
Modified from Oertel and Schulz 17 (2016).
The second approach relates to the groundbreaking genetic discoveries in PD. In fact, a dramatic shift in the strategy for developing a new PD therapy has taken place: pharmaceutical efforts now target alpha-synuclein protein synthesis, degradation (such as autophagia, lysosomal, or proteasomal degradation), protein aggregation, and propagation in the nervous system. Finally, 20 years after the discovery of PARK1, the academic and pharmaceutical industrial scientific community can offer the first candidates with a potential for a disease-modifying effect in PD.
Three different principles of therapeutic action are addressed: (1) active or passive immunotherapy, (2) modulation of alpha-synuclein aggregation, and (3) enhancement of autophagy of alpha-synuclein ( Table 3).
Table 3. Therapy with compounds targeting alpha-synuclein.
| Compound | Indication | Mode of action | Phase of development | Reference |
|---|---|---|---|---|
| Immunotherapy (IT) | ||||
| Active immunization
(Affiris [C]) |
Motor | IT | Phase II ongoing | Schneeberger
et al. 2016
103
Manoutcharian et al. 2016 104 |
| Passive immunization
BIIB054 (Biogen [C]) PRX002 (Parthena/Roche [C]) |
Motor | IT | Phase II in preparation
phase II in preparation |
Weihofen
et al. 2016
105
Bergström et al. 2016 106 Kalia et al. 2015 6 Games et al. 2014 107 Spencer et al. 2016 69 |
|
Alpha-synuclein
aggregation modulators (aSAMs) |
||||
| NPT200-11
(UCB/Neuropore [C]) |
Motor?
likely in de novo Parkinson’s disease (PD) |
aSAM | Phase I in planning | Koike
et al. 2014
74
Szoke et al. 2014 75 |
|
NPT100_18a
(Neuropore [C]) |
Not applicable | aSAM | Preclinical testing | Wrasidlo et al. 2016 75 |
| ANLE 138b
(MODAG [C]) |
Motor?
likely in de novo PD |
aSAM | Phase I in planning | Deeg
et al. 2015
73
Levin et al. 2014 72 Wagner et al. 2013 71 |
|
a-synuclein autophagia
enhancer (aSAE) |
||||
| Nilotinib
Tasigna off-label use (Georgetown University, Washington, DC, USA [C]) |
Motor
non-motor |
“Tyrosine kinase
inhibitor” aSAE |
Investigator initiated trial
– open-label small pilot study randomized controlled trial in planning (MJFF-USA, Cure PD Trust, UK) |
Pagan
et al. 2016
78
Hebron et al. 2014 80 Hebron et al. 2013 81 |
Modified from Oertel and Schulz 16 (2016).
1) The first approach mimics a strategy that has been followed in Alzheimer’s disease for the last decade: active and passive immunizations are being developed as therapeutic approaches. This immunotherapeutic strategy relies on the assumption that (a) alpha-synuclein is accessible in the extracellular space (trans-synaptic spreading), (b) antibodies against alpha-synuclein reach the brain in sufficient quantity, and (c) they trap alpha-synuclein aggregates when these are released (“spread”) into the extracellular synaptic space. Today, active and passive immunization trials are under way in phases I and II. These treatments have passed the safety level testing, and the first data on phase II trials are awaited in 2017 to 2019. One limitation of active and passive immunotherapy, the low amount of antibodies passing the blood-brain barrier, may be overcome by coupling antibodies to the peptide penetratin, as has recently been reported in a mouse PD model 69.
2) Modulating the aggregation of alpha-synuclein aims to block or reduce the aggregation of alpha-synuclein monomers to oligomers or later on to fibrils. Two drugs are close to or under very early development. The first compound is called ANLE138b, and the few articles published demonstrate that this drug is able to reduce the aggregation of alpha-synuclein. In addition, in a mouse model with an A30P alpha-synuclein mutation, the compound extends survival 70– 72. The second drug is called NPT200-11, and only abstracts on its efficacy in preclinical testing are in the public domain 73, 74. This compound again reduces aggregation of alpha-synuclein at least in vitro and according to public information should have reached the very first safety testing in humans. A third compound NPT100-18a has been reported to displace alpha-synuclein from membranes, but is still in the phase of preclinical testing 75. The advantage of these small molecules is that, in variance to antibodies employed in immunotherapeutic attempts, they readily pass the blood-brain barrier.
3) Other newly developed compounds promise to enhance autophagy of alpha-synuclein. They are still in preclinical testing, although screening of libraries of registered compounds may well reveal further potential members of this group 76.
In 2015, a report from a research group at Georgetown University (Washington, DC, USA) may unexpectedly reduce the time to an available registered and reimbursed disease-modifying therapy of PD 77. The neurologists treated 12 patients with PD in advanced stages—including PD patients with cognitive impairment—with the compound nilotinib, a tyrosine kinase inhibitor approved as a therapy for chronic myeloid leukemia. Patients with PD were treated in a safety study with an open-label design (150 or 300 mg daily), and after 6 months of therapy, a clinical improvement was reported. In articles published between 2012 and 2014, nilotinib had been shown to reduce alpha-synuclein levels in protein aggregation models of PD in rodents and to prevent the loss of dopaminergic neurons in a transgenic PD mouse model. Its mode of action is to increase autophagy 78, 79. In the meantime, the Michael J. Fox Foundation (MJFF), together with Cure Parkinson’s disease trust in the UK, picked up this finding, which initially was looked at very skeptically, and decided to test nilotinib in a double-blind controlled study in patients with PD.
In summary, the field has steadily shifted from developments on symptomatic therapy to preventive therapy, with at least five different options (active immunization, passive immunization, two small molecules that function as alpha-synuclein aggregation modulators, and most recently an autophagy enhancer with a known adverse profile, which is already registered in the field of oncology). Thus, for the very first time, the possibility of a disease-modifying therapy appears to be testable in PD.
Search for primary endpoints reflecting the progression of Parkinson’s disease in the prodromal stages
Taking together the discoveries on the genetic background of PD and the Braak staging hypothesis, new avenues for drug development and clinical testing have opened up. For clinical testing—at least in the next few years—potential disease-modifying compounds are and will be tested in the early stage of motor PD; that is, very early de novo PD patients, who never received a symptomatic therapy will be recruited and should present with a unilateral asymmetric very mild motor symptomatology.
However, for “true” neuroprevention (that is, the prevention or delay of the conversion of a prodromal stage to the motor stage of PD), parameters and biomarkers which reflect the progression of the alpha-synucleinopathy in the prodromal stage have to be discovered. In addition, such a parameter must be responsive to therapy, even in the prodromal stage, in order to qualify as a primary endpoint for pivotal registration trials. At present, such a parameter has not been identified. Respective research ranges from studies on biomarkers in the cerebrospinal fluid, peripheral blood, saliva, and sweat and in biopsies of the colonic enteric nervous system, the salivary gland, or the skin 80– 83. Major efforts are placed into different imaging techniques with sophisticated magnetic resonance methods, nuclear medical ligands for the dopamine transporter single-positron emission computed tomography (SPECT) or fluoro-desoxyglucose positron emission tomography 84.
Conclusions
Neurologists have to accept that the majority of patients with PD, even at the very early stage of neurological diagnosis, actually present a late-stage phenotype of an alpha-synucleinopathy. Thus, PD has started at least 20 years before it manifests in the clinic with its motor symptoms. Neurologists will likely have to shift their clinical and diagnostic focus away from the dopaminergic system to symptoms related to different parts of the nervous system, such as the enteric system 62, the brainstem with its autonomic control areas, the locus coeruleus 57, or even the skin. If the dream of a disease-modifying therapy is to come true, neuroscience, drug development, and physician scientists face at least two challenges. First, drug development will target the aggregation and propagation of alpha-synuclein and of related mechanisms as well as mitochondrial dysfunction; second, a major effort has to be made to enhance the diagnostic methodology in order to identify a primary endpoint for clinical neuroprotective trials, not only in early motor PD but also in the prodromal stages of PD 82– 84. It has never been so exciting as today to work in the field of PD, and we should share this belief with the patients we diagnose, treat, and care for.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Peter LeWitt, School of Medicine, Wayne State University, Detroit, MI, USA
Mark Hallett, Human Motor Control Section, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD, USA
Funding Statement
WH Oertel is Hertie Senior Research Professor supported by the Charitable Hertie Foundation, Frankfurt/Main, Germany.
[version 1; referees: 2 approved]
References
- 1. Hughes AJ, Daniel SE, Ben-Shlomo Y, et al. : The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125(Pt 4):861–70. 10.1093/brain/awf080 [DOI] [PubMed] [Google Scholar]
- 2. Lewy FH: Paralysis agitans. I. Pathologische Anatomie.In: Handbuch der Neurologie(Lewandoski M, Hrsg), Springer Berlin. III:920–933. [Google Scholar]
- 3. Spillantini MG, Schmidt ML, Lee VM, et al. : Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–40. 10.1038/42166 [DOI] [PubMed] [Google Scholar]
- 4. Tretiakoff C: Contribution a l'etude l'anatomie pathologique du locus Niger de soemmering: avec quelques déductions relatives à la pathogénie des troubles du tonus musculaire et de la maladie de Parkinson. Jouve Paris.1919. Reference Source [Google Scholar]
- 5. Birkmayer W, Hornykiewicz O: [The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia]. Wien Klin Wochenschr. 1961;73:787–8. [PubMed] [Google Scholar]
- 6. Kalia LV, Kalia SK, Lang AE: Disease-modifying strategies for Parkinson's disease. Mov Disord. 2015;30(11):1442–50. 10.1002/mds.26354 [DOI] [PubMed] [Google Scholar]
- 7. Polymeropoulos MH, Lavedan C, Leroy E, et al. : Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–7. 10.1126/science.276.5321.2045 [DOI] [PubMed] [Google Scholar]
- 8. Braak H, Del Tredici K, Rüb U, et al. : Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. 10.1016/S0197-4580(02)00065-9 [DOI] [PubMed] [Google Scholar]
- 9. Hawkes CH, Del Tredici K, Braak H: Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33(6):599–614. 10.1111/j.1365-2990.2007.00874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martínez-Fernández R, Schmitt E, Martinez-Martin P, et al. : The hidden sister of motor fluctuations in Parkinson's disease: A review on nonmotor fluctuations. Mov Disord. 2016;31(8):1080–94. 10.1002/mds.26731 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Seppi K, Weintraub D, Coelho M, et al. : The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson's disease. Mov Disord. 2011;26 Suppl 3:S42–80. 10.1002/mds.23884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deuschl G, Schade-Brittinger C, Krack P, et al. : A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355(9):896–908. 10.1056/NEJMoa060281 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Schuepbach WM, Rau J, Knudsen K, et al. : Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. 2013;368(7):610–22. 10.1056/NEJMoa1205158 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. de Vries NM, Sturkenboom IH, Bloem BR: Physiotherapy and Occupational Therapy and Mild to Moderate Parkinson Disease. JAMA Neurol. 2016;73(7):893–4. 10.1001/jamaneurol.2016.1277 [DOI] [PubMed] [Google Scholar]
- 15. Bloem BR, de Vries NM, Ebersbach G: Nonpharmacological treatments for patients with Parkinson's disease. Mov Disord. 2015;30(11):1504–20. 10.1002/mds.26363 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. LaHue SC, Comella CL, Tanner CM: The best medicine? The influence of physical activity and inactivity on Parkinson's disease. Mov Disord. 2016;31(10):1444–54. 10.1002/mds.26728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oertel W, Schulz JB: Current and experimental treatments of Parkinson disease: A guide for neuroscientists. J Neurochem. 2016;139 Suppl 1:325–37. 10.1111/jnc.13750 [DOI] [PubMed] [Google Scholar]
- 18. Olanow CW, Kieburtz K, Odin P, et al. : Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13(2):141–9. 10.1016/S1474-4422(13)70293-X [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Hauser RA, Hsu A, Kell S, et al. : Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson's disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol. 2013;12(4):346–56. 10.1016/S1474-4422(13)70025-5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Pahwa R, Lyons KE, Hauser RA, et al. : Randomized trial of IPX066, carbidopa/levodopa extended release, in early Parkinson's disease. Parkinsonism Relat Disord. 2014;20(2):142–8. 10.1016/j.parkreldis.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 21. Stocchi F, Hsu A, Khanna S, et al. : Comparison of IPX066 with carbidopa-levodopa plus entacapone in advanced PD patients. Parkinsonism Relat Disord. 2014;20(12):1335–40. 10.1016/j.parkreldis.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 22. Waters CH, Nausieda P, Dzyak L, et al. : Long-Term Treatment with Extended-Release Carbidopa-Levodopa (IPX066) in Early and Advanced Parkinson's Disease: A 9-Month Open-Label Extension Trial. CNS Drugs. 2015;29(4):341–50. 10.1007/s40263-015-0242-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsu A, Yao HM, Gupta S, et al. : Comparison of the pharmacokinetics of an oral extended-release capsule formulation of carbidopa-levodopa (IPX066) with immediate-release carbidopa-levodopa (Sinemet( ®)), sustained-release carbidopa-levodopa (Sinemet( ®) CR), and carbidopa-levodopa-entacapone (Stalevo( ®)). J Clin Pharmacol. 2015;55(9):995–1003. 10.1002/jcph.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mao ZL, Modi NB: Dose-Response Analysis of the Effect of Carbidopa-Levodopa Extended-Release Capsules (IPX066) in Levodopa-Naive Patients With Parkinson Disease. J Clin Pharmacol. 2016;56(8):974–82. 10.1002/jcph.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yao HM, Hsu A, Gupta S, et al. : Clinical Pharmacokinetics of IPX066: Evaluation of Dose Proportionality and Effect of Food in Healthy Volunteers. Clin Neuropharmacol. 2016;39(1):10–7. 10.1097/WNF.0000000000000126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nausieda PA, Hsu A, Elmer L, et al. : Conversion to IPX066 from Standard Levodopa Formulations in Advanced Parkinson's Disease: Experience in Clinical Trials. J Parkinsons Dis. 2015;5(4):837–45. 10.3233/JPD-150622 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Stocchi F, Borgohain R, Onofrj M, et al. : A randomized, double-blind, placebo-controlled trial of safinamide as add-on therapy in early Parkinson's disease patients. Mov Disord. 2012;27(1):106–12. 10.1002/mds.23954 [DOI] [PubMed] [Google Scholar]
- 28. Schapira AH, Stocchi F, Borgohain R, et al. : Long-term efficacy and safety of safinamide as add-on therapy in early Parkinson's disease. Eur J Neurol. 2013;20(2):271–80. 10.1111/j.1468-1331.2012.03840.x [DOI] [PubMed] [Google Scholar]
- 29. Borgohain R, Szasz J, Stanzione P, et al. : Randomized trial of safinamide add-on to levodopa in Parkinson's disease with motor fluctuations. Mov Disord. 2014;29(2):229–37. 10.1002/mds.25751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borgohain R, Szasz J, Stanzione P, et al. : Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson's disease. Mov Disord. 2014;29(10):1273–80. 10.1002/mds.25961 [DOI] [PubMed] [Google Scholar]
- 31. Cattaneo C, Sardina M, Bonizzoni E: Safinamide as Add-On Therapy to Levodopa in Mid- to Late-Stage Parkinson's Disease Fluctuating Patients: Post hoc Analyses of Studies 016 and SETTLE. J Parkinsons Dis. 2016;6(1):165–73. 10.3233/JPD-150700 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Stocchi F, Torti M: Adjuvant therapies for Parkinson's disease: critical evaluation of safinamide. Drug Des Devel Ther. 2016;10:609–18. 10.2147/DDDT.S77749 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Ferreira JJ, Lees A, Rocha JF, et al. : Opicapone as an adjunct to levodopa in patients with Parkinson's disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol. 2015;15(2):154–165, pii: S1474-4422(15)00336-1. 10.1016/S1474-4422(15)00336-1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Fabbri M, Rosa MM, Ferreira JJ: Clinical pharmacology review of opicapone for the treatment of Parkinson's disease. Neurodegener Dis Manag. 2016;6(5):349–62. 10.2217/nmt-2016-0022 [DOI] [PubMed] [Google Scholar]
- 35. Rocha JF, Ferreira JJ, Falcão A, et al. : Effect of 3 Single-Dose Regimens of Opicapone on Levodopa Pharmacokinetics, Catechol-O-Methyltransferase Activity and Motor Response in Patients With Parkinson Disease. Clin Pharmacol Drug Dev. 2016;5(3):232–240. 10.1002/cpdd.217 [DOI] [PubMed] [Google Scholar]
- 36. Pahwa R, Tanner CM, Hauser RA, et al. : Amantadine extended release for levodopa-induced dyskinesia in Parkinson's disease (EASED Study). Mov Disord. 2015;30(6):788–95. 10.1002/mds.26159 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Pahwa R, Tanner CM, Hauser RA, et al. : Results of a phase 3 efficacy and safety study of ADS-5102 (amantadine HCl) extended-release capsules in Parkinson’s disease patients with Levodopa-induced dyskinesia (EASE LID 3).International Movement Disorder Congress, Berlin, Late breaking abstract 7,2016. [Google Scholar]
- 38. Mizuno Y, Hasegawa K, Kondo T, et al. : Clinical efficacy of istradefylline (KW-6002) in Parkinson's disease: a randomized, controlled study. Mov Disord. 2010;25(10):1437–43. 10.1002/mds.23107 [DOI] [PubMed] [Google Scholar]
- 39. Mizuno Y, Kondo T, Japanese Istradefylline Study Group: Adenosine A 2A receptor antagonist istradefylline reduces daily OFF time in Parkinson's disease. Mov Disord. 2013;28(8):1138–41. 10.1002/mds.25418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kondo T, Mizuno Y, Japanese Istradefylline Study Group: A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin Neuropharmacol. 2015;38(2):41–6. 10.1097/WNF.0000000000000073 [DOI] [PubMed] [Google Scholar]
- 41. Factor S, Mark MH, Watts R, et al. : A long-term study of istradefylline in subjects with fluctuating Parkinson's disease. Parkinsonism Relat Disord. 2010;16(6):423–6. 10.1016/j.parkreldis.2010.02.014 [DOI] [PubMed] [Google Scholar]
- 42. Pourcher E, Fernandez HH, Stacy M, et al. : Istradefylline for Parkinson's disease patients experiencing motor fluctuations: results of the KW-6002-US-018 study. Parkinsonism Relat Disord. 2012;18(2):178–84. 10.1016/j.parkreldis.2011.09.023 [DOI] [PubMed] [Google Scholar]
- 43. Vorovenci RJ, Antonini A: The efficacy of oral adenosine A 2A antagonist istradefylline for the treatment of moderate to severe Parkinson's disease. Expert Rev Neurother. 2015;15(12):1383–90. 10.1586/14737175.2015.1113131 [DOI] [PubMed] [Google Scholar]
- 44. Pinna A: Adenosine A 2A receptor antagonists in Parkinson's disease: progress in clinical trials from the newly approved istradefylline to drugs in early development and those already discontinued. CNS Drugs. 2014;28(5):455–74. 10.1007/s40263-014-0161-7 [DOI] [PubMed] [Google Scholar]
- 45. Stocchi F, Rascol O, Destee A, et al. : AFQ056 in Parkinson patients with levodopa-induced dyskinesia: 13-week, randomized, dose-finding study. Mov Disord. 2013;28(13):1838–46. 10.1002/mds.25561 [DOI] [PubMed] [Google Scholar]
- 46. Petrov D, Pedros I, de Lemos ML, et al. : Mavoglurant as a treatment for Parkinson's disease. Expert Opin Investig Drugs. 2014;23(8):1165–79. 10.1517/13543784.2014.931370 [DOI] [PubMed] [Google Scholar]
- 47. Rascol O, Fox S, Gasparini F, et al. : Use of metabotropic glutamate 5-receptor antagonists for treatment of levodopa-induced dyskinesias. Parkinsonism Relat Disord. 2014;20(9):947–56. 10.1016/j.parkreldis.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 48. Trenkwalder C, Stocchi F, Poewe W, et al. : Mavoglurant in Parkinson's patients with l-Dopa-induced dyskinesias: Two randomized phase 2 studies. Mov Disord. 2016;31(7):1054–8. 10.1002/mds.26585 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Trenkwalder C, Berg D, Rascol O, et al. : A Placebo-Controlled Trial of AQW051 in Patients With Moderate to Severe Levodopa-Induced Dyskinesia. Mov Disord. 2016;31(7):1049–54. 10.1002/mds.26569 [DOI] [PubMed] [Google Scholar]
- 50. Barbe MT, Maarouf M, Alesch F, et al. : Multiple source current steering--a novel deep brain stimulation concept for customized programming in a Parkinson's disease patient. Parkinsonism Relat Disord. 2014;20(4):471–3. 10.1016/j.parkreldis.2013.07.021 [DOI] [PubMed] [Google Scholar]
- 51. Timmermann L, Jain R, Chen L, et al. : 134 VANTAGE Trial: Three-Year Outcomes of a Prospective, Multicenter Trial Evaluating Deep Brain Stimulation With a New Multiple-Source, Constant-Current Rechargeable System in Parkinson Disease. Neurosurgery. 2016;63(Suppl 1):155. 10.1227/01.neu.0000489704.68466.0a 27399413 [DOI] [Google Scholar]; F1000 Recommendation
- 52. McIntyre CC, Anderson RW: Deep brain stimulation mechanisms: the control of network activity via neurochemistry modulation. J Neurochem. 2016;139(Suppl 1):338–45. 10.1111/jnc.13649 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Cummings J, Isaacson S, Mills R, et al. : Pimavanserin for patients with Parkinson's disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533–40. 10.1016/S0140-6736(13)62106-6 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Trenkwalder C, Chaudhuri KR, Martinez-Martin P, et al. : Prolonged-release oxycodone-naloxone for treatment of severe pain in patients with Parkinson's disease (PANDA): a double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2015;14(12):1161–70. 10.1016/S1474-4422(15)00243-4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Mathias CJ, Senard JM, Braune S, et al. : L-threo-dihydroxyphenylserine (L-threo-DOPS; droxidopa) in the management of neurogenic orthostatic hypotension: a multi-national, multi-center, dose-ranging study in multiple system atrophy and pure autonomic failure. Clin Auton Res. 2001;11(4):235–42. 10.1007/BF02298955 [DOI] [PubMed] [Google Scholar]
- 56. Hauser RA, Hewitt LA, Isaacson S: Droxidopa in patients with neurogenic orthostatic hypotension associated with Parkinson's disease (NOH306A). J Parkinsons Dis. 2014;4(1):57–65. 10.3233/JPD-130259 [DOI] [PubMed] [Google Scholar]
- 57. Espay AJ, LeWitt PA, Kaufmann H: Norepinephrine deficiency in Parkinson's disease: the case for noradrenergic enhancement. Mov Disord. 2014;29(14):1710–9. 10.1002/mds.26048 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Dafsari HS, Reddy P, Herchenbach C, et al. : Beneficial Effects of Bilateral Subthalamic Stimulation on Non-Motor Symptoms in Parkinson's Disease. Brain Stimul. 2016;9(1):78–85. 10.1016/j.brs.2015.08.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Hernandez DG, Reed X, Singleton AB: Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J Neurochem. 2016;139(Suppl 1):59–74. 10.1111/jnc.13593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hoglinger GU, Alvarez-Fischer D, Arias-Carrion O, et al. : A new dopaminergic nigro-olfactory projection. Acta Neuropathol. 2015;130(3):333–48. 10.1007/s00401-015-1451-y [DOI] [PubMed] [Google Scholar]
- 61. Kordower JH, Chu Y, Hauser RA, et al. : Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14(5):504–6. 10.1038/nm1747 [DOI] [PubMed] [Google Scholar]
- 62. Sampson TR, Debelius JW, Thron T, et al. : Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell. 2016;167(6):1469–1480.e12. 10.1016/j.cell.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Schenck CH, Montplaisir JY, Frauscher B, et al. : Rapid eye movement sleep behavior disorder: devising controlled active treatment studies for symptomatic and neuroprotective therapy--a consensus statement from the International Rapid Eye Movement Sleep Behavior Disorder Study Group. Sleep Med. 2013;14(8):795–806. 10.1016/j.sleep.2013.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Iranzo A, Fernández-Arcos A, Tolosa E, et al. : Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One. 2014;9(2):e89741. 10.1371/journal.pone.0089741 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Migdalska-Richards A, Schapira AH: The relationship between glucocerebrosidase mutations and Parkinson disease. J Neurochem. 2016;139(Suppl 1):77–90. 10.1111/jnc.13385 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Berg D, Postuma RB, Adler CH, et al. : MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2015;30(12):1600–11. 10.1002/mds.26431 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Kingsbury AE, Bandopadhyay R, Silveira-Moriyama L, et al. : Brain stem pathology in Parkinson's disease: an evaluation of the Braak staging model. Mov Disord. 2010;25(15):2508–15. 10.1002/mds.23305 [DOI] [PubMed] [Google Scholar]
- 68. Hellenbrand W, Seidler A, Robra BP, et al. : Smoking and Parkinson's disease: a case-control study in Germany. Int J Epidemiol. 1997;26(2):328–39. 10.1093/ije/26.2.328 [DOI] [PubMed] [Google Scholar]
- 69. Spencer B, Williams S, Rockenstein E, et al. : α-synuclein conformational antibodies fused to penetratin are effective in models of Lewy body disease. Ann Clin Transl Neurol. 2016;3(8):588–606. 10.1002/acn3.321 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Wagner J, Ryazanov S, Leonov A, et al. : Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson's disease. Acta Neuropathol. 2013;125(6):795–813. 10.1007/s00401-013-1114-9 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Levin J, Schmidt F, Boehm C, et al. : The oligomer modulator anle138b inhibits disease progression in a Parkinson mouse model even with treatment started after disease onset. Acta Neuropathol. 2014;127(5):779–80. 10.1007/s00401-014-1265-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Deeg AA, Reiner AM, Schmidt F, et al. : Anle138b and related compounds are aggregation specific fluorescence markers and reveal high affinity binding to α-synuclein aggregates. Biochim Biophys Acta. 2015;1850(9):1884–90. 10.1016/j.bbagen.2015.05.021 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Koike MA, Price DL, White BM, et al. : The novel alpha-synuclein stabilizer NPT200-11 improves behavior, neuropathology and biochemistry in the murine thy1-ASYN transgenic model of Parkinson’s disease. Society for Neuroscience Congress, Washington D.C. USA - poster 411.06/M1,2014. Reference Source [Google Scholar]
- 74. Szoke B, Wrasidlo W, Stocking E, et al. : Biophysical characterization of the interaction of NPT200-11 with alpha-synuclein. Society for Neuroscience, Congress Washington D.C., USA - poster, 411.04/L11,2014. Reference Source [Google Scholar]
- 75. Wrasidlo W, Tsigelny IF, Price DL, et al. : A de novo compound targeting α-synuclein improves deficits in models of Parkinson's disease. Brain. 2016;139(pt 12):3217–36. 10.1093/brain/aww238 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Höllerhage M, Moebius C, Melms J, et al. : High-throughput screening of FDA-approved drugs reveals PDE1 inhibition to protect against α-synuclein toxicity in vitro and in an in vivo mouse model of PD. Acta Neuropathol.(submitted). [Google Scholar]
- 77. Pagan F, Hebron M, Valadez EH, et al. : Nilotinib Effects in Parkinson's disease and Dementia with Lewy bodies. J Parkinsons Dis. 2016;6(3):503–17. 10.3233/JPD-160867 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Hebron ML, Lonskaya I, Moussa CE: Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of α-synuclein in Parkinson's disease models. Hum Mol Genet. 2013;22(16):3315–28. 10.1093/hmg/ddt192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hebron ML, Lonskaya I, Olopade P, et al. : Tyrosine Kinase Inhibition Regulates Early Systemic Immune Changes and Modulates the Neuroimmune Response in α-Synucleinopathy. J Clin Cell Immunol. 2014;5:259. 10.4172/2155-9899.1000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Donadio V, Incensi A, Leta V, et al. : Skin nerve α-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology. 2014;82(15):1362–9. 10.1212/WNL.0000000000000316 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Doppler K, Ebert S, Uçeyler N, et al. : Cutaneous neuropathy in Parkinson's disease: a window into brain pathology. Acta Neuropathol. 2014;128(1):99–109. 10.1007/s00401-014-1284-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Antelmi E, Donadio V, Incensi PG, et al. : Skin nerve phosphorylated α-synuclein deposits in idiopathic REM sleep behavior disorder. Neurology.(In Press). [DOI] [PubMed] [Google Scholar]
- 83. Doppler K, Jentschke HM, Schulmeyer L, et al. : Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson's disease. Acta Neuropathol. 2017;1–11. 10.1007/s00401-017-1684-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Meles SK, Vadasz D, Renken RJ, et al. : Brain metabolic and dopaminergic imaging versus olfaction in rapid eye movement sleep behavior disorder. Mov Disord.(submitted). [Google Scholar]
- 85. Zangaglia R, Stocchi F, Sciarretta M, et al. : Clinical experiences with levodopa methylester (melevodopa) in patients with Parkinson disease experiencing motor fluctuations: an open-label observational study. Clin Neuropharmacol. 2010;33(2):61–6. 10.1097/WNF.0b013e3181c5e60c [DOI] [PubMed] [Google Scholar]
- 86. Fasano A, Bove F, Gabrielli M, et al. : Liquid melevodopa versus standard levodopa in patients with Parkinson disease and small intestinal bacterial overgrowth. Clin Neuropharmacol. 2014;37(4):91–5. 10.1097/WNF.0000000000000034 [DOI] [PubMed] [Google Scholar]
- 87. Ferreira JJ, Rocha JF, Falcão A, et al. : Effect of opicapone on levodopa pharmacokinetics, catechol- O-methyltransferase activity and motor fluctuations in patients with Parkinson's disease. Eur J Neurol. 2015;22(5):815–25, e56. 10.1111/ene.12666 [DOI] [PubMed] [Google Scholar]
- 88. Michel A, Downey P, van Damme X, et al. : Behavioural Assessment of the A 2a/NR2B Combination in the Unilateral 6-OHDA-Lesioned Rat Model: A New Method to Examine the Therapeutic Potential of Non-Dopaminergic Drugs. PLoS One. 2015;10(8):e0135949. 10.1371/journal.pone.0135949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hauser RA, Olanow CW, Kieburtz KD, et al. : Tozadenant (SYN115) in patients with Parkinson's disease who have motor fluctuations on levodopa: a phase 2b, double-blind, randomised trial. Lancet Neurol. 2014;13(8):767–76. 10.1016/S1474-4422(14)70148-6 [DOI] [PubMed] [Google Scholar]
- 90. Perez-Lloret S, Merello M: Two new adenosine receptor antagonists for the treatment of Parkinson's disease: istradefylline versus tozadenant. Expert Opin Pharmacother. 2014;15(8):1097–107. 10.1517/14656566.2014.903924 [DOI] [PubMed] [Google Scholar]
- 91. Hacksell U, Burstein ES, McFarland K, et al. : On the discovery and development of pimavanserin: a novel drug candidate for Parkinson's psychosis. Neurochem Res. 2014;39(10):2008–17. 10.1007/s11064-014-1293-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chung KA, Lobb BM, Nutt JG, et al. : Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology. 2010;75(14):1263–9. 10.1212/WNL.0b013e3181f6128c [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Ravina B, Putt M, Siderowf A, et al. : Donepezil for dementia in Parkinson's disease: a randomised, double blind, placebo controlled, crossover study. J Neurol Neurosurg Psychiatr. 2005;76(7):934–9. 10.1136/jnnp.2004.050682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wills AM, Eberly S, Tennis M, et al. : Caffeine consumption and risk of dyskinesia in CALM-PD. Mov Disord. 2013;28(3):380–3. 10.1002/mds.25319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Postuma RB, Lang AE, Munhoz RP, et al. : Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology. 2012;79(7):651–8. 10.1212/WNL.0b013e318263570d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bhattacharyya S, Bakshi R, Logan R, et al. : Oral Inosine Persistently Elevates Plasma antioxidant capacity in Parkinson's disease. Mov Disord. 2016;31(3):417–21. 10.1002/mds.26483 [DOI] [PubMed] [Google Scholar]
- 97. Ascherio A, LeWitt PA, Xu K, et al. : Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol. 2009;66(12):1460–8. 10.1001/archneurol.2009.247 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Simuni T, Biglan KM, Lowell J, et al. : An Update on STEADY-PD III. a phase 3 study of isradipine as a disease modifying agent in patients with early Parkinson’s disease. Baseline characteristics of the enrolled cohort. International Movement Disorder Society Congress, Berlin, Germany – poster 2085.2016. Reference Source [Google Scholar]
- 99. Parkinson Study Group: Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson's disease (STEADY-PD). Mov Disord. 2013;28(13):1823–31. 10.1002/mds.25639 [DOI] [PubMed] [Google Scholar]
- 100. Oertel WH, Brittinger C, Kamp C, et al. : The NIC-PD-study – baseline data of a randomized, placebo-controlled, double-blind, multi-centre trial to assess the disease-modifying potential of transdermal nicotine in early Parkinson‘s disease in Germany and the USA. International Movement Disorder Congress, Berlin, Germany - abstract 1982,2016. Reference Source [Google Scholar]
- 101. Quik M, O'Leary K, Tanner CM: Nicotine and Parkinson's disease: implications for therapy. Mov Disord. 2008;23(12):1641–52. 10.1002/mds.21900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hong D, Fink AL, Uversky VN: Smoking and Parkinson's disease: does nicotine affect alpha-synuclein fibrillation? Biochim Biophys Acta. 2009;1794(2):282–90. 10.1016/j.bbapap.2008.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schneeberger A, Tierney L, Mandler M: Active immunization therapies for Parkinson's disease and multiple system atrophy. Mov Disord. 2016;31(2):214–24. 10.1002/mds.26377 [DOI] [PubMed] [Google Scholar]
- 104. Manoutcharian K, Perez-Garmendia R, Gevorkian G: Recombinant Antibody Fragments For Neurodegenerative Diseases. Curr Neuropharmacol. 2016. 10.2174/1570159X01666160930121647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Weihofen A, Patel H, Huy C, et al. : Human-derived α-synuclein antibody BIIB054 binds pathologic forms of α-synuclein and attenuates transmission of α-synuclein in vitro and in vivo . International Movement Disorder Society Congress, Berlin, Germany - oral presentation,2013. Reference Source [Google Scholar]
- 106. Bergström AL, Kallunki P, Fog K: Development of Passive Immunotherapies for Synucleinopathies. Mov Disord. 2016;31(2):203–13. 10.1002/mds.26481 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 107. Games D, Valera E, Spencer B, et al. : Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson's disease-like models. J Neurosci. 2014;34(28):9441–54. 10.1523/JNEUROSCI.5314-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]