Abstract
Excessive ethanol consumption alters the neuroimmune system and particularly impacts the cytokine milieu of the CNS. Cytokine dysregulation has been shown to underlie addictive-like behaviors including alcohol abuse; however, many studies focus primarily on the proinflammatory cytokine profile and alcohol dependence. The current study furthers this research by determining the impact of excessive ethanol consumption on interleukin-10 (IL-10) and interleukin-4 (IL-4) activity in a model of non-dependent binge consumption called the “drinking in the dark” (DID) paradigm. Furthermore, the ability of IL-10 to modulate ethanol consumption was tested using site-directed pharmacology. Immunohistochemistry analyses determined that ethanol decreased IL-10 by 50% in the basolateral amygdala (BLA) but had no effect on IL-4. Neither IL-10 nor IL-4 were altered seen in the central amygdala (CEA). Enzyme linked immunosorbent assays confirmed that IL-10 was decreased in the amygdala but not in the serum, suggesting changes of this cytokine with the DID paradigm is restricted to the central nervous system. Finally, bilateral infusions of IL-10 into the BLA, but not CeA, reduced binge-like drinking and corresponding blood ethanol concentrations without impacting either locomotor activity or anxiety-like behavioral correlates. Together, these data support the idea that alcohol abuse dysregulates specific anti-inflammatory cytokines; however, ameliorating alcohol-induced effects on cytokines, like IL-10, may prove to be an effective therapy in curbing excessive consumption.
Keywords: interleukin-10, interleukin-4, drinking in the dark paradigm, binge drinking, alcohol abuse, neuroimmune system, cytokines, amygdala
Introduction
The link between the neuroimmune system and alcohol use disorders (AUDs) generally focus on the implications of neuroinflammation on alcohol-induced brain damage; however, not all individuals with an AUD have neuronal damage. Moreover, AUDs are a continuum of maladaptive behaviors, including episodic binge-drinking without signs of alcohol dependence, so it is imperative to also elucidate the impact of binge drinking on the neuroimmune system. Binge-drinking is a pattern of drinking that results in blood ethanol concentrations (BECS) that exceed the legal limit of 80 mg/dL (NIAAA 2004). This can occur after 4-5 drinks in a two hour window. Although repeated bouts of binge drinking can result in neuroplastic changes that contribute to the development of alcohol use disorders (AUDs) (Der-Avakian and Markou 2012), research indicates that the neurobiological changes that occur during acute intoxication as a result of binge drinking not only mimic those of chronic abuse but may also inherently alter ethanol consumption (Sprow and Thiele 2012). Understanding the neurobiochemistry of binge ethanol consumption may lead to the development of novel therapeutics that could curb excessive drinking and prevent many of the detrimental issues that occur following the motor-impairment and general disinhibition caused by ethanol. The “drinking in the dark” (DID) paradigm is a model of binge-like drinking that is well-suited to understand the biological phenomena that occur after binge drinking prior to dependence (Rhodes et al. 2005; Thiele and Navarro 2014). While there are a plethora of neuroplastic events that occurs as a result of the promiscuity of ethanol, this study focuses on the implications of alcohol-induced alterations to the neuroimmune system, specifically two cytokines with significant anti-inflammatory properties, interleukin-10 (IL-10) and interleukin-4 (IL-4).
Both clinical and preclinical studies concur that excessive ethanol consumption disrupts normal neuroimmune function, but these studies were conducted in dependent animals or after a lifetime of alcohol abuse in individuals suffering from an AUD (Crews and Vetreno 2014; He and Crews 2008; Marshall et al. 2013; Zou and Crews 2012). Although these studies all indicate increased neuroimmune function induced by ethanol, there were slight differences in the extent of the response depending on the duration of ethanol exposure. Determining the neuroimmune response following binge-like drinking in the absence of dependence will tease apart the acute contributions of binge ethanol consumption on neuroimmune responses from those associated with neurodegeneration or other comorbidities associated with ethanol misuse. Recently, it has been shown that binge-like ethanol consumption during the DID paradigm is sufficient to elicit an increase in IL-1β in the amygdala indicating that even acute excessive ethanol consumption can cause dysregulation of the neuroimmune system (Marshall et al. 2016a). However, the influence of alcohol on anti-inflammatory cytokines, like IL-10 and IL-4, have yet to be explored. Determining the impact of ethanol on both aspects of the neuroimmune response provides a better viewpoint from which to understand the neuroimmune system as a potential target for AUD therapy.
Not only does excessive ethanol consumption cause changes to the neuroimmune system, but manipulating the neuroimmune response alters ethanol consumption (Blednov et al. 2014; Blednov et al. 2012). Recently, it was determined that modulation of IL-1 signaling within the amygdala with curbs binge-like drinking (Marshall et al. 2016a). This study seeks to determine if that effect was unique to IL-1 signaling or if other cytokines also modulate ethanol consumption. Given the lack of significant alcohol mediated effects on IL-4, the experiments herein focused on whether IL-10 administration modulates binge-like ethanol consumption. IL-10 treatments have previously been implicated in ameliorating pain, depressive and anxiety-like behaviors, and neurodegeneration (Kwilasz et al. 2015), all of which are thought to be underlying comorbidities associated with the progression of an AUD (Goldstein et al. 2012). Given the link between IL-10 and behaviors associated with alcoholism, we hypothesized that IL-10 signaling in the amygdala also modulates binge-like drinking. The current experiments tests these hypotheses by determining: a) the effects of binge-like drinking on IL-10 and IL-4 immunoreactivity b) IL-10 enzyme linked immunosorbent assay (ELISA) determined protein concentrations as well as c) the effects of site-directed Il-10 administration on ethanol consumption.
Methods
Animals
Male C57BL /6J 6-8 weeks old mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were housed individually in an AALAC accredited vivarium maintained at 22°C with a reversed 12:12 hour light:dark cycle. Mice had ad libitum access to either Prolab® RMH 3000 (Purina LabDiet®; St. Louis, MO) or Teklad Diet® 2920X (Harlan Laboratories Inc.; Indianapolis, IN) and water, unless otherwise stated (Marshall et al. 2015). Prior to any experimental procedures, animals were allotted at least a week to fully acclimate to the environment. The procedures used in this study were all approved by the University of North Carolina Institutional Animal Care and Use Committee and followed the Guidelines for the Care and Use of Laboratory Animals (NRC 2011).
“Drinking in the Dark” Procedures
Binge drinking was modeled using a 4 day DID paradigm exactly as previously described (Marshall et al. 2015; Rhodes et al. 2005). Three hours into the dark cycle, home cage water bottles were removed and replaced with a single sipper tube container. The sipper tube bottles were filled with 20% (v/v) ethanol, 3% (w/v) sucrose, or tap water. On the first three days mice were allotted two hour access to the sipper tubes, but on the fourth day, also called the test day, the total consumption (g/kg) was measured over two or four hours depending on the experimental design. Each 4-day period of access or cycle, was separated by three days for mice undergoing multiple cycles of DID.
Tail blood samples (≈60μL) were taken immediately following the DID procedure to determine BECs for animals used in the consummatory and IHC studies; whereas, trunk bloods were collected for animals used in the ELISA study. Serum was obtained by centrifugation of blood samples collected. Each sample was run in duplicate on the AM1 Alcohol Analyzer (Analox, London, UK) and averaged for BEC determination (mg/dL).
Immunohistochemistry
Mice used for immunohistochemical (IHC) analysis underwent one or three, 4-day cycles of DID receiving either ethanol or sucrose or 3 cycles with water (Ethanol n= 9/subgroup; Sucrose n= 9-10/subgroup; H2O n= 9). To collect brains for IHC procedures, mice were overdosed with a 0.15mL intraperitoneal (i.p.) injection of an anesthetic cocktail of ketamine and xylazine (66.67mg/mL; 6.67mg/mL;0.9% saline) immediately following the last cycle of the DID. Animals were then perfused transcardially using 0.1M phosphate buffer saline (PBS; pH=7.4) and 4% paraformaldehyde in PBS (pH=7.4). After extraction, brains were post-fixed in 4% paraformaldehyde for 24 hours before being sliced coronally on a vibratome (Leica VT1000S; Wetzlar, Germany) to obtain 40μm sections. Sections were collected in a 1:4 series to be stored in cryopreserve at −4°C until further processing.
Every fourth section collected was used for IL-10 or IL-4 immunoreactivity detection. Adjacent sections have previously been analyzed for IL-1β (Marshall et al. 2016a). Briefly, sections were rinsed in 0.1M PBS before endogenous peroxidases were quenched with 0.6% H2O2. After serial PBS washes, antigen retrieval was performed using citrate buffer (10mM citric acid; 0.05% Tween 20; pH= 6.0) at 65°C for 1 hour. A series of washes was followed by thirty minutes in goat block (PBS/0.1% triton-X/3% serum; Vector Labs; Burlingame, CA) to reduce background staining. Sections were then incubated in rabbit IL-10 (1:1000; R&D Systems; Minneapolis, MN) or goat IL-4 (1:2000; LifeSpan BioSciences; Seattle, WA) primary antibody for 48 hours at 4°C. After serial washes in goat block to remove the primary, a complex was formed with the antigen by incubation in biotinylated rabbit anti-goat or goat anti-rabbit secondary antibody (Vector Labs) for IL-10 and IL-4 IHC, respectively. Following secondary incubation, the signal was amplified using avidin-biotin-peroxidase complex (ABC elite kit, Vector Labs) and the chromagen, 3,3’-diaminobenzidine tetrahydrochloride (Polysciences; Warrington, PA). Sections were then mounted onto glass slides and coverslipped using SHUR/Mount™ (Triangle Biomedical Sciences; Durham, NC).
Images of slides were taken at 100× magnification using a Zeiss Axio Zoom V16 microscope (Jena, Germany) installed on an HP Z820 Workstation. Slide images were coded during quantification to avoid any experimenter biases. The basolateral amygdala (BLA) and the central nucleus of the amygdala (CeA) were separately traced on sections imaged between Bregma −0.70mm and −2.06mm (Paxinos and Franklin 2004). IL-10 or IL-4 immunoreactivity was measured using the Zeiss Zen Pro 2012 analysis program.
Enzyme Linked ImmunoSorbent Assay
A separate cohort of animals were used for the ELISA experiments, but similar to the mice used for IHC, mice were subjected to 1 or 3 DID cycles of 20% ethanol (n=20) or 3% sucrose (n=20) or water (n=10) to assess IL-10. Immediately following the last cycle of the DID mice were euthanized by rapid decapitation and brains were immediately snap frozen by immersion in clear frozen section compound on dry ice. Bilateral punches of tissue (1mm × 1mm cylindrical punch) were taken from the amygdala (Bregma −0.70mm) and manually homogenized by pipetting in 100 μl of lysis buffer [320 mM sucrose, 1% SDS, 5 mM HEPES buffer, and 1% Halt 100× Protease/Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific, Rockford, IL)]. Animals were paired according to BEC (Ethanol group) or average consumption (water and sucrose group) to pool homogenates and serum samples to obtain a sufficient sample for duplicate runs of ELISA and BCA analyses. A BCA Protein Assay Kit (Thermo Fisher Scientific) was run to determine the total protein in each sample. IL-10 content was determined with an ELISA kit using the methodology described by the manufacturer (R&D Systems product #M1000B). Absorbance was measured at 450nm with a wavelength correction at 540nm with Gen5 data analysis software on an Epoch Microplate Spectrophotometer (BioTek, Winooski, VT). Cytokine concentrations are reported as pg of IL-10/mg of protein.
Surgeries & IL-10 Administration
Animals used to manipulate IL-10 signaling were subjected to one cycle of the 4-day DID with 20% ethanol procedure prior to surgery. For cannulae placement surgery, animals were given i.p. injections (1.5mL/kg) of an anesthetizing cocktail of xylazine (10mg/kg) and ketamine (100mg/kg). Using an Angle II™ Stereotax (Leica Instruments, Buffalo Grove, IL), bilateral guide cannulae (Plastics One; Roanoke, VA) were lowered into the BLA (n=10; AP:−1.22, ML:±3.01, DV: −4.75) (Paxinos and Franklin 2004). Mice were allowed to recover for a week before undergoing cycles of the DID paradigm. A 100ng dose of murine IL-10 (PeproTech, Rocky Hill, NJ) was chosen based on its neurobiological or behavioral effects in other rodent models (Bluthe et al. 1999; Liesz et al. 2009). Using a 2×2 Latin-square Design, 100ng or 0ng dose of IL-10 dissolved in a 0.9% saline was administered 30 minutes prior to access to either alcohol or sucrose. Infusions occurred at a rate of 0.15μL/min for two minutes using a Hamilton syringe (Reno, NV) attached to a Harvard Apparatus PHD 2000 infusion pump (Holliston, MS). After infusion, injectors remained in guide cannulae for an additional 3 minutes for diffusion before access to alcohol or sucrose on the test day. To determine the specificity of the results observed with IL-10 BLA administration, a separate group of animals were cannulated in the CeA (n=11; AP:−1.06, ML:±2.50, DV: −4.64) and administered 100ng or 0ng of IL-10 in a Latin-square design and subjected to the DID paradigm. At the conclusion of all behavioral analyses injection placements were histologically verified using an identical volume of Alcian blue dye (0.3μL/injection site) as used with IL-10.
Open Field Tests & Elevated Plus Maze
A separate cohort of mice (n=10) were used to determine if IL-10 administration in the BLA had an effect on locomotor activity or anxiety-like behaviors using an open-field test (OFT), as previously described (Cox et al. 2013; Prut and Belzung 2003). Briefly, animals were administered 100ng of IL-10 or saline into the BLA and subjected to open-field locomotor activity tests in an open-field arena identical to previous descriptions (Fee et al. 2004). Animal movement was tracked using the VersaMax® software program (AccuScan Instruments, Inc., Columbus, OH) over a two hour period with five minute bin outputs. Total distance traveled was measured to assess the effect of IL-Ra on locomotor activity; whereas, center distance and center time were recorded to determine the anxiolytic effects of IL-10 (Prut and Belzung 2003).
The elevated plus maze test, a common tool used to measure anxiety-like behavior, was used to further explore the impact of IL-10 on behavior (Walf and Frye 2007). The same cohort of mice used in the OFT were placed at the intersection of the elevated plus maze (Med Associates Inc., St. Albans, VT) following administration of 100ng of IL-10 or saline in the BLA. The total time spent in the open arena within the five minute test was recorded. Increased time within the open arm is associated with an anxiolytic-like behavior (Walf and Frye 2007). Both behavioral tasks were performed three hours into the dark cycle to coincide with the DID procedures and reduce the impact of circadian rhythm differences on locomotor activity.
Statistical Analysis
Prism Version 6.07 (GraphPad Software, Inc. La Jolla, Ca) was used to analyze and graph all data reported herein. One-way analyses of variance (ANOVAs) were used to assess the effects of ethanol and sucrose consumption on IL-10 or IL-4 immunoreactivity. However, for all other analyses a two-way ANOVA was used to determine the effect of treatment. Bonferroni post-hoc tests or t-tests were only conducted if a significant interaction or main effect of treatment was observed. All data are reported as the mean ± standard error of the mean and considered significant if p<0.05, two-tailed.
Results
Binge-Like Ethanol Consumption Decreased IL-10 but Not IL-4 Immunoreactivity
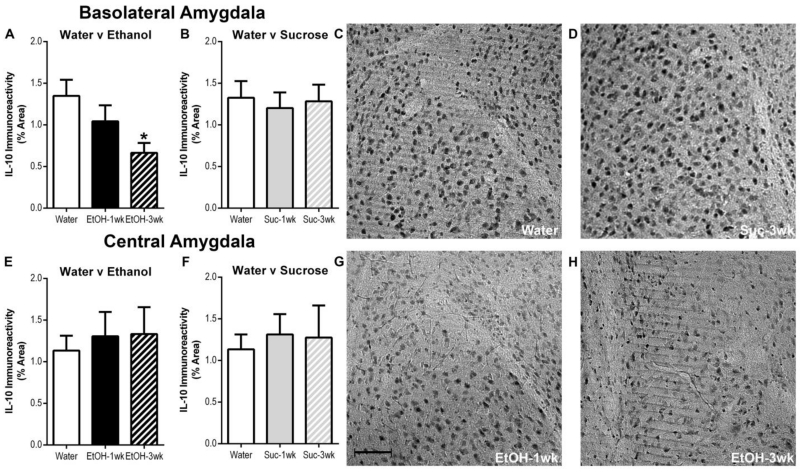
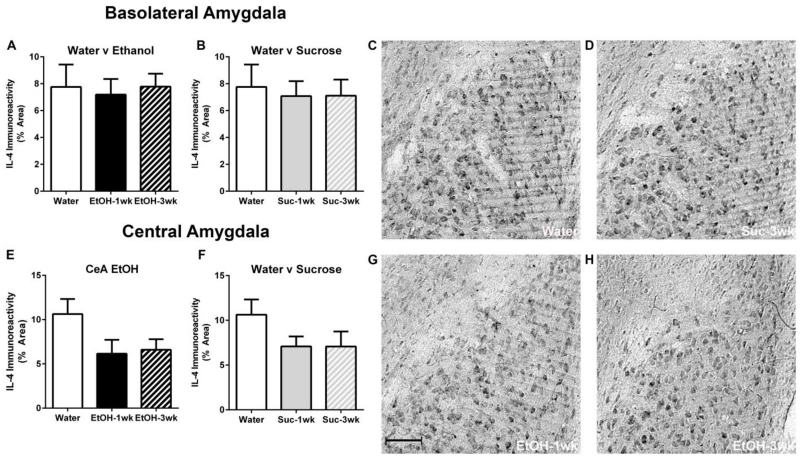
T-tests indicated no significant differences following 1 or 3 DID cycles in either ethanol consumption [t(17) =0.02, p=0.98] or BECs [t(17) =1.57, p=0.14] (Table 1; Immunohistochemistry). Photomicrographs indicated both IL-10 and IL-4 immunoreactivity were present in the amygdala of all treatment groups (Figure 1, 2). Binge-like ethanol drinking resulted in decreased IL-10 immunoreactivity compared with the water drinking control group in the BLA (Figure 1a). A one-way ANOVA revealed a main effect of group for mice drinking ethanol relative to those drinking water [F(2,25)=3.89, p=0.034] but sucrose consumption did not alter IL-10 immunoreactivity relative to controls [F(2,25)=0.11, p=0.90] (Figure 1a, 1b). Post-hoc Bonferroni tests indicated a significant decrease in IL-10 immunoreactivity in mice that experienced 3 DID ethanol cycles compared with water drinking mice (p<0.05). In the CeA, a one-way ANOVA did not reveal any statistically significant differences in IL-10 immunoreactivity between groups drinking ethanol versus water [F(2,25)=0.15, p=0.86] or those drinking sucrose versus water [F(2,25)=0.11, p=0.90] (Figure 1e, 1f). In regards to IL-4, one-way ANOVAs indicated that neither ethanol or sucrose had a significant effects within the BLA ([F(2,25)=0.07, p=0.93]; F(2,25)=0.83, p=0.92]) or CeA ([F(2,25)=2.63, p=0.09]; F(2,25)=1.83, p=0.18]), respectively (Figure 2).
Table 1.
ETHANOL INTAKE AND BLOOD ETHANOL LEVELS FROM ELISA and IMMUNOHISTOCHEMISTRY EXPERIMENTS
| Experiment | Time | Consumption (g/kg/4hr) |
BEC (mg/dL) |
|---|---|---|---|
| ELISA | 1-DID Cycle | 5.2±0.4 | 114.1±14.1 |
| 3-DID Cycles | 4.7±0.4 | 122.4±19.2 | |
| Immunohistochemistry* | 1-DID Cycle | 4.2±0.4 | 54.10±13.3 |
| 3-DID Cycles | 4.7±0.4 | 91.33±16.3 |
Previously published in Marshall et al. 2016
Fig. 1.
Ethanol induced a decrease in IL-10 Iimmunoreactivity within the BLA after 3 cycles compared with the water control (a); however, no statistically significant differences were seen within the CeA between water and ethanol drinking groups (e). Further, no statistically significant differences were evident between water and sucrose drinking groups in either the BLA (b) or CeA (f). Representative photomicrographs of the BLA of mice exposed to water (c), 3 sucrose DID cycles (d), 1 ethanol DID cycle (g), and 3 ethanol DID cycles (H) are shown. The scale bar = 20μm (g). All data are presented as mean ± SEM. *p<0.05 compared to water group
Fig. 2.
Ethanol had no effect on IL-4 immunoreactivity within the BLA (a) or CeA (e) between the water control groups and either ethanol drinking cohorts. Further, no statistically significant differences were evident between water and sucrose drinking groups in either the BLA (b) or CeA (f). Representative photomicrographs of the BLA of mice exposed to water (c), 3 sucrose DID cycles (d), 1 ethanol DID cycle (g), and 3 ethanol DID cycles (H) are shown. The scale bar = 20μm (g). All data are presented as mean ± SEM. *p<0.05 compared to water group
Binge-Like Ethanol Consumption Decreased IL-10 Protein within the Amygdala in ELISA Assay
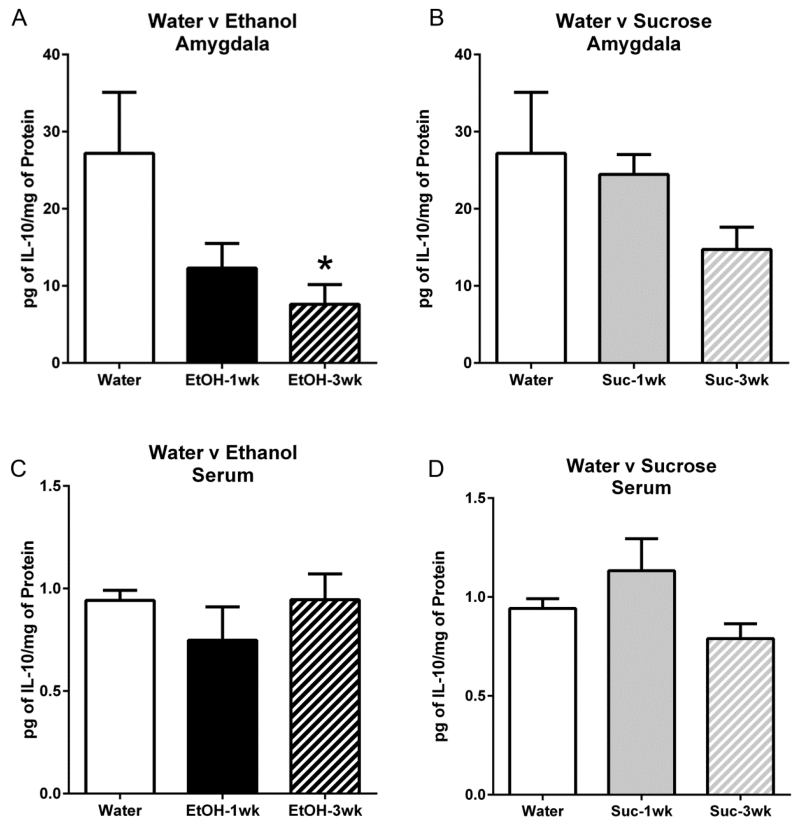
Similar to IHC, t-tests indicated no significant differences in consumption (t(18)=0.84, p=0.41) or BECs (t(18)=0.34, p=0.74) between animals that had 1 or 3 cycles of DID (Table 1; ELISA). Ethanol exposure resulted in decreased IL-10 protein in the amygdala when compared with the water drinking control group according to a one-way ANOVA [F(2,12)=3.96, p=0.048] (Figure 3a). Post-hoc Bonferroni analysis indicated that IL-10 protein concentrations after 3 cycles of ethanol exposure were significantly lower than the water control group. However, no detectable differences were seen in IL-10 protein between the water and sucrose groups [F(2,12)=3.11, p=0.09] (Figure 3b). Using the serum as an assessment of the peripheral system cytokine concentrations, one-way ANOVAs indicated no difference in IL-10 protein following various cycles of ethanol [F(2,12)=0.86, p=0.45] or sucrose [F(2,12)=2.60, p=0.12] consumption compared to the water control group (Figure 3c, 3d).
Fig. 3.
ELISAs revealed that three cycles of ethanol DID induced a decrease in IL-10 protein in the amygdala (a) but DID cycles of sucrose did not significantly alter IL-10 protein compared with water controls (b). In the serum, no differences in IL-10 protein content were seen between animals given water compared to various cycles of ethanol (c) or sucrose (d). All data are presented as mean ± SEM. *p<0.05 compared to water group
IL-10 Administration in the BLA Reduced Binge-Like Ethanol Consumption
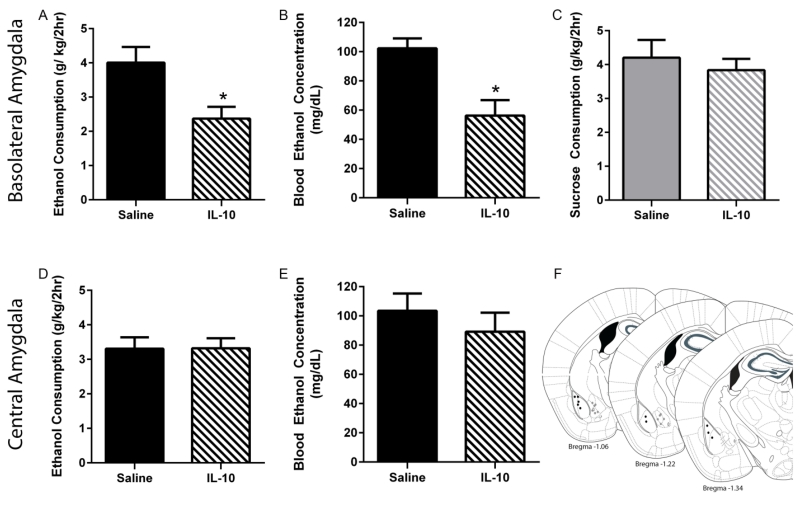
With respect to the BLA, a two-way repeated-measures (RM) ANOVA (drug × order of drug presentation) on ethanol consumption indicated a main effect of IL-10 treatment [F(1,8)=6.80, p=0.03] but no main effect of drug order [F(1,8)=0.25, p=0.64] or interaction between order and treatment [F(1,8)=0.07, p=0.06] (Figure 4a). Likewise, a two-way RM ANOVA (drug × order of drug presentation) of BECs indicated a main effect of drug treatment [F(1,8)=12.96, p=0.0007] but no main effect of drug order [F(1,8)=0.68, p=0.43] or interaction between order and treatment [F(1,8)<0.01, p=0.97] (Figure 4b). The effects of administration of IL-10 in the BLA were specific to ethanol consumption as a two-way RM ANOVA of sucrose consumption among the groups did not indicate any significant effect of treatment [F(1,8)=1.03, p=0.34], drug presentation order [F(1,8)=0.34, p=0.57], or any interaction [F(1,12)=0.38, p=0.56] (Figure 4c). Moreover the effect of IL-10 administration was specific to the BLA, as two-way ANOVAs indicated no interaction or main effects of either IL-10 administration in the CeA or order of presentation for either consumption [F(1,9)=3.74, p=0.09; F(1,9)=0.05, p=0.82; F(1,9)=0.29, p=0.60] or BECs [F(1,9)=0.38, p=0.55; F(1,9)=2.57, p=0.14; F(1,9)=0.05, p=0.83], respectively (Figures 4d, 4e).
Fig. 4.
Effects of site-directed infusion of IL-10 on consumption and BECs. IL-10 infusion into the BLA significantly reduced ethanol consumption (a) during the DID session resulting in BECs (b) below 80mg/dL that were significantly lower compared with saline treatment. However, IL-10 administration into the BLA did not alter sucrose consumption (c). No significant effect of CeA infusion of IL-10 was observed in either consummatory data (d) or in BECs (e). Approximate injection sites are shown (f). Black circles represent cannulae placement within the BLA while grey circles represent those in the CeA. All data are presented as mean ± SEM. *p<0.05 compared to saline group
IL-10 Infusion into the BLA had No Effects on Open-Field Test or Elevated Plus Maze
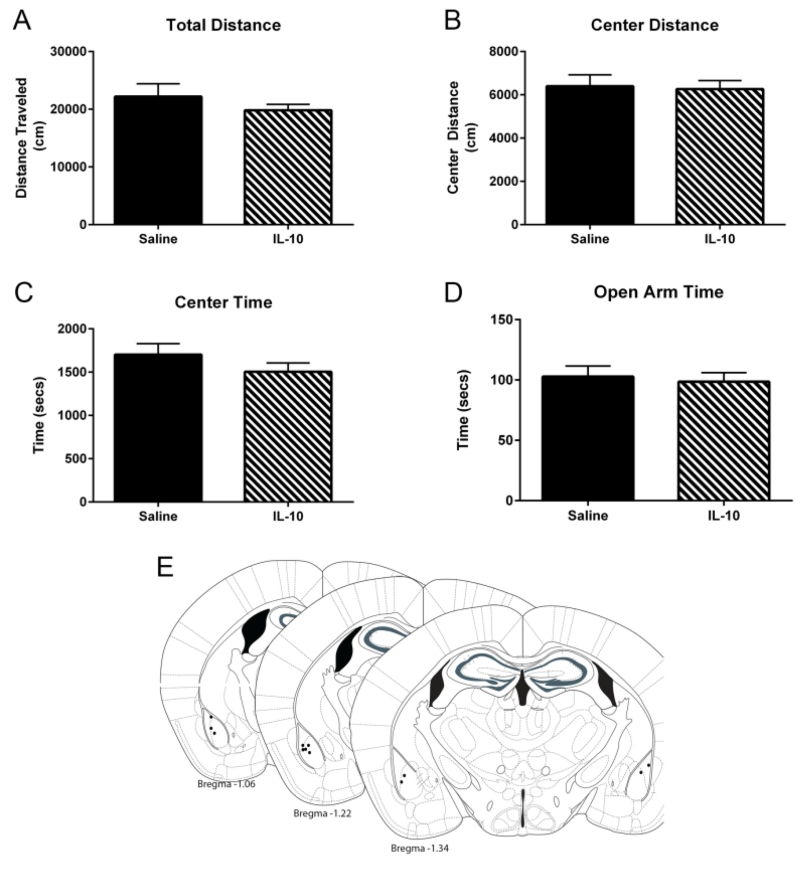
Locomotor activity in the OFT was not different between mice that received 100ng of IL-10 into the BLA compared with those that were administered saline. Two-way RM ANOVAs only indicated a significant main effect of time [F(23,184)= 5.92, p<0.001; F(23,184)= 3.39, p<0.001] but not treatment [F(1,8)= 0.95, p=0.36; F(1,8)= 0.04, p=0.84] or any interaction [F(23,184)=0.84, p=0.68; F(23,184)=0.88, p=0.62] on total distance traveled (Figure 5a) or distance traveled in center (Figure 5b), respectively. Moreover, no significant main effect of treatment, time, or interactions were found when analyzing total time spent in the center of the open-field chamber [F(23,184)=0.84, p=0.68; F(23,184)=0.88, p=0.62]. In the elevated plus maze data, an additional test of anxiety-like behavior, there was no difference in time spent in the open arm based on a t-test [t(7) =0.38, p=0.72] between treatment groups.
Fig. 5.
Within the OFT, IL-10 administration into the BLA did not significantly affect locomotor activity (a) or any measures of anxiety-like behavior [Center Distance (b); Center Time (c)]. Moreover, BLA IL-10 treatment did not alter time spent in the open arm (d) in the elevated plus maze. Approximate injection sites are represented by black circles (e). All data are presented as mean ± SEM
Discussion
The current study builds upon a growing body of literature that implicates the neuroimmune system in ethanol consumption. Specifically, this report highlights IL-10, but not IL-4, as a factor in binge-like ethanol consumption. The data herein provides evidence that: a) binge-like ethanol consumption decreased IL-10 protein in the amygdala without altering peripheral concentrations, b) the decrease in anti-inflammatory cytokines appears to be specific to IL-10 and centralized in the BLA, and c) administration of IL-10 into the BLA reduces binge-like ethanol consumption. Collectively, these studies suggest that alcohol-induced decreases of IL-10 signaling in the BLA may modulate excessive ethanol consumption which can be ameliorated by IL-10 administration.
Increased pro-inflammatory chemokines and cytokines have been observed in the parenchyma of post-mortem alcoholics as well as serum samples from individuals with a history of alcohol misuse (Gonzalez-Quintela et al. 1999; He and Crews 2008). Changes in cytokines have even been proposed as a biomarker for alcoholism (Achur et al. 2010). Various pre-clinical models of alcohol-induced neurodegeneration concur with the observation in humans showing increased TNF-α and IL-1β and suggest that the neuroimmune response is contingent upon the extent of alcohol exposure (Marshall et al. 2013; McClain et al. 2011; Qin and Crews 2012; Qin et al. 2008). Recent evidence shows that even short-term binge-like ethanol drinking stemming from the DID model can cause persistent upregulation of pro-inflammatory cytokines (Marshall et al. 2016a), but the present study specifically looks at the impact of alcohol on an anti-inflammatory state. The data herein concurs with our previous finding showing that short-term binge-like ethanol drinking influences the neuroimmune system, specifically eliciting a decrease in the anti-inflammatory cytokine IL-10 but not IL-4. A decrease in anti-inflammatory molecules was expected as an increase in proinflammatory cytokines often coincides with a decline of anti-inflammatory cytokines and neurotrophic factors in other neuropathologies and in alcohol models (Patterson 2015; Qin et al. 2008; Schiepers et al. 2005). The specificity in which IL-10 but not IL-4 was dysregulated may seem superficially odd since they are both primarily anti-inflammatory cytokines. However, it is important to denote that although the terms “pro” and “anti-inflammatory are often considered distinct, the role of cytokines are somewhat nuanced making understanding their function within the context of a pathology critical (Cavaillon 2001; Steinman 2008). In the peripheral system, IL-10 seems to suppress a proinflammatory response more than IL-4 (Hart et al. 1995; Morita et al. 2001), but the literature comparing IL-10 and IL-4 within the CNS is sparse. The impact of ethanol on cytokines deserves further consideration, but these data suggest that ethanol may only dysregulated discrete immunomodulatory molecules, specifically IL-10 shown herein.
ELISA data confirmed that IL-10 was decreased following ethanol exposure in the amygdala and indicates that the alcohol-induced diminished IL-10 is constrained to the CNS as no differences were determined in the blood sample. Moreover, IHC analyses suggest that the focal point of IL-10 immunoreactivity depression was the BLA and not the CeA. Whether IL-10 immunoreactivity depression persists in abstinence is still to be determined, but other AUD models suggest that this effect is transient as protracted alcohol abstinence results in upregulation of IL-10 (Marshall et al. 2013; Schunck et al. 2015; Suryanarayanan et al. 2016). Most importantly, this data indicates that excessive ethanol consumption elicits a neuroimmune response independent of, and prior to, peripheral immune system dysregulation observed in clinical studies of alcoholics (McClain and Cohen 1989; Nikou et al. 2016; O’Halloran et al. 2016). Peripheral immune reactions combined with the neuroimmune response are hypothesized as a mechanism of alcohol-induced brain damage (Crews and Vetreno 2014), but these data suggest that neuroimmune dysregulation occurs without peripheral influences (Marshall et al. 2016b; Marshall et al. 2013). The fact that only the CNS cytokines are impacted supports the idea that cytokine responses stemming from acute binge-like ethanol drinking are involved with signaling rather than neurodegeneration (Crews and Vetreno 2014; Marshall et al. 2016a). This distinction parses apart the impact of the IL-10 on mediating brain damage following chronic exposure, which has not been observed following binge-like drinking in non-dependent animals, from its role in neurotransmission.
Considering the fact that IL-10 receptors are expressed on neurons (Lim et al. 2013; Sharma et al. 2011) and central IL-10 administration is known to modulate behavior (Barak et al. 2002; Bluthe et al. 1999; Lim et al. 2013; Perkeybile et al. 2009), direct manipulation of IL-10 receptor signaling, using central murine IL-10 administration, was used to determine the impact of this cytokine on ethanol consumption. Peripheral administration of immunomodulatory drugs alter ethanol consumption with proinflammatory agents generally increasing ethanol intake (Bell et al. 2013; Blednov et al. 2011; Blednov et al. 2012); however, the present studies specifically identified that IL-10 administration blunts ethanol consumption and corresponding BECs. Importantly, IL-10 reduced BECs below the binge threshold of 80mg/dL that is associated with so many of the problems of alcohol misuse (NIAAA 2004; Sacks et al. 2015). These studies also indicate a central pharmacological role of immunomodulators rather than as secondary effects of peripheral actions. The ability of IL-10 to modulate ethanol consumption was specific to the BLA, much like IL-1 receptor antagonism (Marshall et al. 2016a). Intra-BLA IL-10 administration did not affect other caloric-rewards or inhibit locomotor activity. Moreover, the dose used in these studies did not affect anxiety-like measures although others have shown that IL-10 can be anxiolytic. The specificity of intra-BLA IL-10 to ethanol consumption and lack of effects in other behavioral correlates is promising when considering therapeutic agents.
The mechanism of action for reduced binge-like drinking is unknown given the current studies. Although IL-10 does not independently modulate glutamatergic signaling (Bachis et al. 2001), IL-10 can inhibit the production of pro-inflammatory cytokines like IL-1β and IL-6 (Thompson et al. 2013). Moreover, IL-1 receptor antagonism has previously been shown to reduce both ethanol consumption and ethanol-induced glutamatergic signaling (Bajo et al. 2015a; Bajo et al. 2015b; Marshall et al. 2016a). Reduced IL-6 expression can also inhibit the ethanol associated depression of excitatory signaling potential (Hernandez et al. 2016). Given that both IL-6 and IL-1 modulate ethanol depression of excitatory signaling and their production is altered by IL-10, it is perceivable that IL-10 indirectly reduces glutamatergic signaling mimicking the actions of acute alcohol and therein reduce binge-like drinking (Kalk and Lingford-Hughes 2014; Lovinger et al. 1989). More directly, recent evidence suggests that IL-10 regulates GABAergic transmission and that IL-10 can ameliorate alcohol-induced deficits in the loss of righting reflex (Suryanarayanan et al. 2016). Regardless of the mechanism, this study concurs with many that the neuroimmune system modulates ethanol consumption, specifically that the promotion of an anti-inflammatory state, like the central IL-10 administration used herein, reduces ethanol consumption .
Regulation of the immune system is a recurring issue in various pathologies and has been a concern for behavioral correlates for some time (Kelley and McCusker 2014). This study highlights the impact of experimental, albeit problematic, binge-like ethanol drinking on cytokine dysregulation. The persisting nature of reduced IL-10 within the amygdala is not specified herein, but these data do argue that, acutely, IL-10 modulates binge-like ethanol consumption. Moreover, IL-10 disruption is specific to the brain, and its effects are mediated centrally, specifically within the BLA. In summary, these studies suggest that therapies that promote an anti-inflammatory milieu in the CNS, especially those that promote the production of IL-10, may be effective in AUD treatment.
Acknowledgments
The authors thank Rhiannon D. Thomas, Sonia Sabater, Timothy P. Gilliam, and Suzahn Ebert for their technical assistance.
Funding
This work was supported by National Institutes of Health grants AA022048, AA013573, AA015148, AA021611, AA011605, DA034721, & GM000678.
Footnotes
Disclosure:
The authors declare no conflict of interest.
References
- Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol. 2010;5:83–91. doi: 10.1007/s11481-009-9185-z. doi:10.1007/s11481-009-9185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Colangelo AM, Vicini S, Doe PP, De Bernardi MA, Brooker G, Mocchetti I. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J Neurosci. 2001;21:3104–3112. doi: 10.1523/JNEUROSCI.21-09-03104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, et al. Role of the IL-1 receptor antagonist in ethanol-induced regulation of GABAergic transmission in the central amygdala. Brain Behav Immun. 2015a;45:189–197. doi: 10.1016/j.bbi.2014.11.011. doi:10.1016/j.bbi.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, et al. IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala. Frontiers in pharmacology. 2015b;6:49. doi: 10.3389/fphar.2015.00049. doi:10.3389/fphar.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak O, Goshen I, Ben-Hur T, Weidenfeld J, Taylor AN, Yirmiya R. Involvement of brain cytokines in the neurobehavioral disturbances induced by HIV-1 glycoprotein120. Brain Res. 2002;933:98–108. doi: 10.1016/s0006-8993(02)02280-1. [DOI] [PubMed] [Google Scholar]
- Bell RL, Lopez MF, Cui C, Egli M, Johnson KW, Franklin KM, Becker HC. Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addict Biol. 2013 doi: 10.1111/adb.12106. doi:10.1111/adb.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Harris RA. Inhibition of phosphodiesterase 4 reduces ethanol intake and preference in C57BL/6J mice. Frontiers in neuroscience. 2014;8:129. doi: 10.3389/fnins.2014.00129. doi:10.3389/fnins.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25(Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. doi:S0889-1591(11)00013-4 [pii] 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. doi:10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe RM, et al. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology. 1999;24:301–311. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Cavaillon JM. Pro- versus anti-inflammatory cytokines: myth or reality. Cell Mol Biol (Noisy-le-grand) 2001;47:695–702. [PubMed] [Google Scholar]
- Cox BR, et al. Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol Clin Exp Res. 2013;37:1688–1695. doi: 10.1111/acer.12145. doi:10.1111/acer.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP. Neuroimmune basis of alcoholic brain damage. Int Rev Neurobiol. 2014;118:315–357. doi: 10.1016/B978-0-12-801284-0.00010-5. doi:10.1016/B978-0-12-801284-0.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. doi:10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee JR, Sparta DR, Knapp DJ, Breese GR, Picker MJ, Thiele TE. Predictors of high ethanol consumption in RIIbeta knock-out mice: assessment of anxiety and ethanol-induced sedation. Alcohol Clin Exp Res. 2004;28:1459–1468. doi: 10.1097/01.ALC.0000141809.53115.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RB, Dawson DA, Chou SP, Grant BF. Sex differences in prevalence and comorbidity of alcohol and drug use disorders: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of studies on alcohol and drugs. 2012;73:938–950. doi: 10.15288/jsad.2012.73.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Quintela A, Vidal C, Lojo S, Perez LF, Otero-Anton E, Gude F, Barrio E. Serum cytokines and increased total serum IgE in alcoholics. Ann Allergy Asthma Immunol. 1999;83:61–67. doi: 10.1016/S1081-1206(10)63514-4. [DOI] [PubMed] [Google Scholar]
- Hart PH, Ahern MJ, Smith MD, Finlay-Jones JJ. Comparison of the suppressive effects of interleukin-10 and interleukin-4 on synovial fluid macrophages and blood monocytes from patients with inflammatory arthritis. Immunology. 1995;84:536–542. [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental neurology. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RV, et al. Transgenic mice with increased astrocyte expression of IL-6 show altered effects of acute ethanol on synaptic function. Neuropharmacology. 2016;103:27–43. doi: 10.1016/j.neuropharm.2015.12.015. doi:10.1016/j.neuropharm.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalk NJ, Lingford-Hughes AR. The clinical pharmacology of acamprosate. Br J Clin Pharmacol. 2014;77:315–323. doi: 10.1111/bcp.12070. doi:10.1111/bcp.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, McCusker RH. Getting nervous about immunity. Semin Immunol. 2014;26:389–393. doi: 10.1016/j.smim.2014.01.011. doi:10.1016/j.smim.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology. 2015;96:55–69. doi: 10.1016/j.neuropharm.2014.10.020. doi:10.1016/j.neuropharm.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. doi:10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Lim SH, Park E, You B, Jung Y, Park AR, Park SG, Lee JR. Neuronal synapse formation induced by microglia and interleukin 10. PLoS One. 2013;8:e81218. doi: 10.1371/journal.pone.0081218. doi:10.1371/journal.pone.0081218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Marshall SA, Casachahua JD, Rinker JA, Blose AK, Lysle DT, Thiele TE. IL-1 receptor signaling in the basolateral amygdala modulates binge-like ethanol consumption in male C57BL/6J mice. Brain Behav Immun. 2016a;51:258–267. doi: 10.1016/j.bbi.2015.09.006. doi:10.1016/j.bbi.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Geil CR, Nixon K. Prior Binge Ethanol Exposure Potentiates the Microglial Response in a Model of Alcohol-Induced Neurodegeneration. Brain Sci. 2016b;6 doi: 10.3390/brainsci6020016. doi:10.3390/brainsci6020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol Dis. 2013;54:239–251. doi: 10.1016/j.nbd.2012.12.016. doi:S0969-9961(13)00007-7 [pii]10.1016/j.nbd.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Rinker JA, Harrison LK, Fletcher CA, Herfel TM, Thiele TE. Assessment of the Effects of 6 Standard Rodent Diets on Binge-Like and Voluntary Ethanol Consumption in Male C57BL/6J Mice. Alcohol Clin Exp Res. 2015 doi: 10.1111/acer.12773. doi:10.1111/acer.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. doi:S0270913989000613 [pii] [DOI] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun. 2011;25(Suppl 1):S120–128. doi: 10.1016/j.bbi.2011.01.006. doi:S0889-1591(11)00011-0 [pii]10.1016/j.bbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, et al. Differential in vitro effects of IL-4, IL-10, and IL-13 on proinflammatory cytokine production and fibroblast proliferation in rheumatoid synovium. Rheumatol Int. 2001;20:49–54. doi: 10.1007/s002960000074. [DOI] [PubMed] [Google Scholar]
- NIAAA . NIAAA Council Approves Definition of Binge Drinking. Winter edn. National Institute of Health; Bethesda, MD: 2004. [Google Scholar]
- Nikou T, et al. Alteration in the concentrations of Interleukin-7 (IL-7), Interleukin-10 (IL-10) and Granulocyte Colony Stimulating Factor (G-CSF) in alcohol-dependent individuals without liver disease, during detoxification therapy. Drug Alcohol Depend. 2016 doi: 10.1016/j.drugalcdep.2016.03.022. doi:10.1016/j.drugalcdep.2016.03.022. [DOI] [PubMed] [Google Scholar]
- NRC . Guide for the care and use of laboratory animals. 8th edn. National Academies Press; Washington, D.C.: 2011. [PubMed] [Google Scholar]
- O’Halloran EB, Curtis BJ, Afshar M, Chen MM, Kovacs EJ, Burnham EL. Alcohol. Vol. 50. Fayetteville, NY: 2016. Alveolar macrophage inflammatory mediator expression is elevated in the setting of alcohol use disorders; pp. 43–50. doi:10.1016/j.alcohol.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL. Immune dysregulation and cognitive vulnerability in the aging brain: Interactions of microglia, IL-1beta, BDNF and synaptic plasticity. Neuropharmacology. 2015;96:11–18. doi: 10.1016/j.neuropharm.2014.12.020. doi:10.1016/j.neuropharm.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Compact 2nd edn. Elsevier Academic Press; Amsterdam; Boston: 2004. [Google Scholar]
- Perkeybile AM, Schiml-Webb PA, O’Brien E, Deak T, Hennessy MB. Anti-inflammatory influences on behavioral, but not cortisol, responses during maternal separation. Psychoneuroendocrinology. 2009;34:1101–1108. doi: 10.1016/j.psyneuen.2009.02.014. doi:10.1016/j.psyneuen.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J Neuroinflammation. 2012;9:130. doi: 10.1186/1742-2094-9-130. doi:1742-2094-9-130 [pii],10.1186/1742-2094-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. doi:1742-2094-5-10 [pii] 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. doi:10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 National and State Costs of Excessive Alcohol Consumption. American journal of preventive medicine. 2015;49:e73–79. doi: 10.1016/j.amepre.2015.05.031. doi:10.1016/j.amepre.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. doi:10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schunck RV, et al. Protracted alcohol abstinence induces analgesia in rats: Possible relationships with BDNF and interleukin-10. Pharmacology, biochemistry, and behavior. 2015;135:64–69. doi: 10.1016/j.pbb.2015.05.011. doi:10.1016/j.pbb.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Sharma S, Yang B, Xi X, Grotta JC, Aronowski J, Savitz SI. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res. 2011;1373:189–194. doi: 10.1016/j.brainres.2010.11.096. doi:10.1016/j.brainres.2010.11.096. [DOI] [PubMed] [Google Scholar]
- Sprow GM, Thiele TE. The neurobiology of binge-like ethanol drinking: evidence from rodent models. Physiol Behav. 2012;106:325–331. doi: 10.1016/j.physbeh.2011.12.026. doi:10.1016/j.physbeh.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. Nuanced roles of cytokines in three major human brain disorders. J Clin Invest. 2008;118:3557–3563. doi: 10.1172/JCI36532. doi:10.1172/JCI36532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayanan A, Carter JM, Landin JD, Morrow AL, Werner DF, Spigelman I. Role of interleukin-10 (IL-10) in regulation of GABAergic transmission and acute response to ethanol. Neuropharmacology. 2016;107:181–188. doi: 10.1016/j.neuropharm.2016.03.027. doi:10.1016/j.neuropharm.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Navarro M. Alcohol. Vol. 48. Fayetteville, NY: 2014. “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in non-dependent mice; pp. 235–241. doi:10.1016/j.alcohol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CD, Zurko JC, Hanna BF, Hellenbrand DJ, Hanna A. The therapeutic role of interleukin-10 after spinal cord injury. J Neurotrauma. 2013;30:1311–1324. doi: 10.1089/neu.2012.2651. doi:10.1089/neu.2012.2651. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. doi:10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Crews FT. Inflammasome-IL-1beta Signaling Mediates Ethanol Inhibition of Hippocampal. Neurogenesis Frontiers in neuroscience. 2012;6:77. doi: 10.3389/fnins.2012.00077. doi:10.3389/fnins.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]