Abstract
The aim of the study was to evaluate the levels of physical activity in individuals with primary Sjögren’s syndrome (PSS) and its relationship to the clinical features of PSS. To this cross-sectional study, self-reported levels of physical activity from 273 PSS patients were measured using the International Physical Activity Questionnaire-short form (IPAQ-SF) and were compared with healthy controls matched for age, sex and body mass index. Fatigue and other clinical aspects of PSS including disease status, dryness, daytime sleepiness, dysautonomia, anxiety and depression were assessed using validated tools. Individuals with PSS had significantly reduced levels of physical activity [median (interquartile range, IQR) 1572 (594–3158) versus 3708 (1732–8255) metabolic equivalent of task (MET) × min/week, p < 0.001], but similar levels of sedentary activity [median (IQR) min 300 (135–375) versus 343 (223–433) (MET) × min/week, p = 0.532] compared to healthy individuals. Differences in physical activity between PSS and controls increased at moderate [median (IQR) 0 (0–480) versus 1560 (570–3900) MET × min/week, p < 0.001] and vigorous intensities [median (IQR) 0 (0–480) versus 480 (0–1920) MET × min/week, p < 0.001]. Correlation analysis revealed a significant association between physical activity and fatigue, orthostatic intolerance, depressive symptoms and quality of life. Sedentary activity did not correlate with fatigue. Stepwise linear regression analysis identified symptoms of depression and daytime sleepiness as independent predictors of levels of physical activity. Physical activity is reduced in people with PSS and is associated with symptoms of depression and daytime sleepiness. Sedentary activity is not increased in PSS. Clinical care teams should explore the clinical utility of targeting low levels of physical activity in PSS.
Electronic supplementary material
The online version of this article (doi:10.1007/s00296-016-3637-6) contains supplementary material, which is available to authorized users.
Keywords: Primary Sjögren’s syndrome, Physical activity, Patient registry, Patient-reported outcomes, Fatigue
Introduction
Musculoskeletal pain and fatigue are key symptoms of primary Sjögren’s syndrome (PSS) and can adversely impact on levels of physical activity. Indeed, individuals with PSS have reported that the disease impacts on their physical activity levels [1]. A physically active lifestyle is associated with decreased risk of cardiovascular disease, stroke, hypertension, type 2 diabetes, obesity, some cancers as well as all-cause mortality [2–4]. It is possible that people with PSS are at excess risk of secondary disease as a result of reduced physical activity in addition to the primary clinical diagnosis.
Very little research has been undertaken to evaluate physical activity levels in PSS patients. To date, a Dutch study has reported physical activity in people with PSS as its primary outcome. A report of 223 PSS patients and 67 controls revealed that PSS patients reported less moderate and vigorous physical activity, but similar levels of walking to matched controls without PSS [5]. Although an important study, the data are limited by the relatively small control group and is missing data on body mass index (BMI). Matching for BMI is important as BMI is related to physical activity [6, 7]. Another report from Sweden also suggested that physical activity is reduced in PSS, although physical activity was not the primary outcome and the study is limited by a small sample size (n = 51) and use of tool that is not validated [8]. Furthermore, it has been known that physical activity behaviour varies greatly across different countries in population-based studies [9, 10], and to our knowledge, physical activity in PSS has not been assessed in the UK. Interestingly, no studies have reported whether people with PSS spend more time sedentary, despite the powerful role of sedentary behaviour in health and well-being.
The International Physical Activity Questionnaire-short form (IPAQ-SF) is the most widely used self-reported questionnaire for population or cohort studies of habitual physical activities which is easy to complete, and its reliability and validity have been established in different countries [11, 12].
The primary aim of this study was to assess the level of physical activity and sedentary behaviour in a large cohort of PSS patients and people without chronic disease individually matched for age, gender and BMI. The secondary aims were to explore the relationships between physical activity and sedentary behaviour with clinical features of PSS.
Patients and methods
To this cross-sectional study, PSS patients and healthy control subjects were recruited from September 2009 to September 2011. Self-reported levels of physical activity from PSS patients were compared with healthy controls. Fatigue and other clinical aspects of PSS including disease status, dryness, daytime sleepiness, dysautonomia, anxiety and depression were also assessed.
Subject groups
All PSS patients are participants of the United Kingdom primary Sjögren’s syndrome registry (UKPSSR, www.sjogrensregistry.org) [13] and were recruited from 30 participating centres in the UK. Embedded within the design of the UKPSSR are several optional sub-studies aiming to address different clinical questions, one of which is the level of physical activity among PSS patients. Participants of the UKPSSR were given the IPAQ-SF questionnaire which they could choose whether to complete or not. Each PSS participant with complete IPAQ-SF data matched case by case (1:1) by sex, age (±3 years) and BMI (±3 kg/m2) was recruited from a community control cohort of 800 subjects without clinical diagnosis of chronic disease based on self-reported history established by co-author Trenell.
Inclusion criteria were as follows: age over 18, fulfil the American European Consensus Group (AECG) classification criteria [14] and those with complete datasets for physical activity assessment and matched controls. Patients without matched controls and body mass index (BMI) data were excluded.
Research ethical approval was granted by the North West Research Ethics Committee. Informed consent was obtained from all patients according to the principles of the Helsinki Declaration.
Measures
All clinical and laboratory data were collected prospectively at the time of recruitment, and the instruments used were: IPAQ-SF, 100-mm visual analogue scale to determine overall fatigue, Profile of Fatigue (ProF, measures fatigue in PSS) [15], Epworth Sleepiness Scale (ESS, measures daytime sleepiness) [16], Orthostatic Grading Scale (OGS, measures orthostatic intolerance) [17], Composite Autonomic Symptom Scale (COMPASS, measures autonomic symptoms) [18], EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI, measures disease activity) [19], EULAR Sjögren’s Syndrome Patient-Reported Index (ESSPRI, measures overall burden of symptoms) [20], EULAR Sicca Score (EULAR-SS, measures overall severity of dryness) [20], Hospital Anxiety and Depression Scale (HADS, measures anxiety and depressive symptoms) [21], EuroQol-5 Domain (EQ5D, measures health-related quality of life) [22] and Comorbidity–Polypharmacy Score (CPS, quantify the magnitude of comorbid conditions) [23, 24]. To score the CPS, all the known comorbidities and medications that a patient has been taking were considered.
Physical activity was measured using IPAQ-SF. The nine-item IPAQ-SF records the time spent on physical activity of three intensity levels (vigorous, moderate and walking) as well as the time spent on sitting (referred to as sedentary in this study) in the past week. The data were processed and analysed according to the guidelines published by the IPAQ research committee [25]. In brief, the metabolic equivalent of task (MET) values for each intensity level of physical activity, derived from the IPAQ Reliability Study [12], were multiplied by the time spent (in minutes/week) for each intensity level of physical activity to obtain the total MET values for each intensity levels of physical activity. The total physical activity (PA) score is the sum of total MET values for vigorous, moderate and walking activities. Sedentary time (median) was reported in minutes/week.
Statistical analysis
All data were analysed using IBM SPSS Statistics 19. As most variables were not normally distributed, Wilcoxon matched pairs/Mann–Whitney test was performed for comparisons between PSS and control groups. The PA scores were substantially positively skewed and were therefore log transformed. Pearson’s and Spearman’s correlations were used for correlation analysis for nonparametric and parametric data, respectively. To identify independent predictors for physical activity, stepwise linear regression analysis was performed using log-transformed total PA score as the dependent variable.
Results
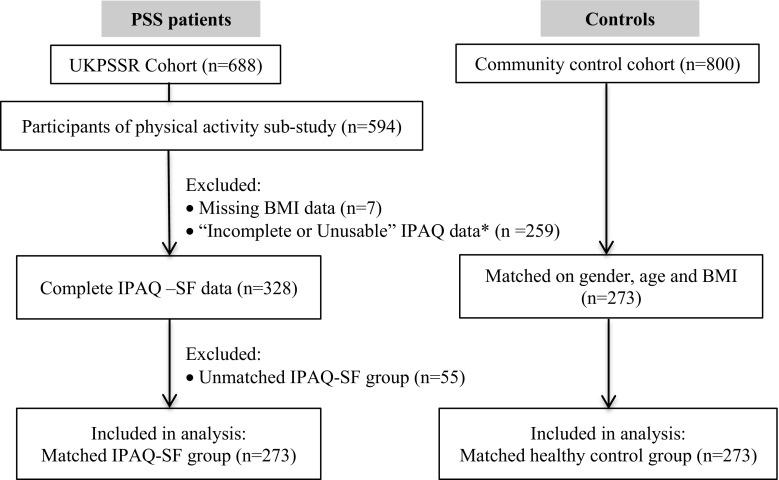
Among 688 patients recruited to the UKPSSR, 594 patients (86.3%) participated in the physical activity sub-study. Only those with complete datasets for physical activity assessment and matched controls (n = 273) were included in the analysis. Figure 1 summarizes the participant flow through this study.
Fig. 1.
Summary of the participant flow of the study. *The majority (~70%) of the “Incomplete/unusable” data were “unusable” because the participants had responded “Don’t know/Not sure” to the question on “how much time spent on the physical activity”, the remaining were “unusable” because data on the number of days or hours/minutes spending on the physical activity were missing, unclear or contradictory
Patient characteristics
The clinical characteristics of the PSS cohort used in this study and the demographics of the age-, sex- and BMI-matched healthy controls are summarized in Table 1. The study cohort (“matched IPAQ-SF” group, (n = 273) differed clinically from the remaining UKPSSR cohort (n = 415), with the study cohort being younger, with better quality of life, fewer comorbidities and less overall symptom burden (ESSPRI), fatigue, pain, symptoms of anxiety, depression and daytime sleepiness (Online Resource, Supplementary table 1). There were no significant differences in the physical activity measures between the study group and those with complete IPAQ-SF datasets but without matched controls (“unmatched IPAQ-SF” group, n = 55), with the exception of age and BMI, which were expected because the more extreme values of the age and BMI in the unmatched IPAQ-SF group were the reasons for matching not being achieved (Online Resource, Supplementary table 2). The “matched IPAQ-SF” group also has lower CPS score than the “unmatched IPAQ-SF” group.
Table 1.
Characteristics of the primary Sjögren’s syndrome (PSS) cohort and matched healthy control
| PSS cohort | Healthy control | p | |
|---|---|---|---|
| Sample size | 273 | 273 | |
| Female/male, n | 254/19 | 254/19 | |
| Age | 57 (47–65) | 58 (47–65) | 0.310 |
| Body mass index (kg/m2) | 25 (23–28) | 25 (23–27) | 0.285 |
| Sitting time (min) | 300 (135–375) | 343 (223–433) | 0.454 |
| Vigorous PA (MET × min/wk) | 0 (0–480) | 480 (0–1920) | <0.001 |
| Moderate PA (MET × min/wk) | 0 (0–480) | 1560 (570–3930) | <0.001 |
| Walking (MET × min/wk) | 792 (396–2079) | 990 (462-3020) | 0.012 |
| Total PA score (MET × min/wk) | 1572 (594–3158) | 3708 (1732–8255) | <0.001 |
All values are presented as medians (interquartile ranges)
kg/m 2 kilogram per metre squared, min minutes, MET metabolic equivalent of task, min/wk minutes per week
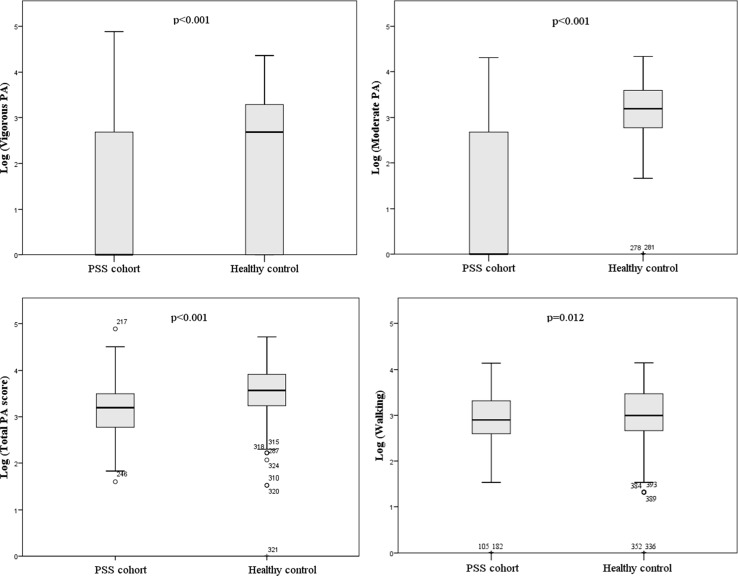
Levels of physical activity were significantly reduced in PSS patients
The median total PA score in the PSS group was <50% compared to the controls (Table 1). Moderate and vigorous physical activities were markedly reduced among PSS patients compared to the matched control group (Fig. 2). Levels of sedentary activity (sitting time) were similar between PSS patients and controls. Female patients were less active than male patients, but there was no difference in the control group (Online Resource, Supplementary table S3).
Fig. 2.
Vigorous, moderate, total and walking physical activity levels in primary Sjögren’s syndrome (PSS) cohort and healthy controls. PA physical activity
Relationships between physical activity levels and clinical features of PSS
To explore the relationships between levels of physical activity and clinical features of PSS, we first performed a correlation analysis between total PA score and a range of pre-specified parameters based on potential biological links and data from previous studies. Total PA score correlated weakly but statistically significantly with physical fatigue (r = −0.159), mental fatigue (r = −0.135), symptoms of depression (r = −0.146), orthostatic intolerance (r = −0.124) and quality of life (r = −0.200), but none of the clinical features correlated with sitting time (Online Resource, Supplementary table S4). Stepwise linear regression analysis identified symptoms of depression and daytime sleepiness as independent predictors of total PA score (Table 2). However, these two predictors accounted for only approximately 4.5% of the variance in total PA score (p = 0.047). Fatigue was a predictor of vigorous and moderate intensities of physical activity, whereas symptoms of depression, anxiety and daytime sleepiness were predictors of physical activity of moderate intensity. Symptoms of dryness, depression and BMI predicted walking activity (Table 2).
Table 2.
Stepwise regression analysis of independent correlations of total and various intensity levels of physical activity of primary Sjögren’s syndrome cohort
| Coefficients | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unstandardized | Standardized | |||||||
| B | SE | Beta | t | p | R | R 2 | Adj. R 2 | |
| (a) Total PA score | 0.211 | 0.045 | 0.036 | |||||
| (Constant) | 3.191 | 0.075 | 54.148 | <0.001 | ||||
| Depression (HADS) | −0.031 | 0.010 | −0.218 | −3.105 | 0.002 | |||
| Daytime sleepiness (ESS) | 0.016 | 0.008 | 0.140 | 2.000 | 0.047 | |||
| (b) Vigorous PA | 0.308 | 0.095 | 0.087 | |||||
| (Constant) | 3.019 | 0.484 | 6.234 | <0.001 | ||||
| Physical fatigue (ProF) | −0.210 | 0.55 | −0.244 | −3.848 | <0.001 | |||
| Age | −0.024 | 0.008 | −0.189 | −2.977 | 0.003 | |||
| (c) Moderate PA | 0.346 | 0.120 | 0.104 | |||||
| (Constant) | 1.047 | 0.222 | 4.709 | <0.001 | ||||
| Overall fatigue (VAS) | −0.012 | 0.004 | −0.228 | −2.790 | 0.006 | |||
| Daytime sleepiness (ESS) | 0.073 | 0.021 | 0.246 | 3.452 | 0.001 | |||
| Depression (HADS) | −0.093 | 0.034 | −0.248 | −2.781 | 0.006 | |||
| Anxiety (HADS) | 0.086 | 0.027 | 0.264 | 3.209 | 0.002 | |||
| (d) Walking | 0.289 | 0.084 | 0.071 | |||||
| (Constant) | 3.321 | 0.320 | 10.382 | <0.001 | ||||
| EULAR Sicca Score | 0.065 | 0.018 | 0.238 | 3.538 | <0.001 | |||
| Depression (HADS) | −0.029 | 0.012 | −0.161 | −2.390 | 0.018 | |||
| Body mass index | −0.028 | 0.012 | −0.149 | −2.321 | 0.021 | |||
PA physical activity, HADS Hospital Anxiety and Depression Scale, ESS Epworth Sleepiness Scale, ProF Profile of Fatigue, VAS visual analogue scale
Discussion
In this study, we showed that self-reported physical activity levels were significantly lower among PSS patients, particularly for physical activities of vigorous and moderate intensities, compared to age-, sex- and BMI-matched healthy controls. However, levels of sedentary activity (sitting time) were not increased in PSS patients. Reduced levels of total physical activity score were independently associated with symptoms of depression and daytime sleepiness.
Our data reveal a decrease in physical activity in people with PSS against a well-matched control group. The data build on previous reports, supporting the association of PSS with reduced moderate and vigorous physical activity [5], but add further by demonstrating that this is not confounded by BMI. Our patients were recruited from 30 centres (with a mixture of teaching hospitals and district general hospitals) across the UK, increasing the ecological validity of the data. Sedentary activity is associated with adverse health effects independent of those from decreased levels of overall physical activity or higher-intensity physical activity [26, 27]. Interestingly, the decrease in physical activity in the present study was not accompanied by an increase in sedentary behaviour. The similarity in sedentary behaviour between the PSS and control groups does not support the belief that PSS patients spend more time sitting. Combined, these data suggest that patients are moving as much as their healthy counterparts, but when they are active they are not working as hard. As such, patients with PSS may be exposed to excess risk of developing secondary chronic disease as a result of their low levels of physical activity and this should be targeted therapeutically.
Despite the weak association, our study is the first to demonstrate the relationship between physical activity and daytime sleepiness in PSS, which together with symptoms of depression, predicted lower levels of physical activities, mainly those at moderate intensity levels. In a previous study, excessive daytime sleepiness, defined as the propensity to fall asleep at a time when the individual would usually be awake and alert, was more prevalent in PSS patients than in healthy controls and was associated with mental and physical fatigue. Sleep disturbances in PSS, anxiety, nocturia and sicca problems, were also more prevalent, but only insomnia correlated with daytime sleepiness and depression had some impact on daytime sleepiness [28]. In a study by Zafar et al., PSS patients had higher level of daytime sleepiness and twice the frequency of obstructive apnoeas and hypopneas compared with control subjects but no significant correlations were found between these parameters and sleepiness scores [29]. Walker et al. [30] found that PSS patients have more severe symptoms of daytime sleepiness than patients with osteoarthritis, independent of nocturia. Daytime sleepiness in PSS is also associated with impaired functional status [31], autonomic symptoms [32] and decreased quality of life [33]. However, daytime sleepiness and depression can be interrelated and should be viewed concurrently.
Depression is a key determinant of EQ5D utility values in a large PSS cohort in the UK and also has a significant correlation with fatigue [33]. Depression is an independent predictor of cognitive symptoms [34], strongly correlates with functional disability [31] and leads to more physician visits and work disability in PSS patients [35]. Strömbeck et al. [8] did not find an association between depression and an indirect measure of aerobic capacity. In contrast, our study demonstrated that depression may be an independent predictor of decreased levels of self-reported physical activity in PSS. Our data suggest that identifying excessive daytime sleepiness and depressive symptoms as well as their contributing factors may be important in achieving a more physically active lifestyle for some people living with PSS.
The inverse relationship between fatigue and moderate/vigorous physical activity levels is perhaps unsurprising and has been reported in healthy populations and other chronic conditions [36–39]. Whether there is a causal link between fatigue and physical activity levels remains unclear and cannot be addressed based on the data generated from this study. Strömbeck et al. reported that women with PSS have decreased physical capacity and experienced more pain during the shoulder mobility test. Furthermore, diminished aerobic capacity correlated with symptoms of fatigue experienced [8]. Interestingly, in a small group of PSS patients, Nordic walking for 45 min thrice weekly for 12 weeks improved aerobic capacity and reduced symptoms of fatigue and depression, but not quality of life [40]. More recently, Wouters et al. [5] have shown that in PSS patients’ lower physical activity, higher activity avoidance and somatic focus were associated with more severe symptoms of fatigue. These observations implied that programmes designed to increase the levels of physical activity may ameliorate the symptoms of fatigue and depression.
The association between symptoms of dryness and physical activity levels is a novel finding and could be a consequence of the increased walking time compared with vigorous and moderate activities in patients with higher symptoms of dryness. Further studies are needed to explore the underlying mechanisms of association between sicca symptoms and physical activity levels.
In this study, symptoms of depression and daytime sleepiness accounted for merely 4.5% of the variance of total physical activity levels. Symptoms of pain, autonomic dysfunction and anxiety are well described among PSS patients and may also affect physical activity levels. However, although fatigue and orthostatic intolerance inversely correlated with total physical activity score, they were not identified as independent predictors in regression analysis. Given the link between reduced physical activity levels and adverse health outcomes [2–4] as well as diminished quality of life [41–43], further studies to improve our understanding of the contributing factors to reduced physical activity in PSS and to devise strategies to increase physical activity levels in PSS are warranted.
The present study has limitations. Formal sample size calculation was not performed; nevertheless, our sample is greater than the previous study [5] and is matched case by case by sex, age and BMI. Direct measurements of physical activity were not performed; however, IPAQ-SF scores have been shown to correlate well with actual physical activity levels [11, 12]. Furthermore, since physical activity levels of both study groups were estimated using the same instrument, we anticipate that our observation of reduced physical activity levels in PSS relative to their matched healthy controls remains valid upon objective measurements. Only ~40% of the entire cohort had complete and matched IPAQ-SF data for analysis, and the study cohort differed from the remaining PSS cohort—with the study cohort having better quality of life and lower levels of fatigue, disease activity, fewer comorbidities, less symptom burden, dryness, orthostatic intolerance, anxiety, symptoms of depression and being younger. Since fatigue, depression and orthostatic intolerance are inversely correlated with the levels of physical activity, we believe that the physical activity levels of PSS patients of the entire cohort might be even lower. External validation of our findings with an independent cohort has not been conducted.
In conclusion, physical activity is reduced in people with PSS without a concomitant increase in sedentary activity. Symptoms of depression and daytime sleepiness may be predictors of physical activity in PSS. Given the risks of developing secondary chronic disease as a result of low levels of physical activity, clinicians should explore the clinical utility of increasing physical activity as part of a holistic management package of PSS.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all the patients who have participated in this study.
WFN, SJB and BG are investigators of the UKPSSR. The other UKPSSR members (as of 1 January 2013) include, in alphabetical order of their affiliations: Frances Hall (Addenbrooke’s Hospital, Cambridge); Elalaine C Bacabac, Robert Moots (Aintree University Hospitals); Kuntal Chakravarty, Shamin Lamabadusuriya (Barking, Havering and Redbridge NHS Trust); Michele Bombardieri, Constantino Pitzalis, Nurhan Sutcliffe (Bart and the London NHS Trust); Nagui Gendi, Rashidat Adeniba (Basildon Hospital); John Hamburger, Andrea Richards (Birmingham Dental Hospital); Saaeha Rauz (Birmingham & Midland Eye Centre); Sue Brailsford (University Hospitals Birmingham); Joanne Logan, Diarmuid Mulherin (Cannock Chase Hospital); Jacqueline Andrews, Paul Emery, Alison McManus, Colin Pease (Chapel Allerton Hospital, Leeds); Alison Booth, Marian Regan (Derbyshire Royal Infirmary); Theodoros Dimitroulas, Lucy Kadiki, Daljit Kaur, George Kitas (Dudley Group of Hospitals NHS Foundation Trust); Mark Lloyd, Lisa Moore (Frimley Park Hospital); Esther Gordon, Cathy Lawson (Harrogate District Foundation Trust Hospital); Monica Gupta, John Hunter, Lesley Stirton (Gartnavel General Hospital, Glasgow); Gill Ortiz, Elizabeth Price (Great Western Hospital); Gavin Clunie, Ginny Rose, Sue Cuckow (Ipswich Hospital NHS Trust); Susan Knight, Deborah Symmons, Beverley Jones (Macclesfield District General Hospital & Arthritis Research UK Epidemiology Unit, Manchester); Shereen Al-Ali, Andrew Carr, Katherine Collins, Ian Corbett, Christine Downie, Suzanne Edgar, Marco Carrozzo, Francisco Figuereido, Heather Foggo, Katherine James, Dennis Lendrem, Iain Macleod, Philip Mawson, Sheryl Mitchell, Andini Natasari, Philip Stocks, Jessica Tarn (Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University); Adrian Jones, Peter Lanyon, Alice Muir (Nottingham University Hospital); Paula White, Steven Young-Min (Portsmouth Hospitals NHS Trust); Susan Pugmire, Saravanan Vadivelu (Queen’s Elizabeth Hospital, Gateshead); Annie Cooper, Marianne Watkins (Royal Hampshire County Hospital); Anne Field, Stephen Kaye, Devesh Mewar, Patricia Medcalf, Pamela Tomlinson, Debbie Whiteside (Royal Liverpool University Hospital); Neil McHugh, John Pauling, Julie James, Nike Olaitan (Royal National Hospital for Rheumatic Diseases); Mohammed Akil, Jayne McDermott, Olivia Godia (Royal Sheffield Hospital); David Coady, Elizabeth Kidd, Lynne Palmer (Sunderland Royal Hospital); Bhaskar Dasgupta, Victoria Katsande, Pamela Long (Southend University Hospital); Charles Li (Royal Surrey Hospital); Usha Chandra, Kirsten MacKay (Torbay Hospital); Stefano Fedele, Ada Ferenkeh-Koroma, Ian Giles, David Isenberg, Helena Maconnell, Stephen Porter (University College Hospital & Eastman Dental Institute); Paul Allcoat, John McLaren (Whyteman’s Brae Hospital, Kirkcaldy).
Funding
This work was supported by the Medical Research Council (G0800629 to WFN, SJB & BG) and the British Sjögren’s Syndrome Association. This project also received infrastructure support from the Northumberland, Tyne and Wear Musculoskeletal Comprehensive Local Research Network, the Newcastle Clinical Research Facilities and The National Institute for Health Research (NIHR) Biomedical Research Centre on Ageing and Age-Related Diseases. MT is supported by a Senior Fellowship from the NIHR. Samira Tatiyama Miyamoto was funded by CAPES Foundation (Proc. n. BEX 8831/14-9). None of the funders contributed to the design or interpretation of the results of this study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Samira Tatiyama Miyamoto: CAPES Foundation scholar—Proc. n. BEX 8831/14-9.
Contributor Information
Wan-Fai Ng, Phone: +44-191-2228125, Email: Wan-fai.Ng@ncl.ac.uk.
on behalf of the UK Primary Sjögren’s Syndrome Registry:
Frances Hall, Elalaine C. Bacabac, Robert Moots, Kuntal Chakravarty, Shamin Lamabadusuriya, Michele Bombardieri, Constantino Pitzalis, Nurhan Sutcliffe, Nagui Gendi, Rashidat Adeniba, John Hamburger, Andrea Richards, Saaeha Rauz, Sue Brailsford, Joanne Logan, Diarmuid Mulherin, Jacqueline Andrews, Paul Emery, Alison McManus, Colin Pease, Alison Booth, Marian Regan, Theodoros Dimitroulas, Lucy Kadiki, Daljit Kaur, George Kitas, Mark Lloyd, Lisa Moore, Esther Gordon, Cathy Lawson, Monica Gupta, John Hunter, Lesley Stirton, Gill Ortiz, Elizabeth Price, Gavin Clunie, Ginny Rose, Sue Cuckow, Susan Knight, Deborah Symmons, Beverley Jones, Shereen Al-Ali, Andrew Carr, Katherine Collins, Ian Corbett, Christine Downie, Suzanne Edgar, Marco Carrozzo, Francisco Figuereido, Heather Foggo, Katherine James, Dennis Lendrem, Iain Macleod, Philip Mawson, Sheryl Mitchell, Andini Natasari, Philip Stocks, Jessica Tarn, Adrian Jones, Peter Lanyon, Alice Muir, Paula White, Steven Young-Min, Susan Pugmire, Saravanan Vadivelu, Annie Cooper, Marianne Watkins, Anne Field, Stephen Kaye, Devesh Mewar, Patricia Medcalf, Pamela Tomlinson, Debbie Whiteside, Neil McHugh, John Pauling, Julie James, Nike Olaitan, Mohammed Akil, Jayne McDermott, Olivia Godia, David Coady, Elizabeth Kidd, Lynne Palmer, Bhaskar Dasgupta, Victoria Katsande, Pamela Long, Charles Li, Usha Chandra, Kirsten MacKay, Stefano Fedele, Ada Ferenkeh-Koroma, Ian Giles, David Isenberg, Helena Maconnell, Stephen Porter, Paul Allcoat, and John McLaren
References
- 1.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273(5):402–407. doi: 10.1001/jama.1995.03520290054029. [DOI] [PubMed] [Google Scholar]
- 2.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 3.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segal B, Bowman SJ, Fox PC, Vivino FB, Murukutla N, Brodscholl J, et al. Primary Sjogren’s syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes. 2009;7:46. doi: 10.1186/1477-7525-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wouters EJ, van Leeuwen N, Bossema ER, Kruize AA, Bootsma H, Bijlsma JW, et al. Physical activity and physical activity cognitions are potential factors maintaining fatigue in patients with primary Sjogren’s syndrome. Ann Rheum Dis. 2012;71(5):668–673. doi: 10.1136/ard.2011.154245. [DOI] [PubMed] [Google Scholar]
- 6.Hemmingsson E, Ekelund U. Is the association between physical activity and body mass index obesity dependent? Int J Obes. 2007;31(4):663–668. doi: 10.1038/sj.ijo.0803458. [DOI] [PubMed] [Google Scholar]
- 7.Hagstromer M, Oja P, Sjostrom M. Physical activity and inactivity in an adult population assessed by accelerometry. Med Sci Sports Exerc. 2007;39(9):1502–1508. doi: 10.1249/mss.0b013e3180a76de5. [DOI] [PubMed] [Google Scholar]
- 8.Strombeck B, Ekdahl C, Manthorpe R, Jacobsson LT. Physical capacity in women with primary Sjogren’s syndrome: a controlled study. Arthritis Rheum. 2003;49(5):681–688. doi: 10.1002/art.11384. [DOI] [PubMed] [Google Scholar]
- 9.Guthold R, Ono T, Strong KL, Chatterji S, Morabia A. Worldwide variability in physical inactivity a 51-country survey. Am J Prev Med. 2008;34(6):486–494. doi: 10.1016/j.amepre.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Bauman A, Bull F, Chey T, Craig CL, Ainsworth BE, Sallis JF, et al. The international prevalence study on physical activity: results from 20 countries. Int J Behav Nutr Phys Act. 2009;6:21. doi: 10.1186/1479-5868-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Poppel MN, Chinapaw MJ, Mokkink LB, van Mechelen W, Terwee CB. Physical activity questionnaires for adults: a systematic review of measurement properties. Sports Med. 2010;40(7):565–600. doi: 10.2165/11531930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 13.Ng WF, Bowman SJ, Griffiths B, UKPSSR study group United Kingdom Primary Sjogren’s Syndrome Registry—a united effort to tackle an orphan rheumatic disease. Rheumatology (Oxford) 2011;50(1):32–39. doi: 10.1093/rheumatology/keq240. [DOI] [PubMed] [Google Scholar]
- 14.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowman SJ, Booth DA, Platts RG, Situnayake D, Gordon C, Hanu-Cernat L, et al. Measurement of fatigue and discomfort in primary Sjogren’s syndrome using a new questionnaire tool. Rheumatology. 2004;43(6):758–764. doi: 10.1093/rheumatology/keh170. [DOI] [PubMed] [Google Scholar]
- 16.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Schrezenmaier C, Gehrking JA, Hines SM, Low PA, Benrud LM, Sandroni P. Evaluation of orthostatic hypotension: relationship of a new self-report instrument to laboratory-based measures. Mayo Clin Proc. 2005;80(3):330–334. doi: 10.4065/80.3.330. [DOI] [PubMed] [Google Scholar]
- 18.Suarez GA, Opfer-Gehrking TL, Offord KP, Atkinson EJ, O’Brien PC, Low PA. The autonomic symptom profile a new instrument to assess autonomic symptoms. Neurology. 1999;52(3):523–528. doi: 10.1212/WNL.52.3.523. [DOI] [PubMed] [Google Scholar]
- 19.Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, et al. EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis. 2010;69(6):1103–1109. doi: 10.1136/ard.2009.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seror R, Ravaud P, Mariette X, Baron G, Tzioufas A, Theander E, et al. EULAR Sjogren’s Syndrome Patient Reported Index (ESSPRI): development of a consensus patient index for primary Sjogren’s syndrome. Ann Rheum Dis. 2011;70(6):968–972. doi: 10.1136/ard.2010.143743. [DOI] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.EuroQol Group EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 23.Evans DC, Cook CH, Christy JM, Murphy CV, Gerlach AT, Lindsey DE, et al. Comorbidity-polypharmacy scoring may improve outcome prediction in older trauma patients. J Am Coll Surgeons. 2010;211(3):S59. doi: 10.1016/j.jamcollsurg.2010.06.152. [DOI] [PubMed] [Google Scholar]
- 24.Evans DC, Gerlach AT, Christy JM, Jarvis AM, Lindsey DE, Whitmill ML, et al. Pre-injury polypharmacy as a predictor of outcomes in trauma patients. Int J Crit Illn Inj Sci. 2011;1(2):104–109. doi: 10.4103/2229-5151.84793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Committee IR (2005) Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)—short and long forms. https://www.researchgate.net/file.PostFileLoader.html?id=56f92d66615e27d49a658031&assetKey=AS%3A344600888791041%401459170662924. Accessed 25 Sep 2016
- 26.Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–666. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 27.Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008;31(2):369–371. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 28.Theander L, Strömbeck B, Mandl T, Theander E. Sleepiness or fatigue? Can we detect treatable causes of tiredness in primary Sjögren’s syndrome? Rheumatology. 2010;49:1177–1183. doi: 10.1093/rheumatology/keq023. [DOI] [PubMed] [Google Scholar]
- 29.Usmania ZA, Hlavac M, Rischmueller M, Heraganahally SS, Hilditch CJ, Lester S, et al. Sleep disordered breathing in patients with primary Sjögren’s syndrome: a group controlled study. Sleep Med. 2012;13(8):1066–1070. doi: 10.1016/j.sleep.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Walker J, Gordon T, Lester S, Downie-Doyle S, McEvoy D, Pile K, et al. Increased severity of lower urinary tract symptoms and daytime somnolence in primary Sjögren’s syndrome. J Rheum. 2003;30(11):2406–2412. [PubMed] [Google Scholar]
- 31.Hackett K, Newton J, Frith J, Elliott C, Lendrem D, Foggo H, et al. Impaired functional status in primary Sjogren’s syndrome. Arthritis Car Res. 2012;64(11):1760–1764. doi: 10.1002/acr.21738. [DOI] [PubMed] [Google Scholar]
- 32.Newton JL, Frith J, Powell D, Hackett K, Wilton K, Bowman S, et al. Autonomic symptoms are common and are associated with overall symptom burden and disease activity in primary Sjogren’s syndrome. Ann Rheum Dis. 2012;71:1973–1979. doi: 10.1136/annrheumdis-2011-201009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lendrem D, Mitchell S, McMeekin P, Bowman S, Price E, Pease CT, et al. Health-related utility values of patients with primary Sjogren’s syndrome and its predictors. Ann Rheum Dis. 2014;73(7):1362–1368. doi: 10.1136/annrheumdis-2012-202863. [DOI] [PubMed] [Google Scholar]
- 34.Segal BM, Pogatchnik B, Holker E, Liu H, Sloan J, Rhodus N, et al. Primary Sjogren’s syndrome: cognitive symptoms, mood, and cognitive performance. Acta Neurol Scand. 2012;125(4):272–278. doi: 10.1111/j.1600-0404.2011.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westhoff G, Dörne T, Zink A. Fatigue and depression predict physician visits and work disability in women with primary Sjögren’s syndrome: results from a cohort study. Rheumatology. 2012;51(2):262–269. doi: 10.1093/rheumatology/ker208. [DOI] [PubMed] [Google Scholar]
- 36.Bonner A, Wellard S, Caltabiano M. The impact of fatigue on daily activity in people with chronic kidney disease. J Clin Nurs. 2010;19(21–22):3006–3015. doi: 10.1111/j.1365-2702.2010.03381.x. [DOI] [PubMed] [Google Scholar]
- 37.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(1):123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 38.Neuberger GB, Aaronson LS, Gajewski B, Embretson SE, Cagle PE, Loudon JK, et al. Predictors of exercise and effects of exercise on symptoms, function, aerobic fitness, and disease outcomes of rheumatoid arthritis. Arthritis Rheum. 2007;57(6):943–952. doi: 10.1002/art.22903. [DOI] [PubMed] [Google Scholar]
- 39.Valentine RJ, Woods JA, McAuley E, Dantzer R, Evans EM. The associations of adiposity, physical activity and inflammation with fatigue in older adults. Brain Behav Immun. 2011;25(7):1482–1490. doi: 10.1016/j.bbi.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Strombeck BE, Theander E, Jacobsson LT. Effects of exercise on aerobic capacity and fatigue in women with primary Sjogren’s syndrome. Rheumatology (Oxford) 2007;46(5):868–871. doi: 10.1093/rheumatology/kem004. [DOI] [PubMed] [Google Scholar]
- 41.Anokye NK, Trueman P, Green C, Pavey TG, Taylor RS. Physical activity and health related quality of life. BMC Public Health. 2012;12:624. doi: 10.1186/1471-2458-12-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown DR, Carroll DD, Workman LM, Carlson SA, Brown DW. Physical activity and health-related quality of life: US adults with and without limitations. Qual Life Res. 2014;23(10):2673–2680. doi: 10.1007/s11136-014-0739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klavestrand J, Vingard E. Retracted: the relationship between physical activity and health-related quality of life: a systematic review of current evidence. Scand J Med Sci Sports. 2009;19(3):300–312. doi: 10.1111/j.1600-0838.2009.00939.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.