Abstract
Invasive allergenic plant species may have severe health-related impacts. In this study we aim to predict the effects of climate change on the distribution of three allergenic ragweed species (Ambrosia spp.) in Europe and discuss the potential associated health impact. We built species distribution models based on presence-only data for three ragweed species, using MAXENT software. Future climatic habitat suitability was modeled under two IPCC climate change scenarios (RCP 6.0 and RCP 8.5). We quantify the extent of the increase in ‘high allergy risk’ (HAR) areas, i.e., parts of Europe with climatic conditions corresponding to the highest quartile (25%) of present day habitat suitability for each of the three species. We estimate that by year 2100, the distribution range of all three ragweed species increases towards Northern and Eastern Europe under all climate scenarios. HAR areas will expand in Europe by 27–100%, depending on species and climate scenario. Novel HAR areas will occur mostly in Denmark, France, Germany, Russia and the Baltic countries, and overlap with densely populated cities such as Paris and St. Petersburg. We conclude that areas in Europe affected by severe ragweed associated allergy problems are likely to increase substantially by year 2100, affecting millions of people. To avoid this, management strategies must be developed that restrict ragweed dispersal and establishment of new populations. Precautionary efforts should limit the spread of ragweed seeds and reduce existing populations. Only by applying cross-countries management plans can managers mitigate future health risks and economical consequences of a ragweed expansion in Europe.
Keywords: Aeroallergen, Habitat suitability, MAXENT, Public health, Urban heat islands, Allergy, A. artemisiifolia, Species distribution models, Invasive species, Climate change
Introduction
Climate is one of the most important determinants of species distributions at regional and global scales (Parmesan & Yohe, 2003; Burrows et al., 2011). Human-induced climate change is affecting global vegetation patterns, ecosystem functions and the distribution range of multiple terrestrial and aquatic species (Cramer et al., 2001; Gonzalez et al., 2010). One dramatic effect of climate change is the shift in species distribution range and phenological interactions (Parmesan & Yohe, 2003; Visser & Both, 2005), which can change the pollen season and lead to increases in local pollen production (Rogers et al., 2006). Allergic responses such as rhinitis (hayfever) and asthma, caused by plant pollen are therefore globally an increasing public health concern (Albertine et al., 2014; Essl et al., 2015; Hamaoui-Laguel et al., 2015; Schindler et al., 2015).
Among allergenic plants, ragweeds (Ambrosia L., Asteraceae) are considered to be among the most potent aeroallergens. Ambrosia is distributed from Mexico to Canada and contains at least 40 species (Essl et al., 2015). Ragweed species flower from July to October and each plant is able to produce millions of wind-dispersed pollen grains that may be transported over long distances, with reports ranging to over 1,000 km (Belmonte et al., 2000; Bullock et al., 2013; Hamaoui-Laguel et al., 2015). Ragweed pollen is a significant cause of allergic reactions (Frenz, 2001; Ziska et al., 2011). The threshold for provoking allergic rhinitis in ragweed pollen sensitized patients is below 20 grains m−3, and in sensitive patients be as low as 1–5 grains m−3 (Déchamp et al., 1997; Šikoparija et al., 2009) compared to 50 grass pollen grains (Davies & Smith, 1973). Ragweed pollen furthermore induces asthma about twice as often as other types of pollen (Skjøth et al., 2010). In this regard, common ragweed (A. artemisiifolia), perennial ragweed (A. psilostachya) and giant ragweed (A. trifida) are of special concern as they rank among the most widespread allergenic plants (Bassett & Crompton, 1982; Ghosh et al., 1994; Rich, 1994; Frenz, 2001). The medical cost of allergies in the United States is estimated to an annual cost in excess of $18 billion a year (Centers for Disease Control and Prevention, 2011). Ragweed is the major cause of late summer allergic rhinitis (hay fever) in the United States and Canada (Arbes Jr et al., 2005), and is also a significant problem in Southern and Southeastern Europe where allergy is the most common chronic disease (Papadopoulos et al., 2012). Estimated medical costs of ragweed-related allergies are substantial in the affected countries. In Hungary, where 25% of the population is allergic to ragweed, Kazinczi et al. (2008) estimated the annual cost to exceed €110 million per year; in Austria Gerber et al. (2011) put the cost around €88 million per year (for a comprehensive review, see Task 5 in Bullock et al. (2013)).
Ragweed populations remain relatively rare in Northern Europe, where low autumn temperatures prevent ragweed species from flowering and reaching reproductive maturity (Chapman et al., 2014). However, global warming is predicted to facilitate a northwards expansion of ragweed species (Storkey et al., 2014; Essl et al., 2015). This can accelerate the establishment and pollen production of self-propagating ragweed populations, and extend the local pollen season in regions currently climatically unsuitable (Gerber et al., 2011; Cunze, Leiblein & Tackenberg, 2013). Increasing amounts of anthropogenically released carbon dioxide (CO2) also has the potential to increase pollen production (Sheffield, Weinberger & Kinney, 2011). Studies have shown an increase of up to 61% in ragweed pollen production in a CO2 enriched atmosphere, both in climate-controlled greenhouses and in field based experiments (Ziska et al., 2003; Rogers et al., 2006). Furthermore, Patz & Olson (2006) described how especially plants in urban areas are exposed to elevated CO2 concentrations, i.e., from industry and dense traffic. Cities are therefore prone to a disproportionate increase in ragweed pollen production, with serious consequences for public health (Ziska et al., 2003). This raises concern about the consequences of the increasing frequency of which ragweed species have been registered across continental Europe, where they are now considered bioinvaders (Fernández-Llamazares et al., 2012). Over future decades, ragweed may become a major source of pollen allergy in Northern Europe, with severe medical and economic implications (Bullock et al., 2013).
In this paper, we use species distribution modeling (SDM) to explore which areas of Europe are most susceptible to successful invasion of common ragweed, perennial ragweed and giant ragweed by year 2100 under two different IPCC climate scenarios (RCP 6.0 and RCP 8.5). We show that all three ragweed species are expected to expand and shift their potential distribution across Europe by year 2100, and that ragweed species by the end of the century may cause large-scale health related impacts on the European continent.
Methods
Study species and occurrence records
We focused our analyses on the three most widespread ragweed species in Europe: common (Ambrosia artemisiifolia), perennial (A. psilostachya) and giant (A. trifida) ragweed. Common ragweed is widespread in large parts of Eurasia and North America and is considered highly invasive across the globe (GISD, 2012). Outside North America, perennial ragweed is found in counties across Europe and in Australia, and giant ragweed is widely recorded in Europe, Asia and South America. A map and table displaying the distribution of the three species in Europe is provided in Fig. S1 and Table S1. Ragweed species have been known in Europe since the 19th century and are now naturalized in more than 20 European countries (Gerber et al., 2011; GISD, 2012; Essl et al., 2015).
All three studied ragweed species are native to North America. Geo-referenced occurrence records from North America (Table 1) were therefore downloaded for each species from the Global Biodiversity Information Facility online database (GBIF; http://www.gbif.org). All records with obviously erroneous coordinates (e.g., records located in sea) were excluded. To ensure that their northern distribution limits were well represented in the data, all non-georeferenced records from Canada that had sufficiently precise locality information were geo-referenced using Google Earth and added to the dataset. To verify the precision of the occurrence data, the elevation of each record was extracted in ArcGIS 10.1 (ESRI, Redlands, CA, USA) using a topographical map at 10 arc-minutes resolution obtained from the WorldClim database version 1.4 (http://worldclim.org; Hijmans et al., 2005). If the difference between the extracted elevation and that cited in the original record exceeded 100 m then the occurrence point was removed from the dataset to avoid inaccurate assessment of climate variables at the collection site due to imprecise geo-referencing (Skov & Borchsenius, 1997).
Table 1. Distribution records used for modeling of common ragweed (Ambrosia artemisiifolia), perennial ragweed (A. psilostachya) and giant ragweed (A. trifida).
| Common ragweed | Perennial ragweed | Giant ragweed | |
|---|---|---|---|
| North American occurrences | 2,284 | 1,531 | 1,985 |
| European occurrences | 4,127 | 248 | 364 |
| Total | 6,411 | 1,779 | 2,349 |
Notes.
The dataset include North America distribution records from the GBIF online database (http://data.gbif.org). MAXENT automatically removed duplicated presence records defined as records in the same 10’ grid cell.
Bioclimatic variables
Three of the most important climatic determinants of global vegetation patterns are minimum temperature, growing season and water balance (Prentice et al., 1992; Normand, Svenning & Skov, 2007). We therefore selected these three variables to model occurrence patterns of ragweed over the next century: growing degree days (GDD), absolute minimum temperature of the coldest month (Tmin) and water balance (WBAL) (for further information on the climatic variables see Appendix S1). We used two IPCC AR5 climate change scenarios, including a high-end (RCP 8.5) and a moderate (RCP 6.0) climate change scenario, to represent future climate conditions by year 2100 (projection period 2070–2099).
Because MAXENT implicitly assumes that grid cells have the same size when choosing random samples, equal area projections are preferable (Elith et al., 2011). Therefore, prior to analyses, we reprojected the climatic layers onto a Behrmann’s equal area projection, using a bilinear interpolation, with datum WGS1984 and a grid size resampled to 10 km2. We used ArcGIS 10.1 for resampling and reprojection of the environmental layers.
Species distribution modeling
The potential future distribution models were based on the occurrence records from North America. This approach is considered the most conservative estimate for the habitat suitability, and therefore is generally applied to model the distribution of invasive species (Guisan & Thuiller, 2005; Pearson, 2007). Ragweed species potentially undergo niche shifts after introduction to Europe. In this case, distribution models based on the native range only (i.e., North America) may underestimate the potential distribution in Europe. Therefore, we also built models based only on occurrence records from the invasive range in Europe and on combined records from North America and Europe, and we present results from those models in Table S2 and Figs. S2 and S3. Preliminarily results based on AUC values indicated that all models performed as adequate modeling tools (AUC > 0.7) (Table 2 and Table S2). However, when comparing the results of the models we found models trained on the native range made the best prediction of where the Ambrosia species currently occur abundantly in Europe (Essl et al., 2015). Models based on combined invasive and native range or invasive range only data tended to overpredict habitat suitability towards the north of Europe, e.g., areas such as Southern Scandinavia, where flowering and seeding of these species is currently almost absent (Figs. S2 and S3). Therefore, we base our main findings on models built with occurrence records from native range only (i.e., North America).
Table 2. Model predictive ability under current climate based on median Area Under the Curve (AUC) values for common ragweed (Ambrosia artemisiifolia), perennial ragweed (A. psilostachya) and giant ragweed (A. trifida).
AUC values were derived from average test AUC values for MAXENT models of 15 replicates based on all occurrence records from the native range (see Table 1).
| Model AUC (based on test records in native range) | |||
|---|---|---|---|
| Common ragweed | Perennial ragweed | Giant ragweed | |
| Median ± SD | 0.797 ± 0.013 | 0.806 ± 0.014 | 0.817 ± 0.012 |
| Min–Max | (0.774–0.825) | (0.787–0.827) | (0.797–0.837) |
After building models based on current climate conditions, we projected the models into the projected climatic conditions for Europe (defined as areas 34–72°N and 11°W–32°E) to predict future potential ranges of the three species in that continent. Models for all three ragweed species were modeled using MAXENT v3.3.3k (http://www.cs.princeton.edu/ schapire/maxent), a machine-learning method that uses the principle of maximum entropy to approximate the unknown probability distribution of a species based on presence-only data (Phillips, Anderson & Schapire, 2006). The output of MAXENT consists of values proportional to the expected number of species occurrences in each grid cell (relative occurrence rate or ‘habitat suitability’). We chose to use MAXENT because it has been shown to outperform other presence-only methods (Elith et al., 2006; Phillips & Dudik, 2008; Elith et al., 2011). To balance model fit and predictive ability, we conducted species-specific tuning of MAXENT model settings using AICc and the R package ENMeval (Muscarella et al., 2014) (for further details on MAXENT settings see Appendix S1).
We used MAXENT’s jackknife procedure to assess the relative importance of each variable (i.e., WBAL, Tmin and GDD). Briefly, the jackknife tool excludes one variable at a time before running a model, thus testing model performance when different combinations of variables are included. MAXENT then compares the different model results and includes the variables that provide strong individual effects in the final model, and quantifies the relative importance of each variable in the final model.
Projecting models based on current climatic conditions may involve extrapolating model predictions beyond the observed data. To determine the extent to which our models were extrapolating, we conducted a multivariate environmental similarity surfaces (MESS) analysis (Elith, Kearney & Phillips, 2010). MESS analysis compares the reference climate (defined as the background regions) with the projected region by assigning negative values to sites where at least one input variable has a value outside of the reference climate (Elith et al., 2011; Webber et al., 2011).
We assessed model performance using Area Under the Curve (AUC) as calculated by MAXENT for the test data (Phillips, Anderson & Schapire, 2006; Ward, 2007). AUC values range from 0.5 (i.e., random) for models with no predictive ability to 1.0 (i.e., perfect discrimination between suitable and unsuitable cells) for models with a perfect prediction. According to the classification of Swets (1988), an AUC score of >0.7 (“useful” discrimination ability), >0.8 (“good” model performance) and >0.9 (“very good” model performance) was used. Since models were built for presence-only data, the background data constitute pseudo-absences for the AUC calculations (Phillips & Dudik, 2008).
To estimate the potential future allergy problems caused by ragweed expansion in Europe, we identified the areas, which under the models receive a future climate corresponding to the highest quartile (25%) of present day climate suitability for each of the three species. We refer to these as ‘high allergy risk’ (HAR) areas. The rationale for this approach was two-fold. First, the highest quartile of present-day climate suitability largely correspond to those areas in Europe where high levels of ragweed induced allergies are reported today (based on Déchamp, Méon & Reznik (2009) and Essl et al. (2015)). Second, we consider this approach to be a conservative one relative to predicting future potential allergy risk, which is preferable given the uncertainties that are always associated with forecasts based on modelling approaches. To estimate the potential future expansion of HAR areas, we reclassified raster maps into two classes defined by a break value corresponding to the lower limit of the highest quartile of present day habitat suitability. The reclassified raster maps were then used to calculate the change in HAR area between the present and the future under the RCP 6.0 and RCP 8.5 scenarios. We conducted these analyses in R (R Core Team, 2015) using the dismo (Hijmans et al., 2011) and raster (Hijmans & Van Etten, 2013) packages.
Results
Based on all data points from the native range (Table 1), the median AUC values ranged from 0.80 to 0.82, depending on the species, indicating ‘good’ performance (AUC > 0.8) of all models for all three species (Table 2).
Future species distribution in Europe
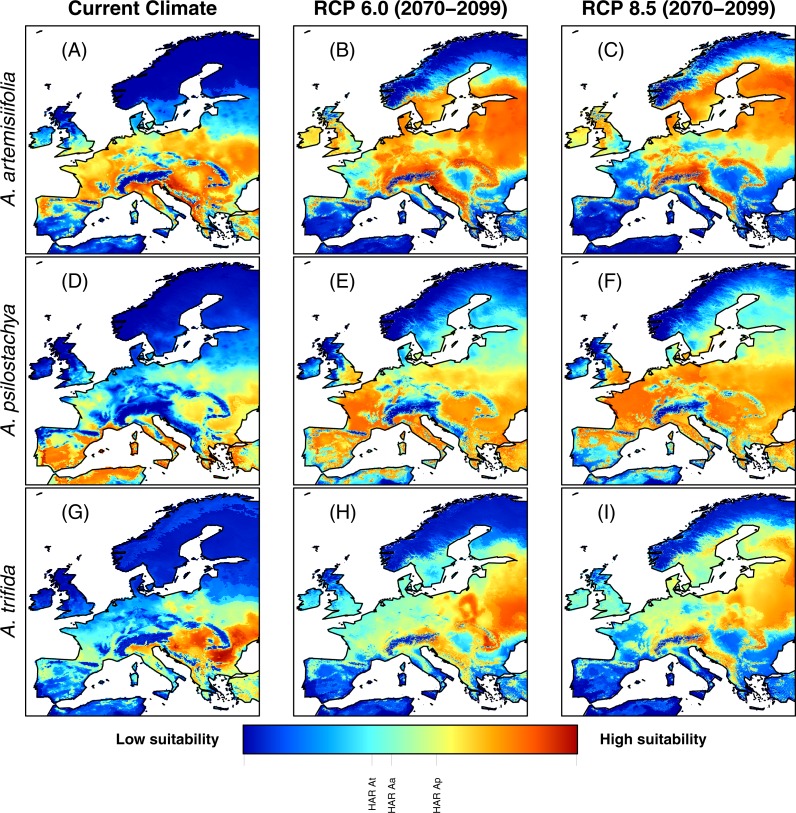
In Europe, all models predicted that climatically suitable areas for ragweed will become more widespread on the continent by year 2100 (following IPCC climate change scenarios RCP 6.0 and RCP 8.5) (Fig. 1). At present, the ragweed ‘high allergy risk’ (HAR) areas are found mostly in Southern Europe (Fig. 1 and Fig. S4). The models show a substantial increase of HAR areas across Europe by 2100 (Table 3). For common ragweed the HAR areas with maximum habitat suitability increased 100% (RCP 6.0) and 45% (RCP 8.5), respectively, with most new areas located in Northern (e.g., Denmark, United Kingdom) and Eastern Europe (e.g., the Baltic countries) (Fig. 1 and Table 3). For perennial ragweed models predicted an increase of 127% (RCP 6.0) and 189% (RCP 8.5), with new areas located across most of Central and Eastern Europe (Fig. 1 and Table 3). For giant ragweed, the HAR area increased by 97% for the RCP 6.0 projections but decreased by 69% for the RCP 8.5 projections. New habitats occurred especially in Russia, the Baltic countries and Southeastern Europe (Fig. 1 and Table 3).
Figure 1. Habitat suitability of common ragweed (A. artemisiifolia) (A–C), perennial ragweed (A. psilostachya) (D–F) and giant ragweed (A. trifida) (G–I) in Europe under current climate conditions, and future climates (projections for years 2070–2099) assuming RCP 6.0 and RCP 8.5.
Maps show average MAXENT values, derived from 15 replicates. Thresholds for HAR areas for each species are indicated graphically on the color bar (e.g., “HAR Aa” shows the HAR threshold value for A. artemisiifolia).
Table 3. The area (km2) in future ‘high allergy risk’ (HAR) areas of common ragweed (Ambrosia artemisiifolia), perennial ragweed (A. psilostachya) and giant ragweed (A. trifida) in Europe under current and future climate (year 2100) conditions assuming RCP 6.0 and RCP 8.5 climate change scenarios (IPCC, 2014).
| Species | Highest habitat suitability (km2) | ||
|---|---|---|---|
| Current | RCP 6.0 | RCP 8.5 | |
| Common ragweed | 89,460 | 178,960 | 129,320 |
| Perennial ragweed | 41,660 | 52,760 | 78,650 |
| Giant ragweed | 38,220 | 75,290 | 26,530 |
Effects of environmental variables
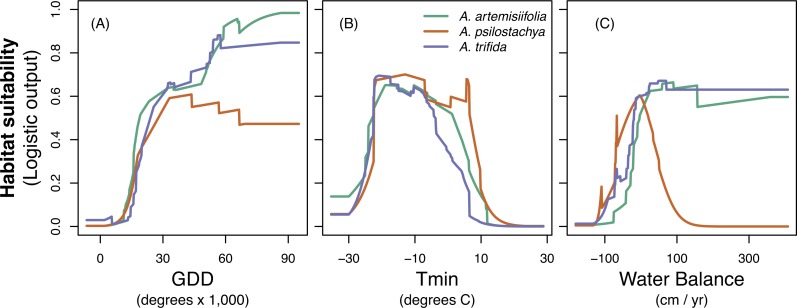
The jackknife evaluation procedure suggested that GDD was the most important predictor for all three species. Tmin was the second most important variable for perennial and giant ragweed, and WBAL was the second most important variable for common ragweed (Table 4 and Fig. S5). The response of the variables showed somewhat different tendencies (Fig. 2). Habitat suitability for all species tended to increase with GDD. Species showed similar response to Tmin. Habitat suitability of common ragweed and giant ragweed showed a similar increasing response to WBAL, whereas habitat suitability for perennial ragweed showed a hump-shaped relationship with WBAL values ranging from ca. −1,000 to 1,000 mm. Across species, WBAL had the strongest impact for common ragweed (Table 4). All species had their highest estimated habitat suitability for Tmin values ranging from approximately −30 to 10 °C (Fig. 2). MESS comparison of the environmental factors in Europe indicated very little extrapolation outside the range of the native conditions. Specifically, more than 99% of grid cells for both the present-day and future climate conditions fell within the range of the native climate conditions (Fig. S6).
Table 4. Explanatory power of three climate variable (growing degree days, GDD; minimum temperature, Tmin; water balance, WBAL) deviated from the jackknife analysis on native range models of 15 replicates.
Original jackknife analysis output of the model are found in Fig. S5.
| Parameter | Species | ||
|---|---|---|---|
| Common ragweed | Perennial ragweed | Giant ragweed | |
| GDD | 0.425 | 0.456 | 0.548 |
| Tmin | 0.215 | 0.306 | 0.252 |
| WBAL | 0.36 | 0.238 | 0.2 |
Figure 2. Response curves for common ragweed (A. artemisiifolia), perennial ragweed (A. psilostachya) and giant ragweed (A. trifida) to the three climatic variables; (A) growing degree days (GDD), (B) absolute minimum temperature (Tmin), and (C) water balance.
Habitat suitability (given in MAXENT logistic output values) is averaged across 15 replicates.
Discussion
Understanding the future distribution of allergenic plant species is crucial for predicting the consequences of the associated health related issues. Our study shows that three allergenic ragweed species have considerable potential to successfully expand their distribution range in Europe by the end of the century. The predicted ‘high allergy risk’ (HAR) areas, i.e., areas with climatic conditions corresponding to the highest quartile (25%) of present day habitat suitability for each of the three species, may expand extensively in Europe, depending on species and future climate change (RCP 6.0/8.5) scenarios (Table 3).
Our results for common ragweed agree with those of Cunze, Leiblein & Tackenberg (2013) in predicting an increased habitat suitability of common ragweed in Europe, particularly towards Northern and Eastern Europe. These authors, however, did not quantify the extent of the increase in areas where allergenic problems may be expected as a consequence of climatic changes. The majority of ragweed-studies so far have exclusively investigated common ragweed. However, considering that pollen of ragweed species is morphologically similar and give similar allergenic reactions (Frenz, 2001; Ziska et al., 2011; Sikoparija et al., 2016), it is important to consider multiple ragweed species and climate scenarios when predicting future increases in habitat suitability of allergenic ragweeds. Specifically, depending on the species and RCP used, our models estimated a change in ragweed HAR areas ranging from a loss of 11,690 km2 (giant ragweed, RCP 8.5) to an increase of 89,500 km2 (common ragweed RCP 6.0), affecting in particular Great Britain, Denmark, Sweden and the Baltic countries. Surprisingly, the more severe climate change scenario (RCP 8.5) did not facilitate an expansion of ragweeds further north compared to the milder scenario (RCP 6.0). In fact, the largest extent of suitable habitats was found under RCP 6.0 for both common and giant ragweed. We note that the northward expansion of ragweed species will be controlled by biological constraints in addition to climate variables (Deen, Hunt & Swanton, 1998). For instance, ragweeds are short-day plants and their flowering is induced by a dark period of approximately eight hours (Deen, Hunt & Swanton, 1998). Ragweeds are also sensitive to frost, and will not continue to flower when temperatures drop below freezing (Deen, Hunt & Swanton, 1998). In Northern Europe, growing season length and dark period duration may become insufficient for ragweed species to reproduce despite relatively favorable climatic conditions. Additionally, the lower extent of high habitat suitability for these species under RCP 8.5 could also be associated with drought. We found that both common and giant ragweed perform well under relatively wet conditions, whereas perennial ragweed performs better in drier habitats (Fig. 2). Prolonged drought and dry periods are increasingly expected under RCP 8.5, hence water scarcity provides one potential explanation why perennial ragweed will become most abundant under RCP 8.5 compared to both common and giant ragweed. Overall, we recommend that further work examining ragweed species distributions may be improved by considering important non-climatic variables including, for example, day length.
In addition to a geographical increase in areas with high climate suitability, a warmer climate may facilitate flowering and pollen production in areas where flowering and seed maturation currently is limited by low temperatures. Thus, allergenic pollen load could also be intensified within the current realized range of the three ragweed species. These changes impose substantial health related risks, as evident from current HAR areas in Southern Europe. For example, a prick-test study of ragweed allergens showed levels of pollen-allergic patients ranging from 30–40% in the Rhône Valley (France), to 70% in Northern Italy and more than 80% in Hungary (Rybnícek & Jäger, 2001). Another health related concern is cross-reactivity between species within the genus Ambrosia as well as with other allergenic species, such as mugwort (Artemisia L.) species (White & Bernstein, 2003; D’Amato et al., 2007). This combined with the late flowering of ragweeds, that will prolong the local pollen season (D’Amato et al., 2007; Vogl et al., 2008), are likely to entail medical and socio-economical implications for the public in novel HAR areas.
In the context of allergenic plants, urban areas are of main concern due to their high population densities (higher exposure risk) and elevated CO2 concentrations that are known to facilitate biomass and pollen production (Ziska et al., 2003). Our models indicate that future ragweed HAR areas will overlap with major cities in Europe including St. Petersburg, Hamburg and Paris. Ragweeds future distribution may therefore affect large populations across Europe. A higher overall abundance of ragweed species also increases the risk of long-distance transport of allergenic pollen (Grewling et al., 2016). Ragweed pollen can disperse 100’s of kilometers (Bullock et al., 2013), thus affecting allergic persons in areas without established ragweed populations. This has already been shown using complex cluster backward trajectories (Makra et al., 2016), and Hamaoui-Laguel et al. (2015) estimated that airborne ragweed pollen concentration will increase about four times by 2050, dispersing pollen across large regions of Europe. The low atmospheric concentration of ragweed pollen necessary to induce allergic reactions in sensitive patients (∼5 grains m−3) (Déchamp et al., 1997) combined with the long-distance wind dispersal potential, makes urban ragweed populations important sources for pollen induced allergies across large spatial areas (Belmonte et al., 2000; Essl et al., 2015; Hamaoui-Laguel et al., 2015).
While the results of this study can inform future management strategies addressing climate change and increasing human disturbance, there is a need to acknowledge the caveats embedded in all climate model studies (Araujo & Guisan, 2006). For instance, our model does not take into consideration anthropogenic factors like disturbance, land use and propagule pressure, which will be crucial in controlling range filling within the potential range set by the climatic variables. Including such parameters in future model-work will allow us to expand knowledge and predictability of the distribution of invasive allergenic species (Chapman et al., 2016). Local adaptation is another parameter that may affect the distribution of species and their response to climate change. Intra-population local adaption has been shown to allow species to survive in otherwise unfavorable conditions (Li et al., 2015). Thus, incorporation of physiological performance/phenology of ragweed throughout their distribution would be beneficial in future work to estimate range expansion at regional or local scales (Chapman et al., 2014). For now, our results demonstrate that future climate changes could result in a large expansion of Ambrosia related allergy problems in Europe, unless precautionary efforts are made to limit further expansion of the three species across Europe (Pearson, 2007; Webber et al., 2011; Chapman et al., 2016). In Northern and Eastern Europe, focus should primarily be on restricting further invasion and establishment of ragweed (Dullinger et al., 2009; Cunze, Leiblein & Tackenberg, 2013; Storkey et al., 2014). The most important factor for successful invasion by ragweed is propagule pressure, both in terms of number of introductions and number of propagules released (Lockwood, Cassey & Blackburn, 2005; Gladieux et al., 2011). Ragweeds are species that propagate exclusively by seeds. Long-distance dispersal of ragweed seeds is mostly dependent on anthropogenic forces, including import of contaminated birdseeds and crops (e.g., maize and soya beans), transport of contaminated soil, and seed infested agricultural machines (Essl et al., 2015; Chapman et al., 2016). Seeds may remain dormant for as long as 40 years (Bassett & Crompton, 1975) if conditions are unfavorable, calling for a long term effort in controlling ragweed invasion. All these aspects illustrate the importance of cross-countries management planning in order to prevent and control future spread of these allergenic bioinvaders.
Supplemental Information
Maps showing occurrence records of A. artemisiifolia, A. psilostachya and A. trifida. Points represent the ‘cleaned’ species occurrence records (see main text). The points within the outlined frame illustrate the native dataset, whereas all points illustrate the global dataset.
Habitat suitability of common ragweed (A. artemisiifolia) (A–C), perennial ragweed (A. psilostachya) (D–F) and giant ragweed (A. trifida) (G–I) in Europe under current climate conditions, and future climates (projections for years 2070–2099) assuming RCP 6.0 and RCP 8.5. Maps show average MAXENT values, derived from 15 replicates.
Habitat suitability of common ragweed (A. artemisiifolia) (A–C), perennial ragweed (A. psilostachya) (D-F) and giant ragweed (A. trifida) (G-I) in Europe under current climate conditions, and future climates (projections for years 2070-2099) assuming RCP 6.0 and RCP 8.5. Maps show average MAXENT values, derived from 15 replicates.
High allergy risk’ (HAR) areas of common ragweed A. artemisiifolia) (A–C), perennial ragweed (A. psilostachya) (D–F) and giant ragweed (A. trifida) (G–I) in Europe under current climate conditions, and projected future climates (for years 2070–2099) under RCP 6.0 and RCP 8.5. Letters indicate locations of major cities (a, Madrid; b, London; c, Paris; d, Hamburg; e, Rome; f, Berlin; g, Vienna; h, Bucharest; i, Istanbul; j, Saint Petersburg).
Original jackknife model output.
Figure displaying the MESS analysis results.
Supplementary material presenting additional information on (1) the method and (2) extended results not included in the paper.
Acknowledgments
The authors thank Signe Normand and Anne Blach Overgaard for assistance with climate variables. Peder Klith Bøcher is acknowledged for GIS assistance.
Funding Statement
RM was supported by NSF-DBI 1401312. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Karen Rasmussen conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Jakob Thyrring analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Robert Muscarella analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Finn Borchsenius conceived and designed the experiments, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplemental Information.
References
- Albertine et al. (2014).Albertine JM, Manning WJ, DaCosta M, Stinson KA, Muilenberg ML, Rogers CA. Projected carbon dioxide to increase grass pollen and allergen exposure despite higher ozone levels. PLOS ONE. 2014;9(11):e111712. doi: 10.1371/journal.pone.0111712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo & Guisan (2006).Araujo MB, Guisan A. Five (or so) challenges for species distribution modelling. Journal of Biogeography. 2006;33:1677–1688. doi: 10.1111/j.1365-2699.2006.01584.x. [DOI] [Google Scholar]
- Arbes Jr et al. (2005).Arbes Jr SJ, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the Third National Health and Nutrition Examination Survey. Journal of Allergy and Clinical Immunology. 2005;116:377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Bassett & Crompton (1975).Bassett IJ, Crompton CW. The biology of Canadian weeds.: 11. Ambrosia artemisiifolia L. and A. psilostachya DC. Canadian Journal of Plant Science. 1975;55:463–476. doi: 10.4141/cjps75-072. [DOI] [Google Scholar]
- Bassett & Crompton (1982).Bassett IJ, Crompton CW. The biology of Canadian weeds.: 55. Ambrosia trifida L. Canadian Journal of Plant Science. 1982;62:1003–1010. doi: 10.4141/cjps82-148. [DOI] [Google Scholar]
- Belmonte et al. (2000).Belmonte J, Vendrell M, Roure JM, Vidal J, Botey J, Cadahía Á. Levels of Ambrosia pollen in the atmospheric spectra of Catalan aerobiological stations. Aerobiologia. 2000;16:93–99. doi: 10.1023/A:1007649427549. [DOI] [Google Scholar]
- Bullock et al. (2013).Bullock JM, Chapman D, Schafer S, Roy D, Girardello M, Haynes T, Beal S, Wheeler B, Dickie I, Phang Z, Tinch R, Čivić K, Delbaere B, Jones-Walters L, Hilbert A, Schrauwen A, Prank M, Sofiev M, Niemelä S, Räisänen P, Lees B, Skinner M, Finch S, Brough C. Assessing and controlling the spread and the effects of common ragweed in Europe. Final report: ENV.B2/ETU/2010/0037, Natural Environment Research Council, UK. 2013 456 p. https://circabc.europa.eu/sd/d/d1ad57e8-327c-4fdd-b908-dadd5b859eff/FinalFinalReport.pdf .
- Burrows et al. (2011).Burrows MT, Schoeman DS, Buckley LB, Moore P, Poloczanska ES, Brander KM, Brown C, Bruno JF, Duarte CM, Halpern BS, Holding J, Kappel CV, Kiessling W, O’Connor MI, Pandolfi JM, Parmesan C, Schwing FB, Sydeman WJ, Richardson AJ. The pace of shifting climate in marine and terrestrial ecosystems. Science. 2011;334:652–655. doi: 10.1126/science.1210288. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2011).Centers for Disease Control and Prevention Allergies — gateway to health communication. 2011. https://www.cdc.gov/healthcommunication/toolstemplates/entertainmented/tips/allergies.html https://www.cdc.gov/healthcommunication/toolstemplates/entertainmented/tips/allergies.html
- Chapman et al. (2014).Chapman DS, Haynes T, Beal S, Essl F, Bullock JM. Phenology predicts the native and invasive range limits of common ragweed. Global Change Biology. 2014;20:192–202. doi: 10.1111/gcb.12380. [DOI] [PubMed] [Google Scholar]
- Chapman et al. (2016).Chapman DS, Makra L, Albertini R, Bonini M, Páldy A, Rodinkova V, Šikoparija B, Weryszko-Chmielewska E, Bullock JM. Modelling the introduction and spread of non-native species: international trade and climate change drive ragweed invasion. Global Change Biology. 2016;22:3067–3079. doi: 10.1111/gcb.13220. [DOI] [PubMed] [Google Scholar]
- Cramer et al. (2001).Cramer W, Bondeau A, Woodward FI, Prentice IC, Betts RA, Brovkin V, Cox PM, Fisher V, Foley JA, Friend AD, Kucharik C, Lomas MR, Ramankutty N, Sitch S, Smith B, White A, Young-Molling C. Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Global Change Biology. 2001;7:357–373. doi: 10.1046/j.1365-2486.2001.00383.x. [DOI] [Google Scholar]
- Cunze, Leiblein & Tackenberg (2013).Cunze S, Leiblein MC, Tackenberg O. Range expansion of Ambrosia artemisiifolia in Europe is promoted by climate change. ISRN Ecology. 2013;2013 doi: 10.1155/2013/610126. Article 610126. [DOI] [Google Scholar]
- D’Amato et al. (2007).D’Amato G, Cecchi L, Bonini S, Nunes C, Annesi-Maesano I, Behrendt H, Liccardi G, Popov T, Van Cauwenberge P. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62:976–990. doi: 10.1111/j.1398-9995.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- Davies & Smith (1973).Davies RR, Smith LP. Forecasting the start and severity of the hay fever season. Clinical & Experimental Allergy. 1973;3:263–267. doi: 10.1111/j.1365-2222.1973.tb01332.x. [DOI] [PubMed] [Google Scholar]
- Déchamp, Méon & Reznik (2009).Déchamp C, Méon H, Reznik S. Ambrosia artemisiifolia L. an invasive weed in Europe and adjacent countries: the geographical distribution (except France) before2009. In: Déchamp C, Méon H, editors. Ambroisie: the first international ragweed review. AFEDA; Saint-Priest: 2009. [Google Scholar]
- Déchamp et al. (1997).Déchamp C, Rimet ML, Meon H, Deviller P. Parameters of ragweed pollination in the Lyon’s area (France) from 14 years of pollen counts. Aerobiologia. 1997;13:275–279. doi: 10.1007/BF02694495. [DOI] [Google Scholar]
- Deen, Hunt & Swanton (1998).Deen W, Hunt T, Swanton CJ. Influence of temperature, photoperiod, and irradiance on the phenological development of common ragweed (Ambrosia artemisiifolia) Weed Science. 1998;46:555–560. [Google Scholar]
- Dullinger et al. (2009).Dullinger S, Kleinbauer I, Peterseil J, Smolik M, Essl F. Niche based distribution modelling of an invasive alien plant: effects of population status, propagule pressure and invasion history. Biological Invasions. 2009;11:2401–2414. doi: 10.1007/s10530-009-9424-5. [DOI] [Google Scholar]
- Elith et al. (2006).Elith J, Graham CH, Anderson RP, Dudik M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, Overton JM, Peterson AT, Phillips SJ, Richardson K, Scachetti-Pereira R, Schapire RE, Soberon J, Williams S, Wisz MS, Zimmermann NE. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29:129–151. doi: 10.1111/j.2006.0906-7590.04596.x. [DOI] [Google Scholar]
- Elith, Kearney & Phillips (2010).Elith J, Kearney M, Phillips S. The art of modelling range-shifting species. Methods in Ecology and Evolution. 2010;1:330–342. doi: 10.1111/j.2041-210X.2010.00036.x. [DOI] [Google Scholar]
- Elith et al. (2011).Elith J, Phillips SJ, Hastie T, Dudik M, Chee YE, Yates CJ. A statistical explanation of MaxEnt for ecologists. Diversity and Distributions. 2011;17:43–57. doi: 10.1111/j.1472-4642.2010.00725.x. [DOI] [Google Scholar]
- Essl et al. (2015).Essl F, Biro K, Brandes D, Broennimann O, Bullock JM, Chapman DS, Chauvel B, Dullinger S, Fumanal B, Guisan A, Karrer G, Kazinczi G, Kueffer C, Laitung B, Lavoie C, Leitner M, Mang T, Moser D, Muller-Scharer H, Petitpierre B, Richter R, Schaffner U, Smith M, Starfinger U, Vautard R, Vogl G, Von der Lippe M, Follak S. Biological flora of the British Isles: Ambrosia artemisiifolia. Journal of Ecology. 2015;103:1069–1098. doi: 10.1111/1365-2745.12424. [DOI] [Google Scholar]
- Fernández-Llamazares et al. (2012).Fernández-Llamazares Á, Belmonte J, Alarcón M, López-Pacheco M. Ambrosia L. in Catalonia (NE Spain): expansion and aerobiology of a new bioinvader. Aerobiologia. 2012;28:435–451. doi: 10.1007/s10453-012-9247-1. [DOI] [Google Scholar]
- Frenz (2001).Frenz DA. Interpreting atmospheric pollen counts for use in clinical allergy: allergic symptomology. Annals of Allergy, Asthma & Immunology. 2001;86:150–158. doi: 10.1016/S1081-1206(10)62683-X. [DOI] [PubMed] [Google Scholar]
- Gerber et al. (2011).Gerber E, Schaffner U, Gassmann A, Hinz HL, Seier M, Müller-Schärer H. Prospects for biological control of Ambrosia artemisiifolia in Europe: learning from the past. Weed Research. 2011;51:559–573. doi: 10.1111/j.1365-3180.2011.00879.x. [DOI] [Google Scholar]
- Ghosh et al. (1994).Ghosh B, Rafnar T, Perry MP, Bassolino-Klimas D, Metzler WJ, Klapper DG, Marsh DG. Immunologic and molecular characterization of Amb p V allergens from Ambrosia psilostachya (western Ragweed) pollen. The Journal of Immunology. 1994;152:2882–2889. [PubMed] [Google Scholar]
- Gladieux et al. (2011).Gladieux P, Giraud T, Kiss L, Genton BJ, Jonot O, Shykoff JA. Distinct invasion sources of common ragweed (Ambrosia artemisiifolia) in Eastern and Western Europe. Biological Invasions. 2011;13:933–944. doi: 10.1007/s10530-010-9880-y. [DOI] [Google Scholar]
- GISD (2012).Global Invasive Species Database Ambrosia artemisiifolia. 2012. http://www.iucngisd.org/gisd/species.php?sc=1125. [accessed 10 December 2012]. http://www.iucngisd.org/gisd/species.php?sc=1125
- Gonzalez et al. (2010).Gonzalez P, Neilson RP, Lenihan JM, Drapek RJ. Global patterns in the vulnerability of ecosystems to vegetation shifts due to climate change. Global Ecology and Biogeography. 2010;19:755–768. doi: 10.1111/j.1466-8238.2010.00558.x. [DOI] [Google Scholar]
- Grewling et al. (2016).Grewling Ł, Bogawski P, Jenerowicz D, Czarnecka-Operacz M, Šikoparija B, Skjøth CA, Smith M. Mesoscale atmospheric transport of ragweed pollen allergens from infected to uninfected areas. International Journal of Biometeorology. 2016;60:1493–1500. doi: 10.1007/s00484-016-1139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisan & Thuiller (2005).Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecology Letters. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- Hamaoui-Laguel et al. (2015).Hamaoui-Laguel L, Vautard R, Liu L, Solmon F, Viovy N, Khvorostyanov D, Essl F, Chuine I, Colette A, Semenov MA, Schaffhauser A, Storkey J, Thibaudon M, Epstein MM. Effects of climate change and seed dispersal on airborne ragweed pollen loads in Europe. Nature Climate Change. 2015;5:766–771. doi: 10.1038/nclimate2652. [DOI] [Google Scholar]
- Hijmans et al. (2005).Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- Hijmans et al. (2011).Hijmans RJ, Phillips S, Leathwick J, Elith J. Package ‘dismo’. 2011. http://cran.r-project.org/web/packages/dismo/index.html http://cran.r-project.org/web/packages/dismo/index.html
- Hijmans & Van Etten (2013).Hijmans RJ, Van Etten J. raster: geographic data analysis and modeling. http://CRAN.R-project.org/package=raster 2013
- IPCC (2014).IPCC IPCC, Geneva, SwitzerlandClimate change 2014: synthesis report. Contribution of working groups I, II and III To the fifth assessment report of the intergovernmental panel on climate change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)] 2014 151 pp.
- Kazinczi et al. (2008).Kazinczi G, Béres I, Pathy Z, Novák R. Common ragweed (Ambrosia artemisiifolia L.): a review with special regards to the results in Hungary: II. Importance and harmful effect, allergy, habitat, allelopathy and beneficial characteristics. Herbologia. 2008;9:93–118. [Google Scholar]
- Li et al. (2015).Li XM, She DY, Zhang DY, Liao WJ. Life history trait differentiation and local adaptation in invasive populations of Ambrosia artemisiifolia in China. Oecologia. 2015;177:669–677. doi: 10.1007/s00442-014-3127-z. [DOI] [PubMed] [Google Scholar]
- Lockwood, Cassey & Blackburn (2005).Lockwood JL, Cassey P, Blackburn T. The role of propagule pressure in explaining species invasions. Trends in Ecology & Evolution. 2005;20:223–228. doi: 10.1016/j.tree.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Makra et al. (2016).Makra L, Matyasovszky I, Tusnady G, Wang YQ, Csepe Z, Bozoki Z, Nyul LG, Erostyak J, Bodnar K, Sumeghy Z, Vogel H, Pauling A, Paldy A, Magyar D, Manyoki G, Bergmann KC, Bonini M, Sikoparija B, Radisic P, Gehrig R, Seliger AK, Stjepanovic B, Rodinkova V, Prikhodko A, Maleeva A, Severova E, Scevkova J, Ianovici N, Peternel R, Thibaudon M. Biogeographical estimates of allergenic pollen transport over regional scales: common ragweed and Szeged, Hungary as a test case. Agricultural and Forest Meteorology. 2016;221:94–110. doi: 10.1016/j.agrformet.2016.02.006. [DOI] [Google Scholar]
- Muscarella et al. (2014).Muscarella R, Galante PJ, Soley-Guardia M, Boria RA, Kass JM, Uriarte M, Anderson RP. ENMeval: an R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods in Ecology and Evolution. 2014;5:1198–1205. doi: 10.1111/2041-210X.12261. [DOI] [Google Scholar]
- Normand, Svenning & Skov (2007).Normand S, Svenning JC, Skov F. National and European perspectives on climate change sensitivity of the habitats directive characteristic plant species. Journal for Nature Conservation. 2007;15:41–53. doi: 10.1016/j.jnc.2006.09.001. [DOI] [Google Scholar]
- Papadopoulos et al. (2012).Papadopoulos NG, Agache I, Bavbek S, Bilo BM, Braido F, Cardona V, Custovic A, Demonchy J, Demoly P, Eigenmann P, Gayraud J, Grattan C, Heffler E, Hellings PW, Jutel M, Knol E, Lotvall J, Muraro A, Poulsen LK, Roberts G, Schmid-Grendelmeier P, Skevaki C, Triggiani M, Vanree R, Werfel T, Flood B, Palkonen S, Savli R, Allegri P, Annesi-Maesano I, Annunziato F, Antolin-Amerigo D, Apfelbacher C, Blanca M, Bogacka E, Bonadonna P, Bonini M, Boyman O, Brockow K, Burney P, Buters J, Butiene I, Calderon M, Cardell LO, Caubet JC, Celenk S, Cichocka-Jarosz E, Cingi C, Couto M, Dejong N, Del Giacco S, Douladiris N, Fassio F, Fauquert JL, Fernandez J, Rivas MF, Ferrer M, Flohr C, Gardner J, Genuneit J, Gevaert P, Groblewska A, Hamelmann E, Hoffmann HJ, Hoffmann-Sommergruber K, Hovhannisyan L, Hox V, Jahnsen FL, Kalayci O, Kalpaklioglu AF, Kleine-Tebbe J, Konstantinou G, Kurowski M, Lau S, Lauener R, Lauerma A, Logan K, Magnan A, Makowska J, Makrinioti H, Mangina P, Manole F, Mari A, Mazon A, Mills C, Mingomataj E, Niggemann B, Nilsson G, Ollert M, O’Mahony L, O’Neil S, Pala G, Papi A, Passalacqua G, Perkin M, Pfaar O, Pitsios C, Quirce S, Raap U, Raulf-Heimsoth M, Rhyner C, Robson-Ansley P, Alves RR, Roje Z, Rondon C, Rudzeviciene O, Rueff F, Rukhadze M, Rumi G, Sackesen C, Santos AF, Santucci A, Scharf C, Schmidt-Weber C, Schnyder B, Schwarze J, Senna G, Sergejeva S, Seys S, Siracusa A, Skypala I, Sokolowska M, Spertini F, Spiewak R, Sprikkelman A, Sturm G, Swoboda I, Terreehorst I, Toskala E, Traidl-Hoffmann C, Venter C, Vlieg-Boerstra B, Whitacker P, Worm M, Xepapadaki P, Akdis CA. Research needs in allergy: an EAACI position paper, in collaboration with EFA. Clinical and Translational Allergy. 2012;2:21. doi: 10.1186/2045-7022-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan & Yohe (2003).Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Patz & Olson (2006).Patz JA, Olson SH. Climate change and health: global to local influences on disease risk. Annals of Tropical Medicine and Parasitology. 2006;100:535–549. doi: 10.1179/136485906X97426. [DOI] [PubMed] [Google Scholar]
- Pearson (2007).Pearson RG. Species’ distribution modeling for conservation educators and parctitioners. 2007. http://ncep.amnh.org. [15 September 2012]. http://ncep.amnh.org
- Phillips, Anderson & Schapire (2006).Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190:231–259. doi: 10.1016/j.ecolmodel.2005.03.026. [DOI] [Google Scholar]
- Phillips & Dudik (2008).Phillips SJ, Dudik M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008;31:161–175. doi: 10.1111/j.0906-7590.2008.5203.x. [DOI] [Google Scholar]
- Prentice et al. (1992).Prentice IC, Cramer W, Harrison SP, Leemans R, Monserud RA, Solomon AM. A global biome model based on plant physiology and sominance, soil properties and climate. Journal of Biogeography. 1992;19:117–134. doi: 10.2307/2845499. [DOI] [Google Scholar]
- R Core Team (2015).R Core Team . R Foundation for Statistical Computing; Vienna: 2015. [Google Scholar]
- Rich (1994).Rich TCG. Ragweeds (Ambrosia L.) in Britain. Grana. 1994;33:38–43. doi: 10.1080/00173139409427454. [DOI] [Google Scholar]
- Rogers et al. (2006).Rogers CA, Wayne PM, Macklin EA, Muilenberg ML, Wagner CJ, Epstein PR, Bazzaz FA. Interaction of the onset of spring and elevated atmospheric CO2 on ragweed (Ambrosia artemisiifolia L.) pollen production. Environmental Health Perspectives. 2006;114:865–869. doi: 10.1289/ehp.8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybnícek & Jäger (2001).Rybnícek O, Jäger S. Ambrosia (ragweed) in Europe. Allergy & Clinical Immunology International. 2001;13:60–66. doi: 10.1027/0838-1925.13.2.60. [DOI] [Google Scholar]
- Schindler et al. (2015).Schindler S, Staska B, Adam M, Rabitsch W, Essl F. Alien species and public health impacts in Europe: a literature review. NeoBiota. 2015;27:1–23. doi: 10.3897/neobiota.27.5007. [DOI] [Google Scholar]
- Sheffield, Weinberger & Kinney (2011).Sheffield PE, Weinberger KR, Kinney PL. Climate change, aeroallergens, and pediatric allergic disease. Mount Sinai Journal of Medicine. 2011;78:78–84. doi: 10.1002/msj.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikoparija et al. (2016).Sikoparija B, Skjøth CA, Celenk S, Testoni C, Abramidze T, Alm Kübler K, Belmonte J, Berger U, Bonini M, Charalampopoulos A, Damialis A, Clot B, Dahl Å, De Weger LA, Gehrig R, Hendrickx M, Hoebeke L, Ianovici N, Kofol Seliger A, Magyar D, Mányoki G, Milkovska S, Myszkowska D, Páldy A, Pashley CH, Rasmussen K, Ritenberga O, Rodinkova V, Rybníček O, Shalaboda V, Šaulienė I, Ščevková J, Stjepanović B, Thibaudon M, Verstraeten C, Vokou D, Yankova R, Smith M. Spatial and temporal variations in airborne Ambrosia pollen in Europe. Aerobiologia. 2016:1–9. doi: 10.1007/s10453-016-9463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šikoparija et al. (2009).Šikoparija B, Smith M, Skjøth CA, Radišić P, Milkovska S, Šimić S, Brandt J. The Pannonian plain as a source of Ambrosia pollen in the Balkans. International Journal of Biometeorology. 2009;53:263–272. doi: 10.1007/s00484-009-0212-9. [DOI] [PubMed] [Google Scholar]
- Skjøth et al. (2010).Skjøth CA, Smith M, Šikoparija B, Stach A, Myszkowska D, Kasprzykf I, Radišić P, Stjepanović B, Hrga I, Apatini D, Magyar D, Páldy A, Ianovici N. A method for producing airborne pollen source inventories: an example of Ambrosia (ragweed) on the Pannonian Plain. Agricultural and Forest Meteorology. 2010;150:1203–1210. doi: 10.1016/j.agrformet.2010.05.002. [DOI] [Google Scholar]
- Skov & Borchsenius (1997).Skov F, Borchsenius F. Predicting plant species distribution patterns using simple climatic parameters: a case study of Ecuadorian palms. Ecography. 1997;20:347–355. doi: 10.1111/j.1600-0587.1997.tb00379.x. [DOI] [Google Scholar]
- Storkey et al. (2014).Storkey J, Stratonovitch P, Chapman DS, Vidotto F, Semenov MA. A process-based approach to predicting the effect of climate change on the distribution of an invasive allergenic plant in Europe. PLOS ONE. 2014;9(2):e88156. doi: 10.1371/journal.pone.0088156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swets (1988).Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- Visser & Both (2005).Visser ME, Both C. Shifts in phenology due to global climate change: the need for a yardstick. Proceedings of the Royal Society of London B: Biological Sciences. 2005;272:2561–2569. doi: 10.1098/rspb.2005.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl et al. (2008).Vogl G, Smolik M, Stadler LM, Leitner M, Essl F, Dullinger S, Kleinbauer I, Peterseil J. Modelling the spread of ragweed: effects of habitat, climate change and diffusion. European Physical Journal-Special Topics. 2008;161:167–173. doi: 10.1140/epjst/e2008-00758-y. [DOI] [Google Scholar]
- Ward (2007).Ward DF. Modelling the potential geographic distribution of invasive ant species in New Zealand. Biological Invasions. 2007;9:723–735. doi: 10.1007/s10530-006-9072-y. [DOI] [Google Scholar]
- Webber et al. (2011).Webber BL, Yates CJ, Le Maitre DC, Scott JK, Kriticos DJ, Ota N, McNeill A, Le Roux JJ, Midgley GF. Modelling horses for novel climate courses: insights from projecting potential distributions of native and alien Australian acacias with correlative and mechanistic models. Diversity and Distributions. 2011;17:978–1000. doi: 10.1111/j.1472-4642.2011.00811.x. [DOI] [Google Scholar]
- White & Bernstein (2003).White JF, Bernstein DI. Key pollen allergens in North America. Annals of Allergy, Asthma & Immunology. 2003;91:425–435. doi: 10.1016/S1081-1206(10)61509-8. [DOI] [PubMed] [Google Scholar]
- Ziska et al. (2003).Ziska LH, Gebhard DE, Frenz DA, Faulkner S, Singer BD, Straka JG. Cities as harbingers of climate change: common ragweed, urbanization, and public health. Journal of Allergy and Clinical Immunology. 2003;111:290–295. doi: 10.1067/mai.2003.53. [DOI] [PubMed] [Google Scholar]
- Ziska et al. (2011).Ziska L, Knowlton K, Rogers C, Dalan D, Tierney N, Elder MA, Filley W, Shropshire J, Ford LB, Hedberg C, Fleetwood P, Hovanky KT, Kavanaugh T, Fulford G, Vrtis RF, Patz JA, Portnoy J, Coates F, Bielory L, Frenz D. Recent warming by latitude associated with increased length of ragweed pollen season in central North America. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4248–4251. doi: 10.1073/pnas.1014107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Maps showing occurrence records of A. artemisiifolia, A. psilostachya and A. trifida. Points represent the ‘cleaned’ species occurrence records (see main text). The points within the outlined frame illustrate the native dataset, whereas all points illustrate the global dataset.
Habitat suitability of common ragweed (A. artemisiifolia) (A–C), perennial ragweed (A. psilostachya) (D–F) and giant ragweed (A. trifida) (G–I) in Europe under current climate conditions, and future climates (projections for years 2070–2099) assuming RCP 6.0 and RCP 8.5. Maps show average MAXENT values, derived from 15 replicates.
Habitat suitability of common ragweed (A. artemisiifolia) (A–C), perennial ragweed (A. psilostachya) (D-F) and giant ragweed (A. trifida) (G-I) in Europe under current climate conditions, and future climates (projections for years 2070-2099) assuming RCP 6.0 and RCP 8.5. Maps show average MAXENT values, derived from 15 replicates.
High allergy risk’ (HAR) areas of common ragweed A. artemisiifolia) (A–C), perennial ragweed (A. psilostachya) (D–F) and giant ragweed (A. trifida) (G–I) in Europe under current climate conditions, and projected future climates (for years 2070–2099) under RCP 6.0 and RCP 8.5. Letters indicate locations of major cities (a, Madrid; b, London; c, Paris; d, Hamburg; e, Rome; f, Berlin; g, Vienna; h, Bucharest; i, Istanbul; j, Saint Petersburg).
Original jackknife model output.
Figure displaying the MESS analysis results.
Supplementary material presenting additional information on (1) the method and (2) extended results not included in the paper.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplemental Information.