Abstract
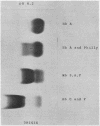
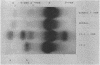
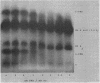
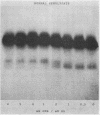
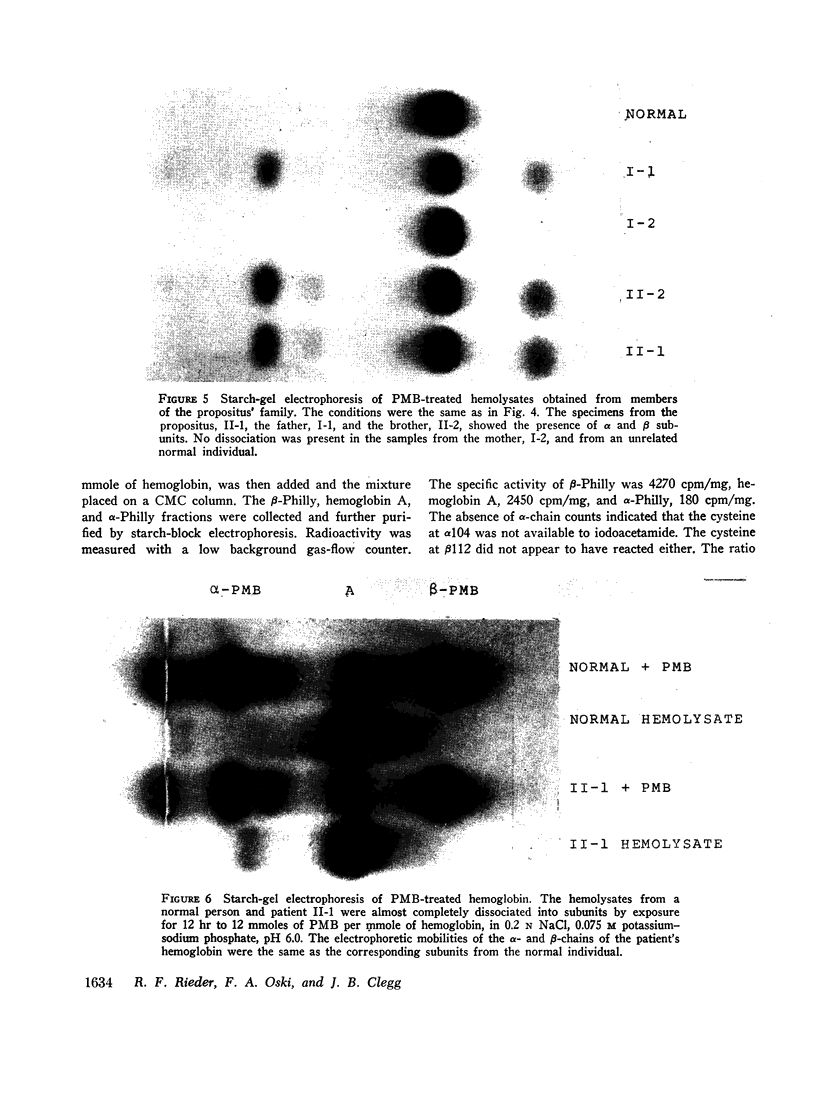
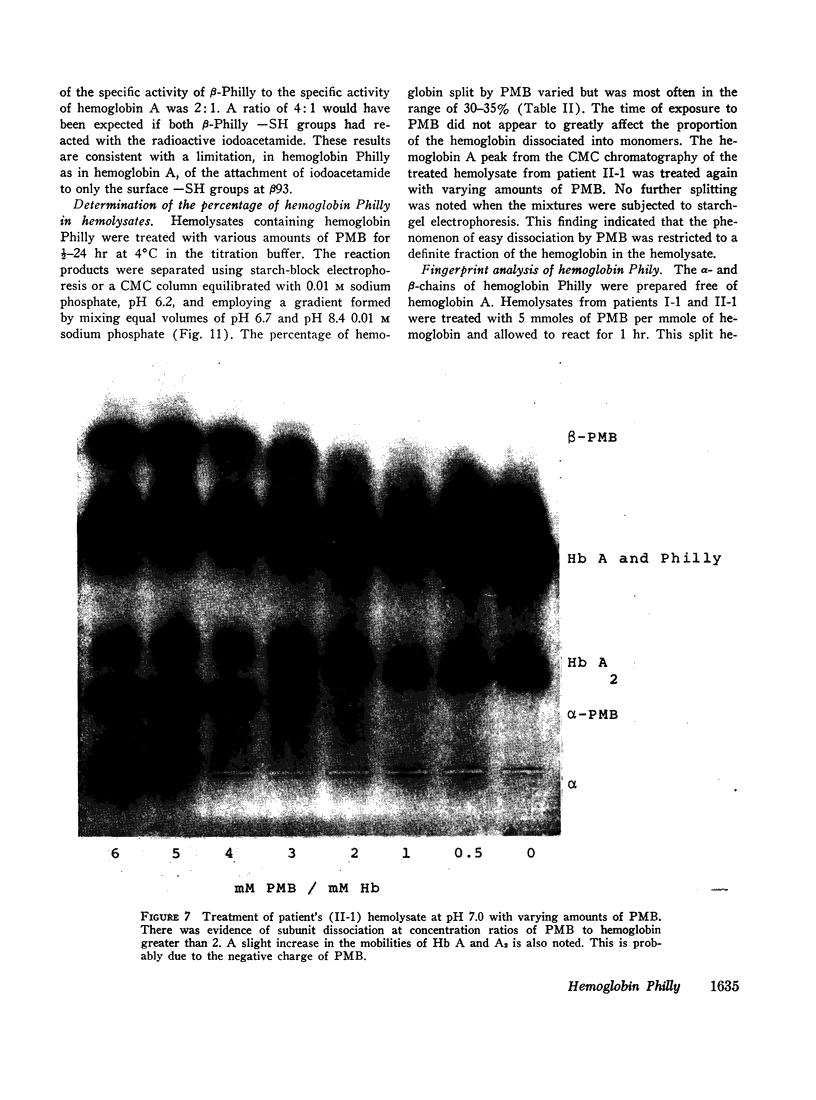
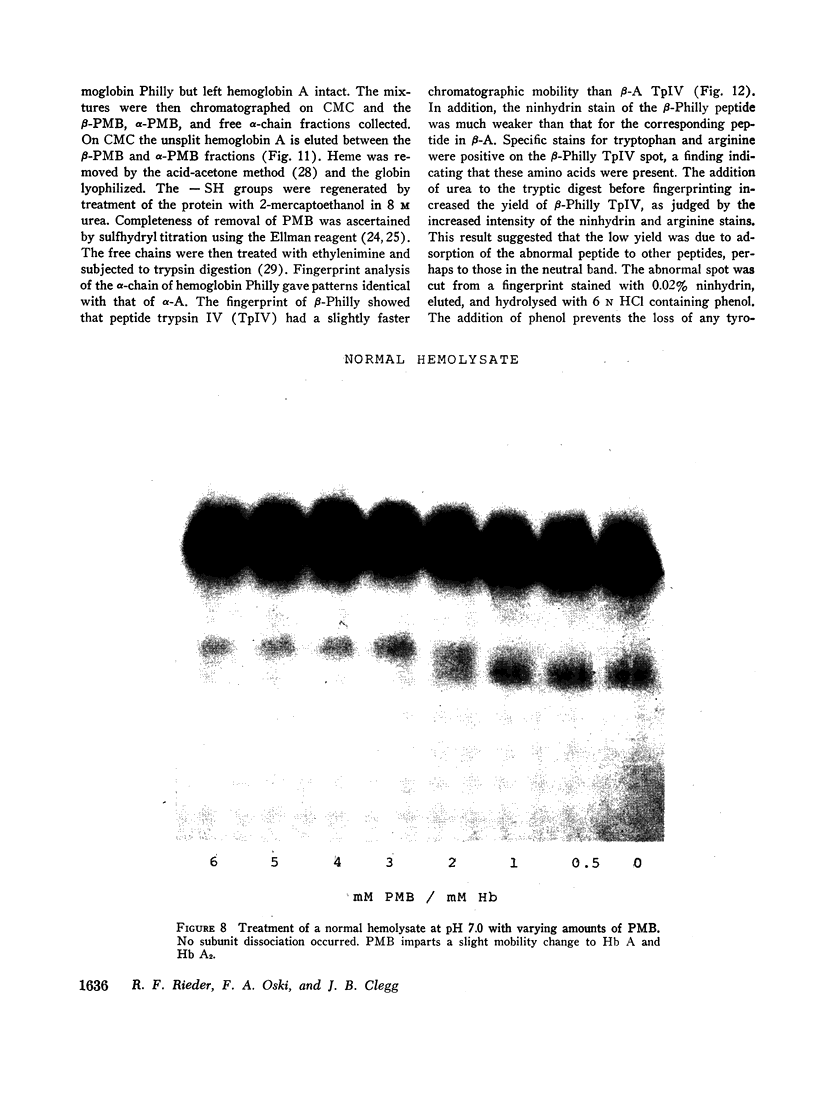
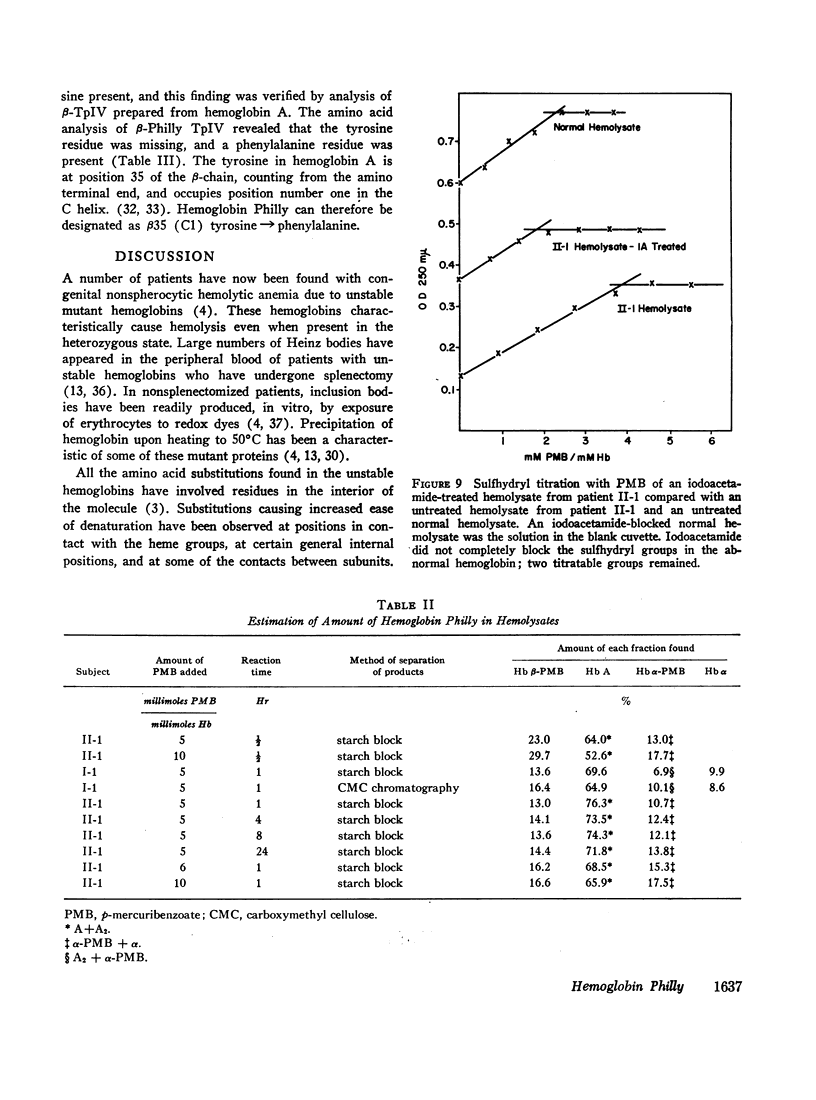
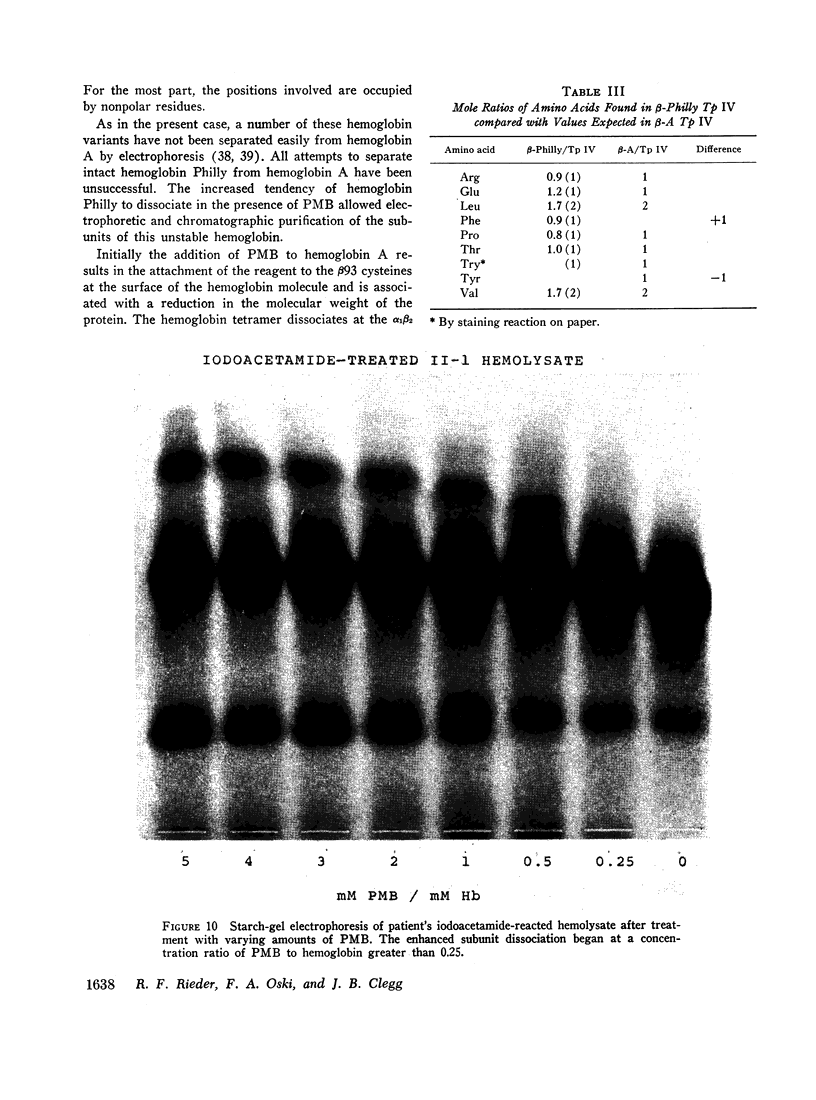
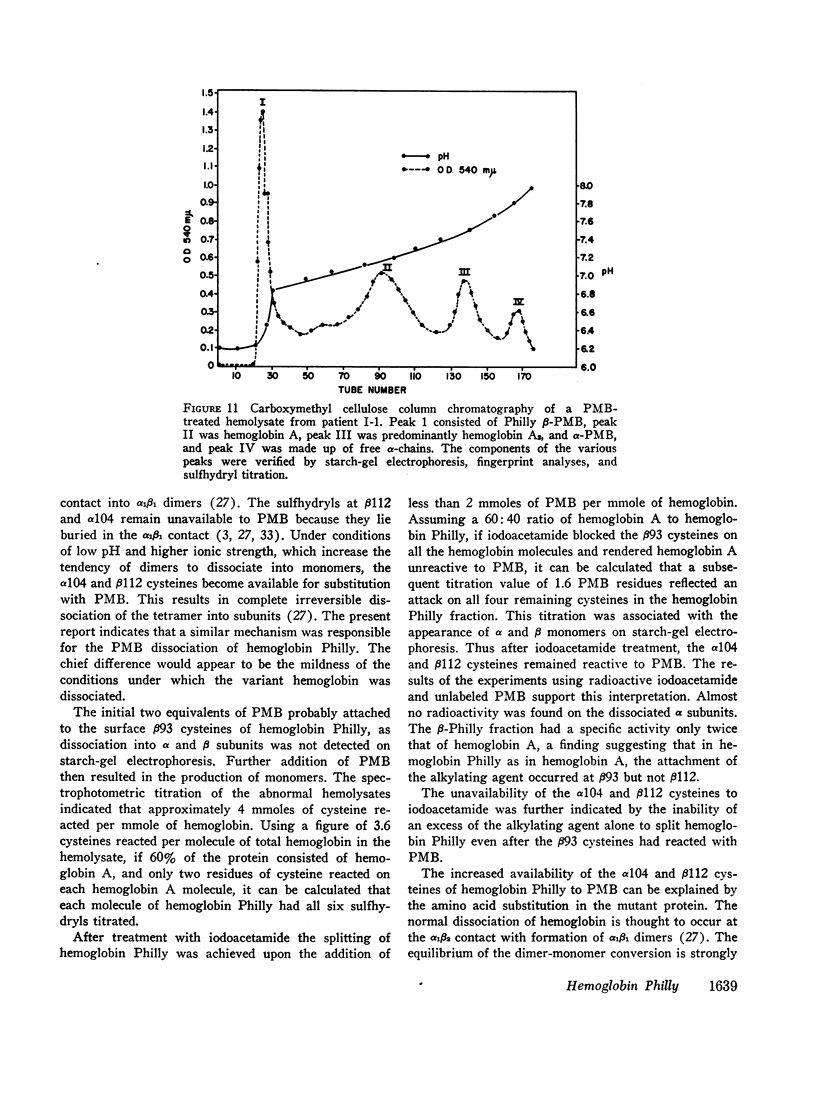
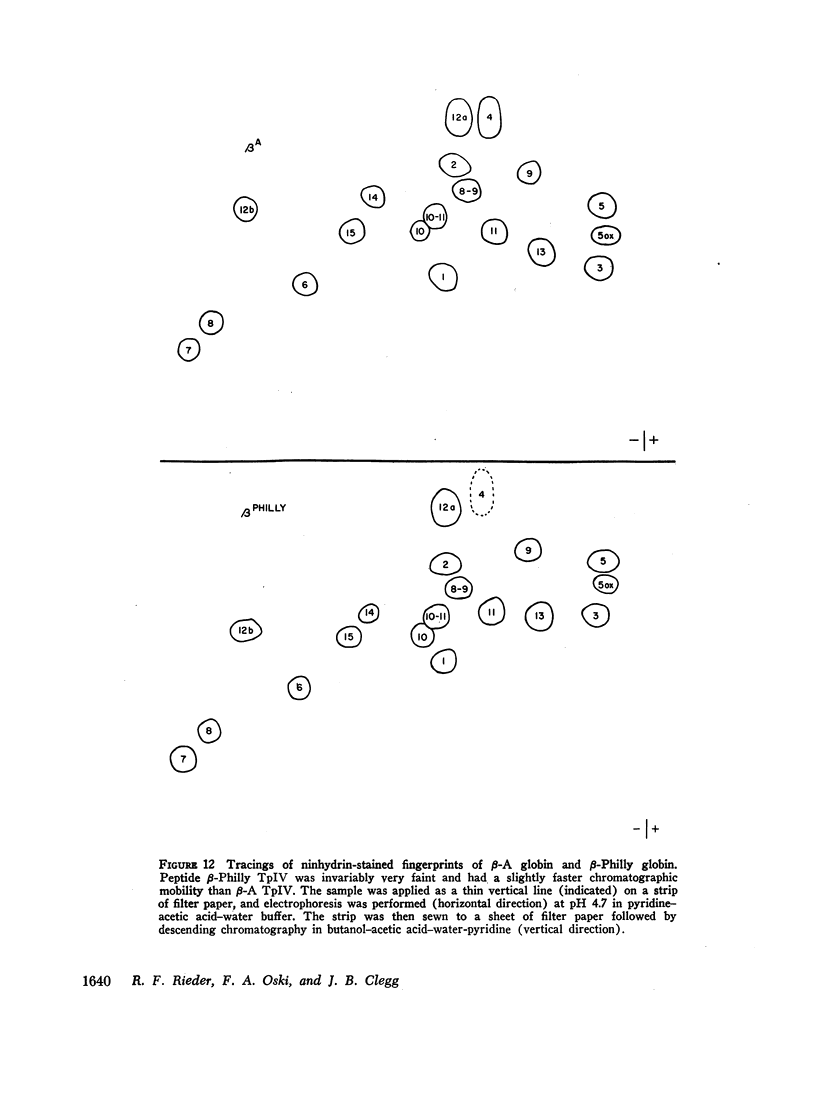
An abnormal unstable hemoglobin, hemoglobin Philly, was found in three members of a family, each of whom had evidence of a chronic hemolytic state. The presence of the mutant protein was suggested by the rapid appearance of inclusion bodies upon incubation of erythrocytes with brilliant cresyl blue and by the increased heat precipitability of the hemoglobin. However, no abnormal hemoglobin could be demonstrated by electrophoresis or column chromatography. Sulfhydryl titration of the hemolysates with p-mercuribenzoate indicated that there was an average of four reactive sulfhydryl groups per hemoglobin molecule instead of the usual two. The total number of hemoglobin sulfhydryl groups was normal; six groups were measured when denatured globin was reacted with 5,5′-dithiobis[2-nitrobenzoic acid]. This indicated that the increased sulfhydryl reactivity was due to an increased availability to p-mercuribenzoate of the usually unreactive hemoglobin cysteines at β112 and α104. After treatment for ½ hr with 4-5 moles of p-mercuribenzoate per mole of hemoglobin, electrophoresis revealed that 30-35% of the hemoglobin had been dissociated into α- and β-chains. Normal hemolysates revealed negligible splitting after 72 hr of similar treatment. The α- and β-chains of hemoglobin Philly were separated from the unsplit hemoglobin A by carboxymethyl cellulose chromatography. Fingerprint and amino acid analyses revealed that tyrosine β35 was replaced by phenylalanine. In hemoglobin Philly there is loss of the normal hydrogen bond between the tyrosine hydroxyl group and the carboxyl group of aspartic acid α126 at the α1β1 contact. This shifts the equilibrium from hemoglobin tetramers toward monomers, exposing the β112 and α104 cysteines. In the cell, precipitation of the unstable monomers may contribute to erythrocyte destruction.
Full text
PDF



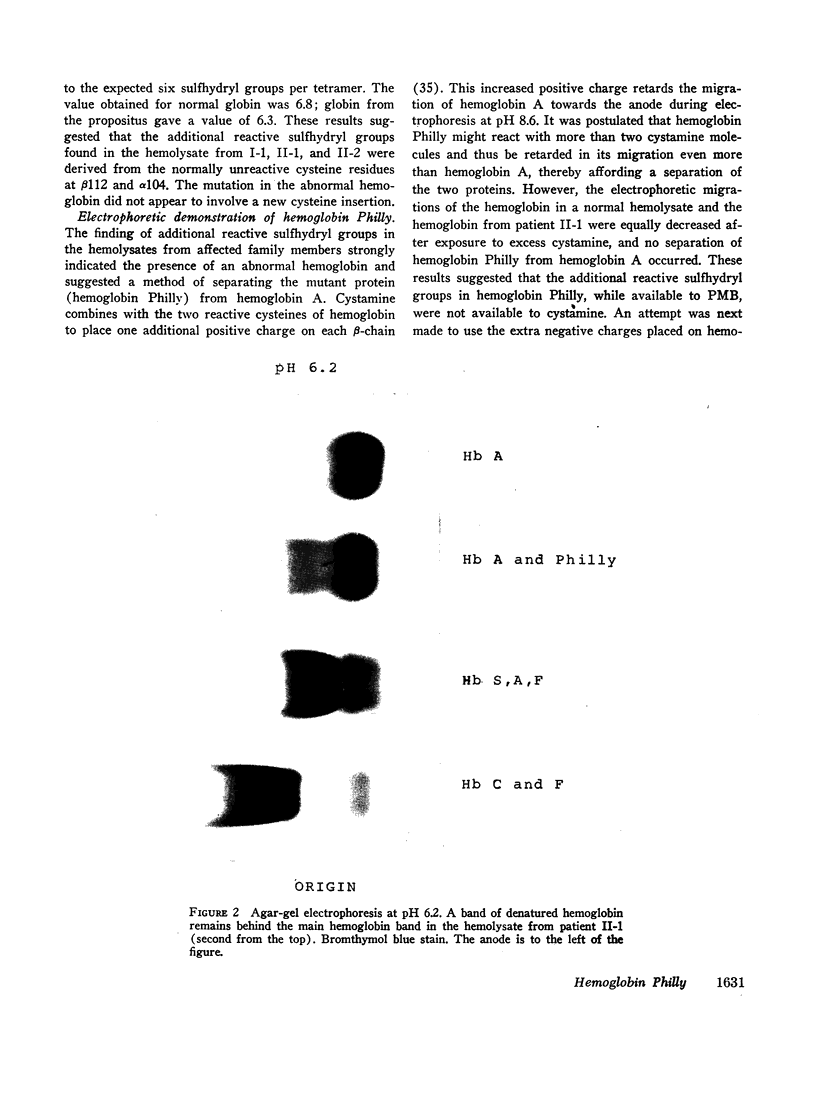
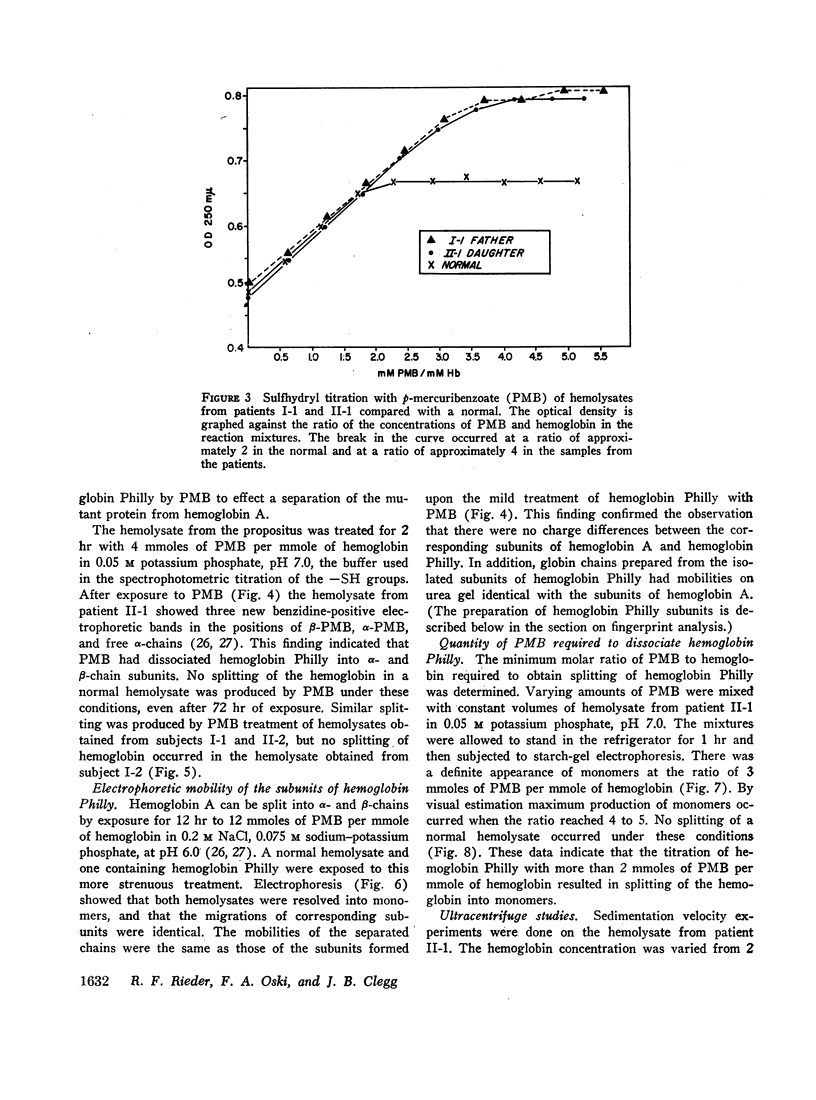
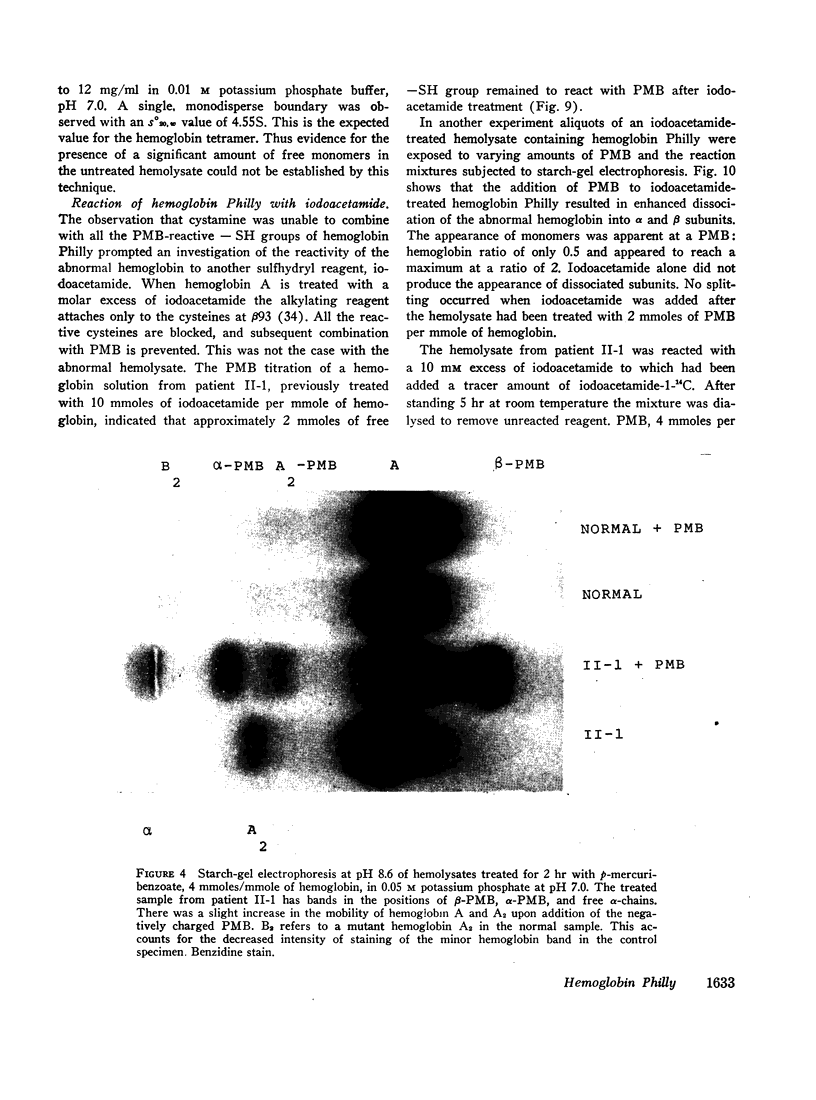


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENESCH R., BENESCH R. E. Determination of--SH groups in proteins. Methods Biochem Anal. 1962;10:43–70. doi: 10.1002/9780470110270.ch2. [DOI] [PubMed] [Google Scholar]
- BETKE K., MARTI H. R., SCHLICHT I. Estimation of small percentages of foetal haemoglobin. Nature. 1959 Dec 12;184(Suppl 24):1877–1878. doi: 10.1038/1841877a0. [DOI] [PubMed] [Google Scholar]
- BRAUNITZER G., HILSE K., RUDLOFF V., HILSCHMANN N. THE HEMOGLOBINS. Adv Protein Chem. 1964;19:1–71. doi: 10.1016/s0065-3233(08)60188-6. [DOI] [PubMed] [Google Scholar]
- BUCCI E., FRONTICELLI C. A NEW METHOD FOR THE PREPARATION OF ALPHA AND BETA SUBUNITS OF HUMAN HEMOGLOBIN. J Biol Chem. 1965 Jan;240:PC551–PC552. [PubMed] [Google Scholar]
- Beretta A., Prato V., Gallo E., Lehmann H. Haemoglobin Torino--alpha-43 (CD1) phenylalanine replaced by valine. Nature. 1968 Mar 16;217(5133):1016–1018. doi: 10.1038/2171016a0. [DOI] [PubMed] [Google Scholar]
- CHAPMAN R. G., HENNESSEY M. A., WALTERSDORPH A. M., HUENNEKENS F. M., GABRIO B. W. Erythrocyte metabolism. V. Levels of glycolytic enzymes and regulation of glycolysis. J Clin Invest. 1962 Jun;41:1249–1256. doi: 10.1172/JCI104587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERNOFF A. I., PETIT N., NORTHROP J. THE AMINO ACID COMPOSITION OF HEMOGLOBIN. V. THE PREPARATION OF PURIFIED HEMOGLOBIN FRACTIONS BY CHROMATOGRAPHY ON CELLULOSE EXCHANGERS AND THEIR IDENTIFICATION BY STARCH GEL ELECTROPHORESIS USING TRIS-BORATE-EDTA BUFFER. Blood. 1965 May;25:646–661. [PubMed] [Google Scholar]
- CHERNOFF A. I., PETTIT N. M., Jr THE AMINO ACID COMPOSITION OF HEMOGLOBIN. 3. A QUALITATIVE METHOD FOR IDENTIFYING ABNORMALITIES OF THE POLYPEPTIDE CHAINS OF HEMOGLOBIN. Blood. 1964 Dec;24:750–756. [PubMed] [Google Scholar]
- CROSBY W. H. Normal functions of the spleen relative to red blood cells: a review. Blood. 1959 Apr;14(4):399–408. [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Dacie J. V., Shinton N. K., Gaffney P. J., Jr, Lehmann H. Haemoglobin Hammersmith (beta-42 (CDI) Phe replaced by ser). Nature. 1967 Nov 18;216(5116):663–665. doi: 10.1038/216663a0. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fessas P., Loukopoulos D., Kaltsoya A. Peptide analysis of the inclusions of erythroid cells in beta-thalassemia. Biochim Biophys Acta. 1966 Aug 24;124(2):430–432. doi: 10.1016/0304-4165(66)90216-9. [DOI] [PubMed] [Google Scholar]
- GRIMES A. J., MEISLER A., DACIE J. V. CONGENITAL HEINZ-BODY ANAEMIA. FURTHER EVIDENCE ON THE CAUSE OF HEINZ-BODY PRODUCTION IN RED CELLS. Br J Haematol. 1964 Jul;10:281–290. doi: 10.1111/j.1365-2141.1964.tb00704.x. [DOI] [PubMed] [Google Scholar]
- GUIDOTTI G., KONIGSBERG W. THE CHARACTERIZATION OF MODIFIED HUMAN HEMOGLOBIN. I. REACTION WITH IODOACETAMIDE AND N-ETHYLMALEIMIDE. J Biol Chem. 1964 May;239:1474–1484. [PubMed] [Google Scholar]
- Guidotti G. Studies on the chemistry of hemoglobin. I. The reactive sulfhydryl groups. J Biol Chem. 1967 Aug 25;242(16):3673–3684. [PubMed] [Google Scholar]
- HUEHNS E. R., SHOOTER E. M. HUMAN HAEMOGLOBINS. J Med Genet. 1965 Mar;2(1):48–90. doi: 10.1136/jmg.2.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTCHISON H. E., PINKERTON P. H., WATERS P., LEHMANN H., BEALE D., DOUGLAS A. S. HEREDITARY HEINZ-BODY ANAEMIA, THROMBOCYTOPENIA, AND HAEMOGLOBINOPATHY (HB KOELN) IN A GLASGOW FAMILY. Br Med J. 1964 Oct 31;2(5417):1099–1103. doi: 10.1136/bmj.2.5417.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller P. Hemoglobinopathic dysfunction of the red cell. Am J Med. 1966 Nov;41(5):799–814. doi: 10.1016/0002-9343(66)90038-6. [DOI] [PubMed] [Google Scholar]
- ITANO H. A. Solubilities of naturally occurring mixtures of human hemoglobin. Arch Biochem Biophys. 1953 Nov;47(1):148–159. doi: 10.1016/0003-9861(53)90444-5. [DOI] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. II. Studies in vivo. J Clin Invest. 1962 Jul;41:1514–1523. doi: 10.1172/JCI104607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V., Carrell R. W., Lehmann H. Abnormal haem binding and globin SH group blockade in unstable haemoglobins. Nature. 1968 Jun 29;218(5148):1214–1217. doi: 10.1038/2181214a0. [DOI] [PubMed] [Google Scholar]
- KUNKEL H. G., CEPPELLINI R., MULLER-EBERHARD U., WOLF J. Observations on the minor basic hemoglobin component in the blood of normal individuals and patients with thalassemia. J Clin Invest. 1957 Nov;36(11):1615–1625. doi: 10.1172/JCI103561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LODER P. B., DEGRUCHY G. C. RED-CELL ENZYMES AND CO-ENZYMES IN NON-SPHEROCYTIC CONGENITAL HAEMOLYTIC ANAEMIAS. Br J Haematol. 1965 Jan;11:21–31. doi: 10.1111/j.1365-2141.1965.tb00080.x. [DOI] [PubMed] [Google Scholar]
- MARDER V. J., CONLEY C. L. Electrophoresis of hemoglobin on agar gels; frequency of hemoglobin D in a Negro population. Bull Johns Hopkins Hosp. 1959 Aug;105(2):77–88. [PubMed] [Google Scholar]
- PAULING L., ITANO H. A. Sickle cell anemia a molecular disease. Science. 1949 Nov 25;110(2865):543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- RIEDER R. F., ZINKHAM W. H., HOLTZMAN N. A. HEMOGLOBIN ZUERICH; CLINICAL, CHEMICAL AND KINETIC STUDIES. Am J Med. 1965 Jul;39:4–20. doi: 10.1016/0002-9343(65)90241-x. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., CAPUTO A. Pure native globin from human hemoglobin: preparation and some physico-chemical properties. Biochim Biophys Acta. 1958 Apr;28(1):221–221. doi: 10.1016/0006-3002(58)90462-1. [DOI] [PubMed] [Google Scholar]
- Rieder R. F., Bradley T. B., Jr Hemoglobin Gun Hill: an unstable protein associated with chronic hemolysis. Blood. 1968 Sep;32(3):355–369. [PubMed] [Google Scholar]
- Rifkind R. A. Destruction of injured red cells in vivo. Am J Med. 1966 Nov;41(5):711–723. doi: 10.1016/0002-9343(66)90032-5. [DOI] [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. Control of glycolysis in the human red blood cell. J Biol Chem. 1966 Nov 10;241(21):4848–4854. [PubMed] [Google Scholar]
- Rosemeyer M. A., Huehns E. R. On the mechanism of the dissociation of haemoglobin. J Mol Biol. 1967 Apr 28;25(2):253–273. doi: 10.1016/0022-2836(67)90141-6. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. CHARACTERIZATION OF GENETIC VARIANTS OF BLOOD PROTEINS. Vox Sang. 1965 May-Jun;10:359–362. [PubMed] [Google Scholar]
- Schrier S. L. Organization of enzymes in human erythrocyte membranes. Am J Physiol. 1966 Jan;210(1):139–145. doi: 10.1152/ajplegacy.1966.210.1.139. [DOI] [PubMed] [Google Scholar]
- Smithies O. Disulfide-bond cleavage and formation in proteins. Science. 1965 Dec 17;150(3703):1595–1598. doi: 10.1126/science.150.3703.1595. [DOI] [PubMed] [Google Scholar]
- ZINKHAM W. H., LENHARD R. E., Jr Metabolic abnormalities of erythrocytes from patients with congenital nonspherocytic hemolytic anemia. J Pediatr. 1959 Sep;55:319–336. doi: 10.1016/s0022-3476(59)80228-6. [DOI] [PubMed] [Google Scholar]