Abstract
Obesity has grown worldwide over the last few decades. In its different degrees, obesity is accompanied by many clinical and biochemical alterations reflecting the pathological condition of various body tissues. Among the mechanisms underlying the pathogenesis of obesity and associated complications, oxidative stress (OS) may be playing an important role. In the present study, we have characterized at systemic level the degree of OS status in a group of morbid obese patients (BMI>40 kg/m2) at basal sate and its modulation during one year after bariatric surgery using the laparoscopic sleeve gastrectomy (LSG) technique. As compared with normal weight subjects matched in age, peripheral blood mononuclear cells (PBMc) of obese patients present a significant reduction of the antioxidant enzyme activities superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) as well as a significant increase of the oxidized/reduced glutathione ratio (GSSG/GSH) in these cells. Lipid peroxidation is significantly increased in the patient group as shown by the increased levels of malondialdehyde (MDA) in PBMc and the amount of F2-Isoprostanes (F2-IsoPs) released in urine. In addition, the DNA damage product 8-oxo-7,8-2′-deoxyguanosine (8-oxo-dG) was also observed to be increased in serum and urine of morbid obese patients as compared with the control group. After LSG, an improvement of their ponderal and metabolic profile was accompanied by a progressive recovery of antioxidant enzyme activities and the decline of oxidative byproducts both in PBMc and biological fluids. The observed changes of urinary 8-oxo-dG levels correlate positively with its serum concentration, the lipid peroxidation products MDA and F2-IsoPs, triglycerides, glucose, insulin, HOMA index and body weight and negatively with the percentage of weight and BMI loss and antioxidant activities. We conclude that the analysis of urinary 8-oxo-dG could be validated as a useful marker for the monitoring of ponderal and metabolic status of morbid obese patients.
Keywords: Morbid obesity; Bariatric surgery; Oxidative stress; Antioxidants; DNA damage; 8-oxo-7,8-2′-deoxyguanosine
Graphical abstract
Highlights
-
•
A study on the clinical and oxidative stress status in morbid obesity is shown.
-
•
Morbid obese patients present an important reduction of antioxidant enzymes.
-
•
This deficiency is accompanied by the increase of lipid peroxidation and DNA damage.
-
•
Bariatric surgery normalizes the metabolic profile and oxidative stress status.
-
•
8-oxo-dG can be use as a clinical marker for the monitoring of morbid obesity.
1. Introduction
In the present days, considered as the epidemic of the XXI century, obesity represents a threat for public health and a social problem, affecting over 300 million people worldwide, which is continuously spreading and confirmed as the fifth leading cause of death globally [1], [2].
Even in countries of the Mediterranean areas obesity exists with high incidence and prevalence percentages as has important consequences. In Spain, the situation is equally alarming with the same epidemic figures and health risk [3].
Overweight and/or obesity are major risk factors for the development of severe diseases, including cardiometabolic alterations and cancer [4], [5]. Among these pathologies, 44% of the diabetes, 23% of the ischemic heart-disease burden, between 7% and 41% of certain cancer burdens, and the metabolic syndrome, are clinical complications attributed to the over-weight [2].
Treatment of obesity is expensive and time-cost with no always the expected or desired results for affected patients. This is special through for those with morbid obesity (BMI>40%). Bariatric surgery is presented as the treatment of choice due to its long effective result and low risk. For this purpose, different surgical techniques have been developed showing different efficiencies [6], [7].
Obesity is considered as a chronic low-grade inflammatory state where adipose tissue appears chronically inflamed, especially in morbid obese patients [8], [9].
Obesity induced inflammation eventually causes many of its clinical complications due to different molecular mechanisms, many of them still unexplained [10].
As a result of macrophages infiltration, due to the systemic pro-inflammatory cytokines and chemokines produced by the excessive adipose tissue, even more pro-inflammatory adipokines are released to the blood stream, such as different interleukins ((IL)-1ß, IL-6, IL-10…), the tumour necrosis factor-alpha (TNF-α), NLR pyrin domain containing-3 (Nlrp3) or the monocyte chemoattractant protein-1 (MCP-1), triggering the formation of highly reactive oxygen species (ROS) and inducing oxidative stress (OS) [8], [10].
ROS produced during normal aerobic metabolism and under the control of specific antioxidant are known to regulate many signal transduction pathways and biological processes. However, overproduction of ROS may lead to increase the oxidation of important macromolecules such as lipids, proteins and nucleic acids, therefore contributing to the distortion of cell and tissue homeostasis. This damaging effect is known as oxidative stress (OS) [11]. Indeed, different OS byproducts have been implicated in the pathophysiology of many diseases [12], [13] and some of them are mutagenic, as lipid peroxidation and DNA damage products [14], [15], [16].
Of special interest, in these terms is the oxidative modified base 8-oxo-7,8-2′-deoxyguanosine (8-oxo-dG), as elevated concentrations of this DNA damage product have been reported in patients with various pathologies, such hypertension [17], [18], [19], diabetes [20], metabolic syndrome [21], obesity [22] and cancer [8], [13], [23], [24], [25], [26].
An association between obesity and cancer incidence has been reported in worldwide populations. Even though it is not equally extensive to all tumour types, increased BMI has been positively associated with increased incidence and mortality from certain types of cancers. Specifically, about 20% of all cancers are estimated to be cause by excess weight [8], while, the underlying mechanism in obesity-induce tumorigenesis is still not clear established. A potential linkage between altered lipid metabolism and cancer may be lie on an excessive oxidative modification to specific molecules due to free radical production.
Although different experimental approaches have been designed to search the role of OS status in human obesity, there are important aspects and metabolites still not investigated. Indeed, in morbid obesity some critical OS byproducts, such as urine F2-Isoprostanes (F2-IsoPs) have not been determined. In addition, a complete study of systemic OS metabolism, including oxidation products and antioxidant enzyme activities in morbid obese patients together with their anthropometric, metabolic and clinical evolution during one year-long after bariatric surgery has not been conducted. In this work we present a comprehensive investigation of OS status in morbid obese patients at basal state and after different time periods (1, 3, 6 and 12 months) post-bariatric surgery and emphasize the usefulness of the determination 8-oxo-dG released by the urine as a marker of the clinical and metabolic status of morbid obese patients.
2. Materials and methods
2.1. Recruitment of subjects and biological samples
A group of 21 morbid obese patients with a mean age of 50±12 years and 100 healthy control volunteers matching in age were included in the study after an exhaustive clinical examination and their signed informed consent to participate on it. The experimental research and protocol was performed according to the declaration of Helsinki and was approved by the Ethics Committee of the University of Valencia. Diagnosis and follow up of morbid obese patients after surgical treatment were performed by medical expertise of the Services of Endocrinology and Digestive surgery respectively. Prior to surgery, all patients were required to follow an appropriate diet and physical exercise program.
Overweight patients with a body mass index ≥40 kg/m2 were classify as morbid obese following the clinical protocol and guidance of World Health Organization [27].
All morbid obese patients showed associated-comorbidities, Anthropometric measures, as well as data from routine biochemical analysis were collected during the study at the established times. Results obtained at the different time-periods were then compared both with the control group and their baseline levels before surgery.
Bariatric surgical was performed by the laparoscopic sleeve gastrectomy (LSG) [28], [29] in the Service of General and Digestive Surgery at the University Hospital of Valencia. Biological samples for the OS study included peripheral blood mononuclear cells (PBMc), serum and urine obtained at fasting conditions from patients and control healthy subjects and processed in the Unit of Oxidative Pathology of the Department of Biochemistry and Molecular Biology in the Faculty of Medicine (Valencia) along with the Clinical Analysis Service. In morbid obese patients samples were obtained at basal conditions (24 h before) and 1, 3, 6 and 12 months after surgery.
2.2. Variation of ponderal, metabolic and anthropometric parameters
The ponderal measurements and blood pressure were collected using standardized procedures: weight (kg), height (m), BMI (kg/m2), and blood pressure (mmHg).To evaluate the pondered evolution of patients, % of changes in weight and BMI were calculated using the following formulas:
-
•
Percentage of Excess weight Loss (%EWL):
100%×(preoperative W - current W)/(preoperative W - ideal W)
-
•
Percentage of excess BMI loss (%EBMIL):
100%×(preoperative BMI – current BMI)/(preoperative BMI - ideal BMI) [30].
Evolution of patient comorbidities as well as the changes of their biochemical parameters were followed along the study. Analysis of biochemical parameters, which include triglycerides (TGs), total cholesterol (TC) and HDL-cholesterol, basal glucose and insulin were determined by standard procedures on automatic analysers after appropriated controls validation. The homeostasis model assessment index (HOMA), which is defined as fasting insulin (in microunits per milliliter)×fasting plasma glucose (in millimoles per liter)/22.5 was used as index of insulin resistance as previously described [31], [32].
2.3. Analysis of selected OS markers in peripheral blood mononuclear cells (PBMc), serum and urine
-
1.
Isolation of PBMc from whole blood
A volume of 10–15 ml of whole blood samples were extracted from patients and collected in EDTA tubes at the specific times. Extracted tubes were kept in a refrigerator at 2–4 °C until they were processed for lymphocytes isolation within the four first hours after extraction. Isolation was carried out by density gradient centrifugation with Hystophaque (Sigma H-1077) at 1700 rpm for 30 min (12 °C), Polymorphonuclear cells (PMNc) attached to the bottom were gently resuspended, transferred to new Eppendorf tubes and stored at −80 °C until use. Prior to assay samples were sonicated for 3–5 s.
-
1.
Serum sample extraction
After 12 h fast, 7 ml of whole blood were drawn from an antecubital vein in tubes with coagulation activator and silicon. Tubes were left to coagulate for 15–30 min at room temperature (RT) and immediately centrifuged at 3000 rpm for 5 min at 4 °C. Resulting serum supernatant was pipetted off, and stored at 4 °C for a maximum of 3 days for clinical chemistry or at −80 °C until use for OS markers analysis.
-
1.
Urine collection
10–15 ml of spot urine (first urine in the morning) were collected in polyethylene bottles and transferred to glass tubes. Urinary samples were centrifuged at 3000 rpm for 5 min to precipitate and remove impurities. Different aliquots were separated and stored at −80 °C until use.
2.3.1. Assay of antioxidant activities and redox state assay in PBMc
SOD, CAT and GPx activities and glutathione levels were analysed by spectrophotometry method following the respective Cayman Assay kits procedures: SOD (n° 706002), CAT (n° 707002), GPx (n° 703102) as well as total GSH and GSSG (n° 703002).
2.3.2. Assay of OS byproducts
-
1.
MDA assay in PBMc by HPLC-UV
PBMc lipoperoxides are hydrolysed by boiling in dilute orthophosphoric acid. MDA-thiobarbituric acid (TBA) reacting adduct products (MDA (TBA))2 were eluted from the column and quantified spectrophotometrically. For MDA quantification, 50 μl of PBMc samples were mixed in a sterile Eppendorf tube with 75 μl H3PO4 0.44 M, 25 μl of TBA solution and heated at 95 °C for 30 min. After adding (on ice) 150 μl of neutralizing solution (1 M NaOH), samples were centrifuged at 10,000 rpm for 5 min. MDA was purified and measured from 100 μl of the resulting supernatant by HPLC-UV using an isocratic elution method with a solution containing 5% of acetonitrile and 50 mM potassium phosphate buffer at pH 5.5. The chromatographic runs were 3 min at a flow rate of 1 ml/min. The quantification of compounds was determined at 532 nm. Total protein content is measured by the Lowry method [33].
-
1.
8-oxo-dG assay in serum by HPLC-EC
Assay of 8-oxo-dG in serum was performed following the method by Li et al. (2013) [20] with slight modification reported by Borrego el at. (2013) [26]. For 8-oxo-dG separation and quantification, a Waters 515 HPLC Pump (model n° 5200ª) with 5 µm Spherisorb ODS2 column (4.6 mm x 250 mm) and electrochemical detectors (HPLC-EC) was used. Serum samples were defrosted immediately before use. A volume of 60 μl was heated at 50 °C for 1 h. Once samples were tempered again at RT, 100 μl of proteinase-k were added, and then incubated at 37 °C for 1 h. After that, 200 μl of the buffer solution KH2PO4/K2HPO4 (50 mM; pH 5.1) were added (same as used in HPLC, mobile phase) followed by centrifugation at 13,000 rpm for 5 min. Aliquots of 200 μl of the resulting supernatant were injected with a syringe of 0.2–40 µm in the HPLC-EC system. Running conditions are based on the method described by Espinosa, et al. maintaining a flow rate of 1 ml/min with 50 mmol/L potassium phosphate (pH 5.1) buffer in 5% of acetonitrile, and a retention time of 7.5 min. Electrochemical detection was achieved with a ESA Coulochem II detector (Chelmsford, MA, USA) equipped with a 5011 high-sensitivity analytical cell (sensibility of 1 μA). This is equipped with coloumetric (electrode 1) and amperometric (electrode 2) electrodes linked in series. For the purpose of this assay, the potentials set for the two electrodes were 0.2 V and 0.4 V, respectively. To assess the optimization and accuracy of the HPLC-EC assay for the isolation and detection of 8-oxo-dG, appropriate chromatograms of both samples and standards were recorded at the beginning of each working day. All samples were analysed in duplicate.
-
1.
8-oxo-dG assay in urine by HPLC-EC
Method used was modified from the one described in Espinosa et al. (2007) [18]. After 1 ml of urine was defrosted, 100 μl of 3 mol/l Tris–EDTA solution (pH 8.6) were added to vortex mixed for 30 s. 1 ml of the resulting was then applied to a Bond Elute C18(OH)SPE (3 ml) column that had been prepared with 3 ml methanol and 3 ml distilled water (HPLC-grade). The column is washed with 3 ml water followed by 3 ml of 2.5% acetonitrile and 1.5% of methanol in 10 mmol/l borate (pH 7.9). The sample was then eluted with 3 ml of the same buffer and applied to a Bond Elute strong cation exchange column (3 ml) prepared with 3 ml of methanol and 3 ml of borate buffer (pH 7.9). The 8-oxo-dG was then eluted with 2 ml of acetonitrile/methanol buffer in borate and then adjusted to pH 6.0 with 1 mol/l HCl. About 4 ml of 50:50 dichloromethane: propane-2-ol was added to the 2 ml of eluent and vortex mixed for 30 s. Samples were then centrifuge for 10 min at 3500 rpm, the upper aqueous layer was aspirated off and 3 ml of organic layer were evaporated to dryness in a vacuum chamber (Concentrator plus; Eppendorf AG, 2331 Hamburg) at 50 °C. Finally, samples were reconstituted in 1 ml HPLC running buffer as above but without acetonitrile, and 50 μl were injected into the HPLC column. Running conditions and EC detection were same as those described for serum samples. 8-oxo-dG values were expressed as the ratio to mmol/mol creatinine quantified with the Cayman Creatinine (urinary) Colorimetric Assay kit (n° 500701) as described by Borrego et al. (2013) [26]. Calibration procedures were those described by Espinosa et al. (2007) [18]. A HPLC-grade water solution of 8-oxo-dG >98% (TLC) purchased from Sigma Aldrich Chemical Com. Saint Luis MO, USA (ref. number H5653) was used as a standard sample. Each working day six different samples with known low and high concentrations of 8-OH-dG were run twice. The intra- and inter-day calculated variability coefficient was 5%.
-
1.
F2-8-Isoprostanes assay
F2-8-Isoprostanes levels in urine were quantified by ELISA assay following the procedure described in the Cayman Assay kit n° 516351, and the results expressed as the ratio to creatinine.
2.4. Statistical analysis
SPSS (Statistical Package for Social Sciences) for Windows 13.0 program has been used for statistical analysis. Unpaired T-test and Pearson’s correlation analysis were used for comparison of data. Results are given as mean±standard deviation and p<0.05 is defined as significant.
3. Results
Table 1 shows the biochemical and anthropometrical parameters of control subjects and morbid obese patients at basal conditions.
Table 1.
Clinical, hematological and anthropometrical parameters of total studied population.
| PARAMETERS | Standard | CONTROLS (n=100) | PRE-SURGERY (BASAL) (n=21) |
|---|---|---|---|
| Reference Values | |||
| TGs (mg/dL) | <150 | 102.24±38.19 | 164.3 ± 67.18** |
| TC (mg/dL) /HDLc (mg/dL) | 3–5 | 3.75±0.72 | 4.91±1.13** |
| INS (mcU/ml) | 5–20 | 7.1±3.3 | 20.6±9.07** |
| HOMA Index | <3 | 1.58±0.9 | 5.18±2.49** |
| GLC (mg/dL) | 70–110 | 95.10±13.07 | 126.57±61.21** |
| WBC (x109/L) | 3.6–11.5 | 7.04±0.7 | 9.00±2.07** |
| RBC (x1012/L) | 3.9–5.8 | 4.2±0.3 | 4.73±0.43** |
| Hgb (g/dL) | 12.1–17.2 | 13.2±1.07 | 13.7±1.24 |
| MCV (fl) | 80–95 | 85.2±3.5 | 88.31±4.73** |
| ALAT (U/L) | 7 –40 | 20.87±15.3 | 29.81±20.44 |
| CRP (mg/L) | Normal: <0.8 | 1.2±3.1 | 2.55±6.4 |
| Low risk: <1.00 | |||
| Average risk: 1.00–3.00 | |||
| High risk: >3.00 | |||
| WGT (kg) | – | 74.89±10.45 | 119.46±16.53 |
| BMI | 18.5–24.9 | 24.2±4.5 | 46.22±5.33 |
Clinical and Anthropometrical characteristics of both control normal weight subjects and morbid obese patients at basal state. Standard reference values are shown. Results are represented as means and ±SD of the total studied population. *p<0.01 comparing values of patients group with control subjects. TGs (Triglycerides); TC/HDL (Total cholesterol/High density lipoprotein); INS (Insulin); HOMA Index (Homeostatic Model Assessment Index); GLC (Glucose); WBC (White Blood Cells); RBC (Red Blood Cells); HgB (Hemoglobin); MCV (medium corpuscular volume); ALAT (Alanine Amino Transferase); CRP (C-Reactive Protein); WGT (Weight); BMI (Body Mass Index).
As can be observed, while the control group maintained their parameters within the standard reference values, morbid obese patients presented significant increases in all their systemic carbohydrate and lipid profiles as well as some haematology parameters. In obese subjects, there is also an increase of ALAT and CRP serum concentrations although without significant differences as compared with the control group. Mean values of weight and BMI in the patients group are those characterized by morbid obesity (Table 1).
The percentage of comorbidities affecting obese patients at basal state and one year after surgical treatment are represented in Table 2. One year after surgery some comorbidities had been restored and others showed an improvement. Highest percentages of total recovery were observed in hypertension (HTN), obstructive sleep apnoea syndrome (OSAS) and type 2 Diabetes Mellitus (DMT2) (Table 2).
Table 2.
One-year effect of bariatric surgery on the comorbidity sates of morbid obese patients.
| % Affected | % Cured | % Improved | |
|---|---|---|---|
| HTN | 61.9 | 69.2 | 15.4 |
| Hyphothyroidism | 23.8 | 20 | – |
| OSAS | 38.1 | 75.0 | 25.0 |
| DMT2 | 52.4 | 54.5 | 36.4 |
| MetS | 4.8 | – | 100 |
| Hyperinsulinemia | 23.8 | – | 100 |
| Asthma | 9.5 | – | 100 |
| Dyslipidemia | 14.3 | 33.3 | 66.7 |
| Steatosis | 9.5 | – | 100 |
Data in the table correspond to % Affected: percentage of comorbidities present in the 21 morbid obese patients at basal state (prior to surgery); %Cured: percentage of patients that after 12 months no longer have the comorbidity and no longer need any treatment; % improved: percentage of patients that just show an improvement of their comorbidity i.e. a reduction of their treatment was reported 12 months after surgery. HTN, hypertension; OSAS, obstructive sleep apnoea syndrome; DMT2, diabetes mellitus type 2; MetS, metabolic syndrome.
3.1. Evolution of ponderal parameters
All patients showed a BMI over 40 kg/m2, concretely, 66,7% of patients presented BMI values between 40–49,9 kg/m2 and a 33,3% had BMI values above 50 kg/m2. Average height was 161±8 cm.
Along the study, all patients showed a progressive decrease of weight as well as of its derived variables: BMI, %EWL and %EBMIL (Table 3).
Table 3.
One-year evolution study of ponderal parameters of morbid obese patients after bariatric surgery.
| Parameters | Basal | 1 m | 3 m | 6 m | 1 year |
|---|---|---|---|---|---|
| WGT (kg) | 119.46±16.53 | 105.6±16 | 95.7±14 | 86.2±12 | 80.2±12 |
| BMI | 46.22±5.33 | 40.9±6 | 37.1±5 | 33.4±5 | 31.2±5 |
| % EWL | 23.1±12 | 39.5±11 | 55.1±12 | 64.6±16 | |
| % EBMIL | 26.2±14 | 44.8±13 | 62.3±15 | 73±19 |
Results are represented as means and ±SD of the patients group. WGT (Weight); BMI (Body Mass Index); %EWL (Excessive Weight Loss); %EBMIL (Excessive Body Mass Index Loss).
One month after surgery there was already a percentage of weight loss of 23.1 kg, from starting mean values of 119.5±17 kg to 105.6±16 kg. Reduction of weight was observed to continue decreasing in all patients at 3, 6 and 12 months-time after bariatric surgery.
Regarding BMI, from starting values above 40 kg/m2, an important decrease was observed. From a 46.2±5 kg/m2 mean BMI value prior to surgery, a mean value of 31.2±5 kg/m2 was observed 1 year after surgery. This final BMI value is lower than the one required to determine that a patient needs surgery (BMI≥35 kg/m2), and away from morbid obesity values (BMI≥40 kg/m2).
Moreover, after 1 year from surgery, mean %EWL values was more than the 60%, but it was at 6 months when the mean percentage EWL (55.1±12%) was already achieved. Along with %EWL values, %EBMIL values also increased from a mean % value of 26.2±14 to 73±19 after 1 year (Table 3).
3.2. Evolution of metabolic parameters
All biochemical parameters analysed also showed an improvement as body weight decreased (Table 4). Reduction of serum TGs to values within the standard normal range was observed at month 3 after surgery and keeping the decreasing tendency during the study period to arrive to those values of the control group. TC/HDL ratio also decreased progressively after surgical treatment. An important improvement was observed in the glucose metabolism profile with a reduction of both serum insulin and glucose values just at first month after bariatric surgery. As a consequence, HOMA index reached its normal value at month 3 post-surgery. All the metabolic indicators in morbid obese patients were close to the control group 1 year after surgery (Table 4).
Table 4.
Time course effect of metabolic recovery of morbid obese patients after bariatric surgery.
| Controls | Basal | 1 m | 3 m | 6 m | 1 year | |
|---|---|---|---|---|---|---|
| TGs (mg/dL) | 102.24±38.19 | 164.33±67.18 | 157.72±70.61 | 138.24±69.36 | 124.72±64.06 | 110.08±54.11 |
| ++ | ++ | |||||
| TC (mg/dL) / HDL (mg/dL) | 3.75±0.72 | 4.91±1.13 | 5.13±1.45 | 4.93±1.38 | 4.63±1.45 | 4.06±1.08 |
| ++ | ++ | ++ | ||||
| INS (mcU/ml) | 7.1±3.3 | 20.62±9.07 | 13.03±8.34 | 10.59±5.08** | 7.65±3.24** | 7.45±4.55** |
| ++ | +* | |||||
| HOMA Index | 1.58±0.9 | 5.18±2.49 | 3.91±3.40 | 2.59±1.95** | 1.87±1.34** | 1.70±1.23** |
| ++ | + | |||||
| GLC (mg/dL) | 95.10±13.07 | 126.57±61.21 | 104.15±29.35 | 97.10±36.35 | 92.38±27.97** | 90.52±17.51** |
| ++ |
Evolution of biochemical parameters in morbid obese patients after bariatric surgery and their comparison with those of control subjects are shown. Results are means and ±SD of 21 different patients and 100 control subjects. Patients values at the stablished times were compared with mean basal values (prior to surgery) representing the level of significance with * for p<0.05 and ** for p<0.01. Significance between patients values evolution and control values is represented with + for p<0.05 and ++ for p<0.01. TGs (Triglycerides); TC/HDL (Total cholesterol/High density lipoprotein); INS (Insulin); HOMA Index (Homeostatic Model Assessment Index); GLC (Glucose).
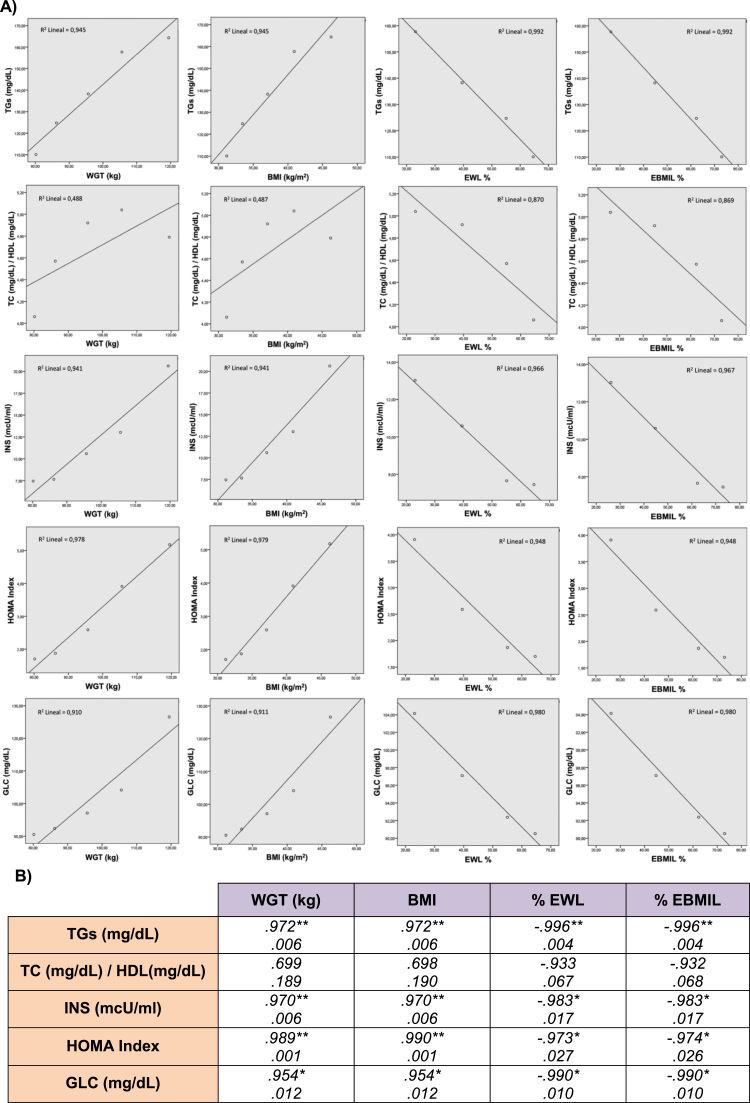
Pearson's correlation study between weight, BMI and metabolic parameters from basal state to 1 year follow up after bariatric surgery and the respective statistical significances is represented in Fig. 1.
Fig. 1.
Correlation study between the ponderal and metabolic profiles of morbid obese patients. Panel A: Graphic representations of correlated parameters. Panel B) Statistical values of Pearson's correlations. *p<0.05 and **p<0.01 are shown.
3.3. Evaluation of OS status
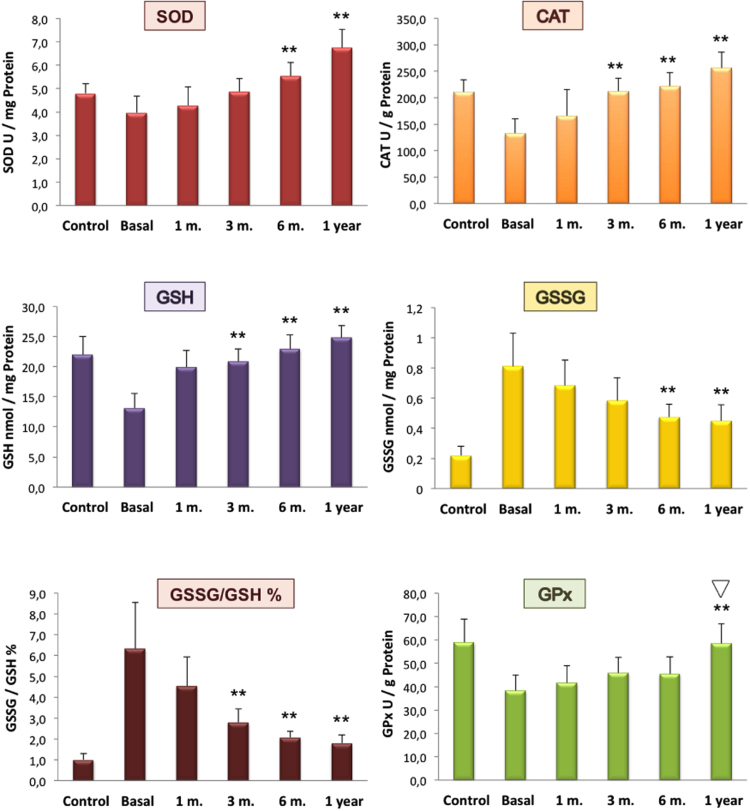
Basal levels (pre-surgery) of the OS by-products analysed on morbid obese patients were significantly elevated while their antioxidant activities appeared diminished compared with those of the non-obese healthy subjects. In both cases, antioxidant systems and oxidation byproducts showed a tendency towards control levels after surgical treatment (Fig. 2, Fig. 3).
Fig. 2.
Antioxidant enzymes activities and thiol redox state in PBMc of control subjects and morbid obese patients. Time-course effect of bariatric surgery. Results are means±SD of 21 different patients and 100 control subjects. Patients values at the established times were compared with mean basal values (prior to surgery). Significance *p<0.05; **p<0.005. * and ** indicate significant differences between indicated value and basal value.  indicates no significant differences between patients and controls values.
indicates no significant differences between patients and controls values.
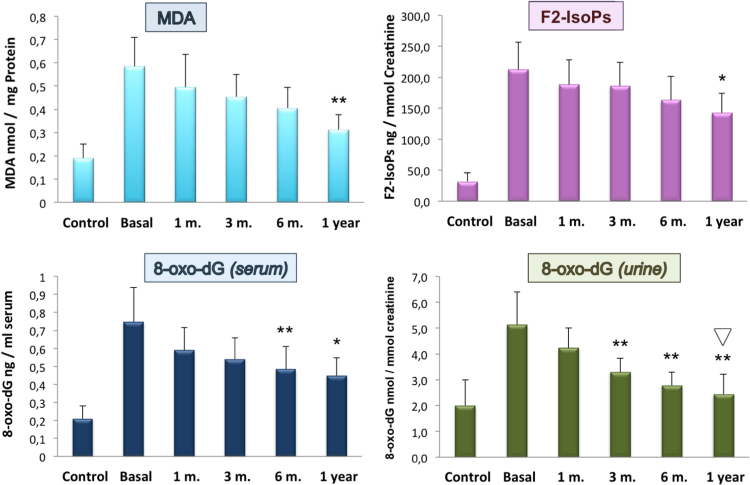
Fig. 3.
Systemic lipid peroxidation and DNA oxidation of control subjects vs. morbid obese patients and the time-course effect of bariatric surgery. Levels of OS markers analysed in PBMc (MDA), serum (8-oxo-dG) and urinary (F2-IsoPs and 8-oxo-dG) are shown. Results are means±SD of 21 different patients and 100 control subjects. Patients values at the established times were compared with mean basal values (prior to surgery). Significance *p<0.05; **p<0.005.  indicates no significant differences between patients and controls values.
indicates no significant differences between patients and controls values.
Regarding the antioxidant enzymes SOD and CAT in PBMc, both showed significant lower activities before surgery as compared with the control group.
One month after surgery, SOD activity values started to rise, arising to values of 4.9±0.7, 6.0±0.7 and 6.8±0.8 U/mg protein at 3, 6 and 12 months respectively. Mean values at the 6th and 12th month were significant (p<0.005) when compared with the basal level.
Mean value for CAT activity prior to surgery was 130±30 U/g protein, showing a marked reduction compared with control subjects (210±20 U/g protein). Levels started to rise after surgery, and at the 3th month mean CAT activity levels in the circulating cells of morbid obese patients already arrived to control levels.
Basal mean value of GSH in morbid obese patients was almost half of the concentration detected in control subjects. Its recovery became significant (p<0.005) at month 3, with a GSH concentration of 21±3 nmols/mg protein, which is close to the control value (22±3 nmols/mg protein). At months 6 and 12, GSH levels were 23±8 and 25±2 nmols/mg protein respectively.
Levels of GSSG were fourfold increased as compared to those of the control group (0.22±0.06 nmols/mg protein). After surgery these values started to decrease from the first months with significant values (p<0.005) at moths 6 (0.48±0.11) and 12 (0.45±0.09).
As expected, %GSSG/GSH ratio was six times higher in obese patients than in control subjects (6±2% vs. 1.0±0.3% respectively). One month after surgery, this ratio decreased to 4.54±1.3%, but it was at the 3th month when values became significant (p<0.005) comparing to the basal levels. At months 6% and 12%GSSG/GSH was further reduced.
An important reduction in the activity of GPx was observed in morbid obese patients compared with the control group (38±9 vs, 60±10 U/g protein). After LSG, the enzyme activity progressively increased being significant at 12 months (p<0.005) compared with the mean basal level and at that time the activity was completely recovered with no statistical differences compared with the control group (Fig. 2). To discard unspecific changes of intracellular protein content, total lactate dehydrogenase (LDH) and creatine kinase (CK-MB) activities were analysed in PBMc of morbid obese patients at basal state and at the end of the study period and no differences were observed (174±33 vs. 166±28 IU/mg prot (n=6) and 6,88±.71 vs. 7,02±1.62 UI/mg prot. (n=6) Respectively).
Oxidation products derived from lipid peroxidation and DNA damage were significantly elevated in morbid obese patients and as in the case of antioxidant activities a progressive normalisation of these values took place after surgical intervention (Fig. 3).
Obese patients showed high levels of MDA in their PBMc compared with mean control levels, 0.58±0.12 and 0.19±0.06 nmol/mg protein respectively. MDA levels decreased after surgery, being significant (p<0.005) at 1 year follow-up compared with basal levels (p<0.005) but without reaching those of the control group.
F2-IsoPs released in urine of morbid obese patients was extensive as shown by the six times higher levels in these patients compared with those of the control group (210±40 vs. 32±14 ng/mmols creatinine). Mean values at 1, 3, 6 and 12 months after surgery were 190±40, 190±40, 160±40 and 140±30 ng/mmols creatinine respectively, showing a significance of p<0.05 at month 12 as compared with the basal level.
Morbid obese patients presented in their serum and urine samples significantly higher amounts of 8-oxo-dG compared with the normal weight group. A mean value almost four times higher than in controls was obtained for 8-oxo-dG levels in serum of obese patients, (0.75±0.14 vs. 0.21±0.07 ng/ml serum). Serum 8-oxo-dG progressively decreased in morbid obese patients after bariatric surgery. At 3, 6 and 12 months mean values were 0.54±0.10, 0.48±0.18 and 0.45±0.12 ng/ml serum respectively with a significant difference observed six months after surgery.
Levels of 8-oxo-dG in urine of the obese group showed a similar tendency to that observed in their serum samples (5.1±0.9 vs 2±1 nmols/mmols creatinine). Levels of 8-oxo-dG started to progressively decrease after surgery, almost reaching controls mean value at month 12. Decrease of mean values showed a significance of p<0,005 from month 3, maintaining that significance at months 6 and 12. At 12 months-time from surgery, there was no significant difference between the obese and the control group (Fig. 3).
3.4. Statistical correlations between the analysed parameters in the morbid obese patients group
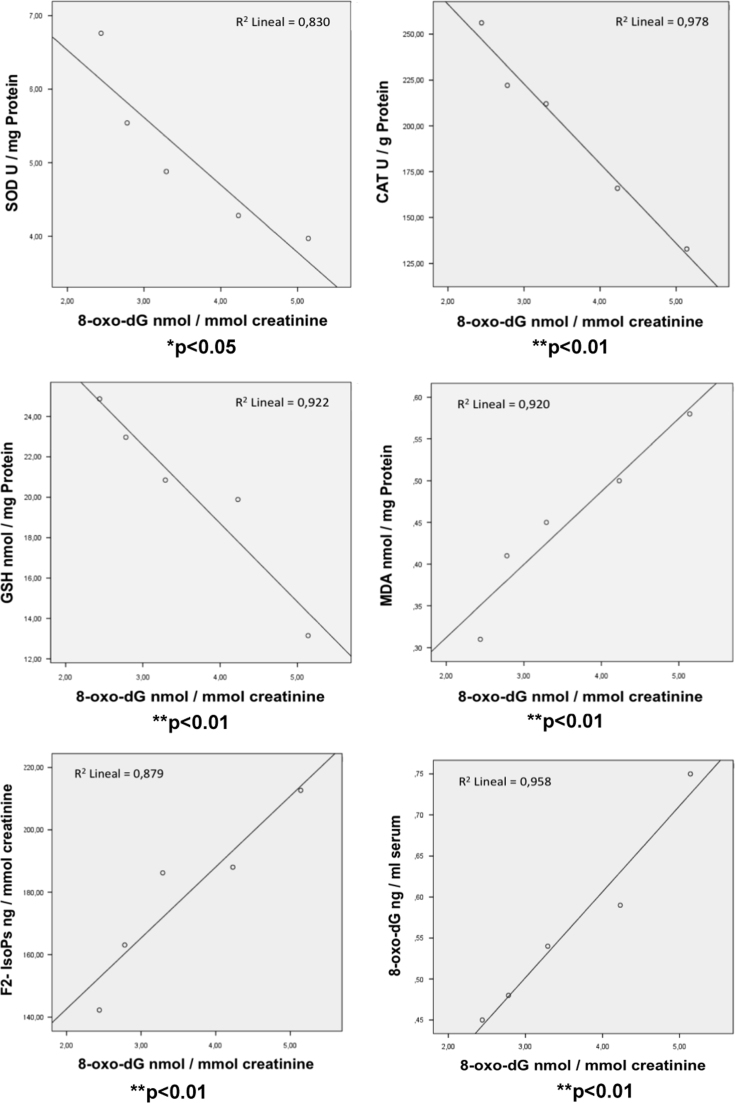
Statistical correlations were calculated between all analysed OS biomarkers. As shown in Fig. 4, the changes in the amount of urinary 8-oxo-dG release in morbid obese patients after bariatric surgery significantly correlates with their activities of SOD (p<0.05) CAT (p<0.01) and the GSH concentration (p<0.01) in PBMc. The same level of significance (p<0.01) was found for GSSG concentration and GSSG/GSH ratio in these cells (results not shown). There was also a good correlation between the evolution of urinary 8-oxo-dG and the lipid peroxidation products MDA in PBMc (p<0.01) and urine F2-IsoPs (p<0.01). A significant correlation (p<0.01) was also found with the 8-oxo-dG levels in the serum of morbid obese patients (Fig. 4).
Fig. 4.
Correlations of the 8-oxo-dG levels released by the urine of morbid obese patients with their antioxidant enzyme activities (SOD and CAT) and the concentrations of GSH and MDA in PBMc, urinary F2-IsoPs and serum 8-oxo-dG at basal state and 1, 3, 6 and 12 months after LSG bariatric surgery. Statistical significances *p<0.05; and ** p<0.01 of correlated parameters are shown.
As shown in Table 5, most of the OS related metabolites significantly correlated with the metabolic markers of morbid obese patients all along the study period but with different degree of significance. TC/HDL ratio only negatively correlated with SOD activity while GPx only negatively correlated with serum TGs concentration. GSH, GSSG, %GSSG/GSH ratio and the levels of 8-oxo-dG both in serum and in urine showed the highest degree of significance in the metabolic correlation study. Pearson's correlation study between ponderal variables and OS byproducts, also showed different statistical significances. Catalase, GSH and GSSG/GSH ratio in PBMc significantly correlate with weight and BMI (p<0.001) and with the percentages of EWL and EBMIL (p<0.05). There was no correlation with GPx. Lipid peroxidation products (MDA in PBMc and urinary F2-IsoPs) significantly correlated with weight and BMI (p<0.01) but not with EWL and EBMIL percentages while the changes in the levels of serum and urinary 8-oxo-dG significantly correlated with all ponderal variables (p<0,01) (Table 5).
Table 5.
Correlations between the Oxidative Stress markers and biochemical and ponderal parameters of the morbid obese patients after one-year follow-up.
|
Biochemical and Ponderal parameters |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TGs | TC/HDL | INS | HOMA Index | GLC | WGT | BMI | % EWL | % EBMIL | ||
| Oxidative Stress markers | SOD | −.978** | −.903 * | NS | −.880* | NS | −.931* | −.930* | .957* | .956* |
| (U/mg Prot.) | .004 | .036 | .049 | .022 | .022 | .043 | .044 | |||
| CAT | −.979** | NS | −.937* | −.976** | −.928* | −.986** | −.986** | .970* | .970* | |
| (U/mg Prot.) | .004 | .019 | .004 | .023 | .002 | .002 | .030 | .030 | ||
| GPx | −.914* | NS | NS | NS | NS | NS | NS | NS | NS | |
| (U/g Prot.) | .030 | |||||||||
| GSH | −.894* | NS | −.987** | −.956* | −.986** | −.970** | −.969** | .972* | .972* | |
| (nmol/mg Prot.) | .041 | .002 | .011 | .002 | .006 | .006 | .028 | .028 | ||
| GSSG | .964** | NS | .976** | .995** | .958* | .998** | .998** | −.995** | −.995** | |
| (nmol/mg Prot.) | .008 | .005 | .000 | .010 | .000 | .000 | .005 | .005 | ||
| %GSSG/GSH | .938* | NS | .982** | .999** | .973** | .985** | .986** | −.966* | −.967* | |
| .018 | .003 | .000 | .005 | .002 | .002 | .034 | .033 | |||
| MDA | .974** | NS | .910* | .936* | .898* | .974** | .973** | NS | NS | |
| (nmol/mg Prot.) | .005 | .032 | .019 | .039 | .005 | .005 | ||||
| 8-Isoprostanes (urine) | .955* | NS | .907* | .915* | .884* | .964** | .963** | NS | NS | |
| (Pg/mmol creatinine) | .011 | .033 | .030 | .046 | .008 | .008 | ||||
| 8-oxo-dG (serum) | .911* | NS | .996** | .979** | .992** | .982** | .982** | −.999** | −.999** | |
| (ng/ml serum) | .031 | .000 | .004 | .001 | .003 | .003 | .001 | .001 | ||
| 8-oxo-dG (urine) | .967** | NS | .971** | .997** | .958* | .996** | .996** | −.990** | −.990** | |
| (nmol/mmol creatinine) | .007 | .006 | .000 | .010 | .000 | .000 | .010 | .010 | ||
Significant correlation *p<0.05 and **p<0.01 are shown; NS represents no significant correlation between the two parameters; - stands for a negative correlation. TGs (Triglycerides); TC/HDL (Total cholesterol/High density lipoprotein); INS (Insulin); HOMA Index (Homeostatic Model Assessment Index); GLC (Glucose); WGT (Weight); BMI (Body Mass Index); %EWL (Excessive Weight Loss); %EBMIL (Excessive Body Mass Index Loss).
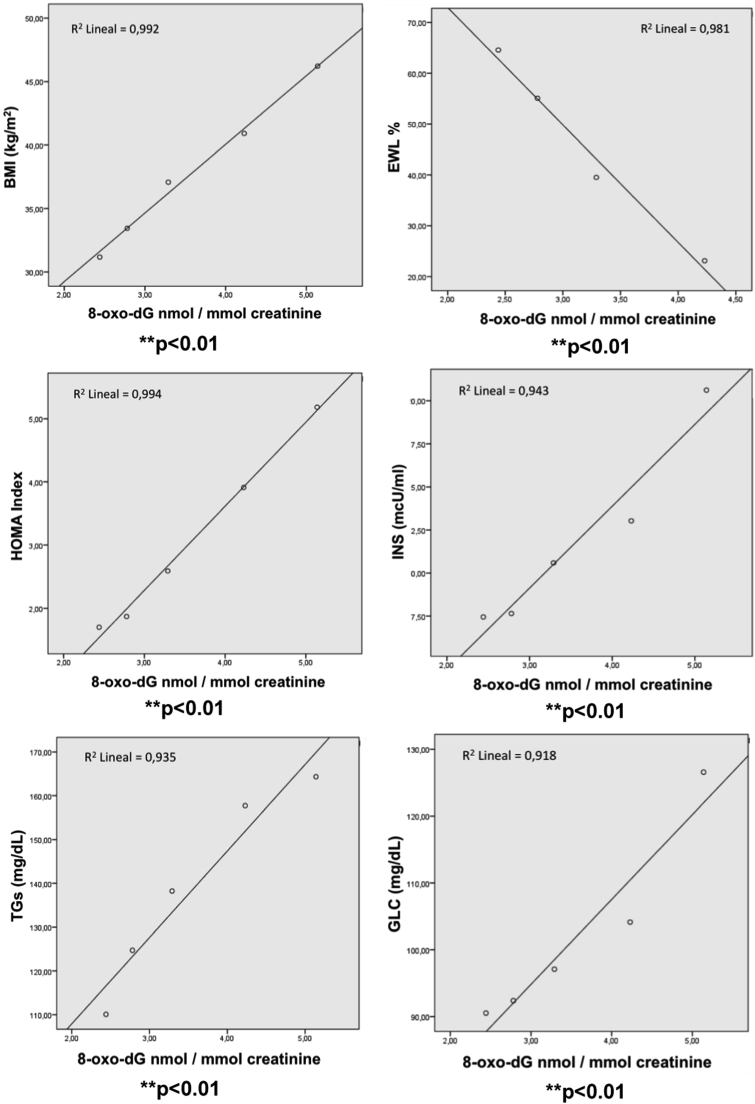
In Fig. 5 results of the correlation study between the changes of urinary levels of 8-oxo-dG and the variations of ponderal and metabolic parameters after LSG are shown. The levels of 8-oxo-dG released by the urine of obese patients significantly (p<0.01) correlated with their weight and %EWL together with their serum metabolic parameters, TGs, glucose, insulin and HOMA index (p<0.01).
Fig. 5.
Correlation of urinary 8-oxo-dG with ponderal and metabolic profile of morbid obese patients at basal state and 1, 3, 6 and 12 months after LSG bariatric surgery. Significant correlation *p<0.05 and **p<0.01 are shown.
To reflect the utility of urine F2-IsoPs and 8-oxo-dG, as potential markers of morbid obesity, the percentages of sensitivity and specificity for each of them were calculated separately and as a whole (F2-IsoPs+8-oxo-dG). Based on the data in Table 6, the highest sensitivity (69.23) was obtained for the modified DNA base 8-oxo-dG.
Table 6.
Comparison of the individual and combined percentages of positive and negative predictive values, sensitivity and specificity of F2-IsoPs and 8-oxo-dG as diagnostic probes for morbid obesity.
| F2-IsoPs | |
|---|---|
| PPV | 29.17% |
| NPV | 100% |
| SEN | 100% |
| SPE | 50.96% |
| 8-oxo-dG | |
|---|---|
| PPV | 39.62% |
| NPV | 100% |
| SEN | 100% |
| SPE | 69.23% |
| F2-IsoPs+8-oxo-dG | |
|---|---|
| PPV | 27.27% |
| NPV | 100% |
| SEN | 100% |
| SPE | 46.15% |
PPV: positive predictive value; NPV: negative predictive value; SEN: sensitivity; SPE Specificity.
4. Discussion
ROS-induced oxidative stress is one of the most important factors implicated in developing a wide variety of pathological conditions by compromising the metabolic homeostasis and the normal regulation of cell growth and differentiation. Therefore, an excessive production of ROS overwhelming antioxidant status in cells and tissues has been implicated as a subjacent pathogenic mechanism including cardiovascular diseases and/or related metabolic disturbances such as dyslipidemia, diabetes, metabolic syndrome and inflammatory processes [11], [34].
Obesity is a complex multifactorial disease which shares many of these alterations by progressing to different cardiometabolic complications and even the development of some type of tumour processes [8]. In fact, it has been widely accepted that there is a strong relationship between OS-induced cell damage and the clinical complications of obese patients [22].
As has been suggested, as a result of excessive fat and nutrients accumulation, adipocytes increase in volume and start to aggregate, then diminishing blood flow and causing hypoxia. Reduced oxygen supply to adipocytes is known to trigger the production and release of different necrotic and apoptotic factors by these cells. Hypertrophic-hyperplastic adipocytes exhibit a lower density of insulin receptors and a higher beta-3 adrenergic receptor, which facilitates the diapedesis of monocytes to visceral adipose stroma, initiating a pro-inflammation cycle between adipocytes and monocytes [35].
Under these circumstances, the release of pro-inflammatory cytokines and chemokines is then significantly increase therefore inducing the production of high amount of ROS and OS associated with cell damage. A vicious cycle is completed by monocyte infiltration to adipose tissue due to the chemo-attractant effect of pro-inflammatory molecules and their further transformation into macrophages which explains the characterized chronic low-grade inflammatory state of obesity [10].
Other mechanisms to explain the increased ROS production in obesity has been also proposed, such as the high metabolic consumption of TGs and their inhibitory effect on mitochondria adenine nucleotides translocation [36].
Based on these facts, it is expected that the therapeutic reduction of adipose tissue may result in an improvement of the OS status and the clinical outcome of affected patients.
In this work we evaluate and characterize the systemic oxidative stress status in a population of morbid obese patients and followed its changes together with their clinical outcome after bariatric surgery. For that purpose, most representative oxidation by-products together with antioxidant enzyme activities have been analysed in different biological samples including PBMC, serum and urine of affected patients and compared with those of a normal healthy population matching in age. A combined study with both oxidation damage products and antioxidant enzyme activities in biological samples of morbid obese patients has not been previously reported.
4.1. . Effectiveness of the bariatric surgery used
Patients in our study, with BMI over 40 kg/m2, were subjected to LSG which has been consolidated as the method of choice for its proved good results in reducing weigh and BMI of extremely obese patients. LSG avoids not only surgery technical difficulties, but also reduces metabolic complications and morbidity compared with other types of interventions such as the duodenal switch (DS) [28], [37] and the laparoscopic adjustable gastric banding (LAGB) [29].
Due to its simplicity and that it has proven to achieve good short and long term results, LSG has gained good acceptance within bariatric surgery groups [38], [39].
Mean BMI value after 1 year from this kind of surgery showed that patients were no longer in the range of that risk BMI value (31.2±5 kg/m2) indicated to undergo surgery [40], therefore supporting the benefits provided by LSG technic.
In a recent report, using a different laparoscopic technique (LAGB) a decrease of BMI and weight values were also achieved but in twice the time (6 months) [41] compared with our results.
We show, a mean %EWL value of 64.6±16% and a mean BMI value of 31.2±5 kg/m2 at 12 months after surgery (Table 3). It is considered that achieving a 50% of EWL is indicator of health risk reduction and improvement of health and related quality of life, which was estimated by the BAROS text with a final score of 4.8 [42], [43]. Additionally, by reducing body weight, all patients showed a partial or total remission of their comorbidities (Table 2).
4.2. . Evolution of biochemical parameters
As expected, all biochemical parameters decreased 1 year after surgery. Serum TGs, glucose and insulin as well as the HOMA index after LSG reduced progressively and in parallel with weight loss, which is a clear sign of health improvement, reaching normal values around 3 months after surgery. (Table 4).
4.3. . Changes of oxidative stress byproducts
According to its definition, oxidative stress in morbid obese patients is evidenced by a significant reduction of antioxidant enzymes and an increase of oxidation byproducts in their blood cells and body fluids. The decrease of antioxidant activities do not occur with the same extent in all analysed enzymes being especially evident and significant in the case of CAT and GPx. Reduced GPx activity has been reported to play an important role as an independent cardiovascular risk factor [44]. Therefore, low GPx activity in these patients might contribute to their increased incidence of cardiovascular events [4], [5].
In addition to the impairment of antioxidant enzyme activities, GSSG/GSH ratio is significantly increased in their PBMc (Fig. 1) indicating an important redox shift to a more oxidative state. Changes in Intracellular redox equilibrium is known to influence a variety of signal transduction pathways implicated in cell growth and differentiation [45], [46] while alterations of the cytosol and/or mitochondrial redox ratio may disrupt the activities of different thiol containing proteins and related enzymes. Furthermore, reduced levels of GSH may also compromise cell defence against oxidative stress since the tripeptide has been shown to act directly as an effective stabilizer (scavenger) of the highly reactive hydroxyl radical (·OH) [47], [48]. Moreover, plasma total thiol content (TTC) have been also observed to be reduced in overweight and/or obese patients, compared with control subjects, emphasizing the role of the –SH group in metabolic regulation and antioxidant protection [49], [50].
We observed an improvement in the antioxidant capability and a decrease of GSSG/GSH ratio in circulating PBMc after bariatric surgery. GPx practically returned to its normal levels as there was no significant difference between its mean activity value and that of the control group one year after surgery. In the case of SOD, CAT and GSH, mean values reached control levels at 3 month post-surgery, and further increases were observed during the following months. This effect suggests some kind of metabolic rebound or overshooting response due to the progressive reduction of weight, inflammation state and ROS production. The changes observed in terms of the antioxidant enzyme response to bariatric surgery are in accordance with a previous reported study [9].
Oxidative stress byproducts MDA and F2-IsoPs were chosen because of their reliability as lipid peroxidation markers, while 8-oxo-dG is a good indicator of DNA oxidative damage [18], [34] Moreover, analysis of 8- oxo-dG in urine stands out for being an easy and non-invasive method for monitoring DNA damage in vivo. In morbid obese patients MDA and F2-IsoPs, are increased in PBMc and urine respectively, confirming an enhanced lipid peroxidation.
High levels of F2-IsoPs were also reported by Vlady O, et al. (2011) [51], where obese children showed higher values of this OS product compared with non-obese controls.
Morbid obese patients present significant higher levels of 8-oxo-dG in their serum and urine samples which suggest an intense rate of DNA and/or free nucleotide oxidation but also an effective response towards its repair. The role of the different mechanisms involved in its repair and release to extracellular fluids has been previously reported and reviewed [52], [53].
The quantification of 8-oxo-dG is especially relevant since in addition to its validity as a reliable indicator of DNA oxidation, its pathogenic implications and potential use as a clinical marker has been also addressed [26].
We have used the methodology previously described in which HPLC-EC has been applied for the separation and quantification of the damage base in both serum and urine. Based on its high sensitivity compared with other available techniques, HPLC-EC has been validated through the ESCODD and ESCULA multicenter project as a method of choice to avoid overestimation when analysing the levels of 8-oxo-dG in normal and pathological situations [20], [54], [55].
In the present study, MDA and 8-oxo-dG progressively decrease in patients undergoing LSG. By using the laparoscopic adjustable gastric band (LAGB) technique, Kocael and cols. reported a decline in the levels of serum and urinary 8-oxo-dG of 20 morbid obese patients at 6 months after bariatric surgery, along with the reduction of weight and BMI values [41]. Using LSG we have shown a further decrease of the damaged base both in serum and urine during the next 6 months after surgery arriving to values closer to those of the control group at month 12. A significant difference in the amount of 8-oxo-dG released by the urine was already observed 3 months after surgery (Fig. 2). The changes of 8-oxo-dG in serum and urine were significant correlated (Fig. 3).
The importance of DNA repair relies on the potential genetic instability and carcinogenic effects of 8-oxodG. Significant increases of 8-oxo-dG levels have been observed, as a common characteristic of tumour cells and body fluids of cancer patients [13], [25], [55], [56], [57], [58]. The relevance of oxidative stress-induced DNA damage as a mechanism in obesity associated carcinogenesis together with other molecular and metabolic interactions has been extensively studied and recently reviewed [8].
We have shown that the increase of lipids and DNA oxidation products and GSSG/GSH ratio and the decreased antioxidant activities in morbid obese patients return to almost normal values after bariatric surgery and that these changes are accompanied by a better clinical outcome of these patients.
As is all known and recently reported, impaired redox balance due to an increased systemic oxidative can cause the development of obesity-related complications [59], [60].
Systemic oxidative stress based on both the increase of oxidation byproducts and the decrease of antioxidants enzymes was recently reported by our group in patients with different cardiovascular risk factors including obesity and related diseases such as hypertension, hypercholesterolemia and the metabolic syndrome. In these subjects, the intervention with a Mediterranean-style diet resulted in the recovery of physiological oxidation status [19], [21] as well as in a significant reduction of cardiovascular events [61].
ROS has been shown to enhance the secretion of pro-inflammatory cytokines, adhesion molecules, and growth factors through redox-sensitive transcription factors, especially NF-κB and NADPH oxidase (NOX) pathways were NOX4 enzyme complex is, in turn, the major source for ROS production in adipocytes [62].
The degree of inflammation in obesity is evidenced by the increase in specific markers such as CRP. In obese patients loosing weight as a response to their effective treatment, the degree of inflammation is also reduced as indicated by the reduction of that marker [63], [64].
In present study we have gone a step forward from what it had been previously reported in morbid obese patients in terms of the number of analysed OS markers and the time of follow up of their changes after bariatric surgery with LSG technique. The good correlations obtained between the evolution of urinary 8-oxo-dG and other OS markers along the study backs up its utility as a marker to represent the systemic OS state (Fig. 3). We observed actual changes in OS by-products and antioxidant molecules when weight loss began to occur, suggesting that they could be used to evaluate in which in-depth there is a metabolic improvement. Indeed, the changes observed in the oxidative stress byproducts in the patients group parallel their ponderal variables as well as their metabolic profile. Of the oxidative stress markers analysed, the levels of 8-oxo-dG in urine reach levels close to the controls at one year after surgical treatment while its changes show a statistical correlation with the serum concentrations of TGs, glucose, insulin and the HOMA index. Urinary 8-oxo-dG also significantly correlates (p<0.01) with patients weight, BMI, and the percentages of EWL and EBMIL (Table 5 and Fig. 5). A similar conclusion could be applied for F2-IsPs based on the significant correlations shown with the rest of oxidative stress byproducts and clinical metabolites. However, data from Table 6 and the differences calculated in terms of specificity supports the effectiveness of 8-oxo-dG in the urine as a useful marker of morbid obesity and the clinical status of affected patients. These results, together with the fact that it represents a bloodless analysis and relatively easy to perform, suggests it usefulness as a clinical marker for morbid obesity patients.
We therefore propose that following the changes of OS status in morbid obese patients after bariatric surgery may add further information on their evolution and clinical outcome. Moreover, the amount of 8-oxo-dG released by the urine, could be validated as a useful marker of their metabolic state.
5. Conclusions
We have characterized the systemic oxidative stress in morbid obese patients by analysing the antioxidant enzyme activities and the degree of lipid peroxidation and DNA damage. As compared with normal weight subjects, an important increase of the oxidative stress status has been observed in the patient group. After bariatric surgery using LSG, the progressive loss of weight is accompanied by the recovery of their comorbidities and metabolic profile which parallel the reduction of their systemic oxidative stress. Based on the obtained results and the statistical correlation studies, we propose the assay of urinary 8-oxo-dG as a valid test for the evaluation of oxidative stress and the clinical monitoring of morbid obese patients.
Conflict of interest
The authors declare that there is no conflict of interest that could be prejudice for the impartiality of this work.
Acknowledgements
Supported by the PI13/01848, PI14/00874, 4/00874, PIR14/00031 projects, funded by the ISCIII and European Regional Development Fund (ERDF) and by research project ACOMPT/2013/039 from Generalitat Valenciana. CIBERDEM is an initiative of FIS and ISCIII founded by ERDF.
Contributor Information
Lidia Monzo-Beltran, Email: Lidia.monzo@gmail.com.
Antonio Vazquez-Tarragón, Email: Tonivaz79@hotmail.com.
Concha Cerdà, Email: Concermi@gmail.com.
Paula Garcia-Perez, Email: Paulagperez88@gmail.com.
Antonio Iradi, Email: Antonio.Iradi@uv.es.
Carlos Sánchez, Email: Carlos.sanchez@uv.es.
Benjamin Climent, Email: Climent_ben@gva.es.
Carmen Tormos, Email: M.Carmen.Tormos@uv.es.
Antonio Vázquez-Prado, Email: Vprado.a@gmail.com.
Javier Girbés, Email: Jagirllo88@gmail.com.
Nuria Estáñ, Email: Estany_nur@gva.es.
Sebastián Blesa, Email: Sebastian.blesa@uv.es.
Raquel Cortés, Email: Raquel.Cortes@uv.es.
Felipe J. Chaves, Email: Felipe.Chaves@uv.es.
Guillermo T. Sáez, Email: Guillermo.saez@uv.es.
References
- 1.Sikaris K. The clinical biochemistry of obesity. Clin. Biochem. Rev. 2004;25:165–181. [PMC free article] [PubMed] [Google Scholar]
- 2.WHO: World Health Organization, Obesity and Overweight, Fact Sheet Nº 311 [updated June 2016], 2013. Available at: 〈http://www.who.int/mediacentre/factsheets/fs311/en/〉.
- 3.Gargallo Fernández Manuel M., Breton Lesmes I., Basulto Marset J., Quiles Izquierdo J., Formiguera Sala X., Salas-Salvadó J. Evidence-based nutritional recommendations for the prevention and treatment of overweight and obesity in adults. The role of diet in obesity treatment (III/III) Nutr. Hosp. 2012;27(3):833–864. doi: 10.3305/nh.2012.27.3.5680. [DOI] [PubMed] [Google Scholar]
- 4.Freedman D.S., Khan L.K., Dietz W.H., Srinivasan S.R., Berenson G.S. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics. 2001;108(3):712–718. doi: 10.1542/peds.108.3.712. [DOI] [PubMed] [Google Scholar]
- 5.Alberti K.G., Zimmet P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Picot J., Jones J., Colquitt J.L., Gospodarevskaya E., Loveman E., Baxter L. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol. Assess. 2009;13(41):1–190. doi: 10.3310/hta13410. [DOI] [PubMed] [Google Scholar]
- 7.Colquitt J.L., Pickett K., Loveman E., Frampton G.K. Surgery for weight loss in adults. Cochrane Database Syst. Rev. 2014;(8):CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerdá C., Sánchez C., Climent B., Vázquez A., Iradi A., El Amrani F. Oxidative stress and DNA damage in obesity-related tumorigenesis. Adv. Exp. Med. Biol. 2014;824:5–17. doi: 10.1007/978-3-319-07320-0_2. [DOI] [PubMed] [Google Scholar]
- 9.Albuali W.H. Evaluation of oxidant-antioxidant status in overweight and morbidly obese Saudi children. World J. Clin. Pediatr. 2014;3(1):6–13. doi: 10.5409/wjcp.v3.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marseglia L., Manti S., D'Angelo G., Nicotera A., Parisi E., Di Rosa G. Oxidative stress in obesity: a critical component in human diseases. Int. J. Mol. Sci. 2014;16(1):378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sies H., Cadenas E. Oxidative stress: damage to intact cells and organs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985;311(1152):617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- 12.Zitka O., Skalickova S., Gumulec J., Masarik M., Adam V., Hubalek J. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012;4(6):1247–1253. doi: 10.3892/ol.2012.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliva M.R., Ripoll F., Muñiz P., Iradi A., Trullenque R., Valls V. Genetic alterations and oxidative metabolisms in sporadic colorectal tumors from a Spanish community. Mol. Carcinog. 1997;18(4):232–243. [PubMed] [Google Scholar]
- 14.Voulgaridou G.P., Anestopoulos I., Franco R., Panayiotidis M.I., Pappa A. DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat. Res. 2011;711(1–2):13–27. doi: 10.1016/j.mrfmmm.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Kuchino Y., Mori F., Kasai H., Inoue H., Iwai S., Miura K. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987;327:77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- 16.Shibutani S., Takeshita M., Grollman A.P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxo-dG. Nature. 1991;349:431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- 17.Redón J., Oliva M.R., Tormos C., Giner V., Chaves J., Iradi A. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension. 2003;41(5):1096–1101. doi: 10.1161/01.HYP.0000068370.21009.38. [DOI] [PubMed] [Google Scholar]
- 18.Espinosa O., Jiménez-Almazán J., Chaves F.J., Tormos M.C., Clapes S., Iradi A. Urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxo-dG), a reliable oxidative stress marker in hypertension. Free Radic. Res. 2007;41(5):546–554. doi: 10.1080/10715760601164050. [DOI] [PubMed] [Google Scholar]
- 19.Fandos M., Corella D., Guillén M., Portolés O., Carrasco P., Iradi A. Impact of cardiovascular risk factors on oxidative stress and DNA damage in a high risk Mediterranean population. Free Radic. Res. 2009;43(12):1179–1186. doi: 10.3109/10715760903247231. [DOI] [PubMed] [Google Scholar]
- 20.Li Y.S., Song M.F., Kasai H., Kawai K. 8-hydroxyguanine in urine and serum as an oxidative stress marker: effects of diabetes and aging. J. UOEH. 2013;35(2):119–127. doi: 10.7888/juoeh.35.119. [DOI] [PubMed] [Google Scholar]
- 21.Mitjavila M.T., Fandos M., Salas-Salvadó J., Covas M.I., Borrego S., Estruch R. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clin. Nutr. 2013;32(2):172–178. doi: 10.1016/j.clnu.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Donmez-Altuntas H., Sahin F., Bayram F., Bitgen N., Mert M., Guclu K. Evaluation of chromosomal damage, cytostasis, cytotoxicity, oxidative DNA damage and their association with body-mass index in obese subjects. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014;771:30–36. doi: 10.1016/j.mrgentox.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Floyd R.A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990;11:1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- 24.Cooke M.S., Evans M.D., Dizdaroglu M., Lunec J. Oxidative DNA damage: mechanisms, mutation and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez M., Torres J.V., Tormos C., Iradi A., Muñiz P., Espinosa O. Impairment of antioxidant enzyme, lipid peroxidation and 8-oxo-2’-deoxyguanosine in advanced epithelial ovarian carcinoma from a Spanish community. Cancer Lett. 2006;233:28–35. doi: 10.1016/j.canlet.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 26.Borrego S., Vazquez A., Dasí F., Cerdá C., Iradi A., Tormos C. Oxidative Stress and DNA Damage in Human Gastric Carcinoma: 8-Oxo-7′8-dihydro-2′- deoxyguanosine (8-oxo-dG) as a possible tumor marker. Int. J. Mol. Sci. 2013;14(2):3467–3486. doi: 10.3390/ijms14023467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO . World Health Organization; Geneva: 2012. World Health Statistics. [Google Scholar]
- 28.Boza C., Gamboa C., Salinas J., Achurra P., Vega A., Pérez G. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: a case-control study and 3 years of follow-up. Surg. Obes. Relat. Dis. 2012;8(3):243–249. doi: 10.1016/j.soard.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 29.Noria S.F., Grantcharov T. Biological effects of bariatric surgery on obesity-related comorbidities. Can. J. Surg. 2013;56(1):47–57. doi: 10.1503/cjs.036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park Y.H., Kim S.M. Laparoscopic sleeve gastrectomy as revisional surgery for adjustable gastric band erosion. J. Laparoendosc. Adv. Surg. Tech. A. 2014;24(9):593–600. doi: 10.1089/lap.2013.0584. [DOI] [PubMed] [Google Scholar]
- 31.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Pedro T., Martinez-Hervas S., Tormo C., Garcia-Garcia A.B., Saez-Tormo G., Ascaso J.F. Oxidative stress andantioxidant enzyme values in lymphomonocytes after an oral unsaturatedfat load test in familial hypercholesterolemic subjects. Transl. Res. 2013;161(1):50–56. doi: 10.1016/j.trsl.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Lowry O.H., Rosenbrough N.J., Farr A.L., Randal R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 34.Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. 4th ed. Oxford University Press; Oxford, UK: 2007. [Google Scholar]
- 35.Deng Y., Scherer P.E. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y Acad. Sci. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández-Sánchez A., Madrigal-Santillán E., Bautista M., Esquivel-Soto J., Morales González A., Esquivel-Chirino C. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011;12(5):3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen N.T., Nguyen B., Gebhart A., Hohmann S. Changes in the makeup of bariatric surgery: a national increase in use of laparoscopic sleeve gastrectomy. J. Am. Coll. Surg. 2013;216(2):252–257. doi: 10.1016/j.jamcollsurg.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Csendes A., Braghetto I., León P., Burgos A.M. Management of leaks afterlaparoscopic sleeve gastrectomy in patients with obesity. J. Gastrointest. Surg. 2010;14:1343–1348. doi: 10.1007/s11605-010-1249-0. [DOI] [PubMed] [Google Scholar]
- 39.Roa P.E., Kaidar-Person O., Pinto D., Cho M., Szomstein S., Rosenthal R.J. Laparoscopic sleeve gastrectomy as treatment for morbid obesity: technique and short-term outcome. Obes. Surg. 2006;16(10):1323–1326. doi: 10.1381/096089206778663869. [DOI] [PubMed] [Google Scholar]
- 40.Ashrafian H., le Roux C.W., Darzi A., Athanasiou T. Effects of bariatric surgery on cardiovascular function. Circulation. 2008;118(20):2091–2102. doi: 10.1161/CIRCULATIONAHA.107.721027. [DOI] [PubMed] [Google Scholar]
- 41.Kocael A., Erman H., Zengin K., Kocael P.C., Korkmaz G.G., Gelisgen R. The effects on oxidative DNA damage of laparoscopic gastric band applications in morbidly obese patients. Can. J. Surg. 2014;57(3):183–187. doi: 10.1503/cjs.008113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van de Laar A., de Caluwé L., Dillemans B. Relative outcome measures for bariatric surgery. Evidence against excess weight loss and excess body mass index loss from a series of laparoscopic Roux-en-Y gastric bypass patients. Obes. Surg. 2011;21(6):763–767. doi: 10.1007/s11695-010-0347-0. [DOI] [PubMed] [Google Scholar]
- 43.Oria H.E., Moorehead M.K. Bariatric analysis and reporting outcome system (BAROS) Obes. Surg. 1998;8(5):487–499. doi: 10.1381/096089298765554043. [DOI] [PubMed] [Google Scholar]
- 44.Blankenberg S., Rupprecht H.J., Bickel C., Torzewski M., Hafner G., Tiret L. AtheroGene investigators. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N. Engl. J. Med. 2003;349(17):1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 45.Sen C.K. Cellular thiols and redox-regulated signal transduction. Curr. Top. Cell Regul. 2000;36:1–30. doi: 10.1016/s0070-2137(01)80001-7. [DOI] [PubMed] [Google Scholar]
- 46.Harris C., Hansen J.M. Oxidative stress, thiols, and redox profiles. Methods Mol. Biol. 2012;889:325–346. doi: 10.1007/978-1-61779-867-2_21. [DOI] [PubMed] [Google Scholar]
- 47.Sáez G.T., Valls V., Muñiz P., Perez-Broseta C., Iradi A., Oliva M.R. The role of glutathione in protection against DNA damage induced by rifamycin SV and copper (II) ions. Free Radic. Res. Commun. 1993;19(2):81–92. doi: 10.3109/10715769309056502. [DOI] [PubMed] [Google Scholar]
- 48.Muñiz P., Valls V., Perez-Broseta C., Iradi A., Climent J.V., Oliva M.R. The role of 8-hydroxy-2′-deoxyguanosine in rifamycin-induced DNA damage. Free Radic. Biol. Med. 1995;18(4):747–755. doi: 10.1016/0891-5849(94)00200-4. [DOI] [PubMed] [Google Scholar]
- 49.Vehapoglu A., Turkmen S., Goknar N., Özer Ö.F. Reduced antioxidant capacity and increased subclinical inflammation markers in prepubescent obese children and their relationship with nutritional markers and metabolic parameters. Redox Rep. 2016;21(6):271–280. doi: 10.1080/13510002.2015.1133035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uzun H., Konukoglu D., Gelisgen R., Zengin K., Taskin M. Plasma protein carbonyl and thiol stress before and after laparoscopic gastric banding in morbidly obese patients. Obes. Surg. 2007;17(10):1367–1373. doi: 10.1007/s11695-007-9242-8. [DOI] [PubMed] [Google Scholar]
- 51.Ostrow Vlady, Wu Shufang, Aguilar Alexandra, Bonner Jr Robert, Suarez Elizabeth, De Luca Francesco. Association between oxidative stress and masked hypertension in a multi-ethnic population of obese children and adolescents. J. Pediatr. 2011;158(4):628–633. doi: 10.1016/j.jpeds.2010.09.081. [DOI] [PubMed] [Google Scholar]
- 52.Cooke M.S., Henderson P.T., Evans M.D. Sources of extracellular, oxidatively-modified DNA lesions: implications for their measurements in urine. J. Clin. Biochem. Nutr. 2009;45:255–270. doi: 10.3164/jcbn.SR09-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J., Braganza A., Sobol R.W. Base excision repair facilitates a functional relationship between guanine oxidation and histone demethylation. Antioxid. Redox Signal. 2013;18(18):2429–2443. doi: 10.1089/ars.2012.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barregard L., Møller P., Henriksen T., Mistry V., Koppen G., Rossner P., Jr. Human and methodological sources of variability in the measurement of urinary8-oxo-7,8-dihydro-2′-deoxyguanosine. Antioxid. Redox Signal. 2013;18(18):2377–2391. doi: 10.1089/ars.2012.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oliva M.R., Iradi A., Garrido F., Ramos M., Oltra A.M., Muñiz P. Oxidative stress induces the expression of the major histocompatibility complex in murine tumor cells. Free Radic. Res. 2001;35(2):119–128. doi: 10.1080/10715760100300661. [DOI] [PubMed] [Google Scholar]
- 56.Oltra A.M., Carbonell F., Tormos C., Iradi A., Sáez G.T. Antioxidant enzyme activities and the production of MDA and 8-oxo-dG in chronic lymphocytic leukemia. Free Radic. Biol. Med. 2001;30(11):1286–1292. doi: 10.1016/s0891-5849(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 57.Collado R., Ivars D., Oliver I., Tormos C., Egea M., Miguel A. Increased oxidative damage associated with unfavorable cytogenetic subgroups in chronic lymphocytic leukemia. Biomed. Res. Int. 2014;2014(686392) doi: 10.1155/2014/686392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cha H.J., Yim H. The accumulation of DNA repair defects is the molecular origin of carcinogenesis. Tumor Biol. 2013;34:3293. doi: 10.1007/s13277-013-1038-y. [DOI] [PubMed] [Google Scholar]
- 59.Kilic E., Özer Ö.F., Erek Toprak A., Erman H., Torun E., Kesgin Ayhan S., Caglar H.G., Selek S., Kocyigit A. Oxidative stress status in childhood obesity:obesity: a potential risk predictor. Med. Sci. Monit. 2016 13;22:3673–3679. doi: 10.12659/MSM.897965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Correia-Costa L., Sousa T., Morato M., Cosme D., Afonso J., Areias J.C., Schaefer F., Guerra A., Afonso A.C., Azevedo A., Albino-Teixeira A. Oxidative stress and nitric oxide are increased in obese children and correlate with cardiometabolic risk and renal function. Br. J. Nutr. 2016;116(5):805–815. doi: 10.1017/S0007114516002804. [DOI] [PubMed] [Google Scholar]
- 61.Estruch R., Ros E., Salas-Salvadó J., Covas M.I., Corella D., Arós F. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 62.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 63.Shargorodsky M.1, Fleed A., Boaz M., Gavish D., Zimlichman R. The effect of a rapid weight loss induced by laparoscopic adjustable gastric banding on arterial stiffness, metabolic and inflammatory parameters in patients with morbid obesity. Int. J. Obes. 2006;30(11):1632–1638. doi: 10.1038/sj.ijo.0803320. [DOI] [PubMed] [Google Scholar]
- 64.Vehapoglu A., Turkmen S., Goknar N., Özer Ö.F. Reduced antioxidant capacity and increased subclinical inflammation markers in prepubescent obese children and their relationship with nutritional markers and metabolic parameters. Redox Rep. 2016;21(6):271–280. doi: 10.1080/13510002.2015.1133035. [DOI] [PMC free article] [PubMed] [Google Scholar]