Abstract
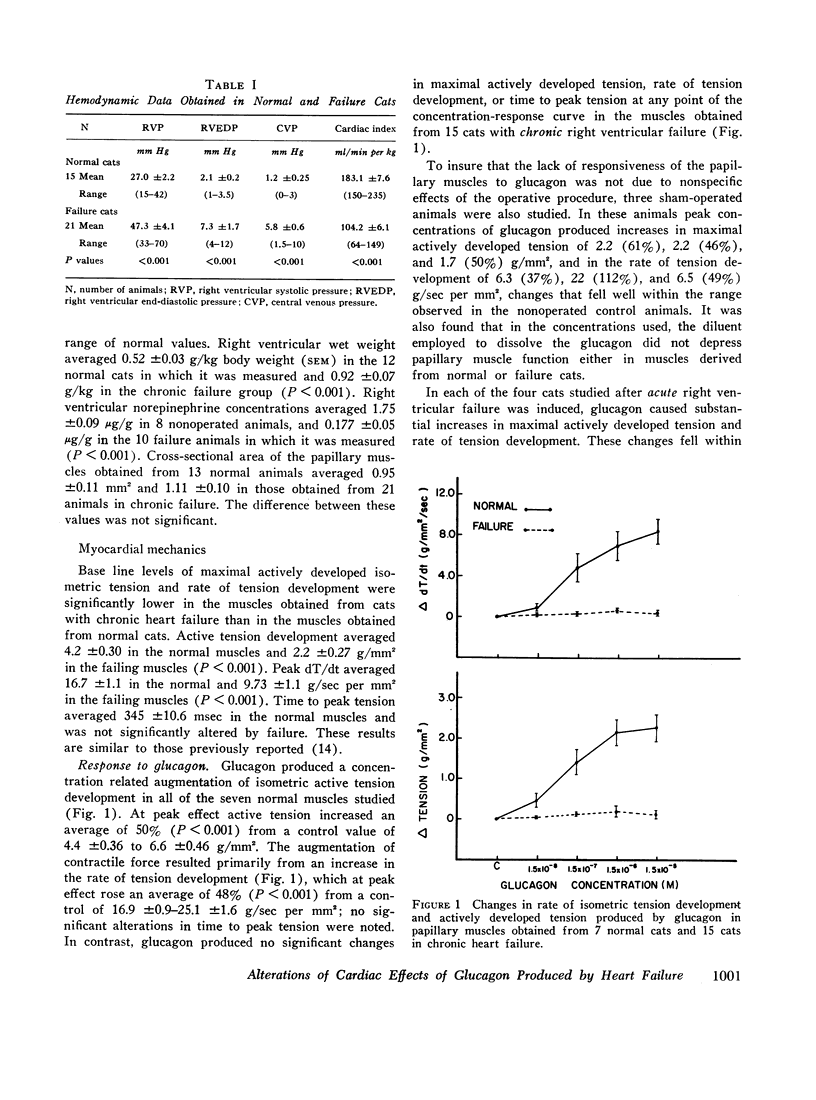
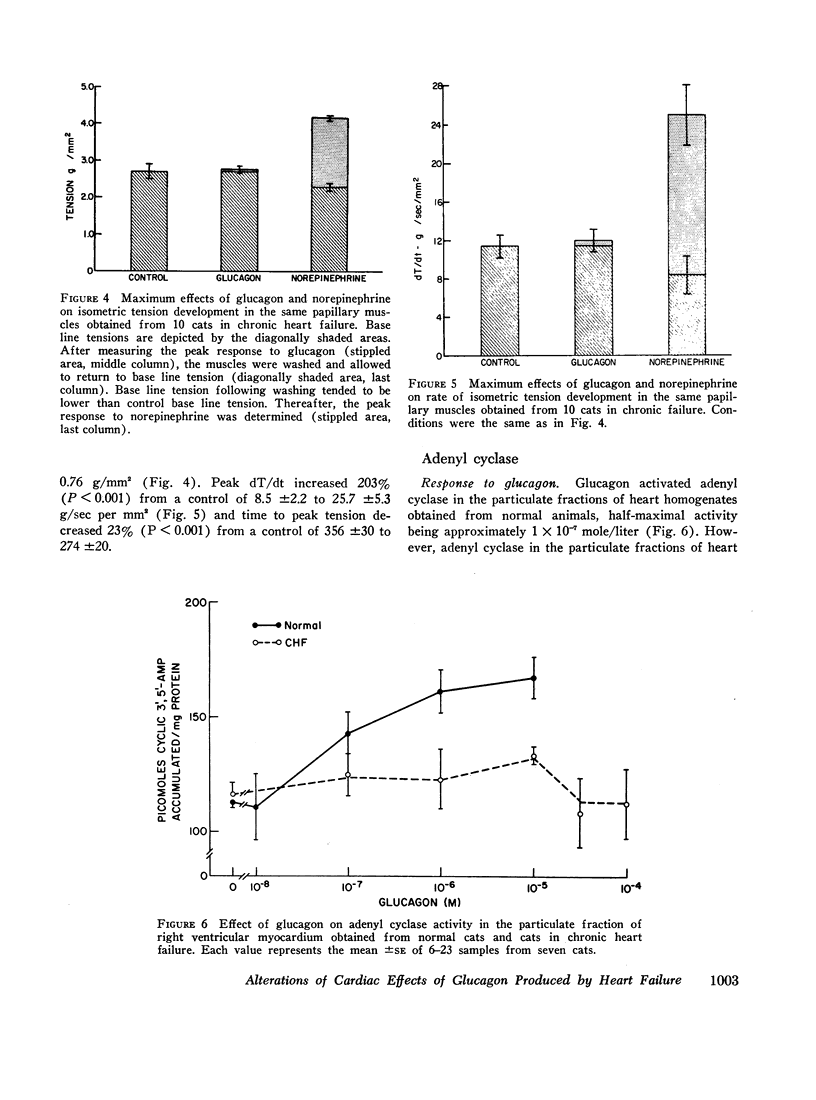
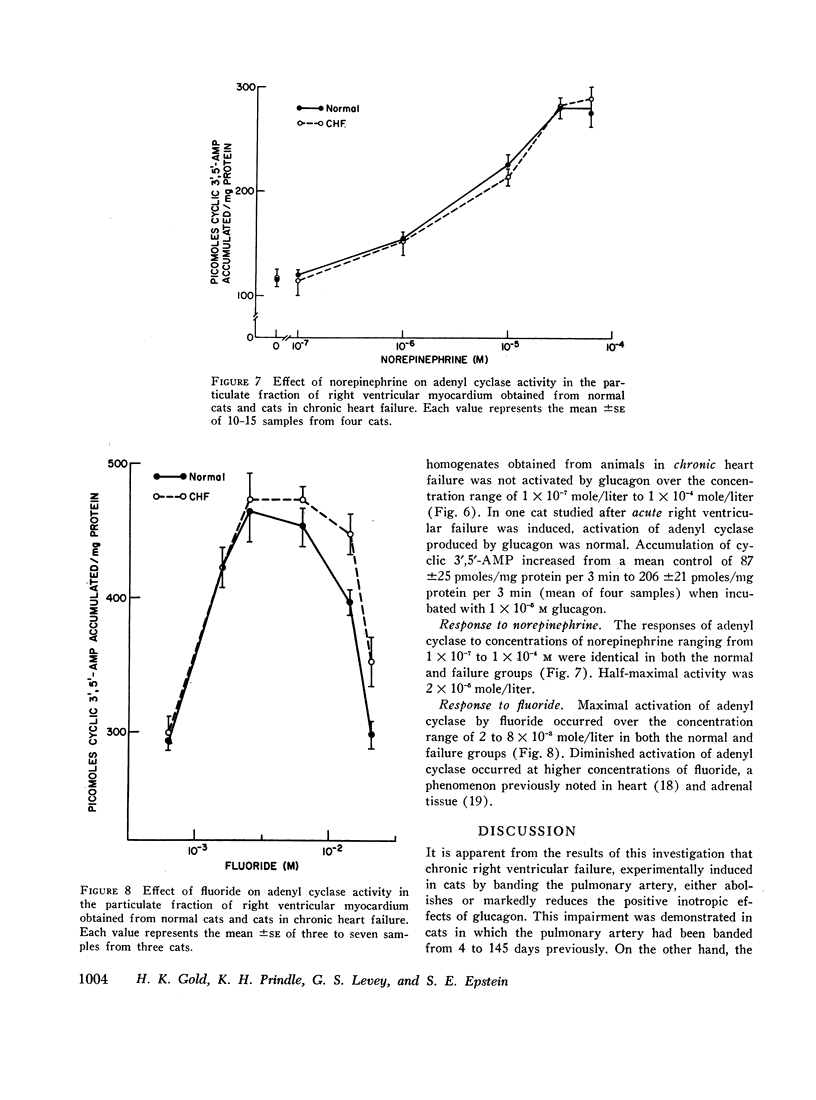
Although glucagon exerts positive inotropic effects in patients with no or mild impairment of cardiac function, similar effects are not consistently observed in patients with chronic heart failure. Accordingly, the inotropic effects of glucagon on papillary muscles from normal cats and cats in which right ventricular failure had been produced for 4-145 days by pulmonary artery banding were compared. At the peak of the concentration-response curve, glucagon increased peak isometric tension (T) in normal muscles from 4.4±0.4 to 6.6±0.5 g/mm2 (P <0.001), and maximum rate of tension development (dT/dt) from 16.9±0.9 to 25.1±1.6 g/sec per mm2 (P < 0.001). In contrast, glucagon produced no significant increases in T or dT/dt in failure muscles. The percentage increases in T and dT/dt caused by norepinephrine were the same in muscles from normal and failing hearts. Since the cardiac effects of glucagon and norepinephrine may be mediated by adenyl cyclase, responsiveness of adenyl cyclase was determined in particulate fractions of the right ventricle. Glucagon activated adenyl cyclase in normal, but had no effect in failure preparations. Norepinephrine-induced activation of adenyl cyclase, however, was unaltered by failure. Thus, in contrast to norepinephrine, glucagon loses the capacity to augment myocardial contractility and activate adenyl cyclase in hearts derived from cats in chronic failure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Entman M. L., Levey G. S., Epstein S. E. Demonstration of adenyl cyclase activity in canine cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun. 1969 Jun 6;35(5):728–733. doi: 10.1016/0006-291x(69)90466-5. [DOI] [PubMed] [Google Scholar]
- FARAH A., TUTTLE R. Studies on the pharmacology of glucagon. J Pharmacol Exp Ther. 1960 May;129:49–55. [PubMed] [Google Scholar]
- Glick G., Parmley W. W., Wechsler A. S., Sonnenblick E. H. Glucagon. Its enhancement of cardiac performance in the cat and dog and persistence of its inotropic action despite beta-receptor blockade with propranolol. Circ Res. 1968 Jun;22(6):789–799. doi: 10.1161/01.res.22.6.789. [DOI] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levey G. S., Epstein S. E. Activation of adenyl cyclase by glucagon in cat and human heart. Circ Res. 1969 Feb;24(2):151–156. doi: 10.1161/01.res.24.2.151. [DOI] [PubMed] [Google Scholar]
- Lucchesi B. R. Cardiac actions of glucagon. Circ Res. 1968 Jun;22(6):777–787. doi: 10.1161/01.res.22.6.777. [DOI] [PubMed] [Google Scholar]
- Parmley W. W., Glick G., Sonnenblick E. H. Cardiovascular effects of glucagon in man. N Engl J Med. 1968 Jul 4;279(1):12–17. doi: 10.1056/NEJM196807042790103. [DOI] [PubMed] [Google Scholar]
- REGAN T. J., LEHAN P. H., HENNEMAN D. H., BEHAR A., HELLEMS H. K. MYOCARDIAL METABOLIC AND CONTRACTILE RESPONSE TO GLUCAGON AND EPINEPHRINE. J Lab Clin Med. 1964 Apr;63:638–647. [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Skelton C. L., Levey G. S., Epstein S. E. Positive inotropic effects of dibutyryl cyclic adenosine 3',5'-monophosphate. Circ Res. 1970 Jan;26(1):35–43. doi: 10.1161/01.res.26.1.35. [DOI] [PubMed] [Google Scholar]
- Sobel B. E., Henry P. D., Robison A., Bloor C., Ross J., Jr Depressed adenyl cyclase activity in the failing guinea pig heart. Circ Res. 1969 Apr;24(4):507–512. doi: 10.1161/01.res.24.4.507. [DOI] [PubMed] [Google Scholar]
- Spann J. F., Jr, Buccino R. A., Sonnenblick E. H., Braunwald E. Contractile state of cardiac muscle obtained from cats with experimentally produced ventricular hypertrophy and heart failure. Circ Res. 1967 Sep;21(3):341–354. doi: 10.1161/01.res.21.3.341. [DOI] [PubMed] [Google Scholar]
- Spann J. F., Jr, Chidsey C. A., Pool P. E., Braunwald E. Mechanism of norepinephrine depletion in experimental heart failure produced by aortic constriction in the guinea pig. Circ Res. 1965 Oct;17(4):312–321. doi: 10.1161/01.res.17.4.312. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacol Rev. 1966 Mar;18(1):145–161. [PubMed] [Google Scholar]
- Taunton O. D., Roth J., Pastan I. Studies on the adrenocorticotropic hormone-activated adenyl cyclase of a functional adrenal tumor. J Biol Chem. 1969 Jan 25;244(2):247–253. [PubMed] [Google Scholar]
- Williams J. F., Jr, Childress R. H., Chip J. N., Border J. F. Hemodynamic effects of glucagon in patients with heart disease. Circulation. 1969 Jan;39(1):38–47. doi: 10.1161/01.cir.39.1.38. [DOI] [PubMed] [Google Scholar]