Abstract
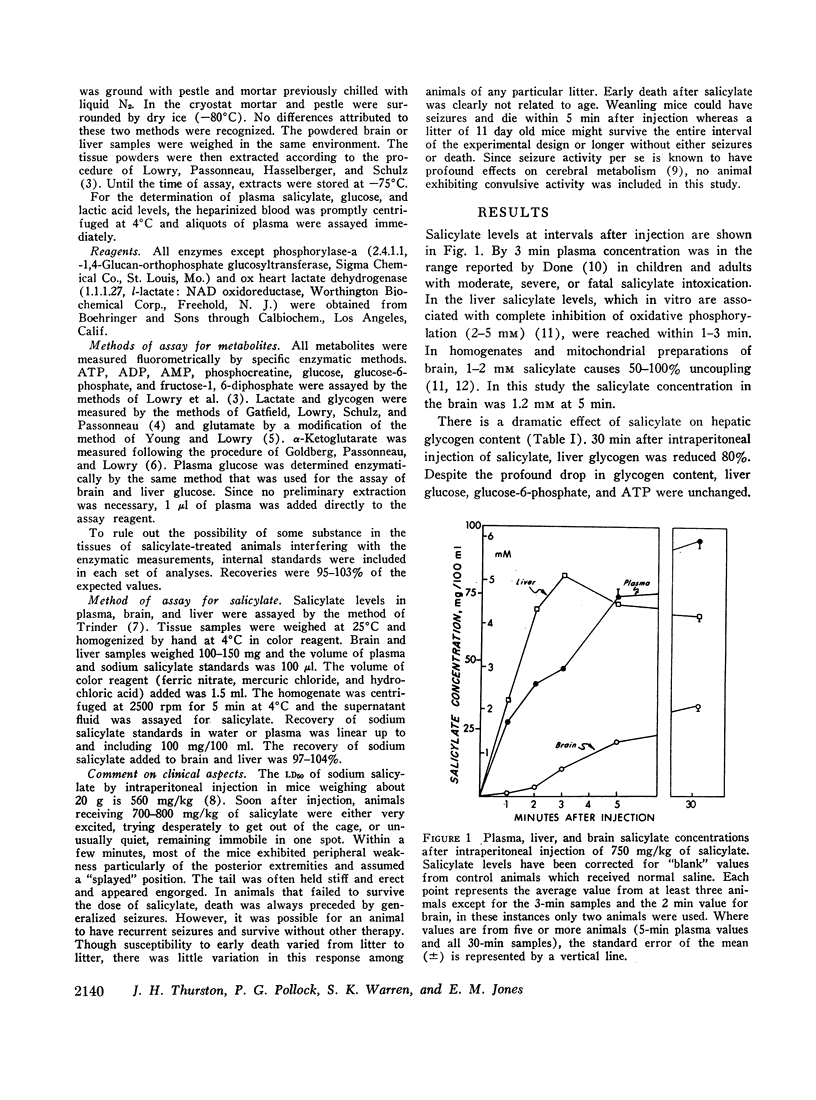
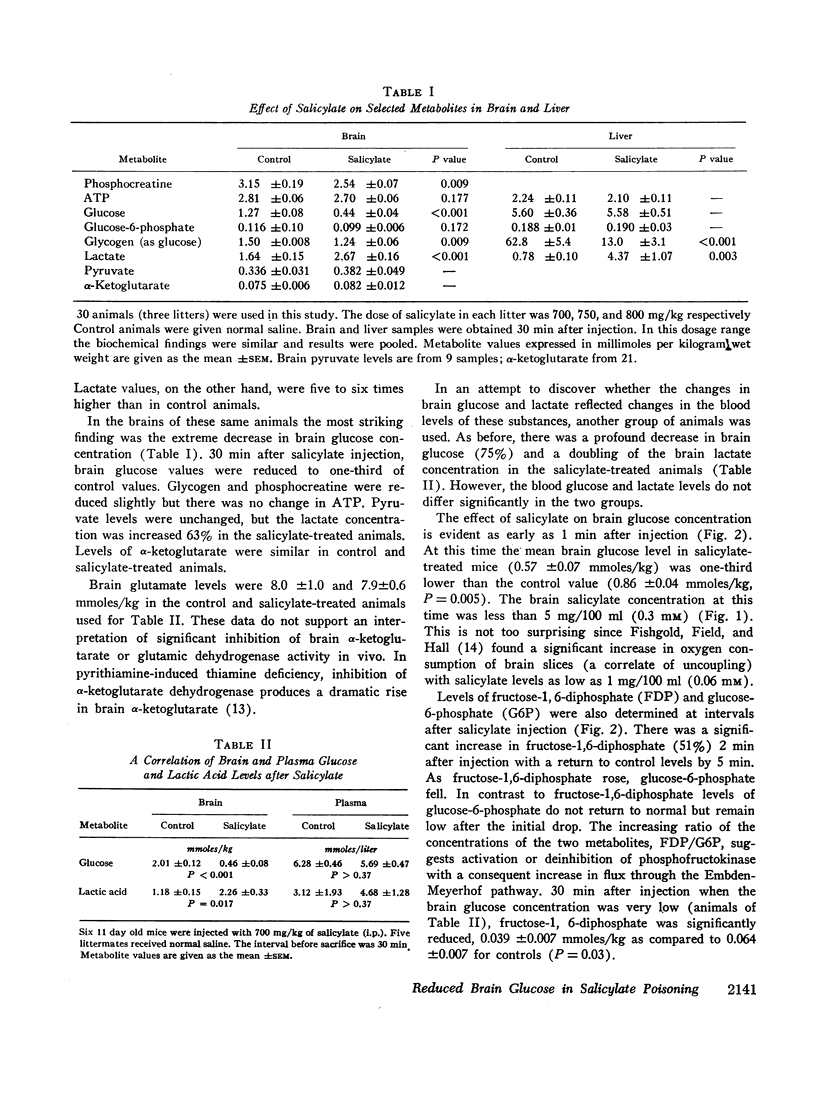
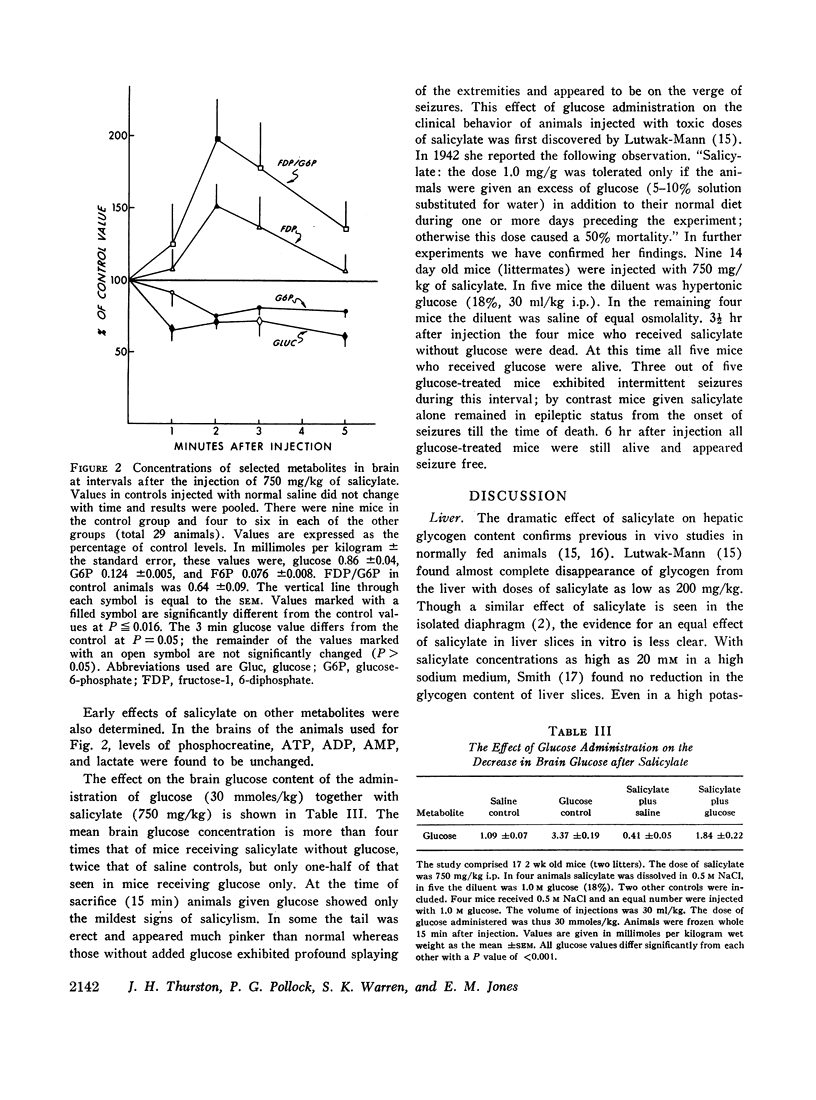
After the intraperitoneal injection into young mice of 700-800 mg/kg of salicylate, brain glucose fell to one-third or less of control values despite normal plasma glucose levels; brain lactate was nearly doubled and there were small decreases in phosphocreatine (18%) and in glycogen (17%). ATP, pyruvate, α-ketoglutarate, and glutamate were unchanged. In liver, glycogen was reduced 79% and lactate was five times higher than in control animals; glucose, glucose-6-phosphate, and ATP were unchanged.
Since salicylate uncouples oxidative phosphorylation, it is postulated that high energy phosphate in the brain is maintained near normal levels by a compensatory increase in cerebral glycolysis. Apparently the brain glucose level falls because the rate of utilization exceeds the rate at which glucose can be supplied from the blood. Concurrent administration of glucose with salicylate elevated brain glucose concentration and was associated with striking improvement in the condition and the increased survival of the animals. These findings stress the fact that in salicylate poisoning the supply of glucose to the brain may be inadequate even when blood glucose levels are normal.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S. C., Smith A. L. Cerebral blood flow and metabolism during acute salicylate intoxication in the goat. J Appl Physiol. 1969 Jun;26(6):745–751. doi: 10.1152/jappl.1969.26.6.745. [DOI] [PubMed] [Google Scholar]
- BRODY T. M. Action of sodium salicylate and related compounds on tissue metabolism in vitro. J Pharmacol Exp Ther. 1956 May;117(1):39–51. [PubMed] [Google Scholar]
- CHARNOCK J. S., OPIT L. J., HETZEL B. S. An evaluation of the effect of salicylate on oxidative phosphorylation in rat liver mitochondria. Biochem J. 1962 Jun;83:602–606. doi: 10.1042/bj0830602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONE A. K. Salicylate intoxication. Significance of measurements of salicylate in blood in cases of acute ingestion. Pediatrics. 1960 Nov;26:800–807. [PubMed] [Google Scholar]
- FEENEY G. C., CARLO P. E., SMITH P. K. Action of salicylates and related compounds on carbohydrate metabolism and on adrenal ascorbic acid and cholesterol concentrations. J Pharmacol Exp Ther. 1955 Jul;114(3):299–305. [PubMed] [Google Scholar]
- FISHGOLD J. T., FIELD J., HALL V. E. Effect of sodium salicylate and acetylsalicylate on metabolism of rat brain and liver in vitro. Am J Physiol. 1951 Mar;164(3):727–733. doi: 10.1152/ajplegacy.1951.164.3.727. [DOI] [PubMed] [Google Scholar]
- Gatfield P. D., Lowry O. H., Schulz D. W., Passonneau J. V. Regional energy reserves in mouse brain and changes with ischaemia and anaesthesia. J Neurochem. 1966 Mar;13(3):185–195. doi: 10.1111/j.1471-4159.1966.tb07512.x. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Passonneau J. V., Lowry O. H. Effects of changes in brain metabolism on the levels of citric acid cycle intermediates. J Biol Chem. 1966 Sep 10;241(17):3997–4003. [PubMed] [Google Scholar]
- Holowach J., Kauffman F., Ikossi M. G., Thomas C., McDougal D. B., Jr The effects of a thiamine antagonist, pyrithiamine, on levels of selected metabolic intermediates and on activities of thiamine-dependent enzymes in brain and liver. J Neurochem. 1968 Jul;15(7):621–631. doi: 10.1111/j.1471-4159.1968.tb08961.x. [DOI] [PubMed] [Google Scholar]
- JEFFREY S. W., SMITH M. J. The effects of salicylate on creatine phosphate and adenosine triphosphate in the isolated rat diaphragm. Biochem J. 1956 Nov;64(3):589–592. doi: 10.1042/bj0640589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALNITSKY G., PENNIALL R., ROUTH J. I. The effects of salicylic acid and related compounds on in vitro rat brain respiration. Arch Biochem Biophys. 1956 Oct;64(2):390–400. doi: 10.1016/0003-9861(56)90283-1. [DOI] [PubMed] [Google Scholar]
- King L. J., Lowry O. H., Passonneau J. V., Venson V. Effects of convulsants on energy reserves in the cerebral cortex. J Neurochem. 1967 Jun;14(6):599–611. doi: 10.1111/j.1471-4159.1967.tb09563.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Lutwak-Mann C. The effect of salicylate and cinchophen on enzymes and metabolic processes. Biochem J. 1942 Dec;36(10-12):706–728. doi: 10.1042/bj0360706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENNIALL R. The effects of salicylic acid on the respiratory activity of mitochondria. Biochim Biophys Acta. 1958 Nov;30(2):247–251. doi: 10.1016/0006-3002(58)90047-7. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., SMITH G. H. Regulation of glucose uptake by muscle. 1. The effects of insulin, anaerobiosis and cell poisons on the uptake of glucose and release of potassium by isolated rat diaphragm. Biochem J. 1958 Nov;70(3):490–500. doi: 10.1042/bj0700490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH M. J. The effects of salicylate and adrenocortical preparations added in vitro upon the glycogen content of liver slices. Biochem J. 1955 Jan;59(1):52–55. doi: 10.1042/bj0590052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPROULL D. H. The glycogenolytic action of sodium salicylate. Br J Pharmacol Chemother. 1954 Mar;9(1):121–124. doi: 10.1111/j.1476-5381.1954.tb00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRINDER P. Rapid determination of salicylate in biological fluids. Biochem J. 1954 Jun;57(2):301–303. doi: 10.1042/bj0570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. L., Lowry O. H. Quantitative methods for measuring the histochemical distribution of alanine, glutamate and glutamine in brain. J Neurochem. 1966 Sep;13(9):785–793. doi: 10.1111/j.1471-4159.1966.tb05873.x. [DOI] [PubMed] [Google Scholar]


