The plant cytoskeleton is a highly dynamic and versatile intracellular scaffold composed of microtubules and actin microfilaments and plays an important role in many aspects of plant cell growth and development, including such fundamental processes as cell division, cell expansion, and intracellular organization and motility (Staiger, 2000; Wasteneys and Galway, 2003). During evolution, plants have developed mechanisms to exploit, survive, or minimize the negative impact of a diverse range of environmental factors, and in many cases the plant cytoskeleton is instrumental in mediating the plant's response. Cytoskeletal elements, for example, translocate chloroplasts under high light conditions (Takagi, 2000), facilitate gravity sensing (Blancaflor, 2002), and direct cellular response to wounding (Foissner et al., 1996; Hush and Overall, 1996). In addition to these abiotic factors, plants also encounter and must deal with a range of other organisms that may be potential partners or pathogens. Once again, the plant cytoskeleton plays a key role. In many ways, biotic factors in the environment present a greater challenge to the plant than do abiotic stresses because living organisms, like their plant hosts, are continually evolving. Potential pathogens develop new ways of avoiding or overcoming existing plant defenses; symbionts may attain aggressive traits or lose beneficial ones. Plants must thus constantly refine existing defenses and develop new strategies to maintain an upper hand in their interactions with other organisms. Changes in the organization of the plant cytoskeleton during plant interactions with microbial and other organisms are complex and varied, and much still remains to be elucidated, especially in terms of the molecules that signal and bring about the dramatic reorganizations that are often observed. This diversity and complexity is, no doubt, a product of many factors, including differences in signal exchanges between the interacting partners and the relative dominance of one or other organism. In many cases, the changes that are observed are likely to be the net result of instructions from both interacting organisms. In this article, we review current understanding of the role of the plant cytoskeleton in defense against invading fungal and oomycete pathogens and in establishing symbiotic relationships with mycorrhizal fungi and bacteria. We also review current information on the targeting of the plant cytoskeleton by viruses to enhance their movement and by signals from the female plant tissues as part of a mechanism of self-incompatibility.
PLANT CYTOSKELETAL RESPONSE TO PATHOGENIC FUNGI AND OOMYCETES
The Role of the Cytoskeleton in Cytoplasmic Aggregation
Cell wall appositions, or papillae, are important barriers formed by plants in defense against attempted penetration by fungal and oomycete pathogens (Aist, 1976). They develop below appressoria, adjacent to intercellular hyphae, and around penetration pegs and haustoria. Prior to the development of papillae, plant cytosol and subcellular components are rapidly translocated to the site of pathogen penetration (Fig. 1A). This cytoplasmic aggregation has been observed in many plant-microbe interactions (see Takemoto et al., 2003) and is a common resistance response to pathogens by both dicotyledonous and monocotyledonous plants to invading filamentous pathogens.
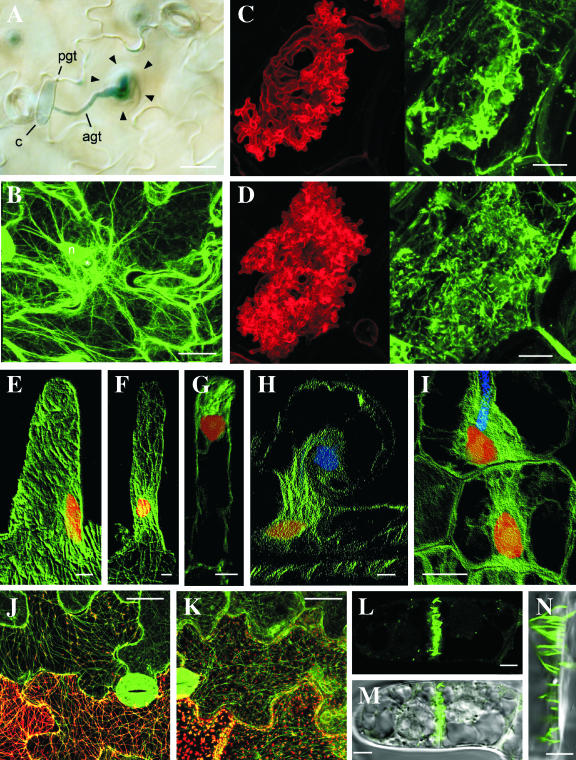
Figure 1.
A, Accumulation of cytoplasm in an Arabidopsis epidermal cell around the attempted penetration site of the nonpathogen, B. graminis f. sp. hordei. Bar = 20 μm. B, GFP-tagged actin microfilaments focusing on the penetration site of B. graminis f. sp. hordei in an Arabidopsis epidermal cell. The fine actin microfilament network beneath the penetration site is likely to be indicative of active exocytosis. The asterisk indicates the attempted penetration site of the nonpathogen. Bar = 20 μm. pgt, Primary germ tube; agt, appressorial germ tube; c, conidum; n, plant nucleus. C and D, Arbuscules of G. versiforme developing in cortical cells of M. truncatula labeled with wheatgerm agglutinin (left) and with anti-tubulin (right). During early development (C), a diffuse fluorescence of anti-tubulin occurs around the developing arbuscular branches. Later in development (D), a dense array of short microtubules lines the perifungal membrane around the arbuscules. Bars = 10 μm. (Reproduced with permission from Blancaflor et al., 2001). E to I, Anti-tubulin labeling of microtubules in root hairs of M. truncatula before (E) and after (F–H) inoculation with rhizobia and in cortical cells (I). The helical array of cortical microtubules in the uninoculated hair (E) is replaced by a dense array connecting the nucleus with the tip of the hair (F and G) or the tip of the infection thread (H). Before the infection thread penetrates the cortical cells, a bridge of cytoplasm, the preinfection thread, forms in line with the advancing infection thread (I). Microtubules, nuclei, and infection threads are shown in green, red, and blue, respectively. E to H, Bars = 5 μm; I, bar = 15 μm. (Reproduced with permission from Timmers et al., 1999). J and K, Localization of wild-type TMV MP (J) and TMV MPR3 (K). Wild-type MP and MPR3 are visualized by tagging with DsRed (red) in transgenic plants expressing GFP-labeled microtubules (green). Bars = 25 μm. (Reproduced with permission from Gillespie et al., 2002). L to N, Fluorescent tubules in a young cross wall of a BY-2 suspension cell formed after expression of GFLV MP tagged with GFP. Bars = 5 μm. (Reproduced with permission from Laporte et al., 2003).
Cytoplasmic aggregation is an example of site-directed cytoplasmic streaming and is dependent upon the action of the actin component of the cytoskeleton, as was shown in the early 1980s using inhibitors of actin polymerization (Tomiyama et al., 1982; Hazen and Bushnell, 1983). Complementing the pharmacological experiments, a variety of cytochemical labeling studies, including recent observations of living cells in which actin microfilaments were labeled with a green fluorescent protein (GFP)-tagged actin-binding protein, have revealed radial focusing of actin microfilaments on the pathogen penetration site in many plant-pathogen interactions (Figs. 1B and 2, A and B; Takemoto et al., 2003). Cytoplasmic aggregation is usually associated with plant resistance. In flax (Linum usitatissimum)-Melampsora and barley (Hordeum vulgare)-Erysiphe interactions, for example, focusing of actin microfilaments at the penetration site is more extensive in resistant plants compared with susceptible hosts (Kobayashi et al., 1992, 1994), suggesting that the accumulation of material at the infection site is related to the degree of host resistance. However, there are some cases in which the intensity of the actin microfilament response does not correlate with the degree of resistance to pathogens. There is no detectable difference in the reorganization of actin microfilaments between nonhost, incompatible, and compatible interactions in Arabidopsis (Arabidopsis thaliana) challenged with oomycete pathogens (Takemoto et al., 2003) and in cowpea infected by Uromyces, a greater number of actin cables accumulated at the infection site in susceptible plants than in resistant plants (Škalamera and Heath, 1998). Assessment of the contribution made by cytoplasmic aggregation to plant resistance may be complicated by an influence of the pathogen on host cell structure and metabolism. As well as redirection by the plant for defense purposes, redeployment of plant materials via cytoskeletal rearrangements could also be orchestrated by an invading pathogen as part of its strategy to obtain nutrients from the plant, as occurs during the development and function of haustoria of biotrophic pathogens. As discussed below, reorganization of plant cytoskeletal systems also takes place during symbiotic interactions and may be necessary for symbiont development and mutually beneficial nutrient exchange (Parniske, 2000).
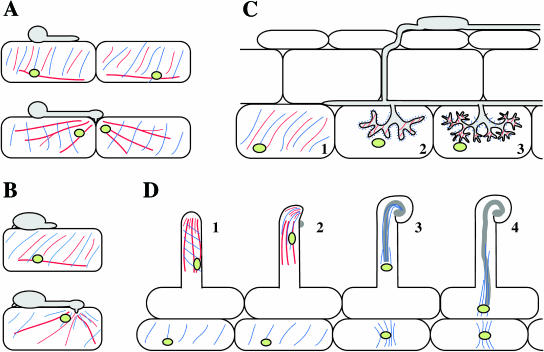
Figure 2.
Diagrammatic representation of the organization of the plant cytoskeleton during different plant-microbe interactions. A, Interaction between a plant and a filamentous pathogen, such as that between Arabidopsis and P. parasitica. Top, Microtubules (blue), actin microfilaments (red), and nuclei (green) before appressorium formation or attempted penetration. Bottom, Actin microfilaments become focused on the penetration site and the nucleus is moved close to the invading pathogen. B, Interaction between a plant and a filamentous pathogen, such as that between barley and Erysiphe. Top, Cytoskeleton and nucleus before appressorium formation. Bottom, Microtubules and actin microfilaments become focused below the infection site and the nucleus also moves to this site. C, Cytoskeletal rearrangements during colonization by an arbuscular endomycorrhizal fungus. Before colonization, microtubules and actin microfilaments are aligned in ordered cortical arrays in cortex cells (C, subsection 1). During arbuscule development (C, subsection 2), actin microfilaments and apparently unpolymerized tubulin occur next to the perifungal membrane around the arbuscule. As the arbuscule matures (C, subsection 3), dense arrays of short microtubules and actin microfilaments line the perifungal membrane. The plant cell nucleus becomes positioned adjacent to the arbuscule. D, Infection thread formation during interaction of rhizobia with legume roots. In uninoculated root hairs (D, subsection 1), microtubules are axial or helically aligned and actin microfilaments are axially aligned in the cortex and endoplasm. The nucleus is positioned about 30 to 40 μm from the tip of the hair. After inoculation (D, subsection 2), dense arrays of microtubules and actin microfilaments form in the hair apex; the nucleus is moved closer to the hair tip and the cytoskeleton becomes focused on the site of growth as the hair begins to curl. As the infection thread grows along the root hair (D, subsections 3 and 4), microtubules line the plasma membrane surrounding the infection thread. A bridge of cytoplasm containing a parallel array of microtubules forms in cortical cells in alignment with the advancing infection thread (D, subsection 4).
Although actin microfilaments are consistently observed to focus on the infection site, even in cases in which the plant fails to stop pathogen ingress, microtubule response to pathogen attack is much more variable in different plant-microbe interactions (Fig. 2, A and B). In barley-Erysiphe and flax-Melampsora interactions, radial arrays of microtubules form beneath the appressorium (Kobayashi et al., 1992, 1994), while in parsley (Petroselinum crispum)- or soybean (Glycine max)-Phytophthora sojae interactions, localized microtubule depolymerization has been observed (Gross et al., 1993; Cahill et al., 2002). In nonhost, incompatible, and compatible interactions of Arabidopsis with P. sojae or different races of Peronospora parasitica, there is neither focusing nor large-scale disappearance of microtubules at the infection site, although diffuse GFP-tubulin fluorescence and a circumferential alignment of microtubules across cell boundaries are observed around the penetration site (Takemoto et al., 2003). Both changes to the microtubular cytoskeleton in Arabidopsis could be due to selective depolymerization of microtubules that were oriented perpendicularly to the penetration site.
Such differences in microtubule behavior may be indicative of a less important role for microtubules in the plant defense response than for actin microfilaments. This conclusion is consistent with results of pharmacological studies with actin or microtubule polymerization/depolymerization inhibitors. It has been shown that in addition to effects on cytoplasmic aggregation and papilla formation (Kobayashi et al., 1997b; Škalamera et al., 1997), cytochalasins (inhibitors of actin polymerization) suppress or delay other resistance reactions such as hypersensitive cell death (Tomiyama et al., 1982; Hazen and Bushnell, 1983; Škalamera and Heath, 1998; Takemoto et al., 1999) and pathogenesis related protein expression (Takemoto et al., 1999). Cytochalasin treatment also permits nonpathogens to penetrate barley coleoptile cells and form secondary hyphae (Kobayashi et al., 1997b) and, in combination with the eds1 (enhanced disease susceptibility1) mutation in Arabidopsis, allows Blumeria graminis (syn. Erysiphe graminis) to complete its lifecycle on the normally nonhost plant (Yun et al., 2003). By contrast, treatment with microtubule inhibitors has only a minor impact on penetration susceptibility (Kobayashi et al., 1997b) and has no effect on callose deposition in papillae (Škalamera et al., 1997), although it delays onset of the hypersensitive response in the flax-Melampsora system (Kobayashi et al., 1997a). Together, these data suggest that microfilaments are the main cytoskeletal element responsible for the penetration resistance based on cytoplasmic aggregation and papilla formation at the infection site.
During cytoplasmic aggregation in response to pathogen attack in Arabidopsis, massive accumulation of endoplasmic reticulum (ER) and preferential residence of Golgi bodies and peroxisomes was observed around the penetration site, consistent with the production and localized secretion of plant material during wall apposition formation (Takemoto et al., 2003; D. Takemoto, unpublished data). It is known that actin microfilaments influence the spatial organization of the ER and are responsible for the trafficking of Golgi bodies and peroxisomes in plant cells (Lichtscheidl and Hepler, 1996; Nebenführ et al., 1999; Mano et al., 2002). In living epidermal cells of Arabidopsis in which ER or the Golgi apparatus was labeled with GFP-fusion proteins, Golgi bodies circulate within the cell but make frequent stops at the penetration site (Takemoto et al., 2003). This is in contrast to the stable accumulation of ER membrane at this location. These observations suggest that movement of ER and Golgi bodies is regulated slightly differently during cytoplasmic aggregation (Takemoto et al., 2003). Similar stop-and-go movement of Golgi bodies has been found in normal cytoplasmic streaming and has been interpreted as a mechanism for site-directed secretion (Nebenführ et al., 1999).
The importance of vesicle transport to and secretion at the infection site has recently been further illustrated by an Arabidopsis mutant, pen1, which has decreased resistance to penetration by the barley pathogen, B. graminis (Collins et al., 2003). The pen1 gene encodes a plasma membrane syntaxin, AtSYP121, which is likely to facilitate membrane fusion during vesicle exocytosis at the infection site, as elsewhere on the plasma membrane. Interestingly, the higher rate of successful penetration by the barley pathogen in the pen1 mutant results in an increased incidence of hypersensitive cell death, a form of defense more typically employed during race-specific resistance (Collins et al., 2003). These data suggest that inhibition of penetration through cytoplasmic aggregation and papilla formation is an early, if not the first, tactic in plant resistance and may be backed up by the hypersensitive response. Reduced papilla formation, as exemplified by less callose deposition around haustoria, in nim1/npr1 (noninducible immunity 1/nonexpressor of pathogenesis related genes 1) mutant of Arabidopsis also leads to enhanced disease susceptibility in already susceptible wild-type plants (Donofrio and Delaney, 2001). Thus, the physical and chemical barrier resulting from actin-dependent cytoplasmic aggregation, secretion, and papilla formation appears to constitute an important and probably ancient form of basal resistance to pathogen attack (Thordal-Christensen, 2003).
A full understanding of the cytoskeletal basis of cytoplasmic aggregation will require identification and characterization of proteins involved in the signaling pathways that induce cytoskeletal rearrangement and of proteins responsible for bringing about cytoskeletal reorganization and function. It is likely that this phenomenon utilizes regulatory proteins that participate in other forms of cytoplasmic streaming, as well as additional factors specific for the localized defense response. Proteins that generate intracellular motility, like myosin for example, are likely to be important components (Shimmen and Yokota, 2004). The activity of plant myosin is inhibited by Ca2+ ions at concentrations of 1 to 10 μm (Yokota et al., 1999), a concentration range comparable to that found in parsley cells after elicitor treatment (Blume et al., 2000). Myosin could thus be a target for stop signals that bring about the temporary residence of Golgi bodies at the penetration site, as described above. Similarly, the formation of thick microfilament bundles focusing on the infection site (Fig. 1B) could involve actin-bundling proteins, such as villin and fimbrin (Vidali et al., 1999; Kovar et al., 2000). In addition, control of localized secretion at the infection site could employ mechanisms similar to those operating during tip growth of plant cells. Vesicle exocytosis at the growing tip of root hairs or pollen tubes is activated by a Ca2+ gradient and involves Ca2+ channels, RAC/ROP G-proteins, and actin depolymerization factor (Hepler et al., 2001; Camacho and Malhó, 2003; Chen et al., 2003). Influx of Ca2+ is also a widespread and early response of plants to pathogen attack (Scheel, 1998) and could be the trigger for site-directed exocytosis of anti-microbial material during cytoplasmic aggregation. Consistent with this idea, a recent study shows that three barley RAC/ROP G-proteins are required for enhanced accessibility, i.e. increased penetration, of B. graminis f. sp. hordei on host barley plants (Schultheiss et al., 2003), although the function of barley RAC/ROP G-protein has not been determined. Plant RAC/ROP G-proteins have been shown to control various cellular processes that include H2O2 production and localized microfilament assembly (Yang, 2002). The expansion of knowledge of plant actin-binding and regulatory proteins in recent years (Wasteneys and Galway, 2003) will undoubtedly help elucidate the role of these proteins in remodeling the actin cytoskeleton during cytoplasmic aggregation and the plant defense response.
PLANT CYTOSKELETAL RESPONSE TO MYCORRHIZAL FUNGI
The establishment of symbiotic associations between plants and mycorrhizal fungi is of widespread occurrence and considerable importance for plant growth. There are two main categories of mycorrhizal symbiosis, ectomycorrhizal and endomycorrhizal associations. Ectomycorrhizal fungi do not penetrate the host cell wall but form a dense layer, called the mantle, on the surface of the root and a network of intercellular hyphae within the root tissues (the Hartig net). In endomycorrhizae, fungal cells penetrate the plant cell wall and elaborate specialized infection structures within the plant cell (as defined by the plant cell walls) although, like intracellular infection hyphae and haustoria of biotrophic fungal pathogens, they remain surrounded and separated from the host cytoplasm by an intact plant plasma membrane.
Increases in expression of tubulin genes and concentrations of plant α-, β-, and γ-tubulins and actin have been observed in a variety of ecto- and endomycorrhizas (Bonfante et al., 1996; Diaz et al., 1996; Niini et al., 1996; Timonen et al., 1996; Timonen and Peterson, 2002). Studies of cytoskeletal behavior in ectomycorrhizal associations are limited but suggest that microtubules and actin microfilaments may be largely unaffected by the proximity of intercellular hyphae except, perhaps, in heavily colonized regions where the density of both cytoskeletal arrays was substantially decreased (Timonen et al., 1993; Timonen and Peterson, 2002; Kuga-Uetake et al., 2004). Studies of endomycorrhizal associations involving arbuscular and orchid mycorrhizae, on the other hand, have revealed dramatic reorganization of the plant cytoskeleton during the development of the symbiotic relationship. In arbuscular mycorrhizas, the invading fungus forms coils and trunk hyphae that develop into progressively finer elements, culminating in a highly branched arbuscule inside the root cell. Orchid symbionts also form coiled hyphal masses, called pelotons, within the host cells. In both cases, after fungal invasion, plant microtubules and actin microfilaments disappear almost entirely from the plant cell cortex and assemble close to the surface of the trunk hyphae, arbuscules, or pelotons (Figs. 1, C and D, and 2C; Genre and Bonfante, 1997; Uetake and Peterson, 1997, 1998; Uetake et al., 1997; Genre and Bonfante, 1998; Matsubara et al., 1999; Blancaflor et al., 2001; Armstrong and Peterson, 2002). In Medicago truncatula, at an early stage of arbuscule development by Glomus versiforme, bright diffuse fluorescence is seen around the arbuscular branches following anti-tubulin labeling (Figs. 1C and 2C, subsection 2; Blancaflor et al., 2001). At later stages of development, short microtubules are closely associated with plasma membrane (perifungal membrane) surrounding the labyrinthine surface of the arbuscule (Figs. 1D and 2C, subsection 3). After arbuscule or peloton senescence, microtubules and actin microfilaments disappear from around the collapsed fungal structures and reappear in the plant cell cortex. In both cases, this cycle can be repeated if the plant cells become reinfected. γ-Tubulin has been showed to be associated with the nuclear envelope and perifungal membrane in tobacco (Nicotiana tabacum) arbuscular mycorrhizas (Genre and Bonfante, 1999). As γ-tubulin is often concentrated in microtubule organizing centers, the presence of γ-tubulin in the perifungal membrane may be indicative of the nucleation of microtubules at this site, rather than their movement from the cell cortex or nucleus.
The role of the perifungal arrays of microtubules and actin microfilaments is still unknown. However, some of the first clues as to the importance of the plant cytoskeleton in the establishment of endomycorrhizal associations have come from a recent study of a symbiosis-defective mutant of Lotus japonicus, Ljsym4-2 (Genre and Bonfante, 2002). In wild-type plants, the symbiotic fungus, Gigaspora margarita, invades and forms small coils in epidermal cells and then grows through to the inner cortex where arbuscules develop. In epidermal cells, cortical arrays of microtubules and actin microfilaments are maintained after hyphal penetration, although additional arrays of both elements surround the invading hyphae and the nucleus that moves to a position close to the invading hyphae. As in other endomycorrhizas, arbuscules in the inner cortex cells are surrounded by a dense network of microtubules and actin. By contrast, in the Ljsym4-2 mutant, the fungus never gets past the epidermis. Localized depolymerization of microtubules and actin microfilaments occurs at the penetration site before the arrays are progressively disassembled. The nucleus fails to move toward the invading hyphae and the epidermal cell dies. Throughout the interaction, cells adjacent to the colonized epidermal cells retain normal cytoskeletal arrays. The authors liken the response in the mutant to that of the hypersensitive response to pathogenic fungi in resistant plants.
THE ROLE OF THE PLANT CYTOSKELETON IN ESTABLISHMENT OF A SYMBIOTIC RELATIONSHIP WITH RHIZOBIA
Changes in cytoskeletal arrays in root hairs and cortical cells occur during the establishment of a symbiotic relationship with certain gram-negative bacteria collectively called rhizobia, and it is clear that microtubules and actin microfilaments play active and necessary roles in root hair curling, growth of the infection thread, and root nodule development.
Changes in the Plant Cytoskeleton in Root Hairs following Inoculation
Rhizobial attachment, or application of host-specific nodulation (Nod) factors (Esseling and Emons, 2004; Riely et al., 2004), causes a localized influx of calcium, depolarization of the plant plasma membrane, alkalinization of the cytoplasm, and curling (or deformation) of the hair (Felle et al., 1998; Cárdenas et al., 2000; Esseling et al., 2003; Shaw and Long, 2003). Before bacterial attachment or Nod factor application, microtubules and actin microfilaments within the root hair usually display an overall axial or helical alignment (Miller et al., 1997; Timmers et al., 1999; Geitmann and Emons, 2000; Sieberer et al., 2002; Ditengou et al., 2003; Weerasinghe et al., 2003) in both cortical and endoplasmic cytoplasm. Microtubules extend between the nucleus and the tip of the hair and are responsible for maintaining the nucleus in a subapical position about 30 to 40 μm behind the apex (Sieberer et al., 2002). Thick bundles of actin microfilaments in vacuolate regions of the cell merge into finer bundles in the subapical cytoplasm (Cárdenas et al., 1998; De Ruijter et al., 1999; Miller et al., 1999) and are responsible for cytoplasmic streaming and localized vesicle exocytosis at the tip of the hair (Staiger et al., 1994; Valster et al., 1997; Miller et al., 1999).
Within minutes of exposure to rhizobia or Nod factors, a localized depolymerization of both microtubules and actin microfilaments occurs at the tip of root hairs (Cárdenas et al., 1998; Cárdenas et al., 2003; Weerasinghe et al., 2003) although in both cases, cytoskeletal arrays similar to those in untreated cells reassemble within about an hour. Prior to root hair curling, the array of longitudinally oriented microtubules between the nucleus and the tip of the root hair increases in density, and the nucleus moves closer to the tip of the hair (Figs. 1, E and F, and 2D, subsections 1 and 2; Timmers et al., 1999). As the root hair begins to curl, entrapping rhizobia between the root hair cell walls, the focus of this microtubule array moves from the tip of the hair to the new growth site (Figs. 1G and 2D subsection 2; Timmers et al., 1999; Timmers, 2000). The plasma membrane adjacent to the bacteria invaginates (Gage and Margolin, 2000; Esseling et al., 2004), initiating infection thread formation that is further delineated by deposition of cell wall material on the outside of the host plasma membrane. Microtubules line the plasma membrane surrounding the infection thread and continue to connect the nucleus to the tip of the infection thread as it grows (Figs. 1H and 2D, subsection 3). In hcl (hair curling) mutants, which are defective in root hair curling, a dense microtubule array forms as usual between the nucleus and tip of the hair and the nucleus migrates to the tip; however, the microtubule array does not adopt the asymmetric arrangement that in wild-type plants would be focused on the new growth point (Catoira et al., 2001). These observations are consistent with a role of microtubules not in root hair tip growth per se, but in regulating the direction of growth, as demonstrated in uninoculated Arabidopsis hairs (Bibikova et al., 1999; Whittington et al., 2001; Sieberer et al., 2002).
Dense arrays of actin microfilaments, similar to those in growing root hairs, also build up in the subapical cytoplasm soon after Nod factor treatment (De Ruijter et al., 1999). Net-axial arrays of microfilaments are present in outgrowths that subsequently emerge from the deformed root hair tip (Miller et al., 1999). If the fine bundles of actin microfilaments in the tip of the hair are depolymerized by treatment with cytochalasin D, deformation but not new outgrowth occurs. These results are consistent with a role of the subapical actin microfilaments in targeting vesicle exocytosis to the growing tip of the hair (Miller et al., 1999).
Changes in the Plant Cytoskeleton in Cortical Cells in Response to Inoculation
Attachment of bacteria or application of Nod factors at the root surface also induces cellular rearrangements and cell divisions in the root cortex and the formation of a nodule primordium. An increase in tubulin gene expression has been detected 18 to 24 h after bacterial inoculation in pea (Pisum sativum), correlating with the induction of cell division in the inner cortex (Stotz and Long, 1999). About the same time as infection thread initiation, the cytoplasm in the cortical cells underlying the infected root hair becomes polarized. In outer cortex cells of temperate legumes that form indeterminate nodules, the normal cortical arrays of transverse, oblique, or longitudinal microtubules disassemble, and parallel, anticlinal arrays of microtubules form within a bridge of cytoplasm, the preinfection thread, that contains the nucleus and is in line with the approaching infection thread (Figs. 1I and 2D, subsections 3 and 4; Timmers et al., 1999). Similar cytoplasmic bridges are also formed during infection of actinorhizal nodules by nitrogen-fixing actinomycetes in the genus Frankia (Berg, 1999). The preinfection thread appears to be analogous to the phragmosome that forms in vacuolate cells before cell division (Panteris et al., 2004); however, the outer cortex cells arrest in G2 and do not divide (Yang et al., 1994). As the infection thread grows across the cortical cells, it is surrounded by a network of microtubules that also connect it to the host cell nucleus (Timmers et al., 1999) and by actin microfilaments (Davidson and Newcomb, 2001a). The hcl mutant, which fails to develop asymmetric microtubule arrays in the root hairs, is also unable to form preinfection threads (Catoira et al., 2001).
When the infection thread reaches the nodule primordium, bacteria, enclosed by the plant plasma membrane, are released into the host cell cytoplasm forming symbiosomes. As the infected cells mature, a stratification of organelles develops and is likely to be important for optimizing nodule function. The arrangement of microtubules and actin microfilaments in these cells is consistent with their role in determining cellular architecture within the nodule, although definitive evidence to support this hypothesis is still lacking.
Cortical microtubules may be responsible for maintaining mitochondria and plastids in the periphery of nodule cells (Timmers et al., 1998; Whitehead et al., 1998; Davidson and Newcomb, 2001b). In alfalfa and pea, radial arrays of microtubules emanating from the cell periphery are likely to orient and position elongated symbiosomes that lie perpendicular to the cell walls (Timmers et al., 1998; Whitehead et al., 1998; Davidson and Newcomb, 2001b). In pea and soybean, actin microfilaments also occur in the cell cortex, parallel to the microtubules, and both cytoskeletal elements form arrays surrounding the nucleus (Whitehead et al., 1998; Davidson and Newcomb, 2001a). In addition, a network of fine actin microfilaments is dispersed throughout the cytoplasm in an arrangement consistent with a role in the spatial organization of the symbiosomes (Whitehead et al., 1998; Davidson and Newcomb, 2001a). Involvement of profilin in restructuring the actin microfilament arrays is suggested by the identification of at least five profilin isoforms in Phaseolus vulgaris root nodules arising from the expression and posttranslational modification of one of two profilin genes in this plant (Guillén et al., 1999).
IS THE PLANT CYTOSKELETON TARGETED BY PATHOGENIC BACTERIA?
Rhizobia and many plant and animal bacterial pathogens possess the type-III secretion system that injects bacterial proteins (effectors) into the host cytoplasm (Cornelis and Van Gijsegem, 2000; Büttner and Bonas, 2003). In animals, the host cytoskeleton, in particular actin microfilaments, is a major target of type-III effectors for pathogen virulence (Cornelis and Van Gijsegem, 2000; Galán and Zhou, 2000; Shao et al., 2002). Type-III effectors YopE and YpkA from Yersinia spp., for example, bind to Rho GTPases that are key regulators of the actin cytoskeleton. Both effectors cause disruption of actin stress fibers (Büttner and Bonas, 2003), and reorganization of the actin cytoskeleton is associated with entry of the bacteria into the animal cell (Galán and Zhou, 2000). Various type-III effectors of plant pathogenic bacteria are virulence factors that suppress plant defense responses such as hypersensitive cell death and expression of defense genes (Jackson et al., 1999; Abramovitch et al., 2003; Hauck et al., 2003). As yet there are no reports of type III effectors targeting plant cytoskeletal elements, although an effector from Xanthomonas campestris, AvrBs3, induces mesophyll cell swelling (Marois et al., 2002), a response that could be indicative of disruption to the plant microtubule cytoskeleton. Genome-wide analysis of Pseudomonas syringae pv. tomato DC3000 has revealed that it contains at least 36 type-III effectors whose function is poorly understood (Collmer et al., 2002). Future research will determine if the plant cytoskeleton is a target for some of these proteins.
THE ROLE OF THE PLANT CYTOSKELETON IN VIRUS INFECTION
In contrast to the plant-microbe interactions described in preceding sections of this article, plant-virus interactions are not mediated across the boundary of the plant plasma membrane but instead occur intracellularly within the host cytoplasm where viral and plant encoded proteins can interact directly. The plant defense response against virus infection incorporates silencing of viral gene expression and salicylic acid mediated resistance (Singh et al., 2004), and in plant-virus interactions, it is the virus that appears to utilize the plant cytoskeleton for disease spread rather than the plant using it to mediate its own defense response.
Recent studies of plant-virus interactions have not only uncovered new information on the mechanisms of virus dissemination but have also contributed to our understanding of the molecular basis of fundamental plant processes, such as intracellular and intercellular trafficking and posttranscriptional gene silencing (Roberts and Oparka, 2003; Lecellier and Voinnet, 2004). Key discoveries in these studies have included the localization of viral movement proteins (MPs) to plasmodesmata and their ability to increase the size exclusion limit of plasmodesmata (Tomenius et al., 1987; Wolf et al., 1989). These results have led to the idea that, in order to spread throughout the host plant, viruses mimic plant proteins and commandeer plant mechanisms for intracellular and intercellular trafficking of macromolecules. Viral MPs have been used as experimental probes to isolate plant factors involved in viral infection and plant transport processes and have identified candidate proteins associated with the nucleus, plasmodesmata, and the cytoskeleton (Oparka, 2004). Since 1995, when it was shown that viral MPs interact with actin microfilaments and microtubules (Heinlein et al., 1995; McLean et al., 1995), evidence has accumulated that both cytoskeletal systems may act as conduits not only for viral RNAs and virions but also for plant macromolecules to reach plasmodesmata (for review, see Lazarowitz and Beachy, 1999; Tzfira et al., 2000; Heinlein, 2002). Nevertheless, current data show that transport of MPs and spread of virus can occur independently of the plant cytoskeleton. The following discussion highlights some of the recent evidence for and against the involvement of the plant cytoskeleton in the development of viral diseases in plants.
Involvement of the Plant Cytoskeleton in Virus Movement
Tobacco mosaic virus (TMV) is a single-strand RNA virus that encodes a 30-kD MP that has been shown to bind to single-strand RNA, actin microfilaments, microtubules, and the ER, and to accumulate at and increase the size exclusion limit of plasmodesmata during TMV infection (Heinlein, 2002). Discovery of the direct interaction of TMV MP with microtubules and actin microfilaments led to the idea that the plant cytoskeleton might function as a scaffold for targeting viruses to plasmodesmata.
MPs of TMV and related tobamoviruses possess a short conserved motif that is similar to a motif in plant α-, β-, and γ-tubulins that mediates lateral association of microtubule protofilaments (Boyko et al., 2000). Single amino acid mutations in this motif can confer temperature sensitivity to the association of MP with microtubules and, at nonpermissive temperatures, reduce intercellular transport of viral RNA, giving evidence of a correlation between the MP's ability to interact with microtubules and cell-to-cell movement of virus (Boyko et al., 2000). Association between TMV MPs and microtubules is resistant to high salt treatment, a feature that could be indicative of integration of the MP into the microtubule lattice. If this were the case, intracellular transport of MP and bound viral RNA might occur through microtubule treadmilling and polymerization-depolymerization dynamics (Boyko et al., 2000). In support of this idea, there are data indicating that MPs can influence microtubule dynamics. TMV MP introduced into mammalian cells associates with microtubule nucleating sites causing microtubule detachment from centrioles, a process that increases microtubule dynamics (Boyko et al., 2000). In addition, a microtubule binding protein encoded by potato virus X (PVX), in conjunction with PVX virions, has been shown to induce microtubule polymerization (Serazev et al., 2003). TMV virions do not have this effect, perhaps because TMV is transported as a viral RNA-protein complex (Heinlein, 2002), whereas PVX moves through plasmodesmata as virion-like filamentous particles (Santa Cruz et al., 1998).
However, despite the evidence of MP binding to microtubules and its apparent mediation of microtubule-based movement of virus, the results of a number of other studies indicate that viral spread and transport of MP can occur independently of plant microtubules. In tobacco, neither drug-induced microtubule depolymerization nor silencing of the α-tubulin gene has any significant effect on the spread of TMV or the accumulation of MP at plasmodesmata (Gillespie et al., 2002; Kawakami et al., 2004). By contrast, intracellular and intercellular movement of TMV replication complexes has been shown to be affected by latrunculin B, an inhibitor of actin polymerization (Kawakami et al., 2004).
A single amino acid change in TMV MP can lead to an association of MP with cortical ER instead of microtubules (Fig. 1, J and K; Gillespie et al., 2002). The GFP-tagged mutated MP, MPR3-GFP, moves from cell-to-cell five times faster that wild-type MP-GFP and shows a higher activity in increasing the plasmodesmatal size exclusion limit. During spread of TMV infection, wild-type MP-GFP appears as a ring of fluorescence at the infection front (Gillespie et al., 2002). This pattern is thought to arise through the breakdown of MP at the center of the infection. MPR3-GFP, on the other hand, appears as a fluorescent disc rather than a ring. Treatment with a proteasome inhibitor led to wild-type MP-GFP displaying a fluorescence pattern similar to that of MPR3-GFP. Building on a previous report of the ubiquitination and subsequent degradation of MP by the 26S proteasome (Reichel and Beachy, 2000), the authors interpret their data as indicating that plant microtubules, rather than being essential for cell-to-cell movement, may be part of a mechanism to degrade TMV MP.
Interaction of Cytoskeleton-Binding Proteins with Viral MPs
Recently, a TMV MP-binding protein, MPB2C, was isolated from tobacco and found to have homologous sequences only in plant species (Kragler et al., 2003). Expression of both TMV MP-GFP and MPB2C-RFP (red fluorescent protein) in tobacco epidermal cells, revealed that MPB2C colocalizes with TMV MP at microtubules but not on the ER. Cell-to-cell movement of MP-GFP was inhibited by overexpression of MPB2C-RFP, whereas that of the ER-associated MPR3-GFP was not affected (Kragler et al., 2003). The authors interpret these results as indicating that MPB2C is a negative effector of microtubule-mediated TMV movement. A protein, At4/1, with weak homologies to myosin and kinesin, has been identified in Arabidopsis and found to interact with the putative MP of tomato spotted wilt virus (von Bargen et al., 2001). At4/1 could mediate cytoskeleton-dependent intracellular trafficking of the virus.
Involvement of the Plant Cytoskeleton in Viral Tubule Distribution
MPs of some viruses, such as grapevine fanleaf virus (GFLV), form tubules that facilitate the movement of virus-like particles through plasmodesmata in infected host plants (Fig. 1, L–N; Ritzenthaler et al., 1995; Lazarowitz and Beachy, 1999). GFP-tagged GFLV MP expressed in tobacco BY-2 cells forms tubules associated with plasmodesmata at cell plates and young cross walls (Laporte et al., 2003; Fig. 1, L–N). Tubule formation is inhibited by brefeldin A, an inhibitor of Golgi-ER membrane trafficking (Nebenführ et al., 2002), but not by cytoskeletal inhibitors, suggesting that tubule formation is reliant on the plant secretory pathway but not on an intact cytoskeleton. However, oryzalin-induced depolymerization of microtubules led to loss of polarity in the distribution of GFP-MP tubules that were found over the whole cell surface rather than preferentially accumulating at young cross walls. Cytochalasin D or latrunculin B, inhibitors of actin polymerization, did not have any effect on tubule formation or their distribution, although simultaneous treatment with oryzalin and latrunculin B resulted in cytosolic localization of tubules. These results indicate that the GFP-MP tubules are transported via a microtubule-dependent pathway, although microfilaments function in the transport process if microtubules are depolymerized.
A ROLE FOR THE CYTOSKELETON IN SELF-INCOMPATIBILITY
During pollination with compatible pollen, growth of the pollen tube is extremely rapid and is dependent upon intact microtubule and actin microfilament cytoskeletons (Hepler et al., 2001; Raudaskoski et al., 2001). Plants employ a number of mechanisms to stop self-pollen from fertilizing the ovule, including the degradation of pollen RNA by stigma RNAases (Kao and Tsukamoto, 2004). In Papaver, growth of self-pollen is rapidly and specifically arrested by stigmatic S (self-incompatibility) proteins via increased levels of cytoplasmic calcium in the pollen tube (Thomas et al., 2003). Treatment with S-protein causes rapid reorganization and depolymerization of endoplasmic actin microfilament bundles in incompatible pollen of Papaver rhoeas, indicating that actin microfilaments are one of the components targeted during arrest of pollen tube growth in this form of self-incompatibility (Fig. 3; Geitmann et al., 2000). Artificial elevation of cytosolic calcium levels increases the G-actin binding activity of pollen profilin, and causes the depolymerization of actin microfilaments (Snowman et al., 2002). In P. rhoeas pollen, a gelsolin-like protein, designated PrABP80, binds to actin microfilaments, causing Ca2+-dependent severing of actin microfilaments and enhancing profilin-mediated actin depolymerization by blocking the barbed ends of actin microfilaments (Huang et al., 2004). These data suggest that PrABP80 could function as the main regulator of actin depolymerization in this self-incompatibility response. In addition to the rapid arrest of pollen tube growth, S-protein treatment can also trigger programmed cell death of self-pollen (Thomas and Franklin-Tong, 2004), giving support for earlier suggestions of overlapping mechanisms in self-incompatibility and hypersensitivity-mediated plant defense against pathogens (Dickinson, 1994).
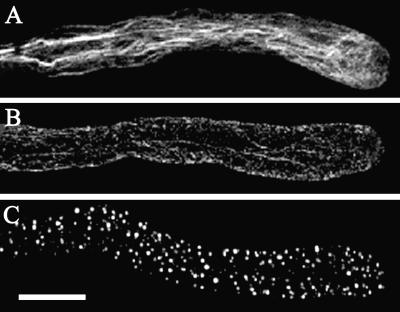
Figure 3.
Depolymerization of actin microfilaments in a pollen tube triggered by a self-incompatibility response. A, Normally growing pollen tube. B, Incompatible pollen tube 5 min after treatment with S-protein. C, Incompatible pollen tube 60 min after treatment with S-protein. Actin filaments were visualized by Alexa-488-phalloidin staining. Bar = 10 μm. (Reproduced with permission from Snowman et al., 2002.)
CONCLUDING REMARKS
In most plant-microbe interactions, the development of structured cytoskeletal arrays at the interaction site is associated with outcomes that are beneficial to the plant. Examples include the radial arrays of actin microfilaments and microtubules that accompany cytoplasmic aggregation during defense responses against invading pathogens, the dense network of actin microfilaments and microtubules that forms around arbuscules and peletons of mycorrhizal fungi, and the lattice of actin microfilaments or radial array of microtubules found among symbiosomes in root nodules. There is evidence of the increased stability of actin to proteolytic degradation in all these circumstances through actin monoubiquitylation (Dantán-González et al., 2001). On the other hand, degradation of the plant cytoskeleton is often associated with outcomes of plant interactions with other organisms that are detrimental to the plant. Both microtubule and actin microfilament arrays are lost during invasion by the Lotus mutant that is unable to develop a functional mycorrhizal symbiosis, and degradation of actin microfilaments results in arrest of self-pollen. Both cytoskeletal arrays are also disrupted or completely depolymerized during feeding cell formation by root-infecting nematodes (de Almeida Engler et al., 2004).
These observations underscore the important role played by the plant cytoskeleton in mediating the plant cell's response to biotic factors. It is clear that remodeling of the plant cytoskeleton is instrumental in achieving structural responses to other organisms, for example, in forming an apoplastic barrier to arrest pathogen ingress. Changes in cytoskeletal organization may also facilitate signaling of the presence of symbionts or pathogens on the plant surface. There is growing evidence that actin and microtubule arrays in plant cells participate in signaling cascades initiated at the plasma membrane, enabling adaption to environmental factors (Abdrakhamanova et al., 2003; Gardiner et al., 2003; Sivaguru et al., 2003; Šamaj et al., 2004a, 2004b). It is likely that research over the next few years will further elucidate the role of the plant cytoskeleton in signal transduction and the plant response to pathogens and symbionts.
Acknowledgments
We thank Dr. D.A. Jones for critical reading of the manuscript, and Drs. E. Blancaflor, C. Ritzenthaler, T. Timmen, and V. Frankin-Tong for allowing us to use their previously published micrographs.
References
- Abdrakhamanova A, Wang QY, Khokhlova L, Nick P (2003) Is microtubule disassembly a trigger for cold acclimation? Plant Cell Physiol 44: 676–686 [DOI] [PubMed] [Google Scholar]
- Abramovitch RB, Kim Y-J, Chen SR, Dickman MB, Martin GB (2003) Pseudomonas type III effector AvrPtoB induces plant disease susceptibility by inhibition of host programmed cell death. EMBO J 22: 60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aist JR (1976) Papillae and related wound plugs of plant cells. Annu Rev Phytopathol 14: 145–163 [Google Scholar]
- Armstrong L, Peterson RL (2002) The interface between the arbuscular mycorrhizal fungus Glomus intraradices and root cells of Panax quinquefolius: a Paris-type mycorrhizal association. Mycologia 94: 587–595 [DOI] [PubMed] [Google Scholar]
- Berg RH (1999) Cytoplasmic bridge formation in the nodule apex of actinorhizal root nodules. Can J Bot 77: 1351–1357 [Google Scholar]
- Bibikova TN, Blancaflor EB, Gilroy S (1999) Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J 17: 657–665 [DOI] [PubMed] [Google Scholar]
- Blancaflor EB (2002) The cytoskeleton and gravitropism in higher plants. J Plant Growth Regul 21: 120–136 [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Zhao L, Harrison MJ (2001) Microtubule organization in root cells of Medicago truncatula during development of an arbuscular mycorrhizal symbiosis with Glomus versiforme. Protoplasma 217: 154–165 [DOI] [PubMed] [Google Scholar]
- Blume B, Nürnberger T, Nass N, Scheel D (2000) Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12: 1425–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante P, Bergero R, Uribe X, Romera C, Rigau J, Puigdomenech P (1996) Transcriptional activation of a maize α-tubulin gene in mycorrhizal maize and transgenic tobacco plants. Plant J 9: 737–743 [Google Scholar]
- Boyko V, Ferralli J, Ashby J, Schellenbaum P, Heinlein M (2000) Function of microtubules in intercellular transport of plant virus RNA. Nat Cell Biol 2: 826–832 [DOI] [PubMed] [Google Scholar]
- Büttner D, Bonas U (2003) Common infection strategies of plant and animal pathogenic bacteria. Curr Opin Plant Biol 6: 312–319 [DOI] [PubMed] [Google Scholar]
- Cahill D, Rookes J, Michalczyk A, McDonald K, Drake A (2002) Microtubule dynamics in compatible and incompatible interactions of soybean hypocotyl cells with Phytophthora sojae. Plant Pathol 51: 629–640 [Google Scholar]
- Camacho L, Malhó R (2003) Endo/exocytosis in the pollen tube apex is differentially regulated by Ca2+ and GTPases. J Exp Bot 54: 83–92 [DOI] [PubMed] [Google Scholar]
- Cárdenas L, Holdaway-Clarke TL, Sánchez F, Quinto C, Feijó JA, Kunkel JG, Hepler PK (2000) Ion changes in legume root hairs responding to Nod factors. Plant Physiol 123: 443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L, Thomas-Oates JE, Nava N, López-Lara IM, Hepler PK, Quinto C (2003) The role of Nod factor substituents in actin cytoskeleton rearrangements in Phaseolus vulgaris. Mol Plant Microbe Interact 16: 326–334 [DOI] [PubMed] [Google Scholar]
- Cárdenas L, Vidali L, Domínguez J, Pérez H, Sánchez F, Hepler PK, Quinto C (1998) Rearrangement of actin microfilaments in plant root hairs responding to Rhizobium etli nodulation signals. Plant Physiol 116: 871–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoira R, Timmers ACJ, Maillet F, Galera C, Penmetsa RV, Cook D, Dénarié J, Gough C (2001) The HCL gene of Medicago truncatula controls Rhizobium-induced root hair curling. Development 128: 1507–1518 [DOI] [PubMed] [Google Scholar]
- Chen CYH, Cheung AY, Wu H-M (2003) Actin-depolymerizing factor mediates Rac/Rop GTPase-regulated pollen tube growth. Plant Cell 15: 237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu JL, Hückelhoven R, Stein M, Freialdenhoven A, Somerville SC, et al (2003) SNARE-protein-mediated disease resistance at the plant cell wall. Nature 425: 973–977 [DOI] [PubMed] [Google Scholar]
- Collmer A, Lindeberg M, Petnicki-Ocwieja T, Schneider DJ, Alfano JR (2002) Genomic mining type III secretion system effectors in Pseudomonas syringae yields new picks for all TTSS prospectors. Trends Microbiol 10: 462–469 [DOI] [PubMed] [Google Scholar]
- Cornelis GR, Van Gijsegem F (2000) Assembly and function of type III secretory systems. Annu Rev Microbiol 54: 735–774 [DOI] [PubMed] [Google Scholar]
- Dantán-González E, Rosenstein Y, Quinto C, Sánchez F (2001) Actin monoubiquitylation is induced in plants in response to pathogens and symbionts. Mol Plant Microbe Interact 14: 1267–1273 [DOI] [PubMed] [Google Scholar]
- Davidson AL, Newcomb W (2001. a) Changes in actin microfilament arrays in developing pea root nodule cells. Can J Bot 79: 767–776 [Google Scholar]
- Davidson AL, Newcomb W (2001. b) Organization of microtubules in developing pea root nodule cells. Can J Bot 79: 777–786 [Google Scholar]
- de Almeida Engler J, Van Poucke K, Karimi M, De Groodt R, Gheysen G, Engler G, Gheysen G (2004) Dynamic cytoskeleton rearrangements in giant cells and syncytia of nematode-infected roots. Plant J 38: 12–26 [DOI] [PubMed] [Google Scholar]
- De Ruijter NCA, Bisseling T, Emons AMC (1999) Rhizobium Nod factors induce an increase in sub-apical fine bundles of actin filaments in Vicia sativa root hairs within minutes. Mol Plant Microbe Interact 12: 829–832 [Google Scholar]
- Diaz EC, Martin F, Tagu D (1996) Eucalypt α-tubulin: cDNA cloning and increased level of transcripts in ectomycorrhizal root system. Plant Mol Biol 31: 905–910 [DOI] [PubMed] [Google Scholar]
- Dickinson H (1994) Self-pollination. Simply a social disease? Nature 367: 517–518 [DOI] [PubMed] [Google Scholar]
- Ditengou FA, Raudaskoski M, Lapeyrie F (2003) Hypaphorine, an indole-3-acetic acid antagonist delivered by the ectomycorrhizal fungus Pisolithus tinctorius, induces reorganisation of actin and the microtubule cytoskeleton in Eucalyptus globulus ssp bicostata root hairs. Planta 218: 217–225 [DOI] [PubMed] [Google Scholar]
- Donofrio NM, Delaney TP (2001) Abnormal callose response phenotype and hypersusceptibility to Peronospora parasitica in defence-compromised Arabidopsis nim1-1 and salicylate hydoxylase-expressing plants. Mol Plant Microbe Interact 14: 439–450 [DOI] [PubMed] [Google Scholar]
- Esseling JJ, Emons AMC (2004) Dissection of Nod factor signalling in legumes: cell biology, mutants and pharmacological approaches. J Microsc 214: 104–113 [DOI] [PubMed] [Google Scholar]
- Esseling JJ, Lhuissier FGP, Emons AMC (2003) Nod factor-induced root hair curling: continuous polar growth towards the point of Nod factor application. Plant Physiol 132: 1982–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esseling JJ, Lhuissier FGP, Emons AMC (2004) A nonsymbiotic root hair tip growth phenotype in NORK-mutated legumes: implications for nodulation factor-induced signaling and formation of a mulitfaceted root hair pocket for bacteria. Plant Cell 16: 933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle HH, Kondorosi É, Kondorosi Á, Schultze M (1998) The role of ion fluxes in Nod factor signalling in Medicago sativa. Plant J 13: 455–463 [Google Scholar]
- Foissner I, Lichtscheidl IK, Wasteneys GO (1996) Actin-based vesicle dynamics and exocytosis during wound wall formation in characean internodal cells. Cell Motil Cytoskeleton 35: 35–48 [DOI] [PubMed] [Google Scholar]
- Gage DJ, Margolin W (2000) Hanging by a thread: invasion of legume plants by rhizobia. Curr Opin Microbiol 3: 613–617 [DOI] [PubMed] [Google Scholar]
- Galán JE, Zhou D (2000) Striking a balance: modulation of the actin cytoskeleton by Salmonella. Proc Natl Acad Sci USA 97: 8754–8761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner J, Collings DA, Harper JDI, Marc J (2003) The effects of the phospholipase D-antagonist 1-butanol on seedling development and microtubule organisation in Arabidopsis. Plant Cell Physiol 44: 687–696 [DOI] [PubMed] [Google Scholar]
- Geitmann A, Emons AMC (2000) The cytoskeleton in plant and fungal cell tip growth. J Microsc 198: 218–245 [DOI] [PubMed] [Google Scholar]
- Geitmann A, Snowman BN, Emons AMC, Franklin-Tong VE (2000) Alterations in the actin cytoskeleton of pollen tubes are induced by the self-incompatibility reaction in Papaver rhoeas. Plant Cell 12: 1239–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genre A, Bonfante P (1997) A mycorrhizal fungus changes microtubule orientation in tobacco root cells. Protoplasma 199: 30–38 [Google Scholar]
- Genre A, Bonfante P (1998) Actin versus tubulin configuration in arbuscule-containing cells from mycorrhizal tobacco roots. New Phytol 140: 745–752 [DOI] [PubMed] [Google Scholar]
- Genre A, Bonfante P (1999) Cytoskeleton-related proteins in tobacco mycorhizal cells: γ-tubulin and clathrin localisation. Eur J Histochem 43: 105–111 [PubMed] [Google Scholar]
- Genre A, Bonfante P (2002) Epidermal cells of a symbiosis-defective mutant of Lotus japonicus show altered cytoskeleton organisation in the presence of a mycorrhizal fungus. Protoplasma 219: 43–50 [DOI] [PubMed] [Google Scholar]
- Gillespie T, Boevink P, Haupt S, Roberts AG, Toth R, Valentine T, Chapman S, Oparka KJ (2002) Functional analysis of a DNA-shuffled movement protein reveals that microtubules are dispensable for the cell-to-cell movement of Tobacco mosaic virus. Plant Cell 14: 1207–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross P, Julius C, Schmelzer E, Hahlbrock K (1993) Translocation of cytoplasm and nucleus to fungal penetration sites is associated with depolymerization of microtubules and defence gene activation in infected, cultured parsley cells. EMBO J 12: 1735–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillén G, Valdés-Lopez V, Noguez R, Olivares J, Rodríguez-Zapata LC, Pérez H, Vidali L, Villanueva MA, Sánchez F (1999) Profilin in Phaseolus vulgaris is encoded by two genes (only one expressed in root nodules) but multiple isoforms are generated in vivo by phosphorylation on tyrosine residues. Plant J 19: 497–508 [DOI] [PubMed] [Google Scholar]
- Hauck P, Thilmony R, He SY (2003) A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proc Natl Acad Sci USA 100: 8577–8582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen BE, Bushnell WR (1983) Inhibition of the hypersensitive reaction in barley to powdery mildew by heat shock and cytochalasin B. Physiol Plant Pathol 23: 421–438 [Google Scholar]
- Heinlein M (2002) The spread of Tobacco mosaic virus infection: insights into the cellular mechanism of RNA transport. Cell Mol Life Sci 59: 58–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinlein M, Epel BL, Padgett HS, Beachy RN (1995) Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science 270: 1983–1985 [DOI] [PubMed] [Google Scholar]
- Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 17: 159–187 [DOI] [PubMed] [Google Scholar]
- Huang S, Blanchoin L, Chaudhry F, Franklin-Tong VE, Staiger CJ (2004) A gelsolin-like protein from Papaver rhoeas pollen (PrABP80) stimulates calcium-regulated severing and depolymerization of actin filaments. J Biol Chem 279: 23364–23375 [DOI] [PubMed] [Google Scholar]
- Hush JM, Overall RL (1996) Cortical microtubule reorientation in higher plants: dynamics and regulation. J Microsc 181: 129–139 [Google Scholar]
- Jackson RW, Athanassopoulos E, Tsiamis G, Mansfield JW, Sesma A, Arnold DL, Gibbon MJ, Murillo J, Taylor JD, Vivian A (1999) Identification of a pathogenicity island, which contains genes for virulence and avirulence, on a large native plasmid in the bean pathogen Pseudomonas syringae pathovar phaseolicola. Proc Natl Acad Sci USA 96: 10875–10880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao T-H, Tsukamoto T (2004) The molecular and genetic bases of S-RNase-based self-incompatibility. Plant Cell (Suppl) 16: S72–S83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami S, Watanabe Y, Beachy RN (2004) Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proc Natl Acad Sci USA 101: 6291–6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I, Kobayashi Y, Hardham AR (1997. a) Inhibition of rust-induced hypersensitive response in flax cells by the microtubule inhibitor oryzalin. Aust J Plant Physiol 24: 733–740 [Google Scholar]
- Kobayashi I, Kobayashi Y, Hardham AR (1994) Dynamic reorganization of microtubules and microfilaments in flax cells during the resistance response to flax rust infection. Planta 195: 237–247 [Google Scholar]
- Kobayashi I, Kobayashi Y, Yamaoka N, Kunoh H (1992) Recognition of a pathogen and a nonpathogen by barley coleoptile cells. III. Responses of microtubules and actin filaments in barley coleoptile cells to penetration attempts. Can J Bot 70: 1815–1823 [Google Scholar]
- Kobayashi Y, Kobayashi I, Funaki Y, Fujimoto S, Takemoto T, Kunoh H (1997. b) Dynamic reorganization of microfilaments and microtubules is necessary for the expression of non-host resistance in barley coleoptile cells. Plant J 11: 525–537 [Google Scholar]
- Kovar DR, Staiger CJ, Weaver EA, McCurdy DW (2000) AtFim1 is an actin filament crosslinking protein from Arabidopsis thaliana. Plant J 24: 625–636 [DOI] [PubMed] [Google Scholar]
- Kragler F, Curin M, Trutnyeva K, Gansch A, Waigmann E (2003) MPB2C, a microtubule-associated plant protein binds to and interferes with cell-to-cell transport of tobacco mosaic virus movement protein. Plant Physiol 132: 1870–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuga-Uetake Y, Purich M, Massicotte HB, Peterson RL (2004) Host microtubules in the Hartig net region of ectomycorrhizas, ectendomycorrhizas, and monotropoid mycorrhizas. Can J Bot 82: 938–946 [Google Scholar]
- Laporte C, Vetter G, Loudes A-M, Robinson DG, Hillmer S, Stussi-Garaud C, Ritzenthaler C (2003) Involvement of the secretory pathway and the cytoskeleton in intracellular targeting and tubule assembly of Grapevine fanleaf virus movement protein in tobacco BY-2 cells. Plant Cell 15: 2058–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz SG, Beachy RN (1999) Viral movement proteins as probes for intracellular and intercellular trafficking in plants. Plant Cell 11: 535–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecellier C-H, Voinnet O (2004) RNA silencing: no mercy for viruses? Immunol Rev 198: 285–303 [DOI] [PubMed] [Google Scholar]
- Lichtscheidl IK, Hepler PK (1996) Endoplasmic reticulum in the cortex of plants. In M Smallwood, JP Knox, DJ Bowles, eds, Membranes: Specialised Functions in Plants. Bios Scientific Publishers, Oxford, pp 383–402
- Mano S, Nakamori C, Hayashi M, Kato A, Kondo M, Nishimura M (2002) Distribution and characterization of peroxisomes in Arabidopsis by visualization with GFP: dynamic morphology and actin-dependent movement. Plant Cell Physiol 43: 331–341 [DOI] [PubMed] [Google Scholar]
- Marois E, Van den Ackerveken G, Bonas U (2002) The Xanthomonas type III effector protein AvrBs3 modulates plant gene expression and induces cell hypertrophy in the susceptible host. Mol Plant Microbe Interact 15: 637–646 [DOI] [PubMed] [Google Scholar]
- Matsubara Y, Uetake Y, Peterson RL (1999) Entry and colonization of Asparagus officinalis roots by arbuscular mycorrhizal fungi with emphasis on changes in host microtubules. Can J Bot 77: 1159–1167 [Google Scholar]
- McLean BG, Zupan J, Zambryski PC (1995) Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco cells. Plant Cell 7: 2101–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DD, De Ruijter NCA, Bisseling T, Emons AMC (1999) The role of actin in root hair morphogenesis: studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J 17: 141–154 [Google Scholar]
- Miller DD, De Ruijter NCA, Emons AMC (1997) From signal to form: aspects of the cytoskeleton-plasma membrane-cell wall continuum in root hair tips. J Exp Bot 48: 1881–1896 [Google Scholar]
- Nebenführ A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA (1999) Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol 121: 1127–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A, Ritzenthaler C, Robinson DG (2002) Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol 130: 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niini SS, Tarkka MT, Raudaskoski M (1996) Tubulin and actin protein patterns in Scots pine (Pinus sylvestris) roots and developing ectomycorrhiza with Suillus bovinus. Physiol Plant 96: 186–192 [Google Scholar]
- Oparka KJ (2004) Getting the message across: how do plant cells exchange macromolecular complexes? Trends Plant Sci 9: 33–41 [DOI] [PubMed] [Google Scholar]
- Panteris E, Apostolakos P, Quader H, Galatis B (2004) A cortical cytoplasmic ring predicts the division plane in vacuolated cells of Coleus: the role of actomyosin and microtubules in the establishment and function of the division site. New Phytol 163: 271–286 [DOI] [PubMed] [Google Scholar]
- Parniske M (2000) Intracellular accommodation of microbes by plants: a common developmental program for symbiosis and disease? Curr Opin Plant Biol 3: 320–328 [DOI] [PubMed] [Google Scholar]
- Raudaskoski M, Åström H, Laitiainen E (2001) Pollen tube cytoskeleton: structure and function. J Plant Growth Regul 20: 113–130 [Google Scholar]
- Reichel C, Beachy RN (2000) Degradation of tobacco mosaic virus movement protein by the 26S proteasome. J Virol 74: 3330–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riely BK, Ané J-M, Penmetsa RV, Cook DR (2004) Genetic and genomic analysis in model legumes bring Nod-factor signaling to center stage. Curr Opin Plant Biol 7: 408–413 [DOI] [PubMed] [Google Scholar]
- Ritzenthaler C, Schmit AC, Michler P, Stussigaraud C, Pinck L (1995) Grapevine fanleaf nepovirus P38 putative movement protein is located on tubules in vivo. Mol Plant Microbe Interact 8: 379–387 [Google Scholar]
- Roberts AG, Oparka KJ (2003) Plasmodesmata and the control of symplastic transport. Plant Cell Environ 26: 103–124 [Google Scholar]
- Šamaj J, Baluška F, Hirt H (2004. a) From signal to cell polarity: mitogen-activated protein kinases as sensors and effectors of cytoskeleton dynamicity. J Exp Bot 55: 189–198 [DOI] [PubMed] [Google Scholar]
- Šamaj J, Baluška F, Menzel D (2004. b) New signalling molecules regulating root hair tip growth. Trends Plant Sci 9: 217–220 [DOI] [PubMed] [Google Scholar]
- Santa Cruz S, Roberts AG, Prior DAM, Chapman S, Oparka KJ (1998) Cell-to-cell and phloem-mediated transport of potato virus X - the role of virions. Plant Cell 10: 495–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel D (1998) Resistance response physiology and signal transduction. Curr Opin Plant Biol 1: 305–310 [DOI] [PubMed] [Google Scholar]
- Schultheiss H, Dechert C, Kogel K-H, Hückelhoven R (2003) Functional analysis of barley RAC/ROP G-protein family members in susceptibility to the powdery mildew fungus. Plant J 36: 589–601 [DOI] [PubMed] [Google Scholar]
- Serazev TV, Nadezhdina ES, Shanina NA, Leshchiner AD, Kalinina NO, Morozov SY (2003) Virions and the coat protein of the potato virus X interact with microtubules and induce tubulin polymerization in vitro. Mol Biol 37: 919–925 [PubMed] [Google Scholar]
- Shao F, Merritt PM, Bao Z, Innes RW, Dixon JE (2002) A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 109: 575–588 [DOI] [PubMed] [Google Scholar]
- Shaw SL, Long SR (2003) Nod factor elicits two separable calcium responses in Medicago truncatula root hair cells. Plant Physiol 131: 976–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmen T, Yokota E (2004) Cytoplasmic streaming in plants. Curr Opin Cell Biol 16: 68–72 [DOI] [PubMed] [Google Scholar]
- Sieberer BJ, Timmers ACJ, Lhuissier FGP, Emons AMC (2002) Endoplasmic microtubules configure the subapical cytoplasm and are required for fast growth of Medicago truncatula root hairs. Plant Physiol 130: 977–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DP, Moore CA, Gilliland A, Carr JP (2004) Activation of multiple antiviral defence mechanisms by salicylic acid. Mol Plant Pathol 5: 57–63 [DOI] [PubMed] [Google Scholar]
- Sivaguru M, Pike S, Gassmann W, Baskin TI (2003) Aluminum rapidly depolymerizes cortical microtubules and depolarizes the plasma membrane: evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol 44: 667–675 [DOI] [PubMed] [Google Scholar]
- Škalamera D, Heath MC (1998) Changes in the cytoskeleton accompanying infection-induced nuclear movements and the hypersensitive response in plant cells invaded by rust fungi. Plant J 16: 191–200 [DOI] [PubMed] [Google Scholar]
- Škalamera D, Jibodh S, Heath MC (1997) Callose deposition during the interaction between cowpea (Vigna Unguiculata) and the monokaryotic stage of the cowpea rust fungus (Uromyces Vignae). New Phytol 136: 511–524 [DOI] [PubMed] [Google Scholar]
- Snowman BN, Kovar DR, Shevchenko G, Franklin-Tong VE, Staiger CJ (2002) Signal-mediated depolymerization of actin in pollen during the self-incompatibility response. Plant Cell 14: 2613–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger CJ (2000) Signalling to the actin cytoskeleton in plants. Annu Rev Plant Physiol Plant Mol Biol 51: 257–288 [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Yuan M, Valenta R, Shaw PJ, Warn RM, Lloyd CW (1994) Microinjected profilin affects cytoplasmic streaming in plant cells by rapidly depolymerizing actin microfilaments. Curr Biol 4: 215–219 [DOI] [PubMed] [Google Scholar]
- Stotz HU, Long SR (1999) Expression of the pea (Pisum sativum L.) α-tubulin gene TubA1 is correlated with cell division activity. Plant Mol Biol 41: 601–614 [DOI] [PubMed] [Google Scholar]
- Takagi S (2000) Roles for actin filaments in chloroplast motility and anchoring. In C Staiger, ed, Actin: A Dynamic Framework for Multiple Plant Cell Functions. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 203–212
- Takemoto D, Jones DA, Hardham AR (2003) GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. Plant J 33: 775–792 [DOI] [PubMed] [Google Scholar]
- Takemoto D, Maeda H, Yoshioka H, Doke N, Kawakita K (1999) Effect of cytochalasin D on defense responses of potato tuber discs treated with hyphal wall components of Phytophthora infestans. Plant Sci 141: 219–226 [Google Scholar]
- Thomas S, Osman K, De Graaf BHJ, Shevchenko G, Wheeler M, Franklin FCH, Franklin-Tong VE (2003) Investigating mechanisms involved in the self-incompatibility response in Papaver rhoeas. Philos Trans R Soc Lond B Biol Sci 358: 1033–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SG, Franklin-Tong VE (2004) Self-incompatibility triggers programmed cell death in Papaver pollen. Nature 429: 305–309 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H (2003) Fresh insights into processes of nonhost resistance. Curr Opin Plant Biol 6: 351–357 [DOI] [PubMed] [Google Scholar]
- Timmers ACJ (2000) Infection of root hairs by rhizobia: infection thread development with emphasis on the microtubule cytoskeleton. In RW Ridge, AMC Emons, eds, Root Hairs. Cell and Molecular Biology. Springer-Verlag, Tokyo, pp 223–239
- Timmers ACJ, Auriac M-C, De Billy F, Truchet G (1998) Nod factor internalization and microtubular cytoskeleton changes occur concomitantly during nodule differentiation in alfalfa. Development 125: 339–349 [DOI] [PubMed] [Google Scholar]
- Timmers ACJ, Auriac MC, Truchet G (1999) Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development 126: 3617–3628 [DOI] [PubMed] [Google Scholar]
- Timonen S, Finlay RD, Söderström B, Raudaskoski M (1993) Identification of cytoskeletal components in pine ectomycorrhizas. New Phytol 124: 83–92 [Google Scholar]
- Timonen S, Peterson RL (2002) Cytoskeleton in mycorrhizal symbiosis. Plant Soil 244: 199–210 [Google Scholar]
- Timonen S, Söderström B, Raudaskoski M (1996) Dynamics of cytoskeletal proteins in developing pine ectomycorrhiza. Mycorrhiza 6: 423–429 [Google Scholar]
- Tomenius K, Clapham D, Meshi T (1987) Localization by immunogold cytochemistry of the virus-coded 30 K protein in plasmodesmata of leaves infected with tobacco mosaic virus. Virology 160: 363–371 [DOI] [PubMed] [Google Scholar]
- Tomiyama K, Sato K, Doke N (1982) Effect of cytochalasin B and colchicine on hypersensitive death of potato cells infected by incompatible race of Phytophthora infestans. Ann Phytopathol Soc Jpn 48: 228–230 [Google Scholar]
- Tzfira T, Rhee Y, Chen MH, Kunik T, Citovsky V (2000) Nucleic acid transport in plant-microbe interactions: The molecules that walk through the walls. Annu Rev Microbiol 54: 187–219 [DOI] [PubMed] [Google Scholar]
- Uetake Y, Farquhar ML, Peterson RL (1997) Changes in microtubule arrays in symbiotic orchid protocorms during fungal colonization and senescence. New Phytol 135: 701–709 [Google Scholar]
- Uetake Y, Peterson RL (1997) Changes in actin filament arrays in protocorm cells of the orchid species, Spiranthes sinensis, induced by the symbiotic fungus Ceratobasidium cornigerum. Can J Bot 75: 1661–1669 [Google Scholar]
- Uetake Y, Peterson RL (1998) Association between microtubules and symbiotic fungal hyphae in protocorm cells of the orchid species, Spiranthes sinensis. New Phytol 140: 715–722 [DOI] [PubMed] [Google Scholar]
- Valster AH, Pierson ES, Valenta R, Hepler PK, Emons AMC (1997) Probing the plant actin cytoskeleton during cytokinesis and interphase by profilin microinjection. Plant Cell 9: 1815–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, Yokota E, Cheung AY, Shimmen T, Hepler PK (1999) The 135kDa actin-binding protein from Lilium longiflorum pollen is the plant homologue of villin. Protoplasma 209: 283–291 [Google Scholar]
- von Bargen S, Salchert K, Paape M, Piechulla B, Kellmann JW (2001) Interactions between the tomato spotted wilt virus movement protein and plant proteins showing homologies to myosin, kinesin and DnaJ-like chaperones. Plant Physiol Biochem 39: 1083–1093 [Google Scholar]
- Wasteneys GO, Galway ME (2003) Remodelling the cytoskeleton for growth and form: an overview with some new views. Annu Rev Plant Biol 54: 691–722 [DOI] [PubMed] [Google Scholar]
- Weerasinghe RR, Collings DA, Johannes E, Allen NS (2003) The distributional changes and role of microtubules in Nod factor-challenged Medicago sativa root hairs. Planta 218: 276–287 [DOI] [PubMed] [Google Scholar]
- Whitehead LF, Day DA, Hardham AR (1998) Cytoskeletal arrays in the cells of soybean root nodules: the role of actin microfilaments in the organisation of symbiosomes. Protoplasma 203: 194–205 [Google Scholar]
- Whittington AT, Vugrek O, Wei KJ, Hasenbein NG, Sugimoto K, Rashbrooke MC, Wasteneys GO (2001) MOR1 is essential for organizing cortical microtubules in plants. Nature 411: 610–613 [DOI] [PubMed] [Google Scholar]
- Wolf S, Deom C, Beachy RN, Lucas WJ (1989) Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science 246: 377–379 [DOI] [PubMed] [Google Scholar]
- Yang W-C, de Blank C, Meskiene I, Hirt H, Bakker J, Van Kammen A, Franssen H, Bisseling T (1994) Rhizobium Nod factors reactive the cell cycle during infection and nodule promordium formation, but the cycle is only completed in primordium formation. Plant Cell 6: 1415–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZB (2002) Small GTPases: versatile signaling switches in plants. Plant Cell (Suppl) 14: S375–S388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota E, Muto S, Shimmen T (1999) Inhibitory regulation of higher-plant myosin by Ca2+ ions. Plant Physiol 119: 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun B-W, Atkinson HA, Gaborit C, Greenland A, Read ND, Pallas JA, Loake GJ (2003) Loss of actin cytoskeletal function and EDS1 activity, in combination, severely compromises non-host resistance in Arabidopsis against wheat powdery mildew. Plant J 34: 768–777 [DOI] [PubMed] [Google Scholar]