Significance
Split GFPs have been used exclusively as imaging tools to study protein–protein interactions, but the irreversible nature of split GFP complementation can be highly perturbative. Dissociating the split GFP into constituent parts with light irradiation not only remedies this problem but also invites the opportunity for noninvasive optical control of biological processes. By elucidating an energetics–function model for split GFP strand photodissociation, we show that photodissociation is achieved by light-activated cis-trans isomerization of the chromophore, a mechanism pervasive among reversibly photoswitchable fluorescent proteins. This observation suggests that these proteins can be engineered to provide active control of in vivo processes as optogenetic tools in addition to their conventional roles as passive imaging reporters.
Keywords: split GFP, potential energy surface, photodissociation, cis-trans isomerization, photosensory protein
Abstract
Split GFPs have been widely applied for monitoring protein–protein interactions by expressing GFPs as two or more constituent parts linked to separate proteins that only fluoresce on complementing with one another. Although this complementation is typically irreversible, it has been shown previously that light accelerates dissociation of a noncovalently attached β-strand from a circularly permuted split GFP, allowing the interaction to be reversible. Reversible complementation is desirable, but photodissociation has too low of an efficiency (quantum yield <1%) to be useful as an optogenetic tool. Understanding the physical origins of this low efficiency can provide strategies to improve it. We elucidated the mechanism of strand photodissociation by measuring the dependence of its rate on light intensity and point mutations. The results show that strand photodissociation is a two-step process involving light-activated cis-trans isomerization of the chromophore followed by light-independent strand dissociation. The dependence of the rate on temperature was then used to establish a potential energy surface (PES) diagram along the photodissociation reaction coordinate. The resulting energetics–function model reveals the rate-limiting process to be the transition from the electronic excited-state to the ground-state PES accompanying cis-trans isomerization. Comparisons between split GFPs and other photosensory proteins, like photoactive yellow protein and rhodopsin, provide potential strategies for improving the photodissociation quantum yield.
Optical techniques for investigating biological processes in vivo can achieve subcellular spatial and millisecond temporal resolution by using genetically encoded light-responsive proteins (1). Photoactivatable proteins are used as either imaging tools, such as reversibly photoswitchable fluorescent proteins (RSFPs), with fluorescence that can be modulated by light (2) or optogenetic switches that convert light input into physiological outputs, such as channelrhodopsins, which can regulate ion flow through membranes in response to light (3). Split GFPs have been widely applied for imaging as fluorescent reporters of cellular processes, because they are small (∼25 kDa), are stable in cytosol, produce chromophores autocatalytically, and are amenable to mutation and circular permutation (4). Typically, the protein is expressed as two or more constituent parts linked to separate proteins that only fluoresce on complementing with one another, offering readouts of protein–protein colocalizaton with low background and high specificity (5). However, this technique can generate misleading results, because the GFP complexes, after being formed and fluorescing, are bound irreversibly, which can be highly perturbative to the processes being studied, especially if the protein interaction being probed is ordinarily reversible (6). Although split GFP complexes essentially do not dissociate spontaneously after they are formed, we have discovered that dissociation into a peptide and a truncated protein can be induced by light irradiation in certain cases (7). Photodissociation introduces the possibility of active control of protein–protein interactions with light. Because split GFPs transduce chromophore excitation to both photon emission and large structural changes, they can be both imaging reporters and optogenetic tools, allowing for additional applications in tracking and delivering small molecules or photocaging enzyme activities using genetically encoded optical switches without relying on exogenous factors (8).
Split GFP photodissociation has not yet been extensively exploited, in part because of its low photodissociation quantum yield (<1%). In this study, we address the origins of low quantum yields by exploring the underlying mechanism and the photochemistry of photodissociation. Using both nonphotoswitchable and photoswitchable circular permutants to examine the dissociation rate dependence on incident light power, we show that photodissociation occurs via light-activated cis-trans isomerization of the chromophore followed by strand dissociation, linking photodissociable split GFPs to RSFPs. This mechanism is supported by point mutations that selectively enhance or suppress the elementary steps. Building on this foundational understanding, the energy landscape was investigated by examining the temperature dependence of photoactivation, strand dissociation, and fluorescence, revealing a barrier on the electronic excited-state energy surface and a photochemical funnel at the transition from the excited to the ground electronic-state potential energy surfaces (PESs). Our results establish an energetics–function model that provides generalizable insights for tuning photoswitchable and photodissociable fluorescent proteins. In the following work, we present the primary results and analysis, whereas more lengthy derivations, results, and side discussions are provided in SI Appendix so as not to interrupt the logical flow. In a number of cases, results are anticipated and then justified by subsequent experiments and analysis. The text is summarized in SI Appendix, section S4 for the convenience of the reader, and a comprehensive list of definitions of states and kinetic parameters involved in photodissociation is provided in SI Appendix, section S5.
Results and Discussion
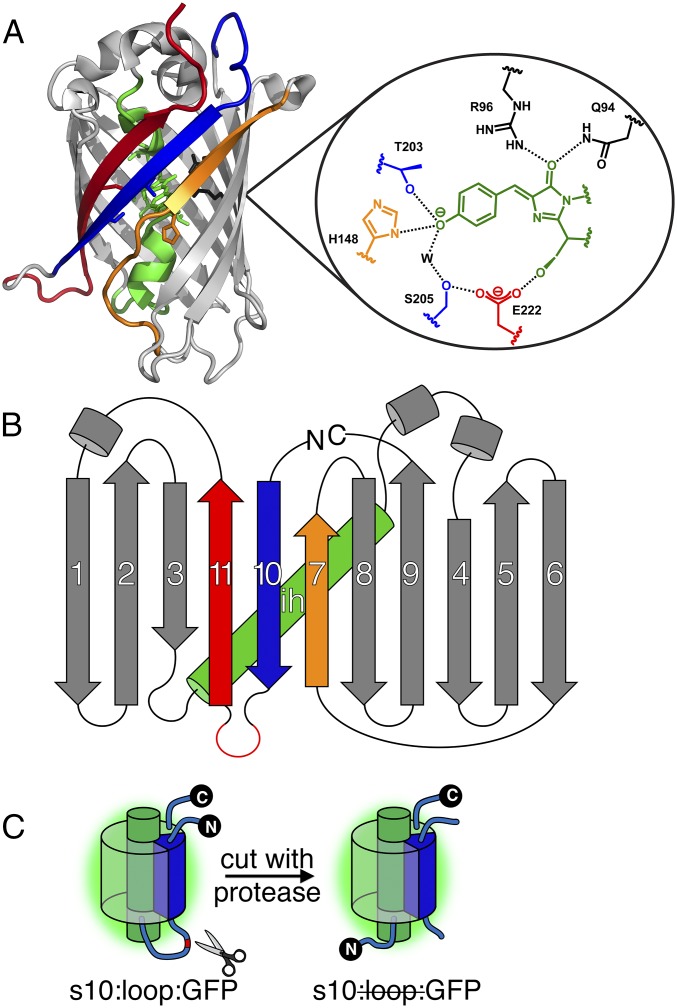
The work described here focuses on the underlying mechanism of photodissociation of split GFP with either its strand 7 (s7) or s10 circularly permuted to the N terminus. These split GFPs, denoted as s7:loop:GFP and s10:loop:GFP, respectively (notation is in Fig. 1 caption), can be prepared by enzymatically cleaving a sacrificial proteolytic loop introduced between the secondary structural element to be photodissociated and the rest of the protein (Fig. 1C).
Fig. 1.
Split GFP structure and preparation. (A) Ribbon structure of Superfolder GFP (Protein Data Bank ID code 2b3p) and the chromophore environment, highlighting residues important to this study. Key structural elements are color-coded as follows: the central helix is green (shown for the native S65 chromophore), β-s7 is orange, β-s10 is blue, and β-s11 is red. Each residue is colored to match its corresponding strand/helix. (B) Split GFP topology shown for the s10:loop:GFP circular permutant. (C) Example preparation scheme using a previously established nomenclature (9). Labels describe elements (demarcated by colons) of GFP progressing from the N terminus to the C terminus when read from left to right. Specific β-strands in the GFP β-barrel are denoted sX, where X is the number of the strand in question. Loop refers to a loop (red; between s10 and s11 here) with proteolytic cleavage sites. GFP refers to the remainder of the protein. A strike through an element indicates that the element has been removed. Synthetic elements are underlined. A dot is used to indicate a noncovalent interaction. For example, s10203Y·s10:loop:GFP denotes a synthetic β-s10 carrying the YFP mutation (T203Y) noncovalently bound to circularly permuted GFP with its original N-terminal s10 and loop removed.
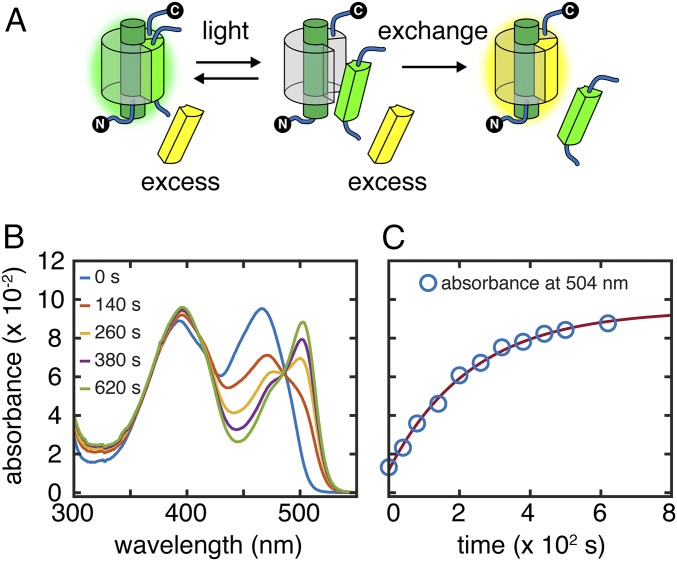
After photodissociation, the corresponding truncated protein can spontaneously reassociate with either the missing strand to form a GFP complex spectrally indistinguishable from the original protein (7) or a point-mutated synthetic strand to form a color-shifted complex that facilitates monitoring the photodissociation process via ultraviolet-visible spectroscopy (UV-vis). For example, replacing threonine 203 on s10 with tyrosine (T203Y) causes a bathochromic shift in the deprotonated chromophore (B state) absorbance, yielding YFPs (a shorthand used in this paper for proteins with the T203Y mutation) (4) (Fig. 1A and SI Appendix, Fig. S3), whereas replacing histidine 148 on s7 with aspartate (H148D) causes a bathochromic shift in protonated chromophore (A state) absorbance (10) (Fig. 1A and SI Appendix, Fig. S4). We can thus directly quantify the extent of strand exchange and obtain the overall photodissociation rate constant koff by performing light irradiation on the split GFP sample in the presence of excess synthetic peptide (s7148D or s10203Y) (Fig. 2A). Isosbestic points from the spectral progression during laser irradiation (Fig. 2B) imply conversion from the original split protein to the new complex without accumulation of intermediates. Time evolution of the absorbance at any wavelength except the isosbestic points fits well with a single-exponential curve and returns the same rate constant.
Fig. 2.
Strand photodissociation and rebinding. (A) Strand photodissociation experimental scheme. After it is photodissociated, a cut strand is replaced with a synthetic strand, inducing an absorbance change in the sample and allowing the dissociation rate to be measured. (B) An example of the scheme in A showing UV-vis spectra of 3 μM s10:loop:GFP mixed with 90 μM s10203Y and irradiated with 79 mW/mL 488-nm laser at 21.9 °C. (C) Time evolution of the absorbance at the YFP peak maximum (504 nm) follows single-exponential kinetics with the rate constant koff of 5.0 × 10−3 s−1.
Power Dependence: Photodissociation as a Two-Step Process.
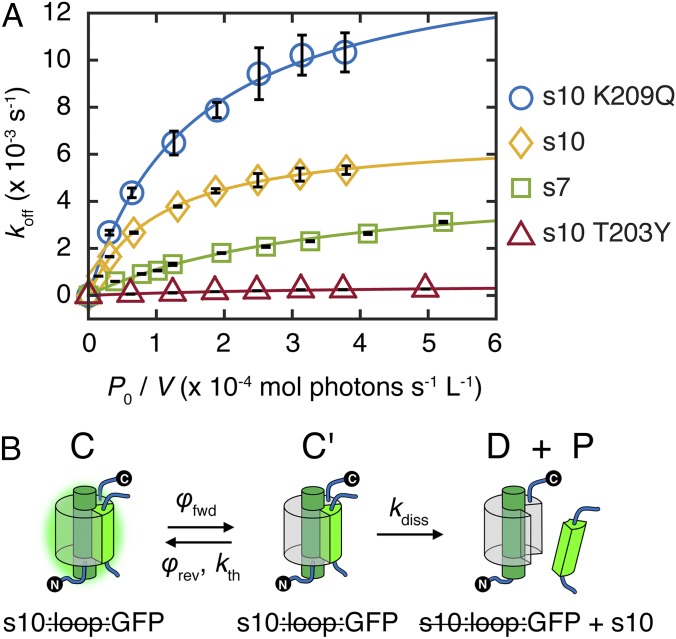
Knowing that light accelerates the strand dissociation of split GFP, we examined the dependence of the dissociation rate on incident light power as the first step in elucidating the underlying mechanism. Both circularly permuted split GFPs show similar behaviors in overall photodissociation rate constants koff with varying incident light power: koff increases linearly at low power and approaches a light-independent asymptote at high power (Fig. 3A). This behavior suggests a two-step process with one single-photon light-driven step (SI Appendix, sections S7 and S10) and one thermal step. The sequence of these steps cannot be determined from these data alone. Experiments discussed below will be used to show that the underlying photodissociation mechanism is a light-activated step followed by a thermal step (vide infra). Accepting for now the kinetic scheme illustrated in Fig. 3B and applying the steady-state approximation to the hypothetical transient intermediate C′ (SI Appendix, section S8), the dependence of the overall photodissociation rate constant koff on the incident light power P0 can be expressed as
| [1a] |
| [1b] |
and
| [1c] |
koff is characterized by the apparent photodissociation quantum yield φapp and the apparent thermal rate constant kapp (full derivation is in SI Appendix, section S7); εC and εC′ are the extinction coefficients of the split GFP before and after light activation at the incident wavelength (488 nm in our case), respectively; V is the sample volume; and is the path length. At low incident power (linear regime), when light activation is the rate-limiting step, φapp can be evaluated from the slope in the plot (Fig. 3A). At high incident power (saturating regime), when thermal dissociation is the rate-limiting step, koff approaches the asymptotic rate constant kapp. These apparent constants are convolved combinations of kinetic parameters from elementary steps. Because of the assumed fast equilibrium of the first step in the saturating regime, kapp (Eq. 1b) is a product of the thermal dissociation rate constant kdiss and the photostationary-state ratio between C and C′; φapp (Eq. 1c) is a product of the photoactivation quantum yield φfwd and the ground-state branching ratio , because C′ can either successfully dissociate into the truncated protein and free strand or thermally relax back to C.
Fig. 3.
Strand photodissociation power dependence and kinetic scheme. (A) Overall photodissociation rate constant koff vs. incident light power corrected for sample volume (P0/V; reported as moles of photons per second per liter) for s7:loop:GFP, s10:loop:GFP (203T), s10:loop:YFP (203Y) (SI Appendix, Fig. S7C), and s10:loop:GFP (209Q) (discussed later), all with E222 retained and irradiated with 488-nm laser at 21.9 °C. Data points are fit well with Eq. 1a. Parameters are listed in SI Appendix, Table S5. (B) Kinetic scheme for the photodissociation process; φfwd and φrev are the forward and reverse quantum yields, respectively, and kth and kdiss are the thermal relaxation and dissociation rate constants, respectively.
Datasets from both circular permutants can be fit well by Eq. 1a with different parameters (Fig. 3A), implying that they share a common mechanism consistent with the kinetic scheme, despite differences in molecular details. Although the apparent thermal rate constant for s7:loop:GFP (5 × 10−3 s−1) is similar to that for s10:loop:GFP (7 × 10−3 s−1), its apparent photodissociation quantum yield is one order of magnitude less (0.01% vs. 0.2%) (SI Appendix, Table S5). The latter agrees with the value estimated previously with 405-nm irradiation of s10:loop:GFP (7), suggesting that the light-activated step is similar even if different protonation states of the chromophore are excited, likely because formation of the I state (also anionic like the B state but with the protonated chromophore environment) by efficient excited-state proton transfer occurs much earlier than photodissociation (11). We use 488-nm irradiation in this study to avoid complications from irreversible E222 decarboxylation (12), which occurs with prolonged irradiation at 405 nm (13). In addition, 488-nm irradiation exclusively excites the B state of the chromophore, avoiding complications from excited-state proton transfer.
E222Q Mutants: Cis-Trans Isomerization as the Light-Activated Step.
Cis-trans isomerization of the GFP chromophore is a well-known structural rearrangement triggered by light irradiation and the basis of RSFPs (2), and we hypothesized that it could be the photoactivating step in the photodissociation process. Our circular permutants do not exhibit spectral changes on light irradiation, and therefore, we introduced the E222Q mutation into s10:loop:GFP. This mutation has been shown by Raman spectroscopy to facilitate photochromic cis-trans isomerization (SI Appendix, Fig. S9) (14, 15), and we believe that this assignment is still applicable to s10:loop:GFP, because circular permutation at the GFP loop region minimally perturbs the local chromophore environment as evidenced by retention of excited-state proton transfer and Förster resonance energy transfer between the only tryptophan and the chromophore (16). The extinction coefficients of the cis and trans species (SI Appendix, Fig. S11) were evaluated from photoswitching kinetics of E222Q mutants and used for spectral deconvolution and population determination (SI Appendix, sections S3 and S6 and Fig. S10) in the following experiments.
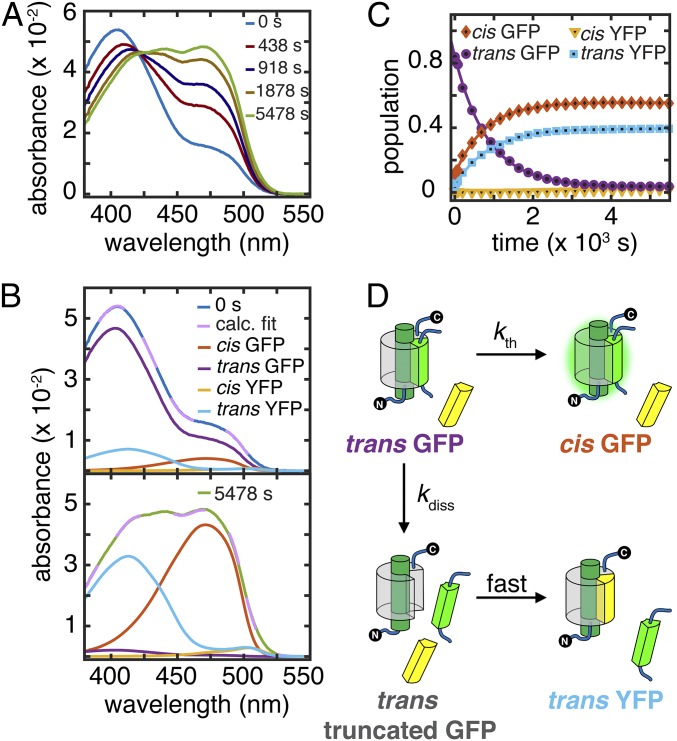
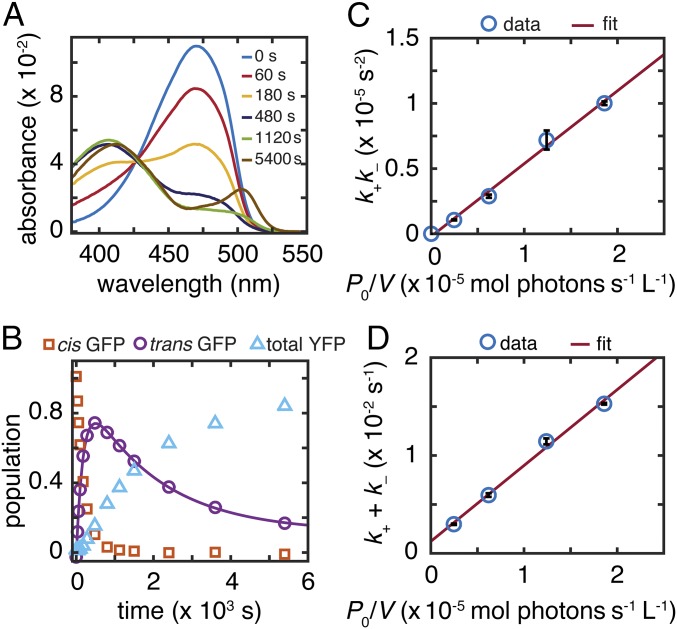
The model predicts that, if light activation corresponds to cis to trans isomerization, then the split protein with the chromophore in the trans state will dissociate in the dark. Photoswitching (1 × 10−2 s−1 under 3 mW/mL) is much faster than photodissociation (6 × 10−4 s−1 under the same conditions) (SI Appendix, Fig. S10), and therefore, brief light irradiation can be used to generate a photostationary mixture of cis and trans s10:loop:GFP E222Q in the presence of excess s10203Y with only minimal s10203Y·s10:loop:GFP E222Q (“YFP”) formation during preparation. The absorption spectrum of a mixture prepared this way was observed to evolve over time without additional irradiation in the dark and exhibited a clear isosbestic point (Fig. 4A). Spectral deconvolution (Fig. 4B) reveals single-exponential growths of both cis GFP and trans YFP at the expense of trans GFP (Fig. 4C), showing that strand dissociation is indeed a thermal step that occurs in the absence of light after photoisomerization of cis GFP (C) to trans GFP (now identified as C′). This result is also qualitatively consistent with the dependence of the photodissociation rate on viscosity (SI Appendix, section S13). Strand dissociation competes with dark relaxation back to cis GFP with rate constants kth = 5.0 × 10−4 s−1 and kdiss = 6.4 × 10−4 s−1 (Fig. 4C). Although the former agrees with the thermal relaxation rate constant measured from s10:loop:GFP E222Q (SI Appendix, Table S4), the latter is much smaller than its E222 counterpart. We measured kth−1 of E222Q YFP to be roughly 3 d, and therefore, formation of cis YFP from trans YFP is not appreciable on the timescale of the experiment. This observation also reveals that photodissociation forms trans truncated protein, which immediately binds to the added s10203Y peptide and creates trans YFP; this interpretation is confirmed by control experiments (SI Appendix, Fig. S13).
Fig. 4.
s10:loop:GFP E222Q dark relaxation after irradiation. (A) UV-vis spectra at representative time points for strand swapping with 90 μM s10203Y in the dark after irradiation of 3 μM s10:loop:GFP E222Q with 3.3 mW/mL 488-nm laser for 400 s at 21.9 °C to create a photostationary state of cis and trans forms. (B) Spectral deconvolution at initial and final time points from A using extinction coefficients from SI Appendix, Fig. S11. Fit curves (broken lilac lines) are linear combinations of basis spectra (SI Appendix, section S3). (C) Population kinetics extracted from A, including more data points than shown in A. Trans YFP, trans GFP, and cis GFP populations follow single-exponential kinetics. (D) Kinetic scheme for C.
In addition to identifying the C′ intermediate in our scheme, this result suggests that our E222 Superfolder GFPs are photoswitchable but have much smaller populations of trans species at their photostationary states (based on the validity of the steady-state approximation for E222 mutants) (SI Appendix, section S8) than other RSFPs (2). Although formation of trans Superfolder GFP is barely detectable by irradiating the GFP and directly observing its absorbance, our approach (photodissociation and trapping the resulting truncated protein with excess peptides) enables tracking this rare population by accumulating newly formed complexes and characterizing the photoswitching rates, provided that the trans protein does dissociate in the dark. In this way, photodissociation is especially useful for probing photoswitching kinetics in GFPs.
When adopting the E222Q mutants to determine the sequence of elementary steps and to identify the C′ intermediate, we assumed that the E222Q mutants would photodissociate according to the same scheme (Fig. 3B) as the E222 counterparts. To confirm that the E222Q mutants do indeed follow this kinetic scheme, the photodissociation kinetics of s10:loop:GFP E222Q were analyzed under continuous laser illumination (Fig. 5). Because a significant amount of trans species is present at the photostationary state, the steady-state approximation is no longer valid, and the kinetic scheme shown in Fig. 3B needs to be solved directly. This result anticipates two timescales for the E222Q dissociation kinetics (as opposed to one in the E222 case) (SI Appendix, section S9), which are supported by the rise and decay of the trans GFP population during the course of irradiation (Fig. 5B). The rate constants k+ and k− corresponding to the two timescales can be extracted by fitting the kinetic trace with double exponentials. The rate constants can be manipulated into linear functions of incident power:
| [2a] |
and
| [2b] |
Because kth and kdiss have already been determined by dark relaxation/dissociation experiments, photoswitching quantum yields can be determined by plotting k+k− and k+ + k− against P0/V (Fig. 5 C and D). The y intercept (kth + kdiss) in Fig. 5D (1.2 × 10−3 s−1) agrees with the value determined independently from the previous dark relaxation experiment (1.1 × 10−3 s−1) (Fig. 4C). The photoactivation quantum yield φfwd is estimated to be 0.5% (Fig. 5C), which is also consistent with φfwd determined independently from the photoswitching kinetics of s10:loop:GFP E222Q (0.6%) (SI Appendix, section S6 and Table S4), suggesting that the kinetic scheme for photodissociation is indeed light-activated cis-trans isomerization followed by thermal dissociation.
Fig. 5.
s10:loop:GFP E222Q strand photodissociation power dependence. (A) UV-vis spectra at representative time points for strand swapping of 3 μM s10:loop:GFP E222Q mixed with 90 μM s10203Y and continuously irradiated with 1.6 mW/mL 488-nm laser at 21.9 °C. Spectral deconvolution of intermediate time points is shown in SI Appendix, Fig. S14. (B) Population kinetics extracted from A. Trans GFP follows double-exponential kinetics. (C) The product and (D) the sum of k+ and k− as functions of P0/V, both including linear fits.
The reduced rate constant κ:
| [3a] |
| [3b] |
and
| [3c] |
can be used to generate the Michaelis–Menten-like relation (Eqs. 1a–1c) and the different power regimes from the two timescales (SI Appendix, Fig. S15). As a photoswitchable protein with an implied higher trans to cis population ratio than nonphotoswitchable proteins, s10:loop:GFP E222Q would be expected to be more readily photodissociable than its E222 counterpart, but this intuition is not correct. The strand photodissociation yield cannot be judged by a single “photoswitchability” parameter. Both φapp and kapp are important, because photodissociation is indeed a two-step power-dependent process. For this E222Q mutant, increases in incident light intensity past 3 mW/mL (compare with ∼100 mW/mL for s10:loop:GFP E222 in Fig. 3A) do not correspond to significant increases in photodissociation rate, because the dissociation rate is limited by kdiss (saturating regime in Fig. 3A and SI Appendix, Fig. S15). Although the E222Q mutant has a larger φfwd than the E222 mutant (0.5% vs. 0.2%), the E222Q mutant has a much smaller kdiss (6.4 × 10−4 s−1 vs. 7 × 10−3 s−1), limiting its maximal photodissociation rate kapp severely compared with that of the E222 mutant (Eqs. 1a and 3a). The observed result is that, although the E222Q mutants do photoswitch better than E222 mutants, their maximum strand photodissociation rate is much smaller. In addition, the slowness of kdiss also strongly penalizes φapp (∼0.3%) via the ground-state branching ratio (Eqs. 1c and 3c), bringing it close to that of E222 (∼0.2%). Accordingly, the E222Q mutant shows that fast photoswitching only enhances photodissociation if the intrinsic dissociation rate constant of its trans species does not act as a bottleneck for photodissociation. This unexpected decrease in kdiss caused by E222Q can possibly be explained by the mutant’s increased ability to accommodate the trans chromophore as evidenced by its photoswitchability. The resulting β-barrel structure is thus more stable than the E222 counterpart, leading to a higher affinity and lower dissociation rate of the cut strand.
Impacts of Mutations on Photodissociation.
After establishing a mechanism for strand photodissociation, we attempted to tune the rates of the elementary steps based on our understanding of their molecular nature.
To increase the thermal dissociation rate for s10:loop:GFP, we introduced K209Q on s10 (SI Appendix, Fig. S7A), which is far from the chromophore and has no impact on absorption. This mutation was expected to break the hydrogen bond between the side chain of K209 and the carbonyl group of H217 on s11, thereby weakening the interaction of s10 with the rest of the protein. Indeed, the asymptotic photodissociation rate constant kapp is enhanced more (2.2- vs. 1.5-fold) than φapp (equivalent to φfwd because of the steady-state approximation) (Fig. 3A and SI Appendix, section S8 and Table S5, measured values). Because K209Q does not change the spectral character of GFP and φfwd is only slightly affected because of the minute structural rearrangement of the barrel on cis-trans isomerization (17), the enhancement in kapp can be attributed to the perturbation in thermal strand dissociation.
In contrast, we can dramatically suppress both the light-activated and thermal steps in s10:loop:GFP circular permutants with the T203Y (YFP) mutation (Fig. 3A). T203Y especially suppresses the light activation process, because the critical π–π stacking between the 203Y residue and the chromophore (18) prevents the chromophore from isomerizing easily (7). In addition, T203Y suppresses the thermal step, because the π–π stacking helps anchor cut s10 to the rest of the protein. This trend can be found in both E222 and E222Q YFP, with fluorescence quantum yields that are noticeably enhanced and photoswitching quantum yields that are largely reduced with the T203Y mutation (SI Appendix, Table S4) relative to their GFP counterparts.
Temperature Dependence: Ground- and Excited-State PESs.
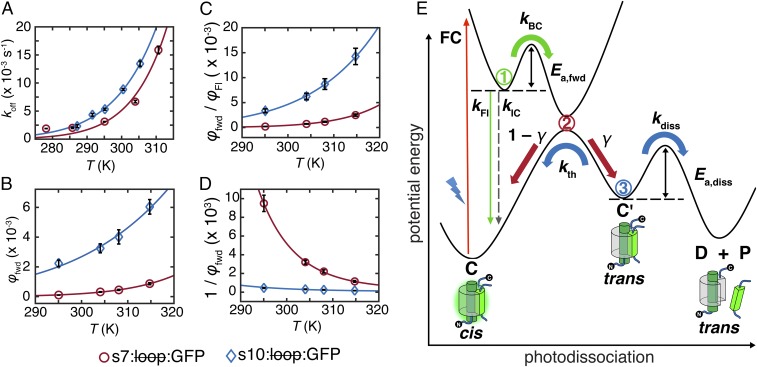
To probe the activation barriers and other features on the ground- and excited-state PESs along the reaction coordinate, we varied the temperature in both the linear and saturating regimes. Both kapp and φapp of the different circular permutants follow the Arrhenius temperature dependence (Fig. 6 A and B, respectively), suggesting that the corresponding processes are governed by thermal barriers. Because φapp is approximately φfwd under the steady-state approximation (SI Appendix, section S8), we can examine the temperature dependence of the light-activated step independently from that of thermal strand dissociation.
Fig. 6.
Temperature relations at 488-nm irradiation and scheme of photodissociation. Temperature dependence of (A) koff at saturating regime (97 mW/mL for s10:loop:GFP and 133 mW/mL for s7:loop:GFP), (B) φapp, (C) φfwd/φFl, and (D) 1/φfwd, with fits shown as solid lines. A–C were fit with the Arrhenius expression [f(x) = Ae−b/x]. D was fit with Eq. 5. (E) Potential energy curves for photodissociation highlighting relevant parameters. Branching points are shown as circled numbers and color-coded to match their associated processes. Branching point 1 represents the excited-state barrier partitioning fluorescence and isomerization. Branching point 2 represents the photochemical funnel, which divides aborted from successful isomerization. Branching point 3 represents branching of strand dissociation and thermal relaxation.
Because strand dissociation is far slower than all excited-state deactivation processes, both C and C′ are expected to be on the ground-state PES. Consequently, the light-activated process should involve three steps: (i) Franck–Condon excitation from C to the excited-state PES, (ii) thermal evolution on the excited-state PES, and (iii) return to the ground-state PES and, eventually, to C′. The electronic absorption spectra of both circular permutants are constant across the accessed temperature range (22 °C to 42 °C), meaning that the Franck–Condon transition cannot be temperature-dependent and that an energy barrier should be located on the excited-state PES before relaxation to the ground state (19, 20) and/or the ground-state PES before the formation of C′. In our minimal model, a barrier on the excited-state PES is more plausible, because, in order to fluoresce, fluorescent molecules should have barriers to competing nonradiative processes that involve significant structural changes on the excited-state PES.
More decisively, the fluorescence and photoactivation quantum yields were found to have temperature dependences that are inversely correlated with one another (Table 1), indicating that an excited-state energy barrier with an activation energy Ea,fwd partitions the two processes. In other words, fluorescence emission and formation of C′ are competing processes (Fig. 6E). Varying the temperature changes the fluorescence quantum yield much more than the photoactivation quantum yield, indicating that population branching (branching point 2 in Fig. 6E) occurs during the relaxation to the ground-state PES, with only some of the molecules reaching C′ and the rest returning to C through unproductive internal conversion. For branching to occur, the local minimum of the excited-state PES along the reaction coordinate should be situated near the local maximum of the ground-state PES. This topology leads to a photochemical funnel that facilitates radiationless relaxation to the ground-state PES (21). Various computational studies suggest that, for fluorescent proteins, this funnel is likely to be a conical intersection (22–25). No assumptions on the nature of the reaction coordinate have been made to acquire the features of the potential energy curves shown schematically in Fig. 6E.
Table 1.
Apparent photoactivation φapp and fluorescence quantum yields φFl for s10:loop:GFP and s7:loop:GFP at different temperatures
| Temperature (K) | s10:loop:GFP | s7:loop:GFP | ||
| φapp (×10−3) | φFl | φapp (×10−4) | φFl | |
| 295.0 | 2.2 ± 0.2 | 0.67 ± 0.04 | 1.0 ± 0.1 | 0.53 ± 0.05 |
| 304.0 | 3.2 ± 0.3 | 0.52 ± 0.03 | 3.1 ± 0.2 | 0.43 ± 0.02 |
| 308.0 | 4.0 ± 0.5 | 0.46 ± 0.01 | 4.5 ± 0.3 | 0.39 ± 0.01 |
| 314.8 | 6.0 ± 0.5 | 0.42 ± 0.03 | 8.7 ± 0.7 | 0.35 ± 0.01 |
Because thermal dissociation is the second step in the photodissociation mechanism, kapp contains parameters from both the light-activated and thermal steps (Eq. 1b). The effective energy barrier extracted from Fig. 6A should, therefore, be interpreted as Ea,diss + Ea,fwd − Ea,rev, where Ea,diss is the actual thermal dissociation activation energy, and Ea,rev is the activation energy associated with the reverse quantum yield φrev from C′ back to C (defined in Fig. 3B), which proceeds along another coordinate (not shown in Fig. 6E). Measuring Ea,rev separately is not straightforward, because C′ cannot be prepared in large quantities for E222 mutants, and therefore, Ea,diss cannot be determined from these measurements.
Analysis of the PESs: Finding Bottlenecks.
Our analysis indicates that strand photodissociation is a low quantum yield process in all of our mutants. Close examination of the processes along the reaction coordinate reveals why. After the Franck–Condon excitation and vibrational relaxation, most of the excited population returns directly back to C via fluorescence or internal conversion. The remainder overcomes the barrier to isomerization in the excited state (branching point 1 in Fig. 6E) and reaches the photochemical funnel. At the funnel (branching point 2 in Fig. 6E), some molecules return back to C through ultrafast internal conversion, and some convert to C′. The population reaching C′ either thermally dissociates into the truncated protein and the split strand or returns to C in competing processes with rates governed by ground-state PES barrier heights (branching point 3 in Fig. 6E).
The excited-state dynamics involve three rate constants: fluorescence kFl, direct internal conversion kIC, and barrier crossing kBC (Fig. 6E). The last one clearly follows the Arrhenius behavior with activation energy Ea,fwd, whereas the first two are likely to be relatively temperature-independent (26, 27) (SI Appendix, section S11). We hypothesize that the branching ratio γ at the photochemical funnel is also temperature-independent or, at most, weakly temperature-dependent within the narrow temperature range that we accessed (28, 29) (SI Appendix, section S12). Consequently, we can write the temperature dependencies of the quantum yields for fluorescence φFl and photoactivation (forward photoswitching) φfwd (30, 31):
| [4a] |
and
| [4b] |
When φfwd is small, barrier crossing is a rare process because of the high energy barrier, and therefore, the contribution of kBC is negligible in the denominator of φfwd, justifying the observation that φfwd obeys Arrhenius behavior (Fig. 6B). However, more accurate values of Ea,fwd can be obtained from an Arrhenius fit of φfwd/φFl vs. temperature without making this approximation (Fig. 6C). The resulting Ea,fwd values are 14 ± 2 and 22 ± 2 kcal/mol for s10 and s7 circular permutants, respectively. These energy barriers experienced by the chromophore during isomerization are caused by the protein environment as shown by model GFP chromophores, which isomerize efficiently instead of fluorescing in fluid solutions unless frozen (32–35). This viewpoint is also supported by various computational studies (24, 36–39). The existence of these large excited-state energy barriers leads to strong fluorescence in GFPs in contrast to other proteins, such as rhodopsin (40) and photoactive yellow protein (PYP) (41), that generically have similar PESs (Results and Discussion, A Unifying Scheme of Fluorescent and Photosensory Proteins) but are optimized for cis-trans isomerization instead of fluorescence.
Using Ea,fwd, the branching ratio γ at the photochemical funnel can be evaluated with Eq. 5, which is a rearrangement of Eq. 4b:
| [5] |
γ values for s10 and s7 circular permutants are estimated to be 0.01 and 0.003, respectively (Fig. 6D). Although most of the excited-state population converts back to C either with or without radiation (∼66% and ∼12%, respectively, for the s10 circular permutant at room temperature), a substantial fraction (∼22%) of the excited protein experiences partial cis-trans isomerization and reaches the photochemical funnel, branching point 2 (Fig. 6E). However, most of it returns back to C, with only a small portion of the total population (∼0.22%) forming C′, which is the precursor to strand dissociation. In other words, for photodissociation to occur, the split protein needs to navigate three branching points upon excitation: barrier crossing, the photochemical funnel, and the ground-state branching (Fig. 6E).
Despite the large excited-state energy barriers, the largest bottleneck to strand dissociation is at the photochemical funnel (branching point 2). According to the Landau–Zener equation (28, 29), for a 1D PES, γ is roughly determined by both the geometry of the funnel (the slope of the PES near the funnel) and dynamics (the velocity of the excited-state population along the reaction coordinate approaching the funnel). Because a large excited-state energy barrier is present on the path toward the funnel, the effect of the dynamic factor is expected to be suppressed, and therefore, it is useful to focus more on the geometric effect of the funnel. If the photochemical funnel involved is a conical intersection, it should be relatively sloped rather than peaked to strongly bias toward aborted isomerization (42–44). This topology is also very distinct from rhodopsin, the epitome of efficient cis-trans isomerization, in which the conical intersection is peaked, facilitating efficient isomerization for its role in vision (45, 46).
A Unifying Scheme of Fluorescent and Photosensory Proteins.
Because nature has optimized fluorescence and photoisomerization quantum yields in proteins, such as GFP and PYP, respectively, we think that we can learn how to tune these properties by examining and comparing the systems with one another. The potential energy curve scheme (Fig. 6E) during light activation is ubiquitous among fluorescent proteins as suggested by transient absorption experiments (47, 48) and should apply broadly to photosensory proteins with chromophores that can readily isomerize on irradiation (23, 37, 44, 49), such as rhodopsin (40, 46, 50, 51) and PYP (52–55). Our photodissociation study suggests that normal GFPs (such as Superfolder GFPs) that are not traditionally considered photoswitchable are actually capable of cis-trans isomerization in the same way as RSFPs. The perceived difference arises from the lack of a spectral change on isomerization and the low forward switching quantum yield of normal GFPs, which is limited by the excited-state energy barrier (as suggested by fluorescence quantum yields) and the branching ratio γ at the photochemical funnel. Dronpa and IrisFP, two well-characterized RSFPs, can be used to illustrate this point (56, 57). Dronpa, IrisFP, and our GFP mutants all show inverse relations between fluorescence and switching quantum yields (Table 2). The reverse switching processes also follow this inverse relation, with the off-states having much smaller fluorescence and much larger switching quantum yields than their on-state counterparts. RSFPs with trans chromophores are generally more flexible proteins than their cis counterparts as determined by NMR spectroscopy and computational studies (58, 59), which leads to lower fluorescence from the excited state of the trans forms, suggesting lower excited energy barriers for the off-to-on than the on-to-off switching processes. This observation extends to a general trend that trans fluorescent proteins are less fluorescent and more photoswitchable than cis proteins (60). Moreover, we speculate based on our observations that it is this flexibility of the trans state, especially from s7–s10, that also allows trans split proteins [or constituent parts linked with a long loop (61)] to dissociate in the dark rather than the motion of cis-trans isomerization itself (i.e., cis-trans isomerization and strand dissociation are not concerted). Whether cis-trans isomerization is achieved by one-bond flip (62) or volume-conserving hula twist (36, 63) is not relevant, because the trans structure does not depend on the pathway of isomerization.
Table 2.
Photoswitching parameters of various fluorescent proteins
| Species | φfwd | φrev | φFl,on(cis) | φFl,off(trans) | γfwd | γrev |
| s10:loop:GFP | (2.2 ± 0.2) × 10−3 | N/A | 0.67 ± 0.04 | N/A | 0.01 ± 0.03 | N/A |
| s7:loop:GFP | (1.1 ± 0.1) × 10−4 | N/A | 0.53 ± 0.05 | N/A | 0.003 ± 0.005 | N/A |
| Dronpa (47, 56) | 3 × 10−4 | 7 × 10−2 | 0.85 | 0.02 | N/A | 0.07 |
| IrisFP (48, 57) | 5 × 10−3 | 1.5 × 10−1 | 0.43 | N/A | N/A | 0.17 |
φfwd, φrev, φFl,on(cis), φFl,off(trans), γfwd, and γrev are forward switching quantum yield, reverse switching quantum yield, on-state (cis) fluorescence quantum yield, off-state (trans) fluorescence quantum yield, forward branching ratio, and reverse branching ratio at the photochemical funnel, respectively. N/A, not available.
Although the natural inverse relation of φfwd (or φrev) and φFl in RSFPs is roughly caused by being partitioned by excited-state energy barriers as suggested by our PES model (Fig. 6E), the exact relation depends on the branching ratio γ at the photochemical funnel (Eqs. 4a and 4b) (60). For these proteins, reverse photoswitching γ values are significantly larger than those of forward switching for our GFP constructs (Table 2), which serve as bottlenecks to strand photodissociation. This observation suggests that it is possible to increase γ appreciably by mutating the residues on the barrel, which will enhance the overall photodissociation quantum yield (see below).
Because we are concerned with photoswitching, we look beyond RSFPs to other proteins that have been naturally optimized solely for photoswitching. In contrast to fluorescent proteins, PYP and rhodopsin have exceedingly low fluorescence quantum yields [2 × 10−3 (64) and 1 × 10−5 (51), respectively], while exhibiting excellent cis-trans isomerization efficiencies [quantum yields 35% (65) and 65% (66), respectively]. Early studies suggest their similarities (52, 67, 68): they have steep Franck–Condon regions (i.e., strong vibronic coupling along the reaction coordinate) and either small or no excited-state barriers, allowing the excited population to rush down to the conical intersection without much impediment, and their conical intersections are relatively more peaked than sloped to increase isomerization quantum yields while suppressing competing internal conversion (45, 46, 53, 69). Although fluorescent proteins and photosensory proteins have drastically different fluorescence and isomerization quantum efficiencies from one another, they can all be described with the same type of PES topology, suggesting that we can learn from photosensory proteins to improve the photodissociation of split GFPs. This knowledge is transferrable, because isomerization efficiencies, in addition to being intrinsic properties of chromophores, are also influenced by protein environments.
The Role of the Protein Environment: Property Tuning and Optimization.
On excitation in solution, the GFP chromophore exhibits almost no fluorescence [quantum yield less than 10−3 (34)], the PYP chromophore shows no detectable photoisomerization (70), and all-trans retinal protonated Schiff base converts into a mixture of 11-cis, 9-cis, and 13-cis with quantum yields of 14%, 2%, and 1%, respectively (51). The protein environment, however, can suppress the unwanted major channels observed in solution (64) and enhance minor reactions, such as all-trans retinal isomerizing into 13-cis (and nothing else) with 64% yield (vs. 1%) in bacteriorhodopsin, by adjusting the potential energy landscape of the embedded chromophores. Protein matrices have major effects on redistributing the quantum yields, which indicates that it is possible to attain desired properties by adjusting the protein environment without changing the chromophore. Through a PES model (Fig. 6E), we can directly link observed functions to energetic contributions from protein environments, allowing us to propose the following property-tuning strategies with a hierarchical structure: color (absorption and fluorescence), fluorescence quantum yield, isomerization quantum yield, and photodissociation.
Color tuning has been studied with fluorescent proteins (71), PYP (72), and retinal proteins (“opsin shift”) (73), which all adjust the energy gaps between the excited and ground states. These energy gaps correlate well with electrostatic interactions experienced by the chromophores (74–76). Fluorescence quantum yields can be increased by raising the excited-state barrier height either sterically (as with T203Y) (35) or electrostatically (77) to suppress undesired internal conversion or isomerization. Because charge transfer is dominant during the isomerization process (36, 55, 70, 78, 79), barrier heights can, in principle, be adjusted systematically via electrostatics (80, 81). Conversely, to enhance the isomerization efficiency instead of fluorescence, one needs to lower the excited-state energy barrier as well as tune the conical intersection toward more peaked topology. Similar to isomerization, the structure at the conical intersection has charge transfer character that could be stabilized via electrostatics, altering the shape of the conical intersection (24, 82). However, because conical intersections can be higher-dimensional structures (e.g., seams), tuning them can also change their accessibility or channel the excited-state population into other unwanted degrees of freedom (49). In addition, the photoisomerization quantum yield can be influenced by the phases and amplitudes of involved vibrational modes because of the coherent nature of the excited-state dynamics, adding another layer of complexity (83, 84). Finally, maximizing the photodissociation efficiency requires preserving the high isomerization quantum yield and increasing the thermal dissociation rate of the subsequent trans species (such as K209Q in our case), and to be useful, spontaneous dissociation of the split strand needs to be suppressed. Thus, improving the net photodissociation involves simultaneous optimization of these three topological structures on the potential energy landscape.
Using this energetics–function property-tuning approach requires careful consideration of two limitations. First, the mutant space is constricted (85), and therefore, different photochemical properties might not be independently tunable (86). This limitation was shown by the E222Q mutation, which was predicted to increase the photodissociation quantum yield. Although E222Q successfully enhances the cis-trans isomerization quantum yield of the s10 circular permutant, it sacrifices thermal dissociation of the trans protein, leaving the overall photodissociation quantum yield slightly better but the maximal rate severely restricted. Furthermore, the overall structure, folding efficiency, and chromophore maturation efficiency have to be preserved on mutations, complicating the protein design problem. Second, structure–energetics knowledge is necessary and can be obtained either from computational predictions using X-ray structures (44, 49) or by characterizing exhaustive mutations on important residues of model systems for specific photochemistry (64, 72).
Concluding Remarks
We have unified normal and photoswitchable GFPs by elucidating the underlying mechanism of split fluorescent protein strand photodissociation. We initially observed a Michaelis–Menten incident light power dependence on strand dissociation from split GFPs measured by strand replacement under pseudo–first-order conditions, suggesting that two steps are involved in photodissociation. The linear low-power light dependence indicates that one step must be one-photon light activation and that the other step must be strand dissociation. We found that light activation precedes thermal dissociation by observing the time evolution of E222Q mutants, which are known to exhibit photochromic cis-trans isomerization under light irradiation. We confirmed our notions of the process by using the T203Y and K209Q mutations to suppress and enhance, respectively, the two elementary steps. Measuring the temperature dependence of the photodissociation rate allowed us to deduce critical features of the potential energy curves for both excited and ground states, establishing an energetics–function relationship. This analysis suggests that the bottleneck is the branching of populations at the photochemical funnel, where successful cis-trans isomerization is much less favorable than aborted isomerization. This model was then used to support the possibility that GFPs and photoreceptors, such as PYP and rhodopsin, exist on two extremes of the same spectrum but exhibit different degrees of cis-trans isomerization and fluorescence.
A structure–function relationship is required to rationally improve photodissociation, but such a relationship can be very difficult to understand intuitively. Given the difficulty of linking structures and functions directly, we took a step back and proposed breaking this problem into two more manageable parts: a structure–energetics relationship and an energetics–function model. The former can be guided by computational studies based on crystallographic structures. In addressing the latter, we found it useful to unify some aspects of different proteins using our PES model to seek energetics insights for tuning photoswitchable and photodissociable fluorescent proteins as genetically encoded tools for monitoring and manipulating protein interactions or functions in cells. Clearly, significant improvements in quantum efficiency can most likely be made by starting with weakly fluorescent photoswitchable fluorescent proteins and performing directed evolution, which is a satisfying solution from an engineering perspective. However, this strategy hardly increases our fundamental understanding of protein function engineering, and therefore, we advocate for our more systematic approach, which can hopefully reduce the complexity of rationally engineering protein function and provide insights for future protein design research.
Supplementary Material
Acknowledgments
We thank the Hesselink Laboratory at Stanford and, particularly, Dr. Yao-Te Cheng for providing access to an argon-ion laser. Prof. Todd Martínez, Dr. Luke Oltrogge, and Sam Schneider provided extensive discussions. C.-Y.L. was supported by a Kenneth and Nina Tai Stanford Graduate Fellowship and the Taiwanese Ministry of Education. K.D. was supported by a John Stauffer Stanford Graduate Fellowship and the Korea Foundation for Advanced Studies. This work was supported, in part, by NIH Grants GM27738 (to S.G.B.) and GM118044 (to S.G.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.A.M. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618087114/-/DCSupplemental.
References
- 1.Kao JPY. Caged molecules: Principles and practical considerations. Curr Protoc Neurosci. 2006;6:6.20. doi: 10.1002/0471142301.ns0620s37. [DOI] [PubMed] [Google Scholar]
- 2.Zhou XX, Lin MZ. Photoswitchable fluorescent proteins: Ten years of colorful chemistry and exciting applications. Curr Opin Chem Biol. 2013;17(4):682–690. doi: 10.1016/j.cbpa.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deisseroth K. Optogenetics. Nat Methods. 2011;8(1):26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 5.Kerppola TK. Visualization of molecular interactions by fluorescence complementation. Nat Rev Mol Cell Biol. 2006;7(6):449–456. doi: 10.1038/nrm1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamura T, Hamachi I. Recent progress in design of protein-based fluorescent biosensors and their cellular applications. ACS Chem Biol. 2014;9(12):2708–2717. doi: 10.1021/cb500661v. [DOI] [PubMed] [Google Scholar]
- 7.Do K, Boxer SG. Thermodynamics, kinetics, and photochemistry of β-strand association and dissociation in a split-GFP system. J Am Chem Soc. 2011;133(45):18078–18081. doi: 10.1021/ja207985w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautier A, et al. How to control proteins with light in living systems. Nat Chem Biol. 2014;10(7):533–541. doi: 10.1038/nchembio.1534. [DOI] [PubMed] [Google Scholar]
- 9.Kent KP, Oltrogge LM, Boxer SG. Synthetic control of green fluorescent protein. J Am Chem Soc. 2009;131(44):15988–15989. doi: 10.1021/ja906303f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shu X, et al. Ultrafast excited-state dynamics in the green fluorescent protein variant S65T/H148D. 1. Mutagenesis and structural studies. Biochemistry. 2007;46(43):12005–12013. doi: 10.1021/bi7009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chattoraj M, King BA, Bublitz GU, Boxer SG. Ultra-fast excited state dynamics in green fluorescent protein: Multiple states and proton transfer. Proc Natl Acad Sci USA. 1996;93(16):8362–8367. doi: 10.1073/pnas.93.16.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Thor JJ, Gensch T, Hellingwerf KJ, Johnson LN. Phototransformation of green fluorescent protein with UV and visible light leads to decarboxylation of glutamate 222. Nat Struct Biol. 2002;9(1):37–41. doi: 10.1038/nsb739. [DOI] [PubMed] [Google Scholar]
- 13.Bell AF, Stoner-Ma D, Wachter RM, Tonge PJ. Light-driven decarboxylation of wild-type green fluorescent protein. J Am Chem Soc. 2003;125(23):6919–6926. doi: 10.1021/ja034588w. [DOI] [PubMed] [Google Scholar]
- 14.Bizzarri R, et al. Single amino acid replacement makes Aequorea victoria fluorescent proteins reversibly photoswitchable. J Am Chem Soc. 2010;132(1):85–95. doi: 10.1021/ja9014953. [DOI] [PubMed] [Google Scholar]
- 15.Abbruzzetti S, et al. Photoswitching of E222Q GFP mutants: “Concerted” mechanism of chromophore isomerization and protonation. Photochem Photobiol Sci. 2010;9(10):1307–1319. doi: 10.1039/c0pp00189a. [DOI] [PubMed] [Google Scholar]
- 16.Oltrogge LM, Wang Q, Boxer SG. Ground-state proton transfer kinetics in green fluorescent protein. Biochemistry. 2014;53(37):5947–5957. doi: 10.1021/bi500147n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao Y-T, Zhu X, Min W. Protein-flexibility mediated coupling between photoswitching kinetics and surrounding viscosity of a photochromic fluorescent protein. Proc Natl Acad Sci USA. 2012;109(9):3220–3225. doi: 10.1073/pnas.1115311109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wachter RM, Elsliger MA, Kallio K, Hanson GT, Remington SJ. Structural basis of spectral shifts in the yellow-emission variants of green fluorescent protein. Structure. 1998;6(10):1267–1277. doi: 10.1016/s0969-2126(98)00127-0. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman G, Chow L-Y, Paik U-J. The photochemical isomerization of azobenzene. J Am Chem Soc. 1958;80(14):3528–3531. [Google Scholar]
- 20.Malkin S, Fischer E. Temperature dependence of photoisomerization. Part II. 1. Quantum yields of cis⇆trans isomerizations in azo-compounds. J Phys Chem. 1962;66(12):2482–2486. [Google Scholar]
- 21.Turro NJ, Ramamurthy V, Scaiano JC. Modern Molecular Photochemistry of Organic Molecules. 1st Ed University Science Books; Sausalito, CA: 2010. [Google Scholar]
- 22.Martin ME, Negri F, Olivucci M. Origin, nature, and fate of the fluorescent state of the green fluorescent protein chromophore at the CASPT2//CASSCF resolution. J Am Chem Soc. 2004;126(17):5452–5464. doi: 10.1021/ja037278m. [DOI] [PubMed] [Google Scholar]
- 23.Martínez TJ. Insights for light-driven molecular devices from ab initio multiple spawning excited-state dynamics of organic and biological chromophores. Acc Chem Res. 2006;39(2):119–126. doi: 10.1021/ar040202q. [DOI] [PubMed] [Google Scholar]
- 24.Levine BG, Martínez TJ. Isomerization through conical intersections. Annu Rev Phys Chem. 2007;58:613–634. doi: 10.1146/annurev.physchem.57.032905.104612. [DOI] [PubMed] [Google Scholar]
- 25.Schäfer LV, Groenhof G, Boggio-Pasqua M, Robb MA, Grubmüller H. Chromophore protonation state controls photoswitching of the fluoroprotein asFP595. PLoS Comput Biol. 2008;4(3):e1000034. doi: 10.1371/journal.pcbi.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strickler SJ, Berg RA. Relationship between absorption intensity and fluorescence lifetime of molecules. J Chem Phys. 1962;37(4):814–822. [Google Scholar]
- 27.Nitzan A. Chemical Dynamics in Condensed Phases. 1st Ed Oxford Univ Press; New York: 2006. [Google Scholar]
- 28.Wang Q, Schoenlein RW, Peteanu LA, Mathies RA, Shank CV. Vibrationally coherent photochemistry in the femtosecond primary event of vision. Science. 1994;266(5184):422–424. doi: 10.1126/science.7939680. [DOI] [PubMed] [Google Scholar]
- 29.Wittig C. The Landau-Zener formula. J Phys Chem B. 2005;109(17):8428–8430. doi: 10.1021/jp040627u. [DOI] [PubMed] [Google Scholar]
- 30.Malkin S, Fischer E. Temperature dependence of photoisomerization. III. Direct and sensitized photoisomerization of stilbenes. J Phys Chem. 1964;68(5):1153–1163. [Google Scholar]
- 31.Rubin MB, Weiner M, Katraro R, Speiser S. The temperature dependence of competing photoisomerization and fluorescence decay. J Photochem. 1979;11(4):287–291. [Google Scholar]
- 32.Niwa H, et al. Chemical nature of the light emitter of the Aequorea green fluorescent protein. Proc Natl Acad Sci USA. 1996;93(24):13617–13622. doi: 10.1073/pnas.93.24.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webber NM, Litvinenko KL, Meech SR. Radiationless relaxation in a synthetic analogue of the green fluorescent protein chromophore. J Phys Chem B. 2001;105(33):8036–8039. [Google Scholar]
- 34.Yang J-S, Huang G-J, Liu Y-H, Peng SM. Photoisomerization of the green fluorescence protein chromophore and the meta- and para-amino analogues. Chem Commun (Camb) 2008;(11):1344–1346. doi: 10.1039/b717714c. [DOI] [PubMed] [Google Scholar]
- 35.Walker CL, et al. Fluorescence imaging using synthetic GFP chromophores. Curr Opin Chem Biol. 2015;27:64–74. doi: 10.1016/j.cbpa.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Toniolo A, Olsen S, Manohar L, Martínez TJ. Conical intersection dynamics in solution: The chromophore of green fluorescent protein. Faraday Discuss. 2004;127:149–163. doi: 10.1039/b401167h. [DOI] [PubMed] [Google Scholar]
- 37.Virshup AM, et al. Photodynamics in complex environments: Ab initio multiple spawning quantum mechanical/molecular mechanical dynamics. J Phys Chem B. 2009;113(11):3280–3291. doi: 10.1021/jp8073464. [DOI] [PubMed] [Google Scholar]
- 38.Vallverdu G, Demachy I, Ridard J, Lévy B. Using biased molecular dynamics and Brownian dynamics in the study of fluorescent proteins. J Mol Struct THEOCHEM. 2009;898(1):73–81. [Google Scholar]
- 39.Koseki J, Kita Y, Nagashima U, Tachikawa M. Theoretical study of the reversible photoconversion mechanism in Dronpa. Procedia Comput Sci. 2011;4:251–260. [Google Scholar]
- 40.Wand A, Gdor I, Zhu J, Sheves M, Ruhman S. Shedding new light on retinal protein photochemistry. Annu Rev Phys Chem. 2013;64:437–458. doi: 10.1146/annurev-physchem-040412-110148. [DOI] [PubMed] [Google Scholar]
- 41.Imamoto Y, Kataoka M. Structure and photoreaction of photoactive yellow protein, a structural prototype of the PAS domain superfamily. Photochem Photobiol. 2007;83(1):40–49. doi: 10.1562/2006-02-28-IR-827. [DOI] [PubMed] [Google Scholar]
- 42.Migani A, Olivucci M. Conical intersections and organic reaction mechanisms. In: Domcke W, Yarkony DR, Köppel H, editors. Advanced Series in Physical Chemistry Volume 15: Conical Intersections. World Scientific; Singapore: 2004. pp. 271–320. [Google Scholar]
- 43.Martínez TJ. Physical chemistry: Seaming is believing. Nature. 2010;467(7314):412–413. doi: 10.1038/467412a. [DOI] [PubMed] [Google Scholar]
- 44.Schapiro I, Melaccio F, Laricheva EN, Olivucci M. Using the computer to understand the chemistry of conical intersections. Photochem Photobiol Sci. 2011;10(6):867–886. doi: 10.1039/c0pp00290a. [DOI] [PubMed] [Google Scholar]
- 45.Frutos LM, Andruniów T, Santoro F, Ferré N, Olivucci M. Tracking the excited-state time evolution of the visual pigment with multiconfigurational quantum chemistry. Proc Natl Acad Sci USA. 2007;104(19):7764–7769. doi: 10.1073/pnas.0701732104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polli D, et al. Conical intersection dynamics of the primary photoisomerization event in vision. Nature. 2010;467(7314):440–443. doi: 10.1038/nature09346. [DOI] [PubMed] [Google Scholar]
- 47.Yadav D, et al. Real-time monitoring of chromophore isomerization and deprotonation during the photoactivation of the fluorescent protein Dronpa. J Phys Chem B. 2015;119(6):2404–2414. doi: 10.1021/jp507094f. [DOI] [PubMed] [Google Scholar]
- 48.Colletier J-P, et al. Serial femtosecond crystallography and ultrafast absorption spectroscopy of the photoswitchable fluorescent protein IrisFP. J Phys Chem Lett. 2016;7(5):882–887. doi: 10.1021/acs.jpclett.5b02789. [DOI] [PubMed] [Google Scholar]
- 49.Gozem S, Melaccio F, Luk HL, Rinaldi S, Olivucci M. Learning from photobiology how to design molecular devices using a computer. Chem Soc Rev. 2014;43(12):4019–4036. doi: 10.1039/c4cs00037d. [DOI] [PubMed] [Google Scholar]
- 50.Kukura P, McCamant DW, Yoon S, Wandschneider DB, Mathies RA. Structural observation of the primary isomerization in vision with femtosecond-stimulated Raman. Science. 2005;310(5750):1006–1009. doi: 10.1126/science.1118379. [DOI] [PubMed] [Google Scholar]
- 51.Schulten K, Hayashi S. Quantum biology of retinal. In: Mohseni M, Omar Y, Engel GS, Plenio MB, editors. Quantum Effects in Biology. Cambridge Univ Press; Cambridge, UK: 2014. pp. 237–263. [Google Scholar]
- 52.Mataga N, et al. Ultrafast photoreactions in protein nanospaces as revealed by fs fluorescence dynamics measurements on photoactive yellow protein and related systems. Phys Chem Chem Phys. 2003;5(11):2454–2460. [Google Scholar]
- 53.Groenhof G, et al. Photoactivation of the photoactive yellow protein: Why photon absorption triggers a trans-to-cis isomerization of the chromophore in the protein. J Am Chem Soc. 2004;126(13):4228–4233. doi: 10.1021/ja039557f. [DOI] [PubMed] [Google Scholar]
- 54.Vengris M, et al. Contrasting the excited-state dynamics of the photoactive yellow protein chromophore: Protein versus solvent environments. Biophys J. 2004;87(3):1848–1857. doi: 10.1529/biophysj.104.043224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu J, et al. Short hydrogen bonds and negative charge in photoactive yellow protein promote fast isomerization but not high quantum yield. J Phys Chem B. 2015;119(6):2372–2383. doi: 10.1021/jp506785q. [DOI] [PubMed] [Google Scholar]
- 56.Habuchi S, et al. Reversible single-molecule photoswitching in the GFP-like fluorescent protein Dronpa. Proc Natl Acad Sci USA. 2005;102(27):9511–9516. doi: 10.1073/pnas.0500489102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adam V, et al. Structural characterization of IrisFP, an optical highlighter undergoing multiple photo-induced transformations. Proc Natl Acad Sci USA. 2008;105(47):18343–18348. doi: 10.1073/pnas.0805949105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mizuno H, et al. Light-dependent regulation of structural flexibility in a photochromic fluorescent protein. Proc Natl Acad Sci USA. 2008;105(27):9227–9232. doi: 10.1073/pnas.0709599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smyrnova D, et al. Molecular dynamic indicators of the photoswitching properties of green fluorescent proteins. J Phys Chem B. 2015;119(36):12007–12016. doi: 10.1021/acs.jpcb.5b04826. [DOI] [PubMed] [Google Scholar]
- 60.Bourgeois D, Adam V. Reversible photoswitching in fluorescent proteins: A mechanistic view. IUBMB Life. 2012;64(6):482–491. doi: 10.1002/iub.1023. [DOI] [PubMed] [Google Scholar]
- 61.Do K, Boxer SG. GFP variants with alternative β-strands and their application as light-driven protease sensors: A tale of two tails. J Am Chem Soc. 2013;135(28):10226–10229. doi: 10.1021/ja4037274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu RSH. Photoisomerization by hula-twist: A fundamental supramolecular photochemical reaction. Acc Chem Res. 2001;34(7):555–562. doi: 10.1021/ar000165c. [DOI] [PubMed] [Google Scholar]
- 63.Zimmer M. Green fluorescent protein (GFP): Applications, structure, and related photophysical behavior. Chem Rev. 2002;102(3):759–781. doi: 10.1021/cr010142r. [DOI] [PubMed] [Google Scholar]
- 64.Philip AF, Eisenman KT, Papadantonakis GA, Hoff WD. Functional tuning of photoactive yellow protein by active site residue 46. Biochemistry. 2008;47(52):13800–13810. doi: 10.1021/bi801730y. [DOI] [PubMed] [Google Scholar]
- 65.van Brederode ME, Gensch T, Hoff WD, Hellingwerf KJ, Braslavsky SE. Photoinduced volume change and energy storage associated with the early transformations of the photoactive yellow protein from Ectothiorhodospira halophila. Biophys J. 1995;68(3):1101–1109. doi: 10.1016/S0006-3495(95)80284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dartnall HJA. The photosensitivities of visual pigments in the presence of hydroxylamine. Vision Res. 1968;8(4):339–358. doi: 10.1016/0042-6989(68)90104-1. [DOI] [PubMed] [Google Scholar]
- 67.Mataga N, et al. Ultrafast photoinduced reaction dynamics of photoactive yellow protein (PYP): Observation of coherent oscillations in the femtosecond fluorescence decay dynamics. Chem Phys Lett. 2002;352(3):220–225. [Google Scholar]
- 68.Horn MA, et al. Femtosecond studies of the initial events in the photocycle of photoactive yellow protein (PYP) In: De Schryver FC, De Feyter S, Schweitzer G, editors. Femtochemistry. Wiley-VCH; Weinheim, Germany: 2001. pp. 381–390. [Google Scholar]
- 69.Lin SW, et al. Vibrational assignment of torsional normal modes of rhodopsin: Probing excited-state isomerization dynamics along the reactive C11=C12 torsion coordinate. J Phys Chem B. 1998;102(15):2787–2806. [Google Scholar]
- 70.Espagne A, Changenet-Barret P, Baudin J-B, Plaza P, Martin MM. Photoinduced charge shift as the driving force for the excited-state relaxation of analogues of the photoactive yellow protein chromophore in solution. J Photochem Photobiol A Chem. 2007;185(2):245–252. [Google Scholar]
- 71.Day RN, Davidson MW. The fluorescent protein palette: Tools for cellular imaging. Chem Soc Rev. 2009;38(10):2887–2921. doi: 10.1039/b901966a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Philip AF, Nome RA, Papadantonakis GA, Scherer NF, Hoff WD. Spectral tuning in photoactive yellow protein by modulation of the shape of the excited state energy surface. Proc Natl Acad Sci USA. 2010;107(13):5821–5826. doi: 10.1073/pnas.0903092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang W, et al. Tuning the electronic absorption of protein-embedded all-trans-retinal. Science. 2012;338(6112):1340–1343. doi: 10.1126/science.1226135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kochendoerfer GG, Lin SW, Sakmar TP, Mathies RA. How color visual pigments are tuned. Trends Biochem Sci. 1999;24(8):300–305. doi: 10.1016/s0968-0004(99)01432-2. [DOI] [PubMed] [Google Scholar]
- 75.Drobizhev M, Tillo S, Makarov NS, Hughes TE, Rebane A. Color hues in red fluorescent proteins are due to internal quadratic Stark effect. J Phys Chem B. 2009;113(39):12860–12864. doi: 10.1021/jp907085p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drobizhev M, et al. Long- and short-range electrostatic fields in GFP mutants: Implications for spectral tuning. Sci Rep. 2015;5:13223. doi: 10.1038/srep13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakabayashi T, Kinjo M, Ohta N. Electric field effects on fluorescence of the green fluorescent protein. Chem Phys Lett. 2008;457(4):408–412. [Google Scholar]
- 78.Chosrowjan H, Mataga N, Shibata Y, Imamoto Y, Tokunaga F. Environmental effects on the femtosecond-picosecond fluorescence dynamics of photoactive yellow protein: Chromophores in aqueous solutions and in protein nanospaces modified by site-directed mutagenesis. J Phys Chem B. 1998;102(40):7695–7698. [Google Scholar]
- 79.Olsen S. Locally-excited (LE) versus charge-transfer (CT) excited state competition in a series of para-substituted neutral green fluorescent protein (GFP) chromophore models. J Phys Chem B. 2015;119(6):2566–2575. doi: 10.1021/jp508723d. [DOI] [PubMed] [Google Scholar]
- 80.Fried SD, Bagchi S, Boxer SG. Extreme electric fields power catalysis in the active site of ketosteroid isomerase. Science. 2014;346(6216):1510–1514. doi: 10.1126/science.1259802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park JW, Rhee YM. Electric field keeps chromophore planar and produces high yield fluorescence in green fluorescent protein. J Am Chem Soc. 2016;138(41):13619–13629. doi: 10.1021/jacs.6b06833. [DOI] [PubMed] [Google Scholar]
- 82.Zgrablić G, Novello AM, Parmigiani F. Population branching in the conical intersection of the retinal chromophore revealed by multipulse ultrafast optical spectroscopy. J Am Chem Soc. 2012;134(2):955–961. doi: 10.1021/ja205763x. [DOI] [PubMed] [Google Scholar]
- 83.Prokhorenko VI, et al. Coherent control of retinal isomerization in bacteriorhodopsin. Science. 2006;313(5791):1257–1261. doi: 10.1126/science.1130747. [DOI] [PubMed] [Google Scholar]
- 84.Schapiro I, et al. The ultrafast photoisomerizations of rhodopsin and bathorhodopsin are modulated by bond length alternation and HOOP driven electronic effects. J Am Chem Soc. 2011;133(10):3354–3364. doi: 10.1021/ja1056196. [DOI] [PubMed] [Google Scholar]
- 85.Sarkisyan KS, et al. Local fitness landscape of the green fluorescent protein. Nature. 2016;533(7603):397–401. doi: 10.1038/nature17995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gozem S, Schapiro I, Ferré N, Olivucci M. The molecular mechanism of thermal noise in rod photoreceptors. Science. 2012;337(6099):1225–1228. doi: 10.1126/science.1220461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.