Significance
Why do humans share information with others? Large-scale sharing is one of the most prominent social phenomena of the 21st century, with roots in the oldest forms of communication. We argue that expectations of self-related and social consequences of sharing are integrated into a domain-general value signal, representing the value of information sharing, which translates into population-level virality. We analyzed brain responses to New York Times articles in two separate groups of people to predict objectively logged sharing of those same articles around the world (virality). Converging evidence from the two studies supports a unifying, parsimonious neurocognitive framework of mechanisms underlying health news virality; these results may help advance theory, improve predictive models, and inform new approaches to effective intervention.
Keywords: sharing, virality, fMRI, valuation, psychological mechanisms
Abstract
Information sharing is an integral part of human interaction that serves to build social relationships and affects attitudes and behaviors in individuals and large groups. We present a unifying neurocognitive framework of mechanisms underlying information sharing at scale (virality). We argue that expectations regarding self-related and social consequences of sharing (e.g., in the form of potential for self-enhancement or social approval) are integrated into a domain-general value signal that encodes the value of sharing a piece of information. This value signal translates into population-level virality. In two studies (n = 41 and 39 participants), we tested these hypotheses using functional neuroimaging. Neural activity in response to 80 New York Times articles was observed in theory-driven regions of interest associated with value, self, and social cognitions. This activity then was linked to objectively logged population-level data encompassing n = 117,611 internet shares of the articles. In both studies, activity in neural regions associated with self-related and social cognition was indirectly related to population-level sharing through increased neural activation in the brain's value system. Neural activity further predicted population-level outcomes over and above the variance explained by article characteristics and commonly used self-report measures of sharing intentions. This parsimonious framework may help advance theory, improve predictive models, and inform new approaches to effective intervention. More broadly, these data shed light on the core functions of sharing—to express ourselves in positive ways and to strengthen our social bonds.
Human social interaction is centered on sharing information with others (1), and this sharing critically affects the reach and impact of news, ideas, and knowledge over time (2–5). The more than 4 billion Facebook messages (6), 500 million tweets (7), and 200 billion e-mails (8) shared daily highlight this phenomenon. However, not all information is equally likely to be shared (9, 10). Although a growing body of research describes large-scale patterns of sharing (11–13), the types of data that are used to describe such patterns cannot speak to the underlying psychological and neurocognitive antecedents of sharing. Furthermore, extant empirical research on the psychological mechanisms of sharing (2, 5) is limited by social desirability bias, memory gaps, and the inaccessibility of unconscious, basic processes inherent in self-report and other commonly used measures (14–16).
To this end, we assess the neurocognitive processes in individuals that translate into population-level sharing of health news articles (i.e., virality, defined as the mass popularity of a piece of information among those with direct access to that information). Real-time measurement of brain activity offers a mechanistic window into the processes underlying sharing decisions, is less biased by the factors noted above (17, 18), and hence may offer a new way to understand and predict virality.
Value-Based Virality
We tested a parsimonious model of virality centered around the value of sharing. Value-based virality posits that (i) two types of inputs—expectations of self-related outcomes and the social impact of sharing—inform an overall computation of the value of sharing a piece of information with others, and (ii) this domain-general value signal translates into population-level information virality. Operationally, we relied on meta-analyses and large-scale studies in social neuroscience and neuroeconomics to define theory-driven brain regions of interest (ROIs) from which to extract neural activity as a proxy for each of the three psychological processes central to value-based virality (Table S1).
Table S1.
ROIs in study 1 and study 2
| Center of mass | ||||
| ROI | Volume, cm3 | x | y | z |
| Self-related processing | ||||
| Ventromedial prefrontal cortex | 0.23 | −4.26 | 56.6 | −3.92 |
| Precuneus/posterior cingulate cortex | 1.93 | −6.68 | −55 | 28.2 |
| Valuation | ||||
| Ventral striatum | 4 | −3 | 10 | −4 |
| Ventromedial prefrontal cortex | 3.59 | 1 | 46 | −7 |
| Social processing | ||||
| Middle-medial prefrontal cortex | 2.4 | 1.91 | 55 | 11.6 |
| Dorsomedial prefrontal cortex | 2.61 | −0.13 | 53.7 | 29.3 |
| Right temporoparietal junction | 3.0 | 54.1 | −52.6 | 23.1 |
| Left temporoparietal junction | 3.0 | −51.7 | −58.3 | 24.8 |
| Right superior temporal lobe | 3.1 | 54.4 | −8.45 | −17.3 |
The x, y, and z coordinates correspond to the MNI standard brain. All neural systems and subclusters are defined based on prior studies as described in Methods.
Information-Sharing Value
Neuroscientists have identified subregions of the ventromedial prefrontal cortex (VMPFC) and ventral striatum (VS) that compute value in various contexts (19). Importantly, prior work has characterized the domain-general nature of the value signal that is computed in this neural system (20, 21). That is, if a decision maker is faced with different types of value (e.g., primary and secondary rewards), the brain’s value system enables direct comparisons by transforming them onto a common scale during decision making. Value-based virality argues that this same mechanism enables sharers to compute an overall value of the act of sharing a specific piece of information based on considerations of the self-related and social consequences of sharing. Operationally, the neural valuation system includes VS and VMPFC subclusters which are linked to preference judgments and valuation in decision making across hundreds of studies (19) and which have been linked to sharing decisions in individuals (22, 23).
Self-Related Outcome Expectations as an Antecedent of Sharing
Value-based virality suggests that expectations of self-related outcomes are one primary antecedent to sharing. In line with work on self-relatedness, this concept assumes thoughts about how sharing information affects “our self-presentation or mental concept” (24). This broad definition encompasses various specific thought processes, for instance about the effects of sharing on one’s self-presentation or its potential to support self-enhancement, which have been studied separately elsewhere (2, 5). Value-based virality suggests that neural activity in the brain’s self-related processing system is the greatest common denominator of these broadly self-related processes, allowing us to capture within one measure a set of related cognitions that can vary across people and contexts. Similar to content that enhances such self-related thoughts (2, 5), information that engages neural activity in regions related to such processes, especially in medial prefrontal cortex (MPFC) (24, 25), has been linked to self-reported intentions to share information (22, 23).
Extant observational evidence further suggests that self-relevant issues are among the most frequent conversation topics (26, 27), especially in social media (28), and that disclosing information about the self may be inherently rewarding (29). Value-based virality suggests that, through this neural mechanism, expectations of positive self-related outcomes of sharing increase the perceived value of information sharing, which in turn increases the likelihood of actual sharing.
Operationally, we focus on a self-related processing ROI consisting of clusters in the MPFC and precuneus/posterior cingulate cortex (PC/PCC), regions commonly activated by the types of self-related judgments detailed above (24, 30).
Social Outcome Expectations as an Antecedent of Sharing
In parallel, value-based virality suggests that expectations of social outcomes of sharing are another primary antecedent of sharing decisions. Sharing is an inherently social process, and social considerations can strongly impact how content is received and acted upon (4, 31). In particular, sharers need to consider others’ mental states (e.g., knowledge, opinions, and interests) to predict the potential reactions of their audience and to share successfully (32, 33). This type of social cognition is called “mentalizing” and involves cognitions or forecasts about the mental states of others (34), for instance, predicting what others are likely to think and feel about the shared information and about the sharer. Value-based virality suggests that neural activity in the brain’s social cognition system constitutes the greatest common denominator of a range of socially relevant thought processes in sharers, including thoughts about the meaning of the information to receivers and the potential for positive social interactions with others. Neurally, activity in the mentalizing system has been linked to sharing decisions in individuals (23), and successful persuaders engage brain regions strongly associated with mentalizing (35) more than unsuccessful persuaders within two-person propagation chains (22).
Furthermore, sharing information with others has been found to be rewarding (36). Value-based virality predicts that, by this mechanism, thoughts about potential positive social outcomes of sharing (e.g., having another person know you better or gaining others’ approval) increase the perceived value of information sharing. This is reflected by positive associations between neural activity in social cognition and value systems.
We operationalize social cognition as defined above with an ROI consisting of clusters in the middle and dorsal MPFC, bilateral temporoparietal junction, and right superior temporal sulcus, regions which are robustly activated by tasks involving mentalizing (35) and which specifically overlap with considerations of whether others’ mental states are rational and social (37).
Current Study
We tested the value-based virality framework empirically by combining data from two fMRI experiments with objectively logged population-level data on the sharing of New York Times (NYTimes) health news articles that were collected using the NYTimes’ Most Popular application programming interface (API) search tool (11). We focused on neural activity in theory-driven ROIs associated with key psychological processes (positive valuation, self-related, and social processing) measured while participants in two samples were exposed to headlines and abstracts of NYTimes health news articles. fMRI participants also provided ratings of the likelihood with which they would share each article with their Facebook friends. To create a more realistic sharing context, participants were informed that they would be asked to act on their self-reported intentions after the fMRI scan by sharing articles they rated positively with actual Facebook friends. Furthermore, several article characteristics, such as positivity and perceived usefulness, were available from a prior content-focused investigation of the articles used here (11). Participants completed similar tasks in the two studies (Fig. S1), and parallel analyses were applied to the two datasets to allow the replication of our results linking neural and population-level data. The population-level framework presented here substantially extends orthogonal analyses of individual-level results based on study 1 data showing that decisions about information sharing engage more activity in value, self-related, and social cognition ROIs than do other types of decisions and that this neural activity scales with self-reported, individual-level sharing preferences (23).
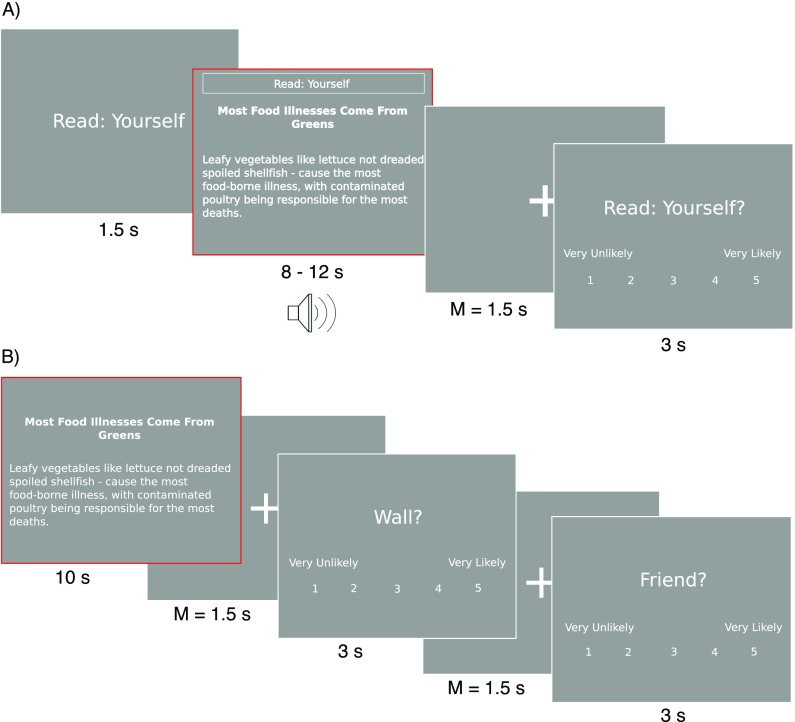
Fig. S1.
fMRI tasks. (A) Reading trial of the article task (study 1). (B) Abstract trial of the article task (study 2). The trial modeled in main analyses is marked in red.
Results
Based on the predictions made by value-based virality (Fig. 1), path models were specified to link percent signal change of brain activity measured in the three theory-driven ROIs while our participants read headlines and abstracts to the population-level sharing counts of each article. The 80 NYTimes articles were shared a total of 117,611 times (mean ± SD, 1,470.1 ± 2,304.3 times; range, 34–12,743 times) via Facebook, Twitter, and email by the NYTimes online reader population within 30 d of each item's publishing date.
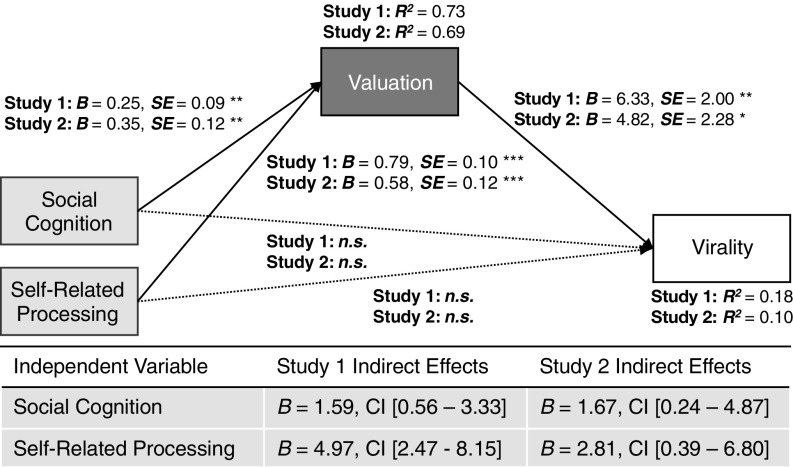
Fig. 1.
Value-based virality path model. The path diagram shows maximum likelihood estimates (unstandardized coefficients). The table presents indirect effect coefficients and bias-corrected, bootstrapped 95% CIs (1,000 replications). As in prior work predicting population-level message effects from neural data (30), all variables were rank-ordered. n = 80 in study 1 and 76 in study 2; *P < 0.05, **P < 0.01, ***P < 0.001, n.s., not significant.
In both samples, we found robust support for value-based virality (Fig. 1 and Table S2). First, articles that had high sharing value indicated by stronger neural activity in the valuation ROI in each of our samples were shared more frequently by NYTimes readers. This result is in line with the idea that, in the context of sharing, the brain’s valuation system encodes the value of sharing information with others. Further, there are commonalities across people in the extent to which information engages this neural system.
Table S2.
Correlation matrices underlying the path models in Fig. 1 (variables 1–4) and Fig. S4 (variables 1–5)
| Variable | 1 | 2 | 3 | 4 | 5 |
| Study 1, n = 80 | |||||
| 1. Self-related processing ROI | 1 | ||||
| 2. Social processing ROI | 0.705*** | 1 | |||
| 3. Valuation ROI | 0.838*** | 0.702*** | 1 | ||
| 4. Population-level virality | 0.240* | 0.253* | 0.387*** | 1 | |
| 5. Self-reported intentions | 0.125 | 0.263* | 0.285* | 0.337** | 1 |
| Study 2, n = 76 | |||||
| 1. Self-related processing ROI | 1 | ||||
| 2. Social processing ROI | 0.822*** | 1 | |||
| 3. Valuation ROI | 0.814*** | 0.770*** | 1 | ||
| 4. Population-level virality | 0.094 | 0.182 | 0.237* | 1 | |
| 5. Self-reported intentions | 0.146 | 0.164 | 0.191 | 0.372*** | 1 |
Asterisks indicate statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001.
In addition, the effects of neural activity in self- and social-cognition systems on population-level virality were fully mediated through value-related activity in both samples. This finding is consistent with the idea that considerations of self-related and social outcomes of sharing impact the overall perceived value of the act of sharing, which in turn directly affects sharing behavior.
These results were robust when using unranked variables (SI Text, Fig. S2, and Table S3). Further, models specifying value-related neural activity as the mediator of the effects of social and self-related processing on virality showed acceptable model fit and outperformed alternative path models (SI Text, Table S4). Finally, following our planned ROI analyses, a whole-brain search for regions associated with population-level virality did not reveal widespread activity outside our ROIs (SI Text, Fig. S3, and Table S5).
Fig. S2.
Value-based virality path model including unranked variables. The path diagram shows maximum likelihood estimates (unstandardized coefficients). The table presents indirect effect coefficients and bias-corrected, bootstrapped 95% CIs (1,000 replications). Population-level virality was log-transformed because of its positively skewed distribution. n = 80 in study 1 and 76 in study 2; *P < 0.05, **P < 0.01, ***P < 0.001, n.s., not significant.
Table S3.
Correlation matrices underlying the path model in Fig. S2 that includes unranked variables
| Variable | 1 | 2 | 3 | 4 |
| Study 1, n = 80 | ||||
| 1. Self-related processing ROI | 1 | |||
| 2. Social processing ROI | 0.717*** | 1 | ||
| 3. Valuation ROI | 0.856*** | 0.758*** | 1 | |
| 4. Population-level virality | 0.236* | 0.235* | 0.352** | 1 |
| Study 2, n = 76 | ||||
| 1. Self-related processing ROI | 1 | |||
| 2. Social processing ROI | 0.868*** | 1 | ||
| 3. Valuation ROI | 0.859*** | 0.851*** | 1 | |
| 4. Population-level virality | 0.107 | 0.163 | 0.256* | 1 |
Population-level virality showed a positively skewed distribution and thus was log-transformed. Asterisks indicate statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001.
Table S4.
Model fit comparison for alternative path structures
| Model | χ2 (df), P | CFI | RMSEA (90% CI) | AIC | BIC |
| Study 1, n = 80 | |||||
| (A) Valuation mediates | 2.36 (2), 0.31 | 0.997 | 0.05 (0.00–0.23) | 1,593.80 | 1,605.71 |
| (B) Self-related processing mediates | 10.63 (2), 0.01 | 0.925 | 0.23 (0.11–0.38) | 1,602.08 | 1,613.99 |
| (C) Social cognition mediates | 10.08 (2), 0.01 | 0.888 | 0.23 (0.10–0.37) | 1,601.53 | 1,613.44 |
| Study 2, n = 76 | |||||
| (A) Valuation mediates | 3.26 (2), 0.20 | 0.986 | 0.09 (0.00–0.26) | 1,457.07 | 1,468.72 |
| (B) Self-related processing mediates | 6.98 (2), 0.03 | 0.955 | 0.18 (0.05–0.34) | 1,460.79 | 1,472.44 |
| (C) Social cognition mediates | 5.09 (2), 0.08 | 0.968 | 0.14 (0.00–0.30) | 1,458.90 | 1,470.56 |
(A) represents a model resembling the path model in Fig.1 excluding the two insignificant effects. (B) represents a version of model A in which the roles of “valuation” and “self-related processing” are switched. (C) represents a version of model A in which the roles of “valuation” and “social cognition” are switched. AIC, Akaike’s information criterion; BIC, Bayesian information criterion.
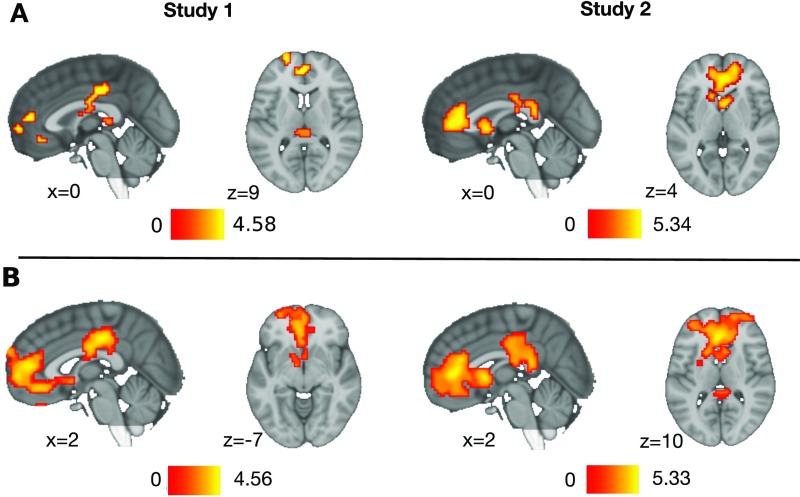
Fig. S3.
Whole-brain analyses of regions associated with each article's rank of population-level sharing counts in study 1 and study 2. Whole-brain maps were thresholded using (A) a nonparametric permutation analysis corrected at FDR-corrected P < 0.05, K ≥10 and (B) a cluster-based approach thresholded at P < 0.005 uncorrected and K ≥320 in study 1 and K ≥296 in study 2, respectively where K is the number of vowels per cluster on a 3dClustSim simulation together corresponding to P < 0.05 corrected.
Table S5.
Whole-brain tables: Clusters significantly associated with population-level virality ranks of the NYTimes articles shown in each trial during reading screen periods (study 1) or abstract trials (study 2)
| Cluster | Nonparametric | |||||||
| Region | R/L | x | y | z | T | K | T | K |
| Study 1 | ||||||||
| Medial prefrontal cortex* | L | −3 | 59 | 1 | 4.52 | 1495 | 4.52 | 90 |
| Anterior cingulate cortex | L | −3 | 47 | 10 | 4.27 | 4.28 | ||
| Caudate† | R | 3 | 8 | −5 | 2.97 | |||
| Dorsomedial prefrontal cortex | L | −12 | 38 | 31 | 4.08 | 4.09 | 14 | |
| Dorsomedial prefrontal cortex† | R | 6 | 65 | 25 | 3.22 | |||
| Dorsolateral prefrontal cortex/superior frontal gyrus | L | −27 | 53 | 31 | 3.28 | 3.28 | 11 | |
| Ventromedial prefrontal cortex | L | −3 | 38 | −11 | 4.23 | 4.24 | 11 | |
| Lateral orbital frontal cortex | L | −21 | 62 | 10 | 4.08 | 4.09 | 48 | |
| Mid cingulate cortex* | L | −6 | −16 | 34 | 4.56 | 549 | 4.57 | 129 |
| Mid cingulate cortex | M | 0 | −22 | 40 | 4.33 | 4.33 | ||
| Precuneus† | L | −18 | −49 | 31 | 4.09 | |||
| Cingulate† | R | 12 | −28 | 28 | 3.84 | |||
| Thalamus | L | −4 | −28 | 7 | — | 3.05 | 32 | |
| Study 2 | ||||||||
| Medial prefrontal cortex | R | 15 | 50 | 1 | 4.76 | 2,698 | 4.77 | 905 |
| Medial prefrontal cortex | L | −15 | 50 | −2 | 4.42 | 4.43 | ||
| Ventromedial prefrontal cortex | R | 3 | 38 | −8 | 3.67 | 3.67 | ||
| Anterior cingulate cortex* | L | −3 | 32 | 10 | 5.33 | 5.34 | ||
| Caudate | R | 3 | 8 | 4 | 4.73 | 4.74 | ||
| Putamen | R | 15 | 8 | −8 | 3.88 | 3.89 | ||
| Caudate | L | −12 | 20 | 1 | 4.59 | 4.61 | ||
| Caudate | R | 12 | 17 | 1 | 3.99 | 4.01 | ||
| Posterior cingulate cortex* | R | 3 | −40 | 19 | 4.48 | 506 | 4.50 | 126 |
| Posterior cingulate cortex | R | 6 | −22 | 31 | 3.99 | 4.00 | ||
| Posterior cingulate cortex | L | −9 | −43 | 19 | 3.70 | 3.72 | ||
Clusters significantly associated with population-level virality ranks of the NYTimes articles shown in each trial during reading screen periods of reading (study 1) or abstract trials (study 2). The x, y, and z coordinates correspond to the MNI standard brain. No suprathreshold clusters were observed that were negatively associated with the parametric modulator. Thresholding: For each study, voxels significant under cluster correction and voxels significant under nonparametric correction are shown. Cluster correction thresholding was performed based on 3dClustSim simulation at P < 0.005 uncorrected and K ≥ 320 in study 1 and K ≥ 296 in study 2; nonparametric thresholding was performed through nonparametric permutation testing and FDR P < 0.05, K >10. Separate clusters in the cluster-corrected map are divided by spaces between rows. df = 1, 38; voxel size = 3 × 3 × 3 mm. K, number of voxels per cluster. L, left; M, medial; R, right.
Peak voxel within cluster.
Peaks that are present only under cluster correction.
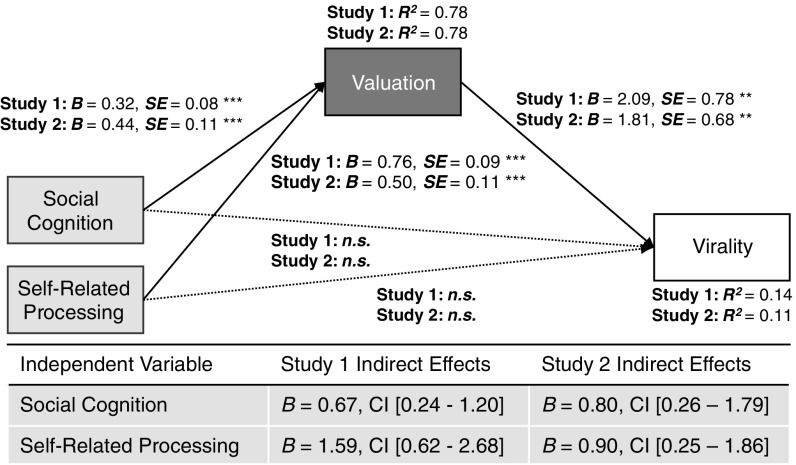
We further compared the predictive power of neural activity in regions predicted by value-based virality with variance explained by commonly used self-report measures (intentions to share each article on Facebook) and tested the robustness of the framework when controlling for the effects of article characteristics that have been associated with news virality in prior work (11, 38). For both the study 1 and study 2 samples, self-reported intentions were significant predictors of population-level sharing (explaining 11.3% and 13.8% of its variance, respectively). Neural activity alone explained 17.5% and 9.6% of the variance in the virality outcome in studies 1 and 2, respectively (Fig. 1). When combined, both self-reported intentions and brain activity remained significant predictors, together explaining 19.2 and 19.1% of the variance in studies 1 and 2, respectively (Fig. S4 and Table S2). In addition, all effects reported in Fig. 1 were robust, even when controlling for any of nine content characteristics available for the article headlines and abstracts (SI Text). Thus, brain activity measured with fMRI can significantly improve the prediction of large-scale sharing behavior beyond other commonly used metrics.
Fig. S4.
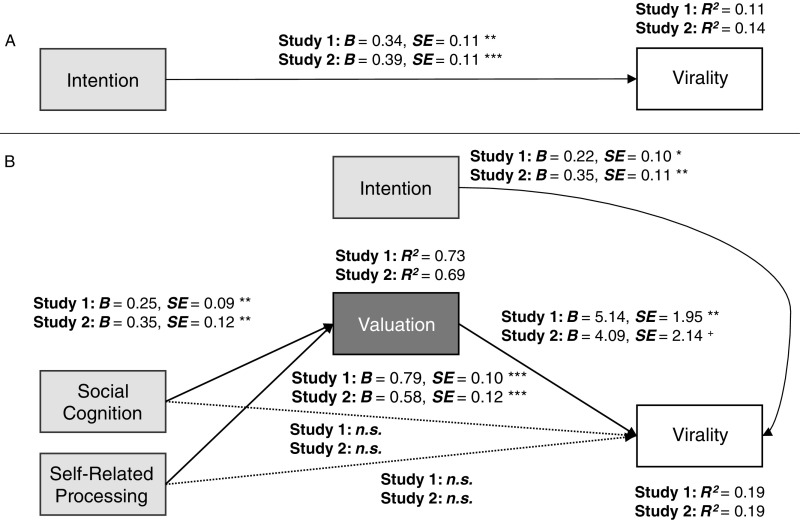
Effects of self-reported intention. (A) Model using intention ratings to predict population-level virality. (B) Model using both intention ratings and value-based virality to predict virality. All variables are rank-ordered; *P < 0.05, **P < 0.01, ***P < 0.001, +P = 0.056, n.s., not significant.
Discussion
Information sharing is an integral part of human nature (1) that enables and accelerates innovation and development in modern societies (3, 39). We iteratively combined neuroimaging data with objectively logged population-level data on hundreds of thousands of shares from the NYTimes API search tool to test a parsimonious, neurocognitive framework of the psychological mechanisms underlying sharing decisions that translate into population-level virality. Specifically, we argue that potential sharers consider a broad range of self-related and social consequences of sharing a piece of information with others. The resulting self-related and social-relevance judgments then serve as inputs to the brain’s valuation system, which converts them to a common scale. This overall value of information sharing is directly predictive of large-scale sharing dynamics.
Consistent with this framework, we found that brain activity in the valuation system (VS and VMPFC) in two groups of participants was associated with virality in the larger population (117,611 total shares of 80 NYTimes articles). That is, articles associated with higher information-sharing value in the brain when individuals first read the headlines and abstracts were shared more frequently by the population of NYTimes readers. Information-sharing value may be a primary psychological motivator and central theoretical concept that guides sharing behavior at scale. Prior work has shown that neural activity in the brain’s valuation system is not only associated robustly with personal preferences (19) but also with the expectation of positive outcomes (40, 41). Brain activity in response to persuasive messages in these regions also is associated with message-consistent behaviors at the individual (22, 42) and population level (30, 43, 44). Our findings show that the predictive validity of neural valuation activity extends to the realm of information virality and highlights the domain-general nature of this brain signal (20, 21). In the case of sharing, value-based virality suggests that considerations of self-related and social consequences of sharing are key inputs in the computation of the value of sharing information, even though the specific nature of the self-related and social inputs that inform that value signal may vary depending on qualities of the information sharer, the receiver, or their relationship.
In line with this argument, we found robust, indirect effects of brain activity in regions associated with self-related processing during article exposure on population-level sharing behavior through value-related activity. Prior evidence has linked a range of self-related judgments to sharing. For example, the promotion of a positive self-image (46, 47) is an important goal in social interactions, and information that allows potential sharers to appear in a more positive light is more likely to go viral (2, 5), perhaps because it increases the perceived value of information sharing. Further, self-disclosure increases activity in the brain’s valuation system, suggesting that providing information about or reflecting about the self might be inherently rewarding (29). Value-based virality brings together prior findings, arguing that self-related neural activity is the greatest common denominator for various self-related thought processes, including reflecting self-concept and self-presentational concerns, and constitutes a primary antecedent of sharing value.
Further, our results show an indirect effect of activity in neural regions associated with social cognition, and in particular mentalizing, on population-level article virality through value-related activity. Existing work has shown that the expectation of positive social outcomes such as positive interactions with others engages the brain’s valuation system (40, 48), and our ROI overlaps with brain regions supporting considerations of whether others’ mental states are rational and whether they are social (37). Social belonging is a basic human need and motivation (49, 50), and relationship maintenance has been suggested as a motivator of information sharing (2, 5). A range of basic social motives focused on understanding others’ minds and forecasting their reactions, and expectations about positive social outcomes of sharing information with others may increase the perceived value of information sharing; in turn, the perceived increase in the value of information increases the potential that the information will go viral. Value-based virality brings together prior findings, arguing that neural activity in areas associated with social cognition is the greatest common denominator for various social thought processes and informs sharing value.
Although we removed voxels within the VMPFC and PCC [regions commonly associated with both self-related and social processing (24, 35, 51)] from our social-processing ROI to ensure statistical validity, self-related and social thoughts are conceptually intertwined. Social psychologists have suggested that one’s sense of self is defined by simple rules that include or exclude an individual from certain social groups and practices, resulting in a “social self” concept (52, 53). In the context of value-based virality, it follows that content that is expected to have positive social outcomes when shared (e.g., because it is helpful to the receiver or results in a positive social interaction) will likely reinforce the perceived positivity of self-related outcomes of sharing (e.g., by making the sharer look charitable and friendly) and vice versa. Nonetheless, our analyses demonstrate that when operationalizations of both self-related and social processing are included in one model, each concept contributes unique variance to the calculation of overall sharing value. In the future, explorations of the relative importance of each cognition and the patterns of their interaction in the calculation of information-sharing value will be valuable.
Finally, in line with prior investigations in other contexts (30, 43–45, 54), we show links between brain activity in small groups of individuals and large-scale virality, even though the perception of the sharing value of the same content might vary across people, and the same content might appear valuable to different people for different reasons. Although what is personally relevant to the self and useful to share with others might differ somewhat across individuals, human societies are characterized by a set of basic common values and social norms that drive behavior across individuals (55, 56). Sharing decisions rely on such basic motives, namely, the pursuit of a positive self-image and social belonging (46, 49). Consequently, similar types of information are likely to be perceived to have high sharing value across individuals. Furthermore, expectations of self-related and social outcomes, two core concepts within value-based virality, are defined broadly as the greatest common denominators of various self-related and social thought processes, respectively. In other words, population-level prediction of virality from neuroimaging of small groups is likely facilitated by broad societal values, the inclusiveness of our theoretical conceptualizations, and the unique information afforded by neuroimaging. Specifically, neuroimaging is optimally situated to identify such high-level, hard-to-articulate cognitions, allowing us to capture relevant cognitions in a parsimonious way despite the variability in the thought processes that different individuals might associate with the same content. Along with this strength, however, we relied on functionally defined ROIs to take optimal advantage of neuroimaging to operationalize these constructs, which are inherently subject to the limitations of reverse inference (57).
The results summarized in this article were robust across several methods of analysis, and the hypothesized model outperformed alternative path structures, although causal inferences are limited by the cross-sectional nature of our data. Additionally, a whole-brain analysis did not provide strong evidence for the involvement of neural regions outside our ROIs in population-level virality. Nevertheless, future work might reveal other basic processes that could complement the theory, for instance as additional inputs to the value signal or its antecedents. Further, our effects were robust, even when controlling for self-reported sharing intentions and various article characteristics. In sum, our data highlight the value of including neural variables in the conceptualization of virality in the context of health news and offer a testable and parsimonious framework that could be extended to virality in other contexts. This mechanistic account of sharing decisions complements insights from previous studies using self-report measures or big data approaches (e.g., 12, 13).
Conclusion
Information that elicits greater brain response in self-, social-, and in turn value-related systems is more likely to be shared. These processes may reflect thoughts about the potential outcomes of sharing to the self and to one’s social relationships. If so, self-related and social processes could serve as targets for content designers aiming to increase the virality potential of their messages. Taken together, our data support a parsimonious neurocognitive model of virality, one of the most prominent social phenomena in the 21st century, and shed light on the core functions of sharing—to express aspects of ourselves and to strengthen our social bonds.
Methods
Neural activity was examined while two samples of participants (study 1 and study 2) completed the article task (Fig. S1) in which participants were exposed to headlines and abstracts of news items taken from the NYTimes website (https://www.nytimes.com/). We then tested for associations between activity within functionally defined, theory-driven ROIs associated with self-relatedness, social processing, and valuation and the number of article retransmissions performed online by NYTimes readers as a population-level indicator of virality.
Similar protocols were administered in both studies, and each group of participants was presented with the same news items. Differences in data collection and processing between the two studies are detailed below. All models and results reported here were derived using parallel statistical approaches across studies. All participants provided informed consent, and all procedures were approved by the Institutional Review Board at the University of Pennsylvania.
Hypothesis Preregistration.
At the onset of study 1, we preregistered our study design (58), and upon completion of data collection we explored the relationship between neural data and population-level article retransmission. Based on the results in study 1, hypotheses specifying the effects of self- and social-processing on value-related neural activity and of activity in the value-related ROI on population-level virality were preregistered before the analysis of study 2 data (59).
Sample NYTimes Article.
During the article task, participants in both samples were exposed to the original headline and abstract of 80 articles from the Health section of the NYTimes website (https://www.nytimes.com/). The articles were chosen from a complete census (excluding certain article categories to preserve homogeneity in article format; see ref. 11 for details) of articles (n = 760) published online in the 7.7 mo between 11 July 2012 and 28 February 2013. Population-level data about the number of retransmissions of each article through email, Twitter, and Facebook were collected via the NYTimes API. The 80 articles were chosen to maximize comparability regarding topic (healthy living and physical activity) and length (for the word count of title and abstract, see SI Text). The 80 articles selected into the final sample were of comparable lengths, i.e., a word count (mean ± SD) of 29.43 ± 3.87 words (range, 21–35 words). To control for reading speed in study 1, we produced audio files in which a female voice read each of the article headlines and abstracts. Depending on word count, each audio file was produced to last 8, 10, or 12 s.
Coded characteristics of each article’s headline and abstract were available as described by Kim (11).
Population-Level Retransmission.
An article’s population-level retransmission count was measured through the NYTimes’ Most Popular API and defined as the sum of retransmissions via Facebook, Twitter, and email using sharing tools available on the NYTimes website within 30 d of the article’s first appearance on the website (mean ± SD, 1,470.14 ± 2,304.32 retransmissions; range, 34–12,743 retransmissions). Retransmission counts for social media (Twitter and Facebook) and email were highly correlated (r = 0.917) and thus are not presented separately, although results remain substantively identical when each type of sharing is considered separately.
Study 1 Participants.
From a larger sample of respondents who participated in a project examining the neural correlates of retransmission and social influence by filling out a short online survey, we selected 43 participants. These 43 participants completed an online screening process and an in-person appointment including a 60-min fMRI scan. To be eligible for the fMRI portion, screened participants had to meet standard fMRI eligibility criteria including no metal in the body, no history of psychiatric or neurological disorders, not currently pregnant or breast-feeding, and not currently taking psychiatric or illicit drugs. All participants were right-handed.
Two participants were excluded from analysis because of data corruption. One participant saw only three of the four conditions during the article task, and one participant showed poor normalization to the template brain. Additionally, for four participants a smaller number of trials was available for analysis because of the loss of data from one run of the article task (n = 1), excessive head motion in one run of the task (n = 2), and technical difficulties in which 23 articles were shown twice, resulting in only 57 trials that qualified as initial exposures to an article (n = 1). The partial data from these participants were included in the analyses. The age of the final sample of 41 participants (29 females) was 20.6 ± 2.1 y (mean ± SD) (range, 18–24 y).
Study 2 Participants.
Forty participants were selected from the pool of respondents used to select the study 1 sample using inclusion criteria that paralleled those in study 1. These participants underwent an fMRI session.
Because of excess head movement during the article task, one participant was removed from all analyses, and one run of the article task was discarded for a second participant. The remaining 39 participants (28 female) were 18–24 y old (mean ± SD, 21.0 ± 2.02 y).
Study 1 Article Task.
Inside the fMRI scanner, study 1 participants completed two runs of the article task consisting of 40 trials each (Fig. S1A). Each trial lasted an average of 14.7 s without fixation. At the beginning of each trial a cue screen indicating the current condition was presented for 1.5 s. Then participants read the article’s title and abstract while considering a condition-specific question. In the four conditions participants were asked to consider (i) whether to read the full text of the article themselves, (ii) whether to share the article via a post on their Facebook wall; (iii) whether to share the article via a private Facebook message to one friend (5-point Likert-type scales from very unlikely to very likely), and (iv) whether age/nutrition/fitness/science/laws/well-being/cancer was the topic of this article (5-point Likert-type scale from certainly not to certainly yes). Conditions were presented in a pseudorandom order based on a Latin-square. To control for reading speed, headlines and abstracts were also presented in auditory format through scanner-compatible headphones while the text was presented on the screen. Article abstracts were categorized in three groups depending on the length of the text. Consequently, the reading screen was presented for 8 (n = 16), 10 (n = 40), or 12 (n = 24) s. Article length was counterbalanced across conditions and task runs. The reading screen was followed by a randomly jittered fixation screen that lasted 1.5 s on average (range, 0.5–4.7 s). Participants then used a button box to indicate their answer to the condition-specific question (3 s). Finally, there was a randomly jittered intertrial interval with an average length of 2 s (range, 1–4.7 s).
In this analysis, we focused on reading trials in which participants viewed the article headlines and abstracts to decide whether they wanted to read the full text of the article (see SI Text for results in other conditions). Furthermore, we only included reading screens within each trial (i.e., periods in which article headlines and abstracts were visible). This task condition closely mimics natural situations in which readers are initially exposed to articles online.
Study 2 Article Task.
Study 2 participants completed two runs (21 trials each) of a modified version of the article task (Fig. S1B). First, each article’s headline and a description of the article were presented on the reading screen for 10 s, and participants were instructed to read the text on the screen. Articles were not presented in auditory format in study 2. Three types of article descriptions were used: Participants saw the original article headline and abstract that also was seen by study 1 participants (i) or saw the original article headline and a Tweet-length message written by a participant in study 1 to be shared either with one Facebook friend (ii) or on the participants’ Facebook wall (iii). The reading screen was followed by a randomly jittered fixation period (mean, 1.5 s; range, 0.3–4.8 s). Afterward, participants provided two ratings per trial: (i) the likelihood they would share the article on their Facebook wall and (ii) the likelihood (on 5-point Likert-type scales paralleling those used in study 1) that they would share the article via a private Facebook message with one friend. Each rating screen was available for 3 s. Rating screens were separated by a short, jittered fixation period (mean, 1.5 s; range, 0.4–4.3 s). Finally, there was a randomly jittered intertrial interval (mean, 2.9 s; range, 0.5–11.5 s). To parallel study 1 analyses closely, only reading screen periods within each trial (i.e., when article headlines and descriptions were visible) and only abstract trials that presented original NYTimes abstracts were analyzed here. The 80 articles used in study 1 were pseudorandomly assigned to experimental conditions for each participant in study 2; however, because of randomization, only 76 articles were presented in the relevant abstract condition across all study 2 participants.
A Priori ROIs.
Three neural masks were constructed as functional ROIs based on extensive prior work in each of the respective subject areas (Table S1). The self-relatedness ROI was defined based on a prior study (30) that collected neural data using a well-validated self-localizer task (60) in which participants judge whether personality traits describe them or not (the self-condition) or whether the adjective shown is positive or negative (the valence condition). Blocks of self-judgments are contrasted with blocks of valence judgments to isolate neural activity associated with self-relatedness.
The social-processing ROI was defined based on a large-scale study that used the well-validated false-belief localizer during which participants engage in mentalizing (35). Trials during which participants judged whether beliefs held by others were true or false were contrasted to trials in which they judged whether physical representations were true or false to retrieve the mask used here. To avoid inflated correlations among activity in the three neural systems, we created a reduced version of the social cognition mask, excluding the clusters in VMPFC and PCC that overlap with the self and value ROIs. This mask is used in all analyses presented here. Models using the full social-cognition ROI instead of the reduced social-cognition ROI yielded very similar results and support identical conclusions.
Finally, the valuation ROI was defined based on a quantitative meta-analysis of 206 studies that reported neural correlates of subjective valuation during decision making. This mask represents the conjunction of several valuation-relevant contrasts, all of which required some form of value-based decision making (figure 9 in ref. 23).
MRI Image Acquisition.
Neuroimaging data were collected using a 3-T Siemens Magnetom Tim Trio scanner equipped with a 32-channel head coil was used for 40 participants in study 1 and 33 participants in study 2, and a Siemens Prisma 3T whole-body MRI with a 64-channel head/neck array was used for one participant in study 1 and six participants in study 2. Identical specifications were used on both scanners, except for the number of slices acquired for T2*-weighted images (54 at the Tim Trio and 52 at the Prisma scanner). This difference was accounted for in the slice-time correction step during preprocessing. Standard parameters used to acquire T2*- (two runs of 500 volumes in study 1 and two runs of 311 volumes in study 2), T2-, and T1-weighted anatomical image sequences are described in detail in the SI Text.
Imaging Data Preprocessing.
For the analysis of data from both studies, we used SPM8 (Wellcome Department of Cognitive Neurology, Institute of Neurology, the University of London), incorporating tools from AFNI (Analysis of Functional NeuroImages) (61) and FSL (FMRIB Software Library) (62) during data preprocessing. The first five volumes of each run were not collected to allow stabilization of the blood oxygenation level-dependent (BOLD) signal. Functional images were despiked using 3dDespike as implemented in AFNI. Slice time correction was performed using Sinc (Stanford University ideal bandlimited) interpolation in FSL. Data then were spatially realigned to the first image and were coregistered in two six-parameter affine stages. First, mean functional images were registered to in-plane T2-weighted images. Next, high-resolution T1 images were registered to the in-plane image. After coregistration, high-resolution structural images were segmented into gray matter, white matter, and cerebral spinal fluid to create a brain mask used to determine the voxels to be included in first- and second-level models. The masked structural images then were normalized to the skull-stripped Montreal Neurological Institute (MNI) template provided by FSL (MNI152_T1_1mm_brain.nii). Finally, functional images were smoothed using a Gaussian kernel (8 mm FWHM). The fMRI data were modeled for each participant using fixed-effects models within the general linear model as implemented in SPM8, using SPM’s canonical difference of gamma hemodynamic response function (HRF). The six rigid-body translation and rotation parameters derived from spatial realignment were also included as nuisance regressors in all first-level models. Data were high-pass filtered with a cutoff of 128 s. Random effects models for the article task were also implemented in SPM8.
Analysis of Study 1 Imaging Data.
We took an itemwise approach to modeling the article task using procedures similar to those used elsewhere (30, 61). Specifically, using a single boxcar function for each trial (i.e., each of the 80 articles) encompassing the 8- to 12-s reading screen, we extracted neural activity in each ROI during each trial compared with the implicit baseline resting state. Activity related to cue and all rating screens was pooled into a separate regressor of no interest each. In addition, the model for one participant who accidentally saw several articles twice included an additional regressor of no interest for each second occurrence of an article. Fixation periods were pooled into the implicit baseline rest.
Analysis of Study 2 Imaging Data.
Study 2 data were analyzed using methods parallel to those applied to study 1 data to yield comparable models. Specifically, using a single boxcar function for each of the 42 trials per participant, encompassing the 10-s reading screen, we extracted neural activity observed during each trial compared with the implicit baseline resting state. A regressor of no interest was included for each of the two rating screens. Fixation periods were pooled into the implicit baseline rest.
Path Models.
For each a priori ROI, average parameter estimates of activity across all voxels within the region were extracted for each participant and each article using Marsbar (63). Each set of parameter estimates was divided by the grand mean to derive estimates of the percent signal change. Percent signal change vectors for each participant were reduced to those trials shown in the reading condition for study 1 and in the abstract condition for study 2. For each participant, these reduced vectors were then z-scored and ranked across articles. As in prior work (30), we then computed the mean ranks of each article across participants and linked these data with the ranked population-level data from the NYTimes API separately.
Specifically, we conducted path analyses using maximum likelihood estimation in lavaan (64) to yield the results presented in Fig. 1. Nonparametric, bias-corrected 95% confidence intervals (CIs) for indirect effects using 1,000 bootstrap samples were further estimated using the mediation package for R (65) to test for indirect effects of self-related processing and social processing on population-level retransmission through valuation (Table S2 for relevant correlation matrices).
Robustness Checks.
To check the robustness of our results, we fit (i) models using unranked variables in which population-level retransmission counts were log-transformed because of the positively skewed distribution (Fig. S2 and Table S3), (ii) models excluding the insignificant direct effects of the exogenous variables shown in Fig. 1 to obtain model fit statistics (SI Text), and (iii) alternative structural models to those estimated in step ii to compare model fit (SI Text and Table S4).
Whole-Brain Analysis.
We conducted exploratory whole-brain searches for regions associated with population-level retransmission ranks in study 1 and study 2 to verify the specificity of our results to our ROIs and to explore whether additional activity outside these ROIs is associated with population-level virality (SI Text).
Models Including Self-Reported Sharing Intentions and Article Characteristics.
We further tested whether the predictions of value-based virality held above and beyond the variance explained by self-reported sharing intentions (Fig. S4 and Table S2) and article characteristics (SI Text).
Study 1 participants provided one rating (intention either to broadcast or narrowcast) for 40 articles. For each article, we computed a mean sharing intention across participants including all available narrowcast and broadcasting ratings.
Study 2 participants provided both narrowcast and broadcasting ratings for all 42 articles shown to them. For trials shown in the abstract condition, we first calculated a mean sharing intention across the two ratings for each article within participants and then computed a mean sharing intention for each article across participants.
First, ranked population-level retransmission was regressed onto sharing intentions to estimate the effect of intentions on virality in each sample. Second, we reestimated the models shown in Fig. 1 with self-reported intentions specified as an additional exogenous variable with a direct effect on population-level retransmission. This step was further repeated for each available article characteristic (SI Text).
SI NY Times Article Sample
We selected 80 articles from the full set of 760 articles analyzed in ref. 11 with the goal of maximizing comparability in topic and length. Specifically, we conducted a keyword search of the full set of 760 articles using the following terms: exercise, fitness, physical activity, running, swimming, skiing, soccer, walking, food (excluding “Food and Drug Administration”), eating, nutrition, nutrient, diet, vitamin, calcium, carbohydrates, gluten, caffeine, cholesterol, obesity, and weight. The search retrieved 143 articles. A closer examination revealed that four articles were irrelevant, and these articles were removed. Of the remaining 139 articles, the 80 that were most similar in length were chosen.
SI Scanning Parameters
We captured neural activity during two runs of the article task (500 volumes in each run in study 1 and 311 volumes in each run in study 2) using a T2*-weighted image sequence [repetition time (TR) = 1.5 s, echo time (TE) = 25 ms, flip angle = 70°, −30° tilt relative to the anterior commissure–posterior commissure (AC–PC) line, 54 slices at the Magnetom Tim Trio scanner, 52 slices at the Prisma scanner, field of view (FOV) = 200 mm, slice thickness = 3 mm, multiband acceleration factor = 2, voxel size = 3 × 3 × 3 mm]. High-resolution T1-weighted anatomical images were collected using a magnetization-prepared rapid gradient-echo (MPRAGE) sequence [inversion time (TI) = 1,110 ms, 160 axial slices, voxel size = 0.9 × 0.9 × 1 mm]. Finally, we collected an in-plane, structural, T2-weighted image (slice thickness = 1 mm, 176 axial slices, voxel size = 1 × 1 × 1 mm) to implement a two-stage coregistration procedure between functional and anatomical images.
SI Robustness Checks
To test the robustness of our main results reported in Fig. 1, we estimated models using unranked variables. These analyses produced results similar to those presented in the main text and supported identical conclusions (Fig. S2 and Table S3). Further, models excluding the insignificant direct effects of the two exogenous variables on virality shown in Fig. 1 were estimated to obtain model fit statistics. Both models revealed satisfactory model fit for the hypothesized structural model, considering its small degrees of freedom (df) and small sample size (66): (2) = 2.36, P = 0.31, comparative fit index (CFI) = 0.997, residual mean square error of approximation (RMSEA) = 0.05, 90% CI (0.00, 0.23) for study 1; (2) = 3.26, P = 0.20, CFI = 0.986, RMSEA = 0.09, 90% CI (0.00, 0.26) for study 2. Additional analyses revealed the model fit for the hypothesized path structure was superior to that of alternative structural models (Table S4), providing additional confidence to our proposal that valuation, taking inputs from self and social considerations, serves as a final common pathway.
SI Study 1 Whole-Brain Analysis
To test the specificity of our results to our theory-driven ROIs, we conducted exploratory whole-brain analyses. We first created first-level models for each participant that included a separate boxcar function for activity across all trials within a certain condition (content, reading, broadcasting, narrowcasting) for the reading screen and the rating screen of the article task, respectively (eight regressors). An additional regressor represented the boxcar function representing the reading screen during reading trials modified by a mean-centered parametric modulator of population-level virality ranks of each article. Population-level virality ranks were derived by ranking all articles presented within the reading condition by their population-level retransmission counts for each participant (range, 1–20). The model also included a boxcar function for activity across all trials within the cue screen and six nuisance regressors to control for motion. Finally, to ensure that only first exposures were modeled in the main regressor of interest, one regressor of no interest was entered to account for trials in which one participant was accidentally presented with an article for a second time. Second, at the group level, neural activity was pooled for all participants to examine the main contrasts of interest: activity during the reading screen in reading trials modulated by population-level retransmission ranks compared with implicit baseline.
To balance the risks of false positives and false negatives, we conducted two different kinds of correction for multiple comparisons to derive whole-brain maps and tables of voxels in which neural activity scales with population-level virality (Fig. S3 and Table S5). The first whole-brain map was thresholded at P < 0.005 and K ≥320, where K is the number of voxels per cluster, to produce a threshold of P < 0.05, corrected using 3dClustSim simulation (version AFNI_16.2.02). Although the type 2 error rate can be expected to be lower for this method of analysis, prior work has shown that cluster correction tends to overestimate the number of significant voxels and thus increases the type 1 error rate (67). Consequently, we also present the results of a more stringent whole-brain correction that controls the number of false positives more efficiently. Specifically, we used nonparametric permutation testing (5,000 iterations) and false-discovery rate (FDR) correction for a voxelwise P-threshold of P < 0.05 and K ≥10 as implemented in the SnPM13 toolbox (68). (Study 1 results for multiple comparisons correction using nonparametric permutation testing corrected at FDR P < 0.05 vary across individual runs of the 5,000 permutations protocol implemented here, because of random elements in this analysis technique. Specifically, although several runs produced maps similar to the map printed in Fig. S3, these results border on P < 0.05. All runs of the permutation protocol for study 1 produced maps that looked very similar to the one printed here at P < 0.06 or P < 0.07. Study 2 results are highly robust across several runs of the permutation protocol, P < 0.05, FDR corrected.)
SI Study 2 Whole-Brain Analysis
To conduct a parallel whole-brain analysis for study 2 participants, we first created first-level models for each participant that included a separate boxcar function for activity across all trials within a certain condition (abstract, narrowcasting, broadcasting) for the reading screen (three regressors) of the article task. Separate regressors for rating screens were further derived depending on the condition presented on the reading screen (six regressors in total). Crucially, an additional regressor specified the boxcar function representing the reading screen during abstract trials modified by a mean-centered parametric modulator of population-level virality ranks of each article. As for study 1, virality ranks were derived by ranking articles shown within the abstract condition by their population-level retransmission counts for each participant (range, 1–14). The model also included six nuisance regressors to control for motion. Second, at the group level, neural activity during the main task was pooled for all participants to examine the main contrasts of interest: activity during the reading screen in abstract trials modulated by population-level virality ranks compared with the baseline resting state. SI Text, Fig. S3, and Table S4 for details and results.
In parallel to study 1 analyses, whole-brain maps were thresholded via 3dClustSim simulation at P < 0.005 and K ≥296 (version AFNI_16.2.02) and nonparametric permutation testing (5,000 iterations) and FDR correction for a voxelwise P-threshold of P < 0.05 and K ≥10 as implemented in the SnPM13 toolbox (68). Results are reported in Fig. S3 and Table S5.
SI Analysis of Other Article Task Conditions
In the main text, we focus on neural activity extracted from reading trials in the study 1 article task (Fig. 1) because the reading condition most closely represents real-world experiences of NYTimes readers who are unlikely to visit the website to find an article to share with somebody. Instead, readers are more likely to browse abstracts and consider reading various articles until one article motivates them to share it with somebody else.
Nonetheless, an additional question to consider is the extent to which task instructions affect the relationship between neural activity during article exposure and population-level sharing. Therefore we examined the relationship between value-related neural activity in our value ROI in response to an article’s headline and abstract and population-level article retransmission data, focusing separately on narrowcasting trials in which participants were primed before each trial via a cue screen to consider sharing articles with one Facebook friend and broadcasting trials in which participants were primed to consider sharing the article on their Facebook wall. Note that this analysis is not possible for study 2 data, because the other two conditions, not analyzed in the main text, are not comparable to those in study 1 and did not include the presentation of original article abstracts.
Results show that value-related neural activity in response to articles shown in a sharing condition is marginally related to population-level virality in the case of narrowcasting trials [r = 0.184, P = 0.10] and is not significantly related to population-level virality in the case of broadcasting trials [r = 0.133, P = 0.24]. Individual-level data from study 1 suggest that explicit instructions to share (i.e., the two sharing conditions) increase the overall level of sharing-relevant brain activity compared with instructions to consider reading the full text of an article (i.e., the reading condition analyzed here; ref. 23). However, we also found that these explicit instructions reduce the variance in value-related activity, which is larger for reading trials (s2 = 5.10) than for narrowcasting (s2 = 4.18) and broadcasting (s2 = 3.24) trials. This ordering of conditions according to variance in information-sharing value corresponds to the condition ordering in terms of the strength of the relationship between value-related activity and population-level virality. If this interpretation is correct, one potential implication could be that sharers are likely to share articles based on “gut” decisions, which are better represented by the reading trials, which did not specifically give participants the goal of sharing in each trial, than by longer elaboration, which is better represented by sharing trials.
SI Article Characteristics
In a content-focused investigation of 760 NYTimes health news articles that included the 80 articles used here, Kim (11) characterized the article headlines and abstracts by analyzing human (i.e., the presence of efficacy information or the mention of diseases or bad health conditions) and computerized (expressed positivity: the difference between the number of positive and negative words; expressed evocativeness/arousal: the sum of positive and negative words) content and with the help of lay human raters (perceived usefulness, induced positivity, perceived controversiality, induced evocativeness/arousal, and perceived novelty). Here we explore the relationship between these content characteristics and concepts within our value-based virality framework as well as population-level virality.
SI Analysis of Article Characteristics
Prior work has shown that content characteristics can impact virality (2, 5), and this argument has been made particularly effectively in the case of news articles (11, 38). Consequently, we explored the role of content characteristics in value-based virality. Specifically, content characteristics might be involved in three different ways. (i) Article characteristics might affect virality directly and independently of variables included in the value-based virality model. If so, it would be of interest whether neural data explain the variance in population-level sharing over and above that explained by article characteristics. (ii) Article characteristics might affect information-sharing value directly or via some other mechanism not currently included in the value-based virality model. (iii) Article characteristics might be antecedents of thoughts regarding the self-related and social outcomes of sharing.
To explore these possibilities, we first checked whether the predictions made by value-based virality (Fig. 1) hold even when controlling for article characteristics. For this purpose, we estimated models identical to the one in Fig. 1 but for the sake of parsimony excluded the insignificant direct effects of self-related and social processing on virality. Each model additionally included a direct effect of one article characteristic on population-level virality. Paralleling other analyses presented in this article, all variables were rank-ordered. In both studies, the effects presented in Fig. 1 were robust when controlling for any of the nine article characteristics considered here. In fact, the only article characteristic that showed a significant effect on population-level virality in these models was the perceived usefulness of an article [B (unstandardized estimate of this parameter) = 0.202, SE = 0.101, P = 0.04] in study 1, but this effect did not replicate in study 2.
Second, we examined the relationships between each of the nine content characteristics available to us and average neural activity in regions associated with self-related and social processing in response to each article using t tests and Pearson correlation where appropriate. Paralleling other analyses presented in this article, all variables were rank-ordered.
In study 1, we found a positive relationship between induced positivity in an article and neural activity in the self-related processing ROI [r = 0.231; P = 0.04]. In addition, articles that mentioned diseases or negative health issues (mean, 9.74) were associated with less self-related processing than articles that did not [mean, 10.70; T(78) = 2.24; P = 0.03] in study 1. However, these effects did not replicate in study 2.
Finally, we explored direct effects of article characteristics on information-sharing value (i.e., average neural activity in our value-related processing ROI) using analytical strategies identical to those explained above. Value-related neural activity was positively related to the extent to which articles induced positivity in human raters [r = 0.309; P = 0.005], and articles that mentioned diseases or bad health conditions (mean, 9.50) engaged less value-related activity than articles that did not [mean, 10.96; T(78) = 3.04; P = 0.003]. However, these effects did not replicate in study 2.
In sum, our results hold, even when controlling for the effects of various article characteristics on virality, suggesting that neural activity contributes information over and above what can be learned from variables commonly used in the literature on virality (11, 38). In contrast to prior work, most article characteristics did not predict population-level sharing. This dissonance with existing studies might be the result of methodological differences among studies. Most notably, previous reports of effects between article characteristics and population-level sharing showed relatively small effect sizes that were identified only in very large samples (e.g., n > 6,000 in ref. 38 and n = 760 in ref. 11). Because of time restrictions in the fMRI scan, we were not able to replicate these article sample sizes. Nonetheless, our ability to predict virality from neural variables even in this small sample of articles speaks to the strength and utility of fMRI.
In addition, we identified selected relationships between individual article characteristics and the extent to which articles engaged neural activity associated with self-related, social, or value-related cognition in study 1. Although these relationships generally did not replicate in study 2, these findings might suggest that content characteristics could be promising candidates in the search for antecedents of the psychological processes that affect sharing. The lack of robustness of these effects might be due to the small sample size and homogeneity of articles. In addition, it is possible that sharing-relevant cognitions are more sensitive to combinations of article characteristics (e.g., the emotional tone in combination with the topic) than to isolated characteristics. However, the specific combination of article characteristics that enhances expectations of positive social or self-related outcomes of sharing might be highly context dependent. An exploration of the large number of potential interaction terms is beyond the scope of this investigation.
Acknowledgments
We thank Erin Maloney and Rosie (Eungyuhl) Bae for support with stimulus materials and the Communication Neuroscience Laboratory for research assistance, particularly Nicole Cooper for advice during data analysis and Lynda Lin and Elizabeth Beard for support with data collection. This work was supported by the Defense Advanced Research Projects Agency (DARPA) Grant D14AP00048 (E.B.F., principal investigator). Additional support was received from NIH New Innovator Award 1DP2DA03515601 (E.B.F., principal investigator). The views, opinions, and/or findings contained in this article are those of the authors and should not be interpreted as representing the official views or policies, either expressed or implied, of DARPA or the Department of Defense.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data and code to replicate the main analyses presented in this manuscript are available at https://github.com/cnlab/viralityPNAS/.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615259114/-/DCSupplemental.
References
- 1.Csibra G, Gergely G. Natural pedagogy as evolutionary adaptation. Philos Trans R Soc Lond B Biol Sci. 2011;366(1567):1149–1157. doi: 10.1098/rstb.2010.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger J. Word of mouth and interpersonal communication: A review and directions for future research. J Consum Psychol. 2014;24(4):586–607. [Google Scholar]
- 3.Rogers EM. Diffusion of Innovations. 5th Ed Free Press; New York: 2003. [Google Scholar]
- 4.Southwell BG, Yzer MC. The roles of interpersonal communication in mass media campaigns. Commun Yearb. 2007;31(1):420–462. [Google Scholar]
- 5.Cappella JN, Kim HS, Albarracín D. Selection and transmission processes for information in the emerging media environment: Psychological motives and message characteristics. Media Psychol. 2015;18(3):396–424. doi: 10.1080/15213269.2014.941112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao L. 2010 Facebook: 350M people using messaging; more than 4B messages sent daily. Available at https://techcrunch.com/2010/11/15/facebook-350m-people-using-messaging-more-than-4b-messages-sent-daily/. Accessed March 11, 2016.
- 7.Krikorian R. 2013 New Tweets per second record, and how! Available at https://blog.twitter.com/2013/new-tweets-per-second-record-and-how. Accessed November 12, 2015.
- 8.Radicati Group 2015 Email statistics report 2015-2019, executive summary. Available at www.radicati.com/wp/wp-content/uploads/2015/02/Email-Statistics-Report-2015-2019-Executive-Summary.pdf. Accessed November 12, 2015.
- 9.Bandari R, Asur S, Huberman BA. 2012 The pulse of news in social media: Forecasting popularity. ArXiv12020332 Phys. Available at https://arxiv.org/abs/1202.0332. Accessed May 11, 2016.
- 10.Southwell BG. Social Networks and Popular Understanding of Science and Health: Sharing Disparities. Johns Hopkins Univ Press; Baltimore: 2013. [Google Scholar]
- 11.Kim HS. Attracting views and going viral: How message features and news-sharing channels affect health news diffusion. J Commun. 2015;65(3):512–534. doi: 10.1111/jcom.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh B, Hong L, Pirolli P, Chi EH. 2010 IEEE Second International Conference on Social Computing (SocialCom) IEEE; New York: 2010. Want to be retweeted? Large scale analytics on factors impacting retweet in Twitter network; pp. 177–184. [Google Scholar]
- 13.Goel S, Anderson A, Hofman J, Watts DJ. The structural virality of online diffusion. Manage Sci. 2016;62(1):180–196. [Google Scholar]
- 14.Krumpal I. Determinants of social desirability bias in sensitive surveys: A literature review. Qual Quant. 2011;47(4):2025–2047. [Google Scholar]
- 15.Wilson TD, Nisbett RE. The accuracy of verbal reports about the effects of stimuli on evaluations and behavior. Soc Psychol. 1978;41(2):118–131. [Google Scholar]
- 16.Wilson TD, Schooler JW. Thinking too much: Introspection can reduce the quality of preferences and decisions. J Pers Soc Psychol. 1991;60(2):181–192. doi: 10.1037//0022-3514.60.2.181. [DOI] [PubMed] [Google Scholar]
- 17.Falk EB, Cascio CN, Coronel JC. Neural prediction of communication-relevant outcomes. Commun Methods Meas. 2015;9(1–2):30–54. [Google Scholar]
- 18.Plassmann H, Venkatraman V, Huettel S, Yoon C. Consumer neuroscience: Applications, challenges, and possible solutions. J Mark Res. 2015;52(4):427–435. [Google Scholar]
- 19.Bartra O, McGuire JT, Kable JW. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy DJ, Glimcher PW. The root of all value: A neural common currency for choice. Curr Opin Neurobiol. 2012;22(6):1027–1038. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy DJ, Glimcher PW. Comparing apples and oranges: Using reward-specific and reward-general subjective value representation in the brain. J Neurosci. 2011;31(41):14693–14707. doi: 10.1523/JNEUROSCI.2218-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falk EB, Morelli SA, Welborn BL, Dambacher K, Lieberman MD. Creating buzz: The neural correlates of effective message propagation. Psychol Sci. 2013;24(7):1234–1242. doi: 10.1177/0956797612474670. [DOI] [PubMed] [Google Scholar]
- 23.Baek EC, Scholz C, O’Donnell MB, Falk EB. Neural correlates of selecting and sharing information. Psychol Sci. doi: 10.1177/0956797617695073. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray RJ, Schaer M, Debbané M. Degrees of separation: A quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci Biobehav Rev. 2012;36(3):1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Northoff G, et al. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Dunbar RI, Marriott A, Duncan ND. Human conversational behavior. Hum Nat. 1997;8(3):231–246. doi: 10.1007/BF02912493. [DOI] [PubMed] [Google Scholar]
- 27.Landis MH, Burtt HE. A study of conversations. J Comp Psychol. 1924;4(1):81–89. [Google Scholar]
- 28.Naaman M, Boase J, Lai C-H. Proceedings of the 2010 ACM Conference on Computer Supported Cooperative Work. Association for Computing Machinery; New York: 2010. Is it really about me?: Message content in social awareness streams; pp. 189–192. [Google Scholar]
- 29.Tamir DI, Mitchell JP. Disclosing information about the self is intrinsically rewarding. Proc Natl Acad Sci USA. 2012;109(21):8038–8043. doi: 10.1073/pnas.1202129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falk EB, et al. Functional brain imaging predicts public health campaign success. Soc Cogn Affect Neurosci. 2016;11(2):204–214. doi: 10.1093/scan/nsv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cascio CN, Scholz C, Falk EB. Social influence and the brain: Persuasion, susceptibility to influence and retransmission. Curr Opin Behav Sci. 2015;3:51–57. [Google Scholar]
- 32.Barasch A, Berger J. Broadcasting and narrowcasting: How audience size affects what people share. J Mark Res. 2014;51(3):286–299. [Google Scholar]
- 33.Clark HH, Murphy GL. Audience design in meaning and reference. Adv Psychol. 1982;9:287–299. [Google Scholar]
- 34.Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50(4):531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Dufour N, et al. Similar brain activation during false belief tasks in a large sample of adults with and without autism. PLoS One. 2013;8(9):e75468. doi: 10.1371/journal.pone.0075468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamir DI, Zaki J, Mitchell JP. Informing others is associated with behavioral and neural signatures of value. J Exp Psychol Gen. 2015;144(6):1114–1123. doi: 10.1037/xge0000122. [DOI] [PubMed] [Google Scholar]
- 37.Tamir DI, Thornton MA, Contreras JM, Mitchell JP. Neural evidence that three dimensions organize mental state representation: Rationality, social impact, and valence. Proc Natl Acad Sci USA. 2016;113(1):194–199. doi: 10.1073/pnas.1511905112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berger J, Milkman KL. What makes online content viral? J Mark Res. 2012;49(2):192–205. [Google Scholar]
- 39.Pentland A. Social Physics: How Good Ideas Spread-the Lessons from a New Science. Penguin; New York: 2014. [Google Scholar]
- 40.Rademacher L, et al. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49(4):3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- 41.Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50(7):1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Cooper N, Tompson S, O’Donnell MB, Falk EB. Brain activity in self- and value-related regions in response to online antismoking messages predicts behavior change. J Media Psychol. 2015;27(3):93–109. doi: 10.1027/1864-1105/a000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falk EB, Berkman ET, Lieberman MD. From neural responses to population behavior: Neural focus group predicts population-level media effects. Psychol Sci. 2012;23(5):439–445. doi: 10.1177/0956797611434964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venkatraman V, et al. Predicting advertising success beyond traditional measures: New insights from neurophysiological methods and market response modeling. J Mark Res. 2015;52(4):436–452. [Google Scholar]
- 45.Berns G, Moore SE. 2010 A neural predictor of cultural popularity. Available at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=1742971. Accessed February 8, 2016.
- 46.Mezulis AH, Abramson LY, Hyde JS, Hankin BL. Is there a universal positivity bias in attributions? A meta-analytic review of individual, developmental, and cultural differences in the self-serving attributional bias. Psychol Bull. 2004;130(5):711–747. doi: 10.1037/0033-2909.130.5.711. [DOI] [PubMed] [Google Scholar]
- 47.Taylor SE, Brown JD. Illusion and well-being: A social psychological perspective on mental health. Psychol Bull. 1988;103(2):193–210. [PubMed] [Google Scholar]
- 48.Fehr E, Camerer CF. Social neuroeconomics: The neural circuitry of social preferences. Trends Cogn Sci. 2007;11(10):419–427. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychol Bull. 1995;117(3):497–529. [PubMed] [Google Scholar]
- 50.Lieberman MD. Social: Why Our Brains Are Wired to Connect. Crown; New York: 2013. [Google Scholar]
- 51.Saxe R, Moran JM, Scholz J, Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Soc Cogn Affect Neurosci. 2006;1(3):229–234. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brewer MB. The social self: On being the same and different at the same time. Pers Soc Psychol Bull. 1991;17(5):475–482. [Google Scholar]
- 53.Bretherton I. In: Pouring New Wine into Old Bottles: The Social Self as Internal Working Model. Self Processes and Development, The Minnesota symposia on Child Psychology. Gunnar MR, Sroufe LA, editors. Vol 23. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ: 1991. pp. 1–41. [Google Scholar]
- 54.Genevsky A, Knutson B. Neural affective mechanisms predict market-level microlending. Psychol Sci. 2015;26(9):1411–1422. doi: 10.1177/0956797615588467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz SH. 2007. Value orientations: Measurement, antecedents and consequences across nations. Measuring Attitudes Cross-Nationally. Lessons from the European Social Survey (Sage Publishing, London), pp 161–193.
- 56.Schwartz SH. A theory of cultural value orientations: Explication and applications. Comp Sociol. 2006;5(2):137–182. [Google Scholar]
- 57.Poldrack RA. Inferring mental states from neuroimaging data: From reverse inference to large-scale decoding. Neuron. 2011;72(5):692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scholz C, Baek EC, O’Donnell MB, Falk EB. 2015 Social networks and neural mechanisms of communication. Open Science Framework. https://dx.doi.org/10.17605/OSF.IO/A8XME.
- 59.Scholz C, Baek EC, O’Donnell MB, Falk EB. 2016 Neural mechanisms of influence and message propagation. Open Science Framework. https://dx.doi.org/10.17605/OSF.IO/4SRW5.
- 60.Schmitz TW, Johnson SC. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neurosci Biobehav Rev. 2007;31(4):585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 62.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 63.Brett M, Anton J-L, Valabregue J-BP. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16(2):497 (abstr). [Google Scholar]
- 64.Rosseel Y. lavaan: An R package for structural equation modeling. J Stat Softw. 2012;48(2):1–36. [Google Scholar]
- 65.Tingley D, Yamamoto T, Kentaro H, Keele L, Imai K. mediation: R package for causal mediation analysis. J Stat Softw. 2014;59(5):1–38. [Google Scholar]
- 66.Kenny DA, Kaniskan B, McCoach DB. The performance of RMSEA in models with small degrees of freedom. Sociol Methods Res. 2015;44(3):486–507. [Google Scholar]
- 67.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Statistical nonParametric Mapping. A toolbox for SPM Available at www2.warwick.ac.uk/snpm. Accessed December 1, 2016.