Abstract
Extracellular calmodulin (ExtCaM) exerts multiple functions in animals and plants, but the mode of ExtCaM action is not well understood. In this paper, we provide evidence that ExtCaM stimulates a cascade of intracellular signaling events to regulate stomatal movement. Analysis of the changes of cytosolic free Ca2+ ([Ca2+]cyt) and H2O2 in Vicia faba guard cells combined with epidermal strip bioassay suggests that ExtCaM induces an increase in both H2O2 levels and [Ca2+]cyt, leading to a reduction in stomatal aperture. Pharmacological studies implicate heterotrimeric G protein in transmitting the ExtCaM signal, acting upstream of [Ca2+]cyt elevation, and generating H2O2 in guard cell responses. To further test the role of heterotrimeric G protein in ExtCaM signaling in stomatal closure, we checked guard cell responses in the Arabidopsis (Arabidopsis thaliana) Gα-subunit-null gpa1 mutants and cGα overexpression lines. We found that gpa1 mutants were insensitive to ExtCaM stimulation of stomatal closure, whereas cGα overexpression enhanced the guard cell response to ExtCaM. Furthermore, gpa1 mutants are impaired in ExtCaM induction of H2O2 generation in guard cells. Taken together, our results strongly suggest that ExtCaM activates an intracellular signaling pathway involving activation of a heterotrimeric G protein, H2O2 generation, and changes in [Ca2+]cyt in the regulation of stomatal movements.
Changes in cytosolic free Ca2+ ([Ca2+]cyt) have been observed during the signal transduction in response to abiotic and biotic stresses (Sanders et al., 2002). In guard cells, [Ca2+]cyt elevations have been shown to be early events in the signaling cascade that results in abscisic acid (ABA)-induced stomatal closure in a number of plant species (McAinsh et al., 1995; Grabov and Blatt, 1998). Accumulating evidence indicates that many stimuli enhance [Ca2+]cyt increase in guard cells (Rudd and Franklin-Tong, 2001); however, the upstream components of calcium signaling are not well understood.
Heterotrimeric G proteins composed of α-, β-, and γ-subunits are a key intracellular signaling molecule in eukaryotic cells. The activation by G-protein-coupled receptor results in conformation change of the Gα protein due to GTP binding and the separation of Gα from the Gβγ dimer. GTP hydrolysis by GTPase activity of Gα results in the reassociation of Gα with Gβγ (Jones and Assmann, 2004). In plants, G protein has been found to be involved in ion-channel regulation (Aharon et al., 1998; Wang et al., 2001), control of seed germination (Ullah et al., 2002), pollen tube elongation (Ma et al., 1999), and responses to ABA (Wang et al., 2001). Genome sequencing revealed the existence of only one prototypical Gα (GPA1) in Arabidopsis (Arabidopsis thaliana; Ma et al., 1990). It was reported that Gα-subunit-null mutants, gpa1-1 and gpa1-2, were insensitive to ABA inhibition of whole-cell inward K+ currents and pH-independent ABA-activation of anion channels (Wang et al., 2001), suggesting Gα is a key component in ABA signaling. It is unknown whether Gα-subunit participates in calcium signaling in ABA regulation of guard cell responses.
Recently, reactive oxygen species (ROS) has been shown to be an important second messenger in signaling to developmental processes, such as polar growth of Fucus rhizoid cells (Coelho et al., 2002) and cell elongation in root growth (Demidchik et al., 2003), responses to environmental stresses (Baxter-Burrell et al., 2002), and guard cell movement (Pei et al., 2000). Evidence indicates that homeostasis of ROS depends on the activity of several enzymes involved in ROS generation as well as the activity of ROS scavenging enzymes (for review, see Mittler, 2002). Recently, guard cell-specific NADPH oxidases AtrbohD (Arabidopsis respiratory burst oxidase homologs d) and AtrbohF have been identified, and the double mutants of atrbohD/F are impaired in ABA-induced ROS generation, [Ca2+]cyt increases, and stomatal closing (Kwak et al., 2003), suggesting that AtrbohD and AtrbohF NADPH oxidases and ROS play an important role in ABA signal transduction in guard cells. The evidence that Ca2+-sensing receptor (CAS) in Arabidopsis plasma membrane, which mediates extracellular Ca2+ induced cytosolic Ca2+ increase in guard cells (Han et al., 2003) suggests that CAS may regulate [Ca2+]cyt status through functioning together with the regulation of Ca2+ influx and release from intracellular Ca2+ stores, Ca2+-ATPase, and Ca2+/H+ antiporter. However, it is unclear whether ROS also acts as a mediator in extracellular Ca2+ receptor mediated stomatal movement.
Calmodulin (CaM), a ubiquitous and abundant intracellular Ca2+ receptor, exists in all eukaryotic cells (for review, see Vetter and Leclerc, 2003). Recently, it has been found that CaM exists extracellularly to exert many functions in both animals and plants. In animals, extracellular CaM (ExtCaM) is present in body fluids, saliva, urine, and milk (Houston et al., 1997), stimulating the proliferation of cultured hepatocytes, melanoma cells, and fibroblasts (Goberdhan et al., 1993). In addition, ExtCaM inhibits tumor necrosis factor-α release and augments neutrophil elastase release, preventing further cytotoxicity (Houston et al., 1997). In plants, extracellular peptides, such as systemin, CLAVATA3, and ENOD40, may act as intercellular signals, regulating some important processes, e.g. wound defense reaction, maintenance of shoot apical meristem, and nodule formation (Pearce et al., 1993; Yang et al., 1993; van de Sande et al., 1996; Trotochaud et al., 2000). ExtCaM has been found in many plant species. For example, ExtCaM was detected in the medium of suspension-cultured cells from Angelica dahurica, carrot, and tobacco (Sun et al., 1994, 1995). The existence of ExtCaM in plants suggests that it may have important functions. Indeed, ExtCaM stimulates proliferation of suspension-cultured cells of A. dahurica, Fenistum typhoides, and Sataria italica by enhancing cell wall regeneration and protoplast division (Sun et al., 1994) and accelerates pollen germination and tube growth (Ma and Sun, 1997; Ma et al., 1999; Shang et al., 2001). Pharmacological studies have implicated several signaling molecules, including heterotrimeric G protein (Ma et al., 1999), phosphoinositide, and cytosolic Ca2+ (Shang et al., 2001) in the signal transduction pathway of ExtCaM-enhanced pollen germination.
Stomatal movements regulate the loss of water to the atmosphere and the entry of CO2 into the plants for photosynthetic carbon fixation. Many factors, such as ABA, CO2, light/darkness, and temperature, are known to modulate stomatal movements (Schroeder et al., 2001). The involvement of intracellular CaM in stomatal movements has also been studied (Cottele et al., 1996). However, the effects of ExtCaM on stomatal movements are not well addressed. In our previous report, we demonstrated that ExtCaM existed in the walls of guard cells and that its exogenous application promoted stomatal closure (Chen et al., 2003). In this report, we investigate the intracellular signaling mechanism by which ExtCaM mediates stomatal movement using combined pharmacological, physiological, and genetic approaches. We have provided convincing evidence that ExtCaM triggers a cascade of intercellular signaling events involving heterotrimeric G protein, H2O2, and Ca2+in the regulation of stomatal closure.
RESULTS AND DISCUSSION
ExtCaM Induces [Ca2+]cyt Increase in Guard Cells
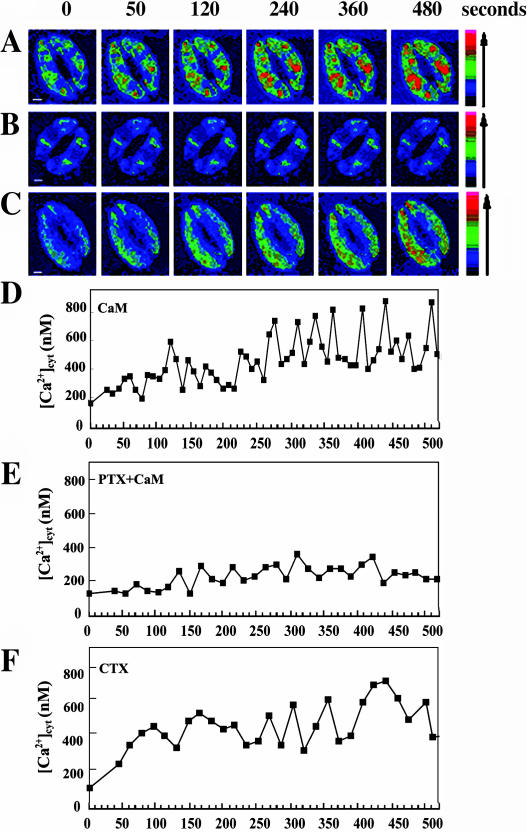
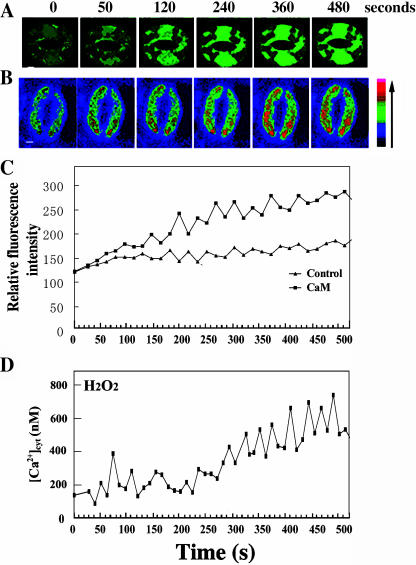
Since [Ca2+]cyt levels and oscillation have been shown to be a key mediator of guard cell movement (Allen et al., 1999, 2001), we were interested in whether ExtCaM has an effect on the dynamic changes of [Ca2+]cyt in guard cells. Vicia faba guard cells have been a favorite model for the study of guard cell movement (Assmann, 1993). In this study, we applied 10−8 m CaM to induce stomata closure (Chen et al., 2003) and used confocal laser scanning microscopy (CLSM) to visualize the fluorescence of fluo-3, which was loaded into guard cells. Among 27 V. faba guard cells, 63% of the cells showed the typical [Ca2+]cyt changes responsive to ExtCaM (Fig. 1A). ExtCaM-induced dramatic elevation in [Ca2+]cyt was found after 280 s incubation of CaM (Fig. 1D), while the control treatment (10−8 m bovine serum albumin) did not cause obvious fluorescent changes in guard cells (data not shown).
Figure 1.
Application of ExtCaM causes the changes of [Ca2+]cyt through the function of G protein in V. faba guard cells. A to C, Fluorescent changes in guard cells preloaded with 10 μm fluo-3 AM and (D–F) quantitative curve of [Ca2+]cyt responsive to the induction of 10−8 m CaM (A and D), pretreatment of 400 ng/mL PTX plus CaM (B and E), and 400 ng/mL CTX (C and F), respectively. [Ca2+]cyt was quantified based the relative fluorescent intensity referred to the standard serial calcium concentrations as described in “Material and Methods.” All the start times in the following figures of this paper reorganize the time point of chemicals/protein treatment as zero time. Bar = 10 μm.
Multiple factors such as ABA, CO2, light/darkness, and temperature regulate stomatal movements and cause guard cell [Ca2+]cyt changes; for example, ABA induces increase in [Ca2+]cyt and subsequently stomatal closure (Grabov and Blatt, 1998; Hamilton et al., 2000). A plasma membrane-localized extracellular CAS has been shown to regulate guard cell [Ca2+]cyt (Han et al., 2003). Our results demonstrate that an apoplast-localized protein, ExtCaM, can regulate [Ca2+]cyt elevation in guard cells. This finding is quite exciting because it supports the hypothesis that guard cells may sense extracellular Ca2+ and regulate intracellular Ca2+ levels via Ca2+-CaM complex. A very important and interesting future question is whether Ca2+-CaM interacts with or acts independent of CAS to regulate [Ca2+]cyt during guard cell movement. Furthermore, considering that the ExtCaM induction of [Ca2+]cyt elevation is similar to that of ABA, it might be possible that a new regulatory factor naturally existing in guard cell walls regulates stomatal movements together with ABA. These findings not only extend the functions of ExtCaM, but also provide clues to understanding the regulatory mechanisms for stomatal movements.
Heterotrimeric G Protein Might Be Involved in ExtCaM Promotion of Stomatal Closure
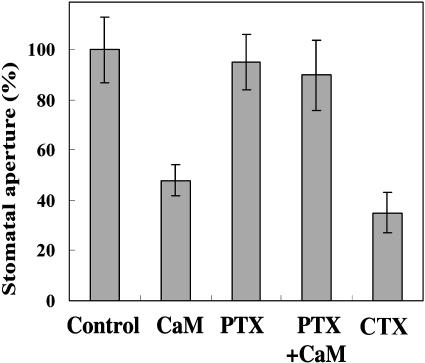
Having observed ExtCaM induction of [Ca2+]cyt elevation and stomatal closure, we next sought to investigate whether other important signaling components might transduce this signal input. Heterotrimeric G proteins have been shown to regulate [Ca2+]cyt by modulating Ca2+ channels in the plasma membrane of animal cells. It was reported that heterotrimeric G protein mediated ExtCaM stimulation of pollen germination (Ma et al., 1999). In guard cells, G protein activators such as cholera toxin (CTX; van Corven et al., 1993) and GTPγS inhibited the influx of K+, and the effect of GTPγS is prevented by buffering cytosolic Ca2+ to a low level, suggesting that activated G proteins may inhibit inward K+ channels via elevation of [Ca2+]cyt (Fairley-Grenot and Assmann, 1991; Wu and Assmann, 1994). Using genetic approaches, Gα has been shown to mediate ABA signaling in regulating inward K+ channels and slow anion channels and stomatal movements in Arabidopsis (Wang et al., 2001; Coursol et al., 2003). Thus, we speculated that heterotrimeric G proteins may also mediate ExtCaM signaling in guard cells. In this study, we used pertussis toxin (PTX), an inhibitor of heterotrimeric G protein α-subunit (Kuryshev et al., 1993), and CTX, an activator of heterotrimeric G protein α-subunit (van Corven et al., 1993) to assess whether heterotrimeric G proteins act as a mediator in ExtCaM promotion of [Ca2+]cyt elevation. As shown in Figure 2, CaM promotion of V. faba stomatal closure was greatly impaired when leaf epidermal strips were pretreated with PTX. In parallel with this effect, when V. faba guard cells were pretreated with PTX, 21 guard cells (n = 30) failed to trigger increase of [Ca2+]cyt responsive to ExtCaM (Fig. 1, B and E). Meanwhile, CTX, an activator of heterotrimeric G protein, induced both stomatal closure (Fig. 2) and [Ca2+]cyt increase in 16 of 22 guard cells during 480-s CTX treatment (Fig. 1, C and F). The effect of CTX on stomatal apertures and elevation in [Ca2+]cyt resembled that of ExtCaM, further supporting the hypothesis that ExtCaM acts through [Ca2+]cyt to regulate stomatal movement. Taken together, these results indicate that heterotrimeric G protein may mediate ExtCaM induction of V. faba stomatal closure by tuning [Ca2+]cyt in guard cells.
Figure 2.
Effect of PTX or CTX on V. faba stomatal closure. Control, Open stomata were kept in MES buffer but minus CaM for 2 h, then the stomatal aperture was treated as 100%; CaM, open stomata were treated with 10−8 m CaM solution for 2 h; PTX, open stomata were treated with 400 ng/mL PTX solution for 2 h; PTX + CaM, open stomata were pretreated with 400 ng/mL PTX for 30 min, washed with MES buffer, then kept in CaM solution for 2 h; CTX, open stomata were treated with 400 ng/mL CTX for 2 h.
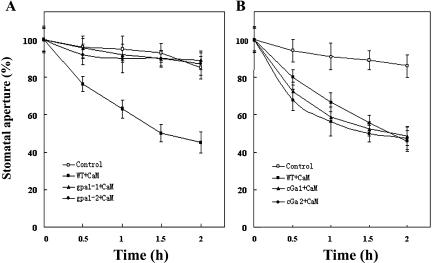
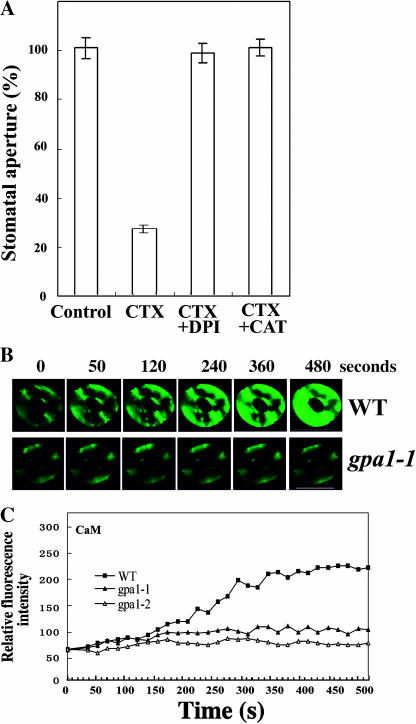
To confirm these pharmacological data, we further used Arabidopsis mutants gpa1-1 and gpa1-2 harboring the recessive T-DNA knockout alleles of AtGPA1, the only one prototypical Gα gene in Arabidopsis genome (Ullah et al., 2001), and Arabidopsis transgenic lines overexpressing cGα (AtGPA1 with a point mutation of Glu-222 to Leu, which locks Gα in the active state once activated; Okamoto et al., 2001), and wild-type Arabidopsis ecotype Wassilewskija (Ws). In Arabidopsis wild-type leaf epidermal strips, ExtCaM induced stomatal closure as in V. faba (Fig. 3A). ExtCaM induction of stomatal closure was completely impaired in the mutants of gpa1-1 and gpa1-2 (Fig. 3A), as the mutant stomata in the presence of ExtCaM behaved exactly like wild-type control in the absence of ExtCaM. In contrast, cGα overexpressing lines showed faster stomatal closure induced by ExtCaM than they did in the wild type, although the final stomatal aperture of the cGα lines was not significantly different from that in the wild type (Fig. 3B). In the meantime, we also checked the effects of PTX and CTX on guard cell responses in gpa1 mutants. Consistent with the above results, our data showed that gpa1 mutants were insensitive to these drugs (data not shown). Therefore our results provide the genetic evidence that Gα is involved in the regulation of ExtCaM action in animals and plants. Together with the pharmacological experiment described above, these results indicate that heterotrimeric G protein acts as a positive regulator of guard cell responses to ExtCaM.
Figure 3.
Application of ExtCaM causes stomatal closure through the function of G protein in Arabidopsis guard cells. A, Mutants gpa1-1 and gpa1-2 impaired stomatal closure induced by 10−8 m CaM, but not in wild type. Control, Epidermal strips of Ws plants were kept in MES buffer; WT (wild type) + CaM, epidermal strips of Ws plants were treated with 10−8 m CaM solution; gpa1-1 + CaM, epidermal strips of gpa1-1 plants were treated with CaM solution; gpa1-2 + CaM, epidermal strips of gpa1-2 plants were kept in 10−8 m CaM solution. B, cGα constitutively overexpressing heterotrimeric G protein α-subunit AtGPA1 stimulated stomatal closure induced by 10−8 m CaM. Control, Epidermal strips of Ws plants were kept in MES buffer; WT + CaM, epidermal strips of Ws plants were treated with 10−8 m CaM solution; cGα1 + CaM, epidermal strips of cGα1 plants were treated with 10−8 m CaM solution; cGα2 + CaM, epidermal strips of cGα2 plants were kept in 10−8 m CaM solution. Each assay was repeated three times. The data were presented as mean ± se (n = 150).
Based on the above results we propose that ExtCaM, perhaps acting as extracellular Ca2+ sensor and activating the receptor of CaM, activates G protein α-subunit, leading to stomatal closure. ExtCaM activation of heterotrimeric G proteins seems to be a common mechanism for the action of ExtCaM, as a similar mechanism was reported for ExtCaM promotion of pollen tube elongation (Ma et al., 1999). In gpa1-1 and gpa1-2 mutants, because of the T-DNA insertion in the predicted seventh intron (gpa 1-1) and in the eighth exon (gpa1-2), four of its five polypeptide loops required for GTP binding, GTPase, and the effector loop have been eliminated (Ullah et al., 2001). The transduction pathway of ExtCaM to stomatal closure has been interrupted in G protein, as the result, stomata failed to close in response to ExtCaM. Thus, Gα is required for ExtCaM-mediated stomatal closure. In AtGPA1 cGα overexpression lines, ExtCaM induction of stomatal closure was accelerated but not constitutive, suggesting that Gα activation is not sufficient for ExtCaM induction of stomatal closure. An interesting question to be addressed in the future is whether there is a functional link between ExtCaM, G protein, and ABA, which is also known to regulate G protein in the regulation of stomatal movement (Wang et al., 2001).
Involvement of H2O2 in ExtCaM-Induced Stomatal Closure
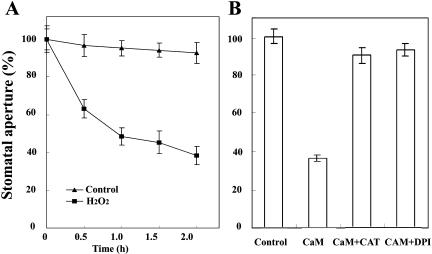
It has been reported that ROS is a key regulator of stomatal movements (Purohit et al., 1994). For instance, H2O2 caused an increase in guard cell [Ca2+]cyt, which was abolished in the presence of EGTA (McAinsh et al., 1996). H2O2 has been shown to be a signal molecule in ABA induction of stomatal closure. In this process, ABA induces H2O2 production in guard cells by activating NADPH oxidases, and then H2O2 causes a [Ca2+]cyt increase by activating Ca2+ channels in the plasma membrane (Pei et al., 2000; Murata et al., 2001; Kwak et al., 2003). In this study, we observed that H2O2 promoted stomatal closure in V. faba (Fig. 4A), which is consistent with the previous reports in Arabidopsis (Pei et al., 2000). To investigate whether H2O2 is involved in ExtCaM-induced stomatal closure, V. faba epidermal strips were incubated in ExtCaM solution containing diphenylene iodonium (DPI) or catalase (CAT), which was either an inhibitor of NADPH oxidases, the key enzyme in the production of H2O2, or the scavenger of H2O2 (Zhang et al., 2001; Qin et al., 2004). Under these conditions, both DPI and CAT abolished the stimulation of stomatal closure by ExtCaM (Fig. 4B), suggesting that stomatal closure induced by ExtCaM requires the production of H2O2, and NADPH oxidases might be involved in the generation of H2O2.
Figure 4.
H2O2 mediates ExtCaM-induced V. faba stomatal closure. A, Effect of 5 × 10−5 m H2O2 in MES buffer on stomatal closure within 2 h. Control indicates no addition of H2O2 except MES buffer. B, Effects of DPI or CAT on stomatal closure-induced by CaM for 2 h. Control, Open stomata were kept in MES buffer under light for 2 h, then the stomatal aperture was treated as 100%; CaM, open stomata were treated with 10−8 m CaM solution; CaM + CAT, open stomata were treated with 100 units/mL CAT plus 10−8 m CaM; CaM + DPI, open stomata were kept in 10 μm DPI plus 10−8 m CaM. Each assay was repeated three times. The data were presented as mean ± se (n = 150).
It has been previously evidenced that H2DCF-DA-based assays are suitable for measurement of H2O2 production in guard cells (Zhang et al., 2001). Using this method we showed that ExtCaM-induced H2O2 production in V. faba guard cells. Among the 25 guard cells, 64% of the cells showed the typical H2O2 response curve to 10−8 m CaM and CTX but not 10−8 m bovine serum albumin (data not shown). The generation rate of H2O2 was rapid during the first 5 min of ExtCaM treatment (Fig. 5, A and C), further suggesting that H2O2 might be a signal molecule involved in the signal transduction pathway of ExtCaM-induced stomatal closure.
Figure 5.
ExtCaM promotes production of H 2O2 that further induces changes of [Ca2+]cyt in V. faba guard cells. A, Fluorescence and (C) quantitative curve of H2O2 changes reflected with 50 μm H2DCF-DA after addition of 10−8 m CaM. B, Fluorescence and (D) quantitative curve of [Ca2+]cyt in V. faba guard cells preloaded with 10 μm fluo-3 AM responsive to the induction of 5 × 10−5 m H2O2. Control indicates no chemical addition in the buffer solution. Bar = 10 μm.
Given that CaM-induced H2O2 production might be a crucial element in the signal transduction pathway of ExtCaM in guard cells, we next assessed H2O2-induced changes in [Ca2+]cyt in V. faba guard cells. Our results showed that 65% guard cells (n = 26) had dramatic elevations of [Ca2+]cyt triggered by 50 μm H2O2 during a 500-s treatment of H2O2 (Fig. 5, B and D).
Heterotrimeric G Protein Mediates ExtCaM-Induced H2O2 Increase in Guard Cells
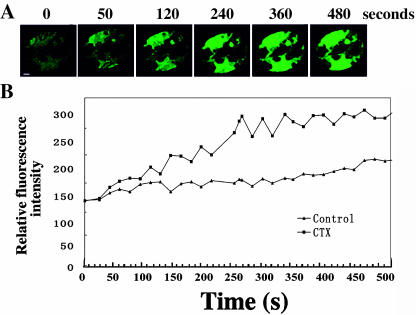
To investigate the relationship among heterotrimeric G protein, H2O2, and Ca2+, we performed the following experiments. First we tested this pathway in V. faba epidermal strips. As shown in Figure 6, A and B, 17 guard cells (n = 25) displayed an increase of H2O2 production induced by CTX. Similarly, CTX induction of stomatal closure was also blocked by either DPI or CAT (Fig. 7A), suggesting that heterotrimeric G protein may act upstream of H2O2 production of the regulation of stomatal closure. Once extracellular Ca2+ was chelated by EGTA, CTX failed to induce H2O2 increase in guard cells (data not shown), suggesting a likely requirement for Ca2+ in the H2O2 production induced by G protein.
Figure 6.
Heterotrimeric G protein activator CTX enhanced H2O2 production in V. faba guard cells. A, Fluorescent changes and (B) quantitative curve of H2O2 reflected with 50 μm H2DCF-DA after addition of 400 ng/mL CTX. Control indicates no chemical addition in the buffer solution. Bar = 10 μm.
Figure 7.
Analysis of functional relationship among heterotrimeric G protein, H2O2, and ExtCaM in guard cells. A, Effects of activator of G protein (400 ng/mL CTX) and inhibitor of NADPH oxidase (10 μm DPI) or scavenger of H2O2 (100 units/mL CAT) on V. faba stomatal closure. Control, Open stomata were kept in MES buffer for 2 h under light, then the stomatal aperture was treated as 100%; CTX, open stomata were treated with 10−8 m CTX solution; CTX + DPI, open stomata were treated with 400 ng/mL CTX plus 10 μm DPI; CTX + CAT, open stomata were pretreated with 400 ng/mL CTX plus 100 units/mL CAT. Each assay was repeated three times. The data were presented as mean ± se (n = 150). B, Fluorescent changes and (C) quantitative curve of H2O2 reflected with 50 μm H2DCF-DA after addition CaM in WT and gap1. Bar = 40 μm.
To confirm the above results, we next used two Gα-subunit-null lines, gpa1-1 and gpa1-2, to assess the role of G protein in the regulation of H2O2 levels in response to ExtCaM. Under the same conditions, gpa1-1 and gpa1-2 showed lower levels of H2O2 than wild-type plants did. Furthermore, gpa1-1 and gpa1-2 showed almost no increase in H2O2 levels when treated with CaM, whereas the wild type had significantly increased in fluorescent intensity within 5 min in the presence of 10−8 CaM (Fig. 7, B and C), indicating that G protein is required for the generation of H2O2.
CONCLUSION
In this study, we have provided strong evidence that ExtCaM stimulates stomatal closure through the activation of heterotrimeric G protein and subsequent promotion of H2O2 production and [Ca2+]cyt elevation. Both genetic and pharmacological studies consistently support the hypothesis that ExtCaM mediating G protein activates the production of H2O2. Pharmacological data also support G protein regulation of ExtCaM-dependent [Ca2+]cyt elevation; this remains to be confirmed by genetic studies. In ABA promotion of stomatal closure, it has been shown that ABA activates the production of H2O2, which in turn activates plasma membrane-localized calcium channels, leading to [Ca2+]cyt elevation (Pei et al., 2000). Given that ExtCaM and G protein activate both H2O2 production and [Ca2+]cyt elevation, it is tempting to propose that in ExtCaM mediated stomatal closure, H2O2 too acts as a second messenger to activate plasma membrane-localized calcium channels and [Ca2+]cyt elevation. However, it is also possible that Ca2+ might also regulate H2O2 production. It has been reported that there are Ca2+ binding sites in NADPH oxidases and that this enzyme may be regulated by heterotrimeric G protein (Keller et al., 1998). The induction of ROS generated by oligogalacturonic acid involves a series of processes including receptor binding (Horn et al., 1989), activation of a heterotrimeric G protein (Legendre et al., 1992), influx of Ca2+ (Chandra et al., 1997), stimulation of phospholipase C (Legendre et al., 1993), and induction of a number of kinases (Chandra and Low, 1995). Nonetheless, future studies should be directed at understanding the functional relationship among G protein, H2O2, and calcium in ExtCaM-mediated stomatal movement.
MATERIALS AND METHODS
Plant Materials
Vicia faba plants were grown in potting mix in a growth chamber under a 12-h-light and 12-h-dark cycle, with a photon flux density of 0.30 mmol m−2 s−1, and day/night temperature cycle of 25°C ± 2°C and 20°C ± 2°C, respectively. Lower epidermis of fully expanded leaves from 3- to 4-week-old V. faba seedlings was used for bioassay and the measurements of cytosolic calcium and ROS. Arabidopsis (Arabidopsis thaliana) plants of cGα overexpressing constitutively active form mutant of heterotrimeric G protein α-subunit AtGPA1, which were obtained from Dr. L.G. Ma (Yale University), were grown in the presence of 70 nm dexamethasone (DEX; Sigma, St. Louis) according to the methods described by Okamoto et al. (2001). T-DNA insertion mutants gpa1-1 and gpa1-2 (from Nottingham Arabidopsis Stock Center) that lack function of G-protein α-subunit (Ullah et al., 2001), and wild-type Ws were cultured as described by Wang et al. (2001). Fully expanded leaves of 4- to 6-week-old Arabidopsis plants were used for epidermal strip bioassay and ROS measurement.
Ca2+-Sensitive Fluorescent Dye Loading
The abaxial epidermal strips from V. faba were peeled gently and incubated in 10 μm 1-[2-amino-5-(2,7-dichloro-6-hydroxy-3-oxo-9-xanthenyl)phenoxy]-2-(2-amino-5-methylphenoxy)ethane-N,N,N′,N′-tetraacetic acid, pentaacetoxymethyl ester (fluo-3 AM) loading buffer (10 mm MES-Tris, pH 6.1) at 4°C for 2 h in darkness. Because the activities of esterases at 4°C were low, fluo-3 AM permeated through the membranes without being hydrolyzed by esterases in cell walls. After washed three times with MES buffer, strips were kept at room temperature for 1 h. During this time, fluo-3 AM inside the cell was hydrolyzed by intracellular esterases and the hydrolyzed form of fluo-3 AM bound to free Ca2+ to indicate dynamic Ca2+ changes in guard cells (Shang et al., 2001).
H2O2-Sensitive Fluorescent Dye Loading
The abaxial epidermal strips from V. faba or Arabidopsis were peeled gently and incubated in 50 μm H2DCF-DA buffer (10 mm MES-Tris, pH 6.1) for 15 min at room temperature and then washed three times before measurement.
CLSM Microscopy
The fluorescence in guard cells was measured using CLSM (Bio-Rad CLSM 1024, Hercules, CA) with the following settings: excitation = 488 nm, emission = 535 nm, frame 512 × 512. Images were recorded every 10 s. When the fluorescence stabilized around 100 s after scanning, the reagents were added directly to the buffer in which the strips were placed, and we treated this agent addition point as zero time in all assays, and fluorescence changes were recorded and the calcium or ROS relative fluorescence intensity was figured by subtracting the basal signal at different time points indicated in figure legends. Using pixel intensity standard curves created by calcium calibration kit (Molecular Probes, Eugene, OR), the calcium concentrations in cells was quantified (Shang et al., 2001). The experiments were repeated at least three times with 7 to 10 cells each time, and one time data were presented to illustrate the changes of fluorescence intensity.
Epidermal Strip Bioassay
After incubated in MES buffer (10 mm MES-Tris, pH 6.1, containing 30 mm KCl and 0.1 mm CaCl2) for 90 min under light to open the stomata, the strips from V. faba or Arabidopsis were treated with the following procedures. For studying the effects of ExtCaM, CTX, or H2O2 on stomatal closure, the strips with open stomata were transferred to the above buffer containing 10−8 m CaM, 400 ng/mL CTX, or 5 × 10−5 m H2O2 solution, separately. For studying the effects of PTX, DPI, or CAT on CaM induction of stomatal closure, the strips with open stomata were either pretreated with 400 ng/mL PTX solution for 30 min and the strips transferred to and incubated in 10−8 m CaM solution for 2 h, or the strips were transferred to and incubated in 10−8 m CaM solution plus10 μm DPI or plus 100 units/mL CAT for 2 h, respectively. For investigating the effect of G protein on the stimulation of H2O2 production by ExtCaM, the strips with open stomata were transferred to and incubated in 400 ng/mL CTX solution plus 10 μm DPI or plus 100 units/mL CAT, respectively. Stomatal apertures were measured under microscope at indicated times with 50 randomly selected stomata. Each assay was repeated three times. The data were presented as mean ± se (n = 150).
Acknowledgments
The authors thank Drs. L.M. Fan and L. Miao for their helpful discussion and critical reading of the manuscript, Nottingham Arabidopsis Stock Center, and Dr. L.G. Ma for providing Arabidopsis seeds.
This work was supported by the Major State Basic Research Program of China (grant no. G1999011704) and by the National Science Foundation of China (grant no. 30370129).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.047837.
References
- Aharon GS, Snedden WA, Blumwald E (1998) Activation of a plant plasma membrane Ca2+ channel by TGα1, a heterotrimeric G protein α-subunit homologue. FEBS Lett 424: 17–21 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI (1999) Arabidopsis abi1-1 and abi1-2 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell 11: 1785–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM (1993) Signal transduction in guard cells. Annu Rev Cell Biol 9: 345–375 [DOI] [PubMed] [Google Scholar]
- Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296: 2026–2028 [DOI] [PubMed] [Google Scholar]
- Chandra S, Low PS (1995) Role of phosphorylation in elicitation of the oxidative burst in cultured soybean cells. Proc Natl Acad Sci USA 92: 4120–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Stennis M, Low PS (1997) Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J Biol Chem 272: 28274–28280 [DOI] [PubMed] [Google Scholar]
- Chen YL, Zhang XQ, Chen J, Wang XC (2003) Existence of extracellular CaM in abaxial epidermis of Vicia faba L. and its role in regulation of stomatal movements. Acta Bot Sin 45: 40–46 [Google Scholar]
- Coelho SM, Taylor AR, Ryan KP, Sousa-Pinto I, Brown MT, Brownlee C (2002) Spatiotemporal patterning of reactive oxygen production and Ca2+ wave propagation in Fucus rhizoid cells. Plant Cell 14: 2369–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottele V, Forestier C, Vavasseur A (1996) A reassessment of the intervention of calmodulin in the regulation of stomatal movement. Physiol Plant 98: 619–628 [Google Scholar]
- Coursol S, Fan LM, Stunff H, Spiegel S, Gilroy S, Assmann SM (2003) Sphingolipid signaling in Arabidopsis guard cells involves heterotrimeric G proteins. Nature 423: 651–654 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies J (2003) Free oxygen radicals regulate plasma membrane Ca2+ and K+ permeable channels in plant root cells. J Cell Sci 116: 81–88 [DOI] [PubMed] [Google Scholar]
- Fairley-Grenot K, Assmann SM (1991) Evidence for G-protein regulation of inward K+ channel current in guard-cells of fava-bean. Plant Cell 3: 1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberdhan NJ, Dawson RA, Freedlander E, Mac Neil SA (1993) Calmodulin-like protein as an extracellular mitogen for the keratinocyte. Br J Dermatol 129: 678–688 [DOI] [PubMed] [Google Scholar]
- Grabov A, Blatt MR (1998) Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc Natl Acad Sci USA 95: 4778–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DW, Hills A, Kohler B, Blatt MR (2000) Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc Natl Acad Sci USA 97: 4967–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tang R, Anderson LK, Woerner TE, Pei ZM (2003) A cell surface receptor mediates extracellular Ca2+ sensing in guard cells. Nature 425: 196–200 [DOI] [PubMed] [Google Scholar]
- Horn MA, Heinstein PF, Low PS (1989) Receptor-mediated endo-cytosis in plant cells. Plant Cell 1: 1003–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DS, Carson CW, Esmon CT (1997) Endothelial cells and extracellular inhibit monocyte tumor necrosis factor release and augment neutrophil elastase release. J Biol Chem 272: 11778–11785 [DOI] [PubMed] [Google Scholar]
- Jones AM, Assmann SM (2004) Plants: the latest model system for G-protein research. EMBO Rep 6: 572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C (1998) A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10: 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryshev YA, Naumov AP, Avdonin PV, Mozhayeva GN (1993) Evidence for involvement of a GTP-binding protein in activation of Ca2+ influx by epidermal growth factor in A431 cells: effects of fluoride and bacterial toxins. Cell Signal 5: 555–564 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom JL, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre L, Heinstein PF, Low PS (1992) Evidence for participation of GTP-binding proteins in elicitation of the rapid oxidative burst in cultured soybean cells. J Biol Chem 267: 20140–20147 [PubMed] [Google Scholar]
- Legendre L, Yueh YG, Crain R, Haddock N, Heinstein PF, Low PS (1993) Phospholipase C activation during elicitation of the oxidative burst in cultured plant cells. J Biol Chem 268: 24559–24563 [PubMed] [Google Scholar]
- Ma H, Yanofsky M, Meyerowitz EM (1990) Molecular cloning and characterization of GPA1, a G protein α subunit gene from Arabidopsis thaliana. Proc Natl Acad Sci USA 87: 3821–3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LG, Sun DY (1997) The effects of extracellular calmodulin on initiation of Hippeastrum rutilum pollen germination and tube growth. Planta 202: 336–340 [Google Scholar]
- Ma LG, Xu XD, Cui SJ, Sun DY (1999) The presence of a heterotrimeric G protein and its role in signal transduction of extracellular calmodulin in pollen germination and tube growth. Plant Cell 11: 1351–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Clayton H, Mansfield TA, Hetherington AM (1996) Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol 111: 1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Webb AAR, Taylor JE, Hetherington AM (1995) Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell 7: 1207–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder J (2001) Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Matsui M, Deng XW (2001) Overexpression of the heterotrimeric G-protein α-subunit enhances phytochrome-mediated inhibition of hypocotyls elongation in Arabidopsis. Plant Cell 13: 1639–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G, Johnson S, Ryan CA (1993) Structure-activity of deleted and substituted systemin, an 18-amino acid polypeptide inducer of plant defensive genes. J Biol Chem 268: 212–216 [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Purohit S, Lumar GP, Laloraya MM (1994) Involvement of superoxide radical in signal transduction regulation stomatal movements. Biochem Biophys Res Commun 205: 30–37 [DOI] [PubMed] [Google Scholar]
- Qin WM, Lan WZ, Yang X (2004) Involvement of NADPH oxidase in hydrogen peroxide accumulation by Aspergillus niger elicitor-induced Taxus chinensis cell cultures. Plant Physiol 161: 355–361 [DOI] [PubMed] [Google Scholar]
- Rudd JJ, Franklin-Tong VE (2001) Unravelling response-specificity in Ca2+ signalling pathways in plant cells. New Phytol 151: 7–33 [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell (Suppl) 14: S401–S417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hogouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Shang ZL, Ma LG, Wang XC, Sun DY (2001) Effect of extracellular calmodulin on the cytosolic Ca2+ concentration in Lily pollen grains. Acta Bot Sin 43: 12–17 [Google Scholar]
- Sun DY, Bian YQ, Zhao BH, Zhao LY, Yu XM, Duan SJ (1995) The effect of extracellular calmodulin on cell wall regeneration of protoplasts and cell division. Plant Cell Physiol 36: 133–138 [Google Scholar]
- Sun DY, Li HB, Cheng G (1994) Extracellular calmodulin accelerates the proliferation of suspension-cultured cells of Angelica dahurica. Plant Sci 99: 1–8 [Google Scholar]
- Trotochaud AE, Jeong S, Clark SE (2000) CLAVATA3, a multimeric ligand for the CLAVATA1 receptor-kinase. Science 289: 613–617 [DOI] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Wang S, Jones AM (2002) Role of G protein in regulation of Arabidopsis seed germination. Plant Physiol 129: 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Young J, Im KH, Sussman MR, Jones AM (2001) Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292: 2066–2069 [DOI] [PubMed] [Google Scholar]
- van Corven EJ, Hordijk PL, Medema RH, Bos JL, Moolenaar WH (1993) Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc Natl Acad Sci USA 90: 1257–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sande K, Pawlowski K, Czaja I, Wieneke U, Schell J, Schmidt J, Walden R, Matvienko M, Wellink J, van Kammen A, et al (1996) Modification of phytohormone response by a peptide encoded by ENOD40 of legumes and nonlegume. Science 273: 370–373 [DOI] [PubMed] [Google Scholar]
- Vetter SW, Leclerc E (2003) Novel aspects of calmodulin target recognition and activation. Eur J Biochem 270: 404–414 [DOI] [PubMed] [Google Scholar]
- Wang XQ, Ullah H, Jones AM, Assman SM (2001) G protein regulation of ion channels and abscisic acid signal in Arabidopsis guard cells. Science 292: 2070–2072 [DOI] [PubMed] [Google Scholar]
- Wu WH, Assmann SM (1994) A membrane-delimited pathway of G protein regulation of the guard-cell inward K+ channel. Proc Natl Acad Sci USA 91: 6310–6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Katinakis P, Hendriks P, Smolders A, deVries F, Spee J, van Kammen A, Bisseling T, Franssen H (1993) Characterization of Gm-ENOD40, a gene showing novel patterns of cell-specific expression during soybean nodule development. Plant J 3: 573–585 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126: 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]